Abstract
Fetal hypoxia is a common risk factor that has been associated with a range of CNS disorders including epilepsy, schizophrenia, and autism. Cellular and molecular mechanisms through which hypoxia may damage the developing brain are incompletely understood but are likely to involve disruption of the laminar organization of the cerebral cortex. Lysophosphatidic acid (LPA) is a bioactive lipid capable of cortical influences via one or more of six cognate G protein-coupled receptors, LPA1–6, several of which are enriched in fetal neural progenitor cells (NPCs). Here we report that fetal hypoxia induces cortical disruption via increased LPA1 signaling involving stereotyped effects on NPCs: N-cadherin disruption, displacement of mitotic NPCs, and impaired neuronal migration, as assessed both ex vivo and in vivo. Importantly, genetic removal or pharmacological inhibition of LPA1 prevented the occurrence of these hypoxia-induced phenomena. Hypoxia resulted in overactivation of LPA1 through selective inhibition of G protein-coupled receptor kinase 2 expression and activation of downstream pathways including Gαi and Ras-related C3 botulinum toxin substrate 1. These data identify stereotyped and selective hypoxia-induced cerebral cortical disruption requiring LPA1 signaling, inhibition of which can reduce or prevent disease-associated sequelae, and may take us closer to therapeutic treatment of fetal hypoxia-induced CNS disorders and possibly other forms of hypoxic injury.
Keywords: lysophospholipids, cortical development, sphingosine 1-phosphate, ischemia
During fetal development, the embryonic brain is susceptible to hypoxic insults (1) that can contribute to a range of neurological and psychiatric abnormalities including autism, schizophrenia, and epilepsy (2–4). These diseases or disorders are associated with cerebral cortical abnormalities in neuronal migration (5–7), which are thought to occur during the neurogenic period to produce disruption (cortical dysplasia) in the laminar organization of the cortex. Despite the identification of fetal hypoxia as a significant risk factor for these and other afflictions of the brain, mechanistic information is lacking on how hypoxia might contribute to these pathologies.
Lysophosphatidic acid (LPA) is a bioactive lysophospholipid that can influence a range of developmental processes within the embryonic cerebral cortex through the activation of one or more LPA receptors (8). As a family, these G protein-coupled lysophospholipid receptors couple to at least four distinct G protein subtypes (Gαq, Gαi, Gαs, and Gα12/13) that activate multiple downstream signaling pathways to influence myriad cellular physiologies (8). LPA receptor 1 (LPA1) gene expression is enriched in the ventricular zone (VZ), a neurogenic region of the embryonic cerebral cortex (9). Overactivation of LPA signaling rapidly displaces mitotic neural progenitor cells (NPCs) from their normal, apical location to more superficial locations as they undergo interkinetic nuclear migration, and these effects are prevented by genetic removal of LPA1,2 (10). Receptor-mediated LPA signaling has been shown to alter the neural expression of cell adhesion molecules such as N-cadherin or β-catenin (11, 12). Importantly, cortical dysplasia after birth has been reported in cell adhesion molecule knockout (N-cadherin, β-catenin) brains, which had shown a similar displacement of mitotic NPCs during fetal life (13, 14). In addition, LPA signaling has been shown to affect neuronal migration in vitro and in vivo (15).
A correlative thread among fetal hypoxia, LPA signaling, and cerebral cortical defects led to an examination of these elements in the early embryonic cortex. Using genetic approaches combined with LPA receptor-specific pharmacological tools, alterations to the embryonic cerebral cortex were assessed both ex vivo and in vivo under hypoxic conditions. Here we report the identification of cellular and molecular links between fetal hypoxia and receptor-mediated LPA signaling in the embryonic cerebral cortex.
Results
Hypoxia Induces Displacement of Mitotic Cells in the Embryonic Cortex via LPA1.
To identify possible relationships between hypoxia and receptor-mediated LPA mechanisms affecting the embryonic brain, an ex vivo method of culturing intact embryonic cerebral cortices (10) was used. This method maintains normal cortical architecture and neurogenic gradients up to 24 h in culture and allows a direct comparison of hemispheres from the same animal. Control explants were grown under normoxia (21% atmospheric oxygen), which allows normal development to occur in culture (10, 16). Cortices cultured under normoxia showed mitotic cells at the apical boundary (ventricular surface) of the cerebral wall that formed an expected band of mitotic-phase NPCs identified by phosphorylated histone-H3 immunolabeling, as observed in vivo on embryonic day 13.5 (E13.5) (Fig. S1A). Matching contralateral hemispheres were grown under hypoxia (1.8% oxygen or a partial pressure of 1.7 kPa) approximating conditions associated with hypoxic neuronal damage in culture (17). These hemispheres displayed superficially (basally) displaced mitotic NPCs (Fig. 1 A–C and Fig. S1B), with displacement commencing between 6 and 12 h of hypoxic exposure and maximal at 1.8% oxygen (Fig. S2). Hypoxia doubled the percentage of basally displaced NPCs (Fig. 1F). Coimmunolabeling with an NPC marker, phospho-vimentin (18), confirmed that these cells were displaced mitotic NPCs rather than another cell population undergoing abnormal mitosis (Fig. S3).
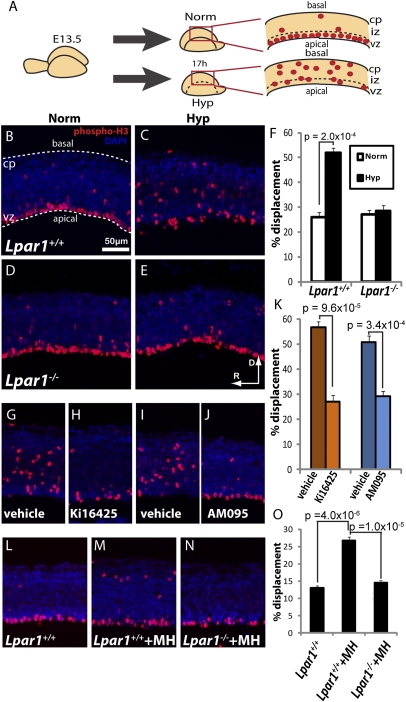
Fig. 1.
Hypoxia induces displacement of mitotic cells in the embryonic cortex via LPA1. (A) E13.5 cortices were cultured in normoxic (Norm) or hypoxic (Hyp) conditions. The red dots depict mitotic cells that were displaced basally upon hypoxic exposure. (B–E) Sagittal sections of cortices were immunolabeled with the mitotic cell marker phospho-histone H3 (phospho H3) and the nuclear stain DAPI. Wild-type (Lpar1+/+) cortices were exposed to normoxia (B) or hypoxia (C) (n = 8 matched pairs). Lpar1−/− cortices were exposed to normoxia (D) or hypoxia (E) (n = 9 matched pairs). Orientation marker indicates rostral (R) to dorsal (D) direction for all panels. (F) Quantification of the displaced mitotic cells. (G–J) Hypoxic cortices were treated with vehicle or the LPA1 antagonist Ki16425 (n = 7 matched pairs) (G and H) or AM095 (n = 11 matched pairs) (I and J). (K) Quantification of G–J. (L–N) Cortices were exposed to maternal hypoxia (MH) in utero. MH-exposed cortices (M) exhibited greater mitotic displacement than controls (L). This effect was absent in Lpar1−/− cortices (N). (O) Quantification of L–N. All statistics were performed on matched cortices using the paired t test (two-tailed). (Scale bar: 50 μm.) cp, cortical plate; vz, ventricular zone.
This hypoxic response was similar to that produced by overactivation of LPA signaling through LPA1 in the embryonic cerebral cortex (10), suggesting a possible mechanistic relationship. This possibility was assessed by hypoxic challenge of cortices from LPA receptor-null mouse mutants. Prior gene-expression studies of the embryonic cerebral cortex identified LPA1, LPA2, and LPA4 as the most highly expressed LPA receptors (19). Therefore, constitutive receptor-null mutants initially were screened, revealing a prominent effect in cortices from mice lacking LPA1 (Lpar1−/− mice). These cortices did not display the increased basal displacement of mitotic NPCs when subjected to hypoxia compared with littermate controls (Fig. 1 D–F), thus linking LPA1 signaling to hypoxic effects on NPC positioning.
It was possible that the constitutive nature of the Lpar1−/− mutant (20) could have produced artifactually altered cellular responses to hypoxia. To eliminate this possibility, wild-type cortices were exposed to two chemically distinct LPA1 antagonists (Fig. 1 G–K and Fig. S4 A and C) followed by hypoxic challenge. Ki16425 is an LPA1- and LPA3-selective antagonist (21), and its exposure to cortices significantly reduced the hypoxia-mediated displacement of mitotic NPCs. Nearly identical results were obtained using a different LPA1-specific antagonist, AM095 (22). Thus, pharmacological LPA1 inhibition phenocopied the results from genetic-deletion studies, supporting LPA1 signaling as an essential component of hypoxia-induced NPC displacement.
To confirm that these observations reflected processes that also could occur in vivo, E13.5 pregnant dams were subjected to 2 h of maternal hypoxia (MH) (9% O2) (23), which is known to produce a 40% reduction in the partial pressure of oxygen (PaO2) of embryonic blood (24) (Materials and Methods). This level of hypoxia represented a less severe insult than that used for ex vivo cultures but was necessary to limit maternal distress (23), and a significant, albeit reduced level of NPC displacement was observed with MH. This mitotic displacement was again absent in the Lpar1−/− cortices from MH-exposed embryos (Fig. 1 L–O). Importantly, this MH model was able to produce live births at a rate similar to controls, underscoring the physiological relevance of these results to existing neurodevelopmental disorders (Table S1).
Hypoxia Induces Altered NPC Position and Migration via LPA1.
Additionally, stereotyped disorganization of the cerebral wall was identified that affected NPCs and young neurons. First, newly postmitotic neurons were displaced neuroanatomically from their normal positions in the cortical plate (future gray matter) as identified by two different markers for postmitotic neurons [neuron-specific class III β-tubulin (Tuj1) and Doublecortin (DCX)] (25). Under hypoxia, these neurons were positioned aberrantly throughout the cerebral wall, consistent with migration defects, as manifested through the abnormal expression patterns revealed by Tuj1 or DCX immunolabeling (Fig. 2 A–D). Importantly, this disorganization was absent in the Lpar1−/− cortices, indicating a dependence on LPA1 (Fig. 2 G–J). Similar ectopic positioning of these cells also was observed in cortices exposed to MH but again was absent in Lpar1−/− cortices, supporting the physiological significance of these results both ex vivo and in vivo (Fig. S5).
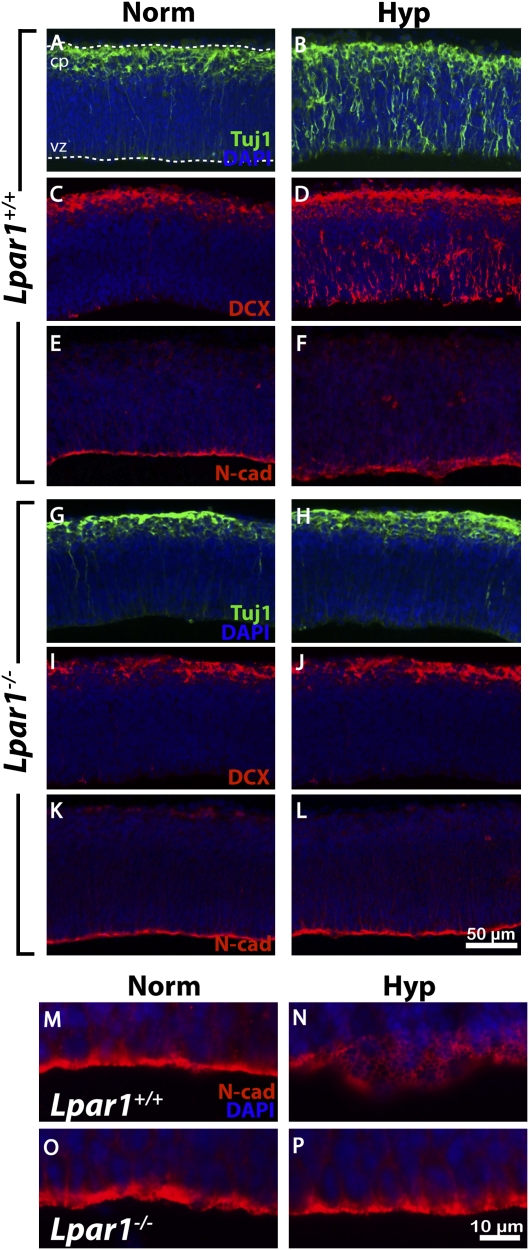
Fig. 2.
Hypoxia induces cortical disorganization via LPA1. (A–L) Sagittal sections of Lpar1+/+ or Lpar1−/− ex vivo cortices subjected to normoxia or hypoxia were immunolabeled for Tuj1 (A and B, G and H; n = 5 and 7 matched pairs, respectively), DCX (C and D, I and J; n = 3 matched pairs in each group), and N-cadherin (E and F, K and L; n = 15 and 5 matched pairs, respectively). (M–P) High-magnification images of N-cadherin immunolabeling in Lpar1+/+ (M and N) or Lpar1−/− (O and P) cortices cultured under normoxic or hypoxic conditions. (Scale bar: 50 μm in A–L, 10 μm in M–P.)
Second, the distribution of the cell adhesion molecule N-cadherin also was disorganized following hypoxia. N-cadherin is expressed on apical NPCs (14), and LPA signaling can alter N-cadherin in neural cells (11). Notably, NPC displacement also was reported in N-cadherin neural-specific null mutants (14). In control wild-type cortices, N-cadherin immunolabeling appeared as a prominent apical band along the ventricular surface (Fig. 2 E and M). Under hypoxia, this N-cadherin band appeared diffuse, forming a honeycomb pattern indicating disruption of normal N-cadherin organization (Fig. 2 F and N). These changes were reduced or absent following hypoxic challenge in LPA1-null cortices or cortices treated with LPA1 antagonists (Fig. 2 K, L, O, and P and Figs. S4B and S6), again identifying LPA1-dependent effects associated with hypoxia.
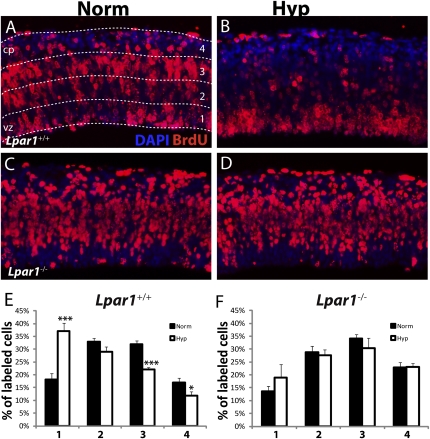
To investigate hypoxic effects on cell migration cortices were pulsed briefly with BrdU to label a subset of NPCs actively undergoing DNA synthesis before normoxia or hypoxia and then were assessed after 17 h in culture. These analyses revealed fewer cells reached their normal postmitotic locations within the cortical plate following hypoxia (Fig. 3 A, B, and E), which is consistent with defects in neuronal migration. This hypoxic effect was absent in the Lpar1−/− cortices, consistent with a dependence on LPA1 (Fig. 3 C, D, and F).
Fig. 3.
Hypoxia induces altered migration of neural cells via LPA1 signaling. (A–D) Matched wild-type (Lpar1+/+) or Lpar1−/− cortices were pulsed with BrdU 1 h before exposure to normoxia or hypoxia for 17 h and subsequently were immunolabeled for BrdU antibody and counterstained with DAPI. (E and F) Quantification of A–D (n = 9 and 6 matched pairs for wild-type and Lpar1-null cortices, respectively). Each cortical section was divided into four equal zones shown in A (1 approximating the VZ, 2 and 3 approximating the subventricular zone, and 4 approximating the intermediate zone/cortical plate), and the percentage of cells in each region was calculated. *P < 0.05; ***P < 0.001.
Hypoxia Selectively Activates LPA1 Downstream Signaling Pathways by Potentiating LPA1 Activity.
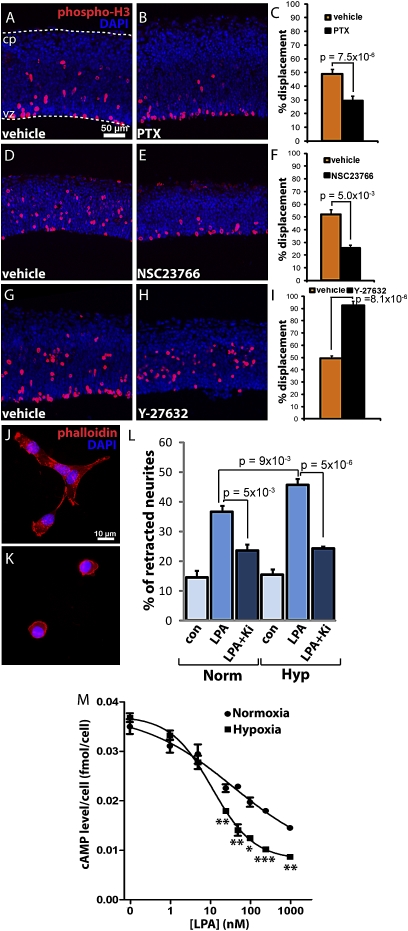
To evaluate the downstream signaling pathways of LPA1-mediated hypoxic effects, pharmacological blockade of known LPA1 downstream effectors was assessed. LPA1 activates G proteins known to influence cell migration, including Gαi, which can be blocked by pertussis toxin (PTX) (26), which in turn can activate the small GTPase Ras-related C3 botulinum toxin substrate 1(Rac1), which can be inhibited by NSC23766 (27). LPA1 also activates Gα12/13 which, in addition to activating Rac1 (28), also can activate the Ras homolog gene family, member A (RhoA) (29), which can be inhibited by the Rho kinase inhibitor Y-27632 (30). These inhibitors were applied to ex vivo cortices under hypoxic conditions; both PTX and NSC23766 prevented NPC displacement as well as disruption of N-cadherin organization, as is consistent with LPA1 activation of Gαi and Rac1, respectively (Fig. 4 A–F and Figs. S4 and S6). In contrast, Y-27632 exacerbated NPC displacement and N-cadherin disruption during hypoxia (Fig. 4 G–I and Figs. S4 and S6), an effect that possibly involves the known antagonistic relationship between RhoA and Rac1 (31) and underscoring the downstream signaling pathway selectivity of the LPA1-hypoxia response. Overall, these data support preferential overactivation of LPA receptor pathways that include Gαi and Rac1 in mediating the effects of hypoxia.
Fig. 4.
Hypoxia activates LPA1 signaling pathways by potentiating LPA1 activity. The Gαi inhibitor PTX (A–C) and the Rac1 inhibitor NSC23766 (D–F) inhibited hypoxia-induced mitotic displacement, whereas treatment with a Rho-associated kinase inhibitor, Y-27632, (G–I) increased displacement of mitotic cells (n = 10, 6, and 7 matched pairs, respectively) compared with cortices treated with vehicle. (J and K) Representative images of LPA-induced neurite retraction. (L) LPA-induced retraction of TSM1 neurites can be blocked partially by inhibiting LPA1 with Ki16425. Other Ki16425-insensitive LPA receptors are expressed by TSM1 cells. This retraction is potentiated by hypoxia and is Ki16425 sensitive (LPA1 specific). (M) cAMP inhibition assay. B103 cells stably expressing LPA1 were treated with increasing LPA concentrations in hypoxia, showing a maximal inhibition at 250 nM (∼50% over normoxia). cAMP levels are normalized to cell number. *P < 0.05; **P < 0.01; ***P < 0.001. (Scale bar: 50 μm in A, 10 μm in J.)
The observed hypoxic effects on NPCs that required LPA1 were consistent with receptor potentiation, resulting in an overactivation of LPA signaling. To examine this possibility, LPA1 activity under hypoxic conditions was assessed by two independent, quantitative assays (Fig. 4 J–M). First, a well-defined LPA response—neurite retraction—was quantified via LPA dose-response under normoxia in a neuronal cell line (TSM1) that endogenously expresses LPA1 (32) (Fig. 4 J–L). This response under normoxia was compared with LPA exposure under hypoxia, which resulted in a 30% increase in neurite retraction that was inhibited by Ki16425, suggesting that hypoxia potentiates LPA1 activity during LPA exposure. Second, heterologous expression of LPA1 in LPA-unresponsive B103 neuroblastoma cells (Fig. S7A) (33) was used to assess Gαi activation through the inhibition of adenylate cyclase and subsequent cAMP production. B103 cells (33) stably expressing LPA1 showed a dose-dependent inhibition of cAMP when exposed to LPA. Under hypoxia, a more potent inhibition of cAMP production was observed at each relevant LPA concentration, reaching a maximum of ∼50% more inhibition relative to normoxia at 250 nM LPA (Fig. 4M). These results support cell-autonomous potentiation of LPA1 by hypoxia.
Hypoxia Potentiates LPA1 Activity by Inhibiting Expression of G Protein-Coupled Receptor Kinase 2.
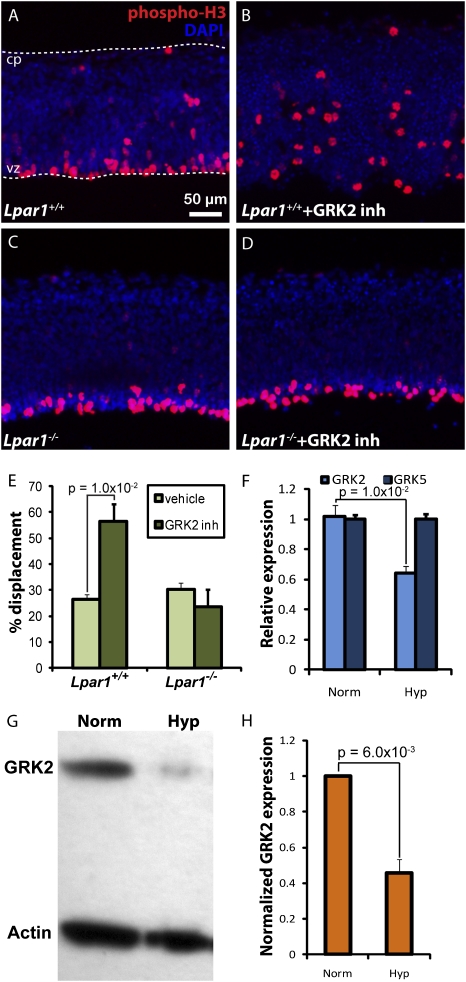
The potentiation of LPA1 signaling by hypoxia could occur through several mechanisms, including increased availability of ligand, increased receptor expression, or altered activity of existing receptors. Unexpectedly, there was no change either in gene expression for LPA1 or in the major LPA-producing enzyme, autotaxin, in hypoxic cortices (Fig. S7B). These results suggested the operation of other receptor mechanisms through which hypoxia may modulate LPA1 activity. One identified mechanism involves inhibition of LPA1 by G protein-coupled receptor kinase 2 (GRK2), implying that inhibition of GRK2 might increase LPA1 signaling (34). To examine this possibility, a GRK2-specific inhibitor (methyl[(5-nitro-2-furyl)vinyl]-2-furoate) was used in ex vivo cortical cultures under normoxic conditions, which resulted in mitotic NPC displacement that also had been observed under hypoxic conditions (Fig. 5 A, B, and E). Heparin, a nonspecific but robust GRK2 inhibitor (35), similarly induced mitotic displacement in the absence of hypoxia (Fig. S8), providing further support that GRK2 inhibition potentiates LPA1 activity. Critically, no significant basal displacement of the mitotic NPCs was observed in LPA1-null mice despite inhibitor exposure (Fig. 5 C–E and Fig. S8). GRK2 also was evaluated by quantitative RT-PCR (qRT-PCR) and Western blot. Hypoxia specifically reduced transcript levels of GRK2 but not GRK5, another major member of the GRK family, consistent with selective GRK2 reduction (Fig. 5F). GRK2 protein levels were reduced significantly upon hypoxia as well (Fig. 5 G and H). These data support transcriptional and translational GRK2 inhibition by hypoxia as a mechanism for LPA1 potentiation.
Fig. 5.
Hypoxia potentiates LPA1 activity by inhibiting GRK2 expression. (A–D) GRK2 inhibitor-treated cortices (B) show an increase in mitotic displacement compared with control hemispheres (A) (n = 15 matched pairs). This effect is absent in Lpar1−/− cortices (C and D) (n = 9 matched pairs). (E) Quantification of A–D. (F) qRT-PCR quantification of GRK2 and GRK5 transcript levels in ex vivo cortices under normoxia or hypoxia (n = 6). (G) Representative Western blot of GRK2 protein in normoxic and hypoxic cortices and (H) corresponding quantitative analysis (n = 4). (Scale bar: 50 μm.)
Prior hypoxia studies in other systems identified transcriptional alterations mediated by hypoxia-inducible factor 1α (HIF-1α) (36), a major downstream effector of hypoxia. To assess the involvement of HIF-1α in our system, we used two potent albeit nonspecific inhibitors of HIF-1α (37, 38). Hypoxic cortices exhibited a significant reduction in the displacement of mitotic NPCs upon treatment with both inhibitors (Fig. S9 A–E). Interestingly, GRK2 expression remained unchanged upon HIF-1α inhibition, suggesting that HIF-1α is downstream of GRK2 inhibition (Fig. S9 F and G).
Discussion
This study identifies LPA signaling as an essential mediator of fetal brain hypoxia, providing functional linkage between lipid signaling and oxygen levels. The hypoxia-induced changes were prevented both by genetic removal of LPA1 and by pharmacological antagonism using two chemically distinct LPA1 antagonists, supporting the direct involvement of LPA1 in the observed cortical effects induced by hypoxia. These pharmacological results also eliminated developmental artifacts hypothetically produced by constitutive deletion of LPA1. Notably, the LPA receptor-dependent hypoxic effects showed selectivity through both proximal pathways (GRK2 vs. GRK5), which appeared to be upstream of HIF-1α and downstream pathways (Gαi and Rac1). Combined with the observed stereotyped cellular responses and their dependence on LPA signaling, these results identified a finite and molecularly accessible set of interactions induced by fetal hypoxia, contrasting with an alternative scenario of nonspecific hypoxic damage.
The stereotyped changes affecting NPCs reported here are similar to defects known to disrupt the normal laminar structure of the cerebral cortex that involve the loss of N-cadherin and β-catenin (13, 14). Phenotypes common to hypoxic challenge and these null mutants include cell adhesion disruption, altered positions of mitotic NPCs, and impaired cell migration. These similarities support the existence of other cellular and molecular changes initiated by fetal hypoxia that could depend on LPA signaling.
The dependence of stereotyped and selective hypoxic changes on LPA signaling, combined with previously reported associations between fetal hypoxia and various CNS disorders (2–4), underscores the therapeutic implications of this study. Targeting LPA receptors by subtype-selective agents and/or their selectively activated downstream pathways may provide ways for interrupting or preventing deleterious sequelae of fetal hypoxia. The feasibility of targeting lysophospholipid receptors such as LPA1 is supported by the recent approval by the Food and Drug Administration of a brain-penetrant medicine (fingolimod) that targets related lysophospholipid receptor family members (39). The relevance of fetal hypoxia–LPA signaling to other forms of hypoxic insult such as stroke suggests that in the future other disease processes could be addressed therapeutically by modulation of lysophospholipid signaling.
Materials and Methods
Cortical Hemisphere Cultures.
Animal protocols were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute and conform to National Institutes of Health guidelines and public law. Ex vivo cortical cultures at E13.5 were performed as described previously (10, 40). Embryos from Lpar1+/− females were genotyped by PCR. Brains of embryos were dissected in serum-free medium: Opti-MEM I (Gibco/BRL) containing 20 mM d-glucose, 55 μM β-mercaptoethanol, and 1% penicillin-streptomycin. The cortical hemispheres of each brain were separated along the midline. One hemisphere was cultured under hypoxia (1.8% O2 or 1.7 kPa) and the other in control medium in 21% O2 in a humidified 5% CO2 chamber as used routinely for explant cultures (16, 41). Hypoxia was achieved in a hypoxia chamber with an attached oxygen sensor (Biospherix), which was calibrated before each experiment. Hemispheres were cultured at 37 °C for 17 h, with shaking at 65 rpm. After 17 h of culture, matched hemispheres were fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.4), cryoprotected, embedded in Tissue-Tek (Sakura), and rapidly frozen on dry ice. Tissue was cut coronally at 20-μm slices on a cryostat and was mounted onto Superfrost Plus slides (Fisher Scientific).
Treatment of Cortical Hemisphere Cultures.
AM095 (Amira Pharmaceuticals, Inc.), an LPA1-specific antagonist (22), was added to the cultures at a final concentration of 1 μM. Ki16425, an LPA1- and LPA3-specific antagonist (Kirin Brewing Co./DebioPharm Group) (21), was added to the cultures at a final concentration of 10 μM 15 min before incubation. NSC23766, a Rac1 inhibitor, was used at a final concentration of 100 μM and was added 2 h before treatment. Y-27632 (Sigma) was added at a concentration of 30 μM 15 min before incubation. PTX (List Biological Laboratories) was used at a final concentration of 100 ng/mL and was added 6 h before hypoxia treatment. A GRK2-specific inhibitor (Calbiochem) and heparin were used at a concentration of 1 mM and 1 μM, respectively.
Immunohistochemistry.
The antibodies used were rabbit anti–phospho-H3 (Upstate Biotechnology), mouse Tuj1 (Covance), and mouse-BrdU (Roche). Primary antibodies were detected with AF568-conjugated donkey anti-rabbit antibody (Invitrogen) and AF488-conjugated anti–mouse antibody (BD Biosciences). Tissue was processed as described previously (10).
Quantification of Mitotic Displacement.
Ventricular mitotic cells were defined as phospho-histone3–positive cells within 5 μm of the ventricular surface. The percentages of displaced and nondisplaced cells then were quantified using ImageJ software. Two-tailed paired t tests were used for all statistical calculations.
BrdU Labeling.
E13.5 timed pregnant BALB/c mice were injected i.p. with BrdU reagent (Invitrogen) (1 mL/100 g body weight) and were killed after 1 h. The brains of embryos then were prepared for cortical ex vivo cultures.
Western Blot.
Cortices were washed in ice-cold 1× PBS before the addition of ice-cold lysis buffer [1× radioimmunoprecipitation assay buffer, complete protease inhibitor mixture (Roche Diagnostics), sodium fluoride, sodium orthovanadate] for 15 min at 4°C on a rotator. The lysate then was centrifuged at 14,000 × g for 15 min and was transferred to a new tube. Then 30 μg of total lysate protein was separated on a 4–12% SDS/PAGE gel, transferred, and blocked overnight. The blot then was incubated with rabbit anti-GRK2 (Santa Cruz Biotechnology, Inc.) diluted 1:200, secondary HRP-conjugated donkey anti-rabbit IgG diluted 1:10,000, and subsequently were visualized using the West Femto kit (Thermo Scientific.).
cAMP Assay.
Cell lines overexpressing HA- LPA1 were generated by transfecting B103 cells with linearized HA-tagged LPA1-pcDNA3.1 (Invitrogen) using Effectene transfection reagent (Qiagen). Stable transfectants were selected using 1 mg/mL Geneticin (Invitrogen) and were clonally expanded. Cells were seeded at 100,000 cells per well and were serum starved overnight and treated with 5 μM forskolin, 0.5 μM 3-isobutyl-1-methylxanthine, and increasing concentrations of LPA. cAMP content was determined according to the protocol supplied by the cAMP ELISA kit (Cayman Chemical). To abate any effects of cell death, the number of viable cells per well was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay of identically treated replicate plates (see below) to allow calculation of cAMP per cell. EC50 values were calculated using the Prism 4.0 program.
Neurite Retraction Assay.
TSM1 cells were seeded at 20,000 cells per well and were serum starved overnight. They then were exposed either to normoxia or hypoxia for 6 h before the addition of 100 nM LPA. After 30 min, the cells were fixed and stained with phalloidin and DAPI for cell morphology. The number of cells with retracted neurites and the number of total cells were counted in three separate fields for each sample, and the percentage of cells with retracted neurites was calculated.
MTT Assay.
To eliminate any effects of cell death on the cAMP assay, a MTT assay was carried out to measure the number of viable cells. B103 cells overexpressing HA-LPA1 were seeded at 100,000 cells per well and after 4 h were serum starved overnight. MTT reagent (1 mg/mL) was added to each well and incubated for 1.5 h, which after the reagent was aspirated, and MTT solvent was added. After 15 min of agitation on a rotator, the absorbance was read on a plate reader at a wavelength of 590 nm. The number of viable cells then was calculated using a standard curve.
Maternal Hypoxia.
E13.5 embryos were exposed to hypoxia in vivo using a modification of a previously described protocol (42). Pregnant C57BL/6J or Balb/cByJ female mice (E13.5) were put in a hypoxia chamber at 9% oxygen for 2 h and then were returned to atmospheric air. After 17 h, the cortices of the embryos were fixed in 4% paraformaldehyde.
The PaO2 in ovine embryos is known to drop from 3.4 kPa to ∼2 kPa when the pregnant dam is subject to 9% O2 (24). Thus, this model of maternal hypoxia is effective in inflicting a hypoxic insult to the embryo. Because the amount of oxygen that actually is delivered to the embryo depends not only on the PaO2 of blood but also on additional physiological factors such as arterial flow rate and hemoglobin saturation (43), it is not possible to compare the level of hypoxia obtained in vivo directly with that generated by our ex vivo model. However, the in vivo hypoxic insult is likely to be much milder in extent and duration than our ex vivo system, in which the cortices are subject to a lower oxygen pressure (1.7 kPa) for 17 h.
Note Added in Proof.
Pathophysiological relevance for LPA receptor overactivation has been recently reported (44).
Supplementary Material
Acknowledgments
We thank Dr. P. Prasit and Amira for the gift of AM095, the Kirin Brewery Co. for the gift of Ki16425, and D. Letourneau for editorial assistance. This work was supported by National Institutes of Health Grants MH051699, NS048478, and HD050685 (to J.C.) and by the Agency of Science, Technology and Research, Singapore (K.J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106129108/-/DCSupplemental.
References
- 1.Arbeille P, Maulik D, Laurini R. Fetal hypoxia. New York: Parthenon Pub. Group; 1999. [Google Scholar]
- 2.Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: A review and integration of findings. Arch Pediatr Adolesc Med. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- 3.Bergamasco B, Benna P, Ferrero P, Gavinelli R. Neonatal hypoxia and epileptic risk: A clinical prospective study. Epilepsia. 1984;25:131–136. doi: 10.1111/j.1528-1157.1984.tb04168.x. [DOI] [PubMed] [Google Scholar]
- 4.Cannon TD, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleeson JG, Walsh CA. Neuronal migration disorders: From genetic diseases to developmental mechanisms. Trends Neurosci. 2000;23:352–359. doi: 10.1016/s0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- 7.Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Choi JW, et al. LPA receptors: Subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 9.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- 11.Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci. 2001;21:7069–7078. doi: 10.1523/JNEUROSCI.21-18-07069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: Signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 13.Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki M, et al. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima N, et al. Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol Cell Neurosci. 2002;20:271–282. doi: 10.1006/mcne.2002.1123. [DOI] [PubMed] [Google Scholar]
- 16.Bultje RS, et al. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashidian J, et al. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:14080–14085. doi: 10.1073/pnas.0500099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–561. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubin AE, Herr DR, Chun J. Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J Neurosci. 2010;30:7300–7309. doi: 10.1523/JNEUROSCI.6151-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta H, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 22.Swaney JS, et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010;160:1699–1713. doi: 10.1111/j.1476-5381.2010.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallak M, Hotra JW, Kupsky WJ. Magnesium sulfate protection of fetal rat brain from severe maternal hypoxia. Obstet Gynecol. 2000;96:124–128. doi: 10.1016/s0029-7844(00)00844-9. [DOI] [PubMed] [Google Scholar]
- 24.Richardson B, et al. Regional blood flow and the endocrine response to sustained hypoxemia in the preterm ovine fetus. Pediatr Res. 1996;40:337–343. doi: 10.1203/00006450-199608000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Francis F, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 26.Hsia JA, Moss J, Hewlett EL, Vaughan M. ADP-ribosylation of adenylate cyclase by pertussis toxin. Effects on inhibitory agonist binding. J Biol Chem. 1984;259:1086–1090. [PubMed] [Google Scholar]
- 27.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kjoller L, Hall A. Signaling to Rho GTPases. Exp Cell Res. 1999;253:166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- 29.Kranenburg O, et al. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: Induction of neurite retraction. Mol Biol Cell. 1999;10:1851–1857. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uehata M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 31.Norman JC, Price LS, Ridley AJ, Koffer A. The small GTP-binding proteins, Rac and Rho, regulate cytoskeletal organization and exocytosis in mast cells by parallel pathways. Mol Biol Cell. 1996;7:1429–1442. doi: 10.1091/mbc.7.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun J, Jaenisch R. Clonal cell lines produced by infection of neocortical neuroblasts using multiple oncogenes transduced by retroviruses. Mol Cell Neurosci. 1996;7:304–321. doi: 10.1006/mcne.1996.0023. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima N, Kimura Y, Chun J. A single receptor encoded by vzg-1/lpA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc Natl Acad Sci USA. 1998;95:6151–6156. doi: 10.1073/pnas.95.11.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aziziyeh AI, et al. Dual regulation of lysophosphatidic acid (LPA1) receptor signalling by Ral and GRK. Cell Signal. 2009;21:1207–1217. doi: 10.1016/j.cellsig.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Benovic JL, Stone WC, Caron MG, Lefkowitz RJ. Inhibition of the beta-adrenergic receptor kinase by polyanions. J Biol Chem. 1989;264:6707–6710. [PubMed] [Google Scholar]
- 36.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehen SK, et al. A new method of embryonic culture for assessing global changes in brain organization. J Neurosci Methods. 2006;158:100–108. doi: 10.1016/j.jneumeth.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Dammerman RS, Noctor SC, Kriegstein AR. Extrinsic GABAergic innervation of developing neocortical layer 1 in organotypic slice co-cultures. J Comp Neurol. 2000;423:112–120. doi: 10.1002/1096-9861(20000717)423:1<112::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 42.Golan MH, et al. Impaired migration signaling in the hippocampus following prenatal hypoxia. Neuropharmacology. 2009;57:511–522. doi: 10.1016/j.neuropharm.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 43.Meschia G. Evolution of thinking in fetal respiratory physiology. Am J Obstet Gynecol. 1978;132:806–813. doi: 10.1016/s0002-9378(78)80015-5. [DOI] [PubMed] [Google Scholar]
- 44.Yung YC, et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Science Translat Med. 2011 doi: 10.1126/scitranslmed.3002095. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.