Abstract
β1 integrin tyrosine phosphorylation by oncogenic kinases, such as Src, has been predicted to induce tumorigenesis by disrupting adhesion and modifying integrin signaling. We directly tested this hypothesis by subjecting mice with “nonphosphorylatable” tyrosine-to-phenylalanine substitutions in the conserved β1 cytoplasmic tail NPxY motifs to a model of cutaneous carcinogenesis in the presence or absence of elevated Src activity. We found that hydrophobic phenylalanine substitutions of both tyrosines diminished the binding of tail-interacting proteins, including talins and kindlins, resulting in reduced β1-mediated adhesion, focal adhesion kinase (FAK) signaling, and epidermal progenitor cell–derived skin tumors. However, increased Src activity drove tumor formation independent of the phenylalanine substitutions by enhancing FAK activity, which in turn maintained the epidermal progenitor state and blocked keratinocyte differentiation. We conclude that a Src/FAK signaling unit inhibits differentiation to promote tumorigenesis downstream of β1 integrin and independent of β1 integrin tyrosine phosphorylation.
Keywords: adhesion receptor, epidermis, cancer, signal transduction
Integrins are heterodimeric transmembrane cell surface adhesion receptors consisting of α and β subunits (1). The β1 integrins, which represent the largest integrin subfamily, promote fundamental cellular processes such as migration, proliferation, differentiation, and survival and are indispensable for development and tissue homeostasis. Tumor cells also express β1 integrins (2); however, their contribution to tumor growth in the presence of high oncogenic tyrosine kinase activity is poorly understood.
Shortly after the discovery of the β1 integrin subunit, fibroblasts transformed by the avian oncogenes v-Src, v-Fps, v-ErbB, and v-Yes were shown to contain phosphorylated tyrosines in the β1 cytoplasmic tail (3). The β1 tail contains two tyrosine residues (Y783 and Y795) that are part of conserved NPxY motifs essential for recruiting talin and kindlin. These in turn facilitate β1 integrin coupling to the actin cytoskeleton and maintain integrins in an active signaling state (4). v-Src was shown to phosphorylate β1 integrin tails on Y783 and Y795 (5–7). Based mainly on structural and biochemical studies, it is thought that phosphorylated Y783 and Y795 block talin and kindlin binding, respectively (5, 8–10). In support of these studies, it has been demonstrated that v-Src expression in fibroblasts decreases β1 integrin–dependent adhesion, focal adhesion formation, cytoskeletal organization, fibronectin assembly, migration, and chemotaxis (7, 11). Moreover, expression of mutant β1 and β3 integrin in which the tyrosine of the membrane-proximal NPxY motif was substituted with a “nonphosphorylatable” phenylalanine rescued the inhibitory effects of v-Src on integrin function (7, 12). These findings support a model in which β1 integrin inactivation through phosphorylation (9) promotes transformation and anchorage-independent growth (7, 13).
This model has several inconsistencies, however. First, levels of β1 tyrosine phosphorylation are extremely low in nontransformed cells (6) and are difficult to detect even in the presence of v-Src (3, 7). Thus, the significance of phosphorylated cell surface β1 is unclear. Second, substitutions of β1 and β3 integrin tail tyrosines with nonphosphorylatable phenylalanines have been shown to inhibit rather than increase integrin function in the absence of v-Src in vitro (14–16). Third, in sharp contrast to the proposed v-Src–mediated phosphorylation and inactivation of β1 integrins, the growth of cutaneous or mammary gland tumors requires β1 expression and function (17–19).
To determine the role of β1 cytoplasmic tyrosines in vivo, we and others have generated mice harboring Y783F-, Y795F-, or YY783/795FF (YYFF)-substituted β1 integrins (20, 21). Such mice develop and age normally and thus allow testing of whether β1Y-to-F substitutions interfere with tumor formation in the presence or absence of increased Src activity. The epidermis was chosen as a model system because epidermal cells lack β3, have low levels of αv, and predominantly express β1 integrins. Our experiments revealed that phenylalanine substitutions in both, but not in either one, of the β1 NPxY motifs diminish tumorigenesis when Src activity is low, but play no role when Src activity is high.
Results
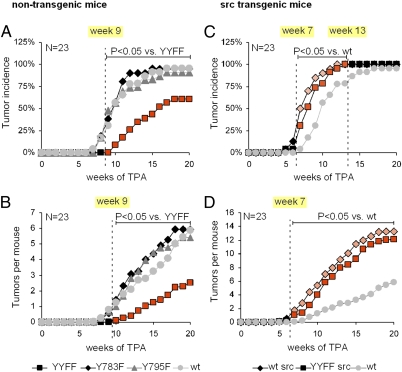
For this study, we crossed mice harboring nonphosphorylatable tyrosine-to-phenylalanine knock-in substitutions at position Y783 and/or Y795 (Y783F, Y795F, and YYFF) of the β1 integrin cytoplasmic domain (21) with a mouse strain carrying a human c-Src transgene controlled by the K5 promotor (22). WT and β1YYFF mice with and without the Src transgene developed normally and showed normal skin morphogenesis and homeostasis irrespective of a 20-fold increased Src protein level, a fourfold increased Src activity, and elevated levels of β1 integrin tyrosine phosphorylation (Fig. S1). We next made use of the two-stage carcinogenesis model involving 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol-13-acetate (TPA) to test the effects of β1Y-to-F substitutions on tumor development in the presence or absence of the Src transgene. In the absence of the Src transgene, β1YYFF mice developed fewer tumors and developed them later than WT animals (Fig. 1 A and B). Single β1Y783F and β1Y795F substitutions showed no difference from WT, however (Fig. 1 A and B). An increase in Src activity through the Src transgene increased tumor incidence and burden to the same extent in WT and β1YYFF mice (Fig. 1 C and D), indicating that Src promotes tumorigenesis independent of β1 phosphorylation.
Fig. 1.
Two-stage carcinogenesis. (A and B) Tumor incidence and burden of nontransgenic animals relative to the start of promotion with TPA. (C and D) The same data depicted for Src transgenic animals. The cohort size was 23 animals per group.
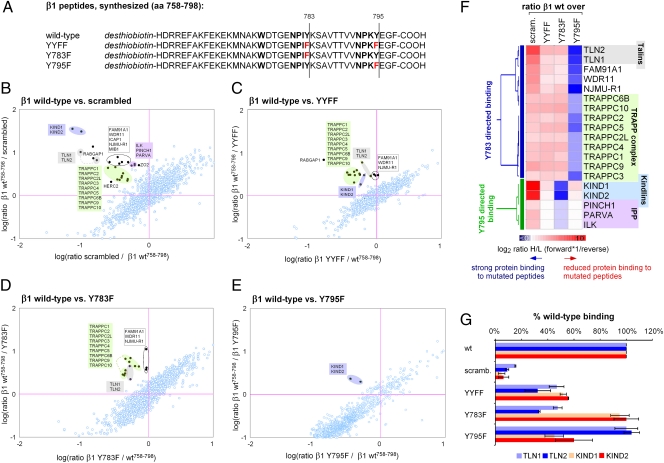
Our results suggest that Y-to-F substitutions interfere with β1 function primarily by affecting the recruitment of β1 tail-binding proteins. To test this hypothesis, we used SILAC-based quantitative proteomics (Fig. S2) and compared the binding affinities of full-length WT and β1Y-to-F cytoplasmic tail peptides (Fig. 2A). To identify nonspecific background binding, we subtracted binding to a scrambled peptide from the pool of proteins pulled down with the WT peptide. A total of 24 proteins, including talins, kindlins and the ILK/PINCH/parvin complex, were found to specifically interact with the WT β1 tail peptide (Fig. 2B). We next compared the binding of specific β1 tail interactors to YYFF-, Y783F-, and Y795F-substituted β1 peptides (Fig. 2 C–E). By performing agglomerative hierarchical clustering, we identified proteins with reduced binding to β1Y783F (β1Y783-directed binding) or to β1Y795F (β1Y795-directed binding) (Fig. 2F). Although binding of the ILK/PINCH/parvin complex was minimally affected by β1Y-to-F substitutions, binding of talins to the β1Y783F mutation and of kindlins to the β1Y795F mutation was reduced by ∼50% compared with WT peptides (Fig. 2G). This is in contrast to β1Y783/Y795A mutations, which almost completely blocked the binding of talins and kindlins, respectively (23).
Fig. 2.
SILAC-based peptide pull-downs. (A) Amino acid sequence of desthiobiotinylated synthesized β1 peptides. (B) The interactome of the β1 WT peptide compared with a scrambled peptide. A total of 24 specific β1 peptide interactors were identified. (C–E) Differential binding of WT peptide vs. YYFF (C), Y783F (D), or Y795F (E). (F) Clustering of β1 tail-interacting proteins to either one of the β1 NPxY motifs based on Y-to-F substituted peptide pull-downs. (G) Y-to-F mutations reduce, but do not abolish, binding of talins and kindlins to the β1 tail.
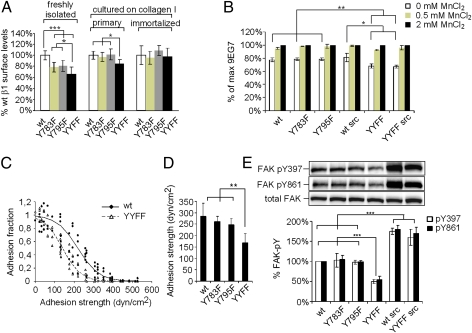
Whereas the β1Y783A and β1Y795A mutations are embryonic lethal (manuscript in preparation), mice harboring β1Y-to-F substitutions are viable and fertile, suggesting that the reduced protein binding to the latter are functionally irrelevant for normal development and physiology. To test whether β1Y-to-F substitutions interfere with β1 function in a cellular context, we performed functional assays with keratinocytes. Freshly isolated β1Y-to-F keratinocytes exhibited a 20–30% reduction in β1 integrin surface levels compared to WT, but reached WT levels after culturing on collagen for 1 wk or when spontaneous immortalization occurred (Fig. 3A). We used keratinocytes with comparable β1 integrin surface levels to quantify the activation epitope 9EG7 (24). We found reduced binding of 9EG7 antibody to keratinocytes carrying a double, but not a single, β1Y-to-F substitution (Fig. 3B). Levels of 9EG7 epitope were unaffected by increased Src activity. The reduced integrin activity conferred by the β1YYFF mutation could be rescued with MnCl2 (Fig. 3B), which shifts integrins into the high-affinity state independent of intracellular cues.
Fig. 3.
Effects of β1Y-to-F substitutions on adhesion and signaling. (A) Whereas β1 surface levels were reduced in β1Y-to-F freshly isolated keratinocytes, they became similar over time in cultured cells propagated on collagen I. (B) Quantification of 9EG7 activation epitope by FACS. (C) Spinning-disk hydrodynamic shear stress assay measuring adhesion strength. A cell detachment profile of β1YYFF and WT keratinocytes is shown as a function of applied shear stress. (D) Mean (50%) ± SD cell detachment force of four independent experiments. (E) FAK-pY397 and FAK-pY861 were assessed in keratinocytes grown on collagen-coated plastic. Results were quantified by densitometry and normalized to total FAK levels. ***P < 0.001; **P < 0.01; *P < 0.05.
We next assessed keratinocyte adhesion by performing a spinning-disk hydrodynamic shear stress assay (Fig. 3C) (25). The adhesion strength of double, but not single, β1Y-to-F keratinocytes was reduced by >40% (Fig. 3D), indicating decreased functional adhesive bonds as a consequence of the double-β1YYFF substitution. In line with these findings, we observed reduced phosphorylation of the β1 integrin effector focal adhesion kinase (FAK) at Y397 and Y861 in double, but not single, β1Y-to-F keratinocytes grown on collagen (Fig. 3E). In keratinocytes carrying the Src transgene, FAK phosphorylation was strongly induced irrespective of the β1YYFF substitution.
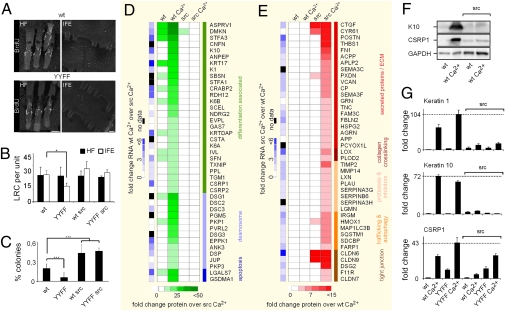
Our data indicate that tumor development correlates with phospho-FAK-Y397/Y861 levels in WT and β1Y-to-F keratinocytes. Because FAK was shown to promote tumorigenesis through epithelial stem cell maintenance (26), we next analyzed epidermal stem cell/long-term resident epidermal progenitor cell numbers with the BrdU label-retention method. We identified similar numbers of label-retaining cells (LRCs) in the bulge of hair follicles from WT and β1YYFF mice regardless of whether or not the Src transgene was present (Fig. 4 A and B). In contrast, LRCs were significantly reduced in the interfollicular epidermis of β1YYFF mice lacking the Src transgene (Fig. 4 A and B). In line with these results, the colony-forming efficacy of freshly isolated β1YYFF keratinocytes was reduced, but was increased in the presence of the Src transgene to the level of WT keratinocytes carrying the Src transgene (Fig. 4C).
Fig. 4.
Differentiation response. (A) Visualization of LRCs of the hair follicle (HF) and interfollicular epidermis (IFE) in WT and β1YYFF tail epidermal whole mounts. (B) Quantification of HF and IFE LRCs in eight epidermal units per mouse per genotype. (C) Colony-forming efficacy in an average of six wells per mouse, three mice per genotype. (Scale bars: 60 μM.) ***P < 0.001; *P < 0.05, unpaired Student t test. (D and E) Proteomics and mRNA changes in response to Ca2+. Experiments were run in duplicates. For protein analysis, cells were exposed to Ca2+ for 48 h. mRNA levels were assessed after 8 h of Ca2+. (F) K10 and CSRP1 levels detected by Western blot analysis in WT and Src transgenic cells before and after Ca2+. (G) RT-PCR levels of K1, K10, and CSRP1 before and after Ca2+ across genotypes.
The increased colony-forming efficacy of the Src transgene in WT and β1YYFF mice suggests that Src may facilitate keratinocyte stem cell maintenance and inhibit differentiation. To test this hypothesis, we determined the proteome and transcriptome of keratinocytes at subconfluence and at confluence before and after exposure to high Ca2+ levels, which is known to trigger keratinocyte differentiation. The global mRNA profile of subconfluent WT and Src transgenic keratinocytes was similar in the absence of high Ca2+, as assessed by Affymetrix GeneChip hybridization and subsequent Pearson correlation analysis across arrays (P ≥ 0.98; Fig. S3); however, their differentiation response at both the protein and mRNA levels differed. The comparison of Ca2+-induced proteins up-regulated in WT over Src transgenic keratinocytes (Fig. 4D) or vice versa (Fig. 4E) revealed that elevated Src activity suppressed the induction of differentiation-associated proteins and induced extracellular matrix components and modifying enzymes. These distinct responses to Ca2+ were confirmed by Western blot analysis (Fig. 4F) and RT-PCR (Fig. 4G). A Src-imposed reduction of the differentiation markers K1, K10, and CSRP1 was not reversed by the β1YYFF substitution (Fig. 4G). A Src-induced inhibition of differentiation also was observed in papillomas derived from mice that underwent two-stage carcinogenesis (Fig. S4 A and B). Moreover, only Src-transgenic papillomas dedifferentiated into carcinomas within a 20-wk period of TPA promotion (Fig. S4 C and D).
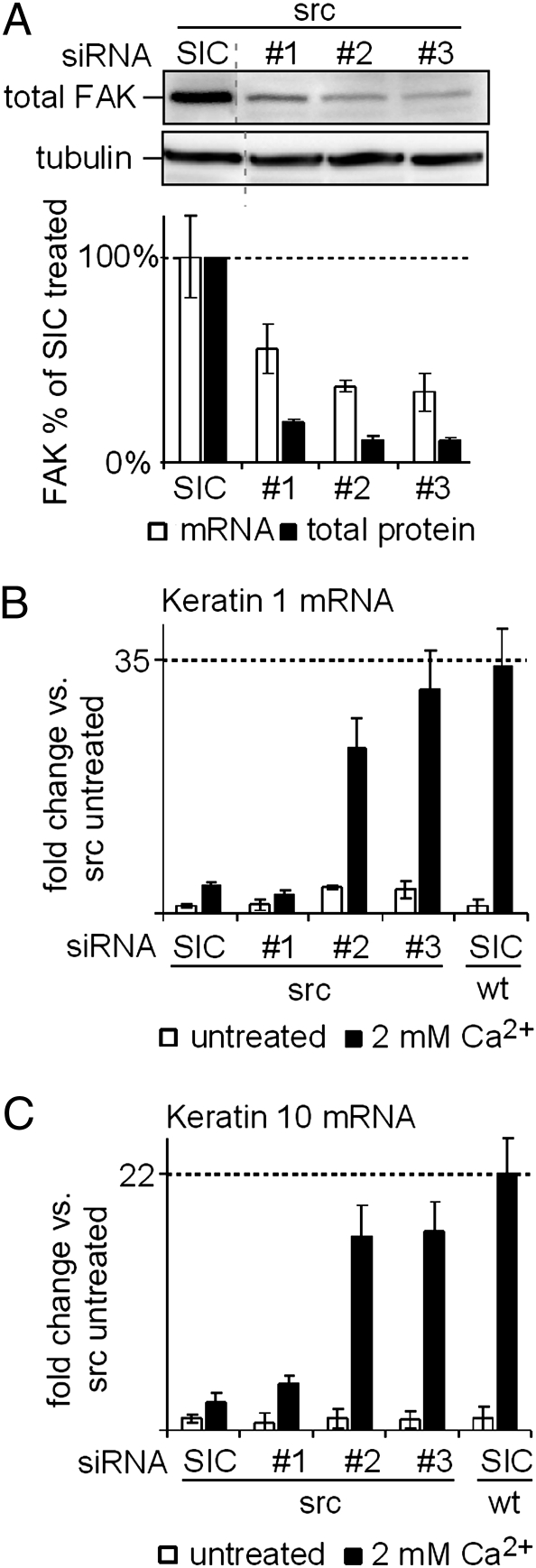
Because FAK activity as assessed by Y397/Y861 phosphorylation increased in response to elevated Src activity (Fig. 3E), we tested whether Src suppresses keratinocyte differentiation via FAK. We depleted FAK mRNA in Src transgenic keratinocytes using three different siRNAs and used a universal negative siRNA as a control (SIC). Whereas siRNA #1 had intermediate activity, siRNAs #2 and #3 reduced total FAK protein levels to <10% of that in the SIC-treated cells (Fig. 5A). When Src transgenic keratinocytes treated with siRNAs #2 and #3 were exposed to Ca2+, the induction of K1 and K10 mRNAs increased to nearly the levels seen in WT mice (Fig. 5 B and C). In contrast, when the less-efficient siRNA #1 was used to deplete FAK, the Src-imposed block on differentiation was relieved much less effectively (Fig. 5 B and C). The specificity of the siRNA-mediated knockdown was confirmed by reexpression of an siRNA-resistant mouse FAK cDNA (Fig. S5).
Fig. 5.
siRNA-mediated depletion of FAK in Src transgenic keratinocytes. (A) Keratinocytes were exposed to a universal negative control siRNA (SIC) or FAK-specific siRNA #1, #2, or #3. siRNA #1 was less efficacious in mediating a FAK depletion compared with siRNAs #2 and #3 at both the mRNA and protein levels. (B and C) Src-imposed blockage of K1 (B) and K10 (C) mRNA up-regulation after 8 h of 2 mM Ca2+ treatment was reversed by FAK depletion with siRNAs #2 and #3, but not by FAK depletion with siRNA #1.
Discussion
Phosphorylation of β1 cytoplasmic tyrosines by Src has been has been predicted to induce transformation and anchorage-independent growth by disrupting adhesion and modifying integrin signaling (7, 11, 13). In sharp contrast, we did not observe abnormal skin tumor growth in mice carrying a β1Y783F substitution or a β1Y795F substitution, regardless of whether or not Src activity was increased. When both β1 tail tyrosines were replaced by phenylalanines, an inhibitory effect on tumorigenesis was seen, but only at endogenous levels of Src activity. When Src activity was increased, the β1YYFF substitution had no detectable effect on tumorigenesis, suggesting that the tumor-promoting properties of Src did not depend on β1 tyrosine phosphorylation.
Previous studies assumed that β1 and β3Y-to-F mutations functionally resemble nonphosphorylated WT integrin and thus the Y-to-F effects were attributed to blocked phosphorylation. However, in our peptide pull-downs, the affinity of a number of β1 tail-interacting proteins, including talins and kindlins, was diminished when β1 cytoplasmic tyrosines were exchanged for phenylalanines, which lack a side-chain phenolic hydroxyl moiety. The binding of talins and kindlins to β1 NPxY motifs shifts the β1 extracellular ligand-binding domain to a high-affinity confirmation, although the extent to which this is induced by the kindlins is currently under debate for β1 heterodimers (27–29). In addition, recruitment of talins and kindlins links integrins to the actin cytoskeleton (4). Consistent with a partial loss of β1 function, β1YYFF integrin exhibited reduced surface 9EG7 epitope and adhesion strength. Interestingly, this was not observed with single Y-to-F substitutions, indicating that both NPxY motifs contribute to adhesive bond formation. Similarly, double, but not single, β1Y-to-F substitutions had a significant impact on FAK phosphorylation and tumorigenesis. FAK phosphorylation on Y397/Y861 has been shown to correlate with the amount of actinomyosin-generated tension applied to the β1 tail (30, 31). Building tension across β1 integrin requires a functional β1 tail talin/kindlin–F-actin interface, which is likely impaired by the β1YYFF substitution and responsible for the reduced FAK activation. Reduced β1 signaling in β1YYFF epidermis could explain a defect in tumor initiation (18, 32), which is highly dependent on β1 function and downstream activation of FAK (17, 19, 33, 34).
The impaired recruitment of talins and kindlins to the β1Y-to-F tails is unexpected and indicates that integrins with such mutations cannot be considered “pure” nonphosphorylateable integrins. An alternative approach would be to engineer mice in which the tyrosines are substituted with a phospho-mimicking acidic residue (β1Y to D/E). But because such substitutions interfere with integrin localization to focal adhesions (35) and thus are seriously flawed, we did not consider them. Despite these limitations, we were able to correlate the extent of Src-induced β1 phosphorylation with integrin function and tumor rates. Most notably, increasing Src activity strongly induced tumor load, and this occurred to the same extent in both WT and β1YYFF mice. This finding clearly suggests that although β1 phosphorylation interferes with talin/kindlin binding much like β1Y-to-F substitutions (8–10), and thus likely interferes with skin tumorigenesis much like β1Y-to-F substitutions, the “tumor-suppressive” effect of β1 phosphorylation at high Src activity is negligible in the face of the tumor-promoting capabilities of Src (36).
Src is not dependent on β1 phosphorylation to boost skin cancer rates. Moreover, Src rescues the inhibitory effects of a functionally impaired β1YYFF integrin on signaling through FAK (Fig. 3E). Because β1 signaling through FAK is a function of extracellular matrix stiffness (18, 30, 31), constitutive high FAK activity induced by oncogenic levels of Src may lead keratinocytes to misinterpret their environment and to trigger context-inappropriate biological responses, for example, to Ca2+ as a physiological epidermal differentiation stimulus. Interestingly, Src-transgenic keratinocytes exhibited constitutive high expression of the otherwise mechanosensitive YAP/TAZ target gene CTGF (Fig. 4E) (37). siRNA-mediated depletion of FAK rescues this aberrant Src-imposed differentiation block. In line with these results, both β1 integrin- and FAK-deficient epidermis and mammary gland are resistant to malignant transformation (17–19, 34) and show reduced numbers of undifferentiated progenitor cells (26, 38). Src transgenic keratinocytes also acquired a secretory phenotype, and a number of proteins were induced that are overexpressed in cutaneous stem cells vs. differentiated progeny (39, 40). In addition, Src-induced proteins are abundantly expressed in many epithelial cancers, including squamous cell carcinomas of the skin (41).
Although the β1YYFF substitution clearly triggered a reduction in integrin activity, the development and postnatal life of β1YYFF mice proceeds without obvious abnormalities (20, 21). This is an interesting observation from a medical standpoint, because it suggests that, contrary to previous assumptions (42), down-regulation of β1 signaling to physiological levels at an early stage of tumor development may be exploited to support antitumor/anticancer chemotherapy without the risk of side effects.
Materials and Methods
Mouse Strains.
Mice with β1YY783/795FF (YYFF), Y783F, or Y795F substitutions (21) were backcrossed for six generations to a FVB/N background. A Src transgene was introduced by crossing F5-generation mutants with FVB/N bovine K5 promoter-driven human WT c-Src transgenic mice (22).
Experimental Procedures on Mice.
Cutaneous two-stage chemical carcinogenesis was performed as described previously (22). Details are provided in SI Materials and Methods.
Tissue Processing and Antibodies.
Skin samples were processed as described previously (21). The antibodies used for immunohistology and Western blot analysis are listed in SI Materials and Methods.
Keratinocyte Culture.
Primary keratinocytes were isolated from adult mice as described previously (43). Colony-forming efficacy was defined as the percentage of cells that formed a colony of three or more cells.
Transcriptome Analysis and RT-PCR.
Total RNA from culture keratinocytes was extracted using the Qiagen RNeasy Kit. Affymetrix 430 2.0 arrays were used for transcriptome analysis. RT-PCR reactions were generated using IQ SYBR Green Supermix (Bio-Rad) and run on an iCycler IQ System (Bio-Rad). Results were normalized to GAPDH. Primers used for RT-PCR are listed in SI Materials and Methods.
SILAC-Based Proteome Analysis.
WT FVB/N keratinocytes that were used as an internal standard were SILAC-labeled by culturing them in Gibco Defined Keratinocyte-SFM (Invitrogen), with the natural lysine and arginine replaced by heavy labeled l-13C615N4-arginine (Arg10) and l-13C615N2-lysine (Lys8) (Cambridge Isotope Laboratories). Lysis was performed with a buffer containing 4% SDS, 100 mM Tris-HCl (pH 7.6), and 100 mM DTT, followed by a 5-min incubation at 95 °C and brief sonication. Proteins were digested by filter aided proteome preparation (FASP), followed by subsequent peptide fractionation (44). LC-MS and data analysis were performed as described previously (45).
SILAC-Based Peptide Pulldowns.
Pull-downs were performed as described previously (46), with modifications. Cell lysates were generated from immortalized WT mouse keratinocytes cultured in the presence of normal or Arg10-, Lys8-labeled medium. Lysis was done in Mammalian Protein Extraction Reagent (Pierce). Then 1 mg of unlabeled or Arg10-, Lys8-labeled supernatant was incubated with either control or experimental peptide overnight at 4 °C. Protein was eluted by incubating the beads in 16 mM biotin (Sigma-Aldrich) in PBS (pH 7.0) for 30 min at 30 °C and then precipitated overnight at −20 °C by adding chilled acetone. The protein pellet was then subjected to a standard tryptic in-solution digest. LC-MS and data analysis were performed as described above.
Spinning-Disk Analysis.
The spinning-disk device and the method have been described in detail and validated previously (25). Suspensions containing 2 × 105 cells were washed and resuspended in serum-free medium. Cells were plated on 25-mm round collagen-coated coverslips for 120 min at 37 °C in a total volume of 400 μL. Cells were spun for 5 min.
siRNA-Mediated FAK Knockdown.
siRNAs were reconstituted in sterile water at a final concentration of 50 μM. Keratinocytes plated in a six-well plate were incubated with 0.6 μM siRNA in 600 μL of OptiMEM supplemented with 12 μL of Lipofectamine 2000 (Invitrogen). After 48 h, the cells were ready for analysis of differentiation properties. Sequences of siRNA duplexes are provided in SI Materials and Methods. The universal negative siRNA SIC001 was obtained from Sigma-Aldrich.
Supplementary Material
Acknowledgments
We thank Tatjana Makhina for helping with the manuscript revision, as well as Roy Zent, Sara Wickström, Kyle Legate, and David Boettiger for carefully reading the manuscript. A.M. is a Mayo Clinic Scholar and is supported by the Mayo Clinic. This work was funded by the EC-FP7 (Metafight) and the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. F.M.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105689108/-/DCSupplemental.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- 3.Hirst R, Horwitz A, Buck C, Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci USA. 1986;83:6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moser M, Legate KR, Zent R, Fässler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 5.Tapley P, Horwitz A, Buck C, Duggan K, Rohrschneider L. Integrins isolated from Rous sarcoma virus–transformed chicken embryo fibroblasts. Oncogene. 1989;4:325–333. [PubMed] [Google Scholar]
- 6.Johansson MW, Larsson E, Lüning B, Pasquale EB, Ruoslahti E. Altered localization and cytoplasmic domain–binding properties of tyrosine-phosphorylated β1 integrin. J Cell Biol. 1994;126:1299–1309. doi: 10.1083/jcb.126.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai T, Jove R, Fässler R, Mosher DF. Role of the cytoplasmic tyrosines of β1A integrins in transformation by v-src. Proc Natl Acad Sci USA. 2001;98:3808–3813. doi: 10.1073/pnas.240456398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxley CL, et al. An integrin phosphorylation switch: The effect of β3 integrin tail phosphorylation on Dok1 and talin binding. J Biol Chem. 2008;283:5420–5426. doi: 10.1074/jbc.M709435200. [DOI] [PubMed] [Google Scholar]
- 9.Anthis NJ, et al. integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J Biol Chem. 2009;284:36700–36710. doi: 10.1074/jbc.M109.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bledzka K, et al. Tyrosine phosphorylation of integrin β3 regulates kindlin-2 binding and integrin activation. J Biol Chem. 2010;285:30370–30374. doi: 10.1074/jbc.C110.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plantefaber LC, Hynes RO. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989;56:281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- 12.Datta A, Huber F, Boettiger D. Phosphorylation of β3 integrin controls ligand binding strength. J Biol Chem. 2002;277:3943–3949. doi: 10.1074/jbc.M109536200. [DOI] [PubMed] [Google Scholar]
- 13.Pylayeva Y, Giancotti FG. Development requires activation but not phosphorylation of β1 integrins. Genes Dev. 2006;20:1057–1060. doi: 10.1101/gad.1432006. [DOI] [PubMed] [Google Scholar]
- 14.Blystone SD, Williams MP, Slater SE, Brown EJ. Requirement of integrin β3 tyrosine 747 for β3 tyrosine phosphorylation and regulation of αvβ3 avidity. J Biol Chem. 1997;272:28757–28761. doi: 10.1074/jbc.272.45.28757. [DOI] [PubMed] [Google Scholar]
- 15.Sakai T, Zhang Q, Fässler R, Mosher DF. Modulation of β1A integrin functions by tyrosine residues in the β1 cytoplasmic domain. J Cell Biol. 1998;141:527–538. doi: 10.1083/jcb.141.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law DA, et al. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 17.White DE, et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuter JA, et al. Modeling inducible human tissue neoplasia identifies an extracellular matrix interaction network involved in cancer progression. Cancer Cell. 2009;15:477–488. doi: 10.1016/j.ccr.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, et al. In vivo β1 integrin function requires phosphorylation-independent regulation by cytoplasmic tyrosines. Genes Dev. 2006;20:927–932. doi: 10.1101/gad.1408306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czuchra A, Meyer H, Legate KR, Brakebusch C, Fässler R. Genetic analysis of β1 integrin “activation motifs” in mice. J Cell Biol. 2006;174:889–899. doi: 10.1083/jcb.200604060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto T, et al. Targeted expression of c-Src in epidermal basal cells leads to enhanced skin tumor promotion, malignant progression, and metastasis. Cancer Res. 2003;63:4819–4828. [PubMed] [Google Scholar]
- 23.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 24.Lenter M, et al. A monoclonal antibody against an activation epitope on mouse integrin chain β 1 blocks adhesion of lymphocytes to the endothelial integrin α6β1. Proc Natl Acad Sci USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boettiger D. Quantitative measurements of integrin-mediated adhesion to extracellular matrix. Methods Enzymol. 2007;426:1–25. doi: 10.1016/S0076-6879(07)26001-X. [DOI] [PubMed] [Google Scholar]
- 26.Guan JL. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life. 2010;62:268–276. doi: 10.1002/iub.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montanez E, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of β-integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284:11485–11497. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manevich-Mendelson E, et al. Loss of Kindlin-3 in LAD-III eliminates LFA-1 but not VLA-4 adhesiveness developed under shear flow conditions. Blood. 2009;114:2344–2353. doi: 10.1182/blood-2009-04-218636. [DOI] [PubMed] [Google Scholar]
- 30.Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: Tethering is required for phosphorylation of FAK Y397. Mol Biol Cell. 2003;14:4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 32.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 33.McLean GW, et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol. 2008;173:1551–1565. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reszka AA, Hayashi Y, Horwitz AF. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 38.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 39.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 40.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nindl I, et al. Identification of differentially expressed genes in cutaneous squamous cell carcinoma by microarray expression profiling. Mol Cancer. 2006;5:30–46. doi: 10.1186/1476-4598-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter A. Integrins as target: First phase III trial launches, but questions remain. J Natl Cancer Inst. 2010;102:675–677. doi: 10.1093/jnci/djq186. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira M, Fujiwara H, Morita K, Watt FM. An activating β1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res. 2009;69:1334–1342. doi: 10.1158/0008-5472.CAN-08-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 45.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 46.Schulze WX, Mann M. A novel proteomic screen for peptide–protein interactions. J Biol Chem. 2004;279:10756–10764. doi: 10.1074/jbc.M309909200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.