Abstract
The lactose permease (LacY) catalyzes galactoside/H+ symport via an alternating access mechanism in which sugar- and H+-binding sites in the middle of the molecule are alternatively exposed to either side of the membrane by opening and closing of inward- and outward-facing cavities. The crystal structures of wild-type LacY, as well as accessibility data for the protein in the membrane, provide strong support for a conformation with a tightly closed periplasmic side and an open cytoplasmic side (an inward-facing conformation). In this study, rates of substrate binding were measured by stopped-flow with purified LacY either in detergent or in reconstituted proteoliposomes. Binding rates are compared with rates of sugar-induced opening of the periplasmic pathway obtained by using a recently developed method based on unquenching of Trp fluorescence. A linear dependence of galactoside-binding rates on sugar concentration is observed in detergent, whereas reconstituted LacY binds substrate at a slower rate that is independent of sugar concentration. Rates of opening of the periplasmic cavity with LacY in detergent are independent of substrate concentration and are essentially the same for different galactosidic sugars. The findings demonstrate clearly that reconstituted LacY is oriented physiologically with a closed periplasmic side that limits access of sugar to the binding site. Moreover, opening of the periplasmic cavity is the limiting factor for sugar binding with reconstituted LacY and may be the limiting step in the overall transport reaction.
Keywords: membrane transport, transport proteins, tryptophan fluorescence
The lactose permease of Escherichia coli (LacY), a paradigm for the major facilitator superfamily of membrane transport proteins (1, 2), catalyzes galactopyranoside/H+ symport across the bacterial membrane. LacY is arguably the most extensively studied membrane transport protein with several crystal structures (3–5), as well as a wealth of biochemical/biophysical data regarding conformational changes associated with the symport mechanism (reviewed in refs. 6 and 7). The X-ray structures reveal an inward-facing conformation with 12 mostly irregular transmembrane helices assembled into two pseudosymmetrical six-helix bundles with a large water-filled cavity open to the cytoplasmic side only and tightly closed on the periplasmic side (Fig. 1A). The structures lead a priori to a postulated mechanism involving a global conformational change with opening of the periplasmic pathway to allow access of substrate to the sugar-binding site. Numerous findings are consistent with the occurrence of multiple conformations during the transport cycle and strongly support an alternating access mechanism in which the sugar-binding site is exposed to either side of the membrane (3, 8–14).
Fig. 1.
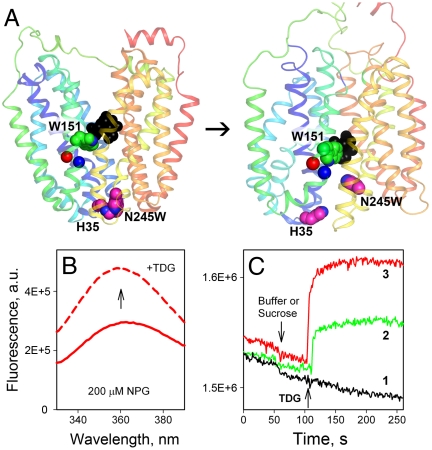
Trp fluorescence as a probe for direct measurement of sugar binding or opening of the periplasmic cavity in LacY. (A) Side view of the LacY in the inward-facing conformation (PDB ID 1PV7) is shown on the left, and the modeled outward-facing conformation (12) is shown on the right. Transmembrane helices are rainbow colored from blue (helix I) to red (helix XII). The arrow indicates the conformational transition resulting in opening of periplasmic cavity and closing of the cytoplasmic cavity. Bound sugar (black spheres) is in close proximity to Trp151 (green spheres). Trp245 introduced in place of Asn245 (helix VII) and its quencher His35 (helix I), are shown as pink spheres. Red and blue spheres represent Cα atoms at positions 154 and 155 on helix V, respectively. The figure was prepared with PyMOL 1.3. (B) Trp151-NPG FRET measured as increase of Trp fluorescence measured after displacement of bound NPG with excess TDG. Trp emission spectra of N245W/A155C LacY mutant recorded at pH 7.5 in the presence of 200 μM NPG before (red solid line) and after (red dashed line) addition of 10 mM TDG. (C) Unquenching of Trp245 fluorescence results from opening of periplasmic cavity after galactoside binding. Time courses of fluorescence changes in WT LacY (1), N245W mutant (2), or N245W/A155C mutant (3) are recorded at pH 6. Arrows indicate addition of 8 mM sucrose or TDG.
In recent years, significant progress has been achieved, particularly with respect to the alternating access mechanism in LacY (7). Because the majority of the studies were carried out with purified protein in detergent, the dynamics of conformational change in the membrane require further investigation. Pre-steady-state kinetic data have been obtained with purified LacY in dodecyl-β-d-maltopyranoside (DDM) (15), thereby allowing direct measurement of sugar-binding rates by FRET, as well as rates of opening and closing of cavities by fluorescence unquenching or quenching of introduced Trp residues (14). Both approaches utilize stopped-flow measurements of Trp fluorescence changes in LacY with all six native Trp residues present. Reconstitution of purified LacY into proteoliposomes yields fully functional protein with transport properties virtually identical to those observed in the native membrane (16). Moreover, as shown with a monoclonal antibody against a specific epitope in periplasmic loop VII/VIII (17), reconstituted LacY adopts the same orientation as in the bacterial membrane (18). Thus, titration of proteoliposomes containing LacY with the antibody demonstrates quantitatively that LacY incorporates into proteoliposomes prepared by dilution (19, 20) with the periplasmic side facing the external medium.
In this paper, rates of sugar binding to LacY in DDM or in proteoliposomes are compared with rates of conformational change due to opening of periplasmic pathway. The results are consistent with earlier studies demonstrating that LacY in proteoliposomes is closed on the periplasmic side. Opening of the periplasmic cavity allows sugar to diffuse into the galactoside-binding site. Furthermore, slow opening of the periplasmic pathway appears to be a limiting step in the alternating access mechanism utilized by LacY because this rate is similar to the overall turnover number for LacY (16).
Results
Design of LacY Mutants.
Intrinsic Trp fluorescence is used here as a probe for direct measurement of sugar binding by FRET from Trp151 to p-nitrophenyl-α-d-galactopyranoside (NPG) (15) and for estimating the rate of opening of the periplasmic cavity by measuring unquenching of Trp fluorescence (14). Trp151 in LacY is an important component of the sugar-binding site (Fig. 1A, green spheres) and the only native Trp residue out of six that is in close proximity to bound substrate (Fig. 1A, black spheres). Binding of NPG places the nitrophenyl moiety within Förster distance of Trp151 and yields galactoside-specific FRET (15) (Fig. 1B). Opening of the periplasmic cavity is accompanied by separation of amino acid residues His35 (helix I) and N245W (helix VII) (Fig. 1A, pink spheres), and fluorescence of a Trp residue in place of Asn245 increases as a result of unquenching (Fig. 1C, compare green and black traces). The fluorescence change is relatively small in the N245W mutant, but can be enhanced by decreasing background fluorescence of the native Trp residues by replacement with Tyr or by introducing His or Cys residues close to the native Trp residues as inner quenchers (described in detail below). An example of an inner quencher is the A155C mutation (Cα atom is shown as blue sphere in Fig. 1A), which significantly increases the signal for unquenching with mutant N245W when sugar is added (compare green and red traces in Fig. 1C). Mutant N245W/A155C combines both intrinsic Trp probes, exhibits approximately 50% of the lactose transport activity of WT LacY (Fig. S1), and was used to assay both sugar binding (Trp151-NPG FRET) and opening of the periplasmic cavity (i.e., unquenching of N245W).
Rates of NPG Binding to WT LacY.
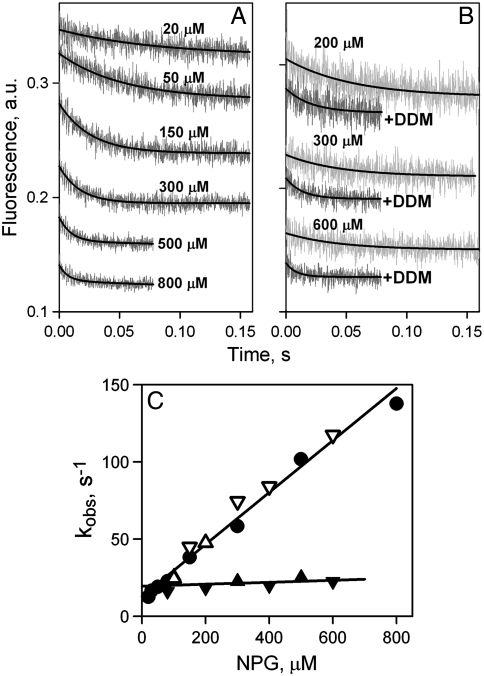
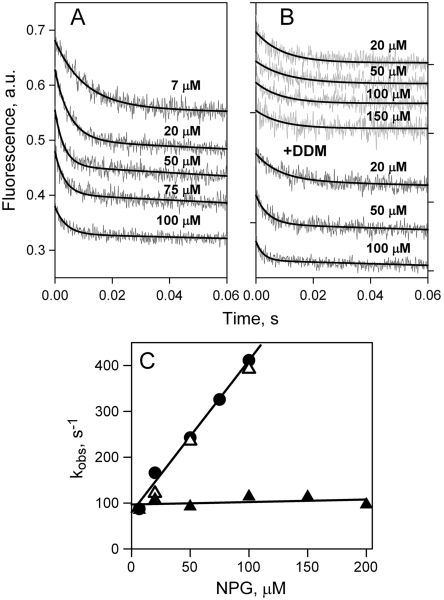
NPG binding rates were measured by stopped flow after mixing NPG with LacY either in DDM or in reconstituted proteoliposomes. In DDM, decreases in fluorescence are clearly observed due to Trp151-NPG FRET (Fig. 2A), and the rates increase with increasing galactoside concentration. NPG binding rates (kobs) estimated from single exponential fits (Fig. 2A, solid lines) vary linearly with sugar concentration (Fig. 2C, filled circles). The linear dependence allows calculation of kinetic parameters for WT LacY in DDM—koff = 13 s-1, kon = 0.2 μM-1 s-1, and Kd = 65 μM (Kd = koff/kon).
Fig. 2.
Rates of NPG binding to WT LacY. (A) Stopped-flow traces of changes in Trp fluorescence recorded after mixing of protein with given concentrations of NPG at pH 7.5. Single-exponential fits are shown as black lines. (B) Stopped-flow traces showing NPG binding to LacY reconstituted into proteoliposomes (light gray traces at three sugar concentrations), and after dissolving of proteoliposomes in 0.3% DDM (dark gray traces). Single-exponential fits are shown as black lines. (C) Concentration dependence of the rates (kobs) of NPG binding measured in DDM (●), reconstituted into proteoliposomes at LPR 10 (▴) or 5 (▾), and after addition of DDM to proteoliposomes at LPR 10 (△) or 5 (▽). Linear fits to the data are shown as solid lines. Reconstituted into proteoliposomes LacY binds NPG with kobs = 21 ± 4 s-1.
Similar experiments performed with LacY reconstituted into proteoliposomes yield remarkable results. The stopped-flow traces also exhibit a decrease in fluorescence as a result of sugar binding due to Trp151-NPG FRET, but the rates are slow over a wide range of concentrations (Fig. 2B, light gray traces). As estimated from single exponential fits, kobs values are not affected by NPG concentration (Fig. 2C, filled triangles), and a slow binding rate of about 20 s-1 is observed with reconstituted LacY at NPG concentrations up to 600 μM. Identical results are obtained using proteoliposomes with different lipid-to-protein ratios (LPR 5 and 10). Addition of DDM to the same proteoliposomes dissolves the membrane and thereby removes the permeability barrier, resulting in increased rates of binding at higher NPG concentrations (Fig. 2B, dark gray traces). The estimated kobs values plotted as a function of NPG concentration are precisely the same as those obtained initially with LacY in DDM (Fig. 2C, open triangles).
NPG binding affinity with reconstituted LacY cannot be estimated directly from measured rates (Fig. 2C, filled triangles) because the kobs is independent of sugar concentration. However, the increase in Trp151 fluorescence observed upon displacement of bound NPG by excess of galactopyranosyl-β-d-galactopyranoside (TDG) from LacY equilibrated with different concentrations of NPG, can be used to calculate Kd (refs. 15 and 21). Higher affinity is observed with LacY in proteoliposomes as opposed to DDM with Kds of 11 and 45 μM, respectively (Figs. S2 and S3A).
Rates of NPG Binding to C154G LacY.
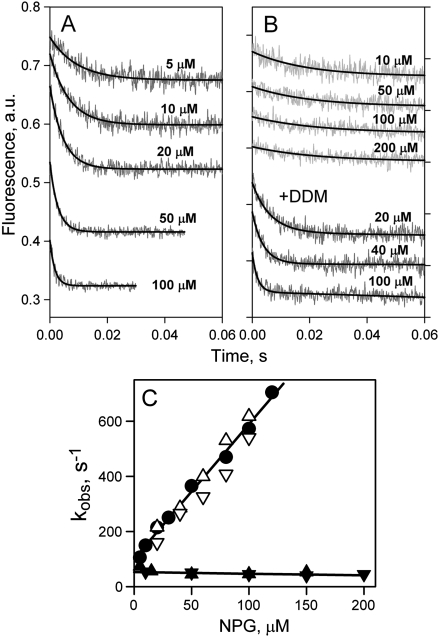
Mutant C154G is severely restricted with respect to transport (Fig. S1) and is arrested in a partially open conformation on the periplasmic side, both in DDM (11, 12) and in right-side-out (RSO) membrane vesicles (22). Rates of NPG binding to C154G LacY in DDM are significantly faster than those measured with WT LacY (compare Figs. 2A and 3A), and a linear dependence of kobs on NPG concentration is observed (Fig. 3C, filled circles). Estimated kinetic parameters are koff = 100 s-1, kon = 5 μM-1 s-1, and Kd = 20 μM. As observed with WT LacY, the rate of NPG binding is independent of concentration with reconstituted C154G LacY. The binding rate is approximately 50 s-1 for all NPG concentrations tested (Fig. 3 B, upper four traces, and C, filled triangles). The same proteoliposomes dissolved in DDM exhibit kinetic parameters for sugar binding that are practically identical to those obtained in DDM (Fig. 3 B, three lower traces, and C, open triangles). The Kd for mutant C154G in proteoliposomes is estimated from displacement experiments as described above for WT LacY. Higher affinity is observed with C154G mutant in proteoliposomes as opposed to DDM with Kds of 8 and 19 μM, respectively (Fig. S3B).
Fig. 3.
Rates of NPG binding to C154G LacY. (A) Stopped-flow traces of Trp fluorescence change were recorded in DDM at indicated sugar concentrations as described in Fig. 2A. Single-exponential fits are shown as black lines. (B) Stopped-flow traces of NPG binding to mutant C154G reconstituted into proteoliposomes are shown at four sugar concentrations (upper four traces). The three lower traces represent binding of NPG to the same proteoliposomes dissolved in 0.3% DDM. Single-exponential fits are shown as black solid lines. (C) Concentration dependence of rates of NPG binding (kobs) to C154G LacY. The rates measured in DDM, proteoliposomes, or dissolved proteoliposomes are presented with symbols described in Fig. 2C. Linear fits to the data are shown as solid lines. Mutant C154G reconstituted into proteoliposomes binds NPG with kobs = 51 ± 10 s-1.
Rates of Sugar Binding to Reconstituted LacY Versus Rates of Opening of the Periplasmic Cavity.
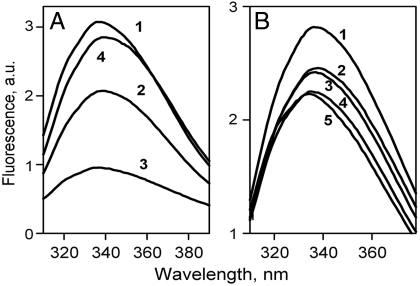
Opening of the periplasmic pathway is triggered by sugar binding to LacY in DDM and results in unquenching of Trp fluorescence in N245W mutant (14). Rather than utilize Trp-less LacY (23), which is unstable in purified form, background fluorescence was reduced by two approaches (see Fig. S4 and Table S1 for details): (i) Some of the native Trp residues were replaced with Tyr. Trp emission spectra of given mutants exhibit a significant decrease of Trp fluorescence intensity (Fig. 4A, traces 2 and 3 compared to 1). The N245W mutation combined with three native Trp (N245W/3Trp) results in stable purified protein suitable for kinetic studies (Fig. 4A, trace 4). (ii) Inner quenchers were introduced in close proximity to native Trp residues. Among several Cys or His replacements (Fig. 4B, Fig. S4, and Table S1), the A155C mutation yields protein with unchanged NPG binding and decreased Trp emission (Fig. 4B, trace 3). Kinetic parameters for sugar binding and opening of the periplasmic cavity for mutant N245W/A155C are described below.
Fig. 4.
Trp emission spectra of LacY mutants with reduced background Trp fluorescence. (A) LacY mutants with different total numbers of native Trp residues replaced with Tyr: 1, WT LacY with six native Trp residues; 2, mutant W78Y/W171Y/W223Y with three Trp replaced; 3, mutant W33Y/W78Y/W171Y/W223Y with four Trp replaced; 4, mutant N245W/W78Y/W171Y/W223Y with Trp in place of Asn245 and three Trp replaced. (B) Mutants with inner quenchers targeting native Trp residues: 1, WT LacY; 2, mutant A88H (Trp171); 3, mutant A155C (Trp 151); 4, mutant N166H (Trp33); 5, mutant N38C (Trp33). The locations of the Trp residues in LacY and positions of inner quenchers introduced are shown in Fig. S4.
NPG binding rates for mutant N245W/3Trp in DDM increase with increasing NPG concentrations (Fig. 5A), and the linear fit (Fig. 5C, filled circles) allows estimation of kinetic parameters: koff = 80 s-1, kon = 3 μM-1 s-1, and Kd = 26 μM. Reconstituted N245W/3Trp mutant exhibits an NPG binding rate of approximately 100 s-1 for all tested NPG concentrations (Fig. 5 B, four upper traces, and C, filled triangles), and dissolving the proteoliposomes in DDM restores the concentration dependence of sugar-binding rates with the same kinetic parameters as observed in DDM (Fig. 5 B, lower three traces, and C, open triangles).
Fig. 5.
Rates of NPG binding to mutant N245W/3Trp. (A) Stopped-flow traces of Trp fluorescence change recorded in DDM and presented as described in Fig. 2A. (B) Stopped-flow traces of NPG binding to mutant N245W/3Trp reconstituted into proteoliposomes are shown at four sugar concentrations (upper four traces). The three lower traces represent binding of NPG to the same proteoliposomes dissolved in 0.3% DDM. Single-exponential fits are shown as black lines. (C) Concentration dependence of rates of NPG binding (kobs) to mutant N245W/3Trp in DDM (●), reconstituted proteoliposomes at LPR 10 (▴), and after dissolving the proteoliposomes in DDM (△). Linear fits to the data are shown as solid lines. Reconstituted mutant N245W/3Trp binds NPG with kobs = 102 ± 12 s-1.
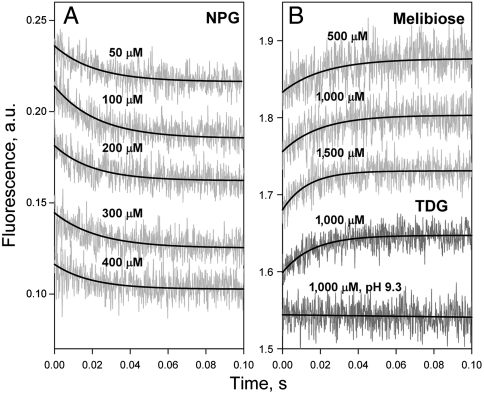
The linear dependence of kobs versus NPG concentrations in DDM and the lack of concentration dependence for rates of sugar binding in proteoliposomes are observed with WT LacY and both mutants (C154G and N245W). Therefore, in proteoliposomes, the slower process, probably opening of the periplasmic cavity, limits sugar binding to LacY. A direct comparison between sugar-binding rates and rates of opening of the periplasmic pathway is possible with the N245W/A155C mutant. Reconstituted into proteoliposomes, this mutant binds sugar with kobs of approximately 56 s-1 at NPG concentrations up to 400 μM (Figs. 6A and 7, filled triangles), and the Kd for binding estimated from displacement experiments is 3.5 μM (Fig. S5). Kinetic parameters measured after dissolving the proteoliposomes in DDM are indistinguishable from those measured in DDM: koff = 49 s-1, kon = 3 μM-1 s-1, and Kd = 16 μM (Fig. 7, filled circles and open triangles; traces shown in Fig. S6) and are similar to those obtained for mutant N245W/3Trp (Fig. 5C).
Fig. 6.
Comparison of the rates of galactoside binding to reconstituted mutant N245W/A155C with the rates of opening of the periplasmic cavity. (A) Binding of NPG to mutant N245W/A155C measured by Trp151-NPG FRET. Stopped-flow traces recorded after mixing of NPG with the mutant reconstituted into proteoliposomes are shown at five sugar concentrations. Single-exponential fits are shown as black lines. (B) Unquenching of Trp245 fluorescence resulting from opening of the periplasmic cavity upon sugar binding. Stopped-flow traces are recorded with the mutant in DDM after mixing with melibiose (three upper traces) or TDG (two lower traces) at pH 6.0, except for the trace at pH 9.3. Final concentrations of sugars are indicated.
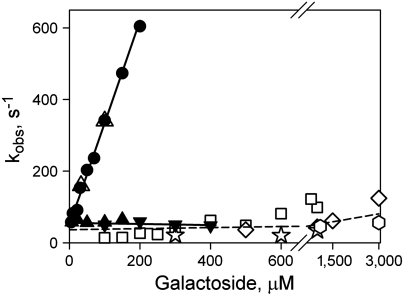
Fig. 7.
Concentration dependence of sugar-binding rates with rates of opening of the periplasmic cavity for mutant N245W/A155C. NPG binding rates measured as Trp151-NPG FRET are shown for purified mutant in DDM (●), reconstituted in proteoliposomes at LPR 10 (▴) or 5 (▾), and after addition of DDM (△). Linear fits to the data obtained for Trp151-NPG FRET are shown as solid lines. Reconstituted mutant N245W/A155C binds NPG with kobs = 56 ± 7 s-1. Rates of unquenching of Trp245 fluorescence resulting from opening of the periplasmic cavity after sugar binding are shown as open symbols: TDG (□); melibiose (⋄); octyl-α-d-galactoside (☆); methyl-α-d-galactoside (hexagon). The rate of opening of periplasmic cavity in DDM is 50–100 s-1 at the higher concentrations of the four galactosides tested.
In order to measure rates of opening of the periplasmic cavity triggered by galactoside binding, four galactosidic sugars that do not change Trp fluorescence—TDG, melibiose, octyl-α-d-galactoside, and methyl-α-d-galactoside—were used (Fig. S7). Unquenching of Trp fluorescence in mutant N245W/A155C due to binding of these galactosides in DDM is shown in Fig. 6B. Stopped-flow traces recorded after mixing the mutant with melibiose (three upper traces) or TDG (two lower traces) demonstrate increasing Trp fluorescence at pH 6.0. Previous results (14) showed that the protonated form of His35 quenches W245 fluorescence (Fig. 1A, pink spheres), and the lack of a TDG-induced fluorescence change at pH 9.3 (Fig. 6B, lower trace) is consistent with quenching by a protonated His residue. Mixing of four different galactosides with mutant N245W/A155C results in essentially the same slow rate of unquenching with little or no dependence on galactoside concentration (Fig. 7, open symbols fitted with broken line). Moreover, the NPG binding rate measured with reconstituted N245W/A155C LacY (Fig. 7, filled triangles) is in the same range as the rate of the opening of periplasmic cavity triggered by binding of different galactosides to the same mutant in detergent.
Discussion
Pre-steady-state kinetics of NPG binding to LacY in DDM is completely different from that observed with reconstituted protein. In DDM, the kobs is linearly dependent on substrate concentration with WT LacY and all three mutants tested (Figs. 2C, 3C, 5C, and 7; filled circles). Thus, in an inward-facing conformation, the sugar-binding site in solubilized LacY is readily accessible, and binding can be described as a single-step reversible process, a pseudo-first-order reaction at [S]≫[E] and 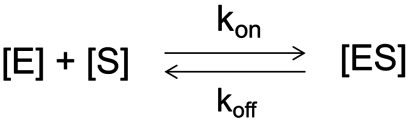 where E is LacY and S is a galactoside. The sugar-binding rate for this reaction depends linearly on ligand concentration (kobs = koff + kon[S]) (24).
where E is LacY and S is a galactoside. The sugar-binding rate for this reaction depends linearly on ligand concentration (kobs = koff + kon[S]) (24).
In marked contrast, LacY reconstituted into proteoliposomes binds NPG at a rate that is independent of NPG concentration (Figs. 2C, 3C, 5C, and 7; filled triangles). This finding suggests that the sugar-binding site is not readily accessible and that the binding reaction includes a limiting step that is likely represented by opening of the periplasmic side of the molecule. LacY molecules reconstituted into proteoliposomes are oriented with the periplasmic side on the outside surface (18). Therefore, as shown in the X-ray structures (3–5), as well as RSO membrane vesicles (22), reconstituted LacY also exists in an inward-facing conformation with a closed periplasmic cavity because sugar-binding rates do not increase even at supersaturating NPG concentrations. Evidently, the periplasmic cavity leading to the sugar-binding site must open first, and this slow opening is likely rate-limiting for substrate binding. This process is described by an equation in which fast binding of sugar to one conformational state [E]open is precluded by slow periplasmic pathway opening (24):  where rc is the rate of conformational change.
where rc is the rate of conformational change.
The rates of conformational change on the periplasmic side, measured in DDM by unquenching of Trp introduced at the periplasmic gate of the N245W/A155C mutant, are 50–100 s-1 for different galactosidic sugars (Fig. 7, open symbols). The rate of Trp unquenching is relatively slow and depends marginally on sugar concentration, although the affinities of LacY for these sugars and the non-galactosyl moieties differ markedly (25) (see Fig. S7C). Essentially the same rate is observed for NPG binding with reconstituted N245W/A155C, and it is also independent of sugar concentration. Therefore, the rate of opening of the periplasmic cavity is evidently a limiting step in the sugar-binding process. Sugar binding in proteoliposomes is likely to represent the rate of spontaneous opening of the periplasmic cavity in the absence of substrate, whereas unquenching of Trp245 in detergent solution represents the rate of opening induced by bound sugar. However, both processes describe opening of the periplasmic side of LacY. The similarity between the two rates suggests that both may be limited by similar conformational constraints.
A turnover number of 16–20 s-1 has been estimated for active lactose transport, as measured with WT LacY in RSO membrane vesicles, as well as inserted into proteoliposomes (16). Very much the same rate is observed here for sugar binding to WT LacY in proteoliposomes (21 ± 3.6 s-1). Therefore, the opening of the periplasmic gate could be a rate-limiting step in the overall transport process catalyzed by WT LacY.
In view of the scenario presented, how does transport of sugar across the membrane occur? In all likelihood, in the absence of external galactoside, opening of LacY on the periplasmic surface occurs with a very low frequency. However, once a sugar is bound, turnover increases markedly.
Methods
Construction of mutants, protein purification, and materials used in this study are described in SI Methods.
Reconstitution of Purified Proteins into Proteoliposomes.
Synthetic phospholipids [1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) ratio 3∶1] were used for protein reconstitution by the dilution method (26). Briefly, purified LacY in 0.02% DDM was mixed with phospholipids dissolved in 1.2% octyl-glucoside at an LPR of 5 or 10 wt/wt, as indicated. The mixture was kept on ice for 20 min and then quickly diluted 50 times in 50 mM NaPi (pH 7.5). The proteoliposomes were collected by centrifugation at 100,000 × g for 1 h and subjected to two cycles of freeze-thaw/sonication before use.
Fluorescence Measurements.
Steady-state Trp fluorescence of 0.5 μM protein was monitored at excitation wavelength 295 nm on a SPEX Fluorolog 3 spectrofluorometer at room temperature as described (14). Time traces were recorded at emission wavelength 335 nm. Stopped-flow measurements were performed at 25 °C on an SFM-300 rapid kinetic system equipped with either a microcuvette (dead time 0.22 ms) or a TC-50/10 cuvette (dead time 1.1 ms), and an MOS-450 spectrofluorimeter (Bio-Logic). Excitation wavelength was 295 nm with a 320-nm cutoff filter for emission. The final concentration of protein after mixing was 0.5–2 μM. Measurements with purified protein in detergent were done in 0.02% DDM and 50 mM NaPi (pH 7.5), citrate/NaPi (pH 6.0), or 3-(cyclohexylamino)-1-propanesulfonic acid (pH 9.3), as indicated. Experiments with proteoliposomes were carried out in 50 mM NaPi (pH 7.5). For dissolving proteoliposomes, DDM was added to a final concentration of 0.3%, and after 10 min, the samples were used in stopped-flow experiments. Typically, 10–20 traces were recorded for each data point, averaged and fitted with an exponential equation using the built-in Bio-Kine32 software package, or by using Sigmaplot 10 (Systat Software). All concentrations given are final after mixing.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants DK51131, DK069463, and GM074929, as well as National Science Foundation Grant 0450970 (to H.R.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112157108/-/DCSupplemental.
References
- 1.Saier MH, Jr, et al. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- 2.Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 3.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 4.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H(+) symport in LacY. EMBO J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of WT lactose permease. Proc Natl Acad Sci USA. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaback HR, Smirnova I, Kasho V, Nie Y, Zhou Y. The alternating access transport mechanism in LacY. J Membr Biol. 2011;239:85–93. doi: 10.1007/s00232-010-9327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaback HR, et al. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie Y, Ermolova N, Kaback HR. Site-directed alkylation of LacY: Effect of the proton electrochemical gradient. J Mol Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie Y, Kaback HR. Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane. Proc Natl Acad Sci USA. 2010;107:9903–9908. doi: 10.1073/pnas.1004515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumdar DS, et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci USA. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirnova I, Kasho V, Sugihara J, Kaback HR. Probing of the rates of alternating access in LacY with Trp fluorescence. Proc Natl Acad Sci USA. 2009;106:21561–21566. doi: 10.1073/pnas.0911434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smirnova IN, Kasho VN, Kaback HR. Direct sugar binding to LacY measured by resonance energy transfer. Biochemistry. 2006;45:15279–15287. doi: 10.1021/bi061632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viitanen P, Garcia ML, Kaback HR. Purified reconstituted lac carrier protein from Escherichia coli is fully functional. Proc Natl Acad Sci USA. 1984;81:1629–1633. doi: 10.1073/pnas.81.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Wu J, Carrasco N, Kaback HR. Identification of the epitope for monoclonal antibody 4B1 which uncouples lactose and proton translocation in the lactose permease of Escherichia coli. Biochemistry. 1996;35:990–998. doi: 10.1021/bi952166w. [DOI] [PubMed] [Google Scholar]
- 18.Herzlinger D, Viitanen P, Carrasco N, Kaback HR. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 2. Binding studies with membrane vesicles and proteoliposomes reconstituted with purified lac carrier protein. Biochemistry. 1984;23:3688–3693. doi: 10.1021/bi00311a018. [DOI] [PubMed] [Google Scholar]
- 19.Newman MJ, Wilson TH. Solubilization and reconstitution of the lactose transport system from Escherichia coli. J Biol Chem. 1980;255:10583–10586. [PubMed] [Google Scholar]
- 20.Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981;256:11804–11808. [PubMed] [Google Scholar]
- 21.Smirnova IN, Kasho VN, Sugihara J, Choe JY, Kaback HR. Residues in the H+ translocation site define the pKa for sugar binding to LacY. Biochemistry. 2009;48:8852–8860. doi: 10.1021/bi9011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie Y, Sabetfard FE, Kaback HR. The Cys154 → Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez-Ibar JL, Guan L, Svrakic M, Kaback HR. Exploiting luminescence spectroscopy to elucidate the interaction between sugar and a tryptophan residue in the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 2003;100:12706–12711. doi: 10.1073/pnas.1835645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fersht A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding. New York: Freeman; 1999. [Google Scholar]
- 25.Sahin-Tóth M, Gunawan P, Lawrence MC, Toyokuni T, Kaback HR. Binding of hydrophobic D-galactopyranosides to the lactose permease of Escherichia coli. Biochemistry. 2002;41:13039–13045. doi: 10.1021/bi0203076. [DOI] [PubMed] [Google Scholar]
- 26.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.