Abstract
Targeting the surface of malignant cells has evolved into a cornerstone in cancer therapy, paradigmatically introduced by the success of humoral immunotherapy against CD20 in malignant lymphoma. However, tumor cell susceptibility to immunochemotherapy varies, with mostly a fatal outcome in cases of resistant disease. Here, we show that lymphoma exosomes shield target cells from antibody attack and that exosome biogenesis is modulated by the lysosome-related organelle-associated ATP-binding cassette (ABC) transporter A3 (ABCA3). B-cell lymphoma cells released exosomes that carried CD20, bound therapeutic anti-CD20 antibodies, consumed complement, and protected target cells from antibody attack. ABCA3, previously shown to mediate resistance to chemotherapy, was critical for the amounts of exosomes released, and both pharmacological blockade and the silencing of ABCA3 enhanced susceptibility of target cells to antibody-mediated lysis. Mechanisms of cancer cell resistance to drugs and antibodies are linked in an ABCA3-dependent pathway of exosome secretion.
Monoclonal antibody-based therapy has evolved as a mainstay of targeted anti-cancer therapy, endowing access of both direct and immunomediated lytic mechanisms to the tumor cells. Anti-CD20 chimeric antibody rituximab was one of the first antibodies with high clinical efficacy, defining standards of immunotherapy in malignant B-cell lymphoma (1). Current immunochemotherapy regimens can provide a cure to significant proportions of patients with aggressive lymphoma and prolong survival in patients with indolent B-cell lymphomas (2–4). However, the prognosis for patients with primary resistant or relapsed aggressive lymphoma is still dismal (recently reviewed in ref. 5).
Rituximab exerts its cytolytic effects after CD20 ligation by direct induction of apoptosis, complement-dependent cytolysis (CDC), as well as antibody-dependent cellular cytotoxicity (ADCC), with variation in the contribution to cytotoxicity depending on the B-cell lymphoma entity (6). Independently of the mechanism, however, initiation of cytolysis always requires binding of the antibody to the tumor cell surface.
Exosomes are defined as microvesicular structures with a mean size of 50–100 nm, released by exocytosis following intracellular assembly in multivesicular bodies (MVB) (review in ref. 7). In normal physiology, exosomes are secreted from erythroid progenitors during progenitor cell maturation, as well as from B-lymphocytes and dendritic cells, with multiple immune functions leading to investigations aiming at vaccinations against malignant disease (8–13). Exosomes have also been detected in the supernatant of several tumor cell lines, such as the T-lymphoblastic cell line Jurkat and the erythroleukemic cell line K562 (14, 15). We and others have recently discovered that the intracellular compartment of exosome assembly, i.e., the MVBs, is modulated by the ATP-binding cassette (ABC) transporter A3 in hematological neoplasm with myeloid differentiation, which is associated with resistance against a broad spectrum of cytostatic drugs (16–18). In addition to its role in leukemia, we also detected ABCA3 levels in aggressive lymphoma even exceeding those in myeloid leukemia (16). Consequently, we have analyzed here exosome release from B-cell lymphomas and found strong exosome production and release from aggressive B-cell lymphoma cells in vitro and in vivo. Such exosomes carried the CD20 target antigen and acted as decoy targets upon rituximab exposure, allowing lymphoma cells to escape from humoral immunotherapy.
Results
Lymphoma-Derived Exosomes Bind Therapeutic Anti-CD20 Antibody.
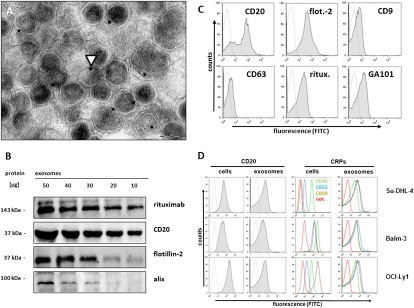
Applying ultracentrifugation techniques described for the isolation of exosomes (19), we recovered monomorphic microvesicular structures of high purity with the typical size and morphology of exosomes in the supernatants from a series of aggressive B-cell lymphoma cell lines (Su-DHL-4, Balm-3, OCI-Ly1) as well as from primary lymphoma cell preparations (Fig. 1A and Fig. S1). The yields of exosomes were comparable to, or even surmounted, the amounts of exosomes harvested from cultures of K562, an erythroleukemic cell line widely used as a model cell line for exosome release (Table S1). Such lymphoma-derived vesicles were positive for the exosome markers flotillin-2, alix, CD9, and CD63 and the GPI-anchored complement regulatory proteins (CRPs) CD55 and CD59. Importantly, the exosomes also carried the B-cell plasmamembrane protein CD20 (Fig. 1 A–D and Fig. S2). The exosomal abundance of CD20 mirrored the expression of this protein in the parental cells, whereas the exosomal membrane levels of CD55, CD59, and CD46 were uniformly high on the exosomes from all cell lines, even when the parental cells showed only low-level expression of the respective CRP (Fig. 1D). Lymphoma cell-derived exosomal CD20 bound the therapeutic anti-CD20 antibody rituximab (Figs. 1 A–D; Figs. S2 A, B, and D and S3A) and thus effectively depleted the soluble antibody from antibody suspensions in vitro (Fig. 2A). In vivo, exosomes also bound the anti-CD20 antibody rituximab in humans who had received the antibody for therapeutic purposes (Fig. 2 B and C, Fig. S3A, and Table S2). Approximately half of all of the plasma rituximab was found to be fixed to exosomes 3 h after the end of the rituximab infusion (rituximab dose of 375 mg/m2, first course of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) immunochemotherapy, Fig. 2C). It is worth noting that these measurements were carried out with an ELISA using the monoclonal anti-idiotypic antibody MB2A4, which detects both soluble and membrane-bound rituximab (20, 21). Thus, our data are comparable to previous pharmacokinetic findings at initiation of rituximab therapy. However, the high fraction of rituximab bound to exosomes indicates that significant proportions of rituximab in the serum are not in a soluble state and thus are not available for lymphoma cell attack.
Fig. 1.
Binding of therapeutic anti-CD20 monoclonal antibody to exosomes from B-cell lymphoma cells. (A) Differential ultracentrifugation of cell culture supernatants from B-cell lymphoma cell line Balm-3 yielded microvesicular structures with the typical size and morphology of exosomes staining positive with anti-CD20 antibody rituximab and labeled with protein A–immunogold. Arrowhead indicates immunogold staining. (Scale bar, 100 nm.) (B) Western blot documented binding of rituximab to exosomal protein preparations, detected with an anti-idiotype monoclonal antibody against rituximab (MB2A4), in parallel to CD20 and exosome markers alix and flotillin-2 (Fig. S2 A and B). Binding of therapeutic monoclonal antibodies rituximab and GA101, as well as CD20, flotillin-2 (flot.-2 in C), CD63, and CD9 was also documented by FACS on purified exosome-labeled beads (Su-DHL-4 in C) (cell lines Balm3, OCI-Ly1 in Fig. S2 C and D; and primary lymphoma in Figs. S1G). (D) For the detection of complement regulatory proteins (CRPs), cells or exosomes coupled to beads were stained with fluorescence-labeled monoclonal antibodies and analyzed by flow cytometry. Expression of CD20 (Left panels), as well as of CRPs [Right panels: CD46, green line; CD55, blue line; CD59, brown line; isotype (isot.) control, red line] differed among lymphoma cell lines, whereas levels of CRPs on the exosome surface were uniformly high.
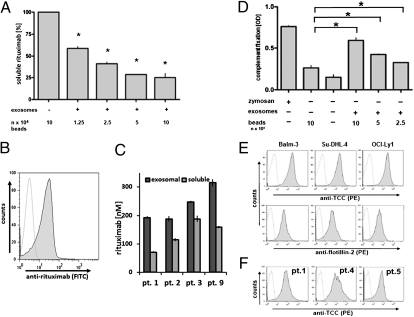
Fig. 2.
Absorption of anti-CD20 antibody rituximab and consumption of complement on lymphoma-derived exosomes in vitro and in vivo. (A) Exosomes from Su-DHL-4 cells were added to medium supplemented with rituximab at an initial concentration of 35 ng/100 μL for 1 h at 21 °C. Soluble rituximab was measured by ELISA, and mean values of triplicates from a representative experiment were expressed as a percentage of controls treated with nonexosome-labeled beads only. Asterisks mark significant reduction, based on repeated measure ANOVA with Bonferroni's post test, for CD20-negative exosomes from K562 leukemia cells as control (Fig. S3B). (B) Exosomes were prepared from the plasma of patients at 3 h after the end of infusion, and rituximab binding was detected with a specific antibody (MB2A4) by flow cytometry (for further examples, see Fig. S3A). (C) For quantification of free versus exosome-bound antibody, rituximab was measured by ELISA, documenting approximately one-third to one-half of plasma rituximab bound to exosomes. Rituximab in the exosomal pellet is represented by dark shading, soluble rituximab from the supernatant by light shading. (E) Latex beads were coated with lymphoma exosomes and exposed to rituximab in the presence of 20% human serum for 30 min. Formation of the terminal complement complex (TCC) was detected with the anti–SC5b-9 primary monoclonal antibody WI3/I5, a phycoerythrin (PE)-labeled secondary antibody and measured by flow cytometry (E, Upper panels). As control for exosome labeling, the beads were stained for the exosome marker flotillin-2 (E, Lower panels). TCC formation was also detected on exosomes from patient samples taken 3 h after exposure to rituximab (F). (D) For an estimate of complement consumption, C3d levels were measured by ELISA using a polyclonal rabbit antibody (I3/15) following addition of beads labeled with exosomes from Su-DHL-4 lymphoma cells in the presence of rituximab (D, bars on right), with PBS and unlabeled beads as negative controls and zymosan as positive control for maximal complement fixation. Error bars indicate SDs of triplicates from a representative experiment of three replicates (repeated measure ANOVA with Bonferroni's post test).
Lymphoma Exosomes Impede CDC.
Rituximab exerts its cytocidal effects after CD20 ligation by initiating direct proapoptotic effects, CDC, and ADCC. Here, binding of rituximab to the exosomes of cell lines led to fixation of complement on the exosome surface, detected by the terminal complement complex (TCC) with the antibody W13/15 on the exosomes, both in vitro and in patients in vivo (Fig. 2 E and F). Moreover, exosomal complement fixation consumed plasma complement levels, measurable as the complement decay product C3d (Fig. 2D). Lymphoma exosomes themselves were largely resistant against complement lysis, associated with their high expression levels of CRPs and in congruence with previous data on exosomes derived from reticulocytes and antigen-presenting cells (see above and Fig. S3 C and D) (22, 23).
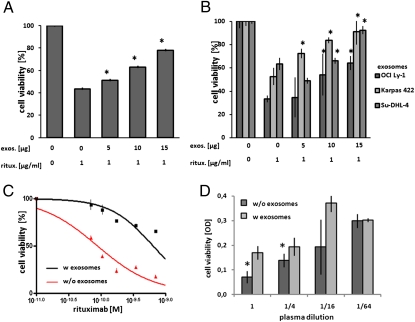
The consumption of both free antibody and complement raised our interest in the effects of exosomes as a third party on rituximab-mediated lymphoma cell lysis. Therefore, we exposed lymphoma cells to rituximab at EC50 in the presence of complement, adding exosomes at increasing concentrations. We observed a dose-dependent protection of the lymphoma cell targets from antibody attack both from autologous exosomes, i.e., exosomes derived from the respective parental cell line, and from allogeneic exosomes derived from other cell lines (Fig. 3 A and B). Importantly, in lymphoma patients treated with rituximab, depletion of exosomes from the patient plasma increased not only the cytolytic efficacy against cell line targets (Fig. 3C), but also the cytolytic activity of the rituximab-containing plasma after infusion against the patient's autologous tumor cells was significantly enhanced by depletion of exosomes (Fig. 3D).
Fig. 3.
Rescue of lymphoma cells from rituximab-mediated CDC by exosomes. Exosomes (exo.) were added to OCI-Ly1 cells, followed by addition of rituximab (ritux.) at EC50 (1 μg/mL), and viability was measured by MTT after an incubation at 37 °C for 1 h. Addition of both autologous (A) and allogeneic (B) exosomes, quantified by total protein, protected target cells from CDC dose-dependently (representative examples are of at least three replicates with SDs of triplicate two-way ANOVA with Bonferroni's post test). (C) And vice versa, patient plasma with or without depletion of exosomes by ultracentrifugation was added to Su-DHL-4 target cells, resulting in higher cytotoxicity of rituximab in the absence of exosomes, two-way ANOVA with Bonferroni's post test, a representative example of three experiments. (D) For the cytolytic acticity of patient plasma dilutions against autologous tumor cells ex vivo directly after rituximab infusion by MTT, we determined that the exosome-depleted plasma revealed higher efficacy (D, two-tailed Student's t test).
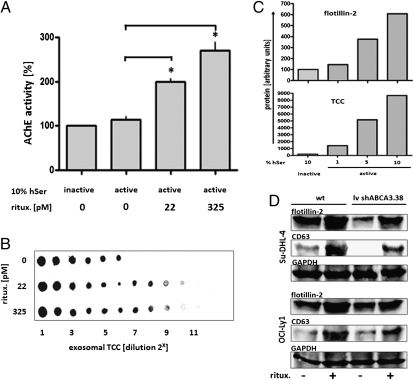
Sublytic complement attack induces a variety of biological effects in the target cells (review in ref. 24). Therefore, we looked for the effects of complement fixation by low levels of rituximab and discovered a significant increase in exosome release from lymphoma under attack (Fig. 4A). Increased exosome secretion was stimulated upon exposure to rituximab at picomolar concentrations, which induced no measurable apoptotic cell death (Fig. S4). Increased exosome release led to a concomitant increased shedding of terminal complement complex, depending on the amounts of both rituximab and of complement in the medium (Fig. 4 B and C). Furthermore, we asked for the impact of ABCA3 on the amounts of rituximab-induced exosome release, comparing wild-type lymphoma cells to variants in which ABCA3 expression had been silenced by two independent shRNA constructs (see below). Rituximab-induced exosome release was effective in both variants, however at decreased levels for both spontaneous and induced exosome release in the ABCA3-silenced variants (Fig. 4D). In vivo, the amounts of plasma exosomes increased following therapeutic rituximab applications, as detected both on the level of whole exosomal protein and by dot blot quantification for the exosomal marker proteins flottilin-2 and CD63 (Fig. S5 A and B). Thus, exosomes provide shielding of target cells against antibody-mediated complement attack both as a constitutive property of lymphoma cells and as an adaptive immune evasive response.
Fig. 4.
Rituximab stimulated shedding of exosomal-bound TCC from lymphoma cells. Su-DHL-4 lymphoma cells (5 × 107) were exposed to sublytic concentrations of rituximab as indicated in the presence of complement (10% human serum, hSer), and exosomes were harvested after 24 h (compare Fig. S4). (A) Yields of exosomes, quantified by AChE activity, increased with addition of rituximab (repeated measure ANOVA with Bonferroni's post test). (B) Accordingly, levels of exosome-bound terminal complement complex (TCC, with 10% active hSer) induced by rituximab were measured by dot blot for W13/15 of exosome dilutions. (C) Likewise, exosome release and fixation of TCC on exosomes increased with addition of active serum at the percentages indicated, quantified by densitrometry (ImageJ, http://rsbweb.nih.gov/ij/) of dot blots (representative result of three experiments). (D) Rituximab (325 pM) was added to Su-DHL-4 and OCI-Ly1 cells, either wild type or the respective variant transduced with an ABCA3-silencing vector (lv shABCA.38; compare Fig. 6). Exosomes were harvested after 48 h, and exosome release was measured by Western blot against flottilin-2 and CD63, and GAPDH from the parental cells was used as reference. Rituximab stimulated exosome release in both the wild type and the shRNA-silenced ABCA3 variants, albeit at lower levels in the ABCA3 knockdown variants.
Enhanced CDC Efficacy by Inhibition of Exosome Release and Silencing of ABC Transporter A3.
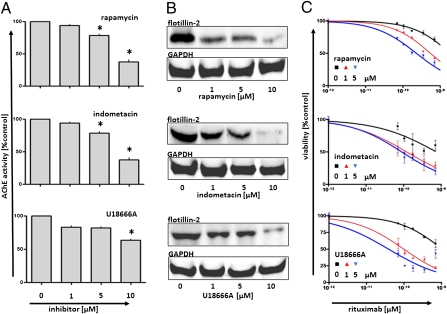
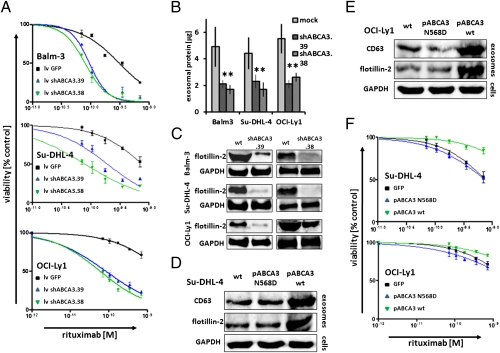
Several mechanisms have been described to interfere with cellular exosome secretion in different biological systems, ranging from agents perturbing MVB biogenesis such as rapamycin, to agents perturbing membrane cholesterol supply such as U18666A (25–27). We confirmed significant inhibitions of exosome release occurring at nontoxic concentrations of the drugs (Fig. 5 A and B and Fig. S6). Diminished exosome release was associated with increased lytic efficacy of rituximab in CDC experiments (Fig. 5C). In addition to the known inhibitors of exosome release, we discovered that the cyclooxygenase type-2 inhibitor indometacin impeded exosome release and that indometacin sensitized the lymphoma cells to CDC (Fig. 5C). Indometacin had been shown to suppress the expression of ABCA3 in acute myeloid leukemia cells, restoring susceptibility to cytostatic therapy (28). Indeed, indometacin strongly diminished ABCA3 expression in our B-cell lymphoma cell lines (Fig. S7) (compare with ref. 16). Suppression of ABCA3 expression by rapamycin and indometacin efficiently suppressed exosome release from the lymphoma cells (Fig. 5 and Fig. S6). Also, silencing of ABCA3 by two separate lentiviral shRNA constructs reduced exosome release from the cell line models Balm-3, Su-DHL-4, and OCI-Ly1 by ∼50% (Fig. 6 B and C). Concordantly, silencing of ABCA3 also increased the susceptibility of the lymphoma cells to CDC-mediated lysis (Fig. 6A). And vice versa, overexpression of ABCA3 alone was also sufficient to enhance exosome release from the cell lines Su-DHL-4 and OCI-Ly1, associated with an increased resistance to rituximab-mediated cell lysis (Fig. 6 D–F). As a control, enforced expression of a nonfunctional ABCA3 mutated in the ATP-binding site (N568D) was not sufficient to induce such effects (Fig. 6 D–F) (16). In addition, increased exosome secretion was also observed after enforced ABCA3 expression in HEK 293 cells, a model system, in which the expression of ABCA3 had been found to induce resistance to a broad spectrum of classical chemotherapy agents (Fig. S7C) (16). Together, these data show that ABCA3 positively modulates exosome release from B-cell lymphoma cells. This modulation is critical for the antibody susceptibility of lymphoma cells and offers a target for pharmacological intervention.
Fig. 5.
Inhibition of exosome shedding and enhanced CDC susceptibility induced by rapamycin, indometacin, and U18666A. OCI-Ly1 Lymphoma cells (5 × 107) were exposed to inhibitors of exosome synthesis or release, exosomes were harvested after a 24 h, and yields were measured by both AChE activity (A) and flotillin-2, with GAPDH as control of protein content in treated parental cells (B). Significant differences (repeated measure ANOVA with Bonferroni's post test) are marked (for equivalent results in the cell lines Balm3 and Su-DHL-4, see Fig. S6). (C) Concomitant incubation of lymphoma cells with inhibitors and rituximab in the presence of 10% active human complement increased the cytolytic activity of the antibody in a dose-dependent manner. Lymphoma cells were exposed to rituximab in the presence of complement. The differences in viabilities between experimental samples (with inhibitors) and controls reached significance at rituximab concentrations above 10−10 M (two-way ANOVA with Bonferroni's post test).
Fig. 6.
Role of ABCA3 for exosome release and anti-CD20–mediated complement-dependent cytolysis. To analyze ABCA3 function in exosome release, both silencing of ABCA3 with two independent shABCA3 constructs (PLKO.1-shRNA.38 and .39) and enforced gene expression with an ABCA3 wild-type plasmid and a nonfunctional mutant (pABCA3 N568D) were applied. The effects on exosome release were measured after incubation for 48 h by whole exosomal protein (B, significance tested by Student's two-sample t test), as well as flotillin-2 Western blot from exosome preparations in the cell culture supernatants (C). RNAi-mediated silencing of ABCA3 increased susceptibility of lymphoma cell lines to CDC (A: lv shABCA3.38, green line; lv shABCA3.39, blue line; two-way ANOVA with Bonferroni's post test). Addressing enforced ABCA3 expression, pABCA3wt and pABCA3N568D were introduced into Su-DHL-4 and OCI-Ly1 by electroporation and cultured for 48 h in exosome-free medium, and the yields of exosomes were harvested from equal amounts of cells probed by Western blot against flottilin-2 and CD63 (D and E). The transfectants expressing competent ABCA3 wt, but not the nonfunctional mutant ABCA3N568D, exhibited reduced susceptibility for CDC-mediated lysis (F). Two-way ANOVA with Bonferroni's post test was used.
Discussion
This study provides in vitro and in vivo evidence for exosome release from B-cell lymphoma cells, the expression of CD20 thereon, the protection of lymphoma cells from rituximab-mediated CDC, and the regulatory role of intracellular ABC transporter A3 for exosome release. These findings have several clinical and immunopathological implications.
First, our data show that CD20 participates in plasma circulation as a protein embedded in the exosomal membrane that intercepts rituximab. Such epitope-specific binding of anti-CD20 antibody outside the plasma membrane was previously not appreciated because neither the transmembrane CD20 protein nor antigenic parts thereof circulate as free molecules (29, 30). The currently available assay system for rituximab pharmacokinetics using the anti-idiotypic antibody MB2A4 detects both soluble and—at high proportions—exosomal-bound antibody (see above and Fig. 2). Notably, rituximab bound to exosomes is not available for plasma membrane attack, thus limiting the availability of cytocidal antibody in situations of high tumor—and tumor exosome—load, i.e., at the start of therapy. Our findings correlate with the clinical observation that starting rituximab antibody therapy requires high doses of antibody to achieve efficient plasma levels (31). In this situation, the absorption of rituximab into circulating exosomes may further contribute to this initial “sink” effect. Thus, exosomal CD20 represents a decoy target for rituximab, reducing the number of antibody molecules effectively reaching the tumor cell (overview in Fig. S7B).
Our data also clearly demonstrate that rituximab bound to exosomes fixes complement in vitro and in vivo and that the consumption of complement impedes the efficacy of rituximab-initiated CDC. Complement consumption occurs in the circulation, but local depletion of complement in the tumor micromilieu may even be of more relevance in vivo, predominantly in proximity to intratumoral blood vessels, where the ratio of antibody and complement passes an optimum for fixation and cell lysis (32). Given the accumulation of exosomes in the interstitial space (Fig. S1C), exosomal complement consumption may contribute to the protection of tumor cells particularly at a distance from the blood vessels. Note that we found that lymphoma-derived exosomes dispose of high amounts of CRPs (Fig. 1 and Fig. S3C), a finding also reported for exosomes from normal B-cells and antigen-presenting cells (23, 33). Furthermore, we found antibody attack to enhance secretion of exosomes at concentrations that were not sufficient to induce apoptosis in our cell line models (22 and 325 pM, Fig. S4). We cannot, at this point, exclude contributions of vesicle formation directly at the plasma membrane to the exosome fractions isolated. The function of the strictly intracellular ABC transporter A3, however, appeared to be critical for both spontaneous and antibody-triggered exosome release (Fig. 4D). Thus, the protective effects of lymphoma-derived exosomes represent both a constitutive property of the tumor cells and a resistance mechanism recruitable as an adaptative response to CDC-associated cellular stress.
Second, with regard to the biogenesis of exosomes, we reveal here a critical role of ABC transporter A3. ABCA3 is known to be an intracellular lipid transporter indispensable for surfactant production (34–37). In murine ABCA3 knock-out models, the pneumocytes of heterozygote animals had fewer lamellar bodies and showed diminished incorporation of radiolabeled substrates into newly synthesized phospholipids (38, 39). Sorting of membrane lipids is essential for MVB biogenesis and exosome release (27). To date, ABC transporters have not been recognized as modulators of exosome biogenesis, but with members of the group A of ABC transporters found mostly associated with lysosome-related organelles (LROs) (37), they can reasonably be expected to modulate this process. We and others have recently discovered that leukemia cells express ABCA3 and that their expression is associated with decreased susceptibility to cytostatic therapy (16–18, 40). Interestingly, ABCA3 protects tumor cells against some of the most efficient cytostatic drugs applied in lymphoma therapy, i.e., vincristine, anthracyclines, and etoposide (16). Aggressive lymphoma cells exceed other hematological malignancies in ABCA3 expression, consistent with their high levels of exosome secretion (Table S1) (16).
The classical concept of ABC transporter-mediated drug resistance describes direct transport of a cytotoxic substrate (41, 42). Our observations document clearly that ABC transporter function may impact on cell structure and metabolism and that such changes may entail a significant mechanism of drug resistance. ABCA3 expression has been described in a variety of tumor entities, so we expect this resistance mechanism to be of relevance beyond leukemia and lymphomas (16–18, 28, 43–46).
Importantly, inhibitors of exosome biogenesis or secretion augmented the cytolytic effects of anti-CD20 antibodies in our in vitro test systems. The known inhibitory mechanisms of the respective substances differ widely, e.g., the inhibition of MVB biogenesis by rapamycin, for which a synergism together with anti-CD20 antibody-mediated lysis has already been described (47). For indometacin, the central mechanism of inhibiting exosome release appears to be the down-regulation of ABCA3 expression at the transcriptional level (Fig. S7A) (28). Anti-cancer effects of indometacin have been consistently observed both clinically and in preclinical model systems (48).
Third, exosomes are under consideration for cancer immunotherapy (49–51). Our data, however, add to the notes of caution, which have shown that, instead of augmenting immune responses, exosomes may eventually also suppress antitumor immune responses (52, 53).
The evidence in this report has a focus on antibody binding and complement consumption. As the other effector mechanisms of rituximab, i.e., induction of apoptosis and ADCC, also depend on plasma membrane binding of rituximab, we extrapolate that such cytocidal mechanisms may also be perturbed by CD20-positive exosomes (54).
In summary, we found strong exosome release in aggressive lymphoma modulated by ABCA3, which leads to exosome-mediated shielding of target cells as a critical determinant of tumor cell susceptibility to antibody therapy.
Experimental Procedures
Cells, Antibodies, Small Molecules, Plasmids, and Vectors.
The cell lines Su-DHL-4, Balm-3, and OCI-Ly1 were used throughout this work, propagated by standard protocols (SI Experimental Procedures).
Exosome Preparation and Quantification.
Exosomes were prepared by differential centrifugation according to standard protocols (modified according to ref. 55). Following incubation of 5 × 107 lymphoma cells for 48 h in complete exosome-free medium, cells and larger debris were removed by centrifugation for 10 min (500 × g, 4 °C). The supernatant was centrifuged again (20 min, 10,000 × g, 4 °C; Beckman L8-55 μLtracentrifuge, rotor Ti32) to remove intermediate-size particles. Subsequently, the supernatant was filtered (0.22 μM Millex GP) and centrifuged (240 min, 120,000 × g, 4 °C; Beckman L8-55 μLtracentrifuge, rotor Ti32) to obtain the exosome pellet, which was washed in PBS and resuspended in 50 μL PBS. Exosomes were quantified by measuring whole protein (BioRad DC-Protein-Assay), Western blot-detecting flotillin-2 and CD63 in comparison with whole cells or control exosome preparations, and acetyl-cholin-esterase (AChE) activity as previously described (15) (SI Experimental Procedures).
Complement Analysis and Rituximab Measurements.
To quantify rituximab in medium and patient serum samples, an ELISA using the anti-idiotypic monoclonal antibody MB2A4 was applied as previously described (20, 21) (SI Experimental Procedures).
Supplementary Material
Acknowledgments
We thank Sabrina Becker of the Flow Cytometry Core Facility at the University of Goettingen. This work was supported by the Deutsche Forschungsgemeinschaft Grant DFG Wu 310/3-1 (to G.G.W.) and by a University Medicine Goettingen Jacob-Henle program grant (to T.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102855108/-/DCSupplemental.
References
- 1.Maloney DG, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466. [PubMed] [Google Scholar]
- 2.McLaughlin P. Treatment of follicular and other indolent lymphomas. Curr Opin Oncol. 1999;11:333–338. doi: 10.1097/00001622-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Keating GM. Rituximab: A review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs. 2010;70:1445–1476. doi: 10.2165/11201110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Gisselbrecht C. Use of rituximab in diffuse large B-cell lymphoma in the salvage setting. Br J Haematol. 2008;143:607–621. doi: 10.1111/j.1365-2141.2008.07383.x. [DOI] [PubMed] [Google Scholar]
- 6.Manches O, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–954. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 7.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 8.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 9.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raposo G, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zitvogel L, et al. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 13.Escudier B, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bard MP, et al. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004;31:114–121. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 15.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 16.Chapuy B, et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia. 2008;22:1576–1586. doi: 10.1038/leu.2008.103. [DOI] [PubMed] [Google Scholar]
- 17.Steinbach D, et al. ABCA3 as a possible cause of drug resistance in childhood acute myeloid leukemia. Clin Cancer Res. 2006;12:4357–4363. doi: 10.1158/1078-0432.CCR-05-2587. [DOI] [PubMed] [Google Scholar]
- 18.Chapuy B, et al. ABC transporter A3 facilitates lysosomal sequestration of imatinib and modulates susceptibility of chronic myeloid leukemia cell lines to this drug. Haematologica. 2009;94:1528–1536. doi: 10.3324/haematol.2009.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 20.Cragg MS, et al. A new anti-idiotype antibody capable of binding rituximab on the surface of lymphoma cells. Blood. 2004;104:2540–2542. doi: 10.1182/blood-2004-05-1733. [DOI] [PubMed] [Google Scholar]
- 21.Hampson G, et al. Validation of an ELISA for the determination of rituximab pharmacokinetics in clinical trials subjects. J Immunol Methods. 2010;360:30–38. doi: 10.1016/j.jim.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Rabesandratana H, Toutant JP, Reggio H, Vidal M. Decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59) are released within exosomes during in vitro maturation of reticulocytes. Blood. 1998;91:2573–2580. [PubMed] [Google Scholar]
- 23.Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33:522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 24.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: Roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27:375–387. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 25.Fader CM, Sánchez D, Furlán M, Colombo MI. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic. 2008;9:230–250. doi: 10.1111/j.1600-0854.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- 26.Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strauss K, et al. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem. 2010;285:26279–26288. doi: 10.1074/jbc.M110.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song JH, Kim SH, Kim HJ, Hwang SY, Kim TS. Alleviation of the drug-resistant phenotype in idarubicin and cytosine arabinoside double-resistant acute myeloid leukemia cells by indomethacin. Int J Oncol. 2008;32:931–936. [PubMed] [Google Scholar]
- 29.Anderson KC, et al. Expression of human B cell-associated antigens on leukemias and lymphomas: A model of human B cell differentiation. Blood. 1984;63:1424–1433. [PubMed] [Google Scholar]
- 30.Einfeld DA, Brown JP, Valentine MA, Clark EA, Ledbetter JA. Molecular cloning of the human B cell CD20 receptor predicts a hydrophobic protein with multiple transmembrane domains. EMBO J. 1988;7:711–717. doi: 10.1002/j.1460-2075.1988.tb02867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiser M, et al. Serum levels and pharmacokinetic of rituximab in bi-weekly R-CHOP in elderly patients with DLBCL treated in the RICOVER-60 trial. Blood. 2006;108:2748. [Google Scholar]
- 32.Press OW, et al. Monoclonal antibody 1F5 (anti-CD20) serotherapy of human B cell lymphomas. Blood. 1987;69:584–591. [PubMed] [Google Scholar]
- 33.Hakulinen J, Junnikkala S, Sorsa T, Meri S. Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol. 2004;34:2620–2629. doi: 10.1002/eji.200424969. [DOI] [PubMed] [Google Scholar]
- 34.Yamano G, et al. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- 35.Mulugeta S, et al. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- 36.Matsumura Y, Sakai H, Sasaki M, Ban N, Inagaki N. ABCA3-mediated choline-phospholipids uptake into intracellular vesicles in A549 cells. FEBS Lett. 2007;581:3139–3144. doi: 10.1016/j.febslet.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 37.Wenzel JJ, Piehler A, Kaminski WE. ABC A-subclass proteins: Gatekeepers of cellular phospho- and sphingolipid transport. Front Biosci. 2007;12:3177–3193. doi: 10.2741/2305. [DOI] [PubMed] [Google Scholar]
- 38.Ban N, et al. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282:9628–9634. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- 39.Cheong N, et al. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282:23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- 40.Efferth T, et al. Expression profiling of ATP-binding cassette transporters in childhood T-cell acute lymphoblastic leukemia. Mol Cancer Ther. 2006;5:1986–1994. doi: 10.1158/1535-7163.MCT-06-0086. [DOI] [PubMed] [Google Scholar]
- 41.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 42.Hedley DW, et al. A novel energy dependent mechanism reducing daunorubicin accumulation in acute myeloid leukemia. Leukemia. 1997;11:48–53. doi: 10.1038/sj.leu.2400538. [DOI] [PubMed] [Google Scholar]
- 43.Hirschmann-Jax C, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasui K, et al. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Liu Y, Zong Z, Tian D. The Rho kinase inhibitor fasudil inhibits the migratory behaviour of 95-D lung carcinoma cells. Biomed Pharmacother. 2010;64:58–62. doi: 10.1016/j.biopha.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Schimanski S, et al. Expression of the lipid transporters ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm Metab Res. 2010;42:102–109. doi: 10.1055/s-0029-1241859. [DOI] [PubMed] [Google Scholar]
- 47.Wanner K, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol. 2006;134:475–484. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- 48.Hull MA, Gardner SH, Hawcroft G. Activity of the non-steroidal anti-inflammatory drug indomethacin against colorectal cancer. Cancer Treat Rev. 2003;29:309–320. doi: 10.1016/s0305-7372(03)00014-8. [DOI] [PubMed] [Google Scholar]
- 49.André F, et al. Tumor-derived exosomes: A new source of tumor rejection antigens. Vaccine. 2002;20(Suppl 4):A28–A31. doi: 10.1016/s0264-410x(02)00384-5. [DOI] [PubMed] [Google Scholar]
- 50.Février B, Raposo G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 51.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: A common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 52.Riteau B, et al. Exosomes bearing HLA-G are released by melanoma cells. Hum Immunol. 2003;64:1064–1072. doi: 10.1016/j.humimm.2003.08.344. [DOI] [PubMed] [Google Scholar]
- 53.Taylor DD, Gerçel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson NA, et al. Diffuse large B-cell lymphoma: Reduced CD20 expression is associated with an inferior survival. Blood. 2009;113:3773–3780. doi: 10.1182/blood-2008-09-177469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.