Abstract
SNARE protein-driven secretion of neurotransmitters from synaptic vesicles is at the center of neuronal communication. In the absence of the cytosolic protein Munc18-1, synaptic secretion comes to a halt. Although it is believed that Munc18-1 orchestrates SNARE complexes, its mode of action is still a matter of debate. In particular, it has been challenging to clarify the role of a tight Munc18/syntaxin 1 complex, because this interaction interferes strongly with syntaxin's ability to form a SNARE complex. In this complex, two regions of syntaxin, the N-peptide and the remainder in closed conformation, bind to Munc18 simultaneously. Until now, this binary complex has been reported for neuronal tissues only, leading to the hypothesis that it might be a specialization of the neuronal secretion apparatus. Here we aimed, by comparing the core secretion machinery of the unicellular choanoflagellate Monosiga brevicollis with that of animals, to reconstruct the ancestral function of the Munc18/syntaxin1 complex. We found that the Munc18/syntaxin 1 complex from M. brevicollis is structurally and functionally highly similar to the vertebrate complex, suggesting that it constitutes a fundamental step in the reaction pathway toward SNARE assembly. We thus propose that the primordial secretion machinery of the common ancestor of choanoflagellates and animals has been co-opted for synaptic roles during the rise of animals.
Neurons, the building blocks of the nervous system, are highly specialized for fast information transmission, which takes place in the form of vesicular neurotransmitter release at specialized junctions, the chemical synapses. Synapses evolved early in animal evolution, and relatively primitive nervous systems can be found in early branching animals, such as jellyfish (1, 2). By contrast, sponges (3) or the placozoan Trichoplax adhaerens (4), appear not to be equipped with bona fide synapses, yet possess several factors related to synaptic function. Hence, it is possible that central features of synaptic transmission evolved early in animal evolution, possibly during the transition to multicellularity.
Choanoflagellates, a group of mostly single-celled eukaryotes that possess a single posterior flagellum surrounded by a collar of actin-based tentacles, are thought to be the closest known sister group to animals (5–8). Although a long period separates the choanoflagellate and animal lineages, it is possible that choanoflagellates have remained similar to the unicellular organism from which all animals evolved (e.g., refs. 7–13). However, it remains unclear which molecular mechanism of the unicellular precursor was fundamental for the development of the neuronal communication apparatus (14)?
To address this matter we focused on a key feature of synapses, the rapid discharge of neurotransmitter-loaded vesicles upon fusion with the presynaptic plasma membrane. In vertebrates, this process is mediated by the neurosecretory soluble NSF attachment protein receptors (SNARE) proteins synaptobrevin 2, syntaxin 1, and SNAP-25. Their assembly between the opposing membranes is thought to drive membrane fusion (15). SNARE function is tightly regulated by Munc18-1, a member of the conserved Sec1/Munc18 (SM) protein family. In the absence of Munc18-1, neurotransmitter release is blocked completely (16–18); note that the homologous factor is referred to as Rop1 in Drosophila melanogaster and Unc18 in Caenorhabitis elegans. The biochemical correlate(s) underlying this positive genetic role remain a matter of debate (e.g., reviewed in refs. 19–22 but see also ref. 23). In brief, it has been challenging to integrate the role of a very tight Munc18/syntaxin 1 complex, because the grip of Munc18 strongly interferes with syntaxin's ability to form a SNARE complex (23–25). It was therefore suggested that the Munc18/syntaxin 1 complex is not universal but plays a special role during neuronal secretion only (20, 21, 26, 27). So far, comparative studies on the other vertebrate Munc18 isoforms, Munc18-2 and Munc18-3, or on the Sacharomyces cerevisiae homolog Sec1 have not been able to clarify this conundrum (28–32). Thus, we decided to tackle the question about the role of the tight Munc18/syntaxin 1 complex from a new angle by comparing the workings of the homologous secretion machinery of the choanoflagellate Monosiga brevicollis with that of animals. Here, we report that M. brevicollis expresses a Munc18 homolog and find that all three secretory SNARE proteins and Munc18 are confined to the apical pole. Further, the mode of interaction of Munc18 and syntaxin 1 from M. brevicollis strikingly resembles that of their animal homologs. Finally, the crystal structure of the M. brevicollis Munc18/syntaxin 1 complex revealed that this high-affinity interaction involves contacts between the N-peptide of syntaxin 1 and its remainder in closed conformation, as it does in the rat. This binding behavior shows that the configuration of the Munc18/syntaxin 1 complex was already in place in the last common ancestor of choanoflagellates and animals.
Results
M. brevicollis Is Equipped with a Single Set of Synapse-Like Core Secretion Proteins.
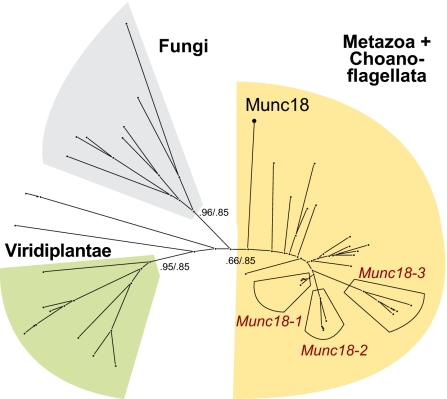
The genome of the choanoflagellate M. brevicollis contains a single set of secretory SNARE proteins (33) and a unique Munc18 homolog, closely related to the ones involved in regulated secretion in animals (Fig. 1). These distributions suggest that the last common ancestor of choanoflagellates and animals was equipped with only one set of core secretion proteins that likely bore a close resemblance to the factors found in the genome of M. brevicollis.
Fig. 1.
Schematic outline of a phylogenetic tree of the SM protein Munc18 demonstrates that the core machinery of the secretory apparatus of M. brevicollis is closely related to animals. Detailed versions of the Munc18 tree and also of the SNAP-25 and syntaxin1 trees are shown in Fig. S1. The major eukaryotic lineages are emphasized by different colors, expansions in the vertebrate lineage are indicated. In addition, the position of Munc18 from M. brevicollis (Munc18) is shown. Whereas M. brevicollis possesses only one Munc18 homolog expansion of Munc18-like factors occurred in vertebrates, giving rise to the three isoforms Munc18-1, -2, and -3. The labels on the major branches represent the Likelihood Mapping (Left) and almost unbiased (AU) support values (Right).
Secretory Components Are Confined to the Apical Pole of M. brevicollis.
Although choanoflagellate genomes can assist in reconstructing the genetic tool kit of the common ancestor of choanoflagellates and animals, their morphology may allow a glimpse at the features of the early animal cell. Indeed, striking morphological similitudes between choanoflagellates and sponge choanocytes have been noted already in the middle of the 19th century (ref. 9; reviewed in ref. 34). The close relationship between choanoflagellates and animals has been confirmed by phylogenetic analysis (7, 8, 10, 11). Hence, studying the localization and interactions of M. brevicollis proteins could shed light on their original role.
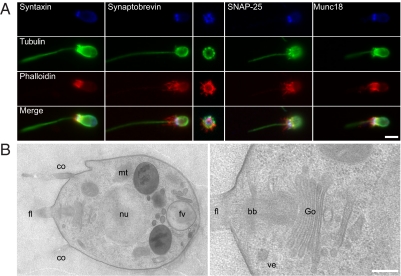
We assessed the subcellular localization of these secretory proteins in M. brevicollis by immunostaining. To this end, we raised polyclonal antibodies against the soluble region of synaptobrevin 1 from M. brevicollis. In addition, we found that several antibodies originally raised against the rat proteins syntaxin 1a, SNAP-25, and Munc18-1 also specifically recognized their respective homologs from M. brevicollis. All four core components of the secretory machinery are confined to the posterior pole of the cell (Fig. 2A), a region that has been suggested to be involved in secretion (35). For example, in the derived lineage of loricate choanoflagellates, the building blocks of the lorica, so-called costal strips, are secreted within the circumfence of the collar base (7). At the ultrastructural level, the basic arrangement of organelles in M. brevicollis (Fig. 2B) resembles the arrangement reported for other choanoflagellates (e.g., refs. 35 and 36). At the posterior pole, the single Golgi apparatus is observed beneath the nucleus, and numerous vesicles of 75–200 nm in diameter are mostly directed to the rear end of the cell, close to the plasma membrane (Fig. 2B, Right).
Fig. 2.
SNAREs, Munc18, and the secretory apparatus in M. brevicollis. (A) Confocal micrographs illustrate immunolabelings for syntaxin 1, synaptobrevin, SNAP-25, or Munc18, all detected at the posterior (apical) pole of the cell. (Center) A cross-section image through the posterior pole shows a circular distribution of synaptobrevin. Colabeling of tubulin and actin allow for identifying the cell's cytoskeleton/architecture. (B) Ultrastructural investigation reveals the presence of the Golgi apparatus (Go) and associated clear vesicles (ve) at the posterior pole of the cell, near the root/basal body of the flagellum (fl) (Right). Note the presence of unrelated, heterogeneous large vesicles, most probably food vacuoles (fv), at the anterior pole (Left). The cell nucleus (nu), mitochondria (mt), and the collar (co) are indicated as well. (Scale bars: A, 1 μm; B, 500 nm.)
Together these data are congruous with the notion that choanoflagellates possess a well-defined secretory machinery that, while relatively simple, may have served as the raw material for the evolution of the intricate machinery found in animal cells.
Conserved Properties of the Core Secretory Components of M. brevicollis.
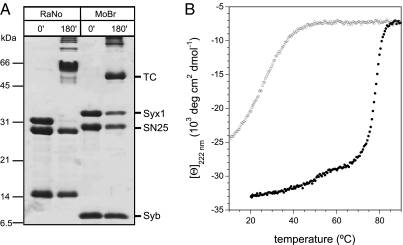
To study the interaction of the choanoflagellate proteins directly, we expressed the proteins in E. coli. We found that the three SNARE proteins form an SDS-resistant complex (Fig. 3A), as the neuronal SNARE complex from several different animal species do (37). The high stability of the core SNARE complex from M. brevicollis was confirmed by circular dichroism (CD) spectroscopy (Fig. 3B).
Fig. 3.
The secretory SNARE proteins from M. brevicollis form a highly stable, SDS-resistant complex. (A) Approximately stoichiometric amounts of SNAP-25, syntaxin 1, and synaptobrevin from R. norvegicus or from M. brevicollis were mixed and incubated for 3 h at RT. Without prior boiling, the secretory SNARE proteins from both species form ternary SDS-resistant complexes (tc) as indicated (37). (B) The core SNARE complex from M. brevicollis exhibits a hysteresis in the unfolding and refolding transitions similar to the one found for the rat complex (41). The core SNARE complex consisting of Syx1 (200-279), Syb (1-75), and SNAP-25 was purified by ion exchange and measured in PBS buffer, pH 7.4. Unfolding of the α-helical complex occurred at Tm ∼ 80 °C (black curve), whereas refolding was observable only at ≈50 °C (gray curve). The transitions were observed at 222 nm.
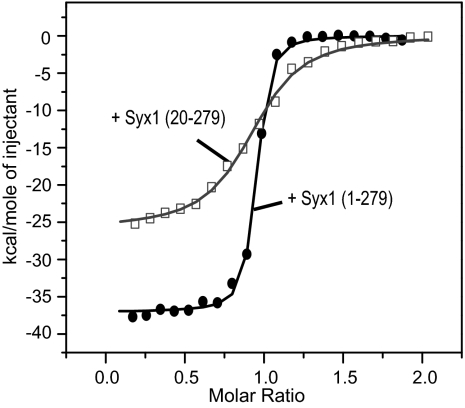
We next studied the interaction of the choanoflagellate Munc18 with the SNARE machinery. We first explored the canonical binary interaction between Munc18 and syntaxin 1 quantitatively by using isothermal titration calorimetry (ITC). We found that the entire cytosolic domain of syntaxin 1, Syx1 (1-279), binds to Munc18 with high affinity (Kd = 3.9 nM; Fig. 4 and Table 1). A comparable affinity has been determined for the interaction of Munc18-1 and syntaxin 1a from rat (23). In addition, the interaction between Munc18 and syntaxin 1 from M. brevicollis led to an increase in intrinsic fluorescence (Fig. S2A) similar to what has been observed for the rat homologs (23), corroborating that both complexes adopt a similar configuration (Fig. S2B). Of note, we found that rat Munc18-1 is able to bind to M. brevicollis syntaxin 1, albeit with reduced affinity (Kd = 80 nM; Fig. S3), confirming the close resemblance of the homologous pairs.
Fig. 4.
Munc18 and syntaxin 1 from M. brevicollis interact with nanomolar affinity. Calorimetric titrations of syntaxin 1 into Munc18 from M. brevicollis. Syx1 (1-279) binds Munc18 with higher affinity and enthalpy than Syx1 (20-279), indicating that the N-peptide participates in binding. The graph displays the integrated areas normalized to the amount of the injectant (kcal·mol−1) versus its molar ratio to Munc18. The solid lines represent the best fit to the data for a single binding site model. The raw data are shown in Fig. S3.
Table 1.
Thermodynamic parameters for Munc18/syntaxin 1 interactions from M. brevicollis
| Munc18 interaction with | Kd, nM | ΔH°, kcal/mole | n |
| Syx1 (1-279) | 3.9 ± 0.6 | −36.7 ± 0.2 | 0.91 |
| Syx1 (1-265) | 5.0 ± 0.9 | −36.8 ± 0.3 | 0.95 |
| Syx1 (1-242) | 450.4 ± 77.3 | −9.6 ± 0.6 | 0.94 |
| Syx1 (1-199) | 558.7 ± 88.6 | −2.8 ± 0.3 | 0.92 |
| Syx1 (1-20) | — | — | — |
| Syx1 (20-279) | 64.1 ± 7.0 | −25.8 ± 0.4 | 0.91 |
| SNARE complex containing Syx1 (1-279) | 571.4 ± 62.6 | −3.0 ± 0.2 | 1.04 |
| SNARE complex containing Syx1 (200-279) | — | — | — |
The corresponding experimental ITC data are shown in Fig. S3. Note that no heat changes were detected when Syx1 (1-20) or the SNARE-complex containing Syx1 (200-279) were mixed with Munc18.
Two distinct regions of syntaxin 1a are known to contribute to the interaction with Munc18-1: The very N-terminal region, called the N-peptide, binds to the outer surface of Munc18-1’s domain 1, whereas the remainder of the syntaxin molecule in a closed conformation binds to a clamp-like structure formed by the three domains of Munc18-1 (23). To test whether a similar configuration may play a role in the interaction of Munc18 and syntaxin 1 from M. brevicollis, we generated several deletion constructs of syntaxin 1. A syntaxin 1 variant, in which the first 19 residues were removed, Syx1 (20-279), showed a clearly reduced affinity to Munc18 (Kd ∼ 64 nM), and a decrease in binding enthalpy (ΔΔH = 10.9 kJ/mol; Fig. 4 and Table 1). However, no binding was detected when the N-peptide alone, Syx1 (1–20), was tested (Table 1 and Fig. S3). Thus, in M. brevicollis as in the rat, the N-peptide and the remaining portion of syntaxin 1 cooperate for high affinity binding to Munc18.
We then tested binding of Munc18 from M. brevicollis to fully assembled SNARE complexes containing different syntaxin constructs. We found that Munc18 binds to the SNARE complex containing Syx1 (1-279) with rather low affinity (Kd ∼ 570 nM; Table 1 and Fig. S3), whereas no binding was detected between Munc18 and a SNARE complex containing syntaxin 1 bearing only its SNARE domain region [Syx1 (200-279)]. This ITC experiment indicates that in M. brevicollis, as in the rat (23), Munc18 does not interact significantly with the extended four-helix bundle SNARE complex.
Crystal Structure of the Munc18/Syntaxin 1 Complex from M. brevicollis.
Altogether, our ITC analysis suggests a mode of interaction between Munc18 and secretory SNAREs from M. brevicollis very similar to that of the vertebrate homologs. To more precisely assess the degree of conservation, we then determined the structure of the complex from M. brevicollis by X-ray crystallography.
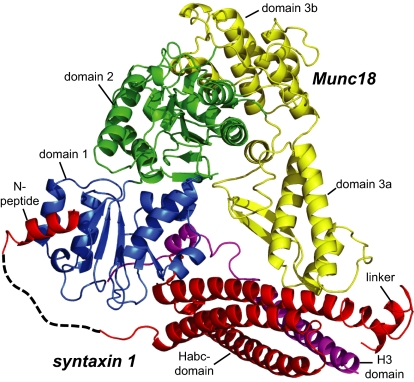
The crystal structure confirmed that two regions of syntaxin 1, the N-peptide and the remainder in closed conformation, bind to Munc18 simultaneously (Fig. 5). Very similar to the structure of the neuronal Munc18-1/syntaxin 1a complex, the N-peptide of the choanoflagellate syntaxin 1 binds to the outer surface of Munc18 domain 1, whereas the remainder of syntaxin 1 interacts with the concave surface formed by domains 1 and 3a of Munc18. In fact, the structures of the choanoflagellate Munc18/syntaxin 1 complex and rat Munc18-1/syntaxin 1a complex can be superimposed with an overall mainchain rmsd of 2.0 Å. Despite the close overall similarity there is a difference to note: The linker helix between syntaxin Habc and H3 domains of M. brevicollis adopts a slightly different conformation than observed in the rat crystal structure. This difference in orientation can be attributed to the lack of a salt bridge between Arg142 of the Hb domain and Glu166 of the linker helix in the R. norvegicus structure (Fig. S4). This is interesting as this interaction is thought to stabilize the closed conformation of rat syntaxin 1a. Notwithstanding, syntaxin 1 from M. brevicollis adopts a closed conformation in the Munc18/syntaxin 1 complex.
Fig. 5.
Crystal structure of the Munc18/syntaxin 1 complex from M. brevicollis. Munc18 domain 1 is formed by residues 1–129, domain 2 by residues 130–237 and 477–616, and domain 3 by residues 238–476. Domain 3 can be further subdivided into a lower half (3a) and an upper half (3b). The domains of Munc18 are colored blue, green, and yellow, respectively. The N-peptide (residues 2–15), the Habc (residues 39–172), the linker helix (183-192) of syntaxin 1 are colored red, and the H3 region (residues 210–261) is colored purple. The dashed line represents residues 16–38 of syntaxin, which were not visible in the electron density maps. Crystallographic data and refinement statistics are given in Table S1. Note that the ordered region of the bound N-peptide of syntaxin 1 is slightly longer in M. brevicollis than in the Munc18-1/syntaxin 1a structure. A more detailed description of the N-peptide is given in Fig. S5.
M. brevicollis Munc18 Controls SNARE Assembly.
For many years, it has been known that tight binding of murine Munc18-1 to syntaxin 1a prevents syntaxin 1a from forming a SNARE complex (24, 25). Recently, we have discovered that binding of the syntaxin N-peptide to the outer surface of Munc18-1 can regulate this process: When the syntaxin 1a N-peptide is bound to Munc18-1, SNARE complex formation is slowed drastically, and removal of the N-peptide enables binding of syntaxin 1a to its partner SNAREs while still bound to Munc18-1 (23).
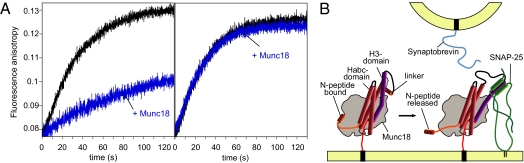
We therefore examined next whether this switch is present in the M. brevicollis Munc18–syntaxin 1 interaction as well. To monitor SNARE complex assembly, we established a fluorescence-based kinetic approach similar to the one that was instrumental to discovering the switch for the murine proteins (23). Indeed, when fluorescently labeled M. brevicollis synaptobrevin was mixed with SNAP-25 and syntaxin 1, with or without N-peptide, from M. brevicollis, an increase in fluorescence anisotropy corresponding to the formation of a ternary SNARE complex was observed. By contrast, premixing of Munc18 with Syx1 (1-279) produced an almost complete block of SNARE complex formation (Fig. 6A, Left). Remarkably, this block was not observed when Syx1 (20-279) was used instead (Fig. 6A, Right), demonstrating that binding of the N-peptide to Munc18 is required to block SNARE complex formation. Similar results were obtained when SNARE complex formation was monitored by SDS/PAGE (Fig. S6).
Fig. 6.
Munc18 from M. brevicollis controls SNARE assembly. Synaptobrevin was labeled with Texas red at Cys58, corresponding to Cys79 of rat synaptobrevin 2 used in our previous study (23). Ternary SNARE complex formation was followed by the increase in fluorescence anisotropy of 50 nM fluorescent Syb1-75 upon mixing with 1 μM syntaxin 1 and 3 μM SNAP-25. In the absence of Munc18, SNARE complexes for both syntaxin 1 variants, Syx1 (1-279) and Syx1 (20-279) formed (A, black curves). In the presence of Munc18 (1 μM), SNARE assembly was almost completely inhibited for Syx 1 (1–279) (Left), but not for Syx1 (20–279) (Right). We noted that the slowdown of SNARE complex formation in the presence of Munc18-1 was not found when a semiquantitative binding assay was used (31). Therefore, it needs to be emphasized here that a kinetic approach is essential to uncover the switch mechanism, whereas binding assays, by nature, are often only suited to uncover an end-product of a binding reaction. (B) Schematic model of how the release of the N-peptide might set off a conformational change that allows binding of SNAP-25 to the Munc18/syntaxin 1 complex, in turn establishing a binding site for the vesicular synaptobrevin.
Discussion
It is widely accepted that zippering of SNARE proteins drives membrane fusion during neuronal exocytosis (15). By contrast, divergent models of how Munc18-1 interacts with the SNARE machinery have been proposed (19–21, 38). In recent years, a model is being favored, in which SM proteins generally clasp an assembled SNARE complex. This scenario seems to reconcile conflicting evidence from in vivo and in vitro studies because it places Munc18-1 at a central position at which it would be able to support membrane fusion. Although the scenario is tempting, it neglects the fact that the tight Munc18-1/syntaxin 1a complex interferes strongly with SNARE complex formation (23–25) and cannot explain how syntaxin 1 is able to escape the tight grip of Munc18. In addition, physiological investigations support a role for Munc18 upstream of the membrane fusion reaction (39).
Despite its extraordinarily stability, it has been argued that the binary Munc18-1/syntaxin 1a complex might only play a supporting role in neuronal secretion (e.g., refs. 20, 21, and 26). The strict structural and functional conservation of the Munc18/syntaxin 1 complex in M. brevicollis, however, brings now to light that the binary complex must have played an important role in the last common ancestor of choanoflagellates and animals and, hence, that it is not a specialization of the neuronal secretion apparatus. In fact, the binding mode with its two spatially separated but linked binding sites makes the binary complex perfectly suited for regulating an important step in the reaction cascade toward SNARE complex assembly (Fig. 6B).
Probably, the primordial machinery of the common ancestor of choanoflagellates and animals served as a starting point for the evolution of the more complex machinery found in animals, in particular in vertebrates. For example, we noted that a prominent expansion of the set of Munc18 genes took place during the evolution of vertebrates, giving rise to three different genes, Munc18-1, -2, and -3. Interestingly, a comparable expansion occurred in the set of secretory SNAREs during vertebrate evolution (33), suggesting that distinct pairs of Munc18 and secretory syntaxins coevolved and may have helped develop a more versatile secretion apparatus. Indeed, those novel factors are largely confined to different subcellular locations or their expression is restricted to particular tissues or different developmental stages (15).
Remarkably, both rat and M. brevicollis syntaxin 1 adopt a closed conformation in complex with Munc18. At first glance, Munc18 appears to lock syntaxin into a conformation that is incompatible with SNARE complex assembly. Therefore, not surprisingly, it is often assumed that syntaxin first needs to leave the tight grip of Munc18. Only then, according to this idea, can syntaxin open up in preparation for SNARE complex assembly (19–21, 38). However, does syntaxin really need to escape to form a SNARE complex? An alternative explanation supported by our findings is that Munc18 might merely reorganize syntaxin in such a way that its conformation is compatible with SNARE complex assembly. Note that this conformation does not necessarily resemble the classical concept of an “open” syntaxin. In fact, we recently found that binding of the N-peptide to the outer surface of Munc18-1 is necessary for Munc18-1 to control the accessibility of syntaxin 1a for its SNARE partners. When syntaxin's N-peptide is removed, the block is relieved, suggesting that syntaxin 1a can form a SNARE complex while still bound to Munc18-1 (23). We found that this handover mechanism is also present in the homologous pair from M. brevicollis, demonstrating that it constitutes a fundamental functional principle of these proteins. It seems therefore that Munc18’s essential role is to steer syntaxin into a configuration suited for SNARE complex assembly. How exactly this transition takes place, whether it involves a conformational change within the binary complex (Fig. 6B), and which other factors control this step will require further study.
Experimental Procedures
Protein Purification.
The soluble portion of synaptobrevin [Syb (1-75)] was amplified by PCR from M. brevicollis cDNA. For specific labeling, a construct containing a cysteine at position 58 was prepared. Codon optimized version of SNAP-25 (1-210), Munc18 (1-649) and the soluble portion of syntaxin 1 [Syx1 (1-279)] of M. brevicollis were prepared by gene synthesis (GenScript). In addition, the following truncated variants were constructed: Syx1 (1-265); Syx1 (1-242); Syx1 (1-199); Syx1 (20-279), and Syx1 (200-279). All constructs were cloned into a pET28a vector and expressed in E. coli. Proteins and SNARE complexes assembled from purified monomers were purified by Ni2+-NTA affinity chromatography followed by ion-exchange chromatography essentially as described (23). A peptide comprising the first 20 residues of syntaxin 1 [Syx1 (1–20)] was synthesized (Biosyntan).
Phylogeny.
Phylogenetic reconstruction was carried out as described (33). To gain insights into the phylogenetic placement of the core factors of the secretory machinery from the choanoflagellate M. brevicollis, we included sequences from 15 animals, 7 fungi, 6 plants, and 4 protists. Detailed information about the sequences and computational approaches used are given in SI Experimental Procedures.
ITC.
ITC was performed on a VP-ITC instrument (GE Healthcare) at 25 °C essentially as described (23). The measured heat released on binding was integrated and analyzed with Microcal Origin 7.0 by using a single-site binding model, yielding the equilibrium association constant Ka, the enthalpy of binding ΔH, and the stoichiometry n. Experimental data are shown in Fig. S3.
Spectroscopy.
Fluorescence measurements were carried out in a Fluorolog 3 spectrometer equipped for polarization (Horiba Scientific). All experiments were performed at 25 °C in 1-cm quartz cuvettetes in PBS buffer. Fluorescence anisotropy, which is used to indicate the local flexibility of the labeled residue, and which increases upon complex formation and decreases upon dissociation, were measured essentially as described (23). CD spectroscopy measurements were performed essentially as described (40, 41) by using the Chirascan instrument (Applied Photophysics). Quartz cuvettetes with a pathlength of 0.1 cm were used. The ellipticity at 222 nm was recorded between 20 and 95 °C at a temperature increment of 30 °C/h.
Crystallization and Data Collection.
For crystallization, a slightly shorter syntaxin 1 construct, Syx1 (1-265), was used. This construct comprises the syntaxin region structured in the homologous Munc18-1/syntaxin 1a crystal structure, i.e., Syx1a (1-248) (23, 42). Syx1 (1-265) was found to bind Munc18 with the same affinity and enthalpy as Syx1 (aa 1–279) (Table 1 and Fig. S3). Crystals were obtained by the sitting drop vapor diffusion method by mixing equal volumes of 17 mg/mL Munc18/Syx1 (1-265) complex with reservoir buffer containing 7.5% PEG 6000 and 0.1 M Tris·HCl at pH 7.0, 4.25% 2-Methyl-2,4-Pentanediol and 15% glycerol. After flash-cooling in liquid nitrogen, diffraction data were collected to a resolution of 2.8 Å at SLS beamline PX2. The crystals belonged to space group P6522 with lattice dimensions of a = b = 146.2 Å and c = 214.8 Å. Procedures for structure determination and refinement are given in SI Experimental Procedures.
Cell culture and Microscopy.
M. brevicollis (50154; American Type Culture Collection) were cultured in artificial sea water mixed with Wards cereal grass medium in a 1:1 ratio, adjusted to a salt concentration of 53 mS/cm and sterile-filtered. Cultures were maintained at 25 °C and diluted 1:100 once a week. For immunofluorescence, the cells were grown to a density of 106 to 107 cells/mL and fixed by adding formaldehyde to a final concentration of 4%. Approximately 0.7 mL of the fixed culture was applied to poly-l-lysine-coated coverslips and left to sediment for 30 min. At room temperature, the coverslips were washed gently four times with PEM (100 mM Pipes at pH 6.9, 1 mM EGTA, and 0.1 mM MgSO4), incubated for 30 min in blocking solution (PEM+: 1% BSA, 0.3% Triton X-100), 1 h in primary antibodies solution (in PEM+), and after further washes (PEM+), 1 h in the dark with fluorescent secondary antibodies (1:100 in PEM+, polyclonal goat anti-mouse-Cy2, and goat anti-rabbit-Cy5; Jackson Immunoresearch) and washed again four times (PEM). A last 15-min incubation in the dark with rhodamine phalloidin (6 U/ml in PEM; Molecular Probes) enabled the visualization of F-actin. After 3 washes (PEM), coverslips were mounted onto slides with Fluorescent Mounting Media (4 μL; Dako). The following primary antibodies have been used: mouse monoclonal antibody against β-tubulin (E7, 1:200; Developmental Studies Hybridoma Bank); rabbit polyclonals against M. brevicollis synaptobrevin (1:100), rat SNAP25 (1:200), rat syntaxin 1 (1:200), and rat Munc18 (1:200). Single-plane confocal images were taken under an inverted TCS-SP2 confocal laser-scanning microscope (Leica Microsystems) with a 63× (NA 1.4) oil objective and a digital zoom factor of 6. Confocal images from dual-labeling experiments were acquired in sequential scanning mode to avoid cross-talk/bleed-through between channels. For electron microscopy, cells were flash-frozen in a Baltec HPM 010 high-pressure freezer (Leica Microsystems). Cryosubstitution and embedding were performed in a Leica EM AFS as described (43). Briefly, specimens were sequentially incubated at low temperature (−90 °C) in 0.1% tannic acid (100 h) and 2% OsO4 (7 h) in acetone. They were progressively brought to room temperature before being embedded in Epon (Electron Microscopy Sciences) and polymerized 24 h at 60 °C. Ultrathin sections were cut and contrasted with uranyl acetate and lead citrate before being observed in a LEO 912 AB (Zeiss).
See SI Experimental Procedures for additional materials and methods.
Supplementary Material
Acknowledgments
We thank Homa Ghalei (Max Planck Institute for Biophysical Chemistry), Gert Weber (AG Strukturbiochemie, Freie Universität Berlin), and the staff at the Swiss Light Source for help with diffraction data collection; Michaela Hellwig for excellent technical assistance; and Monika Abedin and Nicole King, who kindly provided us with M. brevicollis cDNA and culture.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2XHE).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106189108/-/DCSupplemental.
References
- 1.Putnam NH, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 2.Chapman JA, et al. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava M, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–726. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava M, et al. The Trichoplax genome and the nature of placozoans. Nature. 2008;454:955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- 5.King N. The unicellular ancestry of animal development. Dev Cell. 2004;7:313–325. doi: 10.1016/j.devcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado M. Choanoflagellates, choanocytes, and animal multicellularity. Invertebr Biol. 2004;123:1–22. [Google Scholar]
- 7.Leadbeater B. Choanoflagellate evolution: The morphological perspective. Protistology. 2008;5:256–267. [Google Scholar]
- 8.King N, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke HJ. Conclusive proofs of the animality of the ciliate sponges, and of their affinities with the infusoria flagellata. Am J Sci. 1866;2:320–325. [Google Scholar]
- 10.Wainright PO, Hinkle G, Sogin ML, Stickel SK. Monophyletic origins of the metazoa: An evolutionary link with fungi. Science. 1993;260:340–342. doi: 10.1126/science.8469985. [DOI] [PubMed] [Google Scholar]
- 11.Lang BF, O'Kelly C, Nerad T, Gray MW, Burger G. The closest unicellular relatives of animals. Curr Biol. 2002;12:1773–1778. doi: 10.1016/s0960-9822(02)01187-9. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen C. Six major steps in animal evolution: Are we derived sponge larvae? Evol Dev. 2008;10:241–257. doi: 10.1111/j.1525-142X.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 13.Carr M, Leadbeater BS, Hassan R, Nelson M, Baldauf SL. Molecular phylogeny of choanoflagellates, the sister group to Metazoa. Proc Natl Acad Sci USA. 2008;105:16641–16646. doi: 10.1073/pnas.0801667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan TJ, Grant SGN. The origin and evolution of synapses. Nat Rev Neurosci. 2009;10:701–712. doi: 10.1038/nrn2717. [DOI] [PubMed] [Google Scholar]
- 15.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Natl Rev. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 16.Hosono R, et al. The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J Neurochem. 1992;58:1517–1525. doi: 10.1111/j.1471-4159.1992.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 17.Schulze KL, et al. rop, a Drosophila homolog of yeast Sec1 and vertebrate n-Sec1/Munc-18 proteins, is a negative regulator of neurotransmitter release in vivo. Neuron. 1994;13:1099–1108. doi: 10.1016/0896-6273(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 18.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 19.Toonen RF, Verhage M. Munc18-1 in secretion: Lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30:564–572. doi: 10.1016/j.tins.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr CM, Rizo J. At the junction of SNARE and SM protein function. Curr Opin Cell Biol. 2010;22:488–495. doi: 10.1016/j.ceb.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pevsner J, et al. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Deák F, et al. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng RW, Guetg C, Abellan E, Fussenegger M. Munc18b regulates core SNARE complex assembly and constitutive exocytosis by interacting with the N-peptide and the closed-conformation C-terminus of syntaxin 3. Biochem J. 2010;431:353–361. doi: 10.1042/BJ20100145. [DOI] [PubMed] [Google Scholar]
- 29.Latham CF, et al. Molecular dissection of the Munc18c/syntaxin4 interaction: Implications for regulation of membrane trafficking. Traffic. 2006;7:1408–1419. doi: 10.1111/j.1600-0854.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 30.Hu SH, Latham CF, Gee CL, James DE, Martin JL. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc Natl Acad Sci USA. 2007;104:8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu SH, et al. Possible roles for Munc18-1 domain 3a and Syntaxin1 N-peptide and C-terminal anchor in SNARE complex formation. Proc Natl Acad Sci USA. 2011;108:1040–1045. doi: 10.1073/pnas.0914906108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashizume K, Cheng YS, Hutton JL, Chiu CH, Carr CM. Yeast Sec1p functions before and after vesicle docking. Mol Biol Cell. 2009;20:4673–4685. doi: 10.1091/mbc.E09-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloepper TH, Kienle CN, Fasshauer D. SNAREing the basis of multicellularity: Consequences of protein family expansion during evolution. Mol Biol Evol. 2008;25:2055–2068. doi: 10.1093/molbev/msn151. [DOI] [PubMed] [Google Scholar]
- 34.Leadbeater B, Kelly M. Evolution of animals-choanoflagellates and sponges. Water and Atmosphere. 2001;9:9–11. [Google Scholar]
- 35.Laval M. Ultrastructure and feeding mode of the choanoflagellate Salpinogoeca pelagica sp. nov. - comparison with sponge choanocytes (Translated from French) Protistologica. 1971;7:325–336. [Google Scholar]
- 36.Karpov S, Leadbeater B. Cytoskeleton structure and composition in choanoflagellates. J Eukaryot Microbiol. 1998;45:361–367. [Google Scholar]
- 37.Hayashi T, et al. Synaptic vesicle membrane fusion complex: Action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carr CM, Rizo J. At the junction of SNARE and SM protein function. Curr Opin Cell Biol. 2010;22:1–8. doi: 10.1016/j.ceb.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulyás-Kovács A, et al. Munc18-1: Sequential interactions with the fusion machinery stimulate vesicle docking and priming. J Neurosci. 2007;27:8676–8686. doi: 10.1523/JNEUROSCI.0658-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiederhold K, Fasshauer D. Is assembly of the SNARE complex enough to fuel membrane fusion? J Biol Chem. 2009;284:13143–13152. doi: 10.1074/jbc.M900703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fasshauer D, Antonin W, Subramaniam V, Jahn R. SNARE assembly and disassembly exhibit a pronounced hysteresis. Nat Struct Biol. 2002;9:144–151. doi: 10.1038/nsb750. [DOI] [PubMed] [Google Scholar]
- 42.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 43.Möbius W, et al. Electron microscopy of the mouse central nervous system. Methods Cell Biol. 2010;96:475–512. doi: 10.1016/S0091-679X(10)96020-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.