Abstract
The rationale for the pursuit of bacterial type 2 fatty acid synthesis (FASII) as a target for antibacterial drug discovery in Gram-positive organisms is being debated vigorously based on their ability to incorporate extracellular fatty acids. The regulation of FASII by extracellular fatty acids was examined in Staphylococcus aureus and Streptococcus pneumoniae, representing two important groups of pathogens. Both bacteria use the same enzymatic tool kit for the conversion of extracellular fatty acids to acyl-acyl carrier protein, elongation, and incorporation into phospholipids. Exogenous fatty acids completely replace the endogenous fatty acids in S. pneumoniae but support only 50% of phospholipid synthesis in S. aureus. Fatty acids overcame FASII inhibition in S. pneumoniae but not in S. aureus. Extracellular fatty acids strongly suppress malonyl-CoA levels in S. pneumoniae but not in S. aureus, showing a feedback regulatory system in S. pneumoniae that is absent in S. aureus. Fatty acids overcame either a biochemical or a genetic block at acetyl-CoA carboxylase (ACC) in S. aureus, confirming that regulation at the ACC step is the key difference between these two species. Bacteria that possess a stringent biochemical feedback inhibition of ACC and malonyl-CoA formation triggered by environmental fatty acids are able to circumvent FASII inhibition. However, if exogenous fatty acids do not suppress malonyl-CoA formation, FASII inhibitors remain effective in the presence of fatty acid supplements.
Keywords: antibiotics, bacterial phospholipid synthesis, natural products
The frequency of multidrug-resistant Staphylococcus aureus (MRSA) continues to rise, and therapies directed against new targets are needed to combat this prevalent pathogen (1, 2). One such target is bacterial type 2 fatty acid synthesis (FASII) (3). The idea of targeting FASII evolved in Escherichia coli where FASII is essential even though these organisms incorporate environmental fatty acids into phospholipids (4). Extracellular fatty acids cross the outer membrane via the FadL porin and translocate to the cytosolic aspect of the inner membrane where they are activated by acyl-CoA synthetase. Acyl-CoAs are used by the PlsB/PlsC glycerol-PO4 acyltransferase system for phospholipid synthesis. The essentiality of the FASII genes in Gram-negative bacteria arises from the requirement for β-hydroxy-fatty acids to assemble the lipid A core structure of outer membrane lipopolysaccharides (4). Fatty acid supplementation cannot support lipid A synthesis because there is no mechanism to transfer acyl chains from CoA to the acyl carrier protein (ACP) of FASII so that the hydroxyl group can be introduced. Supplementation with hydroxy-fatty acids also is ineffective because the acyltransferases of lipid A biosynthesis use only ACP thioester substrates (5).
A number of natural products target different steps in FASII. These steps include β-ketoacyl-ACP synthase [FabF, thiolactomycin (6, 7), cerulenin (7, 8), platencin (9), and platensimycin (10)], enoyl-ACP reductase [FabI, cephalochromin (11), kalimantacin (12), and aquastatin A (13)], β-ketoacyl-ACP reductase [FabG, macrolactin S (14)], and acetyl-CoA carboxylase [ACC, andrimid (15)]. Several studies in animal models show that these natural products are effective in protecting against bacterial infections in animals (9, 10, 15, 16). These discoveries, coupled with the realization that two widely used antibacterial agents, triclosan (17, 18) and isonaizid (19), target FabI, stimulated the development of small-molecule FabI inhibitors (3). These inhibitors were developed to target S. aureus because other groups of Gram-positive pathogens, exemplified by Streptococcus pneumoniae, do not express an FabI but rather carry out the enoyl-ACP reduction using the unrelated FabK flavoprotein (20). These next-generation FabI inhibitors also are effective in curing S. aureus infections in animal models (21–26).
Brinster et al. (27) have questioned the feasibility of targeting FASII in Gram-positive pathogens based on the finding that FASII is not essential in Streptococcus agalactiae if the bacteria are supplemented with fatty acids or human serum. Whether the findings with S. agalactiae can be extended reasonably to all Gram-positive bacteria has spurred a vigorous debate. Balemans et al. (24) were unable to show that fatty acids rescued S. aureus treated with a FabI inhibitor, but Brinster et al. (28) countered by showing that S. aureus can incorporate exogenous fatty acids and have constructed a S. aureus fabI knockout that is a fatty acid auxotroph. This article addresses this debate by showing that exogenous fatty acids stringently repress acetyl-CoA carboxylase (ACC) activity in Gram-positive bacteria like S. pneumoniae, allowing fatty acid supplements to replace endogenous fatty acids completely. However, S. aureus lacks this regulatory control system, and the inability of exogenous fatty acids to shut off de novo biosynthesis accounts for their sensitivity to FASII inhibitors even in the presence a fatty acid supplement.
Results
Fatty Acid Incorporation into S. aureaus and S. pneumoniae Phospholipids.
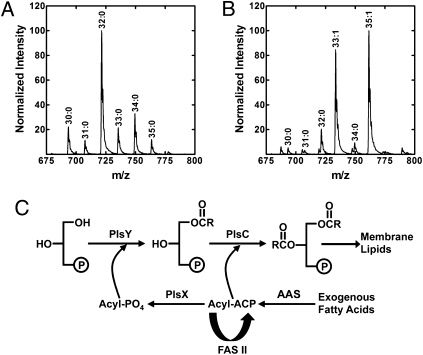
We used oleic acid (18:1Δ9) to study the metabolism of exogenous fatty acids because it is a common mammalian fatty acid and is distinguished easily from the products of FASII. S. aureus grown in medium devoid of fatty acids contained primarily anteiso saturated fatty acids (a15:0 and a17:0) (Table 1). Phosphatidylglycerol (PtdGro) is the major phospholipid of S. aureus, and the principal molecular species was 32:0 consisting of a17:0 (1-position) paired with a15:0 (2-position) (Fig. 1A and Table S1). Growth in 500 μM 18:1Δ9 showed that the a17:0 fatty acid component was replaced by 18:1Δ9 and 20:1Δ11, but the a15:0 content remained at ∼50% (Table 1). The replacement of ∼50% of the acyl chains with 18:1Δ9-derived fatty acids occurred in cells grown in 100 μM 18:1Δ9, and the extent of incorporation did not increase further in cells grown in 4 mM 18:1Δ9. The two new PtdGro molecular species (33:1 and 35:1) in S. aureus grown with 18:1Δ9 corresponded to 18:1Δ9 or 20:1Δ11 (1-position) paired with a15:0 (2-position) (Fig. 1B; Table S2). Although S. pneumoniae (Order: Lactobacillales) and S. aureus (Order: Bacillales) both belong to the Firmicutes phylum, their fatty acid compositions are distinctly different. S. pneumoniae contains straight-chain saturated and monounsaturated fatty acids, and when grown with 18:1Δ9 this organism completely replaced endogenous fatty acids with the exogenous supplement (Table 1). Unlike S. aureus, 18:1Δ9 was not elongated in S. pneumoniae. Thus, there is a limit to the amount of exogenous fatty acid that can be incorporated into S. aureus phospholipids, whereas there is no such barrier in S. pneumoniae.
Table 1.
Fatty acid compositions (weight %) of S. aureus and S. pneumoniae grown in media with or without an 18:1Δ9 supplement
|
S. aureus |
S. pneumoniae |
|||
| Fatty acid | BSA | +18:1 | BSA | +18:1 |
| 14:0 | — | — | 2.4 | 1.8 |
| i15:0 | 7.2 | 4.2 | — | — |
| a15:0 | 32.3 | 26.7 | — | — |
| 16:0 | 3.7 | 3.1 | 39.2 | 4.5 |
| 16:1Δ7 | — | — | 3.3 | — |
| 16:1Δ9 | — | — | 14.3 | 1.9 |
| 16:1Δ11 | — | — | 2.1 | — |
| i17:0 | 8.4 | 1.1 | — | — |
| a17:0 | 24.4 | 4.1 | — | — |
| 18:0 | 7.0 | 3.3 | 6.8 | 1.1 |
| 18:1Δ9 | — | 21.7 | 3.6 | 90.7 |
| 18:1Δ11 | — | — | 27.2 | — |
| 18:1Δ13 | — | — | 1.1 | — |
| i19:0 | 4.4 | tr | — | — |
| a19:0 | 7.8 | 1.6 | — | — |
| 20:0 | 4.8 | 2.1 | — | — |
| 20:1Δ13 | — | 29.7 | — | — |
| 22:1Δ15 | — | 1.6 | — | — |
Base medium was TB for S. aureus and CY medium for S. pneumoniae. BSA, 10 mg/mL; 18:1Δ9, 500 μM. Data are the average of triplicate experiments rounded to 0.1%. tr, <1.0%; —, <0.2%.
Fig. 1.
Assimilation of exogenous fatty acids by S. aureus and S. pneumoniae. (A) Analysis of PtdGro molecular species and positional distribution of acyl chains in S. aureus strain RN4220 grown in tryptone broth (TB). The total fatty acid composition and the distributions between the 1- and 2-positions of the glycerol backbone are reported in Table S1. (B) Analysis of the PtdGro molecular species in S. aureus strain RN4220 grown in TB/BSA/500 μM 18:1Δ9. The total fatty acid composition and the distributions between the 1- and 2-positions of the glycerol backbone are reported in Table S2. (C) Pathway for the activation, elongation, and incorporation of extracellular fatty acids into membrane phospholipids in S. aureus and S. pneumoniae. Fatty acids are ligated to ACP by an acyl-ACP synthetase (AAS). Acyl-ACP is used by PlsC to acylate the 2-position, converted to acyl-PO4 by PlsX, and transferred to the 1-position by PlsY, or elongated by FASII. R, fatty acid alkyl chain.
Although the pathway for incorporation of exogenous fatty acid into phospholipid is established in E. coli (4), Gram-positive bacteria use a different glycerol-PO4 acyltransferase (PlsY) and acyl donor (acyl-PO4) based on research using S. pneumoniae (29). We confirmed that in S. aureus acyl-PO4 is the PlsY substrate, and acyl-ACP is the only PlsC acyl donor (Fig. S1A). Acyl-CoA has no role in S. aureus phospholipid synthesis (Fig. S1A). The elongation of 18:1Δ9 to 20:1Δ11 in S. aureus (Table 1) suggested that, unlike E. coli, exogenous fatty acids have direct access to the FASII system. Acyl-ACP synthetases that ligate fatty acids to ACP are known in bacteria (30, 31), and soluble extracts of both S. aureus and S. pneumoniae exhibited acyl-ACP synthetase activity (Fig. S1B). The genes encoding the acyl-ACP synthetases in these organisms remain to be identified. These data show that both Gram-positive organisms use the same enzymatic tool set for the activation and incorporation of fatty acids into membrane phospholipids via an acyl-ACP intermediate (Fig. 1C).
Evasion of FasII Inhibitors by Exogenous Fatty Acids.
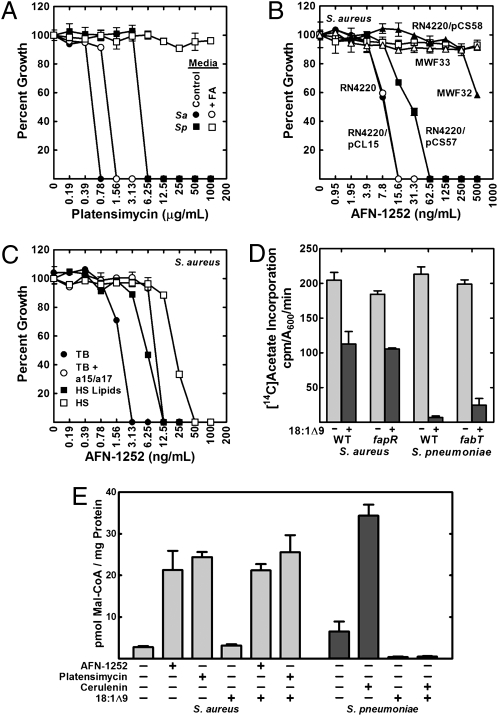
Platensimycin (10) and cerulenin (32) are two natural products that inhibit the condensation step of FASII catalyzed by the β-ketoacyl-ACP synthase II (FabF). FabF is an essential gene in both S. aureus and S. pneumoniae, and the FabF inhibitor platensimycin blocked the growth of both organisms (Fig. 2A). The ability of exogenous fatty acids to overcome FASII inhibitors was tested in S. aureus using medium supplemented with a15:0 plus a17:0 and in S. pneumoniae in medium supplemented with 18:1Δ9. Fatty acid supplementation of S. aureus increased the minimal inhibitory concentration (MIC) for platensimycin, but the FabF inhibitor still blocked the growth of S. aureus. In contrast, FabF inhibition did not prevent S. pneumoniae growth when fatty acids were present. The same results were seen when cerulenin was used as the FabF inhibitor (Fig. S2). These data underscored the distinct difference between S. aureus and S. pneumoniae in the ability of exogenous fatty acids to overcome FASII inhibition.
Fig. 2.
Fatty acid supplementation cannot overcome inhibition of FASII in S. aureus. (A) Platensimycin MIC for S. aureus strain RN4220 (Sa) grown in TB/BSA or TB/BSA plus a15:0/a17:0 (500 μM) (FA) and for S. pneumoniae strain R6 (Sp) grown in CY/BSA or CY/BSA plus 18:1Δ9 (500 μM) (FA). (B) AFN-1252 MIC for S. aureus strains RN4220, RN4220/pCL15(empty), RN4220/pCS57 (FabI), RN4220/pCS58 [FabI(M99T)], MWF32 (fabIM99T), and MWF33 (fabIY147H) grown in TB medium. (C) AFN-1252 MIC for S. aureus strain RN4220 grown in TB, TB/BSA plus a15:0/a17:0 (500 μM), TB/BSA/human serum (HS) lipids, or 50% human serum/50% TB. (D) Metabolic labeling with [14C]acetate (10 μCi/mL) to determine the effect of growth with exogenous 18:1Δ9 and de-repression of fab gene expression on de novo fatty acid biosynthesis. S. aureus strains were RN4220 (WT) and CS34 (ΔfapR). S. pneumoniae strains were D39 (WT) and LYJ4 (ΔfabT). Fatty acid composition of the strains grown and treated as described in D are found in Table S3. (E) Malonyl-CoA levels in S. aureus and S. pneumoniae treated with FASII inhibitors in the presence and absence of exogenous 18:1Δ9 (500 μM). Malonyl-CoA levels were measured by mass spectrometry following a 60-min treatment of strain RN4220 with AFN-1252 (100 ng/mL) or platensimycin (8 μg/mL) or treatment of S. pneumoniae strain R6 with cerulenin (100 μg/mL).
AFN-1252, an S. aureus FabI Inhibitor.
The hydrophobic natural product FASII inhibitors have limited utility as drugs. The recognition of this general problem led to numerous efforts to identify more suitable chemical scaffolds that specifically target FabI (3). AFN-1252 is a potent S. aureus FabI (enoyl-ACP reductase) inhibitor that was optimized to maximize S. aureus efficacy and bioavailability (33). However, few mechanism-of-action data have been published using AFN-1252. We verified that FabI was the relevant in vivo target for AFN-1252 by selecting S. aureus mutants refractory to 40 ng/mL AFN-1252. Colonies arose at a frequency ranging from 1 in 2 × 10−9 and 1 in 1 × 10−10, and the fabI genes from five colonies were sequenced. Four strains had a missense mutation predicted to express FabI[M99T], and one had a mutation encoding FabI[Y147H]. The MIC for AFN-1252 increased from 6.25 to >200 ng/mL in both strains (Fig. 2B). Expression of S. aureus FabI from a plasmid significantly increased the AFN-1252 MIC, and the cells were resistant to >500 ng/mL AFN-1252 when the FabI[M99T] protein was expressed (Fig. 2B). These data established FabI as the relevant in vivo target for AFN-1252. AFN-1252 cannot be used against S. pneumoniae because this bacterium does not have a FabI and thus is refractory to FabI inhibitors, including AFN-1252 (33).
Exogenous fatty acids were unable to overcome FabI inhibition in S. aureus (Fig. 2C), although there was a fourfold increase in the AFN-1252 MIC from 3 to 12 ng/mL Fatty acids also were unable to overcome AFN-1252 action in S. aureus strains SA113, Mu50, and MRSA (Fig. S3), showing that the response of strain RN4220 to AFN-1252 was typical for S. aureus. Human serum significantly increased the MIC but was unable to overcome growth inhibition by AFN-1252 (Fig. 2C). The shift in MIC by serum was caused primarily by AFN-1252 binding to the protein components, because, when the serum lipids were extracted and added at the same concentration as present in human serum, the effect on the AFN-1252 MIC was reduced (Fig. 2C).
Adaptation of S. aureus to Exogenous Fatty Acids.
One concept that was advanced to explain the apparent effectiveness of FASII inhibitors in animal models is that the drugs were administered before S. aureus had time to adapt to the presence of extracellular fatty acids (27). This idea appears to be based on the induction of numerous fatty acid metabolism genes in E. coli and Bacillus subtilis in response to exogenous fatty acids (34). These organisms have fatty acid β-oxidation regulons that are induced by exogenous fatty acids along with the acyl-CoA synthetases required for their activation. Bioinfomatic analysis of the S. aureus genome did not reveal a set of genes for fatty acid degradation, suggesting that elongation by FASII and incorporation into cellular components was their only fate. We confirmed this point by metabolically labeling S. aureus with [14C]18:1Δ9 and determining the recovery of label from the cells and medium (Fig. S4). Quantifying the amount of label remaining in the medium after cell growth showed that 31% of the label was incorporated into the cells. The entire input radioactivity was recovered as fatty acid, demonstrating that S. aureus does not degrade fatty acids. The response of S. aureus to AFN-1252 was the same when S. aureus was adapted to fatty acids by passaging in 18:1Δ9 for 3 d (Fig. S4D), and the proportion of 18:1-derived fatty acids remained at 50%. In summary, we found no evidence for an adaptive response to exogenous fatty acids in S. aureus that affected the extent of fatty acid incorporation or its response to FASII inhibitors.
Differences in Genetic and Biochemical Regulation of FASII in S. aureus and S. pneumoniae.
It is established that S. aureus and S. pneumoniae have different mechanisms for controlling FASII gene transcription. The transcription of FASII and ACC genes in S. pneumoniae is controlled by FabT, a MarR-like repressor that requires long-chain acyl-ACP to bind to DNA (35). In S. aureus, the FASII genes are regulated by FapR, a transcriptional repressor that is released from DNA by malonyl-CoA (36). Unlike FabT regulation in S. pneumoniae, the expression of the acc genes is not regulated by FapR (37). Exogenous fatty acids strongly repressed transcription of the fab/acc genes in S. agalactiae but had no effect on fab gene transcription in S. aureus (24), consistent with the conversion of fatty acids to long-chain acyl-ACP (Fig. 1C), the ligand required for FabT repression of fab/acc genes (35). We compared the suppression of FASII activity by exogenous fatty acids in wild-type and transcription factor-knockout strains to determine if genetic regulation explained the differences in the extent of fatty acid incorporation. In S. aureus grown with 18:1Δ9, the amount of [14C]acetate incorporation was reduced by ∼50% (Fig. 2D), reflecting the amount of 18:1Δ9 incorporated (Table S3). Growth of S. pneumoniae with 18:1Δ9 almost eliminated acetate incorporation (Fig. 2D), leading to 18:1Δ9 predominating in the phospholipids (Table S3). These results were the same in the transcription factor-knockout strains that constitutively expressed high levels of FASII genes (Fig. 2D and Table S3). Thus, the distinct differences in FASII transcriptional regulation did not account for the differences in exogenous fatty acid metabolism. Rather, biochemical regulation of FASII was the key difference.
Exogenous Fatty Acids Repress ACC in S. pneumoniae but Not in S. aureus.
We measured the intracellular levels of malonyl-CoA using electrospray ionization (ESI)-MS/MS to determine if feedback regulation of fatty acid production by exogenous fatty acids occurred at the ACC step. Malonyl-CoA accumulation is a hallmark of FASII elongation cycle inhibition because ACC remains active even though the utilization of malonyl-CoA ceases (37, 38). The treatment of either S. aureus or S. pneumoniae with FASII inhibitors significantly increased the intracellular levels of malonyl-CoA (Fig. 2F). The presence of fatty acids did not decrease the intracellular levels of malonyl-CoA in S. aureus, but fatty acids significantly depressed malonyl-CoA levels in S. pneumoniae (Fig. 2F). Exogenous 18:1Δ9 also suppressed malonyl-CoA levels in S. pneumoniae treated with cerulenin but did not affect malonyl-CoA levels in AFN-1252- or platensimycin-treated S. aureus (Fig. 2F). These data show that exogenous fatty acids potently represses the production of malonyl-CoA in S. pneumoniae, but this mode of biochemical regulation is absent in S. aureus.
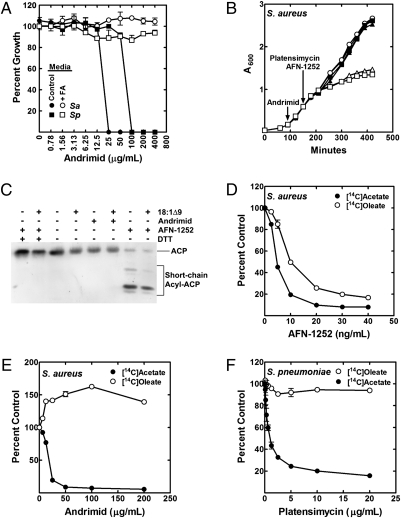
If the lack of regulation at ACC was solely responsible for the inability of S. aureus to circumvent FASII inhibitors using fatty acids, then fatty acid supplements should rescue S. aureus from ACC inhibitors. Andrimid, a natural product ACC inhibitor (15), was effective in blocking the growth of both S. aureus and S. pneumoniae in the absence of fatty acids (Fig. 3A). In contrast to the FASII inhibitors, fatty acids overcame growth inhibition by andrimid in S. aureus as well as S. pneumoniae (Fig. 3A). The addition of either AFN-1252 or platensimycin after an andrimid block did not inhibit S. aureus growth, illustrating the specific action these drugs against FASII (Fig. 3B). These data show that andrimid inhibition of ACC in S. aureus mimics the biochemical regulation of ACC in S. pneumoniae and allows exogenous fatty acids to support cell growth.
Fig. 3.
Extracellular fatty acid metabolism in S. aureus and S. pneumoniae treated with either ACC or FASII inhibitors. (A) Andrimid MIC for S. aureus strain RN4220 (Sa) in TB/BSA (Control) or TB/BSA plus a15:0/a17:0 (500 μM) and for S. pneumoniae strain R6 (Sp) grown in CY/BSA (Control) or CY/BSA plus 18:1Δ9 (500 μM). (B) S. aureus strain RN4220 was grown in TB/BSA/18:1Δ9 (●) and treated with andrimid (75 μg/mL) at the indicated time (○, ■, ▲). Subsequently, either AFN-1252 (80 ng/mL) (■) or platensimycin (6 μg/mL) (▲) was added. Growth inhibition in the absence of andrimid by AFN-1252 (□) or platensimycin (△) is shown. (C) Western blot analysis (12 μg protein) of the S. aureus ACP pool composition using urea gel electrophoresis and anti-ACP antibody treated with either AFN-1252 (60 ng/mL) or andrimid (75 μg/mL) for 15 min in the presence or absence of 500 μM 18:1Δ9. (D) Cells were grown to an A600 of 0.5 and labeled with either 1 μCi/mL [14C]18:1Δ9 or 4 μCi/mL [14C]acetate for 20 min. AFN-1252 inhibition of [14C]acetate and incorporation of [14C]18:1Δ9 into S. aureus strain RN4220 lipids. (E) Andrimid inhibition of [14C]acetate and stimulation of [14C]18:1Δ9 incorporation into S. aureus strain RN4220. (F) [14C]Acetate and [14C]18:1Δ9 incorporation into S. pneumoniae strain R6 in the presence and absence of platensimycin.
FASII Inhibitors Alter Fatty Acid Uptake in S. aureus.
The blockage of FASII in E. coli induces the accumulation of acyl-ACP as chain initiation continues and acyl-ACP accumulates at the inhibited step (39, 40). AFN-1252 triggered acyl-ACP accumulation concomitant with depletion of nonesterified ACP, whereas blocking initiation with andrimid did not (Fig. 3C). The presence of exogenous fatty acid did not prevent the accumulation of short-chain acyl-ACP in AFN-1252–treated cells (Fig. 3C). The faster-migrating bands were thioesters, based on their conversion to ACP by DTT. Strain CS34 (ΔfapR) had 3.6-fold more ACP than strain RN4220 (Fig. S5A) but did not have an altered MIC for AFN-1252 (Fig. S5B), and the elevated level of ACP still was converted almost completely to short-chain acyl-ACP following AFN-1252 treatment (Fig. S5C). Because nonesterified ACP is required for fatty acid incorporation (Fig. S1 A and C), the disappearance of ACP resulting from its conversion to acyl-ACP intermediates in AFN-1252–treated cells suggests that the drug may inhibit the uptake of exogenous fatty acids. AFN-1252 prevents both [14C]acetate and [14C]18:1Δ9 incorporation into S. aureus phospholipids (Fig. 3D). In contrast, andrimid treatment increases the amount of [14C]18:1Δ9 incorporated, concomitant with inhibiting de novo fatty acid synthesis (Fig. 3E). The rate of [14C]18:1Δ9 incorporation into S. pneumoniae is not altered by platensimycin (Fig. 3F). These data show that FASII inhibition triggers the depletion of nonesterified ACP because of the continued initiation and the accumulation of acyl-ACP at the blocked FabI step. This depletion occurred only when ACC was active to supply malonyl-ACP for the initiation of new acyl chains.
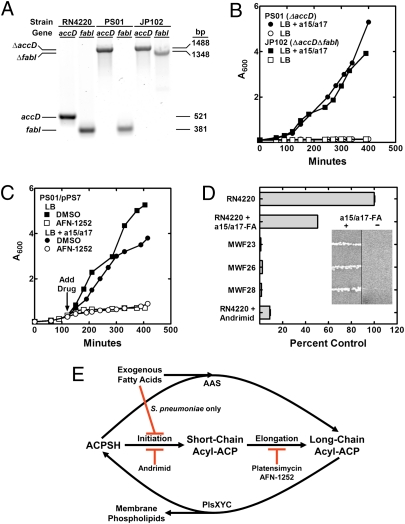
Analysis of S. aureus ΔaccD Mutants Allows Replacement of Endogenous with Exogenous Fatty Acids.
The experiments with andrimid suggest that S. aureus ACC mutants could be isolated as fatty acid auxotrophs. ACC has four protein subunits encoded by four genes (accABCD), and its activity was ablated by knocking out accD. S. aureus strain PS01 (ΔaccD) was constructed by performing all the steps on plates containing a BSA/a15:0/a17:0 mixture (Fig. 4A). Strain PS01 was a fatty acid auxotroph (Fig. 4B) and was complemented by plasmid pPS7 that expressed the accDA operon (Fig. 4C). Plasmid pPS7 was derived from pCL15 and was designed to control gene expression using the Pspac promoter (41). Unfortunately, plasmid pPS7 also supported the growth of the strain PS01 in the absence of a fatty acid supplement and isopropylthio-β-galactoside and thus produced sufficient AccD in the absence of inducer to complement the ACC defect. AFN-1252 had no effect on the growth of strain PS01; however, AFN-1252 did inhibit the growth of the complemented strain PS01/pPS7 in either the presence or absence of a fatty acid supplement (Fig. 4C). These experiments using a targeted genetic approach confirmed the conclusions made using andrimid to inactivate ACC.
Fig. 4.
Phenotypes of S. aureus ΔaccD and ΔaccD ΔfabI mutants. (A) PCR genotyping of strains RN4220 (wild-type), PS01 (ΔaccD), and JP102 (ΔaccD ΔfabI) showing the presence of the gene insertions in the knockout strains using gene-specific primers flanking the insertion site. (B) Strains PS01 (ΔaccD) and JP102 (ΔaccD ΔfabI) were fatty acid auxotrophs. (C) Complementation of S. aureus strain PS01 (ΔaccD) by plasmid pPS7 expressing the accDA operon. The complemented strain was growth inhibited by AFN-1252 (80 ng/mL) in both the presence and absence of fatty acids. (D) FASII activity in three fatty acid auxotrophs (strains MWF23, MWF26, and MWF28) were determined by labeling with [14C]acetate (10 μCi/mL) compared with wild-type strain RN4220 grown with or without an a15:0/a17:0 supplement. Strain RN4220 grown in the presence of andrimid (75 μg/mL) was analyzed also. Inset shows fatty acid auxotroph growth phenotypes on agar plates with and without a15:0/a17:0 supplements. (E) Regulation of FASII activity by fatty acids in S. pneumoniae but not in S. aureus. In S. pneumoniae, fatty acids feedback inhibits acetyl-CoA carboxylase and shuts off FASII. S. aureus lacks this stringent regulatory circuit.
Strain JP102 (ΔaccD ΔfabI) was constructed from strain PS01 (Fig. 4A) and also was a fatty acid auxotroph (Fig. 4B). Our attempts to use the same approach to introduce ΔfabI into the wild-type strain RN4220 were unsuccessful. As expected, strain JP102 was resistant to AFN-1252 inhibition. We were unable to transform strain JP102 with plasmid pPS7, although transformations with the empty vector were successful. In an experiment to address the effect of FabI activity on the ability to transform strain PS01 (ΔaccD) with a plasmid expressing accDA (pPS7), an equal aliquot of cells from the transformation mixture was recovered on medium containing chloramphenicol (to select for the plasmid) and either BSA/a15:0/a17:0 or the same medium containing 40 ng/mL of AFN-1252, and the numbers of colonies recovered were compared. Numerous colonies (64 per plate) appeared when selecting on medium without AFN-1252, but no colonies were recovered in medium containing AFN-1252. These results support the essentiality of FabI in the presence of active ACC.
These genetic experiments suggested how fabI knockouts in S. aureus could be isolated in the laboratory as fatty acid auxotrophs by the acquisition of secondary inactivating mutations in an acc gene. The insertion of a knockout cassette into the target gene is a low-frequency event that usually is selected for stringently using antibiotics. If a secondary mutation is required, the frequency of recovering fabI knockouts would be even lower, consistent with the observation that a ΔfabI allele could not be introduced into a wild-type strain using the same number of cells used to delete fabI in strain PS01. We tested this idea by inactivating FabI in the entire cell population by plating strain RN4220 on medium containing BSA/a15:0/a17:0 plus 40 ng/mL of AFN-1252 and selecting for resistant mutants. The number of colonies arising on the AFN-12522/fatty acid medium was 10-fold higher than when strain RN4220 was selected on medium containing AFN-1252 alone. Two colony types were recovered. The larger colonies (∼7% of the total) did not require a fatty acid supplement and corresponded to lesions in the fabI gene. The smaller colonies (∼93% of the total) were fatty acid auxotrophs. [14C]Acetate labeling of three selected clones showed these strains were more deficient in fatty acid synthesis than andrimid-treated cells (Fig. 4D). The fabI and the four acc genes in each of these strains were sequenced. All strains had wild-type fabI genes. Strain MWF23 had a g232t mutation introducing a stop at codon 78 in accD; strain MWF26 had an a257t mutation in accC predicted to encode an AccC[E86V] protein; and strain MWF28 had a 301-bp deletion in accC. All acc mutant strains were completely refractory to AFN-1252 or platensimycin growth inhibition in the presence of fatty acids. These data illustrate how a knockout in a fab gene can be obtained in S. aureus by selecting on medium containing fatty acids, allowing the selection for inactivating acc mutations, and explain how S. aureus ΔfabI strains can be isolated as fatty acid auxotrophs (28).
Discussion
The differences in the biochemical regulation of ACC by exogenous fatty acids account for the responses of S. aureus and S. pneumoniae to FASII inhibitors (Fig. 4E). In S. pneumoniae fatty acid supplements potently suppress the formation of malonyl-CoA by ACC to eliminate FASII activity. Thus, S. pneumoniae is refractory to growth inhibition by FASII inhibitors in the presence of exogenous fatty acids. The biochemical mechanism for ACC regulation by extracellular fatty acids in S. pneumoniae remains to be determined. Long-chain acyl-ACPs act as feedback regulators of initiation in E. coli (42, 43), but other ligands, such as acyl-PO4, may be involved in S. pneumoniae. Although exogenous fatty acids are incorporated by S. aureus, the regulation of ACC by fatty acids is not stringent enough to suppress FASII. The competition for ACP between FASII and fatty acid activation means that exogenous fatty acids cannot replace the endogenously produced acyl chains completely. When a FASII inhibitor is deployed against S. aureus in the presence or absence of fatty acids, the initiation of new acyl chains continues, leading to the depletion of ACP as the acyl-ACP intermediates accumulate at the inhibited step. The depletion of ACP for fatty acid ligation correlates with diminished exogenous fatty acid incorporation into phospholipid. The identity of the enzymes involved in fatty acid uptake and their regulation remains to be defined.
These data resolve the debate over the utility of FASII inhibitors in treating S. aureus infections. The diversity in the biochemical regulation of FASII in Gram-positive bacteria explains why FASII inhibitors are effective in the presence of fatty acids in some Gram-positive bacteria (S. aureus) but not in others (S. pneumoniae) (Fig. 4E). The isolation of AFN-1252–resistant S. aureus fatty acid auxotrophs with secondary mutations in acc genes coupled with the characteristics of ΔaccD and ΔaccD ΔfabI strains illustrates that FabI is essential in the presence of fatty acids unless ACC is inactivated also. These data provide the mechanistic basis for understanding the anti-staphylococcal activity of fatty acid synthesis inhibitors in animal models (9, 10, 21, 24, 25). Predicting how other Gram-positive bacteria will respond to exogenous fatty acids is problematic. Bacteria with FapR regulation likely will behave like S. aureus, whereas those with FabT-dependent FASII regulation may emulate S. pneumoniae. However, Propionibacterium sp. and Clostridium sp. do not fit either of these paradigms. Determining if FASII is biochemically regulated by extracellular fatty acids is a diagnostic metabolic labeling experiment that could be used to predict the behavior of FASII inhibitors in the presence of fatty acids in these and other pathogens.
The finding that exogenous fatty acids cannot overcome growth inhibition by cerulenin in S. aureus is in apparent disagreement with previously published work (44). These investigators report that growth at the MIC for cerulenin was restored partially by the addition of fatty acids. This observation matches our data showing the restoration of growth at the cerulenin MIC by fatty acids in S. aureus (Fig. S2). The effect of medium composition on MIC can be significant. Fatty acids are delivered as detergent micelles, mixed micelles with another detergent such as Brij-58, or as BSA complexes, and these additives significantly decrease the effective drug concentration. Thus showing fatty acid rescue using the lowest effective drug dose (the MIC) cannot be used as diagnostic. AFN-1252 at the MIC concentration of 3 ng/mL inhibited only [14C]acetate incorporation by ∼20% (Fig. 3D). Thus, exogenous fatty acids should shift the MIC for any FASII inhibitor in S. aureus, which use fatty acid supplements to support ∼50% of phospholipid synthesis. Another complicating factor in interpreting this early study (44) and more contemporary work (24, 27) is that the phospholipid structures of the strains were not examined, and the extent of FASII inhibition in the complex medium was not determined to confirm that the inhibitor completely suppresses FASII in the experiment. Also, triclosan was used to show that serum overcame FASII inhibition in S. agalactiae and other Lactobacilli (24, 27). These experiments are conceptually flawed because there is no FASII target for triclosan in Lactobacilli, which express an FabK instead of an FabI (45). Although our data are consistent with the overall conclusion that Lactobacilli can overcome FASII inhibition by using fatty acid supplements, the observations that fatty acid supplements alleviate the non-FASII effect(s) of triclosan in Lactobacilli (24, 27) illustrate why restoration of growth by the addition of serum or hydrophobic constituents to medium is insufficient to show a specific pathway-rescue effect without additional supporting information.
Materials and Methods
S. aureus and S. pneumoniae strains used in this work are listed in Table S4. Exogenous fatty acids were supplied in the growth medium complexed with 10 mg/mL BSA for S. pneumoniae, and S. aureus fatty acid was delivered with either Brij-58 or BSA. Bacterial lipids were labeled metabolically and extracted using standard techniques, and individual species were separated by thin-layer chromatography. Fatty acid compositions were determined by gas chromatography, and phospholipid molecular species and malonyl-CoA levels were determined by mass spectrometry. MIC values were determined by a microbroth dilution method. Mutants were isolated by selection on the indicated media, and the defects were determined by DNA sequencing of the acc and fabI genes. Details of the experimental procedures can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ying Tu and the St. Jude High-Throughput Analytical Chemistry facility for the purification of andrimid, Steven V. Beer for providing andrimid-producing P. agglomerans, and Nachum Kaplan of Affinium Pharmaceuticals for AFN-1252. This research was supported by National Institutes of Health Grant GM034496, Cancer Center (CORE) Support Grant CA21765, and the American Lebanese Syrian Associated Charities.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109208108/-/DCSupplemental.
References
- 1.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh C. Where will new antibiotics come from? Nat Rev Microbiol. 2003;1:65–70. doi: 10.1038/nrmicro727. [DOI] [PubMed] [Google Scholar]
- 3.Lu H, Tonge PJ. Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc Chem Res. 2008;41:11–20. doi: 10.1021/ar700156e. [DOI] [PubMed] [Google Scholar]
- 4.Cronan JE, Jr, Rock CO. In: Eco-Sal-Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Böck I, et al., editors. Washington, DC: American Society for Microbiology; 2008. [Google Scholar]
- 5.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noto T, Miyakawa S, Oishi H, Endo H, Okazaki H. Thiolactomycin, a new antibiotic. III. In vitro antibacterial activity. J Antibiot (Tokyo) 1982;35:401–410. doi: 10.7164/antibiotics.35.401. [DOI] [PubMed] [Google Scholar]
- 7.Price AC, et al. Inhibition of β-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin. Structure and mechanism. J Biol Chem. 2001;276:6551–6559. doi: 10.1074/jbc.M007101200. [DOI] [PubMed] [Google Scholar]
- 8.Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976;40:681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, et al. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci USA. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 11.Zheng CJ, et al. Cephalochromin, a FabI-directed antibacterial of microbial origin. Biochem Biophys Res Commun. 2007;362:1107–1112. doi: 10.1016/j.bbrc.2007.08.144. [DOI] [PubMed] [Google Scholar]
- 12.Mattheus W, et al. The kalimantacin/batumin biosynthesis operon encodes a self-resistance isoform of the FabI bacterial target. Chem Biol. 2010;17:1067–1071. doi: 10.1016/j.chembiol.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Kwon YJ, Fang Y, Xu GH, Kim WG. Aquastatin A, a new inhibitor of enoyl-acyl carrier protein reductase from Sporothrix sp. FN611. Biol Pharm Bull. 2009;32:2061–2064. doi: 10.1248/bpb.32.2061. [DOI] [PubMed] [Google Scholar]
- 14.Sohn MJ, Zheng CJ, Kim WG. Macrolactin S, a new antibacterial agent with FabG-inhibitory activity from Bacillus sp. AT28. J Antibiot (Tokyo) 2008;61:687–691. doi: 10.1038/ja.2008.98. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Fortin PD, Walsh CT. Andrimid producers encode an acetyl-CoA carboxyltransferase subunit resistant to the action of the antibiotic. Proc Natl Acad Sci USA. 2008;105:13321–13326. doi: 10.1073/pnas.0806873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyakawa S, Suzuki K, Noto T, Harada Y, Okazaki H. Thiolactomycin, a new antibiotic. IV. Biological properties and chemotherapeutic activity in mice. J Antibiot (Tokyo) 1982;35:411–419. doi: 10.7164/antibiotics.35.411. [DOI] [PubMed] [Google Scholar]
- 17.McMurry LM, Oethinger M, Levy SB. Triclosan targets lipid synthesis. Nature. 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 18.Heath RJ, Yu YT, Shapiro MA, Olson E, Rock CO. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J Biol Chem. 1998;273:30316–30320. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- 19.Dessen A, Quémard A, Blanchard JS, Jacobs WR, Jr, Sacchettini JC. Crystal structure and function of the isoniazid target of Mycobacterium tuberculosis. Science. 1995;267:1638–1641. doi: 10.1126/science.7886450. [DOI] [PubMed] [Google Scholar]
- 20.Marrakchi H, et al. Characterization of Streptococcus pneumoniae enoyl-(acyl-carrier protein) reductase (FabK) Biochem J. 2003;370:1055–1062. doi: 10.1042/BJ20021699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne DJ, et al. Discovery of a novel and potent class of FabI-directed antibacterial agents. Antimicrob Agents Chemother. 2002;46:3118–3124. doi: 10.1128/AAC.46.10.3118-3124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller WH, et al. Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI) J Med Chem. 2002;45:3246–3256. doi: 10.1021/jm020050+. [DOI] [PubMed] [Google Scholar]
- 23.Seefeld MA, et al. Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. J Med Chem. 2003;46:1627–1635. doi: 10.1021/jm0204035. [DOI] [PubMed] [Google Scholar]
- 24.Balemans W, et al. Essentiality of FASII pathway for Staphylococcus aureus. Nature. 2010;463:E3. doi: 10.1038/nature08667. discussion E4. [DOI] [PubMed] [Google Scholar]
- 25.Freiberg C, et al. Novel bacterial acetyl coenzyme A carboxylase inhibitors with antibiotic efficacy in vivo. Antimicrob Agents Chemother. 2006;50:2707–2712. doi: 10.1128/AAC.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller JR, et al. A class of selective antibacterials derived from a protein kinase inhibitor pharmacophore. Proc Natl Acad Sci USA. 2009;106:1737–1742. doi: 10.1073/pnas.0811275106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinster S, et al. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature. 2009;458:83–86. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 28.Brinster S, et al. Reply. Nature (London) 2010;463:E4. [Google Scholar]
- 29.Lu Y-J, et al. Acyl-phosphates initiate membrane phospholipid synthesis in Gram-positive pathogens. Mol Cell. 2006;23:765–772. doi: 10.1016/j.molcel.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Chan CH, Cronan JE. The soluble acyl-acyl carrier protein synthetase of Vibrio harveyi B392 is a member of the medium chain acyl-CoA synthetase family. Biochemistry. 2006;45:10008–10019. doi: 10.1021/bi060842w. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, Morgan-Kiss RM, Campbell JW, Chan CH, Cronan JE. Expression of Vibrio harveyi acyl-ACP synthetase allows efficient entry of exogenous fatty acids into the Escherichia coli fatty acid and lipid A synthetic pathways. Biochemistry. 2010;49:718–726. doi: 10.1021/bi901890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Agnolo G, Rosenfeld IS, Awaya J, Omura S, Vagelos PR. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of β-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973;326:155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- 33.Karlowsky JA, Kaplan N, Hafkin B, Hoban DJ, Zhanel GG. AFN-1252, a FabI inhibitor, demonstrates a Staphylococcus-specific spectrum of activity. Antimicrob Agents Chemother. 2009;53:3544–3548. doi: 10.1128/AAC.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita Y, Matsuoka H, Hirooka K. Regulation of fatty acid metabolism in bacteria. Mol Microbiol. 2007;66:829–839. doi: 10.1111/j.1365-2958.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- 35.Jerga A, Rock CO. Acyl-Acyl carrier protein regulates transcription of fatty acid biosynthetic genes via the FabT repressor in Streptococcus pneumoniae. J Biol Chem. 2009;284:15364–15368. doi: 10.1074/jbc.C109.002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schujman GE, et al. Structural basis of lipid biosynthesis regulation in Gram-positive bacteria. EMBO J. 2006;25:4074–4083. doi: 10.1038/sj.emboj.7601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schujman GE, Altabe S, de Mendoza D. A malonyl-CoA-dependent switch in the bacterial response to a dysfunction of lipid metabolism. Mol Microbiol. 2008;68:987–996. doi: 10.1111/j.1365-2958.2008.06202.x. [DOI] [PubMed] [Google Scholar]
- 38.Furukawa H, Tsay JT, Jackowski S, Takamura Y, Rock CO. Thiolactomycin resistance in Escherichia coli is associated with the multidrug resistance efflux pump encoded by emrAB. J Bacteriol. 1993;175:3723–3729. doi: 10.1128/jb.175.12.3723-3729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heath RJ, Rock CO. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J Biol Chem. 1995;270:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 40.Jackowski S, Rock CO. Acetoacetyl-acyl carrier protein synthase, a potential regulator of fatty acid biosynthesis in bacteria. J Biol Chem. 1987;262:7927–7931. [PubMed] [Google Scholar]
- 41.Band L, Yansura DG, Henner DJ. Construction of a vector for cloning promoters in Bacillus subtilis. Gene. 1983;26:313–315. doi: 10.1016/0378-1119(83)90204-4. [DOI] [PubMed] [Google Scholar]
- 42.Davis MS, Cronan JE., Jr Inhibition of Escherichia coli acetyl coenzyme A carboxylase by acyl-acyl carrier protein. J Bacteriol. 2001;183:1499–1503. doi: 10.1128/JB.183.4.1499-1503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heath RJ, Rock CO. Inhibition of β-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:10996–11000. doi: 10.1074/jbc.271.18.10996. [DOI] [PubMed] [Google Scholar]
- 44.Altenbern RA. Cerulenin-inhibited cells of Staphylococcus aureus resume growth when supplemented with either a saturated or an unsaturated fatty acid. Antimicrob Agents Chemother. 1977;11:574–576. doi: 10.1128/aac.11.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heath RJ, Rock CO. A triclosan-resistant bacterial enzyme. Nature. 2000;406:145–146. doi: 10.1038/35018162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.