Abstract
Type 1 and type 2 diabetes result from an absolute or relative reduction in functional β-cell mass. One approach to replacing lost β-cell mass is transplantation of cadaveric islets; however, this approach is limited by lack of adequate donor tissue. Therefore, there is much interest in identifying factors that enhance β-cell differentiation and proliferation in vivo or in vitro. Connective tissue growth factor (CTGF) is a secreted molecule expressed in endothelial cells, pancreatic ducts, and embryonic β cells that we previously showed is required for β-cell proliferation, differentiation, and islet morphogenesis during development. The current study investigated the tissue interactions by which CTGF promotes normal pancreatic islet development. We found that loss of CTGF from either endothelial cells or β cells results in decreased embryonic β-cell proliferation, making CTGF unique as an identified β cell-derived factor that regulates embryonic β-cell proliferation. Endothelial CTGF inactivation was associated with decreased islet vascularity, highlighting the proposed role of endothelial cells in β-cell proliferation. Furthermore, CTGF overexpression in β cells during embryogenesis using an inducible transgenic system increased islet mass at birth by promoting proliferation of immature β cells, in the absence of changes in islet vascularity. Together, these findings demonstrate that CTGF acts in an autocrine manner during pancreas development and suggest that CTGF has the potential to enhance expansion of immature β cells in directed differentiation or regeneration protocols.
Pancreas development initiates at embryonic day (E) 9.5 in the mouse as dorsal and ventral evaginations from the posterior foregut endoderm that undergo branching morphogenesis. Notch/Delta signaling within the ductal epithelium generates a population of endocrine progenitor cells marked by expression of the transcription factor neurogenin3 (Ngn3) (1–4). These progenitors delaminate from the epithelium and differentiate into hormone-positive cells that subsequently proliferate. Islets are complex microorgans responsible for maintaining glucose homeostasis and consist of at least four different endocrine cell types, including insulin-producing β cells and glucagon-producing α cells. Insufficient β-cell mass characterizes both type 1 (autoimmune) and type 2 diabetes. Thus, strategies to generate β cells de novo or increase their number in vivo are a potential approach for the treatment of diabetes and are being widely investigated.
Generation of the correct numbers of the different endocrine cell types requires tight coordination of waves of differentiation and proliferation that are regulated by both paracrine and autocrine signals. The pancreatic vascular endothelium secretes paracrine factors important for pancreas differentiation (5–8). Factors such as retinoic acid, FGFs, and bone morphogenetic proteins (BMPs) regulate the outgrowth of the epithelium, as well as the differentiation of multipotent pancreatic progenitors (9). Signals from the dorsal aorta are necessary for growth of the dorsal pancreas as well as expression of Ptf1a, a transcription factor essential for the development of pancreatic progenitors (7, 10). Furthermore, in vitro coculture experiments demonstrated that recombination of prepatterned dorsal endoderm with aorta endothelium is sufficient to induce differentiation of insulin-expressing cells (8). Blood vessel-derived sphingosine-1-phosphate stimulates growth of the pancreas, and endothelial cells secrete additional factors that have a direct role on the endoderm (11). For example, the concomitant formation of blood vessels and islets involves communication between the endothelial cells and endocrine cells. VEGFA is expressed in islet endocrine cells from very early in development through adulthood and is required for proper development of the islet vasculature (12). Overexpression of VEGFA throughout the entire pancreatic epithelium under the direction of the pancreatic and duodenal homeobox-1 (Pdx-1) promoter leads to an increase in pancreatic vascularization and total islet mass at the expense of exocrine tissue (8). The main VEGF receptor, VEGFR2/flk-1, is not expressed in islets, suggesting that the increase in islet mass is secondary to the increase in vasculature rather than a direct consequence of VEGFA overexpression on the endoderm (8, 12, 13). Thus, although endothelial cells have long been understood to have a role in pancreas growth and endocrine development, the specific secreted factors that mediate its effects on the pancreatic epithelium have not yet been identified.
Although endocrine cell neogenesis generates the majority of the endocrine cells early in development, adequate endocrine cell replication during late gestation (E18.5) and in the neonatal period is necessary to generate the correct number of β cells in the adult. Only a few factors, however, have been shown to regulate embryonic β-cell proliferation in vivo. The eIF2α kinase PERK is important for β-cell proliferation in embryos and neonates but not in the adult (14). The transcription factor Pdx-1 is essential for pancreas organogenesis and β-cell proliferation (15–17). Removal of Pdx-1 specifically in embryonic β cells leads to a significant decrease in β-cell proliferation and a concomitant increase in α-cell proliferation at late gestation (17). Recent evidence has shown that Ngn3-positive proendocrine cells are unipotent; each progenitor cell gives rise to only one endocrine cell type (18). The fact that β- and α-cell proliferation are reciprocally altered when Pdx-1 is inactivated in β cells only suggests that communication between endocrine cell types also plays a role in generating the proper numbers of each different cell type within the islets.
We previously determined that the secreted factor connective-tissue growth factor (CTGF) is required for embryonic β-cell proliferation. CTGF-null embryos die at birth because of skeletal defects (19), but display a significant decrease in β-cell proliferation, specifically at E18.5, and also have defects in pancreatic endocrine lineage allocation and islet morphogenesis (20). In the mouse, CTGF is expressed in the developing pancreas as early as E12.5 and is localized to the developing blood vessels, β cells, ducts, and mesenchyme (20). As development proceeds, CTGF remains highly expressed in ducts and endothelial cells but its expression decreases in β cells. By postnatal day (P) 3, CTGF is no longer detected in β cells but is still expressed in ducts and endothelial cells in adult mice and humans. At early stages, embryos lacking CTGF show normal pancreas development but have an altered endocrine cell ratio beginning at E13.5; α cells are increased without changes in total endocrine area, proliferation, or apoptosis (20).
CTGF is a modular protein that acts in other systems as both an autocrine and paracrine factor via its interactions with integrins, TGF-β, BMPs, and Wnts (21). Because CTGF is expressed by multiple cell types in the pancreas, it is not clear from our previous studies whether CTGF acts in an autocrine or paracrine manner to promote proper endocrine lineage allocation, β-cell proliferation, and islet morphogenesis. We used a conditional null allele of CTGF and used tissue-specific Cre recombinases to inactivate CTGF from the pancreatic epithelium, vasculature, or endocrine progenitors. Here we show that loss of endothelial-derived CTGF results in decreased islet vascularity associated with decreased embryonic β-cell proliferation. Additionally, we find that CTGF produced by the β cells themselves is required for β-cell proliferation, making it unique as an identified autocrine regulator of embryonic β-cell proliferation. We also demonstrate that β cell-specific overexpression of CTGF during embryogenesis using an inducible transgenic system is sufficient to increase proliferation of immature β cells and endocrine cell mass, and that this occurs in the absence of increased vascularity. These studies have implications for the manipulation of stem/progenitor cells in vivo or in vitro to promote pancreatic endocrine differentiation, and enhance β-cell mass expansion for ultimately treating patients with diabetes.
Results
β Cell-Derived CTGF Acts in an Autocrine Manner to Promote Proper Levels of Proliferation During Embryogenesis.
To address how CTGF-mediated autocrine and paracrine communication between the different pancreatic cell types regulates the differentiation of progenitors into endocrine cells and their subsequent proliferation and islet morphogenesis, CTGF was conditionally inactivated in a cell type-specific manner using a previously described conditional-by-inversion (CTGFe2COIN) allele (22) (Fig. S1). In this allele, exposure to Cre recombinase results in the inversion of an otherwise inert insertion into exon 2, thus rendering it a null allele. We determined that the presence of the e2COIN intron did not affect CTGF message levels (Fig. S2A); CTGFe2COIN/+ and CTGFe2COIN/e2COIN mice develop normally. Therefore, CTGFe2COIN/+ and CTGFe2COIN/e2COIN littermates were used interchangeably as controls in all subsequent experiments. We confirmed the Cre-dependent inversion of the CTGFe2COIN allele using PCR on sections of embryonic pancreata (Fig. S2B).
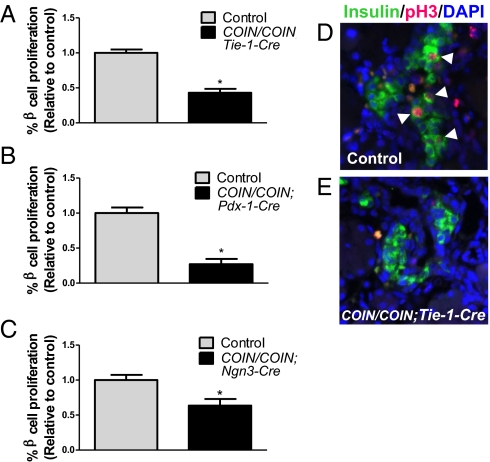
To determine whether CTGF produced by the endothelium promotes β-cell proliferation during embryogenesis, CTGFe2COIN mice were interbred to transgenic mice expressing Cre recombinase from the Tie-1 promoter (23) to generate litters containing embryos in which CTGF is inactivated specifically in the vasculature (CTGFe2COIN/e2COIN;Tie-1-Cre). Previous analysis of the Tie-1-Cre line reveals extremely high specificity for endothelial cells throughout all organs examined, although some recombination has been noted in certain CNS neurons and hematopoietic cells (23–27). β-Cell replication was analyzed in control and CTGFe2COIN/e2COIN;Tie-1-Cre embryos at E18.5 using immunohistochemistry to examine expression of phosphorylated histone H3 (pH3) and insulin. Compared with control embryos, embryos in which CTGF was inactivated in endothelial cells had a significant decrease in β-cell proliferation (Fig. 1 A, D, and E). At this stage, 10.2 ± 0.49% of β cells were proliferating in control embryos, but CTGF mutants had nearly a 60% reduction in β-cell proliferation (4.4 ± 0.56%, P = 0.002). To determine if this was a direct effect of endothelial-derived CTGF on the β-cell population or an indirect effect because of secondary consequences of endothelial CTGF inactivation on the vasculature, we quantified islet blood-vessel density in control and CTGFe2COIN/e2COIN;Tie-1-Cre embryos. We found that indeed, loss of endothelial CTGF resulted in a decrease in islet vascularity (Fig. S3). Thus, at this point we cannot determine whether CTGF is the endothelially derived signal that promotes β-cell proliferation, or if an additional endothelial signal is required that is now reduced as a result of the decreased vasculature. Nonetheless, because CTGF is still produced by the pancreatic epithelium (ducts and β cells) in CTGFe2COIN/e2COIN;Tie-1-Cre mutant embryos, we conclude that the remaining CTGF produced by the epithelium is not sufficient to compensate for loss of the endothelial-derived signal to promote normal β-cell proliferation.
Fig. 1.
CTGF from multiple sources is required for β-cell proliferation during embryogenesis. Quantification of β-cell proliferation normalized to control littermates in CTGFe2COIN/e2COIN;Tie-1-Cre (A), CTGFe2COIN/e2COIN;Pdx-Cre (B), and CTGFe2COIN/e2COIN;Ngn3-CreBAC (C) embryos at E18.5 (A and B) and P1 (C). *P < 0.05 compared with Control. (D and E) Representative images showing decreased β-cell proliferation in CTGFe2COIN/e2COIN;Tie-1-Cre embryos (E) compared with controls (D). Arrowheads indicate proliferating β cells. n = 3–5 animals per genotype. (Magnification: 400×.)
In our previous studies, CTGF expression was examined as early as E12.5 and found to be in the pancreatic trunk epithelium, but absent from branch tips where acinar cells are differentiating (20). As part of the present study, we examined CTGFlacZ/+ embryos at E10.5 and determined that CTGF expression can be detected in scattered cells throughout the pancreatic bud epithelium at this stage (Fig. S4). To address whether CTGF produced by an epithelial source in the pancreas, either the ducts or the β cells, also affects β-cell proliferation, CTGF was inactivated throughout the pancreatic epithelium beginning at E9.5 by interbreeding mice carrying the CTGFe2COIN allele to Pdx-1-Cre mice (4). Analysis of β-cell proliferation at E18.5 indicated that the apparent percentage of replicating β cells in control mice was ∼10-fold lower than in control mice from Tie-1-Cre litters. This difference was determined to be caused by a change in pH3 antibody sensitivity because of lot changes (Fig. S5). Nonetheless, CTGFe2COIN/e2COIN;Pdx-1-Cre embryos displayed a significant reduction in the percentage of replicating β cells compared with control littermates (Fig. 1B). Control embryos displayed 0.5 ± 0.04% β-cell proliferation, but Pdx-1-Cre mutants had 0.14 ± 0.34% (P = 0.001). Thus, CTGF produced by an epithelial source is also required for β-cell proliferation.
Because Pdx-1-Cre removed CTGF function from both the ductal epithelium and β cells, CTGFe2COIN mice were bred to Ngn3-CreBAC mice (28) to inactivate CTGF early from the endocrine lineage. CTGF expression is not localized to any other hormone-expressing cell type in the mouse pancreas, therefore using Ngn3-CreBAC assayed for the role of CTGF in autocrine β-cell signaling. CTGFe2COIN/e2COIN;Ngn3-CreBAC embryos had a significant (36%) reduction in β-cell proliferation at P1 compared with controls (Fig. 1C). Although an average of 0.87 ± 0.06% of β cells were proliferating in controls, CTGFe2COIN/e2COIN;Ngn3-CreBAC mutants had an average of 0.56 ± 0.08% (P = 0.02) proliferating β cells. These data indicate that autocrine CTGF-mediated signaling is required to promote proper levels of embryonic β-cell proliferation. Currently, a Cre driver line with which CTGF can specifically and efficiently be inactivated from embryonic ducts is not available. Therefore, we were unable to directly investigate the role of CTGF produced by the ducts in β-cell proliferation at this time. Inactivating CTGF in endothelial cells, the pancreatic epithelium, or β cells each lead to a defect in β-cell proliferation, suggesting that the function of CTGF produced by each one of these sources is nonredundant with regard to β-cell proliferation.
Different CTGF Sources Function Redundantly to Promote Lineage Allocation and Islet Morphogenesis.
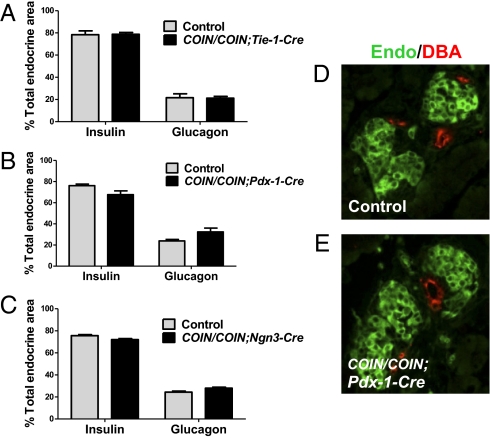
Global inactivation of CTGF leads to defects in endocrine lineage allocation and islet morphology, including an increase in glucagon-positive area, decreased insulin-positive area, failure of islets to separate from the ductal epithelium, and a mixed islet phenotype (20). Therefore, we investigated which sources of CTGF are required for generating the correct proportions of endocrine cell types and for islet morphogenesis. The proportion of endocrine tissue composed of insulin and glucagon-positive area was quantified at P1 in CTGFe2COIN/e2COIN;Tie-1-Cre, CTGFe2COIN/e2COIN;Pdx-1-Cre, and CTGFe2COIN/e2COIN;Ngn3-CreBAC conditional-mutant embryos, as well as in littermate controls. Endocrine composition was not significantly different from controls in any of the tissue-specific mutants (Fig. 2 A–C). The proximity of islets to ducts and islet architecture was also examined in each of the tissue-specific mutants and was found to be similar to controls (Fig. 2 D and E), suggesting that the remaining sources of CTGF compensate for the loss of CTGF from one source with respect to both lineage allocation and islet morphogenesis.
Fig. 2.
Different sources of CTGF function redundantly to promote lineage allocation and islet morphogenesis. The proportion of the endocrine tissue composed of insulin- and glucagon-positive areas in CTGFe2COIN/e2COIN;Tie-1-Cre (A), CTGFe2COIN/e2COIN;Pdx-1-Cre (B), and CTGFe2COIN/e2COIN;Ngn3-CreBAC (C) mutants at P1. n = 3 animals of each genotype. (D and E) Islet morphogenesis comparison in conditional mutants. Sections from P1 pancreata were immunolabeled with insulin and glucagon (Endo, green) and DBA (red). The distance between the endocrine tissue and ducts was measured in control (D) and mutant (E) pancreata. Although only Pdx-1-Cre mutants are shown, mutants from other Cre were analyzed and found not to be significantly different from controls. (Magnification: 400×.)
CTGF Overexpression in Embryonic β Cells Increases Endocrine Cell Mass and Proliferation at P1.
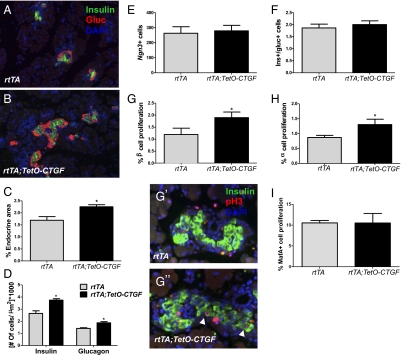
Because loss of CTGF causes a reduction in β-cell proliferation, we assessed whether overexpression of CTGF leads to increased β-cell mass. CTGF was specifically overexpressed in β cells during development using a tetracycline-inducible system. Mice expressing the reverse tetracycline transactivator from the rat insulin promoter (RIP-rtTA) were interbred to a CTGF responder line (TetO-CTGF), and doxycycline was administered continuously in the drinking water of pregnant dams beginning at E9.5. Overexpression of CTGF was confirmed by immunolabeling of pancreas sections at P1 (Fig. S6 A and B) and real-time PCR of pancreata at E16.5 (Fig. S6 C and D). Pancreata from bigenic pups (rtTA;TetO-CTGF) and littermate controls (rtTA) were analyzed for endocrine mass at P1 (Fig. 3 A and B). There was no significant difference in the total pancreatic area between control and bigenic pancreata (Fig. S6E); however, the proportion of the pancreatic area composed of endocrine tissue was significantly increased by ∼25% in bigenic neonates (Fig. 3C) and this was in the absence of any changes in islet vascularity (Fig. S3). Thus, these studies indicate that increased levels of CTGF within β cells are sufficient to increase islet mass. β Cell-derived CTGF increased the number of both α and β cells significantly in bigenic pancreata (Fig. 3D).
Fig. 3.
CTGF overexpression during development led to increased endocrine mass and proliferation. Immunohistochemical analysis of insulin (green) and glucagon (red) in control (A) and bigenic (B) pups at P1 (Magnification: 100×). (C) Quantification of endocrine area at P1 in control (rtTA) and bigenic (rtTA;TetO-CTGF) pups. (D) The number of insulin- and glucagon-positive cells in control and bigenic pups normalized to total pancreatic area. (E) Quantification of Ngn3-positive cells in control and CTGF overexpressing pancreata at E14.5. (F) The ratio of insulin-positive to glucagon-positive cells in control and CTGF overexpressing neonates. (G) The percent of proliferating β cells in control and CTGF overexpressing pups at P1. Representative images of control and bigenic pancreata are shown in G′ and G′′, respectively (Magnification: 400×). Arrowheads indicate proliferating β cells. (H) α-Cell proliferation in control and CTGF overexpressing pups. (I) Quantification of proliferation of MafA-positive cells in control and bigenic pups. n = 3 of each genotype. *P < 0.05 compared with rtTA.
To determine whether the overall increase in endocrine cells observed at P1 was subsequent to earlier changes in endocrine neogenesis, the number of Ngn3-expressing cells was quantified at E14.5. We observed no difference in the number of Ngn3-positive cells in the CTGF overexpressing pancreata compared with control pancreata at this stage (Fig. 3E), suggesting that the increased number of endocrine cells is not caused by an increase in the endocrine progenitor population. The ratio of insulin to glucagon-positive cells was also similar to control pups (Fig. 3F); thus, increased β cell-derived CTGF does not alter lineage allocation of endocrine progenitor cells. We therefore analyzed β- and α-cell proliferation and found that the proportion of proliferating β cells (Fig. 3G) and α cells (Fig. 3H) was significantly increased in bigenic neonates compared with control embryos at P1. Interestingly, the increased number of proliferating insulin-expressing cells was not caused by an increase in the proliferation of the MafA-positive population (Fig. 3I), which represents only about 75% of insulin-positive cells at P1 (29) Thus, CTGF overexpression in embryonic insulin-producing cells has no effect on neogenesis, but rather appears to increase endocrine mass by enhancing proliferation of β and α cells. The increase in β-cell proliferation is primarily caused by increased replication of immature cells. It remains to be seen whether this is also true of the α-cell population.
Discussion
In this study, we investigated the tissue interactions by which CTGF promotes normal pancreatic islet development. We showed that the β cells themselves are a required source of CTGF within the epithelium, making CTGF unique as a secreted β cell-derived factor that has been demonstrated to be required for embryonic β-cell proliferation. We inactivated CTGF from endothelial cells and the entire pancreatic epithelium and found that β-cell proliferation was significantly impaired when CTGF is lost from either source. The changes in islet vascularization observed in embryos lacking endothelial-derived CTGF still leave open the possibility that decreased CTGF itself affects β-cell proliferation. Alternatively, another blood vessel-derived signal may directly impact β-cell proliferation and this signal may be decreased secondarily to the diminished vascularity caused by CTGF inactivation. CTGF promotes endothelial cell migration and angiogenesis in vitro and in vivo in other systems (30); thus, the results we obtained are consistent with known roles of CTGF. However, it is clear that CTGF can have vascular-independent effects on β-cell proliferation because overexpression of CTGF enhances β-cell proliferation without affecting islet vascular density.
Although we were unable to directly assess the role of CTGF produced by pancreatic ducts in this study, it is possible that CTGF produced by the ductal epithelium also contributes to β-cell proliferation. Conversely, embryos in which CTGF is overexpressed specifically in β cells displayed increased endocrine cell proliferation and endocrine mass. Thus, an increase in CTGF levels was sufficient to stimulate β- and α-cell proliferation in vivo, in the absence of any effects of CTGF on islet vasculature. Our results indicate that CTGF overexpression in insulin-expressing cells did not affect endocrine cell neogenesis or the allocation of endocrine progenitors into the α and β lineages. The temporal or spatial pattern of CTGF overexpression in this study may have limited the effects of CTGF to cell proliferation; earlier or broader expression of CTGF in endocrine or pancreatic progenitors may reveal the ability of CTGF to promote neogenesis.
It is noteworthy that although CTGF is a secreted factor, the remaining sources of CTGF were unable to completely compensate for the defect in β-cell proliferation that results when CTGF was lost from a single source. We therefore conclude that the process of β-cell proliferation in the embryo is particularly sensitive to the total level of CTGF to which the β cells are exposed, and that the overall level of CTGF in the pancreas is more important for promoting β-cell proliferation than a specific cellular source. These data are consistent with our previous finding that β-cell proliferation is significantly impaired in CTGF heterozygous mutant embryos, which have a 50% reduction in overall CTGF expression in the pancreas (20). In contrast, the processes of endocrine cell lineage allocation and islet morphology may be less sensitive to the overall levels of CTGF than β-cell proliferation, as they were unaffected when CTGF was removed from only one pancreatic source.
Elegant studies have demonstrated that endothelial cells induce pancreas outgrowth and endocrine differentiation during development, although it may be that the role of endothelial cells differs early in pancreas development compared with after the secondary transition, and may have differential effects on endocrine versus exocrine differentiation (31, 32). In adult islets, the vascular basement membrane produces laminins, which have been shown to promote insulin expression and β-cell proliferation (33). During pregnancy in mice and humans, β-cell mass expands to meet the increased metabolic demand for insulin and in rodents this expansion is caused by a threefold enhancement of β-cell proliferation (34). An increase in vascular growth precedes β-cell proliferation in pregnant rats, suggesting a role for endothelial cells in stimulating β-cell proliferation in response to pregnancy (35). However, to our knowledge, CTGF is unique as a factor identified to be secreted by the endothelium that is required for β-cell proliferation in the embryo. Our studies indicate that the importance of the vasculature–endocrine interaction can be extended to include a role for endothelial cells in embryonic β-cell proliferation.
The fact that CTGF is also highly expressed in adult islet vasculature suggests that CTGF may also promote the proliferation of adult β cells under certain circumstances, although our embryonic data suggest that CTGF does not promote replication of mature β cells. We observe no changes in glucose homeostasis in any of our mutant or overexpressing lines (Fig. S7); however, we previously showed that CTGF is up-regulated in β cells during pregnancy and have preliminary data that CTGF global heterozygotes show impaired glucose tolerance during pregnancy. Therefore, CTGF may act as both an autocrine and paracrine signal in regulating β-cell proliferation in the adult as well as the embryo (20).
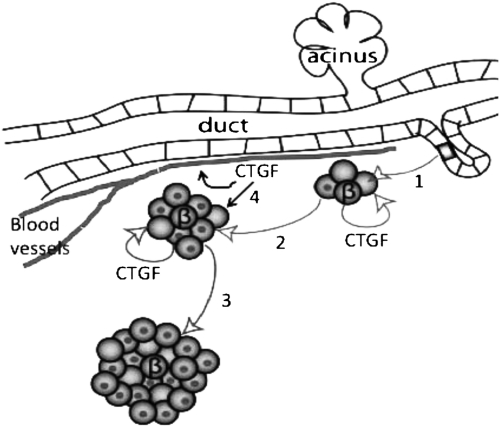
The current approaches to generating transplantation-quality β cells for the treatment of diabetes include the directed differentiation of ES cells or induced pluripotent stem cells down the normal differentiation path from endoderm to insulin-producing cells by adding exogenous factors to the culture medium. These protocols have been successful in generating insulin-producing cells; however, in general, the percentage of β cells in the cultures is relatively low and expansion of these cells has proven difficult (36). Recent studies have sought to identify small molecules or growth factors that are able to increase the yield of β cells (37). Our studies suggest that CTGF is an attractive candidate for inclusion in these directed differentiation protocols, as it is required for proper lineage allocation and embryonic β-cell proliferation and its overexpression in β cells was sufficient to increase endocrine proliferation and mass. We hypothesize that CTGF has the potential to increase the efficiency of the differentiation of stem cells at multiple steps of the protocol, either by increasing the number of endocrine cells that differentiate from endocrine progenitors or by stimulating the proliferation of immature β cells. (See Fig. 4 for a model for CTGF action in endocrine development.) Thus, CTGF is unique as a factor reported to regulate embryonic β-cell proliferation in an autocrine manner, raising the possibility that β cells may be producing other autocrine and paracrine factors that regulate the proportions of different hormone-positive cells during development.
Fig. 4.
Model for CTGF action in endocrine development. Our current and previous studies show that 1, CTGF influences differentiation of delaminating endocrine progenitors to become β cells at the secondary transition. For simplicity, only β cells are shown here. Differentiating β cells begin to produce CTGF. 2, β Cell-derived CTGF acts in an autocrine manner at late gestation to promote replication of MafA−/insulin-positive cells (nonnucleated cells), but not MafA+ mature β cells (nucleated). 3, In adults, the majority of β cells are MafA+ and we hypothesize that these may be refractory to CTGF. 4, Endothelial-derived CTGF may have two roles: an autocrine role for promoting islet vascular development and a paracrine role in stimulating β-cell proliferation.
Materials and Methods
Animals.
The generation of the CTGFe2COIN allele has been previously described (22). Targeted CTGFe2COIN ES cells (Regeneron Pharmaceuticals) were used to generate chimeric male mice at the Transgenic/ESC Shared Resource facility at Vanderbilt University. Chimeras were bred to mice expressing the FLPE recombinase from the Protamine 1 promoter (provided by Chin Chiang, Vanderbilt University, Nashville, TN) for the removal of the HygΔTK selection cassette. The removal of the selection cassette was confirmed by PCR. Mice exhibiting germ-line transmission of the CTGFe2COIN allele were bred to create homozygous CTGFe2COIN/e2COIN mice. Genotyping information is found in the SI Materials and Methods. Tissue-specific inactivation of CTGF was achieved by breeding CTGFe2COIN/e2COIN to the following Cre deleter lines: Tie-1-Cre (provided by Scott Baldwin, Vanderbilt University, Nashville, TN), Pdx-1-Cre (provided by Guoqiang Gu, Vanderbilt University, Nashville, TN) and Ngn3-CreBAC (provided by Christopher Wright, Vanderbilt University, Nashville, TN) (4, 23, 27). Intraperitoneal glucose tolerance tests were performed as previously described (38).
To overexpress CTGF in β cells, transgenic mice were generated in which expression of the CTGF cDNA (Open Biosystems) is driven by the tetracycline operator (TetO; plasmid was a gift from Tim Blackwell, Vanderbilt University, Nashville, TN) rendering transgene expression doxycycline-dependant. TetO-CTGF mice were interbred to homozygous mice expressing the reverse tetracycline transactivator (rtTA) from a fragment of the rat insulin 2 promoter (RIP-rtTA), which were generously provided by Alvin Powers (Vanderbilt University, Nashville, TN) (39). Pregnant mothers were given 2 mg/mL of doxycycline in a 2% Splenda solution in their drinking water beginning on day 9.5 of gestation to expose the embryos to doxycycline before RIP activation, which normally occurs at E11.5.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medial Center.
Statistical Analysis.
Results are expressed as mean ± SEM. For two-group comparison, Student t test was used. P < 0.05 was considered statistically significant.
Methods on PCR genotyping, tissue dissection, histology, morphometric analysis, and quantitative real-time PCR can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank members of the M.G. laboratory for critical reading of the manuscript; Hana Erkou, Rachel Lippert, Nora Kayton, and Brandon McClure for technical assistance; Amanda Ackermann for help with art work; and Guoqiang Gu, H. Scott Baldwin, Christopher V. E. Wright, and Alvin C. Powers for providing reagents. This research involved use of the Vanderbilt Transgenic Mouse/ES Cell Shared Resource and the Functional Genomics Shared Resource (supported in part by National Institutes of Health Grants CA68485 and DK20593), and was supported in part by Vanderbilt Molecular Endocrinology Training Program Grant 5 T 32 DK07563 (to M.A.G.) and Grant 1-2007-548 from the Juvenile Diabetes Research Foundation International (to M.G.).
Footnotes
Conflict of interest statement: A.N.E. is a paid employee and shareholder of Regeneron Pharmaceuticals.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100072108/-/DCSupplemental.
References
- 1.Apelqvist A, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 2.Jensen J, et al. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: A role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49(2):163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- 3.Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 5.Bhushan A, et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- 6.Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Jacquemin P, et al. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290(1):189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 9.Guney MA, Gannon M. Pancreas cell fate. Birth Defects Res C Embryo Today. 2009;87:232–248. doi: 10.1002/bdrc.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 11.Edsbagge J, et al. Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development. 2005;132:1085–1092. doi: 10.1242/dev.01643. [DOI] [PubMed] [Google Scholar]
- 12.Brissova M, et al. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 13.Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol. 1995;9:1760–1770. doi: 10.1210/mend.9.12.8614412. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, et al. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 16.Offield MF, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(6):983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 17.Gannon M, et al. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol. 2008;314(2):406–417. doi: 10.1016/j.ydbio.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development. 2009;136:3567–3574. doi: 10.1242/dev.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivkovic S, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford LA, et al. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23:324–336. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gressner OA, Gressner AM. Connective tissue growth factor: A fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008;28:1065–1079. doi: 10.1111/j.1478-3231.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 22.Canalis E, Zanotti S, Beamer WG, Economides AN, Smerdel-Ramoya A. Connective tissue growth factor is required for skeletal development and postnatal skeletal homeostasis in male mice. Endocrinology. 2010;151:3490–3501. doi: 10.1210/en.2010-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson E, Brakebusch C, Hietanen K, Fässler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci. 2001;114:671–676. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- 24.Bjarnegard M, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnromalities. Development. 2004;131(8):1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 25.Umans L, et al. Inactivation of Smad5 in endothelial cells and smooth muscle cells demonstrates that Smad5 is required for cardiac homeostasis. Am J Pathol. 2007;170(5):1460–1472. doi: 10.2353/ajpath.2007.060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song L, et al. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301(1):276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, et al. Inhibitor of differentiation 1 promotes endothelial survival in a bleomycin model of lung injuury in mice. Am J Pathol. 2007;171(4):1113–1126. doi: 10.2353/ajpath.2007.070226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Artner I, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61) Angiogenesis. 2002;5(3):153–165. doi: 10.1023/a:1023823803510. [DOI] [PubMed] [Google Scholar]
- 31.Sand FW, et al. Growth-limiting role of endothelial cells in endoderm development. Dev Biol. 2011;352:267–277. doi: 10.1016/j.ydbio.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Pierreux CE, et al. Epithelial: Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev Biol. 2010;347:216–227. doi: 10.1016/j.ydbio.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Nikolova G, et al. The vascular basement membrane: A niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: Increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 35.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: Studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 36.D'Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 37.Borowiak M, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackermann Misfeldt A, Costa RH, Gannon M. β-Cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes. 2008;57:3069–3077. doi: 10.2337/db08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milo-Landesman D, et al. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant. 2001;10:645–650. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.