Abstract
The posterior parietal cortex (PPC) of monkeys and prosimian galagos contains a number of subregions where complex, behaviorally meaningful movements, such as reaching, grasping, and body defense, can be evoked by electrical stimulation with long trains of electrical pulses through microelectrodes. Shorter trains of pulses evoke no or simple movements. One possibility for the difference in effectiveness of intracortical microstimulation is that long trains activate much larger regions of the brain. Here, we show that long-train stimulation of PPC does not activate widespread regions of frontal motor and premotor cortex but instead, produces focal, somatotopically appropriate activations of frontal motor and premotor cortex. Shorter stimulation trains activate the same frontal foci but less strongly, showing that longer stimulus trains do not produce less specification. Because the activated sites in frontal cortex correspond to the locations of direct parietal–frontal anatomical connections from the stimulated PPC subregions, the results show the usefulness of optical imaging in conjunction with electrical stimulation in showing functional pathways between nodes in behavior-specific cortical networks. Thus, long-train stimulation is effective in evoking ethologically relevant sequences of movements by activating nodes in a cortical network for a behaviorally relevant period rather than spreading activation in a nonspecific manner.
Keywords: complex movements, nonhuman primate, neocortex
Posterior parietal cortex (PPC) in primates is widely considered to be involved in creating intentions to perform specific motor behaviors, such as grasping, reaching, or directing eyes to new targets (1–5). These intentions are then implemented through connections with primary motor (M1) and premotor (PM) cortex (6–8). Representations of simple movements of various body parts in frontal motor cortex (M1-PM) have been traditionally revealed by use of short bursts of electrical pulses delivered with microelectrodes (9–12). By introducing the use of longer (500 ms) trains of electrical pulses, Graziano et al. (13, 14) and Graziano (15) have identified zones in M1-PM of macaque where grasping, reaching, and other complex, functionally significant movements are evoked. In addition, they have identified matching zones in PPC and M1-PM regions where similar defensive movements of the arm and face can be evoked (16, 17). Our more recent studies with long-train stimulation have identified a series of functional zones in PPC of prosimian galagos (18, 19). These PPC movement zones are preferentially interconnected with functionally matched zones in PM and motor cortex (20). M1 cortex seems to be an essential node in these parietal–frontal networks, because PPC stimulation was ineffective when M1 activity was blocked.
Although long-train stimulation has the potential to reveal much that is new about the functional organization of PPC and the parietal–frontal network that is involved in the production of movements, we do not yet know how long-train effects are mediated and why they are different from short-train stimulation effects. One possibility is that long-train stimulation produces more complex movements by activating the same network as short-train stimulation but more effectively over a longer, behaviorally relevant time. Another possibility is that the longer stimulus train activates more neurons but less specifically because of the spread of activity to involve additional neural pathways. Given the widespread connections of any cortical area (including those connections in PPC) with other areas (21, 22) and thalamic and basal ganglia targets that may directly and indirectly feed back to cortex (23–25), almost any site in the brain seems to have potential of being activated by the long-train stimuli. Without more information, the lack of understanding of the neural effects of long-train stimulation is seen as seriously limiting interpretation of results (26).
Here, we used intrinsic optical imaging to reveal activation patterns in frontal cortex during long-train electrical stimulation of sites in PPC of galagos that evoked classes of complex movements. Because of the shallow intraparietal sulcus (IPS), most of PPC in galagos is accessible on the brain surface, which simplifies the exploration of this region with microelectrodes. Optical imaging provided a global view of how extensively frontal cortex was activated by stimulating sites in PPC and how activation patterns during long-train stimulation compare with those patterns during stimulation with shorter trains of electrical pulses. Frontal cortex in prosimian galagos is well-suited for such imaging studies, because these primates have no central sulcus and the M1-PM region is exposed on the brain surface (12). Optical imaging allows cortical activity, which is reflected by intrinsic signals, to be visualized in a manner similar to functional magnetic resonance imaging (fMRI) signals but at a higher spatial resolution. Our results indicate that long-train stimulation of sites in different functional zones of PPC selectively activates functionally matched zones in motor and PM cortex. Moreover, shorter trains of electrical pulses, including those trains commonly used in mapping studies of motor cortex, activate the same functional zones, although less effectively, and therefore, movement may not occur. We conclude that long-train stimulation, applied at appropriate cortical sites, involves specific neural networks that are functionally distinct and results from long-train stimulation experiments need to be incorporated in theories of PPC organization and function.
Results
Optical imaging was used to reveal zones of activation in M1 and PM cortex of four adult galagos during long-train (500 ms) electrical stimulation of sites in PPC where complex movements were evoked. Activation patterns were compared with those patterns obtained with shorter trains of electrical pulses (60 and 120 ms).
Functional Subdivisions of PPC and M1-PM.
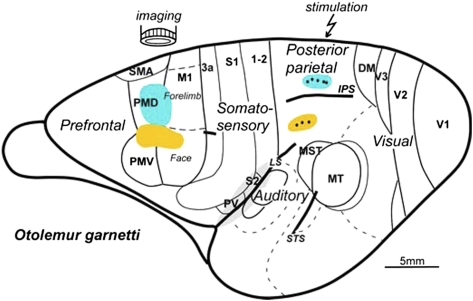
Stimulating electrodes were placed in various locations in PPC around the IPS, and the frontal motor region was imaged. Similar to other primates, the frontal motor region responsive to microstimulation in galagos includes M1 and dorsal (PMD) and ventral (PMV) PM areas. PPC includes cortex between areas 1 and 2 just caudal to the primary somatosensory and dorsomedial visual areas. Electrical stimulation consistently produced movements in the rostral one-half of PPC (PPCr) and not in its caudal one-half. Movements were evoked from the cortex up to the estimated border of areas 1 and 2, and some effective sites may have been slightly across this border (18, 19). By carefully monitoring multiple physiological parameters, including EEG (Materials and Methods), we were able to maintain stable levels of anesthesia. As a result, testing the same site at different times during the experiment produced similar movements.
The stimulation results conformed to a crude somatotopic pattern such that combined hind- and forelimb movements were evoked most medially in PPC and were followed more laterally by regions for forelimb, face, and eye movements (Fig. 1). Long-train electrical stimulation also evoked similar complex movements from M1 and PM cortex, and zones for specific classes of matching movements were aligned in a somatotopic pattern that paralleled those zones of PPC (19).
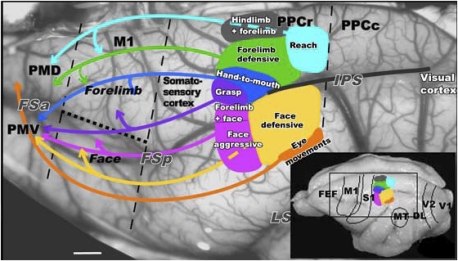
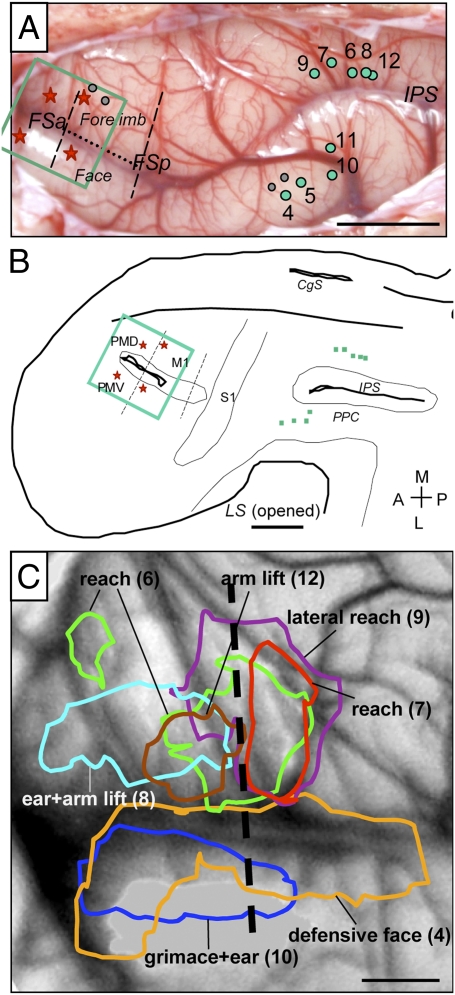
Fig. 1.
Summary of the PPC organization in galago. Functionally distinct movement zones are marked with colors on the exposed left hemisphere. Connections between functional PPC zones and frontal motor region PM-M1 are marked with color-coordinated arrows. This view matches the location of Inset on the schematic of PPC functional zones on a dorsolateral view of the hemisphere (lower right corner). Black dashed lines mark approximate borders of M1 and the border between rostral (PPCr) and caudal PPC (PPCc). The dotted line marks the border between the M1 forelimb and face representations. M1, primary motor cortex; PMD and PMV, dorsal and ventral motor areas; PPCr and PPCc, rostral and caudal posterior parietal cortex; FSa and FSp, anterior and posterior frontal sulci; IPS, intraparietal sulcus; LS, lateral sulcus; FEF, frontal eye field; DL, dorsolateral visual area; MT, middle temporal area; S1, primary somatosensory area; V1, primary visual area; V2, secondary visual area. (Scale bar: 1 mm.)
The optical imaging results presented below also relate to the patterns of connections between PPC and M1-PM cortex that have been revealed by injections of tracers in different motor zones of PPC (21). As expected from the somatotopy of the motor maps in M1 and PM cortex (19), movement zones in PPC that involve the face have more connections in the lateral zones of M1-PM cortex than in the medial movement zones that involve the hand and forelimb (Fig. 1). In galagos, inputs from visual areas are largely limited to the caudal PPC, and caudal PPC is densely interconnected with rostral PPC (21).
Activation Zones in Frontal Cortex.
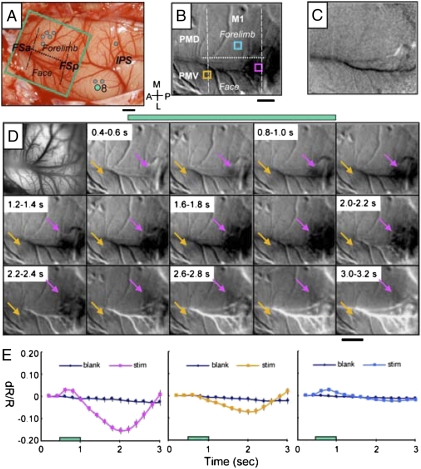
Stimulation of movement sites in rostral PPC activated restricted zones within M1 and PM cortex. In case 08-08, stimulation of three cortical sites in rostral PPC just lateral to the rostral end of the IPS (Fig. 2A) produced face movements, including a grimace with eye squint, ear retraction, and jaw opening, a combination of movements that we classified as defensive (19). When one of those sites (Fig. 2A, green dot 8) was stimulated during optical imaging, a strong hemodynamic response was evoked in the caudal part of lateral M1 (Fig. 2B, dark zone). Short trains of electrical pulses evoke simple face movements from this cortex (12, 27), whereas long trains evoke defensive face and head movements (19). The responsive zone was anterior to the rostral end of the shallow frontal posterior sulcus (FSp), which marks the caudal border of M1. Another less-responsive zone was in PMV near the shallow frontal anterior sulcus (FSa) at the rostral border of M1. When cortex was not stimulated, no activity was apparent in any part of M1 or PM (Fig. 2C).
Fig. 2.
Intrinsic cortical activity in response to electrical stimulation of PPC in galago 08-08. Electrical stimulation (300-μA, 500-ms train) of the face region of ventral PPC evoked a face defensive movement. (A) Pattern of blood vessels on the exposed brain surface. Green rectangle marks imaged M1-PM field. Green dot 8 in face representation of PPC indicates the site of electrical stimulation for illustrated cortical activation. Gray dots mark other stimulation sites. Dashed lines indicate approximate borders of M1, and the dotted line indicates the border between the forelimb and face M1 representations. (B) Intrinsic response (dark region) temporally averaged 1–2 s after stimulus onset. (C) Blank response during the no stimulus condition. (D) Image maps showing time course of activation after stimulation onset. Frame duration is 200 ms. The first frame is the corresponding blood vessel map of images. Activations in M1 and PMV are indicated with pink and yellow arrows, respectively. (E) Time course of changes in reflectance from three regions of interest centered over (Left) M1 (pink box in B), (Center) M1/PMV (yellow box in B), and (Right) dorsal M1 (blue box in B) in trials with electrical stimulation (colored lines) and blank trials (black lines). Stimulation period is indicated by green bars in D and E. Error bars = SD. (Scale bar: 1 mm.) A, anterior; M, medial; L, lateral; P, posterior. Imaged left cortex from 18 trials.
Because the evoked activation was strong enough to be seen in single 0.2-s frames, the time course of the hemodynamic response was determined (Fig. 2D). No response was apparent before the stimulus onset. However, a weak response was already apparent in the first frame, which included the first 100-ms stimulation. The response became more obvious over the full 500-ms duration of the stimulus, and it continued to increase a full 1 s after the end of the stimulus train, after which the response gradually decreased. This amplitude and time course is typical of previously published intrinsic signal time courses recorded from sensory cortical areas during sensory stimulation (28–30).
To further characterize the region of the strong response and compare it with other motor regions during and after the stimulation period, the amplitude and time course of the activation were measured for three small regions of M1-PM (Fig. 2B, colored squares). The reflectance of cortex in the strong response region (Fig. 2B, pink square), overlying the motor face representation in M1, had a peak amplitude of about 0.2% that was approximately 1 s after the end of the stimulus train, which is consistent with the intrinsic signal characteristics (Fig. 2E). The second, more rostral selected region (Fig. 2B, yellow square) was in or near a part of PMV representing the face. At this location, there was a detectable but much weaker response over the same time period (Fig. 2E Center). This weak response is visible in some of the frames in Fig. 2D, yellow arrows. Finally, reflectance changes were measured from a sample of cortex in a more medial portion of M1 representing forelimb movements (Fig. 2B, blue square). In this sample of M1, there was very little change in reflectance during and after stimulation (Fig. 2E Right). Thus, stimulation of a defensive face site in PPC produced a strong focus of activity about 1 mm in diameter in the caudal part of the face representation in M1, while producing only a minor change in the face region of cortex at the M1-PMV junction and no change in forelimb M1. No change was apparent in other parts of M1 or PM cortex either. This finding illustrates the specificity of the long-train electrical stimulation effect.
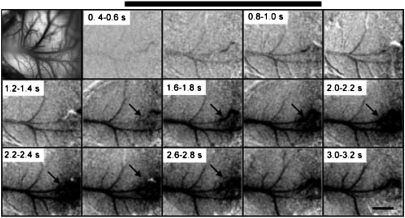
These focused, topographically appropriate responses were evident even in single trials. Although the activation in case 08-08 was most apparent when averaged over 18 trials to reduce noise (Fig. 2), the same pattern could be seen on single trials, although as expected, with weaker signal to noise (Fig. 3). Again, the activated pattern of M1 (dark oval) was apparent, although weaker, toward the end of the stimulus train. It was most pronounced 1 s after the stimulus onset, and it faded to background levels by 2 s after the stimulus ends. At the peak of reflectance change, the focus of activation was about 1 mm in diameter.
Fig. 3.
Time course of cortical activation from an individual trial in case 08-08 (compare with Fig. 2). The top left frame shows a photograph of the imaged cortex. Subsequent frames correspond to a sequence of 200-ms imaging times from a single trial. The black bar represents the stimulus. (Scale bar: 1 mm.)
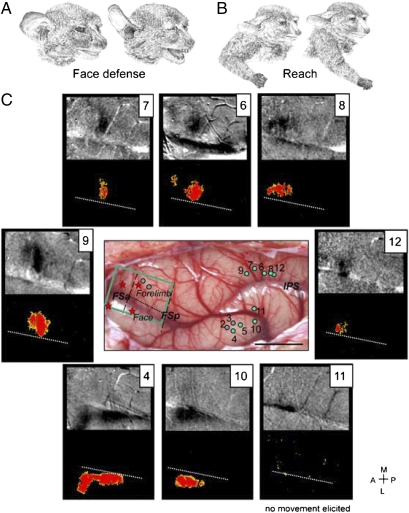
In another galago (08-18), we stimulated sites over a larger PPC region with microelectrodes to compare activation patterns with stimulation of sites in ventral vs. dorsal PPCr (Fig. 4). In agreement with results from case 08-08 and our previous microstimulation studies (18, 19), stimulation of sites located in the ventral PPCr (penetrations 2–5 and 10) evoked defensive face movements, whereas stimulation of sites in dorsal and caudal PPCr (penetrations 6–9 and 12) caused forelimb movements. Defensive face movements consisted of a grimace and backward movement of the ear, sometimes with mouth opening (Fig. 4A). The reaching movements evoked by stimulation of sites 6, 7, and 9 consisted of straightening the arm and extending the opened hand out from the body (Fig. 4B), either forward (sites 6 and 7) or to the side (which we refer to as lateral reach; site 9). Similar upward arm extensions (but without grip opening) evoked by stimulation of sites 8 and 12 were classified here as arm lift.
Fig. 4.
Maps of activation evoked by electrical stimulation of PPC in galago 08-18. Electrical stimulation of PPC (300- to 400-μA, 500-ms trains) that evokes face (A) and arm (B) behaviors produces topographical activation of the M1-PM cortex, which is revealed by intrinsic optical imaging of the hemodynamic response. (C) Activity maps (4–12) recorded for face and forelimb movements. Evoked face movements: 4, defensive face (+ mouth opens); 10, defensive face. Evoked forelimb movements: 6, forward reach; 7, forward reach, 8, slight arm lift + ear; 9, lateral outward reach; 12, arm lift. Stimulation of 11 (face region) did not evoke any movement. For clarity, each intrinsic optical imaging map is paired with a t-test map of cortical motor activity after filtering out random occurring pixels and small clusters of pixels. The t-test map for 11 is presented without filtering to illustrate the level of random significant pixels. White dotted lines in t-test maps mark the borders between forelimb and face representations. Green dots on the dorsolateral view of the exposed cortical surface (C Middle Center) mark the stimulation sites for shown activation maps. Gray dots mark other stimulated sites. Red stars mark lesions. (Scale bar: 5 mm.) Other conventions are as in Figs. 1 and 2.
Activation maps were obtained from stimulation of three sites in ventral PPC and all five sites in dorsal PPC. Defensive face movements were evoked from sites 4 and 10 in ventral PPC. Activation elicited from these stimulation locations were in ventral (but not dorsal) frontal cortex, below the level of frontal sulci FSa and FSp. For both stimulated sites, the focus of activation was in caudal PMV, extending into rostral M1, most clearly for site 4 (Fig. 4C). Stimulation of sites in the dorsal PPC that evoked reaching (or arm lift) movements (penetrations 6–9 and 12) consistently activated a small region of frontal cortex comprising caudal PMD and adjoining parts of M1 (sites 6 and 9) or mainly caudal PMD (sites 12 and 8) or rostral M1 (site 7). Long-train stimulation of the same region of PMD and M1 in galagos evoked reaching movements (19, 21). Stimulation of sites in dorsal PPC that evoked forelimb movements failed to produce foci of activity in ventral M1 or PMV or in other parts of frontal cortex in the imaging field. Thus, only small regions of PMD and M1 seemed to be highly involved in producing the reaching movements. Stimulation of site 11 failed to elicit any noticeable movement. Possibly because of the proximity to the IPS sulcus, the laminar depth of the electrode was not in layer 5, where the electrical stimulation would have been most effective. Because stimulation at site 11 both failed to elicit any movement and failed to produce activation sites in frontal M1 and PM cortex, the site provides an interesting control. We suggest that only stimulations of PPC that produce movement will significantly activate foci in motor and PM cortex.
By stimulating several sites in dorsal and ventral PPC in case 08-18, we were able to compare and contrast the locations of reflectance change in frontal cortex for sites that produce similar or different movements (Fig. 5). Five sites were stimulated in dorsal PPC (Fig. 5 A, 6–9 and 12, and B), with closely positioned sites 6 and 7 producing similar forward reaching movements, more anterior site 9 producing a lateral reach, and sites 8 and 12 (most posterior) producing a slight arm lift; additionally, for site 8, an ear movement, a movement that is often observed together with arm movements (18, 19), was seen. As shown in Fig. 5C, stimulation of both sites 6 (Fig. 5C, green) and 7 (Fig. 5C, red) in the heart of the reach zone produced highly overlapping foci of activation in motor cortex, although an additional small, more rostral focus was produced by stimulation of site 6. Site 9 (Fig. 5C, purple) produced a slightly different reach that was more laterally directed; however, the main focus of activation overlapped those foci of reach sites 6 and 7. The stimulation at most caudal sites 8 (Fig. 5C, light blue) and 12 (Fig. 5C, brown) just outside the PPC reach region evoked more rostral foci of activity in PMD. Defensive face movements were evoked at sites 4 (Fig. 5C, yellow) and 10 (Fig. 5C, dark blue), and after stimulation of these two PPC sites, frontal activation zones in PMV overlapped extensively. The more extensive (involving M1) focus of activation observed after stimulation in point 4 (Fig. 5C, yellow) could reflect the addition of mouth opening in the defense face behavior that differed from a grimace behavior associated with stimulation at point 10 (Fig. 5C, dark blue). Stimulation at site 11 failed to evoke any movement, and no activation of M1 or PM cortex was detected.
Fig. 5.
Areas of activation elicited by electrical stimulation of PPC in case 08-18. (A) Photomicrograph of exposed cortical surface shows the pattern of blood vessels with the marked M1-PM imaged region (green rectangle) and sites of stimulation in PPC (green dots). (B) Reconstructions of the left brain hemisphere shown on the flattened view with stimulated sites (green dots) and areal borders approximated from CO and myelin sections. The green rectangle marks the imaged field. (C) Areas of activation overlaid on a blood vessels map. Stimulus amplitudes were 300–400 μA. Areas activated during face movements were observed laterally, and areas activated during forelimb movements were observed medially. (Scale bars: A and B, 5 mm; C, 1 mm.) Other conventions are the same as in Figs. 1 and 2.
In summary, sites in PPC that evoked movements produced small, 1- to 2-mm foci of activity in frontal cortex, including M1 and PM cortex. The longer focus from site 4 seemed to result from a fusion of foci at the M1/PMV border. Stimulation of PPC sites that produced similar movements activated overlapping regions of frontal cortex, whereas sites that produced different movements (reaching vs. face defensive) activated nonoverlapping regions of frontal cortex.
Shorter Stimulation Trains Activate the Same Sites but Less Effectively.
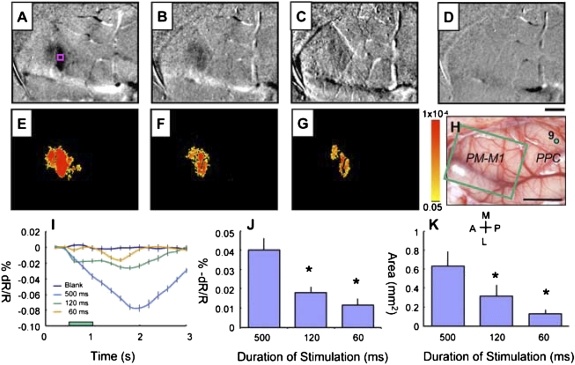
In motor and PM cortex, short (60 ms) trains of electrical pulses reliably produce brief movements (19) that now seem to be the initial part of the more complex movements elicited by the longer (500 ms) trains. In previous (18, 19) and present experiments, 60-ms trains at sites in PPC failed to elicit any movements, and longer 120-ms trains at a few sites tested here produced partial movements. Nevertheless, these shorter trains evoked reflectance changes in frontal cortex at the same sites just like the long-train stimulations. The main difference was that the activation in frontal cortex was reduced in amplitude and spatial extent (Fig. 6). When site 9 of case 08-18 (Fig. 6H, green dot) was stimulated with a 500-ms train of pulses, a characteristic reach to grasp sequence of movements was produced that included extension of the arm, involving both shoulder and elbow movements, accompanied by a dorsal wrist flexion and extension of the digits. The 120-ms train of pulses produced only partial extension involving, mainly, the shoulder. The 60-ms stimulus did not produce a visible movement. The optical imaging results in frontal motor cortex paralleled these behavioral effects. The 500-ms train of electrical pulses produced a dark focus of reduced reflectance in M1-PM, the 120-ms train produced a less dark focus, and the 60-ms train produced a faint focus (Fig. 6 A–C). A blank trial with no electrical stimulation produced no change (Fig. 6D). The difference in the changes produced by these three stimulus conditions is clearly delineated by color-coded response significance maps (Fig. 6 E–G). The activation site at the M1/PMD border remained the same under all three conditions, but the site was less activated for the 120-ms train (Fig. 6F) and only activated slightly above noise for the 60-ms train (Fig. 6G). The time course of response to the 500-ms stimulus rapidly increased to a peak nearly 2 s after stimulus onset and then, decayed over the next 1 s (Fig. 6I; pink square where reflectance changes were measured, Fig. 6A). The activation produced by 120-ms stimulation had the same beginning, but it failed to develop much amplitude and ended sooner. The 60-ms stimulus produced a barely visible cortical response, and this response was delayed and weak. Measurements of response amplitude and areal extent, averaged across stimulation locations, indicate that the focus of changed activity in M1 during the 500-ms train was significantly reduced during the 120- and 60-ms stimulus trains (Fig. 6 J and K).
Fig. 6.
Intrinsic motor cortex activity to different durations of electrical stimulation of PPC in case 08-18. Electrical stimulation (400 μA) of the dorsal PPC (green dot 9 in H) evoked a lateral reaching movement. (A–D) Optical imaging maps and (E–G) t-test maps of motor cortical activity to 500- (A and E), 120- (B and F), and 60-ms (C and G) duration of stimulus. (D) Image map for the no stimulus blank condition. (H) Blood vessels image shows imaged frontal M1-PM region (green rectangle) with site of stimulation in PPC (green dot 9). (I) Change in reflectance for a region of interest (pink square in A) vs. time for different stimulus train durations to stimulation at site 9. (J) Peak percent change in reflectance to 500-, 120-, and 60-ms stimulation for six different sites of stimulation. (K) Area of activation as measured by the number of significant pixels (P < 0.01, uncorrected) to 500-, 120-, and 60-ms stimulation for six different sites of stimulation. *P < 0.01, 500 ms > 120 ms, 500 ms > 60 ms. Clip value = 0.05%. (Scale bars: A–G, 1 mm; H, 5 mm.) Other conventions are the same as in Fig. 2.
Discussion
In the present study, we used optical imaging of M1-PM to reveal regions of activity evoked by 500-ms trains of electrical pulses in various locations in PPC. Such long trains, but not shorter trains, evoked complex, behaviorally meaningful movements even in anesthetized primates (17, 19). These movements were not random or highly variable, because retesting of the same site with long-train stimulation elicited similar movements. Contrary to some expectations (26), the long-train stimulation of PPC did not activate widespread cortical regions, but rather, restricted regions of M1-PM cortex were activated. These regions varied with the locations of the stimulating electrodes in different functional zones of PPC. The activated regions of frontal cortex corresponded closely to the sites of dense interconnections between frontal and parietal cortex that were revealed by injections in functional zones in PPC (21). Shorter trains (120 ms) applied to PPC evoked only the initial movement of the complex sequences, and those trains (60 ms) commonly used for mapping motor cortex (9–12, 31) did not evoke any noticeable movement. Although they produced no movements, such shorter trains, applied to sites in PPC, evoked activity in the same locations in frontal cortex as the long trains, but the amplitude and the areal extent of the activation were greatly reduced. These observations reveal the basic components of the functional circuits of parietal–frontal networks in primates.
Although each functional zone in PPC has widespread connections within PPC as well as other connections with higher-order somatosensory or visual areas (21, 22), which also have connections with frontal cortex, the probable activation of these other direct targets of PPC functional zones did not result in widespread, nonspecific activations of frontal motor areas. Of course, other areas of cortex connected with the stimulated zones in PPC, such as somatosensory areas of the lateral sulcus and prefrontal cortex, were likely activated, but the foci in motor and PM cortex (our field of view) were very restricted. The model that is supported by our observations is that each functional zone in PPC mediates a specific motor response by activating restricted, functionally matched zones in M1-PM cortex. Individual neurons in motor cortex encode fragments of complex movements (32) that are combined to produce complex motor action (33). Functionally matched PM and M1 zones are interconnected (21), with M1 providing most of the subcortical output that is critically important for the evoked behavior. Thus, electrical stimulation of PPC fails to produce movement when the activity of the functionally related portions of M1 is blocked with muscimol. Of course, the visual inputs to PPC do not play a role in visual guidance of movements in anesthetized primates, but somatosensory feedback generated during these movements could help guide the movement sequences, especially at brainstem and spinal cord levels (34).
The present results do not indicate how specific behaviors are selected in awake, behaving primates, but it seems likely that the interconnections between functional zones in PPC are largely suppressive and help resolve conflicts between alternative behaviors as a result of differences in top-down and bottom-up patterns of PPC activation (35). Because comparable functional zones in both frontal parietal cortex and PPC have now been shown in prosimian galagos (19, 21), New World monkeys (20), and Old World macaques (13, 16, 17), the functional parietal–frontal circuits described here likely evolved with or before the first primates and have been retained with modifications in all major branches of the primate radiation, including humans. Our results also show the potential of using optical or fMRI imaging in combination with electrical stimulation to reveal functional pathways in primates and other mammals.
The restricted patterns of activations seen in the present experiments can be considered somewhat surprising given the potential for long-train stimuli at high-current levels to activate wide regions of cortex and other parts of the brain. Physiologically, the functional organization of motor cortex is usually determined in microelectrode stimulation experiments using short-train electrical pulses of 10 μA or less. Such stimuli are thought to activate only a few of the large pyramidal neurons that project to brainstem and spinal cord motoneuron pools that produce movements (36, 37). Higher levels of current stimulate many more neurons over direct and indirect routes (26), and repetitive pulses of stimulation can lead to powerful temporal summation of transneuronal excitation effects (9, 38). Undoubtedly, our long trains of pulses at higher levels of current did stimulate somewhat larger populations of neurons, because shorter trains and less current produced little or no evoked movement from sites in PPC, at least in our anesthetized primates. However, even at high levels of current, the direct effects of electrical stimulation are largely limited to neurons less than 1 mm from the electrode tip (37), although more distant neurons can be activated if their intrinsic axons are near the electrode tip (39). Moreover, a recent study using two-photon calcium imaging to identify activated neurons during electrical stimulation of cortex has shown that the length of the stimulus train had little effect on which neurons in the field of view were activated (39). Thus, long trains may more powerfully activate specific circuits, but they do not necessarily recruit additional circuits. Not only does microstimulation activate mainly neurons and neural processes immediately around the electrode tip but moving the electrode as little as 30 μm can greatly change the pattern of locally activated neurons (39). Finally, the results of microstimulation of cortex in macaque monkeys, when viewed by fMRI, suggest that activity changes are largely limited to structures that are directly interconnected (40–42). Related results suggest that stimulation of a cortical site may suppress the spread of activation beyond those sites of direct connections (43). Thus, electrical stimulation of specific functional zones in PPC likely activates other functional zones in PPC through direct connections, but activation of inhibitory neurons may suppress the outputs of these zones so that they fail to activate their functionally matched zones in PM and motor cortex.
In summary, imaging methods combined with microstimulation reveal parallel parietal–frontal networks that can correspond to results of traditional anatomical labeling and correlate with specific behaviors evoked electrically from PPC.
Materials and Methods
The hemodynamic response of frontal cortex to electrical stimulation of PPC was studied in four adult galagos (Otolemur garnetti) in terminal experiments under anesthesia. Physiological results were later correlated with cortical architectonics revealed in brain sections processed to reveal cytochrome oxidase or myelinated fibers. All procedures were approved by the Vanderbilt Animal Care and Use Committee and followed guidelines of the National Institutes of Health for the care and use of laboratory animals.
Animal Preparation for Stimulation and Imaging.
PPC and frontal cortex were exposed for stimulation and imaging while a galago was anesthetized. Each galago was initially anesthetized with ketamine hydrochloride (10–30 mg/kg, i.m.), and therefore, it could be placed in a stereotaxic frame; then, anesthesia was maintained with 2% isoflurane. The skull was opened to expose parietal and frontal cortex of the left cerebral hemisphere. After dura was retracted, the cortex was covered with a 4% agar solution, and a clear, 0.15-mm-thick glass coverslip was placed over frontal cortex to reduce brain movement. During microstimulation and optical imaging, anesthesia was maintained with ketamine hydrochloride (30–50 mg/kg per h) diluted with physiological saline (1:4), delivered i.v. with an infusion pump, and supplemented every 2–4 h with 0.2–0.5 mg/kg xylazine i.m. Stability of anesthesia was carefully monitored by continuous recording of breathing rate, EEG, muscle tone, and spontaneous movements. We maintained anesthesia at neurosurgical level 2, which is characterized by high-amplitude (100–150 μV) bursts that occur every 1–3 s; these bursts are an accepted indicator of anesthetic plane for human surgery at which electrically evoked movements can still be obtained.
Intracortical Microstimulation.
Stimulation procedures followed those procedures previously described (19). To evoke movements from PPC, a low-impedance tungsten microelectrode was lowered perpendicularly into the cortex to a depth of 1.5–1.8 mm beneath the surface with a micromanipulator. Stimulations at these depths (lower cortical layers) were most effective in evoking movements. Sites of microstimulation were marked on a high-resolution photograph of the exposed cortex. Stimulation was delivered by a Master 8 stimulator (AMPI) with biphasic stimulus isolation (Bak Electronics Inc.). Stimuli consisted of trains of 0.2-ms biphasic pulses, presented at 300 Hz, that extended over the duration of 500 ms, which is long enough to evoke complex movements. Current levels started at 100 μA and were raised to a maximum of 400 μA until consistent movement occurred or no movement was observed (for unresponsive sites). Consecutive stimulus trains were separated by 8 s or more. Threshold was defined by the lowest current level that would evoke the movement or part of the movement for three consecutive trains of stimuli. In some experiments, effects of short trains (60 or 120 ms) of electrical pulses were evaluated. When possible, selected sites were retested to evaluate whether similar motor patterns would be evoked. In each case, optical imaging results were obtained from several stimulated sites, including those sites devoted to different classes of movements. At the end of microstimulation mapping, some sites of interest were marked by electrolytic lesions (10 μA direct current for 10 s) made with the stimulating microelectrode. Although microlesions can clearly be seen in the histological preparations of the brain tissue, we have not found any visible damage from the sites where biphasic stimulation pulses were delivered.
Optical Imaging.
For the optical imaging procedure, a camera was securely positioned directly above the craniotomy. Images of reflectance changes of cortex (intrinsic hemodynamic signals), corresponding to local cortical activity, were acquired using Imager 3001 (Optical Imaging Inc), whereas cortex was illuminated with 630-nm wavelength light provided by fiber optic light guides (28). For each microstimulation train, 20 consecutive image frames were recorded (5-Hz frame rate). To enhance the signal to noise ratio, 30–50 trials of long-stimulation trains were averaged, although single trial results were also considered. Each image frame contained 504 × 504 pixels covering a field of 8 × 8 mm. This view encompassed most of the exposed portions of M1, PMD, PMV, frontal eye field, and adjoining prefrontal cortex and area 3a. Runs of stimulation (500 ms, 300 Hz, biphasic pulse, 300–400 μA) were interleaved with runs of no stimulation for comparison. In some cases, runs of shorter stimulus trains (60 or 120 ms) were interleaved with long-train runs or no stimulus runs. Stimulus onset occurred after the first image frame. Because 60-ms stimulus trains are typically used for mapping the representation of movements in motor cortex (11, 12, 31), we compared activation patterns evoked by stimulus trains of typical length with trains of two different longer lengths.
Data Analysis.
Blood vessel artifact or noise were minimized by (i) high-pass filtering of the images to subtract baseline lighting changes that are a source of motion noise, (ii) blank subtraction of the first frame of each image acquisition, or (iii) frame by frame subtraction of the blank condition image by image. Single condition maps obtained this way represent the percentage of intrinsic signal change compared with an initial (prestimulus) baseline condition. For stimulus-induced intrinsic signals, this value is usually negative (−0.01% to −0.2%). In single condition maps, darker pixels represent higher responses (more negative), and brighter pixels represent lower responses. These maps were used for calculating differential maps and additional quantification.
Within delineated regions, the time course and amplitude of activation were used to further characterize functional response. To delineate the regions of strongest activation, optical maps underwent a thresholding procedure. In this procedure, images were low pass-filtered, pixel distributions were established, and pixels with the strongest activations above a certain value (15%) were identified. Within the area of activation, we centered a region of interest, 150–300 μm in diameter, and used this region to collect the percent change of activation over time. Areas of activation were then aligned on blood vessel maps obtained with 570-nm filter (green light) to compare the locations of activations across stimulus conditions. To measure the sizes of activated regions, we generated T maps and used a P value of 0.01 (uncorrected) to demarcate significant pixels. The number of significant pixels around the functional domain provided a measure of the area of activation.
Histology and Alignment of Cytochrome Oxidase Staining with Optical Maps.
After collecting data, the galago was given a lethal injection of sodium pentobarbital (80 mg/kg or more), perfused with PBS (pH 7.4) followed by 2–4% paraformaldehyde in PBS, and then, given 2–4% paraformaldehyde in PBS with 10% sucrose. After a brain was removed, the cortex was separated from the brainstem, flattened, and immersed in 30% sucrose in PBS. On the next day, it was cut frozen with the surface vascular pattern preserved in the first 100- to 150-μm sections. The vascular pattern in these superficial sections allowed for their accurate alignment with deeper sections and optical imaging results. Deeper sections were cut at 50 μm and stained for cytochrome oxidase (44) or myelin (45) to reveal cortical architecture. Separate sections in the series were aligned with each other using the lumen of blood vessels that crossed sections vertically and marking electrolytic microlesions that were placed at the end of the microstimulation session.
Acknowledgments
The authors thank M. Feurtado for surgical assistance and Y. Chu and L. Trice for technical assistance. O.A.G. was funded by the Natural Sciences and Engineering Research Council of Canada. This work was supported by National Institutes of Health Grants NS 055843 (to I.S.), NS 044375 (to A.W.R.), and NS 16446 (to J.H.K.).
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 15033.
References
- 1.Rizzolatti G, Fogassi L, Gallese V. Parietal cortex: From sight to action. Curr Opin Neurobiol. 1997;7:562–567. doi: 10.1016/s0959-4388(97)80037-2. [DOI] [PubMed] [Google Scholar]
- 2.Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 3.Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- 4.Buneo CA, Andersen RA. The posterior parietal cortex: Sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Andersen RA, Brotchie PR, Mazzoni P. Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol. 1992;2:840–846. doi: 10.1016/0959-4388(92)90143-9. [DOI] [PubMed] [Google Scholar]
- 6.Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: New concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 7.Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- 8.Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage. 2001;14:S27–S32. doi: 10.1006/nimg.2001.0835. [DOI] [PubMed] [Google Scholar]
- 9.Asanuma H, Arnold A, Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res. 1976;26:443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- 10.Strick PL, Preston JB. Multiple representation in the primate motor cortex. Brain Res. 1978;154:366–370. doi: 10.1016/0006-8993(78)90707-2. [DOI] [PubMed] [Google Scholar]
- 11.Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- 12.Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002a;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 14.Graziano MSA, Taylor CSR, Moore T, Cooke DF. The cortical control of movement revisited. Neuron. 2002b;36:349–362. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- 15.Graziano MSA. The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci. 2006;29:105–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- 16.Cooke DF, Taylor CSR, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci USA. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke DF, Graziano MSA. Sensorimotor integration in the precentral gyrus: Polysensory neurons and defensive movements. J Neurophysiol. 2004;91:1648–1660. doi: 10.1152/jn.00955.2003. [DOI] [PubMed] [Google Scholar]
- 18.Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stepniewska I, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J Comp Neurol. 2009;517:765–782. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharbawie OA, Stepniewska I, Burish MJ, Kaas JH. Thalamocortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb Cortex. 2010;20:2391–2410. doi: 10.1093/cercor/bhp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stepniewska I, Cerkevich CM, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Darian-Smith C, Darian-Smith I, Cheema SS. Thalamic projections to sensorimotor cortex in the macaque monkey: Use of multiple retrograde fluorescent tracers. J Comp Neurol. 1990;299:17–46. doi: 10.1002/cne.902990103. [DOI] [PubMed] [Google Scholar]
- 24.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 25.Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP.’. Cereb Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- 26.Strick PL. Stimulating research on motor cortex. Nat Neurosci. 2002;5:714–715. doi: 10.1038/nn0802-714. [DOI] [PubMed] [Google Scholar]
- 27.Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005;490:305–333. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- 28.Chen LM, Friedman RM, Ramsden BM, LaMotte RH, Roe AW. Fine-scale organization of SI (area 3b) in the squirrel monkey revealed with intrinsic optical imaging. J Neurophysiol. 2001;86:3011–3029. doi: 10.1152/jn.2001.86.6.3011. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer MW, Calford MB, Clarey JC, Pettigrew JD, Roe AW. Spontaneous and stimulus-evoked intrinsic optical signals in primary auditory cortex of the cat. J Neurophysiol. 2001;85:1283–1298. doi: 10.1152/jn.2001.85.3.1283. [DOI] [PubMed] [Google Scholar]
- 30.Lu HD, Roe AW. Optical imaging of contrast response in Macaque monkey V1 and V2. Cereb Cortex. 2007;17:2675–2695. doi: 10.1093/cercor/bhl177. [DOI] [PubMed] [Google Scholar]
- 31.Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: A microstimulation study. J Comp Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 32.Hatsopoulos NG, Xu Q, Amit Y. Encoding of movement fragments in the motor cortex. J Neurosci. 2007;27:5105–5114. doi: 10.1523/JNEUROSCI.3570-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leyton SS, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orangutan and gorilla. Q J Exp Physiol. 1917;11:135–222. [Google Scholar]
- 34.Bizzi E, Giszter SF, Loeb E, Mussa-Ivaldi FA, Saltiel P. Modular organization of motor behavior in the frog's spinal cord. Trends Neurosci. 1995;18:442–446. doi: 10.1016/0166-2236(95)94494-p. [DOI] [PubMed] [Google Scholar]
- 35.Capaday C. The integrated nature of motor cortical function. Neuroscientist. 2004;10:207–220. doi: 10.1177/107385403262109. [DOI] [PubMed] [Google Scholar]
- 36.Stoney SD, Jr, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: Effective extent of stimulating current. J Neurophysiol. 1968;31:659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- 37.Andersen P, Hagan PJ, Phillips CG, Powell TP. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon's hand. Proc R Soc Lond B Biol Sci. 1975;188:31–36. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- 38.Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975;249:617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolias AS, et al. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron. 2005;48:901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 41.Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol. 2006;96:512–521. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- 42.Sultan F, Augath M, Logothetis N. BOLD sensitivity to cortical activation induced by microstimulation: Comparison to visual stimulation. Magn Reson Imaging. 2007;25:754–759. doi: 10.1016/j.mri.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Logothetis NK, et al. The effects of electrical microstimulation on cortical signal propagation. Nat Neurosci. 2010;13:1283–1291. doi: 10.1038/nn.2631. [DOI] [PubMed] [Google Scholar]
- 44.Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- 45.Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]