Abstract
Navigation, the ability to reach desired goal locations, is critical for animals and humans. Animal navigation has been studied extensively in birds, insects, and some marine vertebrates and invertebrates, yet we are still far from elucidating the underlying mechanisms in other taxonomic groups, especially mammals. Here we report a systematic study of the mechanisms of long-range mammalian navigation. High-resolution global positioning system tracking of bats was conducted here, which revealed high, fast, and very straight commuting flights of Egyptian fruit bats (Rousettus aegyptiacus) from their cave to remote fruit trees. Bats returned to the same individual trees night after night. When displaced 44 km south, bats homed directly to one of two goal locations—familiar fruit tree or cave—ruling out beaconing, route-following, or path-integration mechanisms. Bats released 84 km south, within a deep natural crater, were initially disoriented (but eventually left the crater toward the home direction and homed successfully), whereas bats released at the crater-edge top homed directly, suggesting navigation guided primarily by distal visual landmarks. Taken together, these results provide evidence for a large-scale “cognitive map” that enables navigation of a mammal within its visually familiar area, and they also demonstrate the ability to home back when translocated outside the visually familiar area.
Keywords: cognitive map, spatial memory, true navigation, movement ecology, global positioning system
Navigation is critical for the survival of animals, and has been extensively studied in animals, mostly in nonmammalian species (1–10). The most advanced type of navigation is the ability to travel directly to a certain destination from any starting point in the environment, regardless of its direction and without relying on familiar routes. Evidence for the existence of such navigational map (11, 12) comes from field and laboratory experiments. In the field, homing experiments in translocated lobsters (13), newts (14) and pigeons (1, 15, 16), for example, showed an ability to navigate from an unfamiliar site to one or more goal locations. Typically, inferences from such homing experiments were based either on animals’ vanishing bearing at the release site, or animals’ reappearance at the goal location. Only recently were translocated pigeons and honey bees tracked continuously (3, 17); yet, to date, no high-resolution movement tracks have been collected from free-ranging mammals homing from translocation distances larger than a few kilometers—and the lack of such data severely limits our understanding of mammalian navigation mechanisms. In the laboratory, studies implementing various experimental approaches suggested the existence of a mental representation of space, or a “cognitive map,” in rodents (2, 18–20); yet, our ability to infer map-like navigation from laboratory experiments on such small spatial scale (in meters) has been questioned (21, 22). Thus, there is a gap in knowledge about mammalian navigation: most of our knowledge about large-scale navigation comes from studies in nonmammalian species, whereas detailed data on mammals’ navigation in the field is scarce, certainly compared with data on birds.
Here, we have set out to close this gap, by examining whether a free-ranging mammal performs map-like navigation on large scales (∼100 km). We equipped cave-dwelling Egyptian fruit bats (Rousettus aegyptiacus) with miniature global positioning system (GPS) dataloggers (Fig. S1), which enabled high-resolution measurements of their flight trajectories over several consecutive nights (Methods). We asked whether bats possess a cognitive map of their visually familiar environment (2), which would be manifested by the ability to perform novel short-cuts within this environment—and whether they are capable of homing back to their cave when translocated outside their visually familiar environment. Our results, combining releases at the roost as well as translocation experiments, suggest that bats are capable of visual-based navigation within the familiar environment, and that they can also home from outside their visually familiar environment. These data thus provide evidence for both kinds of navigational capacities in bats—and evidence for large-scale navigation in a mammal.
Results
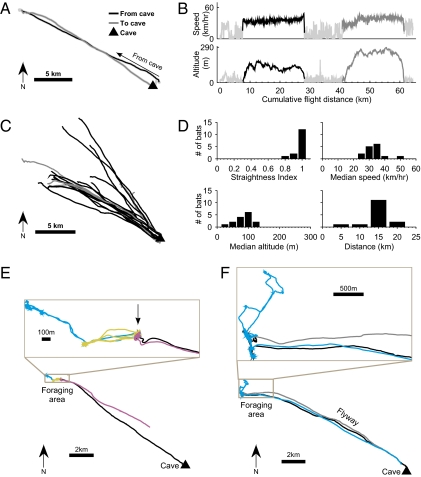
When released near their cave, individual bats commuted to distant fruit trees (Fig. 1 A and B, Fig. S2, and Movie S1) in long, fast, high, and very straight flights (N = 15 bats; mean straightness index ± SD, 0.97 ± 0.02; Fig. 1 B and D, Table 1, and Fig. S3). Commuting flight speeds were typically between 35 and 55 km/h (Fig. 1B, Top), and flight heights were typically at a few hundred meters above ground level (Fig. 1B, Bottom). All but one bat flew straight to a feeding tree without following landscape elements. Bats typically did not fly to the fruit tree nearest to their cave; instead, they flew to remote fruit trees, passing many similar fruiting trees on the way (Fig. S4). When they had arrived at the favored tree, bats typically foraged at this tree and at adjacent trees for the entire night (Movie S1). Moreover, the bats returned to the same fruit tree over several consecutive nights (Fig. 1E, Inset, arrow), often following the same trajectory every night (Fig. 1F, “flyway”). Of the 15 bats for which we collected foraging data, 14 bats had GPS and/or radiotelemetry data from consecutive nights, and 13 of these 14 bats (93%) returned to the same tree in at least two nights within the first three nights from release. Moreover, radiotelemetry tracking of 16 additional fruit bats from the same colony showed that bats foraged on the same tree for as long as 4 mo.
Fig. 1.
GPS tracking of Egyptian fruit bats navigating from their cave. (A) Example of bat 125 leaving the cave, flying locally (light gray line), then taking a long commuting flight to the feeding tree (black line) and back to the cave (dark gray). (B) Speed and altitude above ground level for the same bat as in A as function of its cumulative flight distance during the night. Black, commuting flight from cave; dark gray, back to cave. (C) All commuting flights that started or ended directly at the cave (n = 14); colors as in A; note the very straight flights of all these bats. An additional seven flights were composed of a local flight and then a commuting flight (e.g., the bat in A); their commuting flights were as straight as those depicted here. (D) Flight parameters for the commuting flights of all bats released at the cave (n = 15): shown are straightness index, median speed, median altitude above ground level, and total flight distance to the first feeding tree. (E and F) Bats returned to the same individual tree night after night. Bottom: Full flight path; Top (Inset): Zoom-in view of the feeding trees; colors represent different consecutive nights. (E) Bat 213 returned over four consecutive nights to the same Prunus armeniaca tree (arrow). (F) Bat 243 returned over two nights to the same two trees; note the commuting flyway; black and gray lines represent flight to and from the foraging area on night one.
Table 1.
Summary statistics of data from bats released at the cave and at the 3 translocation points (release sites R1, R2 and R3)
| Characteristic | Cave | R1 | R2 (crater in) | R3 (crater out) |
| No. of bats | 15 | 10 | 7 | 4 |
| Speed, km/h | 33.5 ± 5.5 (26.8–49.3) | 35.3 ± 8.2 (26.2–51.7) | 31.3 ± 2.0 (27.6–34.3) | 48.1 ± 2.2 (45.9–50.8) |
| Altitude above ground level, m | 84.0 ± 27.4 (31.3–122.0) | 55.1 ± 30.3 (13.7–102.2) | 51.4 ± 12.9 (27.6–64.7) | 76.1 ± 20.6 (59.1–103.1) |
| Flight distance, km | 15.0 ± 3.2 (7.1–20.6) | 42.7 ± 15.8 (10.4–62.2) | 50.5 ± 32.4 (5.5–98.5) | 51.8 ± 47.0 (5.3–96.0) |
| Straightness index | 0.97 ± 0.02 (0.93–0.98) | 0.85 ± 0.08 (0.7–0.94) | 0.4 ± 0.18 (0.2–0.7) | 0.88 ± 0.05 (0.82–0.93) |
Data for each bat is from the first night only. For each bat, the “speed” was taken as the bat's median speed over the entire commuting flight, and the “altitude” as the median altitude above ground level (maximum speed and altitude were much higher). Numbers represent mean ± SD, computed over the n bats in each column, as well as (in parentheses) the overall range of the median speeds and altitudes for all the n bats. The sample size shown here includes all 36 bats used for analysis (Methods).
The fast and very straight repetitive flights to the same fruit tree, night after night, might be explained by navigation toward a specific sensory cue [“beaconing” (2)], or navigation via a sequence of landmarks or along a learned vector. Olfactory beaconing toward the tree itself is unlikely caused by the presence of numerous fruit trees of the same species and of similar fruit ripeness along the flyway and in the surroundings (Fig. S4). Visual beaconing to the tree is unlikely because most of the favored trees were not located near any light source to which the bat might beacon; similarly, when flying back to the cave, visual beaconing was unlikely because no light sources are found within 1 km from the cave. Nevertheless, the bats might have beaconed toward the odors of the sea, or toward a distant visual cue in line with the direction of the feeding tree. Such navigation, performed from different starting positions (eg, cave, trees), requires knowledge about the relative geometric locations of multiple goals of interest and multiple distant cues—consistent with map-based navigation (1, 2).
Two other types of navigational strategies should also be considered for interpreting these data: (i) route-based navigation and (ii) path integration [“dead-reckoning” (2)]. To disentangle these possibilities, we carried out homing experiments in which bats were captured in the cave and released in several remote locations outside their familiar area (Methods).
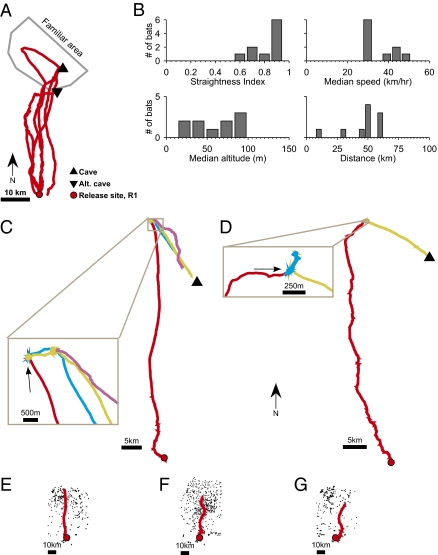
In the first set of homing experiments, we captured 21 bats in the same cave, equipped them with GPS dataloggers, and released them in the Negev Desert, 44 km south of the cave (Fig. 2A, release site R1). This location was well beyond the bats’ familiar area: although the history of each individual bat before capture is unknown, it is unlikely that bats were familiar with this release site, located well outside the foraging area of all bats tracked previously from this colony (N = 38; Fig. 2A, gray polygon; Methods). Furthermore, fruit trees and fruit bats are scarce in this desert area (23). Sixteen of the 21 bats (76%) were found via the radiotelemetry signal in their familiar area in the same night and as long as 1 wk after release; there were no significant differences in probability of returning home as function of sex, body size, body mass, body condition, or GPS datalogger size relative to the bat (Methods). Analysis of full return tracks from all the retrieved GPS dataloggers (n = 10) showed that bats flew directly and rapidly from the release point to a specific familiar goal location (Fig. 2B and Table 1; straightness index, 0.85 ± 0.08). We were able to control for the particular goal location of individual bats by providing two different treatments before release (Methods). Six bats released late at night, after being fed ad libitum, were expected to fly to a cave; five of them did as expected (Fig. 2A). Four bats released early at night, without being fed, were expected to fly to their favored tree; all of them did as expected (Fig. 2 C and D show two of those bats), and also returned to the same tree night after night (Fig. 2 C and D and Fig. S5). These observations significantly match our expectations from the two treatments (Barnard exact test, P = 0.013).
Fig. 2.
Translocation experiments indicate strong homing capacity of Egyptian fruit bats. (A) Homing flights of all bats that homed from release site R1 directly to the cave or to a nearby alternate cave. (B) Flight parameters of all bats (both bats that homed to the cave as in A and bats that flew to trees as in C–G; n = 10); same notation as in Fig. 1D. (C and D) Examples of bats that flew from release site R1 to a tree, and then returned to the same individual tree night after night; colors represent different nights. (C) Bat 230 flew to a Morus alba tree (Inset, arrow) for three consecutive nights. (D) Bat 160 flew to a Diospyros kaki tree (arrow) for three consecutive nights. (E–G) Bats flew to a particular tree (and subsequently returned to the same tree night after night) while ignoring many other trees of the same species on the way (black dots). (E) Bat 230 flew to a M. alba tree. (F) Bat 191 flew to a Ceratonia siliqua tree. (G) Bat 232 flew to a Eriobotrya japonica tree.
These homing experiments further argue against olfactory beaconing to a specific tree as a potential navigational mechanism, because bats bypassed numerous similar trees (Fig. 2 E–G, black dots). They also argue against route-following and path-integration mechanisms, because such mechanisms would lead to disorientation at the unfamiliar release site R1. Our findings suggest that, similar to honeybees and pigeons (3, 15, 16), bats are able to home to one of two goal locations—trees or caves—indicating flexible navigational abilities. However, these results cannot help distinguish between map-based navigation guided by large-scale odor gradients (24) or magnetic gradients (25), versus navigation using cognitive-map mechanisms relying on self-triangulation based on distal visual landmarks (26).
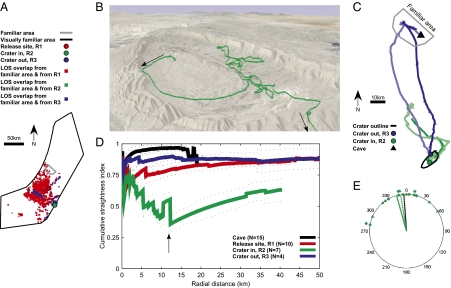
To further elucidate the existence of a navigational map, and to examine the role of self-triangulation based on distal visual landmarks (26), we conducted a second set of homing experiments from a larger distance. This was needed because, in the first set of homing experiments, there was considerable overlap between the visually familiar area (Fig. 3A, large black polygon) that can be visible by bats at the maximum flight altitude recorded within their home range (643 m above ground level) and the area visible from the highest point (115 m above ground) that was reached by a bat within 0.5 km from release point R1 (Fig. 3A, red dots show the line-of-sight overlap between these two locations). To test if the presence of familiar visual landmarks is necessary for large-scale navigation of bats, we repeated the same procedures but released 10 bats at point R2, 84 km from the cave, deep within a large natural erosional crater in southern Israel (Fig. 3B), from which familiar distal landmarks are not visible (Fig. 3A, no small green dots, i.e., no overlap in line of sight between point R2 and the familiar area). Nine of the 10 bats (90%) were detected in their familiar area in the same night and as long as 1 wk after release, based on radiotelemetry tracking; seven of these nine GPS devices were subsequently found (Table 1; examples in Fig. 3 B and C, green lines). As a control group, we also released 11 bats from point R3—the highest mountain at the northwestern rim of the crater, 79 km from the cave—from which familiar distal landmarks are visible (Fig. 3A, small blue dots denote overlap in line of sight between point R3 and the familiar area). Eight of the 11 bats (72%) were found in their familiar area in the same night and as long as 1 wk after release, based on radiotelemetry tracking; six of these eight GPS devices were subsequently retrieved, and four of them had valid data (Methods; examples in Fig. 3C, blue lines). No difference was found in the return-rate probability between bats released within the crater and control bats release on the rim (Barnard exact test, P = 0.49).
Fig. 3.
Bat navigation relies primarily but not exclusively on distal visual landmarks. (A) Line-of-sight calculations: large black polygon represents the visually familiar area, as seen from the highest recorded altitude of bats’ flights (643 m above ground level); small gray polygon represents the familiar area physically visited by foraging bats (Methods). Red squares mark locations seen both from the familiar area (near cave) and from release site R1 (at the highest recorded flight altitude of 115 m). Blue squares represent the same for release site R3 (at the highest flight altitude of 74 m). Note absence of green squares, indicating that bats released within the crater (R2), flying at the highest recorded altitude of 101 m, could not see any familiar visual landmarks. (B) Example of bat 259, released inside the crater; note the tortuous disoriented flight: this bat flew 33.9 km before it left the crater and turned northeast, then northwest toward the familiar area. View from the northeast. (C) Homing flight of two bats (bats 259 and 274, green lines) released inside the crater and two bats (bats 317 and 318, blue lines) released high on the crater rim; light-gray polygon represents the familiar area of the bats. Note that bats released at the crater rim flew north much straighter than bats released inside the crater. (D) Population data showing cumulative straightness index as function of distance from the release site; the four colors represent bats released at the four different release locations (cave, R1, R2, and R3); dotted lines represent median ± SE of the median; shown are only the distances with data from at least three bats. Note the substantially lower cumulative straightness index for within-crater releases (green), indicating strong disorientation when distal landmarks are not visible. (E) Polar display of bats’ vanishing bearings (green circles) and the direction of the bats’ exit points from the crater (triangles) after release at point R2 (inside crater). Green solid and dotted lines represent average directions of the circles and triangles, respectively; black line represents homeward direction (to cave).
Bats released within the crater were fully surrounded by high cliffs, blocking the view of any familiar visual landmark; these bats typically exhibited substantial initial disorientation inside the crater (Fig. 3B, green), but eventually left the crater at the home direction and continued to fly north toward their cave (Fig. 3C, green lines). In contrast, bats released at the high crater rim, only 5.6 km from the within-crater release point, flew straight north (Fig. 3C, blue lines). Analysis of cumulative straightness index (Fig. 3D) showed that bats released within the crater (Fig. 3D, green) flew along a considerably more torturous path compared with all other experimental bat groups. The tortuosity of their path was especially prominent at a 12-km distance (Fig. 3D, arrow; large decrease in straightness index), which corresponds approximately to the length scale of this crater, the size of which is 14 × 6 km, indicating that bats exhibited initial strong disorientation within the crater, and then, after they exited the crater, undertook consistent directional flight toward their familiar area. Thus, the crater release experiments clearly suggest that distal visual landmarks, such as hills or town lights, are important for large-scale homing in Egyptian fruit bats. Notably, however, all bats released at point R2 eventually left the crater at their homeward (northern) direction relative to the release point, as indicated by radiotelemetry-based vanishing bearings (Fig. 3E, green circles; n = 10 bats; mean angle, 341°; Rayleigh test, P < 0.001) and by GPS data of bats’ exit points outside the crater walls (Fig. 3E, green triangles; n = 7; mean angle, 351°; Rayleigh test, P = 0.011). This suggests that visual landmarks are important but not necessary for long-range navigation by these bats. We thus propose that their long-distance homing capacity depends primarily on their ability to extrapolate their position from the geometric configuration of distal visual landmarks, coupled with at least one additional navigational mechanism—magnetic (27, 28), celestial, or olfactory-based navigation—being used when distal landmarks are not visible.
Discussion
Here we studied the navigational capacity of a flying mammal, the Egyptian fruit bat. When GPS-tagged bats were released at their cave, they exhibited high, fast, and very straight commuting flights from their cave to remote fruit trees, and returned to the same tree night after night. Bats displaced 44 km south homed directly to one of two goal locations—familiar fruit tree or cave—ruling out beaconing, route-following, or path-integration mechanisms, and providing evidence for map-like navigation in these mammals.
Previous studies of homing in bats have (i) demonstrated homing after several days or weeks, rather than straight rapid homing flights (29); (ii) demonstrated a clear beaconing strategy in bat navigation, rather than a map-like navigational strategy (30, 31); or (iii) released bats too close to their roost to be able to judge which navigational strategy the bats used (27). Here, we were able to overcome these shortcomings of previous studies by using GPS to precisely measure the straightness and speed of bats’ flights, by releasing bats very far from their familiar area, and by performing manipulations that indicated that Egyptian fruit bats do rely on map-like navigation.
What are the sensory mechanisms used by these bats for long-range navigation? We hypothesized that the bats may use a combination of visual, magnetic, and olfactory-based navigation. To test this, we released bats inside a deep erosional crater or just outside it. Bats released within the crater were initially disoriented, but eventually left the crater toward the home direction and homed successfully, whereas bats released at the crater-edge top homed directly. The crater-release experiments indicate that visual-based navigation may be of particular importance to these bats. The differences in bats’ behavior between the two release points at the crater (disorientation at release point R2 within the crater, vs. straight homing from crater-rim release point R3) are likely a result of differences in availability of visual landmarks—not to differences in celestial cues, magnetic cues, or olfactory cues. Although all these sensory mechanisms were likely used by bats to eventually exit the crater in the homeward direction (Fig. 3E), these mechanisms are unlikely to underlie the clear behavioral differences between the within-crater and crater-rim releases (Fig. 3D, green vs. blue), because of the small differences in magnetic, celestial, or olfactory information between release points R2 and R3. The behavioral differences between points R2 and R3 are unlikely to be caused by differences in exposure to celestial cues, because R2 and R3 releases were done at the same night, and no systematic differences in cloudiness were observed. The dramatic behavioral differences between points R2 and R3 are unlikely to be caused by differences in magnetic-field parameters, because of the very small distance between points R2 and R3 (5.6-km aerial distance) and the relatively small differences in magnetic parameters between the two nearby release points, R2 and R3 (magnetic maps shown in Fig. S6 and ref. 32). Finally, the dramatic behavioral differences between points R2 and R3 are not very likely to be caused by differences in olfactory cues in those two locations. Olfactory navigation, possibly cued by wind-transported odorants originating from abundant orchards at the bats’ familiar area, might be plausible, in principle, as a navigational mechanism, as the typical afternoon breeze from the Mediterranean Sea reaches the study area in the Negev Desert a few hours later (33). However, we consider this mechanism unlikely to explain any behavioral differences between bats released at points R2 and R3 because all bats were in fact released on nights with no winds or very weak winds, indicating that, although the nocturnal breeze could potentially carry odorants from the foraging area, it was not likely to be very effective at the time of release. Nevertheless, further research is needed to examine the roles of these three possible mechanisms in detail. For example, it has been suggested that visual celestial cues near the horizon are important for bird navigation (5, 34), and it might be possible that the lower portion of the sky was occluded by surrounding cliffs for those bats that were released inside the crater. Furthermore, the effects of the crater's complex terrain on wind-mediated odor transport above and within the crater warrants further investigation. However, although it is possible that there was some contribution of magnetic, celestial, and olfactory navigation, the most parsimonious explanation for the dramatic behavioral differences between release point R3 (straight homing) and point R2 (disorientation) is the visual explanation: the availability of distal visual landmarks from point R3 and the lack of familiar distal visual landmarks at point R2. The importance of vision for bat homing has been suggested in several previous studies (e.g., refs. 30, 31). Notably, Egyptian fruit bats are known to have outstanding visual acuity, much better than that of almost all insectivorous bat species (35); therefore, visual-based navigation is certainly plausible in these bats—and although we cannot determine from our experiments which precise landmarks the bats used, our results suggest that the bats used some set of distal visual landmarks for long-range navigation.
In summary, we propose that Egyptian fruit bats use self-triangulation based on multiple distal visual landmarks (26) as their primary large-scale navigational mechanism. This map-based mechanism, proposed previously for rodent navigation in a water maze (20), was studied here in a free-ranging mammal at a spatial scale five orders of magnitude larger. Our study demonstrates the importance of considering all components of the new movement ecology framework (36) for understanding movement phenomena: the internal state determining the strong motivation to move to a specific destination; the motion capacity enabling bats to execute nonstop flapping flights from distant locations; their high navigation capacity—the core component investigated in this study; and the critical role of some specific external factors such as particular fruit trees and distant visual landmarks used for navigation. More specifically, our results also suggest that Egyptian fruit bats use additional navigational mechanisms—possibly based on olfactory, celestial, and/or magnetic cues—when distal landmarks are not visible. These results suggest the ability of bats to navigate within their visually familiar area based on sets of distal visual landmarks—a capacity that could be termed a form of a visually based cognitive map (2, 19). They also demonstrate bats’ ability to eventually home when translocated well outside their visually familiar area—a capacity often called “true navigation” in birds and other animals (13, 14, 24, 25). To our knowledge, this is the first evidence for either of these navigational capacities in bats, and the first evidence for large-scale navigation in a free-ranging wild mammal.
Methods
Research Site and Species.
We studied the navigational strategies of wild Egyptian fruit bats (R. aegyptiacus) from a relatively large colony at the Sgafim cave (location, 31° 40′ N; 34° 54′ E; altitude, 250 m above sea level), located at the Judean lowlands of central Israel; the number of bats in this cave was counted yearround, and was between 400 and 800 individuals. Bats were captured by mist nets upon exiting the cave after sunset, and were kept in a cloth bag until handling. Each bat was sexed and measured for mass and forearm length, a measure of body size. For the GPS tracking experiments, we used a total of 70 adult individuals of both sexes (46 male, 24 female). We used only relatively large bats with body mass of more than 130 g (mean mass ± SD, 150.3 ± 13.8 g; forearm length, 94.6 ± 2.1 mm). Although there was a significant difference in body mass between sexes, no significant difference in any of the flight parameters was found between the sexes; hence, we pooled data from both sexes. In each night, we GPS-tagged and released between one and five individual bats. Experiments were carried out between January 2008 and December 2009; they spanned all seasons, a variety of weather conditions, and all possible moon phases. Experimental procedures were approved by the Israel Nature and Parks Authority and by the institutional animal care and use committees of the Hebrew University of Jerusalem and the Weizmann Institute of Science.
GPS Tracking Device.
Adult Egyptian fruit bats (N = 70) were equipped with a tracking device that included a lightweight GPS datalogger (GiPSy2; Technosmart) plus a radiotelemetry unit (BD-2; Holohil Systems). The average weight of the GPS/radiotelemetry combined pack was 10.9 ± 1.3 g (range, 6.9–13.0 g), including batteries and protective casing. This weight constituted 7.3 ± 1.0% of the bats’ body mass (range, 4.0–9.6%). The dimensions of the GPS/radiotelemetry pack were 48 mm (length) × 23 mm (width) × 11 mm (height).
Device Attachment and Marking of Individual Bats.
Medical skin adhesive (Liquid Bonding Cement; Torbot Group) was used to attach the GPS device to the bat's back, directly above the center of mass of the animal's body. The bat was implanted with a s.c. radiofrequency identification tag for individual identification (Mini-Transponder; UNO Roestvastaal) to verify that all tracked bats were indeed distinct individuals.
GPS Sampling Rate and Time Extent of Data Collection.
The mass and size of the GPS battery limited the device's lifetime, and therefore we modified the GPS sampling rate and activation schedule according to experimental needs, to collect more data. For 66 of the 70 bats, data were collected at high sampling rates (0.1–1 Hz), with most of these data (63 of 66 bats; 95.5%) collected at 1 Hz. The GPS devices were programmed to be active all night and inactive during the day (when bats were inside the cave). Additionally, in some cases, the GPS was activated for only the first part of the night; this saved battery power and allowed GPS recording of bats’ movements for as many as four consecutive nights. Total time extent of data collection ranged from full sampling over one night to 3 h of data per night over as many as four consecutive nights. All bats were also tracked manually by standard radiotelemetry triangulation for purposes of GPS retrieval (typically this tracking was conducted for the first one or two nights after release, and then was intermittently conducted over the following several weeks).
Bat Release.
Before release, bats were given a few milliliters of fruit juice to prevent dehydration and stress related to capture. To prevent group navigation of our experimental bats, we released the bats only after all other bats left the cave (for bats released near the cave), and if several bats were tagged and released on the same night, we released them individually at intervals of more than 20 min. Before release, bats were rotated several times and released from the hand at a random direction.
Homing Experiments.
For homing experiments, we used the same capture and attachment protocol as with cave-released bats. We carried out three sets of homing releases in the Negev Desert, releasing the bats at the following locations: (i) release site R1, Gva'ot Goral (aerial distance of 44 km from capture site; 31° 17’ N; 34° 49’ E; altitude 419 m above sea level); (ii) release site R2, inside HaMakhtesh HaGadol natural erosional crater (aerial distance of 84.5 km from capture site; 30° 55’ N; 34° 58’ E; altitude 400 m above sea level); and (iii) release site R3, outside of HaMakhtesh HaGadol crater (aerial distance of 79 km from capture site; 30° 58’ N; 34° 58’ E; altitude 638 m above sea level). Translocation was done by car, driving the bats total ground distances of 58, 111, and 105 km, respectively. During the entire transport, bats were held inside a cloth bag.
For the release in Gva'ot Goral (site R1), upon arrival to the release location, bats were randomly assigned to one of two treatment groups: (i) 11 bats were released immediately and 10 bats were kept in a closed cage for at least 3 h, given fruits and water ad libitum as well as fed by hand, and only then released (∼3 h before sunrise). For the inside- and outside-crater releases (sites R2 and R3), bats were held in a cloth bag during the drive to the release site, with ad libitum food provided; upon arrival to the site, the bats were released by using the same protocol.
GPS Recovery and Data Download.
GPS/radiotelemetry packs were retrieved after the pack had fallen to the ground (usually after a few weeks). Retrieval of the GPS unit was done by using the radiotelemetry signal. Data download was possible only by physically retrieving the device. In total, we retrieved 51 of the 70 GPS devices (73%) that we deployed. Retrieval rates were 89.3%, 61.9%, 70.0%, and 54.5% for bats released at the cave and sites R1, R2, and R3, respectively. GPS tags of translocated bats (released at R1, R2, or R3) had significantly lower retrieval rates than those of nontranslocated bats released at the cave (χ2 = 6.37, df = 1, P = 0.012). However, there was no significant difference in retrieval success among the three groups of translocated bats (χ2 = 0.531, df = 2, P = 0.767). Therefore, we expect no bias related to tag retrieval success among the three experimental treatments.
Inclusion Criteria for Analysis.
Of the 51 bats whose GPS/radiotelemetry packs were retrieved, we excluded four (three released at the cave and one at R1) that had corrupted data, and two tags (released at R3) that had partial data collected during only a portion of their track as a result of technical failure. Because statistical properties estimated for the same movement path might differ if data points are collected at different sampling rates (37), we narrowed the range of sampling rates by excluding four additional bats, all released at the cave, whose GPS locations were recorded at low sampling rates of approximately 0.017 Hz. Data from five additional bats, three released at the cave and two at R1, were excluded because those bats flew to a fruit tree near (<5 km) the release point and stayed there several hours. We note that the two bats from R1 must have eventually commenced long-distance (unrecorded) homing flights, because their radio signal was detected at the cave on the following morning. The remaining 36 bats formed the basic dataset for all analyses (Table 1). Subcutaneous identification tags verified that all bats were distinct individuals. Of those 36 bats, 21 bats had single-night data, whereas six, seven, and two bats had two, three, and four nights of data, respectively, resulting in a total of 62 nights from 36 bats.
Considering all lost tags and exclusions, the proportion of individuals contributing data to the analyses was not significantly different between translocated and nontranslocated bats (χ2 = 0.008, df = 1, P = 0.931) or among the three groups of translocated bats (χ2 = 2.377, df = 2, P = 0.305). The latter comparison means that there were no differences in data exclusion or tag retrieval success between the three groups of translocated bats, released at sites R1, R2, and R3; this is important because a key comparison in the current study is among the bat groups released at sites R1, R2, and R3: hence, we expect no bias among these three treatments in relation to data exclusion or tag retrieval success.
Data Analysis.
For each bat, we included only data points that had high accuracy, by including only individual points that were based on (i) at least four satellites and (ii) positional dilution of precision less than 12 (this is a standard parameter that quantifies how well the GPS satellites span the sky, which influences the reliability of the GPS reading; see ref. 38).
GPS tracks were segmented into “flight” and “nonflight” portions. A flight segment was defined as a segment in which the bat flew with a ground speed of more than 10 km/h for more than 20 s; all other segments were defined as nonflight. If two flight segments were separated with a nonflight segment whose duration was shorter than 10 min, the two flight segments were merged together.
For all the individual commuting flights, we computed the following trajectory data: (i) altitude above ground level; (ii) flight speed; (iii) total flight distance; (iv) straightness index [defined as D/L, where D is the length of the straight line from the starting-point to the goal and L is the actual total length of the segment flown (39)]; and (v) “first tree stop,” defined as the first stop by the bat at a tree that lasted longer than 10 min; we also physically inspected all the stop locations to identify the tree species and evaluate their fruiting status.
All data analyses of bat trajectories were done by using Matlab (Mathworks). Ground elevation was extracted from a digital terrain model layer with cell size of 25 m2 (created by J. K. Hall, Geological Survey of Israel, Jerusalem, Israel). Statistical tests were done by using the SPSS statistical software (version 17; SPSS); all test results were considered significant if P < 0.05.
The familiar area of the bats (Figs. 2A and 3 A and C, gray polygon) was computed as the 95% convex hull encompassing the positional data from all GPS releases at the cave (n = 19 bats; we included for this particular analysis also GPS data with low sampling rate < 0.1 Hz), as well as positional data from additional foraging bats that were tracked with only radiotelemetry (n = 19)—a total of 38 individual bats, recorded over all seasons.
For line-of-sight calculations, in addition to the familiar area, we computed the visually familiar area, which is based on the notion that, when flying very high up, bats could see visual landmarks from very long distances, and thus may learn the layout of landmarks over a much larger area than the area they physically visited (26). We calculated the visually familiar area (Fig. 3A, large black polygon) by conducting line-of-sight calculation from 100 randomly selected points within the familiar area, at an altitude of 643 m above ground level, which is the highest altitude recorded for foraging bats. The line-of-sight calculation was done by using a digital terrain map with a cell size of 1 km2 (digital terrain map raster file; created by J. K. Hall, Geological Survey of Israel, Jerusalem, Israel), resulting in the large visually familiar area shown by the black polygon in Fig. 3A. A line-of-sight calculation was also done for the translocation release site R1 in the Negev Desert (at an altitude of 115 m above ground level, which is the highest altitude recorded for a translocated bat within 0.5 km distance from release site R1). Red squares in Fig. 3A show the overlap between these two line-of-sight calculations: that is, the locations that could be seen by bats both from their familiar area and from release site R1. This calculation confirmed that many visual landmarks could indeed be seen from both locations, despite the large translocation distance (aerial distance of 44 km). Similar calculations were done for release sites R2 and R3 (at altitudes of 101 m and 74 m above ground level, respectively, the highest altitude recorded for translocated bats within 0.5 km distance from release sites R2 and R3, respectively). The blue squares in Fig. 3A show the locations that could be seen by bats from both their familiar area and release site R3, showing that bats released at site R3 (a high mountain on the crater edge) could potentially use visual landmarks to navigate. Note that, in contrast, there are no green squares in Fig. 3A, that is, there are no locations that could be seen both from the familiar area and from release site R2, which means that bats released at site R2 (within the crater) could not see any familiar visual landmarks.
Cumulative straightness index (Fig. 3D) was calculated for all tracks as follows. For each radial distance l from the release point, we computed the straightness index (as detailed earlier) by using the flight segment that starts at the release location and ends at the first point on the bat's trajectory at which the distance from the release location exceeded l. The cumulative straightness index was computed in 100-m intervals (i.e., l was set to 100, 200, 300 m… up to 50 km; l is shown on the x axis of Fig. 3D).
Supplementary Material
Acknowledgments
We thank K. Cheng, J. Zeil, A. Nirendra, T. Kimchi, T. Merkle, M. Arbib, N. Sapir, W. Wiltschko, and R. Wiltschko for helpful discussions; O. Altstein, D. Shohami, A Zabari, and Y. Yovel for technical assistance in the field; and A. Ben-Nun (GIS Center, Hebrew University) and N. Horvitz for technical assistance in spatial analysis of the data. This study was funded by Israel Science Foundation (ISF) Grants ISF-FIRST 1316/05 and ISF 1259/09 (to R.N.); the Adelina and Massimo Della Pergolla Chair of Life Sciences (R.N.); Forschungskredit Universität Zürich (A.V.); Ornis italica (G.D.); research grants from the Carl and Micaela Einhorn-Dominic Brain Research Institute, Nella and Leon Benoziyo Center for Neurological Diseases, and Mr. and Mrs. Steven Harowitz (N.U.); and fellowships of Bat Conservation International, the Lubee Bat Conservancy, the Zoological Society of Israel, and the Rieger–Jewish National Fund Foundation (A.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 15031.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107365108/-/DCSupplemental.
References
- 1.Wallraff HG. Avian Navigation: Pigeon Homing as a Paradigm. New York: Springer; 2005. [Google Scholar]
- 2.Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- 3.Menzel R, et al. Honey bees navigate according to a map-like spatial memory. Proc Natl Acad Sci USA. 2005;102:3040–3045. doi: 10.1073/pnas.0408550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohmann KJ, Lohmann CM, Ehrhart LM, Bagley DA, Swing T. Animal behaviour: geomagnetic map used in sea-turtle navigation. Nature. 2004;428:909–910. doi: 10.1038/428909a. [DOI] [PubMed] [Google Scholar]
- 5.Cochran WW, Mouritsen H, Wikelski M. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science. 2004;304:405–408. doi: 10.1126/science.1095844. [DOI] [PubMed] [Google Scholar]
- 6.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan MV, Zhang S, Altwein M, Tautz J. Honeybee navigation: Nature and calibration of the “odometer”. Science. 2000;287:851–853. doi: 10.1126/science.287.5454.851. [DOI] [PubMed] [Google Scholar]
- 8.Biro D, Freeman R, Meade J, Roberts S, Guilford T. Pigeons combine compass and landmark guidance in familiar route navigation. Proc Natl Acad Sci USA. 2007;104:7471–7476. doi: 10.1073/pnas.0701575104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorup K, et al. Evidence for a navigational map stretching across the continental U.S. in a migratory songbird. Proc Natl Acad Sci USA. 2007;104:18115–18119. doi: 10.1073/pnas.0704734104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham P, Cheng K. Ants use the panoramic skyline as a visual cue during navigation. Curr Biol. 2009;19:R935–R937. doi: 10.1016/j.cub.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Kramer G. Die sonnenorientierung der vögel. Verh Dtsch Zool Ges. 1953;1:77–84. [Google Scholar]
- 12.Perdeck AC. Two types of orientation in migrating starlings, Sturnus vulgaris and chaffinches, Fringilla coelebs, as revealed by displacement experiments. Ardea. 1958;46:1–37. [Google Scholar]
- 13.Boles LC, Lohmann KJ. True navigation and magnetic maps in spiny lobsters. Nature. 2003;421:60–63. doi: 10.1038/nature01226. [DOI] [PubMed] [Google Scholar]
- 14.Phillips JB, Adler K, Borland SC. True navigation by an amphibian. Anim Behav. 1995;50:855–858. [Google Scholar]
- 15.Lipp H-P. “Columba militaris helvetica”: Biologie und Verhaltensleistungen der Schweizerischen Armeebrieftauben [Biology and spatial behavior of homing pigeons in the Swiss army] Acta Biol Benrodis. 1996;3(Suppl):85–103. (in German) [Google Scholar]
- 16.Baldaccini NE, Benvenuti S, Fiaschi V. Homing behaviour of pigeons confined to a new loft distant from their home. Monitore Zool Ital. 1976;10:461–467. [Google Scholar]
- 17.Lipp H-P, et al. Pigeon homing along highways and exits. Curr Biol. 2004;14:1239–1249. doi: 10.1016/j.cub.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 19.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford Univ Press; 1978. [Google Scholar]
- 20.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 21.Bennett AT. Do animals have cognitive maps? J Exp Biol. 1996;199:219–224. doi: 10.1242/jeb.199.1.219. [DOI] [PubMed] [Google Scholar]
- 22.Ulanovsky N, Moss CF. What the bat's voice tells the bat's brain. Proc Natl Acad Sci USA. 2008;105:8491–8498. doi: 10.1073/pnas.0703550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendelssohn H, Yom-Tov Y. Fauna Palaestina: Mammalia of Israel. Jerusalem: Israel Academy of Sciences and Humanities; 1999. [Google Scholar]
- 24.Wallraff HG. Avian olfactory navigation: Its empirical foundation and conceptual state. Anim Behav. 2004;67:189–204. [Google Scholar]
- 25.Wiltschko R, Wiltschko W. Avian navigation: From historical to modern concepts. Anim Behav. 2003;65:257–272. [Google Scholar]
- 26.Baker RR. Bird Navigation: The Solution of a Mystery? New York: Holmes and Meier; 1984. [Google Scholar]
- 27.Holland RA, Thorup K, Vonhof MJ, Cochran WW, Wikelski M. Navigation: Bat orientation using Earth's magnetic field. Nature. 2006;444:702. doi: 10.1038/444702a. [DOI] [PubMed] [Google Scholar]
- 28.Holland RA, Borissov I, Siemers BM. A nocturnal mammal, the greater mouse-eared bat, calibrates a magnetic compass by the sun. Proc Natl Acad Sci USA. 2010;107:6941–6945. doi: 10.1073/pnas.0912477107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis R. Homing performance and homing ability in bats. Ecol Monogr. 1966;36:201–237. [Google Scholar]
- 30.Williams TC, Williams JM. Radio tracking of homing bats. Science. 1967;155:1435–1436. doi: 10.1126/science.155.3768.1435. [DOI] [PubMed] [Google Scholar]
- 31.Williams TC, Williams JM, Griffin DR. The homing ability of the neotropical bat Phyllostomus Hastatus, with evidence for visual orientation. Anim Behav. 1966;14:468–473. doi: 10.1016/s0003-3472(66)80047-7. [DOI] [PubMed] [Google Scholar]
- 32.Shirman B. Three component magnetic anomaly maps of Israel. Isr J Earth Sci. 2000;49:1–7. [Google Scholar]
- 33.Goldreich Y. The Climate of Israel: Observation, Research, and Application. New York: Plenum; 2003. [Google Scholar]
- 34.Muheim R, Moore FR, Phillips JB. Calibration of magnetic and celestial compass cues in migratory birds—a review of cue-conflict experiments. J Exp Biol. 2006;209:2–17. doi: 10.1242/jeb.01960. [DOI] [PubMed] [Google Scholar]
- 35.Heffner RS, Koay G, Heffner HE. Sound localization in an Old-World fruit bat (Rousettus aegyptiacus): acuity, use of binaural cues, and relationship to vision. J Comp Psychol. 1999;113:297–306. doi: 10.1037/0735-7036.113.3.297. [DOI] [PubMed] [Google Scholar]
- 36.Nathan R, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bovet P, Benhamou S. Spatial analysis of animal's movements using a correlated random walk model. J Theor Biol. 1988;131:419–433. [Google Scholar]
- 38.Kaplan ED, Hegarty CJ. Understanding GPS: Principles and Applications. Boston: Artech House; 2006. [Google Scholar]
- 39.Benhamou S. How to reliably estimate the tortuosity of an animal's path: Straightness, sinuosity, or fractal dimension? J Theor Biol. 2004;229:209–220. doi: 10.1016/j.jtbi.2004.03.016. [DOI] [PubMed] [Google Scholar]