Abstract
Whole organism–based small-molecule screens have proven powerful in identifying novel therapeutic chemicals, yet this approach has not been exploited to identify new cognitive enhancers. Here we present an automated high-throughput system for measuring nonassociative learning behaviors in larval zebrafish. Using this system, we report that spaced training blocks of repetitive visual stimuli elicit protein synthesis–dependent long-term habituation in larval zebrafish, lasting up to 24 h. Moreover, repetitive acoustic stimulation induces robust short-term habituation that can be modulated by stimulation frequency and instantaneously dishabituated through cross-modal stimulation. To characterize the neurochemical pathways underlying short-term habituation, we screened 1,760 bioactive compounds with known targets. Although we found extensive functional conservation of short-term learning between larval zebrafish and mammalian models, we also discovered several compounds with previously unknown roles in learning. These compounds included a myristic acid analog known to interact with Src family kinases and an inhibitor of cyclin dependent kinase 2, demonstrating that high-throughput chemical screens combined with high-resolution behavioral assays provide a powerful approach for the discovery of novel cognitive modulators.
Keywords: acoustic startle response, sensorimotor gating
All organisms, from protozoa to humans, use nonassociative habituation learning as a means to update behavioral responses to sensory input based on recent stimulation history (1). Considered a simple form of learning, habituation reflects a suppressed behavioral response to repeated inconsequential stimulation and serves as a mechanism by which the nervous system filters irrelevant stimuli. Defective habituation not only is indicative of a learning deficit, but also is prevalent in neuropsychiatric conditions, such as schizophrenia, attention deficit hyperactivity disorder, posttraumatic stress disorder, and drug addiction (2–5). Numerous assays have shown that the parameters and rules for habituation learning are similar across phyla (6–9), suggesting conservation of the underlying molecular mechanisms. For example, training session design and stimulation frequency is predictive of the time scale of memory retention. Massed exposure to repeated stimulation at short interstimulus intervals (ISIs) elicits an acquisition of learned information with short-term recall memory, indicated by a change in behavior that does not persist for long after the repeated stimulation is terminated. In contrast, a distributed training protocol with multiple training sessions consisting of stimulation at longer ISIs and rest periods between training blocks induces the acquisition and storage of learned behavior that is capable of being recalled for a more extended period. Thus, intrasession, short-term habituation represents working memory, whereas intersession, long-term habituation includes the storage and retrieval of memory.
Existing strategies to measure vertebrate learning behaviors are time-consuming and difficult to apply to large-scale genetic or small-molecule screens. Despite the abundance of established habituation learning assays for adult rodent and zebrafish models, scaling these assays for systematic approaches is challenging given the inherent complexity and variability of adult behaviors, as well as the time required to train and test large numbers of animals (10–16). Larval zebrafish execute a repertoire of simple, well-defined, and stereotyped sensorimotor behaviors that have accessible and characterized circuitry and provide a vertebrate system amenable to large-scale forward genetic and chemical screening (17–24). Although some studies have explored the capacity of zebrafish larvae for various forms of short-term learning and sensory conditioning, these assays have not been adapted to systematic approaches (25–27). Moreover, whether zebrafish larvae display long-term habituation, and thus the ability for memory storage and retrieval, is unclear.
In the present work, using high-speed video recording and automated behavioral analysis, we demonstrate that larval zebrafish have the capacity for long-term memory recall. Moreover, we show that zebrafish larvae exhibit robust short-term habituation of a kinematically distinct, simple sensorimotor behavior with known underlying circuitry. Using a high-throughput habituation assay to screen libraries of small molecules with identified targets, we find profound pharmacologic conservation of learning between larval zebrafish and adult mammalian vertebrates and reveal additional molecular substrates of nonassociative learning.
Results and Discussion
Zebrafish Larvae Demonstrate Long-Term Habituation to Visual Stimuli.
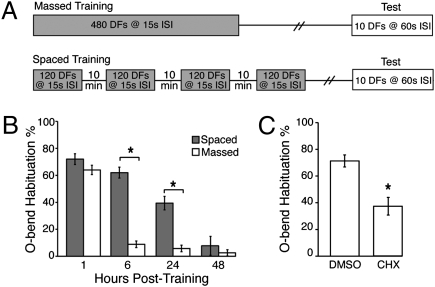
To determine whether larval zebrafish have the capacity to acquire, store, and later recall learned information, we exposed larvae at 6 d postfertilization (dpf) to repetitive visual stimulation and then tested for responsiveness to the trained stimulus. Equilibrating larvae to a uniformly illuminated testing chamber and then abruptly extinguishing the light for 1 s (dark flash) elicits a unique turning maneuver called the O-bend (28). Larvae were exposed to 120 total min of dark flashes with ISIs ranging from 15 s to 60 s in either a massed or spaced dark flash-training format, and then tested for O-bend responsiveness to 10 dark flashes delivered at a 60-s ISI (Fig. 1A). Maximal long-term habituation was observed when dark flashes were delivered at a 15-s ISI during training. Consistent with habituation paradigms for other organisms (29), a spaced training protocol yielded a longer-lasting response decrement, up to 24 h posttraining, compared with only 1 h using a massed training protocol (Fig. 1B).
Fig. 1.
Six-dpf larval zebrafish demonstrate protein synthesis–dependent long-term habituation to visual stimuli. (A) Massed training regimen of 480 total 1-s dark flashes at a 15-s ISI or a spaced training protocol of four sessions, each consisting of 120 1-s dark flashes at a 15-s ISI, with 10 min between sessions. Larvae were tested 1–48 h later for O-bend behavior to a series of 10 1-s dark flashes delivered at a 60-s ISI. (B) Mean O-bend habituation 1–48 h after training with a massed or spaced training paradigm. O-bend habituation percentage was determined by calculating the ratio of O-bend responsiveness during testing to the O-bend responsiveness to the first 10 training stimuli. (C) Mean O-bend habituation at 4 h after CHX exposure during a spaced training paradigm. n = 6 dishes of 20 larvae for all experimental groups. Error bars denote SEM. *P < 0.001, Student t test.
Unlike short-term habituation, long-term habituation requires protein synthesis (29, 30). To determine whether the O-bend habituation after a spaced training protocol requires protein synthesis, we bathed larvae in 10 μM cyclohexamide (CHX) during a spaced training protocol, washed out the CHX after the fourth training session, and tested for O-bend responsiveness 4 h later by delivering 10 dark flashes at a 60-s ISI. CHX treatment significantly reduced the degree of O-bend response attenuation, suggesting that protein synthesis is required for the observed habituation (Fig. 1C). Notably, CHX treatment did not reduce intra–training session habituation, suggesting that short-term habituation is unaffected by CHX treatment (Fig. S1). These results demonstrate that larval zebrafish can store and retrieve visual memory information up to 24 h.
Previous studies in larval zebrafish have shown that retinal circuits can memorize stimulus time intervals; however, the time scale for this memory is ∼20 s (27), and thus this property likely does not account for the memory recall that we observed up to 24 h after training. Here, we demonstrate that 6-dpf larval zebrafish exhibit long-term habituation, consistent with the idea that the neural circuits regulating O-bend behavior are competent for memory storage and retrieval at the larval stage.
Repetitive Acoustic Stimulation Decreases Short-Latency C-Start Responses.
We next asked whether larval zebrafish also exhibit short-term habituation of a simple sensorimotor behavior. Best et al. (25) previously used low–temporal resolution motion analysis to measure a gradual decrement in larval spatial displacement in response to repetitive acoustic stimuli. However, zebrafish larvae demonstrate multiple kinematically different responses in response to acoustic stimuli (18), necessitating the use of high-resolution motion and kinematic analyses to distinguish between these different responses and to focus on a single sensorimotor behavior with known underlying circuitry (24).
Five-dpf zebrafish larvae exhibit a highly stereotyped acoustic startle response characterized by a kinematically unique maneuver known as short-latency C-start (SLC), which is maintained through adulthood (18, 31, 32). To execute the larval and adult SLC behavior, acoustic or tactile sensory input is transmitted directly to the hindbrain, where the Mauthner cells, the command neurons of the SLC response, process the input and subsequently activate contralateral motor neurons and muscle contraction (18, 33–36). Despite the circuit's simplicity, the larval zebrafish's SLC response shows a remarkable capacity for sensorimotor gating processes, such as prepulse inhibition, indicating that the circuit can modulate responsiveness based on experience (18). Moreover, repeated delivery of acoustic stimuli at short ISIs attenuates the SLC response, suggesting that the simple, Mauthner-driven SLC response circuit may have the capacity for nonassociative learning (18).
To examine key kinematic parameters associated with startle attenuation, 5-dpf larvae were exposed to a series of 60 acoustic stimuli of varying intensity and ISI (Fig. 2A). Responses of individually housed larvae were recorded at millisecond resolution and analyzed with automated tracking software for SLC responsiveness (Fig. 2 B and C and Movie S1) (18). First, larvae were exposed to 10 “subthreshold” stimuli (SI Experimental Procedures), delivered at a 20-s ISI, to test startle sensitivity. Stimuli 11–20 (prehabituation phase) consisted of “above-threshold” stimuli (SI Experimental Procedures) with a 20-s ISI to determine various kinematic parameters of the SLC response (including latency, turning angle, and velocity) under nonhabituating conditions. Stimuli 21–50 (habituation phase), consisted again of above-threshold stimuli, now delivered with a 1-s ISI. As shown in Fig. 3A, stimulation at a 1-s ISI elicited a robust SLC response decrement, reaching an asymptotic level by the 10th–15th stimulus at a 1-s ISI (the 30th–35th overall stimulus), suggesting the larvae were habituated. After the 50th stimulus, larvae were given a 3-min rest period, after which they were exposed to an additional set of 10 above-threshold stimuli with a 20-s ISI (recovery phase). During this last phase, larvae had recovered and resumed execution of SLCs at a frequency identical to that observed during the prehabituation phase, consistent with the observation that animals should spontaneously recover from short-term habituation (Fig. 3A) (9).
Fig. 2.
Acoustic startle habituation assay and testing apparatus. (A) Larvae were exposed to 60 repetitive acoustic stimuli delivered at varying intensities and ISIs to evaluate startle sensitivity and nonassociative learning. (B and C) Testing apparatus for the acoustic startle experiments. Using stimulus triggering software (SI Experimental Procedures), we simultaneously controlled the precise intensity and ISI of the acoustic stimuli delivered by a vibrational excitor and triggered video recording to capture larval motor behavior 30 ms before and 90 ms after each acoustic stimulus.
Fig. 3.
Larval zebrafish SLC habituation to acoustic stimuli fits nonassociative learning parametric criteria. (A) Mean SLC response trend of 180 5-dpf larvae to the acoustic stimulation protocol described in Fig. 2A, showing exponential response decrement during habituation phase and spontaneous recovery after a 3-min rest period between stimulus 50 and stimulus 51. (B) Mean degree of SLC habituation is equivalent in 5- to 14-dpf larvae. N larvae shown within each bar in graph. (C and D) Mean SLC response trend (C) and degree of habituation (D) of 30 5-dpf larvae exposed to acoustic stimuli at a 1-s, 5-s, or 20-s ISI during the habituation phase. Mean SLC responses are binned by sets of 10 successive stimuli. SLC habituation is greater and spontaneous recovery is more robust after a 3-min rest period when larvae are stimulated more frequently. *P < 0.001 vs. 20-s ISI group, Student t test. (E) Mean SLC habituation trend of 48 5-dpf larvae during three sets of 20 acoustic stimuli delivered at a 1-s ISI, separated by 3-min rest periods, indicates potentiation of habituation. The degree of habituation at stimuli 2–7 and 15–17 of set 2 and at stimuli 2–12 and 16–18 of set 3 differed significantly from that at the corresponding stimulus during the initial set of 20 stimuli (P < 0.01, Student t test). (F) Mean SLC responsiveness of 10 5-dpf larvae to acoustic stimuli. Larvae were subjected to 30 acoustic stimuli at a 1-s ISI, then a 3-s window during which either no stimulus or a head touch with a hand-held poker was given, followed by a final acoustic stimulus. Larvae dishabituated to the acoustic stimulus via cross-modal, tactile stimulation. Error bars indicate SEM.
Quantitative analysis of 3,590 responses from more than 180 larvae demonstrated that the kinematics of the SLC response, including latency, duration, turning angle, and angular velocity of the C-bend, as well as the overall distance the fish travels as a result of performing a SLC, were identical at all phases of the assay (Fig. S2). To quantify the degree of habituation for each individual tested, we calculated the ratio of the average SLC responsiveness during the last 10 habituation phase stimuli (i.e., stimuli 41–50) to the 10 prehabituation phase stimuli (i.e., stimuli 11–20). The extent of habituation was similar in animals at 5, 10, and 14 dpf (Fig. 3B), demonstrating that our behavioral assay measures behavioral responses of a mature nervous system. Thus, we observe a robust attenuation of a kinematically distinct, simple behavior to acoustic stimulation that is consistent with the idea that our assay measures short-term habituation.
SLC Response Attenuation Assay Measures Nonassociative Learning.
To further validate that the SLC response attenuation assay measures nonassociative learning, we examined additional hallmark criteria of habituation. One such criterion is that shortening the ISI should increase habituation and decrease the time required for the animal to spontaneously recover (9). Indeed, stimulating the larvae at a 1-s ISI elicited a significantly greater degree of habituation, with a shorter time required for complete recovery, compared with stimulation with an ISI of 5 s (Fig. 3 C and D). Habituation also potentiates with repeated sessions of stimulus exposure (9). To validate this criterion, we exposed larvae to three separate sessions of 20 stimuli delivered at a 1-s ISI with 3–60 min between sessions. Quantitative analysis revealed a potentiated increase in the rate of habituation (Fig. 3E). For example, larvae exposed to only one training session reached 80% habituation after 20 stimuli, whereas larvae exposed to two training sessions exhibited 80% habituation already after 13 stimuli, and larvae exposed to a third session reached this level of habituation after only 5 stimuli. Treatment with CHX before and during the assays (as outlined in Figs. 2A and 3E) did not influence SLC attenuation or potentiation, respectively, suggesting that SLC short-term habituation and potentiation do not require protein synthesis (Fig. S3). Notably, further increasing the ISI and/or using a spaced training protocol did not increase SLC habituation or potentiation beyond a 60-min period. This result may not be surprising, given that the innate function of the startle circuit is to mediate escape from predators, and that habituating to a threatening stimulus for an extended period likely constitutes an evolutionary disadvantage.
Finally, to confirm that SLC response attenuation is indeed learning and not a result of sensory or motor fatigue, we tested whether SLC response attenuation is instantly reversible through cross-modal stimulation (9). To test for cross-modal dishabituation, we presented control larvae with 30 acoustic stimuli at a 1-s ISI and then delivered a 31st acoustic stimulus at 3 s after the 30th acoustic stimulus (Fig. 3F). As expected, control larvae displayed SLC response attenuation at the 31st acoustic stimulus. To provide a cross-modal stimulus, between the 30th and 31st acoustic stimuli we applied a brief tactile stimulus that efficiently elicits an SLC response (36). In contrast to control larvae, application of a tactile stimulus with a handheld poker to the larval head during the 3-s period restored SLC responsiveness to the 31st acoustic stimulus, demonstrating that the attenuated SLC response reflects habituation, not fatigue. Consistent with the defined parametric habituation criteria, larvae also habituated to the dishabituating tactile stimulus. Interestingly, replacing the dishabituating tactile stimulus with a visual, dark-flash stimulus was not sufficient to dishabituate acoustic startle habituation; larvae failed to respond to the 31st acoustic stimulus nearly identically to when no dishabituating stimulus was given (Fig. 3F). Dishabituation to acoustic stimulation via dark-flash stimulation may be unlikely, given that the dark-flash–induced O-bend behavior is Mauthner-independent (37), whereas the tactile dishabituating stimulus elicits a Mauthner-mediated response, and thus a tactile stimulus “resets” the appropriate, habituated circuit. Thus, examination of several hallmark criteria for habituation, including modulation of habituation intensity and spontaneous recovery by varying stimulation frequency (Fig. 3 C and D), habituation potentiation (Fig. 3E), and cross-modal dishabituation (Fig. 3F), provides compelling evidence that zebrafish larvae display nonassociative learning. Moreover, these results demonstrate that our assay readily measures short-term habituation at the level of an individual, kinematically distinct behavior.
NMDA-Type Glutamate Receptor Antagonists Reduce Startle Habituation.
Pharmacologic manipulation of glutamate neurotransmission has been shown to modulate habituation in various model systems, and also has proven effective in treating human neuropsychological disorders that manifest with habituation deficits (38–43). To investigate whether key pharmacologic substrates of mammalian habituation are conserved in zebrafish, we tested the effects of two NMDA-type glutamate receptor antagonists, MK-801 and ketamine, on SLC habituation. A 15-min incubation in either MK-801 or ketamine did not alter the kinematic performance of the SLC behavior (Movie S2), nor did it affect the spontaneous initiation of turning or swimming bouts (Fig. S4A). However, MK-801 and ketamine each caused a dose-dependent and reversible reduction (via washout) in startle habituation and increased startle sensitivity (Fig. 4 A and B; data not shown). These results demonstrate that MK-801– and ketamine-treated larvae were able to execute a normal response, but were unable to modulate their responsiveness properly, providing strong evidence that the lack of SLC response attenuation was not attributable to hyperactivity. Thus, brief exposure to known chemical modulators of habituation influences the modulation of the larval zebrafish SLC response without disrupting SLC response performance.
Fig. 4.
Pharmacologic modulation of SLC habituation and SLC response sensitivity in 5-dpf larvae. (A) Mean SLC habituation after a 15-min incubation in 1% DMSO vs. varying doses of MK-801 or ketamine. (B) Mean SLC responsiveness to 10 low-level, subthreshold acoustic stimuli after a 15-min incubation in 1% DMSO vs. 100 μM MK-801 or 500 μM ketamine/1% DMSO. (C–F) Mean SLC responsiveness (binned by responses to 10 successive stimuli) to above-threshold acoustic stimulation (C and E) at a 20-s ISI (prehabituation phase) and a 1-s ISI (habituation phase) and to 10 low-level, subthreshold stimuli (D and F) after a 15-min incubation in hydrastine (C and D) or 12-MDA (E and F) at varying concentrations. (G) Chemical structure of the tested myristic acid analogs. (H) Mean SLC habituation after a 15-min incubation in combinations of varying concentrations of NMDA, ketamine, and/or 12-MDA. The number of larvae are shown in the bars in A and B; n = 32 larvae per group for C–F and H. *P < 0.01; **P < 0.001, Student t test vs. the DMSO group or indicated control group. #P < 0.001 vs. additive effect of the 5-μM 12-MDA and 50-μM ketamine groups, Student t test. Error bars indicate SEM.
Conservation of Neural Substrates of Learning Between Zebrafish and Mammals.
To test the feasibility of our habituation assay for large-scale systematic approaches, we screened two small bioactive compound libraries consisting of 1,760 compounds with defined targets. Five-dpf larvae were incubated in each compound for 15 min before and during the acoustic stimulation assay described in Fig. 2A. Among the 1,760 compounds screened, 11 compounds reduced startle habituation and 19 compounds increased startle habituation (Fig. 4 C–G and Tables S1 and S2). Overall, compounds with similar or common targets often had a comparable influence on habituation rate, whereas compounds with converse effects on identical targets usually caused opposing effects on habituation. Consistent with their high representation in the two chemical libraries, the majority of the compounds affecting habituation were those targeting neurotransmitter systems, including those previously identified to affect mammalian startle modulation (44, 45). For example, compounds antagonizing 5HT-2 serotonin receptors (e.g., pirenperone, ritanserin) or L-type calcium channels (e.g., verapamil, nimodipine) increased habituation, whereas compounds antagonizing glutamate receptors (e.g., MK-801, ketamine, l-701,324) or potassium channels (e.g., linopirdine, meclofenamic acid) reduced habituation. Whereas the adrenergic receptor antagonists BMY 7378 dihyrochloride, prazosin hydrochloride, yohimbine hydrochloride, and verapamil increased habituation, one adrenergic receptor antagonist, phenoxybenzamine, attenuated the habituation rate, suggesting that phenoxybenzamine also may interact with additional targets, such as calmodulin (46), to directly or indirectly antagonize habituation. Finally, compounds agonizing or antagonizing similar targets, such as L-type calcium channels, GABA receptors, and dopamine signaling, exhibited opposite effects on habituation (Tables S1 and S2). For example, the GABA-A receptor antagonist hydrastine reduced habituation, whereas the GABA-A receptor agonists 5-α-THDOC and allopregnan-3α-ol-20-one increased habituation.
The ability to rapidly evaluate phenotypic specificity at the overall activity level, behavioral execution, and behavioral modulation is critical to teasing apart the relationship between molecular and cellular mechanisms underlying behavior. Importantly, none of the compounds reported to affect habituation altered the kinematic performance of the SLC response, including response latency, C-bend turn duration, turning angle, angular velocity, or the distance moved as a result of performing a SLC (selected compounds, Fig. S4 D and E). We noted that many of the compounds reducing habituation also increased startle sensitivity, but did not cause hyperactivity (Table S1). The phenotypic overlap between hypersensitivity and a habituation deficit is consistent with the notion that neural targets and substrates for startle sensitivity and habituation are intricately linked, and that the identification of targets specific for habituation requires behavioral screens designed to instantly distinguish between both processes in vivo. Indeed, we identified several compounds that reduced habituation without increasing startle sensitivity (i.e., hydrastine, SU-9516, and butaclamol; Fig. 4 C and D and Table S1). Finally, our screen was completed in 25 experimental days, which included simultaneous testing of 32 larvae with two behavioral apparatuses, confirming the scalability of our learning assay to large-scale genome-wide or systematic approaches. Thus, using a high-throughput chemical screening assay for short-term habituation modifiers, we have demonstrated a high degree of overlap between the substrates underlying nonassociative learning in larval zebrafish and adult mammals.
Identification of Compounds Regulating Nonassociative Learning.
Our screening identified two classes of compounds previously not known to modulate learning behaviors. First, we identified three compounds targeting cell cycle regulators that modulate SLC habituation. SU-9516, kenpaullone, and indirubin-3′-monoxime are ATP-competitive inhibitors of serine/threonine cyclin-dependent kinase (Cdk) (47–49). SU-9516, an inhibitor of Cdk2 and, to a lesser extent, of Cdk1 and Cdk4 (48), reduced SLC habituation without altering sensitivity, reducing baseline motor activity, or affecting SLC performance kinematics (Table S1 and Fig. S4). In contrast, both indirubin-3′-monoxime and kenpaullone, which inhibit Cdk1, Cdk2, and Cdk5 (47, 49, 50), increased SLC habituation during the prehabituation phase (Table S2). Indirubin-3′-monoxime and kenpaullone also have been reported to inhibit glycogen synthase kinase 3 beta (GSK3B) (51–53), which has known effects on learning as well as on prepulse inhibition of the mammalian acoustic startle response (54). GSK3B hyperactivity is thought to impair memory formation in neuropsychiatric conditions such such as Alzheimer's disease (55). Consistent with this idea, indirubin-3′-monoxime has been shown to reduce learning deficits in Alzheimer's disease models (56), but neither indirubin-3′-monoxime nor kenpaullone has been shown to increase short-term learning in WT animals.
Despite the potential promiscuity of small-molecule kinase inhibitors, the brief exposure to SU-9516, indirubin-3′-monoxime, and kenpaullone during a period in which all neurons of the SLC circuit are postmitotic suggests a possible cell cycle–independent role for these compounds in mediating learning. Consistent with this, many Cdks are expressed in terminally differentiated neurons (57–61), and furthermore, Cdk5, the sole non–cyclin-activated member of the Cdk family, has been shown to regulate synaptic plasticity and learning (62–67). Notably, neither SU-9516 nor its primarily characterized target, Cdk2, has been implicated in synaptic plasticity or learning. However, without direct evidence that SU-9516 is inhibiting Cdks within the SLC circuit, we cannot exclude the possibility that SU-9516 influences learning through Cdk-independent targets.
Second, we identified the myristic acid analog 12-methoxydodecanoic acid (12-MDA), which reduced SLC habituation and increased SLC sensitivity without affecting hyperactivity or SLC kinematic performance (Fig. 4 E and F and Fig. S4). Myristic acid compounds are 13- or 14-carbon saturated fatty acids that are cotranslationally added to the N terminus of membrane-associated proteins and are also common food and cosmetic additives (68). The libraries tested contained two other myristic acid analogs, 4-oxatetradecanoic acid and 2-hydroxymyristic acid, which are structurally similar to 12-MDA (Fig. 4G). Interestingly, neither of these related compounds altered SLC habituation or sensitivity (Fig. S4), suggesting that the position of the oxygen residue within the 12-MDA backbone is important for conferring substrate specificity. 12-MDA has been investigated primarily for its action in inhibiting virus replication (69, 70), although this is unlikely to be the mechanism underlying its effects on habituation.
12-MDA also has been shown to bind and redistribute Src family kinases (SFKs) from the membrane to the cytosol (71). The SFKs Src and Fyn contribute to the scaffolding of the NMDA receptor complex, and modulate synaptic efficacy by regulating postsynaptic glutamate receptor expression (72). Thus, it is conceivable that 12-MDA alters SFK localization, thereby affecting NMDA receptor signaling and thus reducing habituation. To further explore this potential functional link between 12-MDA and NMDA receptor signaling during habituation, we tested whether 12-MDA can modulate the function of NMDA receptors in vivo. We found that coincubation of larvae in 100 μM NMDA with 50 μM 12-MDA reversed the SLC habituation deficit observed after incubation in 50 μM 12-MDA alone (Fig. 4H). Furthermore, coincubation of larvae in subeffective concentrations of 12-MDA (5 μM) and ketamine (50 μM) produced a significantly greater attenuation of SLC habituation than the additive effect of each individual compound at these doses (Fig. 4H). Similarly, the startle hypersensitivity phenotype was suppressed by coincubation of 100 μM NMDA and 50 μM 12-MDA and enhanced by treatment with 5 μM 12-MDA/50 μM ketamine (Fig. S5). Taken together, these results reveal a role for a myristic acid analog in learning, and may suggest new therapeutic approaches to regulating postsynaptic glutamate receptors.
In summary, our high-resolution behavioral assay has shown that larval zebrafish robustly exhibit nonassociative learning, with landmark parametric criteria and conserved pharmacologic characteristics. By scaling our behavioral assay to screen small-molecule libraries with high throughput, we have demonstrated the feasibility of this approach for large-scale genome-wide or systematic approaches that can identify new compounds with specific effects on nonassociative learning in vivo. Several small-molecule screens for basic behaviors, such as sleep/resting and phototactic responses, have been performed in zebrafish (21, 23). Importantly, our assay distinguishes between the effects of a compound on behavioral modulation (e.g., habituation, sensitivity) and alterations in kinematic performance. Given that deficits in modulation of the mammalian acoustic startle response represent an endophenotype common to many neuropsychiatric disorders (73–75), future screening with the assay described here can be applied to distinguish between many kinematic and behavioral processes as the primary target of already available drugs, and also can be applied toward the systematic identification of more “behavior-specific” compounds.
Experimental Procedures
All experiments were performed on zebrafish larvae between 5 and 7 days post fertilization. Fish maintenance, behavioral assays, testing apparatuses, pharmacologic applications, and behavioral scoring methods have been described previously (18, 28, 76). Details and any variations in these methods are provided in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. John Hogenesch for generously providing the LOPAC-1280 chemical library, and Jessica Keel for technical assistance. This work was supported by National Research Science Award postdoctoral Fellowship 5F32EY019434-03 (to M.A.W.) and National Institutes of Health Grant MH092257 (to M.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107156108/-/DCSupplemental.
References
- 1.Christoffersen GR. Habituation: Events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Prog Neurobiol. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 2.Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 3.Jansiewicz EM, Newschaffer CJ, Denckla MB, Mostofsky SH. Impaired habituation in children with attention deficit hyperactivity disorder. Cogn Behav Neurol. 2004;17:1–8. doi: 10.1097/00146965-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 4.McSweeney FK, Murphy ES, Kowal BP. Regulation of drug taking by sensitization and habituation. Exp Clin Psychopharmacol. 2005;13:163–184. doi: 10.1037/1064-1297.13.3.163. [DOI] [PubMed] [Google Scholar]
- 5.Rothbaum BO, Kozak MJ, Foa EB, Whitaker DJ. Posttraumatic stress disorder in rape victims: Autonomic habituation to auditory stimuli. J Trauma Stress. 2001;14:283–293. doi: 10.1023/A:1011160800958. [DOI] [PubMed] [Google Scholar]
- 6.Groves PM, Thompson RF. Habituation: A dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- 7.Rankin CH, et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson RF. Habituation: A history. Neurobiol Learn Mem. 2009;92:127–134. doi: 10.1016/j.nlm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- 10.Blank M, Guerim LD, Cordeiro RF, Vianna MRM. A one-trial inhibitory avoidance task to zebrafish: Rapid acquisition of an NMDA-dependent long-term memory. Neurobiol Learn Mem. 2009;92:529–534. doi: 10.1016/j.nlm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Davis M. Effects of interstimulus interval length and variability on startle-response habituation in the rat. J Comp Physiol Psychol. 1970;72:177–192. doi: 10.1037/h0029472. [DOI] [PubMed] [Google Scholar]
- 12.Griffin JP, Pearson JA. Habituation of the flexor reflex in the rat. J Physiol. 1967;190:3P–5P. [PubMed] [Google Scholar]
- 13.Hunter AJ, Murray TK. Cholinergic mechanisms in a simple test of olfactory learning in the rat. Psychopharmacology (Berl) 1989;99:270–275. doi: 10.1007/BF00442821. [DOI] [PubMed] [Google Scholar]
- 14.Platel A, Porsolt RD. Habituation of exploratory activity in mice: A screening test for memory-enhancing drugs. Psychopharmacology (Berl) 1982;78:346–352. doi: 10.1007/BF00433739. [DOI] [PubMed] [Google Scholar]
- 15.Szabó I, Kolta P. Transitory increase of the acoustic startle reaction during its habituation. Acta Physiol Acad Sci Hung. 1967;31:51–56. [PubMed] [Google Scholar]
- 16.Wong K, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Brockerhoff SE, et al. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA. 1995;92:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess HA, Granato M. The neurogenetic frontier: Lessons from misbehaving zebrafish. Brief Funct Genomics Proteomics. 2008;7:474–482. doi: 10.1093/bfgp/eln039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granato M, et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- 21.Kokel D, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6:231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muto A, et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rihel J, et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolman M, Granato M. Behavioral genetics in larval zebrafish: Learning from the young. Dev Neurobiol. 2011 doi: 10.1002/dneu.20872. 10.1002/dneu.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best JD, et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- 26.Bretaud S, et al. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 27.Sumbre G, Muto A, Baier H, Poo MM. Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature. 2008;456:102–106. doi: 10.1038/nature07351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol. 2007;210:2526–2539. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- 29.Beck CD, Rankin CH. Heat shock disrupts long-term memory consolidation in Caenorhabditis elegans. Learn Mem. 1995;2:161–177. doi: 10.1101/lm.2.3-4.161. [DOI] [PubMed] [Google Scholar]
- 30.Davis HP, Squire LR. Protein synthesis and memory: A review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 31.Eaton RC, Bombardieri RA, Meyer DL. The Mauthner-initiated startle response in teleost fish. J Exp Biol. 1977;66:65–81. doi: 10.1242/jeb.66.1.65. [DOI] [PubMed] [Google Scholar]
- 32.Kimmel CB, Patterson J, Kimmel RO. The development and behavioral characteristics of the startle response in the zebra fish. Dev Psychobiol. 1974;7:47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- 33.Eaton RC, DiDomenico R, Nissanov J. Role of the Mauthner cell in sensorimotor integration by the brain stem escape network. Brain Behav Evol. 1991;37:272–285. doi: 10.1159/000114365. [DOI] [PubMed] [Google Scholar]
- 34.Eaton RC, Emberley DS. How stimulus direction determines the trajectory of the Mauthner-initiated escape response in a teleost fish. J Exp Biol. 1991;161:469–487. doi: 10.1242/jeb.161.1.469. [DOI] [PubMed] [Google Scholar]
- 35.Kohashi T, Oda Y. Initiation of Mauthner- or non–Mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci. 2008;28:10641–10653. doi: 10.1523/JNEUROSCI.1435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 37.Burgess HA, Schoch H, Granato M. Distinct retinal pathways drive spatial orientation behaviors in zebrafish navigation. Curr Biol. 2010;20:381–386. doi: 10.1016/j.cub.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bespalov A, et al. Habituation deficits induced by metabotropic glutamate receptors 2/3 receptor blockade in mice: Reversal by antipsychotic drugs. J Pharmacol Exp Ther. 2007;320:944–950. doi: 10.1124/jpet.106.110684. [DOI] [PubMed] [Google Scholar]
- 39.Bickel S, Lipp HP, Umbricht D. Early auditory sensory processing deficits in mouse mutants with reduced NMDA receptor function. Neuropsychopharmacology. 2008;33:1680–1689. doi: 10.1038/sj.npp.1301536. [DOI] [PubMed] [Google Scholar]
- 40.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 41.Rose JK, Rankin CH. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. J Neurosci. 2006;26:11582–11587. doi: 10.1523/JNEUROSCI.2049-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi E, Niimi K, Itakura C. Impairment of spatial short-term memory following acute administration of the NMDA receptor antagonist in heterozygous rolling Nagoya mice carrying the Ca V 2.1 α1 mutation. Behav Brain Res. 2010;213:121–125. doi: 10.1016/j.bbr.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 43.Venable N, Kelly PH. Effects of NMDA receptor antagonists on passive avoidance learning and retrieval in rats and mice. Psychopharmacology (Berl) 1990;100:215–221. doi: 10.1007/BF02244409. [DOI] [PubMed] [Google Scholar]
- 44.Halberstadt AL, Geyer MA. Habituation and sensitization of acoustic startle: Opposite influences of dopamine D1 and D2 family receptors. Neurobiol Learn Mem. 2009;92:243–248. doi: 10.1016/j.nlm.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leussis MP, Bolivar VJ. Habituation in rodents: A review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Earl CQ, Prozialeck WC, Weiss B. Interaction of α-adrenergic antagonists with calmodulin. Life Sci. 1984;35:525–534. doi: 10.1016/0024-3205(84)90246-7. [DOI] [PubMed] [Google Scholar]
- 47.Hoessel R, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 48.Moshinsky DJ, et al. SU9516: Biochemical analysis of cdk inhibition and crystal structure in complex with cdk2. Biochem Biophys Res Commun. 2003;310:1026–1031. doi: 10.1016/j.bbrc.2003.09.114. [DOI] [PubMed] [Google Scholar]
- 49.Zaharevitz DW, et al. Discovery and initial characterization of the paullones, a novel class of small-molecule inhibitors of cyclin-dependent kinases. Cancer Res. 1999;59:2566–2569. [PubMed] [Google Scholar]
- 50.Marko D, et al. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br J Cancer. 2001;84:283–289. doi: 10.1054/bjoc.2000.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: An update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leclerc S, et al. Indirubins inhibit glycogen synthase kinase-3 β and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease: A property common to most cyclin-dependent kinase inhibitors? J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 53.Polychronopoulos P, et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 2004;47:935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 54.Kapfhamer D, et al. Protein phosphatase 2A and glycogen synthase kinase 3 signaling modulate prepulse inhibition of the acoustic startle response by altering cortical M-type potassium channel activity. J Neurosci. 2010;30:8830–8840. doi: 10.1523/JNEUROSCI.1292-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding Y, Qiao A, Fan G-H. Indirubin-3′-monoxime rescues spatial memory deficits and attenuates β-amyloid-associated neuropathology in a mouse model of Alzheimer's disease. Neurobiol Dis. 2010;39:156–168. doi: 10.1016/j.nbd.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 57.Gomi H, Sassa T, Thompson RF, Itohara S. Involvement of cyclin-dependent kinase-like 2 in cognitive function required for contextual and spatial learning in mice. Front Behav Neurosci. 2010;4:17. doi: 10.3389/fnbeh.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graña X, Claudio PP, De Luca A, Sang N, Giordano A. PISSLRE, a human novel CDC2-related protein kinase. Oncogene. 1994;9:2097–2103. [PubMed] [Google Scholar]
- 59.Hirose T, Tamaru T, Okumura N, Nagai K, Okada M. PCTAIRE 2, a Cdc2-related serine/threonine kinase, is predominantly expressed in terminally differentiated neurons. Eur J Biochem. 1997;249:481–488. doi: 10.1111/j.1432-1033.1997.t01-1-00481.x. [DOI] [PubMed] [Google Scholar]
- 60.Lazzaro MA, Albert PR, Julien JP. A novel cdc2-related protein kinase expressed in the nervous system. J Neurochem. 1997;69:348–364. doi: 10.1046/j.1471-4159.1997.69010348.x. [DOI] [PubMed] [Google Scholar]
- 61.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 62.Bibb JA, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 63.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. J Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawasli AH, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawasli AH, Bibb JA. Alternative roles for Cdk5 in learning and synaptic plasticity. Biotechnol J. 2007;2:941–948. doi: 10.1002/biot.200700093. [DOI] [PubMed] [Google Scholar]
- 66.Wei FY, et al. Control of cyclin-dependent kinase 5 (Cdk5) activity by glutamatergic regulation of p35 stability. J Neurochem. 2005;93:502–512. doi: 10.1111/j.1471-4159.2005.03058.x. [DOI] [PubMed] [Google Scholar]
- 67.Li BS, et al. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci USA. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rioux V, Pédrono F, Legrand P. Regulation of mammalian desaturases by myristic acid: N-terminal myristoylation and other modulations. Biochim Biophys Acta. 2011;1811:1–8. doi: 10.1016/j.bbalip.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Bryant ML, et al. Incorporation of 12-methoxydodecanoate into the human immunodeficiency virus 1 gag polyprotein precursor inhibits its proteolytic processing and virus production in a chronically infected human lymphoid cell line. Proc Natl Acad Sci USA. 1991;88:2055–2059. doi: 10.1073/pnas.88.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pidgeon C, et al. Antiviral phospholipids: Anti-HIV drugs conjugated to the glycerobackbone of phospholipids. J Biol Chem. 1993;268:7773–7778. [PubMed] [Google Scholar]
- 71.Johnson DR, et al. Functional analysis of protein N-myristoylation: Metabolic labeling studies using three oxygen-substituted analogs of myristic acid and cultured mammalian cells provide evidence for protein-sequence–specific incorporation and analog-specific redistribution. Proc Natl Acad Sci USA. 1990;87:8511–8515. doi: 10.1073/pnas.87.21.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 73.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 74.Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- 75.Pfleiderer B, et al. Altered auditory processing in patients with panic disorder: A pilot study. World J Biol Psychiatry. 2010;11:945–955. doi: 10.3109/15622975.2010.490273. [DOI] [PubMed] [Google Scholar]
- 76.Burgess HA, Johnson SL, Granato M. Unidirectional startle responses and disrupted left-right coordination of motor behaviors in robo3 mutant zebrafish. Genes Brain Behav. 2009;8:500–511. doi: 10.1111/j.1601-183X.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.