Abstract
The proper distribution of mitochondria is particularly vital for neurons because of their polarized structure and high energy demand. Mitochondria in axons constantly move in response to physiological needs, but signals that regulate mitochondrial movement are not well understood. Aside from producing ATP, Ca2+ buffering is another main function of mitochondria. Activities of many enzymes in mitochondria are also Ca2+-dependent, suggesting that intramitochondrial Ca2+ concentration is important for mitochondrial functions. Here, we report that mitochondrial motility in axons is actively regulated by mitochondrial matrix Ca2+. Ca2+ entry through the mitochondrial Ca2+ uniporter modulates mitochondrial transport, and mitochondrial Ca2+ content correlates inversely with the speed of mitochondrial movement. Furthermore, the miro1 protein plays a role in Ca2+ uptake into the mitochondria, which subsequently affects mitochondrial movement.
Mitochondria are dynamic organelles that constantly move within cells and undergo morphological changes in response to physiological needs (1–3). In neurons, mitochondria are abundantly present throughout different subcellular compartments. The machineries and signals that transport mitochondria from the cell body (where they are synthesized) to the terminal need to be carefully regulated because of the highly polarized structure and lengthy axon of a neuron (4–6). Defects in transport of mitochondria can cause deleterious effects on mitochondrial functions in different parts of neurons, and hence affect neuronal survival and function (2, 3). Recent efforts to investigate mitochondrial transportation have provided significant new information regarding mitochondrial mobility, especially the mechanical components that modulate mitochondrial transport; however, it remains unclear whether intrinsic signals inside of mitochondria also actively regulate mitochondrial movement.
Mitochondrial transport is mediated by interactions of the mitochondrial adaptor proteins to the kinesin and dynein motors, as well as binding of the motor proteins to the cytoskeleton track (7, 8). It was posited that cytoplasmic Ca2+ level is a key regulator of mitochondrial trafficking in axons and dendrites, and that intracellular Ca2+ influx impedes mitochondrial movement by affecting the overall interactions between the mitochondrial adaptor, motor, and cytoskeleton track (9, 10). Two different mechanisms for Ca2+-mediated stop in mitochondrial trafficking were proposed. Wang and Schwarz suggested that Ca2+ binding to the EF hand motif of the mitochondrial adaptor protein miro1 recruits kinesin-1 motor and, hence, derails kinesin-1 from the microtubule track, thereby stopping mitochondrial transportation in axons (10). Macaskill et al. proposed that following Ca2+ influx induced by glutamate or neuronal activity, Ca2+ binding to the EF hand motif of the miro1 protein causes miro1 to dissociate from the kinesin-1 motor, and hence halts mitochondrial movement (11). Although the mechanisms proposed for this Ca2+-induced arrest in mitochondrial movement are different, both groups agree that cytoplasmic Ca2+ elevation and its subsequent binding to the miro1 protein are key events regulating mitochondrial motility in neurons.
Cytoplasmic Ca2+ has been deemed important for mitochondrial motility in neurons; however, the relationship between mitochondrial Ca2+ content and movement has not been established. It also remains unclear whether mitochondria play a passive or active role in their own transport. Aside from the endoplasmic reticulum, mitochondria are another main source of Ca2+ buffering within the cell. Following intracellular Ca2+ elevation, mitochondria rapidly take up and accumulate Ca2+ to maintain cellular Ca2+ homeostasis (12). Studies also have shown that Ca2+ uptake into mitochondria occurs through the mitochondrial Ca2+ uniporter (12, 13), and that mitochondrial Ca2+ influx can increase ATP production by activating the TCA cycle, as well as enhance the activities of the electron transport-chain enzymes and the ATP synthase complex (14–17). Nevertheless, the relationship between mitochondrial energetic status and mitochondrial movement remains controversial. A report showed that anterogradely moving mitochondria have higher membrane potential than those moving retrogradely (18), yet another group found no difference between the membrane potentials of mitochondria moving in opposite directions or between the stationary and mobile mitochondria (19). The parameters within mitochondria associated with changes in the pattern of mitochondrial motility thus remain unclear. Because Ca2+ in mitochondria is associated with various mitochondrial functions, we hypothesized that mitochondrial Ca2+ may act as a signal that allows mitochondria to actively regulate their own mobility. Here, we show Ca2+ influx through the mitochondrial Ca2+ uniporter modulates mitochondrial transport, and that mitochondrial Ca2+ content correlated inversely with the speed of mitochondrial movement. Furthermore, we demonstrate that the miro1 mutant modulates mitochondrial trafficking by altering the amount of Ca2+ influx into the mitochondria in axons of hippocampal neurons. Taken together, our results imply that mitochondrial matrix Ca2+ is an intrinsic signal that actively regulates mitochondrial transportation in neurons.
Results and Discussion
Visualization of Mitochondrial Ca2+ in Axons.
Detecting small changes in the level of Ca2+ within mitochondria of living neurons have been challenging because of the lack of subcellular specificity provided by synthetic Ca2+ indicators. To test the hypothesis that internal mitochondrial Ca2+ modulates mitochondrial motility, we targeted a genetically encoded GFP-based fluorescent Ca2+ indicator, Case12, to the mitochondria. This targeting was achieved by inserting a mitochondrial signal peptide into the N terminus of the Case12 protein (mito-Case12). Case12 protein allows linear detection of Ca2+ ion concentration within the physiological range and its binding to Ca2+ is rapid and reversible, thus making real-time detection of changes in Ca2+ level within a cell possible (20, 21). Mito-Case12 colocalized with mito-RFP in transfected hippocampal neurons (Fig. 1A, Fig. S1 A and C), thus confirming that mito-Case12 is indeed targeted to the mitochondria. Next, to test the ability of mito-Case12 to respond to increases in Ca2+ content, we treated hippocampal neurons containing mito-Case12 and mito-RFP with calcimycin, a Ca2+ ionophore (Fig. S1A). Following calcimycin treatment, the green fluorescence intensity of mito-Case12 increased significantly within seconds in mitochondria, but the mito-RFP signal remained constant (Fig. S1 A and B). Mito-Case12 signal is dependent on calcium influx into the cell, because calcimycin treatment in calcium-free extracellular solution failed to cause an increase in mito-Case12 signal (Fig. S1 C and D). To account for the difference in the size of mitochondria and thus the amount of fluorescent protein inside of mitochondria, as well as change in focal plane that could occur over time, we also normalized mito-Case12 labeling intensity to that of mito-RFP intensity. Similar to mito-Case12 signal, normalized mito-Case12:mito-RFP ratio increased following calcimycin treatment. Together, our results suggest that (i) mito-Case12 is able to detect Ca2+ elevation within mitochondria, and (ii) Ca2+ is rapidly taken up by mitochondria following intracellular Ca2+ elevation.
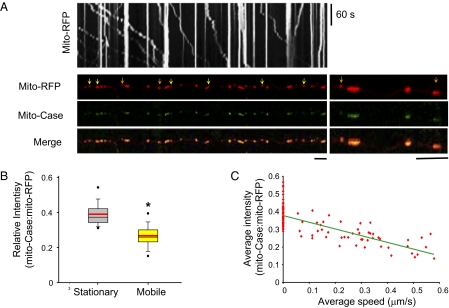
Fig. 1.
Moving mitochondria tend to have lower mitochondrial Ca2+ signal. (A) Kymograph generated from moving mitochondria (Upper) and first frame of live imaging of neuron cotransfected with mito-RFP and mito-Case12. Arrows point to moving mitochondria (yellow and orange) and the region magnified on the right is denoted by the orange arrows. (Scale bars, 10 μm.) (B) Box plot of the mito-Case12:mito-RFP ratio for stationary and mobile mitochondria. Lower and upper boundaries of the box indicate 25th and 75th percentiles, respectively. The black line in the box represents the median, and the red line represents the mean. Whiskers show 10th and 90th percentiles. Dots above and below the box are showing outliers. *P = 2 × 10−15 (C) Average mito-Case12:mito-RFP ratio versus average speed of movement. R2 = 0.58. n > 150 mitochondria from 10 axons for B and C.
Mitochondrial Ca2+ Content Predicts Mitochondrial Mobility.
Having confirmed that mito-Case12 can detect Ca2+ elevation inside of mitochondria, we next investigated if mitochondrial Ca2+ correlates with mitochondrial movement in axons under normal conditions. To visualize mitochondrial movement and Ca2+ content within the mitochondria, time-lapse live imaging of primary hippocampal neurons doubly transfected with mito-RFP and mito-Case12 was performed. Fig. 1A shows a kymograph illustrating mitochondrial movement (x axis) over time (y axis), in which the stationary mitochondria are displayed as straight vertical lines as they are immobile over time, whereas the moving mitochondria are seen as diagonal lines. Interestingly, we noticed that compared with stationary mitochondria, moving mitochondria tend to have lower mito-Case12 fluorescence signal, and hence lower mitochondrial Ca2+ content. To minimize for variability caused by drifts in the focal plane as well as differences in size of mitochondria, we normalized mito-Case12 labeling intensity to that of mito-RFP intensity. Fig. 1 A and B and Fig. S2 show that average mito-Case12:mito-RFP ratio is clearly lower in moving mitochondria (mean = 0.27 ± 0.01), but stationary mitochondria exhibited higher ratio of mito-Case12:mito-RFP (mean = 0.39 ± 0.01), confirming that mitochondria in motion tend to have lower matrix Ca2+ content than stationary mitochondria.
As mitochondria move in opposite directions (anterograde vs. retrograde) and with different speed, we next determined if the direction or speed of mitochondrial movement correlates with mitochondrial Ca2+ content. Analyses of mitochondria moving in opposite directions showed similar mitochondrial Ca2+ content, as determined by the ratio of mito-Case12:mito-RFP (anterograde: 0.24 ± 0.01; retrograde: 0.27 ± 0.02; P = 0.22). We also calculated the average speed of individual moving mitochondria over the period of imaging and plotted against the average mito-Case12:mito-RFP ratio. Fig. 1C shows that the speed of moving mitochondria is between 0.1 μm/s and 0.58 μm/s, within range of values reported previously for mitochondrial movement seen in axons of hippocampal neurons (7). More importantly, Fig. 1C shows that there is a strong correlation between the speed of mitochondrial movement with the level of mitochondrial Ca2+; the lower the mitochondrial matrix Ca2+ content, the faster the movement (Pearson correlation coefficient = −0.76 or −0.67, when including or excluding stationary mitochondria, respectively). Our results imply that mitochondrial Ca2+ does not influence the directionality of movement; rather, mitochondrial Ca2+ content is an important parameter that determines mitochondrial mobility and the speed of mitochondrial movement.
Influx of Ca2+ Through the Mitochondrial Ca2+ Uniporter Alters Mitochondrial Mobility.
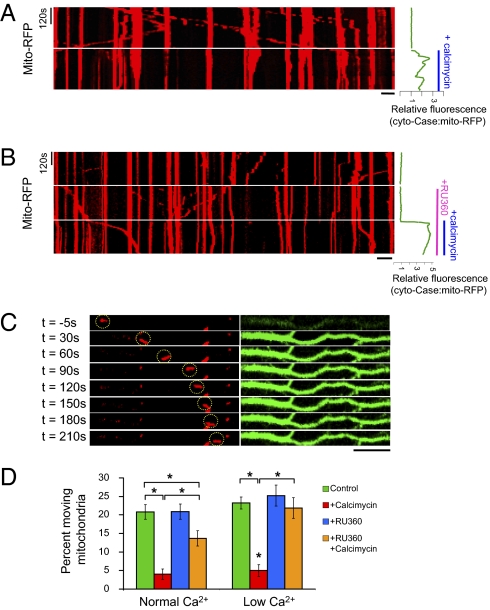
Influx of Ca2+ into the mitochondrial matrix is gated by the mitochondrial Ca2+ uniporter (22, 23), suggesting that it may also play a role in regulating mitochondrial movement. Thus, we next tested the following hypotheses: (i) mitochondrial Ca2+ uniporter modulates mitochondrial transportation in axons; (ii) it is not cytoplasmic Ca2+, but the influx of Ca2+ to mitochondrial matrix that ultimately arrests mitochondrial movement. To this end, we examined mitochondrial mobility following either inhibition or activation of the mitochondrial Ca2+ uniporter. Treatment of neurons with calcimycin, a Ca2+ ionophore, elevates cytoplasmic Ca2+ as detected by Case12 protein in the cytoplasm (cyto-Case12) (Fig. 2A, Right, and Movie S1) and mitochondrial Ca2+ as detected by mito-Case12 (Fig. S1). Neurons treated with calcimycin showed immediate Ca2+ elevation and stop in mitochondrial movement in axons (Figs. 2 A and D). This calcimycin-induced stop in mitochondrial movement is dependent on Ca2+ influx, because calcimycin treatment in the absence of extracellular calcium failed to pause mitochondrial movement (Fig. S3). To assess whether mitochondrial Ca2+ influx into the matrix through the mitochondrial Ca2+ uniporter is necessary for Ca2+-induced stop in mitochondrial movement, we first blocked the mitochondrial Ca2+ uniporter by treating neurons with RU360 (13), then subsequently challenged it with calcimycin. We reasoned that if the mitochondrial Ca2+ uniporter indeed gates mitochondrial Ca2+ influx, and hence affect mitochondrial mobility, blocking the mitochondrial Ca2+ uniporter would allow mitochondrial movement to persist in the presence of high cytoplasmic Ca2+ caused by calcimycin treatment. Fig. 2 B and C and Movie S2 show that mitochondria incubated with RU360 for 15 min before calcimycin treatment maintained their mobility much longer after calcimycin addition, despite the increase in cytoplasmic Ca2+ content indicated by cyto-Case12. Analyses of the percentage of moving mitochondria over the imaging period showed that a significant percentage of mitochondria remained mobile if the neurons were preincubated with RU360 before calcimycin treatment (Fig. 2D, normal calcium). However, RU360 only partially restored the percentage of moving mitochondria to normal, thus suggesting that either cytoplasmic Ca2+ also contributes, or that RU360 did not completely prevent Ca2+ entry into mitochondria. We thus further examined mito-Case12 signal following RU360 and calcimycin treatment (Fig. S4). We found that RU360 delayed Ca2+ entry into mitochondria rather than completely abolishing it (Fig. S4), and that mitochondrial movement correlated inversely with mito-Case12 signal elevation, rather than cyto-Case12 profile. To further reduce calcium entry through the mitochondrial Ca2+ uniporter, we decreased the level of extracellular Ca2+ to 0.18 mM Ca2+ (low Ca2+). Calcimycin treatment in low extracellular calcium was sufficient to stop mitochondrial movement (Fig. S5), but it no longer paused mitochondrial movement in neurons pretreated with RU360 (Fig. 2D and Fig. S6A). Note that we still observed a negligible level of Ca2+ influx into the mitochondria after RU360 and calcimycin treatment (Fig. S6B; mito-Case12 profile), but at a much slower rate and lower level. This result is consistent with report that RU360 is only partially effective in reducing Ca2+ influx through the mitochondrial Ca2+ uniporter (24). We also did not observe redistribution in the percentage of mitochondria undergoing anterograde or retrograde transport, suggesting that RU360 treatment or altering Ca2+ level does not selectively alter directionality of transport (Fig. S7). Taken together, these results imply that a critical level of calcium influx through the mitochondrial Ca2+ uniporter is necessary for the Ca2+-dependent pause in mitochondrial transport in axons.
Fig. 2.
Blocking Ca2+ influx into mitochondria through Ca2+-uniporter delays calcimycin-induced arrest in mitochondrial movement. (A) Kymograph of a neuron cotransfected with mito-RFP and cyto-Case12 before and after addition of calcimycin (2 μg/mL). The rise in Ca2+ is plotted on the right, shown as relative fluorescence of cyto-Case12:mito-RFP ratio. Immediately after calcimycin treatment and Ca2+ elevation, mobile mitochondria became stationary. (B) Kymograph showing mitochondrial movement for neurons cotransfected with mito-RFP and cyto-Case12. Relative intensity of cyto-Case12:mito-RFP is shown on the right following RU360 and calcimycin treatments. Ru360 was applied for 15 min before addition of calcimycin, but only the last 5 min of RU360 treatment is shown. (C) Mito-RFP (red; Left) and cyto-Case12 (green; Right) images after 15 min of RU360 and before (t = −5 s) and after calcimycin treatment. Despite increase in cytoplasmic Ca2+, mitochondria remained mobile (highlighted by yellow circle). (Scale bars in A–C, 10 μm.) (D) Percentage of moving mitochondria over the 5-min imaging period for each condition. Normal Ca2+ indicates 1.8 mM Ca2+ and low calcium indicates 0.18 mM Ca2+ in the extracellular solution. n = 150–380 mitochondria from at least five axons analyzed. Values represent mean ± SEM; *P < 0.01.
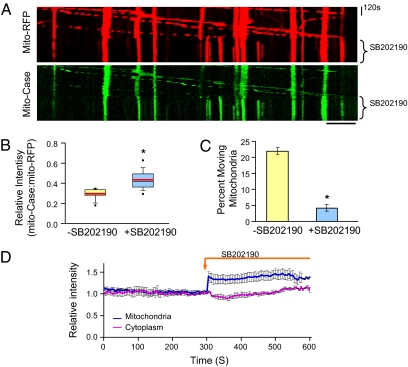
Next, we tested whether elevation of the mitochondrial matrix Ca2+ by activation of the mitochondrial Ca2+ uniporter is sufficient to regulate mitochondrial mobility. Neurons containing mito-RFP and mito-Case12 were treated with a drug that activates the mitochondrial Ca2+ uniporter, SB202190 (25). SB202190 treatment indeed elevated mitochondrial Ca2+ content, as seen by the greater intensity of mito-case12 fluorescence in the kymograph in Fig. 3A. Furthermore, the mito-RFP kymograph shows that mitochondria were mobile before SB202190 treatment, but stopped immediately following SB20190 treatment. This sudden change in mitochondrial movement is accompanied by mitochondrial matrix Ca2+ elevation (Fig. 3 A and D). We also plotted the fluorescence ratio of mito-Case12 to mito-RFP before and after SB202190 treatment for the moving mitochondria (Fig. 3B). Our results clearly demonstrate that the average Ca2+ content in the moving mitochondria is lower initially, but increased significantly after activation of mitochondrial Ca2+ uniporter with SB202190. Analyses of the percentage of moving mitochondria also confirmed that SB202190 treatment, similar to calcimycin treatment, arrested mitochondrial transport (Fig. 3C). Note that SB202190 treatment did not increase cytoplasmic Ca2+, as determined by imaging with cyto-Case12, but significantly elevated mitochondrial Ca2+, as measured by mito-Case12 imaging (Fig. 3D). SB202190 has also been shown to inhibit p38 MAPK (26); thus, it is possible that it may influence mitochondrial movement independently of mitochondrial Ca2+ elevation. However, the observed inverse relationship between mito-Case12 profile and movement, as well as results obtained using RU360, all support the hypothesis that the mitochondrial Ca2+ uniporter regulates mitochondrial movement in axons by gating Ca2+ influx into the mitochondrial matrix.
Fig. 3.
Mitochondrial Ca2+ elevation by activating the mitochondrial Ca2+ uniporter is sufficient to stop mitochondrial movement. (A) Kymograph of time-lapse images of neuron cotransfected with mito-RFP and mito-Case12 before and after SB202190. (Scale bar, 10 μm.) (B) Box plot of mito-Case:mito-RFP intensity ratio before and after SB202190 for the moving mitochondria. n = 40 moving mitochondria from eight axons. Red lines indicate mean. *P = 6 × 10−6. (C) Percentage of moving mitochondria before and after SB202190 treatment. n = 200 mitochondria from eight axons. *P = 9 × 10−10. (D) SB202190 treatment did not alter cytoplasmic Ca2+ level. Relative intensity before and after SB202190 treatment in cytoplasm is measure by Cyto-Case12, and intensity in mitochondria is measured by mito-Case12. To normalize for drifts in z axis during imaging, values were normalized to mito-RFP. Values represent mean ± SEM, n = 8 axons.
Miro1 Protein Alters Ca2+ Entry into Mitochondria.
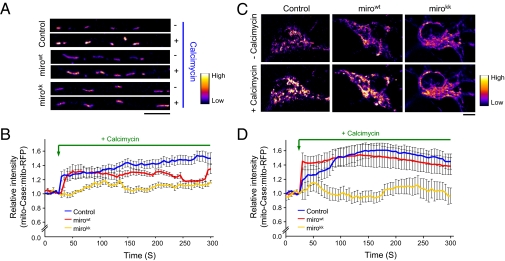
Our findings suggest that mitochondrial Ca2+ influx via the mitochondrial Ca2+ uniporter actively regulates mitochondrial transport. This result prompted us to re-examine the role of miro1 protein in influencing mitochondrial trafficking and test the possibility that miro1 affects transport by altering mitochondrial Ca2+ influx. Previous studies showed that cytoplasmic Ca2+ regulates mitochondrial movement mainly by binding to miro1 EF hand domains, thereby altering miro1 interaction with either the kinesin motor or microtubule track (9–11). Mutations in the EF hand domains of miro1 failed to arrest mitochondrial movement despite high intracellular Ca2+, thus highlighting the importance of miro1 in Ca2+-dependent regulation of mitochondrial movement (9–11). We found that transfection of wild-type miro1 (mirowt) or miro1 EF hand mutations (mirokk) together with mito-RFP and mito-Case12 increased the overall length of mitochondria and the percentage of moving mitochondria, but did not alter the density of mitochondria (number of mitochondria per micrometer) in axons or the average speed of moving mitochondria (Fig. S8). Furthermore, calcimycin treatment failed to stop mitochondrial transport in mirokk-transfected neurons, nor did it change the directionality of mitochondrial movement (Fig. S9). These results are consistent with those reported earlier (9–11). Next, we compared the relative levels of mitochondrial Ca2+ following calcimycin treatment in control neurons and those transfected with mirowt, or mirokk, in addition to mito-RFP and mito-Case12. Representative mito-Case12 signals in axons of neurons before and after calcimycin treatment are shown in Fig. 4A (pseudocolored). Fig. 4B summarizes our findings that mutations in the EF hand domains of miro1 resulted in a significantly lower overall increase in matrix Ca2+ level in the axons (fold-change in signal intensity after 3 min of calcimycin treatment, a time point in which the mito-Case12 signal becomes relatively stable are: control, 1.40 ± 0.06; mirowt, 1.31 ± 0.03; mirokk, 1.11 ± 0.02). Note that the expression of mirowt construct also arrested mitochondrial movement following calcimycin addition (10, 11), and that these axons initially showed Ca2+ influx to the same extent as the control (Fig. 4B). Mirokk mutant, on the other hand, started with low but gradual Ca2+ influx, although never reaching the Ca2+ level comparable to calcimycin treated control or mirowt-transfected neurons (Fig. 4B). To determine if mirokk also influences cytoplasmic Ca2+ influx, we examined the cyto-Case12 profile in control and mirokk-transfected neurons (Fig. S10). We find that mirokk-transfected neurons showed same level of cytoplasmic Ca2+ influx as the control. These results imply that mutations in miro1 EF hand domains affect Ca2+ entry into the mitochondria, which subsequently alter mitochondrial movement. Therefore, it is also plausible that blockage of mitochondrial Ca2+ influx by the miro1 EF mutants also contributes to failure in mitochondrial movement arrest in neurons, as reported previously (9–11). Importantly, our findings confirm that intramitochondrial Ca2+ is an important determinant of mitochondrial movement, and that there is a critical Ca2+ threshold required for pausing mitochondrial movement. Our results differ from a report that examined mitochondrial Ca2+ content in cortical neurons using mitochondrial-targeted aequorin protein, in which they found greater mitochondrial Ca2+ content in miro1 wild-type and EF hand mutants (9). To ensure this difference is not the result of a difference in population of mitochondria examined (mitochondria in axons versus in soma), we directly examined the fold-change in Ca2+ signal in the soma following calcimycin treatment. Fig. 4 C and D shows that mirowt showed similar extent of increase in mitochondrial Ca2+ signal compared with the control, but mirokk displayed a diminished level of Ca2+ influx compared with control or mirowt constructs, similar to what we observed for mitochondria in axons. Thus, it is possible that differences in the neuronal cell type or detection method gave rise to these different results. However, our results and those by Saotome et al. (9) show that miro1 can alter the level of Ca2+ influx into mitochondria.
Fig. 4.
Mutations in miro1 EF hand domains reduce mitochondrial Ca2+ elevation following calcimycin treatment. (A and C) Representative images of mito-Case12 level (pseudocolored) before and after calcimycin treatment in axon and soma, respectively. (Scale bars, 10 μm.) (B and D) Mito-Case12:mito-RFP ratio before and after calcimycin treatment in axon and soma, respectively. Mirokk mutant decreased the level of Ca2+ entering mitochondria. Values represent mean ± SEM. (A and B) n = 6 axons each; (C and D), n = 6 cell bodies each.
We further tested whether elevation in mitochondrial Ca2+ can override the inhibitory effect of mirokk on mitochondrial movement arrest by treating transfected neurons with SB202190. The levels of mitochondrial Ca2+ following SB202190 treatment were similar between mirowt- and mirokk-transfected neurons, and did not differ significantly from that of the control following SB202190 treatment (i.e., at 1 min after SB202190 treatment, the levels of mitochondrial Ca2+ are: control, 1.33 ± 0.10; mirowt, 1.24 ± 0.08; mirokk, 1.22 ± 0.05) (Fig S11A). We found that this increase in mitochondrial Ca2+ content significantly decreased mitochondrial movement in both mirowt and mirokk, albeit the percentage of moving mitochondria in mirowt and mirokk after SB202190 treatment was still slightly higher than that of the control neurons (Fig. S11B). This result is likely caused by a greater percentage of moving mitochondria in mirowt- and mirokk-transfected neurons before SB202190 treatment. We also found that SB202190 treatment did not change the directionality of moving mitochondria (Fig. S12), consistent with our findings that mitochondrial Ca2+ content does not correlate with the direction of mitochondrial movement. Taken together, our results strongly support the hypothesis that intramitochondrial Ca2+ content is a crucial determinant of mitochondrial movement and further imply that miro1 may not directly influence mitochondrial Ca2+ uniporter opening.
Our findings reveal that mitochondrial matrix Ca2+ is an intrinsic signal that actively controls mitochondrial transportation in axons. Although previous reports suggest that cytoplasmic Ca2+ modulates mitochondrial movement through miro1 protein, our data argue that mitochondrial matrix Ca2+ acts as a gatekeeper of mitochondrial mobility. Blocking mitochondrial Ca2+ influx through the mitochondrial uniporter delayed stop in movement, despite intracellular Ca2+ elevation, whereas increasing mitochondrial Ca2+ alone was sufficient to halt mitochondrial transport. Our live imaging studies show that cytoplasmic Ca2+ influx rapidly and invariably leads to mitochondrial Ca2+ elevation (as shown by our mito-Case12 imaging studies in Fig. S1), and interestingly, the average speed of mitochondrial movement correlated inversely with mitochondrial Ca2+ content. Together with our observation that miro1 EF hand mutant blocked Ca2+ entry into the mitochondrial matrix, these results strongly support our conclusion that matrix Ca2+ plays an active role in regulating mitochondrial movement, and further suggests that miro1 may have dual roles both in acting as a cytoplasmic Ca2+ sensor and in affecting the amount of Ca2+ influx into the mitochondria. The molecular components of the mitochondrial Ca2+ uniporter are not well understood; but recent studies show that a newly identified 40-kD inner mitochondrial membrane protein is an essential component of the Ca2+ uniporter, which also interacts with the MICU1 protein, another inner mitochondrial membrane protein that serves as putative regulator of mitochondrial Ca2+ uniporter (27–29). Interestingly, similar to miro1, MICU1 also contains two EF hand domains and a transmembrane domain (29, 30). It is plausible that miro1 may associate with a Ca2+-permeable channel on the outer mitochondrial membrane, such as the mitochondrial voltage-dependent anion channel or other unidentified Ca2+ permeable channel, thus influencing Ca2+ influx into the mitochondria. It will be interesting in the future to examine the mechanism by which intramitochondrial Ca2+ influx through the uniporter in turn affects miro1 interaction with the kinesin motor. As each mitochondrion likely contain multiple miro1 proteins, we speculate that the speed of mitochondrial movement may be regulated by the number of miro1 protein bound to the kinesin motor at a given time, and this miro1-kinesin binding is inversely proportional to the influx of Ca2+ into mitochondria. Once intramitochondrial concentration reaches a critical level, all miro1–kinesin interactions are disrupted, and mitochondria therefore stop moving. Studies have shown that mitochondrial Ca2+ influx into the mitochondria can stimulate the mitochondrial electron transport chain enzymes and TCA cycle to increase ATP production (14–16); it is thus likely that the high Ca2+ concentration inside the stationary mitochondria signals the mitochondria to remain stationary and stimulates the TCA cycle to enhance ATP production, thereby providing energy to the site of high demand. On the other hand, lower Ca2+ content in mitochondria may serve as a signal for mitochondria to remain mobile, thus ensuring redistribution to a different location. Taken together, our data are unique in demonstrating that mitochondrial matrix Ca2+ content can influence mitochondrial movement, and further suggest that mitochondria Ca2+ can be an intrinsic signal that integrates cellular energy demand and ATP production to actively influence subcellular mitochondrial distribution within neurons.
Materials and Methods
Constructs and Reagents.
Mito-RFP used for imaging of mitochondrial movement (pTurboRFP-mito vector) and Case12 protein used for detection of cytoplasmic Ca2+ level (pCase12-Cyto vector) were obtained from Evrogen. To detect mitochondrial Ca2+ level, a mitochondrial targeting sequence derived from subunit VIII precursor of human cytochrome C oxidase was inserted in the N terminus of the Case12 protein. Localization of mito-Case12 in the mitochondria was confirmed by colocalization with Mitotracker Red and Mito-RFP. Human wild-type miro1 construct pRK5Myc-Miro1 (mirowt) and miro1 EF hand mutations pRK5Myc-Miro1E208K,E328K (Mirokk) were obtained from P. Aspenström (Stockholm, Sweden) (9, 11, 31, 32). The following reagents were used: 2 μg/mL calcimycin (3.8 μM; Sigma Aldrich), 10 μM SB202190, and 10 μM RU360 (Calbiochem).
Cell Culture.
Mouse hippocampal neurons were obtained from embryonic day 17 to 18 embryos. Dissociated neurons were plated at 40,000 to 55,000 cells on poly-d-lysine–coated glass bottom plates and maintained in neurobasal medium supplemented with B27 and Glutamax (Invitrogen). Neurons were transfected at days in vitro 4 to 5 using Lipofectamine 2000 (Invitrogen) and imaged at days in vitro 7 to 10.
Live Imaging.
Hippocampal neurons were imaged in CO2-independent Tyrode's buffer (referred to as normal extracellular solution) composed of 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, 5.6 mM Glucose, pH 7.3 at 37 °C. For experiments done in Ca2+-free solution, Tyrode's buffer was used except 1.8 mM CaCl2 was removed and NaCl changed to 136.8 mM to maintain osmolarity. For experiments done in extracellular solution with low Ca2+, CaCl2 in Tyrode's buffer was changed to 0.18 mM CaCl2, and NaCl changed to 136 mM to maintain osmolarity. Time-lapse images were acquired at 5-s intervals using a Leica SP5 scanning confocal microscope at minimum laser intensity to prevent photobleaching (7–20%) and image-acquisition parameters were set to exclude saturation of signals. Images were acquired for 5 min before and after the addition of 2 μg/mL calcimycin and SB202190 (10 μM). Cells were incubated with RU360 (10 μM) for 15 min before calcimycin treatment.
Imaging Analyses.
Fluorescence intensities were measured using ImageJ software and the speed of mitochondrial movement was analyzed using the Manual Tracking Plugin in ImageJ. Dendrites and axons can be distinguished based on morphology, and axons are identified as long, thin processes that extend many times the length of dendrites, which are thicker. To normalize for difference in the size of mitochondria and thus the amount of fluorescent protein inside of mitochondria, as well as photobleaching and shift in focal plane that could occur over time, we normalized mito-Case12 labeling intensity to mito-RFP intensity. To correct for drifts in the z axis, cyto-Case12 signals were also normalized to mito-RFP signals. At least three independent transfections per condition were used for imaging analyses. For more details on image analysis, see SI Materials and Methods.
Statistics.
All values are shown as mean ± SEM, and tested for statistical significance by Student t test.
Supplementary Material
Acknowledgments
We thank Dr. Aspenström for the generous gift of miro1 constructs used in this work, F. Kennedy for assistance with preparation of hippocampal cell cultures, and the Light Microscopy Imaging Center at Indiana University at Bloomington. This work was supported by Indiana University (K.-T.M.) and Grant NS052524 from the National Institutes of Health (to K.T.C).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106862108/-/DCSupplemental.
References
- 1.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang DTW, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:d91–d102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- 5.Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–350. doi: 10.1002/1096-9861(20001120)427:3<340::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: The role of growth factor signaling. J Exp Biol. 2003;206:1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- 7.MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20:102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Cai Q, Sheng Z-H. Mitochondrial transport and docking in axons. Exp Neurol. 2009;218:257–267. doi: 10.1016/j.expneurol.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saotome M, et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macaskill AF, et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabadkai G, Duchen MR. Mitochondria: The hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 13.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 14.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 15.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandecasteele G, Szabadkai G, Rizzuto R. Mitochondrial calcium homeostasis: Mechanisms and molecules. IUBMB Life. 2001;52:213–219. doi: 10.1080/15216540152846028. [DOI] [PubMed] [Google Scholar]
- 18.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 19.Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci. 2008;28:8306–8315. doi: 10.1523/JNEUROSCI.2614-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 21.Souslova EA, et al. Single fluorescent protein-based Ca2+ sensors with increased dynamic range. BMC Biotechnol. 2007;7:37. doi: 10.1186/1472-6750-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csordás G, Hajnóczky G. Plasticity of mitochondrial calcium signaling. J Biol Chem. 2003;278:42273–42282. doi: 10.1074/jbc.M305248200. [DOI] [PubMed] [Google Scholar]
- 23.Vay L, et al. Modulation of Ca(2+) release and Ca(2+) oscillations in HeLa cells and fibroblasts by mitochondrial Ca(2+) uniporter stimulation. J Physiol. 2007;580:39–49. doi: 10.1113/jphysiol.2006.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jesús García-Rivas G, Guerrero-Hernández A, Guerrero-Serna G, Rodríguez-Zavala JS, Zazueta C. Inhibition of the mitochondrial calcium uniporter by the oxo-bridged dinuclear ruthenium amine complex (Ru360) prevents from irreversible injury in postischemic rat heart. FEBS J. 2005;272:3477–3488. doi: 10.1111/j.1742-4658.2005.04771.x. [DOI] [PubMed] [Google Scholar]
- 25.Montero M, Lobaton CD, Moreno A, Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- 26.Karahashi H, Nagata K, Ishii K, Amano F. A selective inhibitor of p38 MAP kinase, SB202190, induced apoptotic cell death of a lipopolysaccharide-treated macrophage-like cell line, J774.1. Biochim Biophys Acta. 2000;1502:207–223. doi: 10.1016/s0925-4439(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 27.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10230. 10.1038/nature 10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10234. 10.1038/nature 10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perocchi F, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fransson A, Ruusala A, Aspenström P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 31.Reis K, Fransson A, Aspenström P. The Miro GTPases: At the heart of the mitochondrial transport machinery. FEBS Lett. 2009;583:1391–1398. doi: 10.1016/j.febslet.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Fransson S, Ruusala A, Aspenström P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.