Abstract
Pierson syndrome is a congenital nephrotic syndrome with ocular and neurological defects caused by mutations in LAMB2, the gene encoding the basement membrane protein laminin β2 (Lamβ2). It is the kidney glomerular basement membrane (GBM) that is defective in Pierson syndrome, as Lamβ2 is a component of laminin-521 (LM-521; α5β2γ1), the major laminin in the mature GBM. In both Pierson syndrome and the Lamb2−/− mouse model for this disease, laminin β1 (Lamβ1), a structurally similar homolog of Lamβ2, is marginally increased in the GBM, but it fails to fully compensate for the loss of Lamβ2, leading to the filtration barrier defects and nephrotic syndrome. Here we generated several lines of Lamβ1 transgenic mice and used them to show that podocyte-specific Lamβ1 expression in Lamb2−/− mice abrogates the development of nephrotic syndrome, correlating with a greatly extended lifespan. In addition, the more Lamβ1 was expressed, the less urinary albumin was excreted. Transgenic Lamβ1 expression increased the level of Lamα5 in the GBM of rescued mice, consistent with the desired increased deposition of laminin-511 (α5β1γ1) trimers. Ultrastructural analysis revealed occasional knob-like subepithelial GBM thickening but intact podocyte foot processes in aged rescued mice. These results suggest the possibility that up-regulation of LAMB1 in podocytes, should it become achievable, would likely lessen the severity of nephrotic syndrome in patients carrying LAMB2 mutations.
Keywords: kidney filtration barrier, albuminuria, kidney disease
Pierson syndrome describes a congenital nephrotic syndrome accompanied by ocular and neurological defects. It is caused by autosomal recessive mutations in LAMB2, the gene encoding the basement membrane protein laminin β2 (Lamβ2) (1, 2). The onset and severity of Pierson syndrome varies, depending on the degree of mutant Lamβ2 function and/or expression (3). The majority of patients with Pierson syndrome are diagnosed with nephrotic-range proteinuria, rapidly develop end-stage renal disease, and die from renal failure as early as 2 wk of age (4).
The kidney glomerular basement membrane (GBM), which consists primarily of laminin-521 (LM-521; α5β2γ1), the type IV collagen α3α4α5 network, nidogen, and the heparan sulfate proteoglycan agrin (5), is one of the major sites affected in Pierson syndrome. The GBM is a sheet-like extracellular matrix meshwork separating two cellular layers, endothelial cells and podocytes. Together, these three layers form the glomerular filtration barrier, which prevents valuable plasma proteins in the blood from leaking into the urine while allowing the efficient flow of water and small molecules. A defect in or injury to any of these three layers can cause albuminuria, demonstrating the importance of all three for maintaining the filtration barrier (6, 7).
Laminin is a cruciform heterotrimer composed of α, β, and γ chains that self-polymerizes into a supramolecular network in the extracellular matrix (8). During glomerulogenesis, laminin trimers are secreted from both podocytes and endothelial cells and deposited into the GBM (9). The laminin composition changes during GBM formation and maturation, from LM-111 (α1β1γ1) at early glomerular stages to LM-521 in the mature GBM; LM-511 (α5β1γ1) is detected transiently during maturation (10). Because LM-521 is the only trimer found in the adult GBM, LAMB2 mutation, as in Pierson syndrome, disrupts the GBM's laminin network. Lamb2−/− mice, which model Pierson syndrome, show congenital albuminuria followed by podocyte foot process effacement; they die at about 3 wk of age with severe neuromuscular defects and nephrotic syndrome (11, 12). Interestingly, Lamb2−/− mice show abnormal accumulation of ectopic laminins such as LM-511, -332, -211, and -111 in the mature GBM; these presumably are expressed in attempt to compensate for the loss of LM-521 (13). Given that basement membranes cannot form without laminin (14), it is likely that the presence of these ectopic laminins allows the GBM's integrity to be maintained.
Transgenic restoration of Lamβ2 specifically at the neuromuscular junction in Lamb2−/− mice rescues the neuromuscular defects, but the mice still succumb to nephrotic syndrome (15), indicating that the ectopic laminin deposition into the GBM is not sufficient to compensate. We therefore concluded that Lamβ2 is functionally unique in the GBM and required for a normal filtration barrier (11, 15). However, an alternative hypothesis is that there is simply an insufficient quantity of laminin in the GBM of Lamb2 null mice, and that a laminin trimer other than LM-521 might function normally in the GBM if its expression level were high enough. LM-511 is the best candidate laminin trimer to functionally compensate for LM-521, because LM-511 shares α and γ chains with LM-521, and the β1 and β2 chains are structurally similar (Fig. 1A, adopted and redrawn from ref. 16). Although LM-511 is increased in the mature GBM of Lamb2 null mice, perhaps the level is simply not sufficient. Furthermore, because the C-terminal LG domain of Lamα5 interacts with integrin α3β1 and other receptors on podocytes and glomerular mesangial cells (17–19), having Lamα5 in a compensating laminin trimer should be beneficial in terms of signals provided to the neighboring cells.
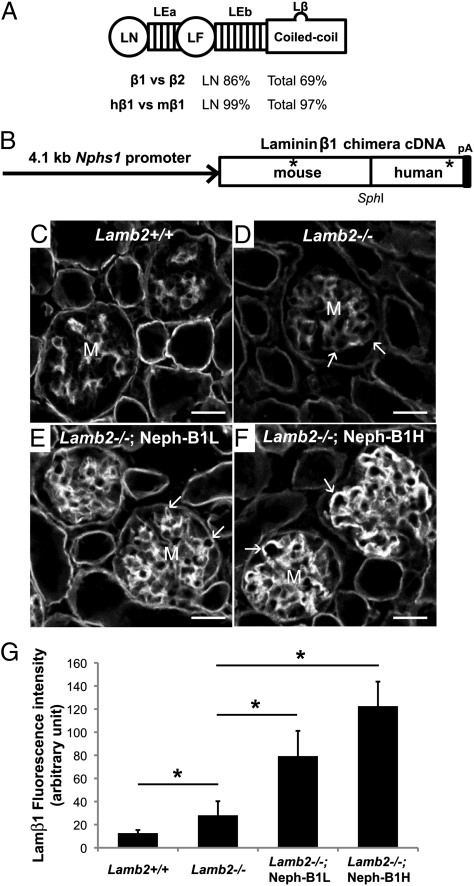
Fig. 1.
Generation and characterization of transgenic mice expressing a chimeric Lamβ1 chain. (A) Shared structure of Lamβ1 and -β2, and percentage of similarities between mouse Lamβ1 and -β2 and between human Lamβ1 (hβ1) and mouse Lamβ1 (mβ1). LN, laminin NH2-terminal domain; LE, laminin EGF-like domain; LF, laminin four domain; Lβ, laminin β-knob. (B) Schematic diagram of the Neph-B1 transgene. The 4.1-kb nephrin (Nphs1) promoter drives expression of the mouse/human chimeric Lamβ1 cDNA with a SV40 polyadenylylation signal sequence (pA). Asterisks indicate the epitopes that the antimouse and antihuman antibodies recognize, respectively. (C–F) Mouse laminin β1 confocal immunofluorescence micrographs. The antibody used recognizes both endogenous Lamβ1 and transgenic Lamβ1. Although wild-type GBM lacks deposition of Lamβ1 and Lamb2−/− GBM shows only a low level of Lamβ1, transgenic mice show linear and higher level deposition of Lamβ1. Arrows indicate GBM, and M indicates mesangial matrix. (G) Quantification of Lamβ1 fluorescence intensity in the GBM. *P < 0.001. (Scale bars, 20 μm.)
Here we used Lamb2−/− mice as a model of Pierson syndrome and tried to ameliorate the nephrotic syndrome by forced transgenic expression of Lamβ1 in podocytes. This led to quantitative replacement of the missing LM-521 network with LM-511. These mice exhibited significantly reduced albuminuria and dramatically increased lifespan. This study shows that Lamβ1 can effectively substitute for Lamβ2 in the GBM when provided quantitatively and suggests a potential therapeutic approach for ameliorating the glomerular filtration defect in Pierson syndrome.
Results
Because Lamb2−/− mice show severe growth retardation due to neuromuscular defects, all mutant mice used in the experiments described herein contained the muscle creatine kinase promoter-driven Lamβ2 (MCK-B2) transgene. The expression of MCK-B2 rescues the neuromuscular junction defects and growth retardation in Lamb2−/− mice without affecting the kidney (15).
Increased Lamβ1 in the GBM of a Pierson Patient.
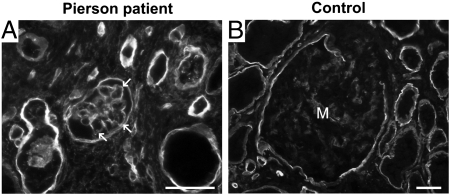
We used a renal biopsy from a 3-mo-old Pierson patient lacking Lamβ2 (20, 21) to determine whether the loss of Lamβ2 in humans leads to the increased Lamβ1 observed in the GBM of Lamb2−/− mice (11). Indeed, Lamβ1, which is in the mesangial matrix of normal glomeruli, was detected in the GBM of the patient (Fig. 2). This suggests a similar imperfect compensatory response to the lack of Lamβ2 in both human and mouse glomeruli.
Fig. 2.
Increased Lamβ1 in the GBM in human Pierson syndrome. Immunofluorescence analysis of Lamβ1 in human kidney sections. A 3-mo-old Pierson syndrome patient's specimen (A) shows linear staining for Lamβ1 in the GBM (arrows), whereas a normal adult control (B) shows weak mesangial staining and the absence of staining in the GBM. (Scale bars, 50 μm.)
Generation of Transgenic Mice That Express Lamβ1 in Podocytes.
To test the hypothesis that albuminuria in Lamb2−/− mice stems from a shortage of laminin in the GBM rather than due to the lack of Lamβ2/LM-521 per se, we decided to force overexpression of Lamβ1 in podocytes so that Lamβ1-containing trimers would be secreted into the GBM and contribute to laminin network formation. Because podocytes normally synthesize laminin α5 and γ1, the Lamβ1 should assemble with them to form LM-511 trimers. A mouse/human chimeric Lamb1 cDNA was designed to be expressed in podocytes under the control of the nephrin promoter (22) (Neph-B1 transgene; Fig. 1B). We generated several different lines of Neph-B1 transgenic mice, and they expressed the transgene and incorporated Lamβ1 in the GBM at various levels (Fig. S1). Based on quantitative confocal immunofluorescence analysis, the transgenic lines were divided into two groups: Neph-B1L (low expressors) and Neph-B1H (high expressors).
Lamβ1 is not found in the GBM of wild-type mice, but it can be detected in the GBM of Lamb2−/− mice (11) (Fig. 1 C and D). When the Neph-B1 transgenes were introduced onto the Lamb2−/− background, a higher level of linear Lamβ1 deposition in the GBM was observed (Fig. 1 E and F). Quantitative analyses showed that expression of transgene-derived Lamβ1 increased total Lamβ1 in the mutant GBM by two- to fourfold compared with the total Lamβ1 observed in the nontransgenic Lamb2−/− GBM (Fig. 1G).
Increased Lamβ1 in the GBM Is Sufficient for Long-Term Survival and Maintenance of Glomerular Filtration Barrier Function.
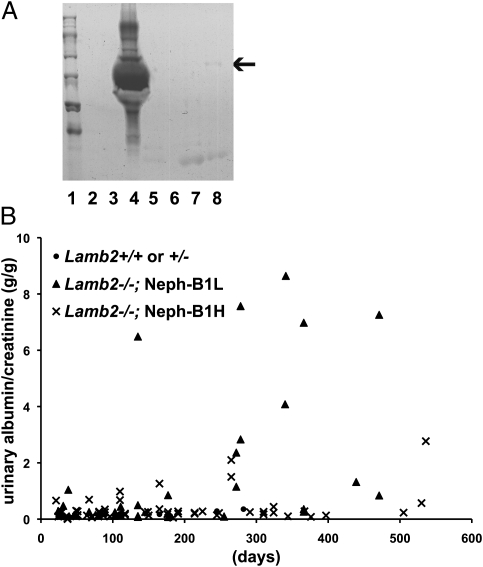
To investigate how the increased Lamβ1 in the GBM affected the glomerular filtration barrier, mouse urine was assayed by SDS/PAGE and Coomassie staining. Compared with the heavy, nephrotic-range albuminuria in Lamb2−/− urine (Fig. 3A, lane 4), albumin was not detected in the urine of 3-wk-old Lamb2−/−; Neph-B1 mice (Fig. 3A, lane 3), and only very little albumin was detected in the urine of 1-y-old Lamb2−/−; Neph-B1 mice (Fig. 3A, lane 8). By calculating urinary albumin-to-creatinine ratios (ACRs) in the different lines at different ages (Fig. 3B), we found that the more Lamβ1 was expressed, the less albuminuria was observed. Fig. 3B also shows the significantly increased lifespan in both low- and high-expressing Lamb2−/−; Neph-B1 mice, which survived longer than 1 y, compared with nontransgenic Lamb2−/− mice, which survive for ∼1 mo.
Fig. 3.
Forced Lamβ1 expression in podocytes of Lamb2−/− mice prevents nephrotic syndrome. (A) Reduced albuminuria in Lamb2−/−; Neph-B1 mice. SDS/PAGE/Coomassie blue analysis of 1 μL of urine from mice of the following genotypes: lane 3, Lamb2−/−; Neph-B1H; lane 4, Lamb2−/−; lane 5, Lamb2+/−; Neph-B1H (lanes 3–5 were from 3-wk-old mice); lane 7, Lamb2+/−; Neph-B1L; lane 8, Lamb2−/−; Neph-B1L (lanes 7 and 8 were from 10-mo-old mice). Lane 1, markers. Arrow indicates the size of albumin. (B) Graph showing albumin-to-creatinine ratios (g/g) in urine of Lamb2−/−; Neph-B1 and control mice. Lamb2−/−; Neph-B1 mice survived more than 1 y and had greatly reduced albuminuria compared with Lamb2−/− mice, which survive 1 mo with ∼100 g urinary albumin/g creatinine. Note that the low Lamβ1-expressing mice (Neph-B1L) showed heavier albuminuria than the high expressing mice (Neph-B1H).
Normal Renal Architecture and Absence of Podocyte Injury in Lamb2−/−; Neph-B1 Mice.
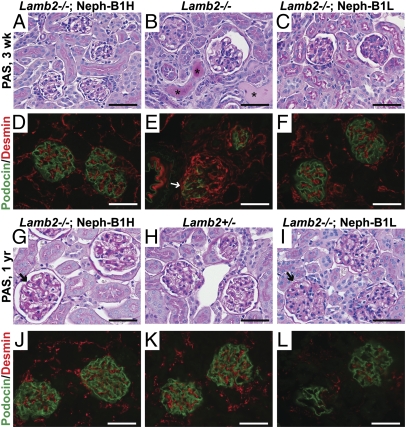
Lamb2−/− mice at 3 wk of age show focal mesangial sclerosis and dilated tubules with protein casts, which is indicative of proteinuria (Fig. 4B). However, neither Lamb2−/−; Neph-B1 mice nor heterozygous controls showed these features (Fig. 4 A and C and Fig. S2), which is consistent with the absence of albuminuria. As Lamb2−/−; Neph-B1 mice became older, PAS staining of glomeruli revealed mild mesangial expansion and segmental thickening of the GBM (Fig. 4 G and I). Notably, the appearance of mesangial expansion and focal thickening of the GBM was correlated with the development of mild, nonnephrotic-range albuminuria by 1 y of age (Fig. 3 A and B). Proximal tubules retained a normal brush border, and the occurrence of sclerotic glomeruli was low in Lamb2−/−; Neph-B1 mice (Fig. 4 G and I).
Fig. 4.
Histological analysis reveals maintenance of podocyte phenotype in Lamb2−/−; Neph-B1 kidneys. (A–C and G–I) PAS staining of kidneys from 3-wk-old (A–C) and 1 y-old (G–I) mice. Asterisks in B indicate protein casts in tubules of a nephrotic Lamb2−/− mouse, which were not observed in Lamb2−/−; Neph-B1 mice (A and C). Arrows in G and I indicate thickening of the GBM at 1 y in Lamb2−/−; Neph-B1 mice. (D–F and J–L) Immunofluorescence analysis of podocin (green) and desmin (red) in kidney sections from 3-wk-old (D–F) and 1-y-old (J–L) mice. Arrow in E indicates an injured podocyte expressing both podocin and desmin; desmin is confined to mesangial cells in normal glomeruli, as observed in the Lamb2−/−; Neph-B1 and Lamb2+/− panels. (Scale bars, 50 μm.)
To examine whether forced expression of Lamβ1 impacted podocyte phenotype and/or protected podocytes from damage in Lamb2−/− mice, we stained kidneys with antibodies to desmin, a known marker of podocyte injury (23) normally restricted to mesangial cells, and to podocin, a crucial slit diaphragm-associated protein whose localization and levels can change in injured podocytes with effaced foot processes. First, Lamβ1 expression itself did not alter podocyte phenotype on the Lamb2+/− background, demonstrated by the absence of desmin in podocytes (Fig. S2). In 3-wk-old Lamb2−/− mice, which have widespread foot process effacement (11, 13), there were glomerular tuft segments without linear podocin staining, together with increased desmin staining on the outer aspects of the tuft, presumably where the podocyte cell bodies are located (Fig. 4E). In contrast, rescued Lamb2−/−; Neph-B1 mice did not usually show alterations in either podocin or desmin at 3 wk of age, or even at 1 y of age (Fig. 4 D and F and J–L).
Restoration of Lamα5 Levels in the GBM by Transgenic Lamβ1 Expression in Lamb2−/− Mice.
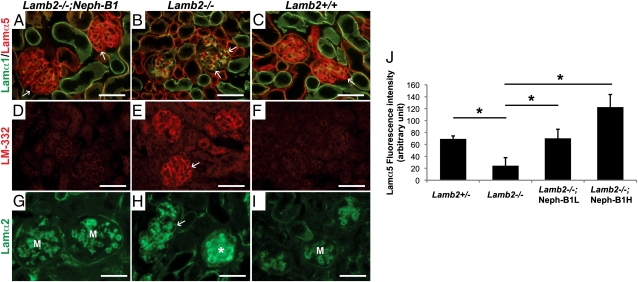
To investigate how the overall laminin composition of the GBM was changed by the forced expression of Lamβ1 in the absence of Lamβ2, we assayed the expression and localization of different laminin chains in 3-wk-old mouse kidneys. Lamα5 was of particular interest, as Lamα5 bears a major podocyte and mesangial cell integrin ligand and appeared low in the GBM of Lamb2−/− mice (13) (Fig. 5B). The increased podocyte expression of Lamβ1 in Lamb2−/− mice restored the level of Lamα5 in the GBM to near wild type (Fig. 5 A, C, and J and Fig. S3). With the restoration of Lamα5, ectopic expression of Lamα1 was not detected in Lamb2−/−; Neph-B1 mice at 3 wk of age (Fig. 5A), indicating that overexpression of Lamβ1 did not drive the formation of LM-111, but rather LM-511. Formation of LM-511 was also supported by the observation that the Lamb2−/−; Neph-B1H mice showed a higher Lamα5 level than the Lamb2−/−; Neph-B1L mice did (Fig. S3). LM-332 was not deposited in the GBM of rescued mice, although it was present in Lamb2−/− GBM (Fig. 5 D–F).
Fig. 5.
Normalization of laminin composition in the GBM of Lamb2−/−; Neph-B1 mice. Immunofluorescence analysis of deposition of (A–C) Lamα1 (green) and Lamα5 (red); (D–F) laminin-332; and (G–I) Lamα2 in the GBMs of 3-wk-old mice. Ectopic deposition of Lamα1, LM-332, and Lamα2 in the Lamb2−/− GBM (B, E, and H) did not occur in the Lamb2−/−; Neph-B1 GBM (A, D, and G). Arrows indicate GBM; Lamα2 is normally present in the mesangium (M) (G and I). Asterisk in H indicates a sclerotic glomerulus. (J) Lamα5 fluorescence intensity in the GBM was quantified using images in Fig. S3. Transgenic Lamβ1 expression increased Lamα5 in the GBM to a level comparable to that of the control. (J) *P < 0.001. (Scale bars, 50 μm.)
The appearance of Lamα1 and Lamα2 in the GBM has been observed in Alport (Col4a3−/−) mice (24–26) and in other models of nephropathy. It is unclear whether ectopic accumulation of laminins in the GBM causes renal malfunction or not. Lamb2−/−; Neph-B1 mice did not show Lamα1 or Lamα2 deposition in the GBM at 3 wk. However, at 1 yr of age some Lamb2−/−; Neph-B1 mice did show focal segmental deposition of Lamα1 and Lamα2 in the GBM (Fig. 5 G–I and Fig. S4), but without albuminuria or kidney pathology, implying that Lamα1 and Lamα2 are not necessarily pathogenic. When collagen α2(IV) and collagen α4(IV) were stained to show mesangial matrix and GBM, respectively, transgenic expression of Lamβ1 was not found to alter the deposition of collagen IV chains (Fig. S5).
Intact Podocyte Foot Processes and Occasional Knob-Like Subepithelial GBM Thickening in Lamb2−/−; Neph-B1 Mice.
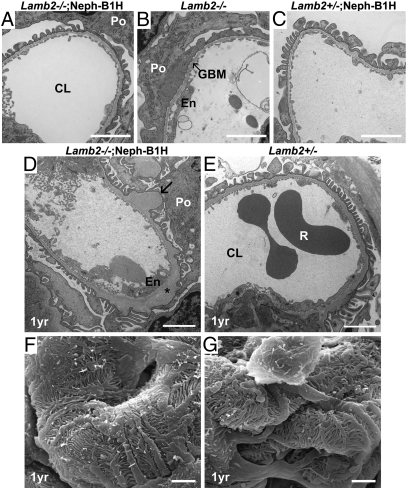
Because rescued null mice did not show major abnormalities other than the appearance of ectopic laminins, we examined the ultrastructure of glomeruli to determine how the increased Lamβ1 in the GBM affected the maintenance of podocyte foot processes and slit diaphragms. Consistent with data presented above, at 3 wk of age there were no noticeable differences between rescued mice and wild-type mice, whereas Lamb2−/− mice showed severe foot process effacement (Fig. 6 A–C). At 1 y of age, the GBM of rescued mice showed occasional subepithelial thickening and electron-lucent, “moth-eaten” areas (Fig. 6 D and E). This thickening was observed in a subset of capillary loops in most glomeruli. These moth-eaten GBM lesions were found only in the rescued mutants, and the Lamb2−/−; Neph-B1L mice showed the more severe defects (Fig. 6D and Fig. S6B). (Interestingly, the podocyte foot processes in Lamb2−/−; Neph-B1 mice were as intact as those in Lamb2+/− mice, even those juxtaposed to the GBM segments showing the moth-eaten pattern.) We confirmed these findings using scanning electron microscopy (Fig. 6 F and G, and Fig. S6 C and D). These results show that transgenic Lamβ1 expression prevents podocyte foot process effacement in Lamb2−/− mice.
Fig. 6.
Ultrastructural analysis of glomerular capillary walls. (A–E) Transmission electron micrographs of glomerular capillary loops from 3-wk-old (A–C) and from 1-y-old (D and E) mice. Note that the severe podocyte foot process effacement observed in Lamb2−/− mice (B) was not observed in young or old Lamb2−/−; Neph-B1 mice (A and D). Arrow in D indicates segmental thickening of the GBM. Asterisk indicates electron lucent areas in the expanded lamina densa. (F and G) Scanning electron micrographs of glomeruli from 1-y-old mice. Podocyte foot processes in Lamb2−/−; Neph-B1 mice were intact. (Scale bar, 2 μm.) Po, podocyte; CL, capillary lumen; En, endothelial cell; R, red blood cell.
Discussion
The importance of laminin for the GBM has been underscored by the discovery of human LAMB2 mutations that cause congenital nephrotic syndrome in Pierson syndrome, together with similar features of Lamb2−/− mice (2, 11). Here we designed experiments to attempt to rescue the fatal renal failure in Lamb2 null mice by increasing the level of Lamβ1 and LM-511 in the mutant GBM. Our data show that transgenic Lamβ1 expression in podocytes is sufficient to rescue the albuminuria and early lethality of Lamb2 null mice. Transgenic Lamβ1 compensates for the loss of Lamβ2 by assembling with Lamα5 and Lamγ1 to generate levels of secreted LM-511 that are sufficient to reconstitute the otherwise defective GBM.
Previously we showed that Lamb2 null mice develop albuminuria at birth and exhibit podocyte foot process effacement by 2 wk (11). Although Lamβ1 is increased in the GBM of Lamb2 null mice, the level of Lamβ1 is apparently insufficient for the establishment of a fully functional barrier to albumin (11). Similarly, here we showed that Lamβ1 is increased in the GBM of a Pierson syndrome patient, which was also insufficient for a normal filtration barrier. According to our recent studies of a pathogenic LAMB2 missense mutation (R246Q) that inhibits laminin-521 secretion, Lamβ1 is also increased in the GBM of mice expressing the R246Q mutant, but apparently not at levels sufficient to prevent proteinuria (27).
The main cause of albuminuria in Lamb2−/− mice is the imperfect GBM, as albuminuria initially occurs without the loss of foot processes and slit diaphragms (13); this emphasizes the GBM's crucial role in the glomerular filtration barrier. Here we demonstrated that albuminuria could be prevented or significantly delayed (and at a much reduced level) in Lamb2−/− mice by the incorporation of transgenic Lamβ1-containing trimers into the GBM's laminin network. We believe that this beneficial role of Lamβ1 in the GBM is achieved in two ways: (i) increased supply of a laminin β chain and (ii) the resulting quantitative recovery of Lamα5-containing trimers. Because the level of transgenic Lamβ1 is much higher than the level of endogenous Lamβ1 in the Lamb2−/− GBM, this increased supply of Lamβ1 provides for a tighter glomerular filtration barrier. Moreover, linear Lamα5 deposition in the GBM was restored to near normal levels by the transgenic Lamβ1, indicating that Lamβ1 is secreted from podocytes mostly as part of LM-511 rather than LM-111, because Lamα1 was not detected in the GBM at early ages and was only rarely observed in a spotty pattern in the GBMs of aged rescued mutant mice. As Lamα5 is the major laminin α chain found in the adult GBM (28) and is essential for GBM maturation and maintenance (29, 30), the increased Lamα5 imparts the laminin network with a structure that is similar to the normal GBM laminin network (LM-521), while also providing ligand for the integrin α3β1 receptor on the adjacent podocytes (31, 32).
Lamb2−/− mice develop heavy albuminuria with eventual foot process effacement, but it is unclear what causes the effacement. As the GBM provides ligands (such as laminin-521) for receptors on podocytes (such as integrin α3β1), the absence of proper signals from the extracellular matrix might lead to the podocyte abnormalities, although these signals seem not to be necessary for initial podocyte maturation. Alternatively, heavy albumin in the urinary or subpodocyte space could be injurious to podocytes (33). Here, the compensating LM-511 network presumably both sends proper extracellular matrix signals to podocytes and allows the GBM to be an effective filtration barrier capable of shielding podocytes from high concentrations of plasma proteins. Moreover, because laminin, collagen IV, and other ECM components are interconnected within the GBM, a change in one component sometimes affects the others (34). For example, deletion of collagen α3(IV) results in expression of ectopic laminin chains (24–26), but Lamb2 null mice, with or without a rescuing transgene, show normal expression of collagen IV chains in glomeruli (Fig. S5 and ref. 11).
Our study can, to some extent, answer the question as to whether it is the quality or the quantity of laminin that is important for the glomerular filtration barrier. First, different transgene expression levels in the different transgenic lines showed that the quantity of Lamβ1 chain and LM-511 trimer impacted the function of the GBM: the more Lamβ1 there was in the GBM, the less albuminuria was observed. (This is consistent with our R246Q-mutant Lamβ2 study, in which the amount of laminin β2 correlated inversely with the level of proteinuria) (27). Interestingly, expression of even the lowest level of transgenic Lamβ1 in Lamb2 null mice was beneficial enough to delay, if not prevent, nephrotic syndrome and extend the lifespan of Lamb2−/− mice, although mild albuminuria was evident. Second, we conclude that Lamβ2 must have a unique qualitative function that Lamβ1 lacks, on the basis of the following observation: the level of LM-511 in Lamb2−/−; Neph-B1 mice was as high as or higher than the level of LM-521 in wild type, as judged by quantitative Lamα5 fluorescence analysis; however, the GBM of Lamb2−/−; Neph-B1 mice was not completely normal, as it showed occasional knob-like subepithelial thickening associated with mild albuminuria by 1 y of age. The uniqueness of Lamβ2 might be conferred in part by the COOH-terminal 20 amino acids, which modulate the binding affinity of laminin α5-containing trimers to the integrin α3β1 receptor (35).
Analogous to our study, Gawlik and colleagues (36–38) reported that transgenic overexpression of Lamα1 rescues both the congenital muscular dystrophy and the peripheral neuropathy caused by the lack of Lamα2, despite the fact that endogenous Lamα1 is not normally expressed in skeletal muscle or peripheral nerve (39). Interestingly, on the basis of a comprehensive phylogenetic analysis of laminin chains, the homologies between Lamα1 and Lamα2 and between Lamβ1 and Lamβ2 were found to be the highest within the laminin α and β chain subfamilies, respectively (40). Lamα1 and Lamα2 show 65% similarity across the full-length proteins but 92% similarity between their laminin NH2-terminal (LN) domains, which are crucial for trimer–trimer interactions and laminin polymerization (41). Lamβ1 and Lamβ2 show 69% similarity in total and 86% similarity between their LN domains. These significant similarities, especially between the LN domains, are consistent with the notion that these laminin pairs might be at least partially functionally redundant under some circumstances, as our data and that of Gawlik et al. (36–38) suggest. Because Lamβ1 is naturally found in Pierson GBM and expressed by podocytes during development, it may be more feasible to increase its production compared with activating production of Lamα1 de novo in congenital muscular dystrophy. Lamb1 has been shown to be up-regulated by retinoic acid in the F9 teratocarcinoma cell line (42), and its promoter contains a retinoic acid-responsive element (43). It remains to be determined whether Lamb1 could be up-regulated by retinoic acid treatment of podocytes or glomerular endothelial cells. Intravenous infusion of human LM-511 is also a potential therapy, as it will not be rejected by the host immune system but should improve glomerular permselectivity with successful incorporation into the GBM. Interestingly, intramuscular injection of laminin-111 protein into mdx mice, a model of Duchenne muscular dystrophy, was shown to ameliorate muscular dystrophy by increasing integrin α7 (44), although, paradoxically, transgenic expression of Lamα1 in mdx muscle fails to improve dystrophic features (45).
Taken together, our results suggest a potential therapy for congenital nephrotic syndrome due to LAMB2 mutation. Increased expression of LAMB1 in podocytes (or perhaps glomerular endothelial cells) and the resulting increased deposition of LM-511 into the GBM should be beneficial for reducing albuminuria and significantly increasing the time to end-stage renal disease.
Materials and Methods
Human Tissues.
We sectioned a frozen kidney fragment from a previously described patient (patient 3 in ref. 21) who had a therapeutic unilateral nephrectomy at 2.5 mo of age. Informed consent and parental permission were obtained. The normal adult kidney was obtained from the Washington University Center for Kidney Disease Research Center, Kidney Translational Research Core. This study was approved by the appropriate institutional review boards.
Animals.
Lamb2 mutant mice and muscle creatine kinase promoter-driven rat laminin β2 (MCK-B2) transgenic mice were previously described (12, 15). The Neph-B1 transgene was engineered from mouse and human LAMB1 cDNAs and the previously described mouse 4.1-kb nephrin promoter (22). Details of the construct are described in SI Materials and Methods. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Antibodies and Histology.
Immunofluorescence analysis was performed with 8-μm frozen sections of unfixed kidney as previously described (15). Antibodies used for immunostaining are described in SI Materials and Methods. Light and electron microscopic analyses were performed as described (13).
Urinalysis.
Urine was collected from mice and assayed by mouse albumin ELISA (Bethyl Laboratories). Albumin–creatinine ratios were obtained by urinalysis with a Cobas Mira Plus analyzer (Roche). Urinary albumin was visualized on gels as described (15).
Confocal Microscopy and Image Analysis.
Immunostained slides were viewed on a Nikon d-eclipse C1plus confocal microscope (Nikon), and images were obtained as described (46). The intensities of the brightest three spots of stained GBM from multiple confocal images were measured using the ImageJ program (National Institutes of Health). Two-tailed, unpaired, and unequal-variance Student's t tests were used for the determination of statistical significance in measurements.
Supplementary Material
Acknowledgments
We thank Gloriosa Go, Jeanette Cunningham, Jennifer Richardson, the Pulmonary Morphology Core, and the Mouse Genetics Core for assistance and Drs. Susan Quaggin, Corinne Antignac, Dale Abrahamson, Takako Sasaki, and Peter Marinkovich for gifts of antibodies and plasmids. This work was supported by National Institutes of Health (NIH) Grants R01DK078314 (with American Recovery and Reinvestment Act of 2009 supplement 3R01DK078314-02S1) and R01GM060432 (to J.H.M.) and by the Washington University Center for Kidney Disease Research (NIH P30DK079333). Scanning electron microscopy was performed in the Washington University Nano Research Facility, a member of the Nanotechnology Infrastructure Network supported by the National Science Foundation (ECS-0335765).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108269108/-/DCSupplemental.
References
- 1.Pierson M, Cordier J, Hervouuet F, Rauber G. An unusual congenital and familial congenital malformative combination involving the eye and kidney. J Genet Hum. 1963;12:184–213. [PubMed] [Google Scholar]
- 2.Zenker M, et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 3.Matejas V, et al. Mutations in the human laminin β2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenker M, Pierson M, Jonveaux P, Reis A. Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am J Med Genet A. 2005;138:73–74. doi: 10.1002/ajmg.a.30894. [DOI] [PubMed] [Google Scholar]
- 5.Miner JH. Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol. 2011;26:1413–1417. doi: 10.1007/s00467-011-1785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarad G, Miner JH. Update on the glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:226–232. doi: 10.1097/mnh.0b013e3283296044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miner JH. Organogenesis of the kidney glomerulus: Focus on the glomerular basement membrane. Organogenesis. 2011;7:75–82. doi: 10.4161/org.7.2.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71:349–356. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- 9.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 10.Miner JH. Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens. 1998;7:13–19. doi: 10.1097/00041552-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Noakes PG, et al. The renal glomerulus of mice lacking s-laminin/laminin β 2: Nephrosis despite molecular compensation by laminin β 1. Nat Genet. 1995;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- 12.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β 2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 13.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyth N, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miner JH, Go G, Cunningham J, Patton BL, Jarad G. Transgenic isolation of skeletal muscle and kidney defects in laminin β2 mutant mice: Implications for Pierson syndrome. Development. 2006;133:967–975. doi: 10.1242/dev.02270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- 17.Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin α5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–196. doi: 10.1083/jcb.200211121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin α3 β1. J Biol Chem. 1998;273:15854–15859. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- 19.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: Laminin-10/11 are recognized by α 3 β 1, α 6 β 1 and α 6 β 4 integrins. J Cell Sci. 2000;113:869–876. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 20.VanDeVoorde R, Witte D, Kogan J, Goebel J. Pierson syndrome: A novel cause of congenital nephrotic syndrome. Pediatrics. 2006;118:e501–e505. doi: 10.1542/peds.2005-3154. [DOI] [PubMed] [Google Scholar]
- 21.Wühl E, et al. Neurodevelopmental deficits in Pierson (microcoria-congenital nephrosis) syndrome. Am J Med Genet A. 2007;143:311–319. doi: 10.1002/ajmg.a.31564. [DOI] [PubMed] [Google Scholar]
- 22.Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE. Glomerular-specific gene excision in vivo. J Am Soc Nephrol. 2002;13:788–793. doi: 10.1681/ASN.V133788. [DOI] [PubMed] [Google Scholar]
- 23.Yaoita E, Kawasaki K, Yamamoto T, Kihara I. Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol. 1990;136:899–908. [PMC free article] [PubMed] [Google Scholar]
- 24.Abrahamson DR, Prettyman AC, Robert B, St John PL. Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int. 2003;63:826–834. doi: 10.1046/j.1523-1755.2003.00800.x. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove D, et al. Integrin α1β1 and transforming growth factor-β1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashtan CE, et al. Abnormal glomerular basement membrane laminins in murine, canine, and human Alport syndrome: Aberrant laminin α2 deposition is species independent. J Am Soc Nephrol. 2001;12:252–260. doi: 10.1681/ASN.V122252. [DOI] [PubMed] [Google Scholar]
- 27.Chen YM, Kikkawa Y, Miner JH. A missense LAMB2 mutation causes congenital nephrotic syndrome by impairing laminin secretion. J Am Soc Nephrol. 2011;22:849–858. doi: 10.1681/ASN.2010060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miner JH. Renal basement membrane components. Kidney Int. 1999;56:2016–2024. doi: 10.1046/j.1523-1755.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 29.Miner JH, Li C. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–289. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg S, Adair-Kirk TL, Senior RM, Miner JH. Maintenance of glomerular filtration barrier integrity requires laminin α5. J Am Soc Nephrol. 2010;21:579–586. doi: 10.1681/ASN.2009091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreidberg JA, Symons JM. Integrins in kidney development, function, and disease. Am J Physiol Renal Physiol. 2000;279:F233–F242. doi: 10.1152/ajprenal.2000.279.2.F233. [DOI] [PubMed] [Google Scholar]
- 32.Korhonen M, Ylänne J, Laitinen L, Virtanen I. The α 1-α 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990;111:1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida S, Nagase M, Shibata S, Fujita T. Podocyte injury induced by albumin overload in vivo and in vitro: Involvement of TGF-β and p38 MAPK. Nephron, Exp Nephrol. 2008;108:e57–e68. doi: 10.1159/000124236. [DOI] [PubMed] [Google Scholar]
- 34.Kanwar YS, Danesh FR, Chugh SS. Contribution of proteoglycans towards the integrated functions of renal glomerular capillaries: A historical perspective. Am J Pathol. 2007;171:9–13. doi: 10.2353/ajpath.2007.070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniguchi Y, et al. The C-terminal region of laminin β chains modulates the integrin binding affinities of laminins. J Biol Chem. 2009;284:7820–7831. doi: 10.1074/jbc.M809332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gawlik K, Miyagoe-Suzuki Y, Ekblom P, Takeda S, Durbeej M. Laminin α1 chain reduces muscular dystrophy in laminin α2 chain deficient mice. Hum Mol Genet. 2004;13:1775–1784. doi: 10.1093/hmg/ddh190. [DOI] [PubMed] [Google Scholar]
- 37.Gawlik KI, Durbeej M. Transgenic overexpression of laminin α1 chain in laminin α2 chain-deficient mice rescues the disease throughout the lifespan. Muscle Nerve. 2010;42:30–37. doi: 10.1002/mus.21616. [DOI] [PubMed] [Google Scholar]
- 38.Gawlik KI, Li JY, Petersén A, Durbeej M. Laminin α1 chain improves laminin α2 chain deficient peripheral neuropathy. Hum Mol Genet. 2006;15:2690–2700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- 39.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sztal T, Berger S, Currie PD, Hall TE. Characterization of the laminin gene family and evolution in zebrafish. Dev Dyn. 2011;240:422–431. doi: 10.1002/dvdy.22537. [DOI] [PubMed] [Google Scholar]
- 41.Colognato H, Yurchenco PD. Form and function: The laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 42.Wang SY, LaRosa GJ, Gudas LJ. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985;107:75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- 43.Vasios GW, Gold JD, Petkovich M, Chambon P, Gudas LJ. A retinoic acid-responsive element is present in the 5′ flanking region of the laminin B1 gene. Proc Natl Acad Sci USA. 1989;86:9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooney JE, Gurpur PB, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2009;106:7991–7996. doi: 10.1073/pnas.0811599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gawlik KI, Oliveira BM, Durbeej M. Transgenic expression of Laminin α1 chain does not prevent muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Am J Pathol. 2011;178:1728–1737. doi: 10.1016/j.ajpath.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abrahamson DR, St John PL, Isom K, Robert B, Miner JH. Partial rescue of glomerular laminin α5 mutations by wild-type endothelia produce hybrid glomeruli. J Am Soc Nephrol. 2007;18:2285–2293. doi: 10.1681/ASN.2007020207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.