Abstract
A major class of bacterial small, noncoding RNAs (sRNAs) acts by base-pairing with mRNAs to alter the translation from and/or stability of the transcript. Our laboratory has shown that Hfq, the chaperone that mediates the interaction of many sRNAs with their targets, is required for the virulence of the enteropathogen Yersinia pseudotuberculosis. This finding suggests that sRNAs play a critical role in the regulation of virulence in this pathogen, but these sRNAs are not known. Using a deep sequencing approach, we identified the global set of sRNAs expressed in vitro by Y. pseudotuberculosis. Sequencing of RNA libraries from bacteria grown at 26 °C and 37 °C resulted in the identification of 150 unannotated sRNAs. The majority of these sRNAs are Yersinia specific, without orthologs in either Escherichia coli or Salmonella typhimurium. Six sRNAs are Y. pseudotuberculosis specific and are absent from the genome of the closely related species Yersinia pestis. We found that the expression of many sRNAs conserved between Y. pseudotuberculosis and Y. pestis differs in both timing and dependence on Hfq, suggesting evolutionary changes in posttranscriptional regulation between these species. Deletion of multiple sRNAs in Y. pseudotuberculosis leads to attenuation of the pathogen in a mouse model of yersiniosis, as does the inactivation in Y. pestis of a conserved, Yersinia-specific sRNA in a mouse model of pneumonic plague. Finally, we determined the regulon controlled by one of these sRNAs, revealing potential virulence determinants in Y. pseudotuberculosis that are regulated in a posttranscriptional manner.
Keywords: Ysr29, RybB, stress, Illumina-Solexa, 2D-DIGE
Historically it was assumed that only proteins act as regulators of gene expression. There has been increasing recognition, however, that small, noncoding RNAs (sRNAs) serve as major components of diverse regulatory circuits in bacteria. sRNAs are RNA molecules that typically are encoded in intergenic regions, are independently transcribed, contain their own promoters and ρ-independent terminators, and range from 50–500 nt in length (1). Many were discovered initially by computational methods involving homology searches of closely related bacterial species and more recently have been identified by a variety of experimental approaches (i.e., genetic screens, deep sequencing, tiling microarrays, coimmunoprecipitation with proteins) (2). Most sRNAs control gene expression at the posttranscriptional level by base-pairing with target mRNAs, resulting in alterations in mRNA target translation or half-life (3, 4). The predominant outcome of the sRNA–target mRNA interaction is the down-regulation of gene expression [e.g., repression of outer membrane protein synthesis by the sRNAs MicA and RybB (5)], but positive regulation by sRNAs also has been described [e.g., regulation of the stationary phase Sigma factor RpoS by the sRNAs DsrA and RprA (6)]. In most cases, the RNA chaperone protein Hfq is required, presumably to stabilize the sRNA–mRNA interaction, because the sRNA contact on the target typically is short and imperfect (7).
Like protein regulators of gene expression, sRNAs act to integrate extracellular signals that aid bacteria in adjusting to their environment. This regulation includes the adaptation of pathogenic bacteria to the host and the coordination of expression of virulence determinants. sRNAs can regulate the expression of virulence genes directly [e.g., RNAIII regulation of staphylococcal protein A and the α-toxin gene mRNAs in Staphylococcus aureus (8, 9)] or control global regulators [e.g., quorum regulatory sRNAs regulate the hemagglutinin/protease regulator hapR mRNA in Vibrio cholerae (10)]. The end result is the fine-tuning of metabolic requirements of pathogenic bacteria to endure the stress imposed by the host and the synthesis of virulence factors.
There are three pathogenic Yersinia species that cause disease in humans: Y. pestis, Y. pseudotuberculosis and Y. enterocolitica. Y. pestis is thought to have evolved from Y. pseudotuberculosis ∼1,500–20,000 y ago. Y. enterocolitica is more distantly related, having diverged from a common ancestral Yersinia species 41–186 million y ago (11, 12). Y. pestis is the causative agent of the disease plague. The bubonic form of the disease occurs following the transmission of Y. pestis via fleabite and multiplication of bacteria in the lymph nodes; pneumonic plague, the most severe form of disease, occurs if the bacteria colonize and multiply in the lungs (13). Y. pestis can be spread from person to person by infectious respiratory droplets, and without early treatment, pneumonic plague is 100% fatal.
In contrast to Y. pestis, the closely related Y. pseudotuberculosis is a soil- and water-borne enteropathogen that primarily infects wild animals and birds. In humans it causes a mild, self-limiting gastrointestinal disease called “yersiniosis” and is transmitted by the fecal–oral route. Yersiniosis caused by Y. pseudotuberculosis is characterized by ileitis and mesenteric lymphadenitis, as well as fever and diarrhea (14). Although Y. pseudotuberculosis and Y. pestis are highly genetically related (they share >97% identity in 75% of their chromosomal genes), they differ radically in their pathogenesis and disease etiologies (15).
Despite these differences, recent evidence indicates that the sRNA chaperone Hfq is required for the full virulence of both Y. pestis (16) and Y. pseudotuberculosis (17) in mouse models of infection (s.c. or i.v. and intragastric, respectively) and suggests a role for sRNAs in the regulation of pathogenesis. The arsenal of sRNAs expressed by Y. pestis and Y. pseudotuberculosis that control gene expression has not been examined experimentally on a global level, however. Thus, we sought to gain a greater understanding of the roles that sRNAs play in manipulating the synthesis of virulence factors in these bacteria. To do so, we used a deep sequencing approach to perform a transcriptome-wide analysis to identify sRNAs encoded by the chromosome and plasmids of Y. pseudotuberculosis strain IP32953. We identified a total of 150 unannotated sRNAs, the majority of which are Yersinia specific. The deletion of multiple sRNAs led to the attenuation of Y. pseudotuberculosis in a mouse model of yersiniosis, suggesting that these sRNAs regulate factors involved in the pathogenesis of this species. In addition, we found that the deletion of one of these virulence-associated, Yersinia-specific sRNAs that is conserved between Y. pseudotuberculosis and Y. pestis resulted in the reduced virulence of the plague pathogen in an intranasal mouse model of infection. These results may have implications for the evolution of mechanisms that regulate virulence in Yersinia.
Results
Global Identification of sRNAs Expressed by Y. pseudotuberculosis.
Our goal was to identify sRNAs expressed by Y. pseudotuberculosis in an unbiased fashion. For this purpose we generated sRNA libraries of Y. pseudotuberculosis grown to early-log, mid-log, late-log, and stationary phase in broth culture at 26 °C and 37 °C and subjected these libraries to deep sequencing based on the Illumina-Solexa platform (18). This analysis resulted in ∼2.4–17 million 36-nt-long reads that were aligned exactly to the Y. pseudotuberculosis chromosome and plasmids. The mapped reads were categorized into mRNA, ribosomal RNA (rRNA), tRNA, tmRNA, and miscellaneous (misc.) RNAs, and the remainder of the reads was classified as intergenic RNA sequences. The distribution of mapped reads into categories at the two temperatures is shown in SI Appendix, Fig. S1. The miscellaneous RNAs category contains all previously annotated sRNAs in Y. pseudotuberculosis (the annotation of which was based on sequence homology with Escherichia coli and Salmonella typhimurium sRNAs), and our analysis was able simultaneously to confirm and map the changing expression patterns of these sRNAs over time (Fig. 1A). Spot 42 (Spf) is a previously annotated sRNA for which the numbers of deep sequencing reads correlated well with its expression as determined by Northern blot (Fig. 1B). Additionally, the sensitivity of Illumina-based deep sequencing in identifying sRNAs was validated, because low-abundance sRNAs such as SraG, which was represented by as few as 191 reads and could not be detected by Northern blot using as much as 10 μg of total RNA, are detected by this method. Notably, the major RNA species in the cell, rRNA, was reduced to ∼0.01–0.7% of total reads by rRNA depletion with MicrobExpress (Ambion). The proportion of tRNA reads in the total also is small (0.3–19%) compared with previous global sRNA identification efforts (19). The intergenic reads mapped to the chromosome of Y. pseudotuberculosis (29.6–43.3% of total reads) contain the 5′ and 3′ UTRs of transcribed mRNAs, cis-encoded antisense RNAs, and putative trans-encoded sRNAs. To identify these sRNAs specifically, the intergenic reads were grouped further into clusters at least 50 nt in length (e.g., this grouping yielded ∼3,200 clusters for the 6-h time-point at 37 °C). A filtering algorithm was developed to eliminate the majority of the 5′ and 3′ UTRs in the clustered intergenic groups by analyzing the expression level of clusters compared with the surrounding ORFs. The clusters whose expression levels differed from the expression levels of a neighboring ORF by less than threefold were removed from further analysis. The remaining intergenic clusters (e.g., 1,313 clusters for the 6-h time point at 37 °C) then were assessed using the Integrated Genome Browser (20). Computational prediction of putative promoter and ρ-independent terminator sequences, which are characteristic features of bacterial sRNAs (21), led to the identification of 150 putative unannotated sRNAs in Y. pseudotuberculosis. Analysis of the intergenic clusters did not reveal any potential sRNAs on the Y. pseudotuberculosis plasmid pYptb and identified only a single sRNA on the plasmid pYV.
Fig. 1.
Experimental identification of annotated sRNAs in Y. pseudotuberculosis. (A) All previously annotated Y. pseudotuberculosis sRNAs were identified by Illumina-Solexa sequencing. Percent of total reads for annotated RNAs identified at 37 °C shows a flux in sRNA levels over the course of bacterial growth. (B) The expression levels of the sRNA spf (Spot 42) as determined by deep sequencing reads (red bars in A) correlate with the levels of the sRNA as measured by Northern blot analysis.
Yersinia-Specific sRNAs.
The 150 clusters that satisfied the criteria for presumed sRNAs are referred to hereafter as “Ysrs” (for Yersinia small RNAs). The predicted genomic coordinates and sizes, orientation with respect to surrounding ORFs, and numbers of deep sequencing reads generated at the different temperatures and time points for all the Ysrs are listed in Dataset S1. For 32 Ysrs, BlastN analysis revealed orthologous sequences in the E. coli and S. typhimurium genomes; these sequences include the previously characterized sRNAs MicA (Ysr7), FnrS/Stnc520 (Ysr11), RprA (Ysr40), GcvB (Ysr45), RybB (Ysr48), MicM (Ysr145), RyhB (Ysr146.1 and Ysr146.2), GlmY (Ysr147), GlmZ (Ysr148), and OmrA/B (Ysr149) (3). Seventy-nine percent (118/150) of the Ysrs are specific to Y. pseudotuberculosis and Y. pestis in that they do not show sequence conservation with other bacterial species (Fig. 2, highlighted in red). Ninety-two Ysrs also are absent from other members of the Yersinia genus such as Y. ruckeri, Y. frederiksenii, and Y. intermedia and also from the closely related genus Photorhabdus (Dataset S1, sheet 2). We identified six Ysrs that are Y. pseudotuberculosis specific (Ysr29, Ysr53, Ysr70, Ysr84, Ysr94, Ysr118), having no homologous sequences in the genome of Y. pestis. In addition, 63/144 Ysrs encoded by both Y. pseudotuberculosis and Y. pestis contain single or multiple differences in sequence (i.e., mismatches, deletions, or insertions highlighted in yellow in Fig. 2).
Fig. 2.
Yersinia small RNAs (Ysrs). The sequences of sRNAs identified by deep sequencing in Y. pseudotuberculosis strain IP32953 were compared with the genomes of Y. pestis strain CO92, Y. enterocolitica 8081, E. coli strain K12 (substrain MG1655), and Salmonella enterica serovar Typhimurium strain LT2 by BlastN analysis. Ysrs present in the genome and identical in sequence are shown in green, and Ysrs present in the genome with sequence differences are shown in yellow. For an sRNA to be considered present in a genome, at least 50 nt of its sequence had to contain homology in the genome of interest. Ysrs absent from the genome are shown in red. Detailed information for each Ysr is given in Dataset S1.
At 26 °C, the majority of identified sRNAs are not expressed in earlier stages of growth (based on number of deep sequencing reads) and start to accumulate at later time points (SI Appendix, Fig. S2). The exceptions are Ysr8, Ysr32, and Ysr45/GcvB. Also, at this temperature, Ysr3 levels peak in late-log phase and then decrease in stationary phase. Although there are more exceptions to this trend at 37 °C than at 26 °C, a large proportion of sRNAs accumulate over the growth curve (SI Appendix, Fig. S2). The most notable exception at 37 °C is Ysr45/GcvB, the levels of which are highest at the start of growth and decrease over time. The most abundant sRNA at 26 °C is Ysr45/GcvB, whereas at 37 °C, the most sequencing reads were obtained for Ysr7/MicA and Ysr149/OmrA/B (SI Appendix, Fig. S2).
Verification of sRNA Expression.
Northern blot analysis was used to detect newly identified sRNAs in Y. pseudotuberculosis. We performed the analysis for 49 Ysrs and detected 29 of these RNAs using biotinylated oligonucleotide probes (Dataset S1). For most of the sRNAs detected, the band sizes correlated with the lengths predicted by the deep sequencing (i.e., Ysr4, Ysr7, and Ysr11). Some Ysrs, however, yielded bands corresponding to larger transcripts (i.e., Ysr1, Ysr2, and Ysr9). For nine of these Ysrs, we performed simultaneous 5′ and 3′ RACE to map the boundaries of primary transcripts and to distinguish them from the processing of longer transcripts. The genomic coordinates and lengths of these sRNAs are listed in Dataset S1, and for eight of the nine Ysrs, the coordinates are identical to or fall within 20 nt of the borders predicted by deep sequencing.
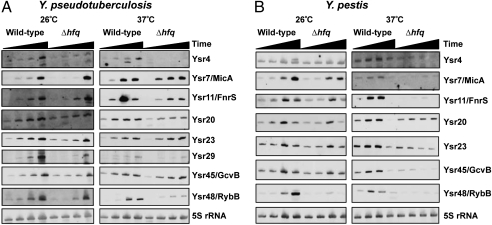
Because many genes in Yersiniae are under thermal regulation and are expressed at either 26 °C or 37 °C (22), it was of interest to examine the expression of Ysrs over the time course of growth at both these temperatures. Additionally, because most sRNAs identified in enteric bacteria are dependent on Hfq for their functions, we examined whether the absence of hfq from the Y. pseudotuberculosis genome led to differential expression and/or stability of the Ysr in question. The expression levels of all Ysrs tested increase over time and culminate in stationary phase at 26 °C (Fig. 3A). At this temperature only Ysr4 shows strong dependence on Hfq for its expression or stability. All the Ysrs tested at 37 °C are expressed similarly at 26 °C, with the exception of Ysr11, which exhibits the highest expression level at late-log phase and then decreases in stationary phase. This sRNA displayed a similar pattern of expression when examined in S. typhimurium (STnc520) (23). Unlike S. typhimurium, however, the abundance of Ysr11 is not dependent on Hfq at either temperature. Ysr4 and Ysr48/RybB are the only sRNAs examined whose expression levels are Hfq dependent at 37 °C. For all the sRNAs tested at both temperatures, only the expression pattern of Ysr45/GcvB at 37 °C by Northern blot appears to be different from that predicted by the numbers of deep sequencing reads.
Fig. 3.
Verification of Ysr expression. (A) Northern blot analysis was performed to examine the expression of identified Ysrs. (B) Northern blot analysis of Y. pseudotuberculosis and Y. pestis wild-type and Δhfq strains. Representative blots of at least two replicate reproducible experiments are shown. Black triangles above the blots indicate different time points on the bacterial growth curve at which the cultures were collected for RNA isolation (i.e., early-log, mid-log, late-log, and stationary phase). 5S rRNA is shown as a loading control.
Because all but six sRNAs identified in Y. pseudotuberculosis contain an at least partially homologous sequence in the Y. pestis genome, Northern blot analysis was used to examine the expression of the seven sRNAs tested above in Y. pestis grown under the same conditions (26 °C and 37 °C, wild-type and hfq mutant) (Fig. 3B). In Y. pestis, at 26 °C the sRNAs examined show mostly steady-state levels over time and little dependence on Hfq. At 37 °C, however, these Ysrs are expressed differently than in Y. pseudotuberculosis: Most show stable levels or accumulation over time with a peak at late-log phase and are almost undetectable in stationary phase. Additionally, unlike in Y. pseudotuberculosis, all the sRNAs tested require Hfq for their stability/expression.
Ysr29 is one of six Y. pseudotuberculosis specific sRNAs. Interestingly, this sRNA is unique in that it is specific for the IP32953 strain of Y. pseudotuberculosis and is absent from other sequenced Y. pseudotuberculosis isolates. It is encoded in the intergenic region between the adenylate cyclase gene cyaA and antisense to a portion of the 3′ end of YPTB0186 (encoding a putative transposase for IS285). Even though there are multiple copies of this transposase in the genomes of Y. pseudotuberculosis and Y. pestis, the Ysr29 locus is the only occurrence of this sRNA sequence. The Ysr29 transcript accumulates in stationary phase of growth at both 26 °C and 37 °C, with slightly elevated levels at the optimal growth temperature for Y. pseudotuberculosis (Fig. 3A). This sRNA may be classified as Hfq dependent, because its levels decrease in the absence of hfq, particularly at 26 °C.
Contribution of Newly Identified Yersinia sRNAs to Virulence.
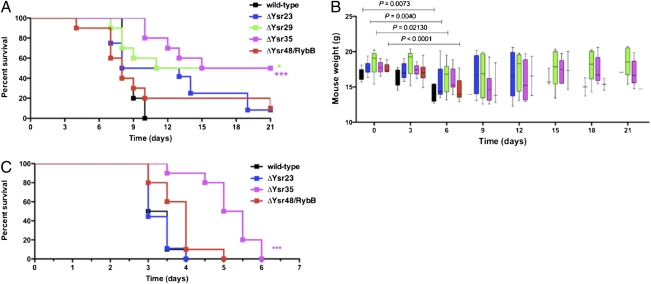
Previous work from our laboratory has established that Hfq is critical for the pathogenesis of Y. pseudotuberculosis, and the deletion of hfq results in an ∼10,000-fold attenuation of the bacteria in a mouse model of yersiniosis (17). This result suggests that Hfq, together with sRNAs, regulates essential virulence determinants in Y. pseudotuberculosis. To test this hypothesis, the Hfq-dependent sRNAs Ysr29 and Ysr48/RybB and the Hfq-independent sRNAs Ysr23 and Ysr35 (SI Appendix, Fig. S3A) were deleted from the Y. pseudotuberculosis chromosome by homologous recombination. Deletions of these Ysrs were confirmed by PCR (SI Appendix, Fig. S3C). The strains carrying deletions of Ysr23, Ysr29, Ysr35, and Ysr48/RybB grew comparably to the wild-type strain at both 26 °C and 37 °C (SI Appendix, Fig. S3E and F). In addition, the deletion of Ysr23, Ysr29, Ysr35, and Ysr48/RybB did not alter significantly the expression of the surrounding ORFs as measured by quantitative RT-PCR (qRT-PCR) (SI Appendix, Fig. S4). To determine whether these sRNAs contribute to the virulence of Y. pseudotuberculosis, wild-type and Ysr-deleted strains were used to infect BALB/c mice via the intragastric route. Although 90% of mice infected with 2.0 × 105 cfu of wild-type Y. pseudotuberculosis succumbed by day 8 postinfection, 10% of mice infected with ΔYsr48/RybB and 50% of mice infected with ΔYsr29 or with ΔYsr35 survived for the duration of the experiment (Fig. 4A). Infection of mice with the ΔYsr23 strain did not result in a significant difference in survival compared with the wild-type but showed a trend toward attenuation. We also measured the weights of the animals as an indicator of their overall health. Mice infected with wild-type bacteria displayed significant weight loss by day 6 before succumbing to infection, whereas the animals infected with Ysr mutants that survived the infection were able to recover after an initial weight decline and continued to maintain or gain weight (Fig. 4B).
Fig. 4.
Contribution of Ysrs to the virulence of Y. pseudotuberculosis and Y. pestis. (A) Groups of 10 mice were inoculated via oral gavage with Y. pseudotuberculosis wild-type, ΔYsr23, ΔYsr29, ΔYsr35, and ΔYsr48/RybB strains (∼2.0 × 105 cfu). Survival of infected mice was monitored over 21 d. P values were determined by Mantel–Cox survival analysis log-rank test. P = 0.2254 for ΔYsr23 compared with wild-type (not significant); *P = 0.0202 for ΔYsr29 compared with wild-type; ***P = 0.0002 for ΔYsr35 compared with wild-type; P = 0.9154 for ΔYsr48/RybB compared with wild-type (not significant). Data are representative of three independent experiments. (B) Body weight over 21 d of mice infected with Y. pseudotuberculosis. The plot shows median weight, indicated by a solid line; boxes represent the 25th and 75th percentiles, and whiskers represent the range. Significance was calculated by student's unpaired t-test. (C) Groups of 10 mice were inoculated intranasally with Y. pestis wild-type, ΔYsr23, ΔYsr35, and ΔYsr48/RybB strains (∼1.0 × 104 cfu). Survival of infected mice was monitored over 7 d. P values were determined by Mantel–Cox survival analysis log-rank test. P = 0.9066 for ΔYsr23 compared with wild-type and P = 0.0946 for ΔYsr48/RybB compared with wild-type (both not significant); ***P < 0.0001 for ΔYsr35 compared with wild-type. Data are representative of two independent experiments.
Ysr35 is 339 nt long, and the ExPasyTranslate Tool (24) predicts that a small ORF may be encoded within the sRNA. To determine if a peptide is synthesized from the predicted ORF, we generated by allelic replacement a version of the ORF with the influenza HA epitope tag on the predicted C-terminal end. Under the conditions in which Ysr35 is expressed, we were unable to detect a HA-tagged peptide by immunoblot (SI Appendix, Fig. S3B).
Because Ysr23, Ysr35, and Ysr48/RybB (but not Ysr29) also are encoded in the genome of Y. pestis and are transcribed (Dataset S1 and Fig. 3), we deleted these sRNAs from Y. pestis (SI Appendix, Fig. S3D) and examined their contributions to the development of pneumonic plague. Similarly to Y. pseudotuberculosis, the inactivation of both Ysr23 (although affecting the expression of one of the neighboring ORFs, ybcI) (SI Appendix, Fig. S4) and Ysr48/RybB does not affect the ability of Y. pestis to cause disease and the subsequent death of mice when delivered via the intranasal route (Fig. 4C). However, much as in Y. pseudotuberculosis, the deletion of Ysr35 results in the attenuation of Y. pestis following intranasal infection, significantly delaying the time to death of mice as compared with the fully virulent, wild-type strain (Fig. 4C). This observation suggests that Ysr35 may play a role in virulence that is conserved between the two Yersinia species.
Identification of sRNA-Regulated Proteins.
Considering the uniqueness of Ysr29 to the IP32953 strain of Y. pseudotuberculosis and its contribution to virulence, we performed a proteomic analysis using 2D differential gel electrophoresis (2D-DIGE) to determine the regulated targets of this sRNA. Protein profiles from whole-cell lysates of wild-type Y. pseudotuberculosis were compared with those of the ΔYsr29 strain grown to stationary phase at 26 °C, the time point at which this sRNA is most abundant. The comparison of protein profiles between the wild-type and ΔYsr29 strains showed 16 spots with 1.5-fold or more difference in fluorescence intensity (Fig. 5A). Thirteen spots were analyzed successfully by MALDI-TOF mass spectroscopy and correspond to eight proteins: 30S ribosomal protein S1 (RpsA), outer membrane protein A (OmpA), the chaperonin GroEL, glutathione-S-transferase (GST), the molecular chaperone DnaK, peroxidase (AhpC), ribosome recycling factor (RRF), and urease (UreC) (SI Appendix, Table S1). Ysr29 does not appear to regulate the identified proteins at the level of transcription, because qRT-PCR analysis shows that the transcript levels for the genes in question are equivalent in the wild-type and ΔYsr29 strains (Fig. 5B).
Fig. 5.
Posttranscriptional regulation of targets by Ysr29. (A) Proteomic comparison of Y. pseudotuberculosis wild-type and ΔYsr29 strains by 2D-DIGE. Wild-type proteins were labeled with Cy3, and ΔYsr29 mutant proteins were labeled with Cy5. The gel image shows 1.5× or greater differences in spot volume ratio for 16 marked spots. Blue indicates spots with a Cy3/Cy5 ratio >0 (increased expression in ΔYsr29 compared with the wild-type); red indicates spots with a Cy3/Cy5 ratio <0 (increased expression in wild-type compared with ΔYsr29). Molecular masses in kilodaltons, indicated by the numbers to the right of the image, are approximate. (B) Expression of Ysr29 targets. Cultures of wild-type and ΔYsr29 strains were grown to stationary phase at 26 °C, and transcript levels of targets identified by 2D-DIGE were examined by qRT-PCR. The fold change in RNA levels is relative to wild-type, which was set to 1. (C) Western blots of whole-cell lysates from wild-type and ΔYsr29 strains expressing chromosomal HA fusions of GroEL, OmpA, RpsA, and GST. (Upper) Anti-HA antibody. (Lower) Anti-RpoA antibody (loading control). (D) The effect of Ysr29 overexpression on GroEL and GST. ΔYsr29 strains with the GroEL-HA and GST-HA fusions were electroporated with either the pACY177 vector or a Ptac-Ysr29 overexpression construct. (Upper) Northern blots showing overexpression of Ysr29 upon addition of 1 mM IPTG to the growth medium. Note: The band in the pACYC177 samples is nonspecific and migrates at a slightly higher molecular weight than Ysr29. 5S rRNA is shown as a loading control. (Lower) Immunoblots showing the effect of Ysr29 overexpression on GST-HA, GroEL-HA, and RpoA (loading control). (E) IntaRNA predictions of base-pairing between Ysr29 and the target mRNAs. The software predicts at least hepta-nucleotide pairing of Ysr29 with the 5′ UTRs of all four target mRNAs.
Therefore, we examined the effects of Ysr29 at the posttranscriptional level by generating chromosomal in-frame fusions of the GST, RpsA, OmpA, and GroEL coding regions with the HA-epitope tag in both wild-type and ΔYsr29 strains. Levels of fusion proteins were measured by immunoblot analysis using an anti-HA antibody (Fig. 5C). In accordance with 2D-DIGE, we found that GST is more abundant in the ΔYsr29 strain than in the wild-type background, whereas RpsA, OmpA, and GroEL are elevated in the wild-type compared with the ΔYsr29 strain, demonstrating posttranscriptional regulation by this sRNA. Because the overexpression of sRNAs can result in more dramatic effects on regulated target protein levels than sRNA deletions, we generated an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Ysr29 plasmid and introduced this construct into the ΔYsr29 strains carrying the GST-HA and GroEL-HA fusions. As expected, upon induction of Ysr29 expression with IPTG (Fig. 5D, Upper), the abundance of GroEL-HA was elevated, and the levels of GST-HA were reduced (Fig. 5D, Lower). Because Ysr29 expression is not fully repressed in the GST-HA strain (Ptac-Ysr29, 0-min Northern blot in Fig. 5D), GST-HA protein is not detected even at the 0-min time point, demonstrating the substantial effect of extended Ysr29 overexpression on GST synthesis. Finally, we used a computational approach to predict if Ysr29 could interact with the 5′ UTRs of these target mRNAs. The IntaRNA alignment tool (25) predicts at least 7-nt-long base-pairing interactions of Ysr29 with the 5′ UTRs of all four validated targets (Fig. 5E).
Discussion
Although small regulatory RNAs have become increasingly recognized as major modulators of gene expression in bacteria (26), only a limited number of studies have sought to identify the global set of expressed sRNAs, particularly in bacterial pathogens (27–29). Additionally, the expression of only a small number of sRNAs has been verified in Yersiniae relative to other enteric bacteria (3). Here, we describe the deep sequencing-based identification of sRNAs in the human gastrointestinal pathogen Y. pseudotuberculosis. A similar approach has been used to identify sRNAs and mRNA targets that interact with Hfq in S. typhimurium (23) and in an RNomic analysis of Burkholderia cenocepacia (30). Analysis of sRNA libraries generated from Y. pseudotuberculosis grown at 26 °C and at 37 °C led to the identification of 150 putative sRNAs as well as confirming the expression of 15 previously annotated sRNAs. The ability to identify all the “miscellaneous” RNAs in an impartial manner validated the deep sequencing approach for discovery of previously unidentified sRNAs. The number of putative sRNAs is much smaller than the 1,478 sRNAs predicted for the closely related Y. pestis by Livny et al. (31) who used a computer algorithm that relies on conservation of intergenic sequences and ρ-independent terminators, but is comparable to the number of sRNAs predicted and verified in other enteric bacteria (3). It is possible, however, that additional sRNAs may be expressed under conditions not examined here, such as upon host-cell contact or during animal infection.
Thirty-two identified sRNAs are orthologs of potential sRNAs encoded in the genomes of E. coli and S. typhimurium. This finding is not surprising, because sequence conservation in intergenic regions of closely related bacteria was one of the original criteria used to identify sRNAs (32). The unexpected observation is that many of the well-characterized sRNAs from E. coli and S. typhimurium are not encoded by the genome of Y. pseudotuberculosis as determined by BlastN alignment analysis. These sRNAs include MicC, OxyS, ArcZ, and DsrA, among others. Although informative, this type of analysis also was unable to identify SgrS in the genome of Y. pseudotuberculosis, an sRNA that has been described previously in this species (33, 34). Interestingly, the filtering algorithm we used to identify sRNAs from our deep sequencing data also did not reveal SgrS, but when the parameters were relaxed (i.e., the position of the cluster with respect to any ORF was moved within 1 kb on the same strand), the deep sequencing reads for SgrS were indeed present (Ysr150 in Dataset S1). This observation suggests that using multiple tools may enhance the comparison of sRNA sequences between species and that adjusting the parameters of deep sequencing data analysis could reveal additional sRNAs encoded in the genome of Y. pseudotuberculosis.
Given the limitations discussed above, it is interesting to hypothesize how Y. pseudotuberculosis may have evolved to regulate the expression of genes that are conserved between the enteric bacteria. The fact that Y. pseudotuberculosis may not express a DsrA ortholog, for example, raises the question of how Y. pseudotuberculosis regulates the stationary phase Sigma factor σS synthesis. One possibility is that some of the Yersinia-specific sRNAs that we have identified functionally replace the above-mentioned enteric sRNAs. Alternatively, RpoS in Yersiniae may not require regulation by sRNAs for its synthesis. Another difference is that Y. pseudotuberculosis encodes two distinct copies of the iron level-regulated sRNA RyhB (Ysr146.1 and Ysr146.2). Because the expression levels of these sRNAs in Y. pseudotuberculosis are low in iron-replete conditions, it would be interesting to examine the expression of the two RyhB copies in Y. pseudotuberculosis in the iron-limiting conditions that are known to induce RyhB in E. coli (26, 35).
We also identified 118 Yersinia-specific sRNAs (78.7%) without a sequence match in available databases. Similarly, an analysis of S. typhimurium-specific genetic islands by Padalon-Brauch et al. (36) led to the identification of 28 candidate sRNAs, 19 of which were verified. These observations suggest that related bacterial species have evolved distinct species-specific regulatory networks that rely on sRNAs that are required for the adaptation to a particular niche or, in the case of pathogens or symbionts, for the interaction with the host. Additionally, we identified sRNAs that are conserved between Y. pseudotuberculosis and Y. pestis, two species of Yersiniae that share 97% identity in 75% of their protein-coding genes (15). Remarkably, a substantial number of these sRNAs (43.75%) contain nucleotide mismatches, insertions, or deletions. Although these hypotheses are not yet tested, it is interesting to speculate that these differences in sRNA sequences might alter the secondary structure of the molecules or that a single-nucleotide mismatch between the sRNA and its target mRNA might affect the outcome of the interaction. To determine whether the nucleotide differences present in Ysrs in Y. pseudotuberculosis and Y. pestis are localized or are spread across the entire sequence of the sRNA, we performed ClustalW2 alignments of all Ysrs containing mismatches between the two species (SI Appendix, Table S2). The differences do not localize to either the 5′ or the 3′ end of Ysrs but rather are spread throughout the length of the sRNAs. Thus, although the mismatches in the conserved sRNAs could lead to differential regulation of targets by the two closely related species, it also is possible that these differences may have little or no effect on sRNA function.
We also observed that sRNAs conserved between Y. pseudotuberculosis and Y. pestis demonstrated divergent expression patterns. Most Ysrs accumulate over time in Y. pseudotuberculosis, but in Y. pestis the levels of those same Ysrs peak in late-log phase and decrease in stationary phase. This difference suggests that the temporal regulation of Ysr gene expression or stability may affect the outcomes of Ysrs in modulating target mRNA expression. This hypothesis is supported by the work of Bai et al. (37), which showed that the deletion of hfq causes a severe growth defect at 37 °C in Y. pestis but a less drastic effect in Y. pseudotuberculosis. Thus, our findings, together with those of Bai et al. (37), further support the hypothesis that these two genetically linked species could differentially regulate gene expression via sRNAs to result in divergent disease manifestations.
Most trans-acting sRNAs in bacteria require the RNA chaperone Hfq for their functions. Although the exact role of Hfq is not fully understood, it appears that the chaperone promotes base-pairing interactions of the sRNA and the mRNA target by increasing the rate of sRNA–mRNA association in addition to protecting the sRNAs from degradation (38, 39). Here we note another difference between Y. pseudotuberculosis and E. coli and Salmonella. In E. coli and Salmonella, MicA and GcvB are known to rely on Hfq for proper expression/stability and also for the regulation of their mRNA targets (40, 41), but in Y. pseudotuberculosis, expression levels of Ysr7/MicA, Ysr20, Ysr23, and Ysr45/GcvB do not differ between the wild-type and Δhfq strains. Our finding that the majority of sRNAs tested in Y. pseudotuberculosis do not require Hfq for their expression and/or stability suggests the possibility of an alternative sRNA chaperone. Y. pseudotuberculosis may rely on SmpB, an Sm-like chaperone of tmRNA (42), or an as yet unidentified protein to stabilize sRNAs and promote interaction with mRNAs.
Hfq is known to play a role in the virulence of Y. pseudotuberculosis (17), and in other species a majority of sRNAs require Hfq for their function (reviewed in ref. 3). Therefore, we examined the contribution of several sRNAs, whose stability/expression either relies on or does not require Hfq, to the virulence of Y. pseudotuberculosis. The deletion of both Ysr29 and Ysr35 resulted in increased survival of mutant-infected mice compared with those infected with wild-type bacteria, whereas the deletion of Ysr23 delayed the overall time to death of mice but did not impact overall survival. Interestingly, deletion of the Hfq-dependent sRNA Ysr48/RybB did not result in a significant decrease in virulence of Y. pseudotuberculosis, suggesting that not all Hfq-binding sRNAs play a direct role in virulence. The attenuation of individual sRNA-deleted strains is not as dramatic as that of a Δhfq strain (17), suggesting that in the absence of Hfq the dysregulation of multiple sRNA-dependent targets may result in the severe attenuation of Y. pseudotuberculosis. It is possible that a double mutant of Ysr35 and Ysr29 may have a phenotype that more closely resembles that of the Δhfq mutant.
Because Ysr35 is a Yersinia-specific sRNA that is conserved between Y. pseudotuberculosis and Y. pestis, we deleted this sRNA from the genome of the plague bacillus and tested its role in virulence. The Ysr35 deletion is attenuated in a mouse model of pneumonic plague (Fig. 4C), suggesting that the sRNA may play a similar role in virulence in Y. pseudotuberculosis and Y. pestis. It will be of interest to determine whether Ysr35 regulates the same or distinct targets in the two species. In addition, although we were unable to detect the production of a Ysr35-encoded peptide by immunoblot in vitro, our results do not discount the possibility that the ORF still may be translated under other conditions.
Hfq-binding sRNAs in E. coli and S. typhimurium exert a variety of physiological roles mediated by the base-pairing interactions of the sRNA to the mRNA target. We sought to determine how Ysr29 may control virulence in Y. pseudotuberculosis by identifying the regulated targets of this unique sRNA. Proteomic analyses of wild-type and ΔYsr29 strains resulted in identification of eight putative targets of Ysr29, although it is not yet known whether Ysr29 regulates these factors directly or acts indirectly to alter their synthesis. Nevertheless, Ysr29 may act on these targets posttranscriptionally, because the absence of the sRNA does not affect their transcript levels but instead alters the levels of protein. This finding suggests that 2D-DIGE accompanied by target-fusion (or direct Western blot analysis, if antibodies are available) is an appropriate approach for validating target gene expression, because transcriptional profiling by microarray probably would not reveal the targets identified by our analysis.
All the Ysr29-regulated targets validated by immunoblot analysis have the potential to be involved in the virulence of Y. pseudotuberculosis. For example, GST participates in protecting cells against the damage of oxidative stress (43, 44), and Ysr29 repression of GST levels may prevent an aberrant response to this stress. In addition, OmpA is an outer membrane protein known to play a role in the pathogenesis of E. coli K1 and acts as an adhesin, invasin, and immune evasin (45–47). Interestingly, in E. coli, the expression of OmpA is posttranscriptionally repressed by MicA (48). It is not known if MicA acts similarly in Y. pseudotuberculosis, but we show here that Ysr29 acts to enhance OmpA levels. There are other examples of targets being differently regulated by multiple sRNAs [e.g., RpoS regulation by DsrA, RprA, ArcZ, and OxyS (49, 50)] and also of multiple targets under the control of one sRNA [e.g., RybB regulation of outer membrane protein synthesis (51)]. In sum, the global identification of sRNAs in Y. pseudotuberculosis provides an opportunity to discover mechanisms of virulence gene regulation in this pathogenic bacterium, particularly in comparison with closely related species such as Y. pestis and in contrast to other bacteria such as E. coli and Salmonella, the prototype species for studying sRNAs.
Materials and Methods
RNA Isolation.
Overnight cultures of Y. pseudotuberculosis strain IP32953 were subcultured in brain heart infusion (BHI) broth supplemented with 2.5 mM CaCl2 to an OD620 of 0.1 and grown to early-log (OD620 0.3, 1.5 h), mid-log (OD620 0.65, 3.5 h), late-log (OD620 4.3, 6 h), and stationary phase (OD620 7.6, 11 h) at either 26 °C or 37 °C. Five OD equivalents were collected, and RNA was stabilized with the RNAprotect Bacteria reagent (Qiagen). RNA enriched for sRNAs was extracted using the RiboPure Bacteria kit (Ambion) with modifications. Specifically, the provided columns (which are designed to eliminate small RNAs) were omitted, and instead the RNA was precipitated overnight with isopropanol. Isolated RNA was treated with DNase I (Ambion), and the quality was assessed using the Experion automated electrophoresis system (Bio-Rad). RNA also was isolated as above from Y. pestis strain CO92 Δpgm for experiments comparing the expression of identified sRNAs by Northern blot analysis. For Y. pestis RNA isolation, overnight cultures diluted to an OD620 of 0.1 were grown to early-log (OD620 0.2, 2.5 h), mid-log (OD620 0.8, 5.5 h), late-log (OD620 1.8, 8.5 h), and stationary phase (OD620 4.5, 15 h) at 26 °C and at 37 °C.
sRNA Library Preparation and Illumina-Solexa Sequencing.
Ribosomal RNA was eliminated by the MicrobExpress kit (Ambion), and the integrity of the RNA was reanalyzed by Experion electrophoresis. sRNA libraries were generated using the Illumina small RNA v1.5 kit with the following modifications: RNA was treated with 5′ RNA polyphosphatase (Epicentre) followed by the ligation of RNA adapter oligonucleotides, cDNA synthesis, and PCR amplification for 12 cycles. The library then was separated on 6% acrylamide gels, and fragments between 90–500 nt were excised. cDNA was eluted from the gels and precipitated. Cluster generation was performed according to the manufacturer's protocols (Illumina), and 36-nt single-end reads were generated on a Solexa Genome Analyzer at the Institute for Genomics and Systems Biology (Argonne National Laboratory, Argonne, IL). The Solexa reads that passed the purity filtering and had a unique alignment were mapped to the Y. pseudotuberculosis IP32953 reference chromosome (NC_006155.1) and plasmids (pYV, NC_006154; pYtb, NC_006153).
Bioinformatics.
Solexa sequencing reads that overlapped the annotated miscellaneous RNA, mRNA, rRNA, tmRNA, and tRNA genes (based on the above GenBank records of Y. pseudotuberculosis IP32953 chromosome and plasmids) were extracted and counted. The reads not overlapping these annotations were considered to be intergenic and include 5′ and 3′ UTRs, cis-encoded antisense RNAs, and potential trans-encoded sRNAs. Clusters of at least 50 bp in length that form a continuous region of coverage were extracted from the intergenic category of reads on each strand of the chromosome or plasmids. These clusters were analyzed further by calculating the expression level in reads per kilobase for each intergenic cluster and all miscellaneous RNA, mRNA, rRNA, tmRNA, and tRNA genes. To aid in eliminating 5′ and 3′ UTRs from the analysis of intergenic clusters, intergenic clusters differentially expressed with respect to nearby ORFs were determined. The candidate clusters were required to have a difference in expression level above or below a threshold of at least threefold with respect to any ORF within 1 kb on the same strand. Generated cluster sets then were analyzed using the Integrated Genome Browser (http://www.bioviz.org/igb/). Predicted sRNAs were inspected for the presence of promoters and ρ-independent terminators using the BProm and TermFind/RNAFold programs (Softberry).
Animal Experiments.
All procedures involving animals were carried out in compliance with protocols approved by the Northwestern University institutional animal care and use committee. For Y. pseudotuberculosis infections, 8-wk-old female BALB/c mice were purchased from Harlan Laboratories and allowed to acclimate in the animal facility for 5–7 d before infection. To prepare the inocula, Y. pseudotuberculosis strains were cultured overnight in BHI broth at 26 °C, diluted to an OD620 of 0.1 in BHI broth, and incubated at 26 °C with shaking (250 rpm) to an OD620 of 0.6. The cells were harvested by centrifugation, washed once with sterile PBS, and diluted to the appropriate OD620 in PBS. Groups of 10 mice were inoculated intragastrically using a 22-gauge feeding needle with ∼2 × 105 cfu of Y. pseudotuberculosis and monitored for 21 d. The weights of individual mice were recorded every third day as an indicator of health status. Input colony-forming units were determined by serial plating onto BHI agar. Animals infected with wild-type Y. pseudotuberculosis that demonstrated weight loss at any point during the infection were included in the analysis. For Y. pestis infections, pathogen-free 6- to 8-wk-old female C57BL/6 mice were obtained from the Jackson Laboratory and allowed to acclimate as above. Bacteria were grown in BHI broth as described in SI Appendix, SI Materials and Methods, washed once with sterile PBS, and maintained at 37 °C. Groups of 10 mice were anesthetized lightly with ketamine and xylazine and inoculated by the intranasal route with wild-type, fully virulent Y. pestis or Ysr deletion strains (1 × 104 cfu) diluted in PBS as described previously (52). Mice were monitored twice daily for 7 d. All animal infections were repeated at least twice, unless stated otherwise. Statistical analysis was performed using the log-rank (Mantel–Cox) test. The weights of infected animals were compared using the student's unpaired t test. A P value of ≤0.05 was considered significant.
sRNA Target Identification.
Overnight cultures of wild-type and ΔYsr29 Y. pseudotuberculosis were diluted to an OD620 of 0.1 in BHI broth and grown to stationary phase (OD620 ∼7, 11 h) at 26 °C. Ten OD equivalents of culture were collected, bacteria were centrifuged, washed once in ice-cold PBS, and incubated with lysozyme (10 mg/mL) for 30 min on ice. Cells were sonicated (three 20-s pulses) on ice, and whole-cell lysates were clarified by centrifugation. Protein content of the lysates was quantified by the Bradford assay (Bio-Rad). The wild-type sample was labeled with Cy3 dye, and the ΔYsr29 sample was labeled with Cy5 dye using the CyDye DIGE Fluor Labeling Kit (GE Healthcare) according to the manufacturer's instructions. Both samples were run in a single polyacrylamide gel but were imaged separately at a wavelength of 570 nm for Cy3 and at a wavelength of 670 nm for Cy5. Protein spots were selected based on >1.5-fold differential expression between the wild-type and ΔYsr29 mutant and were subjected to robotic spot excision, trypsin digestion, and MALDI-TOF. Protein identification was based on highly accurate masses and MS/MS sequence data from multiple tryptic peptides. The 2D-DIGE and subsequent analyses were performed at the W. M. Keck Foundation Biotechnology Resource Laboratory (Yale University, New Haven, CT).
Additional Methods.
Bacterial strains and plasmids (SI Appendix, Table S3), oligonucleotides (SI Appendix, Table S4), and growth conditions and reagents, as well as methods for sRNA and hfq deletions, Northern blot, 5′/3′ RACE, qRT-PCR, sRNA target verification, and Ysr29 overexpression are detailed in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Brian Fritz for guidance and technical support and Marc Domanus for Illumina-Solexa sequencing. We also thank Terence Wu for assistance with 2D-DIGE and mass spectroscopy analysis. We thank Lauren Bellows for outstanding technical assistance and Hank Seifert for helpful discussions. This work was sponsored by the Northwestern University Feinberg School of Medicine and the National Institutes of Health/National Institute of Allergy and Infectious Diseases Regional Center of Excellence (RCE) Research Program for Bio-defense and Emerging Infectious Diseases. The authors acknowledge membership within and support from the Region V ‘Great Lakes’ RCE (National Institutes of Health Award U54-AI-057153).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 15029.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101655108/-/DCSupplemental.
References
- 1.Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma CM, Vogel J. Experimental approaches for the discovery and characterization of regulatory small RNA. Curr Opin Microbiol. 2009;12:536–546. doi: 10.1016/j.mib.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman S, Storz G. Bacterial small RNA regulators: Versatile roles and rapidly evolving variations. Cold Spring Harb. Perspect Biol. 2010 doi: 10.1101/cshperspect.a003798. 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman S. The small RNA regulators of Escherichia coli: Roles and mechanisms. Annu Rev Microbiol. 2004;58:303–328. doi: 10.1146/annurev.micro.58.030603.123841. [DOI] [PubMed] [Google Scholar]
- 5.Vogel J, Papenfort K. Small non-coding RNAs and the bacterial outer membrane. Curr Opin Microbiol. 2006;9:605–611. doi: 10.1016/j.mib.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.McCullen CA, Benhammou JN, Majdalani N, Gottesman S. Mechanism of positive regulation by DsrA and RprA small noncoding RNAs: Pairing increases translation and protects rpoS mRNA from degradation. J Bacteriol. 2010;192:5559–5571. doi: 10.1128/JB.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jousselin A, Metzinger L, Felden B. On the facultative requirement of the bacterial RNA chaperone, Hfq. Trends Microbiol. 2009;17:399–405. doi: 10.1016/j.tim.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Huntzinger E, et al. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J. 2005;24:824–835. doi: 10.1038/sj.emboj.7600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morfeldt E, Taylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 1995;14:4569–4577. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenz DH, et al. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Achtman M, et al. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skurnik M, Peippo A, Ervelä E. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Mol Microbiol. 2000;37:316–330. doi: 10.1046/j.1365-2958.2000.01993.x. [DOI] [PubMed] [Google Scholar]
- 13.Perry RD, Fetherston JD. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer L, Greenstein AJ. Acute yersinial ileitis: A distinct entity. Am J Gastroenterol. 1976;65:548–551. [PubMed] [Google Scholar]
- 15.Chain PS, et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 2004;101:13826–13831. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng J, et al. Involvement of the post-transcriptional regulator Hfq in Yersinia pestis virulence. PLoS ONE. 2009;4:e6213. doi: 10.1371/journal.pone.0006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiano CA, Bellows LE, Lathem WW. The small RNA chaperone Hfq is required for the virulence of Yersinia pseudotuberculosis. Infect Immun. 2010;78:2034–2044. doi: 10.1128/IAI.01046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivatsan A, et al. High-precision, whole-genome sequencing of laboratory strains facilitates genetic studies. PLoS Genet. 2008;4:e1000139. doi: 10.1371/journal.pgen.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JM, et al. Experimental discovery of sRNAs in Vibrio cholerae by direct cloning, 5S/tRNA depletion and parallel sequencing. Nucleic Acids Res. 2009;37:e46. doi: 10.1093/nar/gkp080. 10.1093/nar/gkp080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicol JW, Helt GA, Blanchard SG, Jr, Raja A, Loraine AE. The Integrated Genome Browser: Free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25:2730–2731. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottesman S. Micros for microbes: Non-coding regulatory RNAs in bacteria. Trends Genet. 2005;21:399–404. doi: 10.1016/j.tig.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Straley SC, Perry RD. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 1995;3:310–317. doi: 10.1016/s0966-842x(00)88960-x. [DOI] [PubMed] [Google Scholar]
- 23.Sittka A, et al. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet. 2008;4:e1000163. doi: 10.1371/journal.pgen.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasteiger E, et al. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busch A, Richter AS, Backofen R. IntaRNA: Efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics. 2008;24:2849–2856. doi: 10.1093/bioinformatics/btn544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massé E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci USA. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohn C, et al. Experimental discovery of small RNAs in Staphylococcus aureus reveals a riboregulator of central metabolism. Nucleic Acids Res. 2010;38:6620–6636. doi: 10.1093/nar/gkq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar R, et al. Identification of novel non-coding small RNAs from Streptococcus pneumoniae TIGR4 using high-resolution genome tiling arrays. BMC Genomics. 2010;11:350. doi: 10.1186/1471-2164-11-350. 10.1186/1471-2164-11-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postic G, et al. Identification of small RNAs in Francisella tularensis. BMC Genomics. 2010;11:625. doi: 10.1186/1471-2164-11-625. 10.1186/1471-2164-11-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoder-Himes DR, et al. Mapping the Burkholderia cenocepacia niche response via high-throughput sequencing. Proc Natl Acad Sci USA. 2009;106:3976–3981. doi: 10.1073/pnas.0813403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livny J, Brencic A, Lory S, Waldor MK. Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2. Nucleic Acids Res. 2006;34:3484–3493. doi: 10.1093/nar/gkl453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001;15:1637–1651. doi: 10.1101/gad.901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wadler CS, Vanderpool CK. Characterization of homologs of the small RNA SgrS reveals diversity in function. Nucleic Acids Res. 2009;37:5477–5485. doi: 10.1093/nar/gkp591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horler RS, Vanderpool CK. Homologs of the small RNA SgrS are broadly distributed in enteric bacteria but have diverged in size and sequence. Nucleic Acids Res. 2009;37:5465–5476. doi: 10.1093/nar/gkp501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massé E, Vanderpool CK, Gottesman S. Effect of RyhB small RNA on global iron use in Escherichia coli. J Bacteriol. 2005;187:6962–6971. doi: 10.1128/JB.187.20.6962-6971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padalon-Brauch G, et al. Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence. Nucleic Acids Res. 2008;36:1913–1927. doi: 10.1093/nar/gkn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai G, Golubov A, Smith EA, McDonough KA. The importance of the small RNA chaperone Hfq for growth of epidemic Yersinia pestis, but not Yersinia pseudotuberculosis, with implications for plague biology. J Bacteriol. 2010;192:4239–4245. doi: 10.1128/JB.00504-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohanty BK, Maples VF, Kushner SR. The Sm-like protein Hfq regulates polyadenylation dependent mRNA decay in Escherichia coli. Mol Microbiol. 2004;54:905–920. doi: 10.1111/j.1365-2958.2004.04337.x. [DOI] [PubMed] [Google Scholar]
- 39.Brennan RG, Link TM. Hfq structure, function and ligand binding. Curr Opin Microbiol. 2007;10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Figueroa-Bossi N, et al. Loss of Hfq activates the sigmaE-dependent envelope stress response in Salmonella enterica. Mol Microbiol. 2006;62:838–852. doi: 10.1111/j.1365-2958.2006.05413.x. [DOI] [PubMed] [Google Scholar]
- 41.Pulvermacher SC, Stauffer LT, Stauffer GV. Role of the Escherichia coli Hfq protein in GcvB regulation of oppA and dppA mRNAs. Microbiology. 2009;155:115–123. doi: 10.1099/mic.0.023432-0. [DOI] [PubMed] [Google Scholar]
- 42.Wower J, Zwieb CW, Hoffman DW, Wower IK. SmpB: A protein that binds to double-stranded segments in tmRNA and tRNA. Biochemistry. 2002;41:8826–8836. doi: 10.1021/bi0201365. [DOI] [PubMed] [Google Scholar]
- 43.Allocati N, Federici L, Masulli M, Di Ilio C. Glutathione transferases in bacteria. FEBS J. 2009;276:58–75. doi: 10.1111/j.1742-4658.2008.06743.x. [DOI] [PubMed] [Google Scholar]
- 44.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 45.Shin S, Lu G, Cai M, Kim KS. Escherichia coli outer membrane protein A adheres to human brain microvascular endothelial cells. Biochem Biophys Res Commun. 2005;330:1199–1204. doi: 10.1016/j.bbrc.2005.03.097. [DOI] [PubMed] [Google Scholar]
- 46.Prasadarao NV, et al. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvaraj SK, Prasadarao NV. Escherichia coli K1 inhibits proinflammatory cytokine induction in monocytes by preventing NF-kappaB activation. J Leukoc Biol. 2005;78:544–554. doi: 10.1189/jlb.0904516. [DOI] [PubMed] [Google Scholar]
- 48.Rasmussen AA, et al. Regulation of ompA mRNA stability: The role of a small regulatory RNA in growth phase-dependent control. Mol Microbiol. 2005;58:1421–1429. doi: 10.1111/j.1365-2958.2005.04911.x. [DOI] [PubMed] [Google Scholar]
- 49.Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G. A small, stable RNA induced by oxidative stress: Role as a pleiotropic regulator and antimutator. Cell. 1997;90:43–53. doi: 10.1016/s0092-8674(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 50.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci USA. 2010;107:9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papenfort K, et al. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol Microbiol. 2006;62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lathem WW, Crosby SD, Miller VL, Goldman WE. Progression of primary pneumonic plague: A mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci USA. 2005;102:17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]