Abstract
The effect of the Cretaceous-Paleogene (K-Pg) (formerly Cretaceous–Tertiary, K–T) mass extinction on avian evolution is debated, primarily because of the poor fossil record of Late Cretaceous birds. In particular, it remains unclear whether archaic birds became extinct gradually over the course of the Cretaceous or whether they remained diverse up to the end of the Cretaceous and perished in the K–Pg mass extinction. Here, we describe a diverse avifauna from the latest Maastrichtian of western North America, which provides definitive evidence for the persistence of a range of archaic birds to within 300,000 y of the K–Pg boundary. A total of 17 species are identified, including 7 species of archaic bird, representing Enantiornithes, Ichthyornithes, Hesperornithes, and an Apsaravis-like bird. None of these groups are known to survive into the Paleogene, and their persistence into the latest Maastrichtian therefore provides strong evidence for a mass extinction of archaic birds coinciding with the Chicxulub asteroid impact. Most of the birds described here represent advanced ornithurines, showing that a major radiation of Ornithurae preceded the end of the Cretaceous, but none can be definitively referred to the Neornithes. This avifauna is the most diverse known from the Late Cretaceous, and although size disparity is lower than in modern birds, the assemblage includes both smaller forms and some of the largest volant birds known from the Mesozoic, emphasizing the degree to which avian diversification had proceeded by the end of the age of dinosaurs.
Following their remarkable diversification in the Early Cretaceous (1, 2), birds underwent a major evolutionary transition between the Cretaceous and the Paleogene. Archaic birds (i.e., outside the crown clade Neornithes), such as Enantiornithes and basal ornithurines, failed to persist beyond the Cretaceous, and identifiable members of most modern orders make their first appearances in the Paleocene and Eocene (1, 3–8). There is very little fossil evidence for modern birds in the Cretaceous (1–5, 7). The only definitive neornithine known from the Cretaceous is the anseriform Vegavis (9); Teviornis may also represent an anseriform (10), although its affinities have not yet been examined in the context of a phylogenetic analysis.
These patterns have been interpreted as the result of a mass extinction of archaic birds at the Cretaceous–Paleogene (K–Pg) (formerly Cretaceous–Tertiary, K–T) boundary and the subsequent adaptive radiation of surviving Neornithes in the Paleogene (3–5). The K–Pg mass extinction was a severe, global, and rapid extinction coinciding with an extraterrestrial impact (11) and resulted in major extinctions in terrestrial ecosystems. Nonavian dinosaurs and pterosaurs became extinct, and major extinctions also occurred among mammals, reptiles, insects, and plants (8, 12–14). It would be remarkable if birds survived the K–Pg event unscathed; however, the hypothesis of an avian mass extinction at the K–Pg boundary (3–5) has been debated. This is largely because the timing of extinction of archaic birds is not well constrained (1, 2, 6, 15–17); it has been unclear whether archaic birds remained a diverse component of the avifauna up to the K–Pg boundary or whether many groups were already declining in diversity or extinct at the time of the Chicxulub asteroid impact. Furthermore, molecular clock studies provide conflicting signals. Many studies imply “mass survival” among birds, with numerous Neornithine lineages crossing the K–Pg boundary (18, 19), although one study found evidence for limited Cretaceous diversification followed by explosive diversification in the Paleogene (20). Regardless, molecular studies cannot determine whether archaic lineages persisted until the end of the Cretaceous; only the fossil record can provide data on the timing of their extinction.
The only diverse avian assemblage that can be confidently dated to the end of the Maastrichtian, and which can therefore be brought to bear on this question (SI Appendix), is from the late Maastrichtian (Lancian land vertebrate age) beds of the Western Interior of North America (21–23). Here, birds are known from three formations: the Hell Creek Formation of Montana, North Dakota, and South Dakota, the Lance Formation of Wyoming, and the Frenchman Formation of Saskatchewan (SI Appendix). These rocks were deposited during the final 1.5 million years of the Cretaceous, but most of the fossils described here can be correlated to magnetochron c29r (24, 25), placing them within 300,000 y of the K–Pg boundary (26).
The relationships of these birds are unclear, in part because the fossils consist almost exclusively of isolated bones, but more importantly, they have never been subjected to phylogenetic analysis. Instead, species have been shoehorned into modern orders on the basis of overall similarity (21–23). The diversity of the assemblage is also poorly understood. Species have often been erected on the basis of nonoverlapping elements (22, 23), meaning that some species may have been named several times. As a result, these potentially significant fossils have played little role in discussions of avian evolution and extinction. Several archaic birds are clearly present in the formation, including the enantiornithine Avisaurus (27) and a putative hesperornithiform (28); however, they have been interpreted as representing a minor component of the fauna (23).
Here, we reexamine the birds from the Late Maastrichtian of western North America to assess the relationships of these fossils and the diversity of the assemblage. We focused on the most commonly preserved element, the coracoid, to avoid counting the same taxon several times on the basis of nonoverlapping material. Bird coracoids show minimal variation within species or even genera; thus different morphotypes can confidently be identified as different species (29). Rather than naming new species, we identify morphotypes on the basis of unique combinations of apomorphies and plesiomorphies, differences in shape, and overall size. To determine their relationships, we conducted a cladistic analysis (SI Appendix) on the basis of previously published data (30) and new characters. However, two tarsometatarsus morphs were also included, because they appear to represent species not known from coracoids.
Results
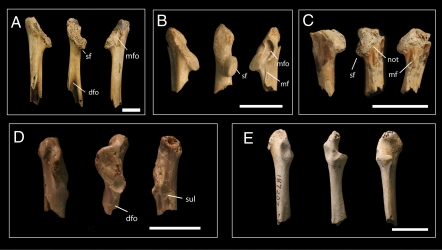
A total of 15 distinct coracoid morphotypes are identifiable; full descriptions of each are given in the SI Appendix. One enantiornithine has previously been recognized from the assemblage (27), but three species are identified here (Fig. 1 A–C). The largest (Fig. 1A) most likely represents the giant enantiornithine Avisaurus archibaldi (27). Enantiornithine features (31) include a dorsal fossa, a posteriorly projecting coracoid boss, a dorsally oriented glenoid, and a medial fossa, but the coracoid lacks a supracoracoideus nerve foramen or a medial flange and groove. Enantiornithine A (Fig. 1B) is a smaller taxon, characterized by a deep medial fossa, a thin medial flange, and a subtriangular coracoid neck. The smallest form, Enantiornithine B (Fig. 1C), is differentiated by a bulbous, medially notched scapular condyle, a robust medial flange, and an elliptical coracoid neck.
Fig. 1.
Coracoids of stem avians from the late Maastrichtian of western North America. Left coracoids (right coracoids reversed) in lateral, dorsal, and medial views. (Scale bars, 10 mm.) (A) cf Avisaurus archibaldi YPM 57235. (B) Enantiornithine A NMC 9528. (C) Enantiornithine B YPM 57823. (D) Palintropus retusus YPM 2076. (E) Ornithurine D UCMP 187207. YPM, Yale Peabody Museum; NMC, Canadian Museum of Nature; UCMP, University of California Museum of Paleontology.
Two archaic ornithurines are represented by coracoids. The first, Palintropus retusus (Fig. 1D), was previously identified as a galliform (23). Our phylogenetic analysis instead recovers it as sister taxon to the archaic ornithurine Apsaravis (32); the two are united by the presence of dorsal and medial grooves, loss of the procoracoid, and a flange-like, ventrally curving glenoid. The second, Ornithurine D (Fig. 1E), is recovered as a member of Ichthyornithes. Features shared with Ichthyornis include a dorsally bowed coracoid shaft, a ventrally hooked procoracoid, a weakly hooked acrocoracoid, and a glenoid lateral to the scapular facet.
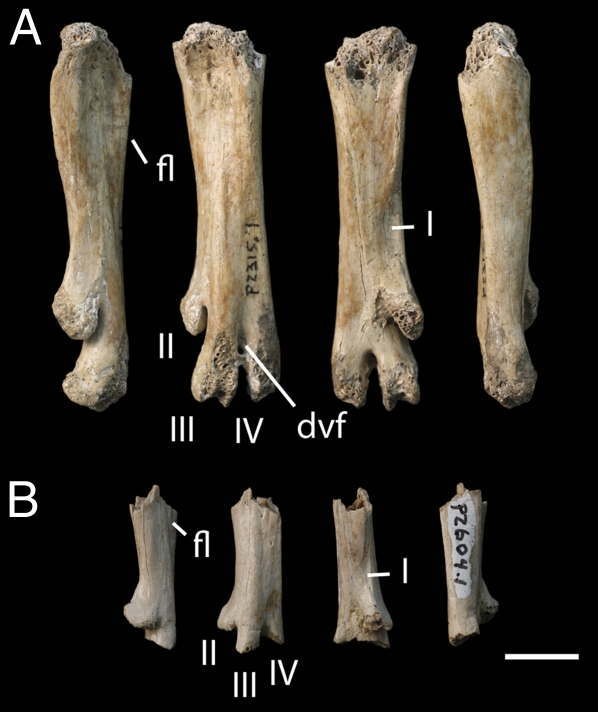
Two species of small Hesperornis-like diving birds are identified on the basis of tarsometatarsi (Fig. 2). Although a putative hesperornithiform has previously been reported from this assemblage (28), this referral was not supported by phylogenetic analysis; the material identified here is therefore unique definitive evidence of Hesperornithes from the end of the Maastrichtian. Synapomorphies of Hesperornithes include a short, caudally displaced metatarsal II, a long, anteriorly displaced metatarsal IV, and a fourth metatarsal with an anteroposteriorly expanded shaft. These forms are more primitive than Baptornis and Hesperornis in lacking a laterally compressed metatarsus or the twisting of the distal metatarsus relative to the proximal metatarsus, but they are almost identical to a small hesperornithiform from freshwater deposits in Mongolia (33). The two species described here differ only in size; the tarsometatarsus of Hesperornithiform B would be roughly two-thirds the length of that of Hesperornithiform A. However, Hesperornithiform B exhibits complete fusion of the element, arguing that it is an adult of a smaller species, and not a juvenile of Hesperornithiform A.
Fig. 2.
Tarsometatarsi of Hesperornithes from the late Maastrichtian of western North America. Left tarsometatarsi in medial, dorsal, plantar, and lateral view. (A) Hesperornithiform A RSM P2315.1. (B) Hesperornithiform B RSM P2604.1. I, facet for metatarsal I; II, metatarsal II; III, metatarsal III; IV, metatarsal IV; dvf, distal vascular foramen; fl, dorsal flange of metatarsal IV; RSM, Royal Saskatchewan Museum. (Scale bar, 1 cm.)
The remaining coracoids belong to derived Ornithurae (i.e., birds closer to Neornithes than to Ichthyornis). These birds and Neornithes are united by an anteriorly displaced glenoid and a long, medially hooked acrocoracoid. However, the available material is inadequate to determine whether any of these species are members of Neornithes as previously asserted (23) or whether they fall outside the clade. Four previously unrecognized forms are present. Ornithurine A (Fig. 3A), previously referred to Cimolopteryx rara (22, 23), is characterized by an ear-shaped glenoid, a circular scapular cotyle, a ventrally positioned supracoracoideus nerve foramen, and an anteriorly projected procoracoid. Ornithurine B (Fig. 3B) is characterized by a narrow glenoid, a slender neck, and a shallow acrocoracoid fossa. Ornithurine C (Fig. 3C), the largest ornithurine in the assemblage, is characterized by a broad glenoid, a massive, bulbous acrocoracoid, a deep acrocoracoid fossa, and a medially extended scapular cotyle. Ornithurine F is characterized by an anteriorly expanded glenoid and an enlarged scapular cotyle (Fig. 3J). Six previously recognized species (22, 23) were examined, and we confirm that they are distinct. These include an unnamed taxon we designate Ornithurine E (Fig. 3E), Ceramornis major (Fig. 3J), and four species placed in Cimolopteryx: C. rara (Fig. 3I), Cimolopteryx minima (Fig. 3G), Cimolopteryx petra (Fig. 3H), and Cimolopteryx maxima (Fig. 3D).
Fig. 3.
Coracoids of derived Ornithurae from the late Maastrichtian of western North America. Left coracoids and right coracoids reversed for comparison. (A) Ornithurine A UCMP 53963. (B) Ornithurine B UCMP 129143. (C) Ornithurine C SDSM 64281. (D) “Cimolopteryx” maxima UCMP 53973. (E) Ornithurine E AMNH 13011. (F) Ceramornis major UCMP 53959. (G) “Cimolopteryx” minima UCMP 53976. (H) “Cimolopteryx” petra AMNH 21911. (I) Cimolopteryx rara YPM 1805. (J) Ornithurine F UCMP 53957. acf, acrocoracoid fossa; lf, lateral fossa, str, strut; UCMP, University of California Museum of Paleontology; SDSM, South Dakota School of Mines; AMNH, American Museum of Natural History; YPM, Yale Peabody Museum. (Scale bar, 1 cm.)
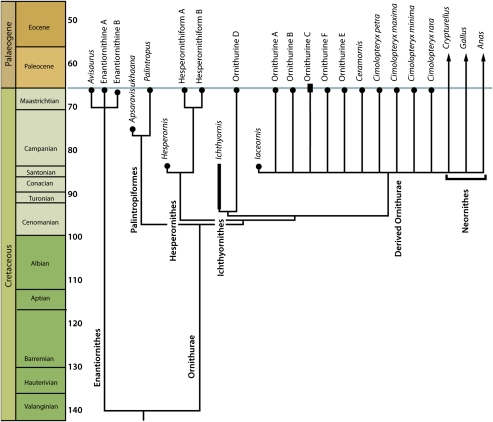
We can therefore identify 15 species on the basis of the coracoid. Most are known from a single specimen, suggesting that the assemblage is undersampled, which is confirmed by rarefaction analysis (SI Appendix). None of the coracoids appear to represent Hesperornithes (Fig. 4), so if the hesperornithiform tarsometatarsi are included, there are 17 species, making this the most diverse known Late Cretaceous avian assemblage. Other species previously described from the Lancian include Lonchodytes estesi, Lonchodytes pterygius, Graculavus augustus, Potamornis skutchi, and a parrot-like form (23). We take a conservative approach by excluding them from our taxon count because we cannot rule out the possibility that they belong to species identified from coracoids. However, it is possible that at least some of these represent additional species not included in our analysis.
Fig. 4.
Phylogeny showing relationships and stratigraphic distribution of late Maastrichtian birds (bold) and other avians. Note that the extension of neornithine branches into the mid Late Cretaceous is the result of an unresolved polytomy; the earliest fossil evidence of Neornithes is Maastrichtian (9). See SI Appendix for full results and details of the analysis.
Body Size.
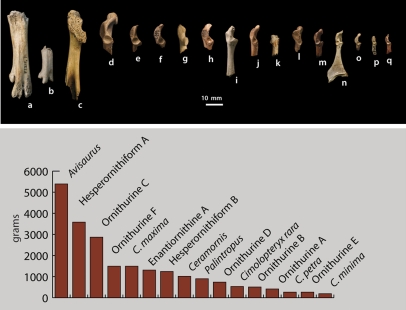
The fossils also show that by the Late Cretaceous, birds had diversified to exploit a wide range of body sizes (Fig. 5), but still do not appear to have exploited the full range of sizes seen in living birds. Avisaurus and Ornithurine C, at roughly 5 and 3 kg, respectively (SI Appendix), are among the largest volant birds known from the Mesozoic. Surprisingly, however, very large (∼10 kg) birds—comparable in size to the extant Trumpeter Swan, Kori Bustard, and White Pelican (34)—are conspicuously absent, although larger birds should be well represented due to taphonomic and collecting biases. Strikingly, smaller birds are also absent; the smallest Lancian bird weighs ∼200 g, whereas many extant finches and sparrows weigh just 10–20 g (34). This pattern may be exaggerated by preservational biases, but given the number of small bones recovered through screenwashing from these deposits, it may not be entirely artifactual.
Fig. 5.
Size range in late Maastrichtian birds. A, Hesperornithiform A; B, Hesperornithiform B; C, cf Avisaurus archibaldi; D, Ornithurine C; E, Ornithurine F; F, Cimolopteryx maxima; G, Enantiornithine A; H, Ceramornis major; I, Ornithurine D; J, Ornithurine B; K, Enantiornithine B; L, Palintropus retusus; M, Ornithurine A; N, Cimolopteryx rara; O, Cimolopteryx petra; P, Ornithurine E; Q, Cimolopteryx minima.
Discussion
The fossils described here show that rather than disappearing gradually over the course of the Cretaceous, at least four separate lineages of archaic birds persisted up to the K–Pg boundary: Enantiornithes, Hesperornithes, Ichthyornithes, and Palintropiformes. These four clades are a major part of the fauna, comprising 7 of the 17 species (41%) recognized here. Definitive fossils of archaic birds have never been reported from the Paleogene (7), and our examination of Paleocene fossils from North America (SI Appendix) failed to identify any archaic birds.
Significantly, enantiornithines are not the dominant members of this fauna. Although it has been argued that enantiornithines dominated Mesozoic terrestrial ecosystems (3, 4), this assemblage is actually dominated by ornithurines (23) (Fig. 4). In particular, many of these birds were found to represent advanced ornithurines, i.e., closer to the crown than Ichthyornis. We can therefore document the existence of a major radiation of advanced ornithurines before the end of the Cretaceous. However, we could not definitively refer any of these fossils to the avian crown; thus claims of a major neornithine radiation in the Cretaceous are not at present supported by the fossil record. One of these species, Ornithurine C, is known from the Paleocene and therefore represents the only Maastrichtian bird known to cross the K–Pg boundary.
The North American record is critical to understanding the dynamics of the K–Pg transition because these fossils can be constrained to the final part of the Cretaceous. Outside of North America, only a handful of archaic birds can be constrained to the last half of the Maastrichtian (9, 35). Nevertheless, a wide range of archaic birds are now known from the Late Cretaceous of Asia (32, 33, 36, 37), Europe (35, 38), South America (31, 39), and Madagascar (40) (SI Appendix). The lack of temporal constraint makes it difficult to be certain that these birds were part of an abrupt extinction coinciding with the K–Pg boundary, yet these fossils do emphasize that the Late Cretaceous harbored an avian fauna that differed radically from that of the Cenozoic. Worldwide, Late Cretaceous avifaunas contain a wide range of archaic forms, including Enantiornithes and basal Ornithurae, which are replaced by neornithines in the Paleogene. Thus, whereas the fossil record outside of North America may not allow us to infer a mass extinction of archaic birds at the K–Pg boundary, it is entirely consistent with it, and consistent with the idea that the catastrophic extinction seen in North America was a global event. We predict that as our understanding of Late Cretaceous avian diversity improves, and as it becomes possible to constrain the ages of these fossils more tightly, the patterns seen in North America will be revealed to be part of a worldwide extinction event.
In conclusion, the persistence of archaic birds up to the K–Pg boundary in North America and the absence of identifiable members of modern orders show that this latest Cretaceous avifauna was still far from modern, and they underscore the extent to which the end-Cretaceous mass extinction has shaped avian diversity. All available fossil evidence is consistent with a major extinction of archaic birds coinciding with the K–Pg boundary, which may have provided an ecological release, permitting the radiation of modern birds in the Paleogene.
Materials and Methods
Phylogenetic analysis was undertaken using a modified version of a previously published matrix (30). Twenty-two characters from the coracoid and tarsometatarsus were added for a total of 227 characters and 46 taxa. Missing data made it impossible to produce a resolved tree and so we estimated the consensus by using the heuristic search algorithm of PAUP* 4.10 b10 (41) to find 100,000 most parsimonious trees and construct a consensus (SI Appendix). To determine whether the fauna was well sampled, we rarefied data for coracoids using the PAST program (42). Finally, to estimate mass, we measured glenoid length and tarsometatarsus length from osteological preparations (SI Appendix) against body mass (34) and fit a reduced major axis (RMA) regression to log-transformed data.
Supplementary Material
Acknowledgments
We thank Marilyn Fox, Pat Holroyd, Mark Norell, Carl Mehling, and Chris Norris for access to and assistance with specimens; Tom Stidham, Kevin de Quieroz, and Jacques Gauthier for discussions; Evgeny Kurochkin for photographs of fossils; Julia Clarke for constructive comments on this manuscript; and the Yale Peabody Museum field crew who collected Avisaurus and Enantiornithine B. N.R.L. was funded by the Yale Institute for Biospheric Studies and D.J.F. was funded by a Natural Sciences and Engineering Research Council Canada Graduate Scholarship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110395108/-/DCSupplemental.
References
- 1.Chiappe LM. Glorified Dinosaurs. Hoboken, NJ: Wiley; 2007. p. 263. [Google Scholar]
- 2.Chiappe LM, Dyke GJ. The Mesozoic radiation of birds. Annu Rev Ecol Syst. 2002;33:91–124. [Google Scholar]
- 3.Feduccia A. Explosive evolution in Tertiary birds and mammals. Science. 1995;267:637–638. doi: 10.1126/science.267.5198.637. [DOI] [PubMed] [Google Scholar]
- 4.Feduccia A. The Origin and Evolution of Birds. New Haven, CT: Yale Univ Press; 1996. [Google Scholar]
- 5.Feduccia A. 'Big Bang' for Tertiary birds? Trends Ecol Evol. 2003;18:172–176. [Google Scholar]
- 6.Chiappe LM. The first 85 million years of avian evolution. Nature. 1995;378:349–355. [Google Scholar]
- 7.Mayr G. Paleogene Fossil Birds. Berlin: Springer; 2009. p. 262. [Google Scholar]
- 8.Novacek M. 100 million years of land vertebrate evolution: The Cretaceous-Early Tertiary transition. Ann Mo Bot Gard. 1999;86:230–258. [Google Scholar]
- 9.Clarke JA, Tambussi CP, Noriega JI, Erickson GM, Ketcham RA. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005;433:305–308. doi: 10.1038/nature03150. [DOI] [PubMed] [Google Scholar]
- 10.Kurochkin E, Dyke GJ, Karhu AA. A new presbyornithid (Aves, Anseriformes) from the Late Cretaceous. Am Mus Novit. 2002;3386:1–11. [Google Scholar]
- 11.Schulte P, et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science. 2010;327:1214–1218. doi: 10.1126/science.1177265. [DOI] [PubMed] [Google Scholar]
- 12.Archibald JD, Bryant LJ. Sharpton VL, Ward PD, GSA Special Paper, editors. Differential Cretaceous–Tertiary extinction of nonmarine vertebrates; evidence from northeastern Montana. Global Catastrophes in Earth History: An Interdisciplinary Conference on Impacts, Volcanism, and Mass Mortality. 1990 247549–562. [Google Scholar]
- 13.Labandeira CC, Johnson KR, Lang P. Preliminary assessment of insect herbivory across the Cretaceous-Tertiary boundary: Major extinction and minimum rebound. GSA Special Paper. 2002;361:297–327. [Google Scholar]
- 14.Nichols DJ, Johnson KR. Plants and the K-T Boundary. Cambridge: Cambridge Univ Press; 2008. p. 280. [Google Scholar]
- 15.MacLeod N, et al. The Cretaceous–Tertiary biotic transition. J Geol Soc London. 1997;154:265–292. [Google Scholar]
- 16.Padian K, Chiappe LM. The origin and early evolution of birds. Biol Rev Camb Philos Soc. 1998;73:1–42. [Google Scholar]
- 17.Buffetaut E. Giant ground birds at the Cretaceous-Tertiary boundary: Extinction or survival? GSA Special Paper. 2002;356:303–306. [Google Scholar]
- 18.Hedges SB, Parker PH, Sibley CG, Kumar S. Continental breakup and the ordinal diversification of birds and mammals. Nature. 1996;381:226–229. doi: 10.1038/381226a0. [DOI] [PubMed] [Google Scholar]
- 19.Cooper A, Penny D. Mass survival of birds across the Cretaceous-Tertiary boundary: Molecular evidence. Science. 1997;275:1113. doi: 10.1126/science.275.5303.1109. [DOI] [PubMed] [Google Scholar]
- 20.Ericson PGP, et al. Diversification of Neoaves: Integration of molecular sequence data and fossils. Biol Lett. 2006;2:543–547. doi: 10.1098/rsbl.2006.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh OC. Notes on Mesozoic vertebrate fossils. Am J Sci, Series. 1892;3 55:171–175. 173 plates. [Google Scholar]
- 22.Brodkorb P. Proceedings of the XIII International Ornithological Congress. NY: Ithaca; 1963. Birds from the Upper Cretaceous of Wyoming. 1955–70. [Google Scholar]
- 23.Hope S. The Mesozoic record of Neornithes (modern birds) In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. CA: University of California Press, Berkeley; 2002. pp. 339–388. [Google Scholar]
- 24.Wilson GP, Dechesne M, Anderson IR. New latest Cretaceous mammals from Northeastern Colorado with biochronologic and biogeographic implications. J Vertebr Paleontol. 2010;30:499–520. [Google Scholar]
- 25.McIver EE. The paleoenvironment of Tyrannosaurus rex from southwestern Saskatchewan, Canada. Can J Earth Sci. 2002;39:207–221. [Google Scholar]
- 26.Hicks JF, Johnson KR, Obradovich JD, Tauxe L, Clark D. Magnetostratigraphy and geochronology of the Hell Creek and basal Fort Union Formations of southwestern North Dakota and a recalibration of the age of the Cretaceous-Tertiary boundary. GSA Special Paper. 2002;361:35–56. [Google Scholar]
- 27.Brett-Surman MK, Paul GS. A new family of bird-like dinosaurs linking Laurasia and Gondwanaland. J Vertebr Paleontol. 1985;5:133–138. [Google Scholar]
- 28.Elzanowski A, Paul GS, Stidham TA. An avian quadrate from the Late Cretaceous Lance Formation of Wyoming. J Vertebr Paleontol. 2000;20:712–719. [Google Scholar]
- 29.Longrich NR. An ornithurine-dominated avifauna from the Belly River Group (Campanian, Upper Cretaceous) of Alberta, Canada. Cretac Res. 2009;30:161–177. [Google Scholar]
- 30.Zhou Z, Clarke J, Zhang F. Insight into diversity, body size and morphological evolution from the largest Early Cretaceous enantiornithine bird. J Anat. 2008;212:565–577. doi: 10.1111/j.1469-7580.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiappe LM, Walker CA. Skeletal morphology and systematics of the Cretaceous Euenantiornithes. In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 241–267. [Google Scholar]
- 32.Norell MA, Clarke JA. Fossil that fills a critical gap in avian evolution. Nature. 2001;409:181–184. doi: 10.1038/35051563. [DOI] [PubMed] [Google Scholar]
- 33.Kurochkin EN. Mesozoic birds of Mongolia and the former USSR. In: Benton MJ, Shishkin MA, Unwin DM, Kurochkin EN, editors. The Age of Dinosaurs in Russia and Mongolia. Cambridge, UK: Cambridge Univ Press; 2000. pp. 533–559. [Google Scholar]
- 34.Dunning JB. Handbook of Avian Body Masses. 2nd Ed. FL: CRC, Boca Raton; 2007. p. 672. [Google Scholar]
- 35.Dyke GJ, et al. Europe's last Mesozoic bird. Naturwissenschaften. 2002;89:408–411. doi: 10.1007/s00114-002-0352-9. [DOI] [PubMed] [Google Scholar]
- 36.Bell AK, et al. Description and ecologic analysis of Hollanda lucera, a Late Cretaceous bird from the Gobi Desert (Mongolia) Cretac Res. 2010;31:16–26. [Google Scholar]
- 37.Dyke GJ, Malakhov DV, Chiappe LM. A re-analysis of the marine bird Asiahesperornis. Cretac Res. 2006;27:947–953. [Google Scholar]
- 38.Wang X, Csiki Z, Osi A, Dyke GJ. The first definitive record of a fossil bird from the Upper Cretaceous (Maastrichtian) of the Hateg Basin, Romania. J Vertebr Paleontol. 2011;31:227–230. [Google Scholar]
- 39.Clarke JA, Chiappe LM. A new carinate bird from the Late Cretaceous of Patagonia (Argentina) Am Mus Novit. 2001;3323:1–23. [Google Scholar]
- 40.O'Connor PM, Forster CA. A Late Cretaceous (Maastrichtian) avifauna from the Maevarano Formation, Madagascar. J Vertebr Paleontol. 2010;30:1178–1201. [Google Scholar]
- 41.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) MA: Sinauer, Sunderland; 2002. 4.0b10. [Google Scholar]
- 42.Hammer Ø, Harper D. Paleontological Data Analysis. Oxford: Blackwell; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.