Abstract
Stereopsis, the perception of depth based on the disparity of the images projected to the retinas of the two eyes, is an important process in our three-dimensional world; however, 3–5% of the population is stereoblind or has seriously impaired stereovision. Here we provide evidence for the recovery of stereopsis through perceptual learning, the repetitive practice of a demanding visual task, in human adults long deprived of normal binocular vision. We used a training paradigm that combines monocular cues that were correlated perfectly with the disparity cues. Following perceptual learning (thousands of trials) with stereoscopic gratings, five adults who initially were stereoblind or stereoanomalous showed substantial recovery of stereopsis, both on psychophysical tests with stimuli that contained no monocular cues and on clinical testing. They reported that depth “popped out” in daily life, and enjoyed 3D movies for the first time. After training, stereo tests with dynamic random-dot stereograms and band-pass noise revealed the properties of the recovered stereopsis: It has reduced resolution and precision, although it is based on perceiving depth by detecting binocular disparity. We conclude that some human adults deprived of normal binocular vision can recover stereopsis at least partially.
Keywords: stereo training, strabismus/amblyopia, adult plasticity
Stereoblindness and stereoanomaly often result from strabismus (a turned eye) or amblyopia (lazy eye) during early childhood. If not treated early enough, strabismus and amblyopia may result in reduced or no stereopsis. Treatment is seldom undertaken in adults; however, Sue Barry's (1) transformative journey from the many visual, social, and psychological challenges of strabismus early in life, to the sudden enrichment of her perceptions of the world following successful unconventional visual therapy begun at 48 years of age suggests that recovery of stereopsis in adults may be possible. Much of Barry's training focused on getting her eyes into alignment, so that she could take advantage of any stereo mechanisms that may have been present but not useful because of the eye turn.
In the present study, we used a stereoscope to aid eye alignment and perceptual learning (PL) to train stereopsis in five adults who were stereoblind or stereoanomalous. Recent studies suggest PL may provide an important method for recovery of vision in adults with amblyopia (2), leading to improvement in Vernier acuity (3, 4), position discrimination (5), spatial interaction (6), contrast detection (7), and letter recognition (8–10). In a few instances, improvement of stereopsis appears to be a side benefit of improving monocular vision through PL in juvenile amblyopia (11) or by reducing suppression (12). Recently, Nakatsuka et al. (13) reported that adult monkeys with mild stereo deficiencies (i.e., that required a larger depth cue than normal) improved their stereoacuity through PL after 10,000–20,000 trials. The purpose of the present study was to test whether the recovery of stereopsis can be induced through PL, in human observers who have suffered longstanding stereoblindness and, if so, the nature of the recovered stereopsis. To induce stereopsis, we used a PL paradigm that combines monocular cues that are correlated perfectly with the disparity cues (see “Rationale for Using Monocular Cues in Training Sessions” in Discussion and Methods for details).
Results
Stereo Training.
Stereo training began by first establishing binocular fusion and alignment (14, 15) (Fig. 1A) by reducing the contrast of the dominant eye's (DE's) frame (Fig. 1A, Left) until both frames (Fig. 1A, Left and Center) were visible through a stereoscope and adjusting the vertical and horizontal positions of the two frames separately. Reducing the frame contrast of the DE enables fusion and also may benefit the training through the push–pull effect (16). Before stereo training, our observers with abnormal binocular vision had already achieved binocular fusion and alignment through our previous binocular combination study (15).
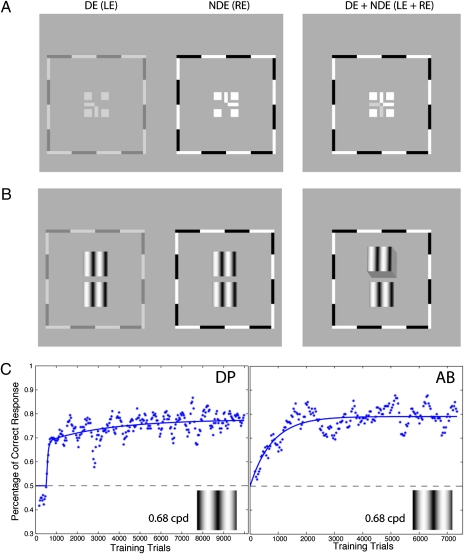
Fig. 1.
Stimuli and results for experiment 1. (A) Each training trial began with binocular-fusion-assisting frames. By decreasing the contrast of the frame of the DE (the left eye, LE) until both frames (Left and Middle) were visible and adjusting the vertical and horizontal positions of the two frames separately, strabismic/amblyopic observers were able to achieve binocular fusion and alignment (Right). RE, right eye. (B) Once fusion was achieved, vertically aligned target (Upper) and reference (Lower) gratings were presented to the two eyes stereoscopically until a response was given. Each stimulus patch had square envelopes in both the horizontal and vertical directions. (C) Results: percentage of correct responses vs. the number of training trials. The horizontal dashed line indicates the chance level (50%). Each point represents the performance based on 180 trials, updated in increments of 36 trials.
In the first experiment, the stimuli were sine-wave gratings with identical contrast (24%) and spatial frequency [0.68 cycles per degree (cpd)] presented to the two eyes (Fig. 1B). The target was shifted horizontally to produce either a crossed (Near) or uncrossed (Far) edge disparity [±165 arc seconds (arcsec)]. The observer's task was to report whether the upper grating appeared in front of or behind the lower grating. Our normal-sighted observers performed perfectly on this task (100% correct responses). Fig. 1C shows the results for two stereoblind observers with the percentage of correct responses as a function of the number of training trials. The horizontal dashed line indicates the chance level (50%). Both stereoblind observers showed substantial improvement (albeit at different rates) from chance performance to ∼80% correct.
Encouraged by the results of the first experiment, we designed a second experiment for further training observers DP and AB, in which the stereo threshold was measured repeatedly. To be able to measure a subpixel stereo threshold, Gaussian-enveloped sine-wave gratings with phase disparities (from −165 arcsec to 165 arcsec) were used in the training. To challenge any latent stereopsis (17), we tried sine-wave gratings with higher contrast (96%) and finer stripes (5.44 cpd), shown as insets in Fig. 2. After training, both observers showed further small (∼50%) but significant (P < 0.05) improvement with time constants of ∼200 and 800 trials, respectively, similar to the learning curves of experienced normal observers but with higher thresholds.
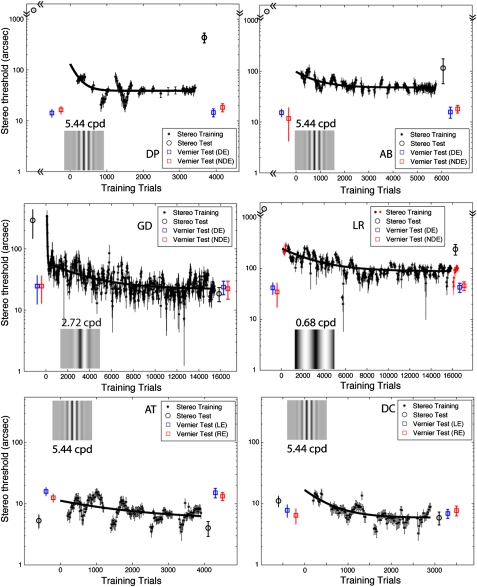
Fig. 2.
Results for experiment 2 showing stereo threshold as function of the number of training trials. The data were grouped by different days. For each day, the initial 180 trials were used to estimate the first threshold, and subsequent thresholds were estimated after every 36 trials based on the previous 180 trials. DP and AB (Top Row) had no stereo perception before training but achieved it through experiment 1. GD (Middle Row Left) already had stereo perception before training. LR (Middle Row Right) had no stereo perception and no stereo training beforehand. AT and DC (Bottom Row) were normal observers who, like observers DP, AB, GD, and LR, had experience in viewing through the stereoscope in binocular combination tasks before training. Each measured point (black asterisk) was the average of thresholds in both Near and Far directions, estimated by fitting a psychometric function (Fig. 7). Because of the large response bias, the first-day stereo threshold for observer LR could not be measured in the Near direction and was taken only in the Far direction (red asterisks on day 1; for comparison, the stereo threshold for the last day also is specified in this way). Stereo tests (circles) with stimuli that contained no monocular cues (PDT achieved by adding substantial positional jitter in the range from −827 to +827 arcsec to the target) were given before and after training. Before training, strabismics LR, AB, and DP were stereoblind (>1,320 arcsec, represented by circles without error bars at the top of the plots). Monocular Vernier tests (squares), using the same setup and the same stimuli as in stereo training, also were given before and after training. Error bars: ± 1 SE.
We used the same sine-wave gratings to train two different abnormally sighted observers, one (GD) with anisometropic amblyopia who had degraded stereopsis before training and one (LR) with strabismus who was stereoblind before training. Neither showed learning effects using gratings at 5.44 cpd. For observer GD, decreasing the spatial frequency to 2.72 cpd (Fig. 2 Middle Left, Inset) resulted in a rapid improvement in stereo performance (time constant ∼210 trials) followed by a slow learning phase (time constant ∼3,500 trials) before reaching plateau. This improvement in an anisometropic amblyope with degraded stereopsis is consistent with a recent case report documenting similar improvements in two anisometropic adults (18). For the strabismic observer LR, we reverted to stimuli (Fig. 2 Middle Right, Inset) that were identical to those used in experiment 1. On the first day of training her stereo threshold was out of the range of measurement in the Near direction because of her strong depth bias, and thus the threshold was measured only in the Far direction (red asterisks in Fig. 2) (see Fig. 7). However, the bias diminished with training, and her stereopsis improved slowly, with a time constant of ∼3,000 trials to reach plateau.
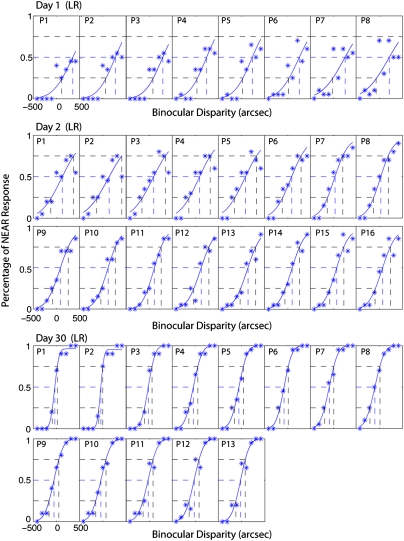
Fig. 7.
Example psychometric functions for stereo training in experiment 2 showing percentage of NEAR response as function of binocular disparities.
The bottom row of Fig. 2 shows results for observers with normal vision. Like our abnormal observers, normal observers AT and DC had participated in our previous binocular combination project; therefore, they had experience viewing through a stereoscope before training. Their stereo performance improved slightly (∼50%) and reached plateau by the third training day.
Pre- and Posttraining Stereo Tests.
Before and after PL, we performed stereo tests with stimuli that contained no monocular cues [pure disparity test (PDT) indicated by circles in Fig. 2]. Observers with abnormal binocular vision improved their stereo performance substantially. Indeed, three strabismics who were stereoblind before training (circles without error bars at the tops of plots) achieved stereopsis through stereo training. Interestingly, although the training stimuli contained monocular position cues, the improvement of stereovision was not a consequence of improved monocular position discrimination. After training, monocular Vernier acuities (squares in Fig. 2; asterisks and plus markers in Fig. 3A) remained unchanged for all observers. Thus, the improvement of stereovision through stereo training was not likely the result of an improvement in monocular vision (11). We also noticed that abnormal observers who had strabismus had poorer stereo performance when using stimuli without monocular cues. Only observer, GD, who had anisometropia but no strabismus, like normal observers, achieved similar stereoacuity with and without monocular cues.
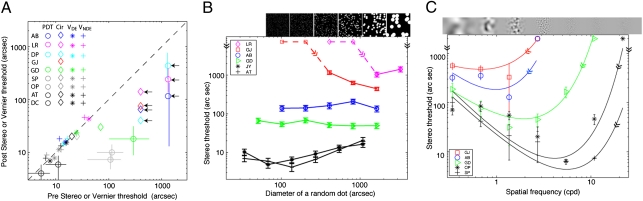
Fig. 3.
Results for stereo tests. (A) Comparison of stereo or Vernier threshold before and after stereo training. The data points below the diagonal dashed line indicate improvement after training. The arrows on the right demonstrate that the real thresholds before training were larger (i.e., performance was poorer) than indicated by the individual marks and approached the indicated values through training. The error bars at measurable values in PDT represent 95% confidence intervals. Abnormal observers: AB, LR, DP, GJ, and GD (colored marks); experienced normal observers: AT and DC (black marks); naïve normal observers: SP and OP (gray marks); PDT, pure disparity test; Cir, clinical circle test (Randot); VDE, Vernier test on dominant eye or left eye; VNDE, Vernier test on nondominant eye or right eye. (B) Results of tests with DRS showing stereo threshold as a function of dot size and density. Dot density decreases when dot size increases (size*density remains constant). Insets show samples of stimuli. Error bars: ± 1 SE. (C) Results of tests with BN, stereo threshold as a function of spatial frequency. Insets show samples of stimuli. Error bars: ± 1 SE.
Fig. 3A shows a comparison of test results before and after training for both stereo and Vernier thresholds. All observers with abnormal binocular vision (AB, LR, DP, GJ, and GD) achieved substantial stereo improvements (points below the 1:1 line) in both psychophysical tests (PDT, colored circles) and clinical circle tests (Cir, colored diamonds). Error bars at measurable values in the PDT tests indicate 95% confidence intervals, demonstrating that these improvements were significant in abnormal observers.
Importantly, before training our strabismic observers (AB, LR, DP, and GJ) were well adapted to viewing through a stereoscope and already had learned how to achieve binocular fusion and alignment through our previous binocular combination project (about 5,000–20,000 trials) (15). However, except for observer GJ, who achieved stereopsis without further stereo training, the three other strabismics failed both clinical and psychophysical stereo tests before training even though they were able to align and fuse the two eyes’ images. For anisometropic observer GD, who also had extensive experience viewing through a stereoscope (about 20,000 trials) and had normal alignment of the two eyes (clinical cover test), after a fast learning phase, stereo performance also improved slowly (about 15,000 training trials) but significantly through training on both clinical and psychophysical tests.
Normal observers (AT and DC) with similar experience in our previous binocular combination project, whose stereo performance on PDT already was excellent (∼10–15 arcsec) before training, improved only slightly after training. Their performance on the clinical circle test was already at the ceiling level before training. We note that for naïve observers, stereo performance might be much poorer initially when viewing through a stereoscope than when seeing real objects in depth (19), and the learning effect may reflect, in part, adaptation to viewing virtual depth through a stereoscope (19). To evaluate this adaptation effect, we tested two naïve normal observers SP and OP (Table 1). Indeed, before training, their performance on PDT was much poorer than that of the experienced normal observers (∼100 arcsec), although their performance on the clinical circle test already was at the ceiling level (20 arcsec). Through training, their performance improved quickly to reach the plateau (10 arcsec) with a time constant of ∼1,000 trials. Unlike the anisometropic observer GD, after the fast-learning phase the naïve normal observers showed no further improvements in the total of about 7,000 trials.
Table 1.
Stereo test results
| Stereo before training |
Stereo after training |
||||||
| Observer | Circles | PDT | Circles | DRS | Sine | PDT | BN |
| Strabismic (“stereoblind”) | |||||||
| DP | >400” | >1,320” | 40′′ | None | 36′′ | 435′′ | None |
| AB | >400” | >1,320” | 70′′ | 135′′ | 48′′ | 116′′ | 185” 1.0 cpd |
| LR | >400” | >1,320” | 140′′ | 1,043′′ | 88′′ | 241′′ | None |
| GJ* | >400” | ? | 70′′ | 444′′ | 142′′ | 315′′” | 475” 0.7 cpd |
| Anisometropic (“stereoanomalous”) | |||||||
| GD | 70′′ | 293′′ | 30′′ | 49′′ | 22′′ | 18′′ | 47” 1.9 cpd |
| Normal | |||||||
| SP† | 25′′ | 108′′ | 20” | 5.1′′ | 7.3′′ | 4.4′′ 5.5 cpd | |
| OP† | 20′′ | 115′′ | 20′′ | 13′′ | 10′′ | 7.4′′ 4.1 cpd | |
| AT | 20′′ | 5.3′′ | 20′′ | 7.6′′ | 5.5′′ | 4.0′′ | |
| DC | 20′′ | 11′′ | 20′′ | 9.6′′ | 6.0′′ | 5.9′′ | |
BN, psychophysical test with band-pass noise (no monocular cues). Peak acuity and spatial frequency are shown. Circles, clinical circle test (Randot), total 10 levels from 20–400 arcsec. The smallest stereo threshold that this test can measure is 20 arcsec. DRS, psychophysical test with dynamic random-dot stereogram. PDT, pure disparity test, a psychophysical test with sine waves that contained no monocular cues (target's horizontal positions jittered in the range from −827 to +827 arcecs); Sine, psychophysical test with sine waves that contained correlated monocular cues.
*Strabismic observer GJ regained stereopsis through our previous binocular combination project and did not participate in training sessions in this project. Before participating in our previous project, no PDT was run for him.
†Normal observers SP and OP had no experience in viewing through a stereoscope to perform any binocular task before this stereo training project.
Nature of Recovered Stereo.
Some consider the appreciation of depth in random-dot stereograms to be the gold standard for stereopsis because the stereograms contain no monocular information (20, 21). However, none of our strabismic observers were able to detect binocular disparities in clinical random-dot stereograms (Randot), consistent with previous studies on small-angle strabismics who had stereovision but still were stereoblind when using clinical random-dot stereograms (22, 23). We reasoned that this failure of detection may have occurred because, in the clinical test, the dots are small and dense, low in contrast, and static, making them less than optimal for a strabismic observer to detect depth. To test this idea, we developed a psychophysical stereo test with high-contrast, dynamic random-dot stereograms (DRS) that eliminate any possible monocular cues. Except for the strabismic observer DP, all observers were able to detect depth in our DRS test (Fig. 3B), although observers LR and GJ could do so only when the dots were large enough. To explore further the nature of the stereopsis in these observers, we also used bandpass noise stimuli (without any monocular cues) and found that the cutoff spatial frequency of recovered stereopsis in adult strabismics was much lower than in normal observers and that the peak performance was more than 20 times worse (Fig. 3C) than in normal observers. Therefore, the stereopsis recovered through PL in adult strabismics is different from that of persons with normal vision. Although the posttraining stereo tests with stimuli that contained no monocular cues revealed that the recovered stereopsis is not an artifact of monocular cues, the monocular cues might play an important role in the stereoprocessing of strabismics. For normal and anisometropic observers, stereo performance is identical whether monocular cues are present or not. For our strabismic observers, however, stereo performance is less precise without monocular cues (i.e., with the PDT).
Table 1 summarizes the stereo test results for all observers. The observers with strabismus showed poorer stereoacuity in DRS and for bandpass noise (BN) stimuli, which contain only disparity information, than in the sine-wave grating test. Only observer DP, who had the largest ocular deviation, was unable to appreciate depth using DRS or BN.
Discussion
It has long been known that stereoacuity can be improved through PL in normal human adults (24, 25). Here we document recovery of stereopsis through PL in human adults who are stereoblind or severely stereodeficient. Our results are consistent with the recent report of Nakatsuka et al. (13) that adult monkeys with mild stereo deficiencies (i.e., that required a larger depth cue) improved their stereoacuity through PL. However, these investigators were unable to train monkeys with severe deficits (e.g., that had no response to depth cues).
Alignment and Fusion Training.
Achieving binocular alignment and fusion might be the first step in the recovery of stereopsis and seems to play a key role in the stereo training. Typically, a strabismic observer views the world monocularly, viewing through the DE or switching between two eyes and suppressing one eye, rather than fusing the two eyes’ images. For stereopsis, however, viewing the two eyes’ images simultaneously and achieving binocular alignment and fusion are essential. For this purpose, we designed a dichoptic cross with binocular fusers (Fig. 1A), a surrounding high-contrast frame, and four luminance squares to assist binocular alignment and fusion in our stereoscope. Our custom four-mirror stereoscope was adjusted carefully for each observer to enable the observer to align the nonius lines easily (both vertically and horizontally).
Initially, our strabismic observers seldom perceived the two eyes’ images simultaneously. By reducing the contrast of the DE's image, it became possible to perceive the two eyes’ images, but the percept was unstable, and for much of the time only the DE's image was seen. Initially the observer might take several minutes to perceive both eyes’ images simultaneously, and the percept was fleeting, lasting only 2 or 3 s. With practice, however, the latency to see both images became shorter, and the duration became longer. After thousands of trials, observers achieved stable binocular fusion; the dichoptic cross appeared stable and continuous.
Our three strabismic observers, DP, AB, and LR, had achieved stable binocular fusion through these binocular combination tasks but still remained stereoblind before beginning the stereo training. They achieved stereopsis only with specific stereo training. However, one observer, GJ, achieved stereopsis through the binocular combination tasks alone, with no stereo training. Before performing any binocular tasks, he showed no stereovision on the clinical circle test (Randot). However, after running more than 10,000 binocular combination trials (15), he achieved stereovision without any further stereo training. We suspect that this alignment-fusion training benefits binocular vision (at least for one of our strabismic observers). However, we are not sure if binocular fusion is possible for strabismics in natural viewing circumstances, even though they reported seeing 3D objects in daily life. From the literature it is clear that binocular disparity can be detected without binocular fusion.
Interocular Contrast Ratio and Monocular Controls.
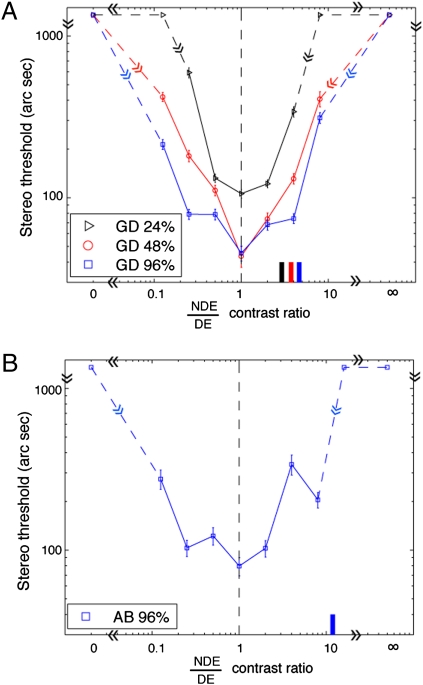
For normal observers, the best stereo performance occurs when the two eyes are presented with identical contrast (26). However, when strabismic and/or amblyopic observers are presented with identical contrast, the nondominant eye (NDE) typically fails to see images because of strong suppression by the DE. To perceive the two eyes’ images equally, the DE's contrast should be decreased (15, 27). Typically, at balanced vision, the NDE's contrast is ∼2–10 times that of the DE's (15). Before stereo training, we performed a preliminary experiment to test how the stereo performance depended on NDE/DE contrast ratio in an observer (GD) with degraded stereo perception. The stimuli were identical to those used in the PDT at 0.68 cpd of spatial frequency, except that the interocular contrast ratio of stimuli varied from trial to trial. We found that at all base contrasts the best stereo performance occurred when the two eyes were presented with identical physical contrast (Fig. 4A), not at perceptually balanced contrast (indicated by short colored bars along the abscissa). Therefore, we used stimuli with identical contrast in the two eyes for both stereo training and tests, although the frame contrasts (Fig. 1A) required for fusion were different. After stereo training, we performed the same experiment using the stimuli shown in Fig. 5A at spatial frequency of 0.68 cpd on an observer (AB) who achieved stereopsis from the training. Again, her performance was best when her two eyes were presented with identical physical contrast (Fig. 4B), whereas at perceptually balanced contrast (indicated by the short blue bar at the bottom), her stereo performance was very poor. It seems that the strong suppression of the NDE by the DE has little effect on the stereo performance in the recovered stereopsis for our amblyopic/strabismic observers. This surprising result may be relevant to the neurophysiological data reported by Nakatsuka et al. (13), which imply that the improvement of disparity sensitivity in prism-reared monkeys occurs primarily beyond cortical area V1.
Fig. 4.
Results for PDT at different interocular contrast ratios when the spatial frequency was 0.68 cpd and the base contrast (the higher contrast in two eyes) was 96% (blue), 48% (red), or 24% (black). (A) Results for observer GD. (B) Results for observer AB. For each base contrast, when the NDE/DE contrast ratio was <1, the DE's contrast remained constant at the base contrast, and the contrast ratio was increased by increasing the NDE's contrast. When the NDE/DE contrast ratio was >1, the NDE's contrast remained constant at the base contrast, and the contrast ratio was increased by decreasing the DE's contrast. Marks without an error bar at the top of the panel indicate that no depth could be detected under these conditions. An NDE/DE contrast ratio at 0 or ∞ represents a monocular control condition (only one eye was presented with stimuli). A short colored bar along the abscissa indicates the NDE/DE contrast ratio at which both eyes perceived stimuli equally. Error bar: 1 SE.
Fig. 5.
Stimuli for stereo training and stereo testing (PDT). (A) Stimuli with sharp edges at a lower spatial frequency. (B) Stimuli with Gaussian envelopes at a higher spatial frequency. In a training session, the sine-wave gratings were in phase, and the top and bottom patches were aligned vertically (see Fig. 1B). In a session for PDT, the position of sine-wave gratings was jittered from trial to trial.
For the monocular control conditions (only one eye was presented with stimuli, NDE/DE = 0 or ∞), no depth could be detected (indicated by symbols without error bars at the top of the figure); therefore, our PDT contained no useful monocular cues for depth perception.
Rationale for Using Monocular Cues in Training Sessions.
A special feature of our PL paradigm is that the stimuli used in training sessions contained monocular position cues that were correlated perfectly with the binocular disparities. The rationale for including monocular position cues was twofold. First, if stereoblind observers were presented repeatedly with a stimulus that contained only a pure disparity cue and were asked to judge the relative depth, they became frustrated and gave up or responded randomly. On the other hand, if a stereoblind observer was presented a stimulus with perfectly correlated disparity and relative monocular position information and was given response feedback, the observer could learn to associate the monocular information with the appropriate depth response. Second, and more importantly, Wilcox et al. (28) have shown that there is a monocular input to stereoscopic depth mechanisms. We reasoned that through repeated practice with stimuli that contained correlated monocular and depth information, stereoblind observers might learn to perceive depth from binocular disparity.
Benefit in Everyday Life.
Stereoblind or stereodeficient individuals who recover stereopsis may gain substantial benefit in everyday life (1). After achieving stereopsis, our observers reported that the depth “popped out,” which they found very helpful and joyful in their everyday life. The anisometropic observer GD noticed “a surge in depth” one day when shopping in a supermarket. While playing table tennis, she feels that she is able to track a ping-pong ball more accurately and therefore can play better. Strabismic observer AB is more confident now when walking down stairs because she can judge the depth of the steps better. Strabismics AB, DP, and LR, are able to enjoy 3D movies for the first time, and strabismic GJ finds it easier to catch a fly ball while playing baseball.
Conclusions
Here we have documented the recovery through PL of stereopsis in humans with strabismus. Although these findings may suggest learning-induced improvements in cortical disparity processing, we cannot exclude the possibility that the observed recovery of function is caused, at least in part, by improvements in vergence control. Future studies will be required to disentangle the role of vergence (if any) in this improvement. We conclude that PL may provide a useful method for treating stereoblindness and stereoanomalies and that the recovered stereopsis may improve the quality of life in persons with stereodeficiencies. Determining the clinical utility of this approach will require a randomized clinical trial.
Methods
Observers.
Nine observers (four with normal binocular vision, four strabismics, and one anisometropic) participated in the experiments. Clinical details are shown in Table 2. Before training, two normal observers had no experience in viewing through a stereoscope. The other seven observers already had been adapted to viewing through a stereoscope and had achieved binocular fusion through other (nonstereoscopic) binocular tasks (5,000–20,000 trials) (15). Indeed, one strabismic observer, GJ, achieved stereopsis just from this fusion training (>10,000 trials) and did not receive any stereopsis training. Thus, we show only his test results.
Table 2.
Clinical details
| Observer | Age (y) | Sex | Condition | Eye | Refractive error | Letter acuity (Snellen) |
| Strabismic (“stereoblind”) | ||||||
| DP | 23 | F | Alternating XT >25 Δ | R( NDE) L (DE) | +1.00 +1.00 | 20/16+1 20/12.5−1 |
| AB | 24 | F | Alternating ET 9 Δ R hyT 8 Δ | R (NDE) L (DE) | −3.75 −6.25 | 20/20 20/20 |
| LR | 28 | F | Alternating XT 5 Δ | R (NDE) L (DE) | −0.75 −4.00 | 20/20 20/20 |
| GJ | 25 | M | R ET 5 Δ | R (NDE) L (DE) | +3.00 –0.25 | 20/40−1 20/16−1 |
| Anisometropic (“stereoanomalous”) | ||||||
| GD | 46 | F | Anisometropia | R (DE) L (NDE) | +0.25 +3.75 | 20/12.5 20/50+2 |
| Normal | ||||||
| SP | 20 | M | Normal | R L | −5.25 −5.0 | 20/16−2 20/16−2 |
| OP | 20 | F | Normal | R L | −0.75 −1.25 | 20/16+1 20/12.5 |
| AT | 27 | F | Normal | R L | −6.50 −6.25 | 20/20 20/20 |
| DC | 23 | F | Normal | R L | Plano Plano | 20/20 20/20 |
| JY | 20 | F | Normal | R L | −1.25 −1.50 | 20/20 20/20 |
XT, exotropia; ET, esotropia; hyT, hypertropia; Δ, prism dioptres.
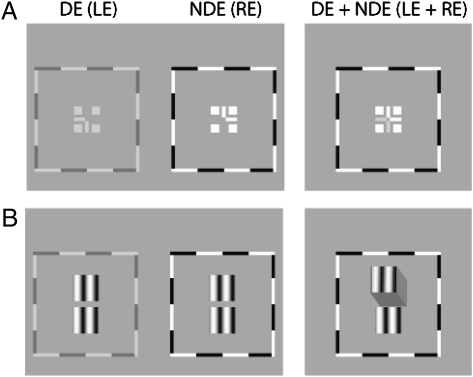
Binocular-Fusion-Assisting Frames.
Fig. 1A shows binocular-fusion-assisting frames that were viewed through a custom stereoscope. The panel on the left shows two frames that were presented to the two eyes at the beginning of each trial. The two frames were identical except that the half-cross, the “7” image without the corner was presented to the left eye or DE, and the other half-cross, the “L” image without the corner was presented to the right eye or NDE. With correct vergence, a whole cross with a blank square in its center was perceived, as shown in the panel on the right. To assist vergence, a high-contrast surrounding frame and four squares also were presented binocularly. By decreasing the contrast of the DE's frame until both frames were visible and adjusting the vertical and horizontal positions of the two frames separately, strabismic/amblyopic observers were able to achieve binocular fusion and alignment (Right). Anisometropic observer GD achieved a stable perception of dichoptic cross when the frame contrast in the DE was reduced to 3.8 times that in the NDE. Strabismic observer AB began to be able to view the dichoptic cross for a short duration when the frame contrast in the DE was 15.8 times lower than in the NDE. Strabismic observers GJ, DP, and LR, could view the dichoptic cross for a short duration under the identical-contrast condition. After fusion training (through our previous binocular combination project), all observers reported a stable perception of dichoptic cross. In the following training and test sessions, the contrast of the DE's frame was held constant for each observer.
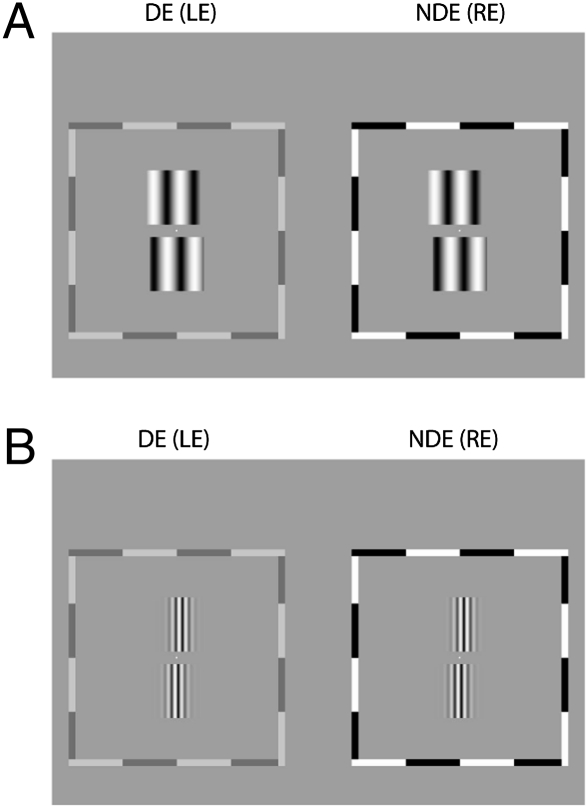
Stimuli for Stereo Training.
Two kinds of sine-wave gratings were used for stereo training, one with sharp edges (Fig. 5A) and one with Gaussian envelopes (Fig. 5B). For each eye, two vertical sine-wave gratings, aligned vertically, were presented (Fig. 1B). The bottom pair served as a reference with zero disparity, i.e., in the same plane as the surrounding frame. The top pair served as a target with a crossed or an uncrossed disparity.
The sharp-edged stimuli (Fig. 1B) were used to train stereoblind observers (DP and AB in experiment 1 and LR in experiment 2). The four sine-wave gratings had identical contrast (24%) and spatial frequency (0.68 cpd). The observation distance was 68 cm. Each sine-wave patch, extending 3 × 3, had square envelopes in both horizontal and vertical directions, rising or falling rapidly at the edges. The target pair had a crossed or uncrossed shift to produce edge disparity (±165 arcsec in experiment 1; −412 to +412 arcsec in experiment 2). The gap between the top and bottom patches was 20 arc minutes (arcmin).
The Gaussian-enveloped stimuli (Fig. 5B) were used to train observers in experiment 2 who had normal, degraded (observer GD) or recovered stereopsis from training of experiment 1 (observers DP and AB). The four gratings had identical contrast (96%) and spatial frequency, 5.44 cpd (observation distance = 136 cm) for normal observers and strabismic observers DP and AB, and 2.72 cpd (observation distance = 68 cm) for anisometropic observer GD. Each sine-wave patch, extending 1.5 × 1.5 or 3 × 3, had Gaussian (σ = 0.18° or 0.37°) and square envelopes in horizontal and vertical directions, respectively. The bottom (reference) pair always had zero disparity (in the same plane of the surrounding frame), and the top (target) pair had a phase disparity, selected randomly from five uncrossed and five crossed phase shifts (either from −165 arcsec to 165 arcsec or from −330 arcsec to 330 arcsec). The gap between the top and bottom patches was 10 arcmin or 20 arcmin. Because these stimuli contained no edge disparity, we hypothesized that they would be more challenging to the observers.
Stimuli for Stereo Tests.
Both clinical (Randot) and psychophysical (viewing through a stereoscope) stereo tests were given to all observers before and after training.
Clinical tests.
Stimuli for clinical tests were polarizing 3D images, provided by Randot Stereotests (Stereo Optical Co., Inc.). Wearing polarized 3D glasses, an observer was asked to detect the depth of a target. In the clinical circle test, at each level of disparity (total of 10 levels from 20–400 arcsec), there were three contoured circles of which only one had crossed disparity. The observer was asked to tell which one seemed to float forward (having crossed disparity). In the clinical random dot stereogram test, at each of the two levels of disparity (250 and 500 arcsec), there were four Randot patches, three of which had a simple geometric form in the center. The observer was asked to identify the forms.
PDT.
Fig. 5 shows the stimuli used for the psychophysical PDT before and after stereo training. The stimuli were identical to those used in the training sessions except that, instead of being vertically aligned with the reference, the target horizontal position was jittered (uniformly distributed in the range from −827 to +827 arcsec) to render any monocular cues useless. The position jitter was identical in the two eyes. Thus, it rendered any monocular disparity information useless but did not add any disparity noise. Test results (Fig. 4) in control conditions with only one eye's image presented show that these tests contained no useful monocular cues.
For strabismic observers AB, DP, and LR, sharp-edged gratings (Fig. 5A) at different binocular disparities (maximum 1,320 arcsec at 90° relative phase shift) were used as test stimuli. For the anisometropic observer GD and normal observers, the test stimuli were the Gaussian-enveloped gratings (Fig. 5B). However, for strabismic observer GJ, who performed only a binocular combination task (i.e., with no stereo training), we performed only clinical tests before his training. The stimuli used in his “after-training” tests were identical to those in experiment 1 with (Fig. 1B, Sine) or without (Fig. 5A, PDT) monocular cues.
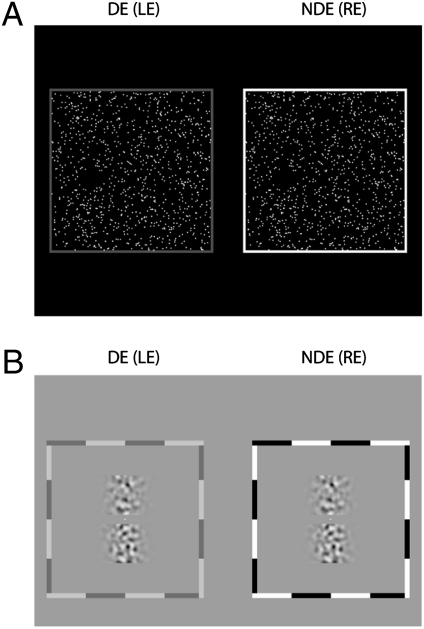
DRS.
Fig. 6A shows the stimuli for posttraining DRS. Circular bright dots (83 cd/m2) on a dark background (0.03 cd/m2), varied in size (34–3,260 arcsec in diameter) and density (63.9–0.67 dots per arc degree) but constant in size*density (∼2,175), were distributed randomly in a 13.7 × 13.7 field (observation distance = 68 cm) and were updated every 200 ms. The target consisted of dots presented in a central area of 3.4 × 3.4 having nonzero binocular disparity (from −1,600 to 1,600 arcsec). The dots in the surround served as a reference, always having zero disparity. The observation distance was 68 cm for strabismic observers LR, GJ, and AB, 136 cm for anisometropic observer GD, and 204 cm for normal observers JY and AT. A demonstration movie is available online (Movie S1). To rule out the possibility that an observer might use binocular correlation to detect depth, we ran a control experiment in which we combined the two eyes’ random-dot patterns into a single monocular stimulus for two normal and two abnormal observers. All four observers failed to detect the depth in this combined monocular stimulus; their performances were around the chance level (50%).
Fig. 6.
Stimuli DRS (A) and tests with BN (B).
Psychophysical tests with BN.
Fig. 6B shows stimuli for posttraining tests with BN. BN was produced by filtering a 2D binary random noise with a 2D isotropic bandpass filter (29, 30). In the spatial frequency domain, the isotropic bandpass filters are defined as
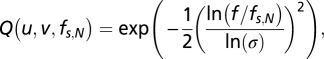 |
where u and v are the dimensions of a 2D Cartesian spatial-frequency coordinate system, f is defined as 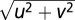 , fs,N is the center spatial-frequency, and σ determines the bandwidth of the filter. The four BN patches had identical maximum contrast and central spatial frequency (0.34, 0.68, 1.36, 2.72, 5.44, 10.88, or 21.76 cpd) with 1.26 octaves of half-amplitude bandwidth. The observation distance was either 136 cm or 68 cm. Each BN patch, extending 4.5 × 1.5 or 9 × 3, had Gaussian (σ = 0.37° or 0.74°) and square envelopes in the horizontal and vertical directions, respectively. The disparity values for each trial were selected randomly from nine values (four uncrossed, four crossed, and one with zero disparities). Gaussian envelopes of the top and bottom patches always were aligned vertically, and the disparities were produced from relative horizontal shifts of noise patterns in top patches.
, fs,N is the center spatial-frequency, and σ determines the bandwidth of the filter. The four BN patches had identical maximum contrast and central spatial frequency (0.34, 0.68, 1.36, 2.72, 5.44, 10.88, or 21.76 cpd) with 1.26 octaves of half-amplitude bandwidth. The observation distance was either 136 cm or 68 cm. Each BN patch, extending 4.5 × 1.5 or 9 × 3, had Gaussian (σ = 0.37° or 0.74°) and square envelopes in the horizontal and vertical directions, respectively. The disparity values for each trial were selected randomly from nine values (four uncrossed, four crossed, and one with zero disparities). Gaussian envelopes of the top and bottom patches always were aligned vertically, and the disparities were produced from relative horizontal shifts of noise patterns in top patches.
Procedure.
For each trial, the observer pressed a key to initiate the trial only after the dichoptic cross (Fig. 1A) appeared stable. After the key press, the target (top) and reference (bottom) patches were presented to each eye (Figs. 1B, 5, and 6) and remained until the observer responded in a training session or for 2 s in a test session. During a training trial, the observer was allowed to move his/her eyes to take advantage of monocular cues during the training, but during a test trial the observer was asked to focus on the fixation point. The observer's task was to indicate whether the top patch was closer or farther than the bottom patch or, in the DRS test, whether the dots in central square area were closer or farther than the dots in surrounding area. Audible feedback was given following the response in a training session, but no feedback was given in a test session. After the response, preparation for the next trial began with the presentation of a dichoptic nonius cross surrounded by a high-contrast frame (Fig. 1A).
For each training day, 180–1,000 trials (about 1–2 h) were given to an observer depending on his/her convenience. Typically, an abnormal observer spent more time on a trial and became fatigued more easily.
Psychometric Functions.
Stereo thresholds were estimated by fitting a psychometric function (a cumulative Gaussian distribution) to the percentage of Near responses vs. binocular disparity.
Fig. 7 shows sample psychometric functions for one observer (LR) in the first two training days (day 1 and day 2) and the last training day (day 30). Uncrossed binocular disparities are designated as negative values on the abscissa, and crossed binocular disparities are designated as positive values. Each psychometric function was fitted to 180 trials (20 trials for each disparity level), in increments of 36 trials. For each panel, the vertical dashed line at 50% Near responses indicates the stereo bias, and the half of the interval between the two vertical dashed lines at 25% and 75% Near responses indicates the stereo acuity, an average threshold in both the Near and Far directions. Because of the large bias, if the performance at the largest near disparity levels did not exceed 75% Near responses, the stereo acuity was taken as the interval between 25% and 50%, i.e., the acuity was measured only in the Far direction because the acuity was out of measurement range in the Near direction.
On day 1, run 1 (Fig. 7, Top) LR's performance was strongly biased, so that even with the large Near (crossed) disparities, she reported that the target was farther than the reference on most trials, and her “Near” responses never exceeded 50%. The disparity threshold was out of measurement range in the Near direction and was measured only in the Far direction, as represented by red asterisks in Fig. 2. As shown in Fig. 7, the disparity bias diminished with training; the disparity threshold became measurable in both the Near and Far directions on day 2 and improved further during the course of the day. With further training (thousands of trials), her disparity threshold improved substantially (day 30).
Supplementary Material
Acknowledgments
The authors thank Dr. Marty Banks for his critical reading and comments; Dr. Stanley Klein and Dr. Thom Carney for their useful suggestions; Dr. Roger Li and Mr. Charlie Ngo for observer recruitment; and Ms. Jennifer Luu for her reading and correction of the manuscript. This work was supported by National Eye Institute Grant R01EY01728 and by a grant from the James S. McDonnell Foundation—Collaborative Network for Critical Period Re-Examination (Brain CPR).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 15035.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105183108/-/DCSupplemental.
References
- 1.Barry SR. Fixing my Gaze: A Scientist's Journey into Seeing in Three Dimensions. New York: Basic Books; 2009. [Google Scholar]
- 2.Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Res. 2009;49:2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci USA. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi DM, Polat U, Hu YS. Improvement in Vernier acuity in adults with amblyopia. Practice makes better. Invest Ophthalmol Vis Sci. 1997;38:1493–1510. [PubMed] [Google Scholar]
- 5.Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. J Vis. 2004;4:476–487. doi: 10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- 6.Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci USA. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Levi DM. Perceptual learning in adults with amblyopia: A reevaluation of critical periods in human vision. Dev Psychobiol. 2005;46:222–232. doi: 10.1002/dev.20050. [DOI] [PubMed] [Google Scholar]
- 9.Chung ST, Li RW, Levi DM. Identification of contrast-defined letters benefits from perceptual learning in adults with amblyopia. Vision Res. 2006;46:3853–3861. doi: 10.1016/j.visres.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung ST, Li RW, Levi DM. Learning to identify near-threshold luminance-defined and contrast-defined letters in observers with amblyopia. Vision Res. 2008;48:2739–2750. doi: 10.1016/j.visres.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest Ophthalmol Vis Sci. 2007;48:5046–5051. doi: 10.1167/iovs.07-0324. [DOI] [PubMed] [Google Scholar]
- 12.Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: Antisuppression therapy. Optom Vis Sci. 2010;87:697–704. doi: 10.1097/OPX.0b013e3181ea18e9. [DOI] [PubMed] [Google Scholar]
- 13.Nakatsuka C, et al. Effects of perceptual learning on local stereopsis and neuronal responses of V1 and V2 in prism-reared monkeys. J Neurophysiol. 2007;97:2612–2626. doi: 10.1152/jn.01001.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Sperling G. A gain-control theory of binocular combination. Proc Natl Acad Sci USA. 2006;103:1141–1146. doi: 10.1073/pnas.0509629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Klein S, Levi D. Binocular combination in amblyopic vision. J Vis. 2009;9(8):274. [Google Scholar]
- 16.Xu JP, He ZJ, Ooi TL. Effectively reducing sensory eye dominance with a push-pull perceptual learning protocol. Curr Biol. 2010;20:1864–1868. doi: 10.1016/j.cub.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess RF, Mansouri B, Thompson B, Gheorghiu E. Latent stereopsis for motion in depth in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2009;50:5006–5016. doi: 10.1167/iovs.09-3551. [DOI] [PubMed] [Google Scholar]
- 18.Astle AT, McGraw PV, Webb BS. Recovery of stereo acuity in adults with amblyopia. BMJ Case Reports. 2011 doi: 10.1136/bcr.07.2010.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKee SP, Taylor DG. The precision of binocular and monocular depth judgments in natural settings. J Vis. 2010;10(10):1–13. doi: 10.1167/10.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julesz B. Stereopsis and binocular rivalry of contours. J Opt Soc Am. 1963;53:994–999. doi: 10.1364/josa.53.000994. [DOI] [PubMed] [Google Scholar]
- 21.Julesz B. Foundations of Cyclopean Perception. Chicago: Univ of Chicago Press; 1971. [Google Scholar]
- 22.Cooper J, Feldman J. Random-dot-stereogram performance by strabismic, amblyopic, and ocular-pathology patients in an operant-discrimination task. Am J Optom Physiol Opt. 1978;55:599–609. doi: 10.1097/00006324-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Rutstein RP, Eskridge JB. Stereopsis in small-angle strabismus. Am J Optom Physiol Opt. 1984;61:491–498. doi: 10.1097/00006324-198408000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Fendick M, Westheimer G. Effects of practice and the separation of test targets on foveal and peripheral stereoacuity. Vision Res. 1983;23:145–150. doi: 10.1016/0042-6989(83)90137-2. [DOI] [PubMed] [Google Scholar]
- 25.Gantz L, Patel SS, Chung ST, Harwerth RS. Mechanisms of perceptual learning of depth discrimination in random dot stereograms. Vision Res. 2007;47:2170–2178. doi: 10.1016/j.visres.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legge GE, Gu YC. Stereopsis and contrast. Vision Res. 1989;29:989–1004. doi: 10.1016/0042-6989(89)90114-4. [DOI] [PubMed] [Google Scholar]
- 27.Huang CB, Zhou J, Lu ZL, Feng L, Zhou Y. Binocular combination in anisometropic amblyopia. J Vis. 2009;9(3):11–16. doi: 10.1167/9.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcox LM, Harris JM, McKee SP. The role of binocular stereopsis in monoptic depth perception. Vision Res. 2007;47:2367–2377. doi: 10.1016/j.visres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Rainville SJ, Kingdom FA. Scale invariance is driven by stimulus density. Vision Res. 2002;42:351–367. doi: 10.1016/s0042-6989(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 30.Ding J, Sperling G. Binocular Combination: Measurements and a Model. New York: Cambridge Unv Press; 2007. [Google Scholar]