Abstract
Caspase-8 (casp8) is required for extrinsic apoptosis, and mice deficient in casp8 fail to develop and die in utero while ultimately failing to maintain the proliferation of T cells, B cells, and a host of other cell types. Paradoxically, these failures are not caused by a defect in apoptosis, but by a presumed proliferative function of this protease. Indeed, following mitogenic stimulation, T cells lacking casp8 or its adaptor protein FADD (Fas-associated death domain protein) develop a hyperautophagic morphology, and die a programmed necrosis-like death process termed necroptosis. Recent studies have demonstrated that receptor-interacting protein kinases (RIPKs) RIPK1 and RIPK3 together facilitate TNF-induced necroptosis, but the precise role of RIPKs in the demise of T cells lacking FADD or casp8 activity is unknown. Here we demonstrate that RIPK3 and FADD have opposing and complementary roles in promoting T-cell clonal expansion and homeostasis. We show that the defective proliferation of T cells bearing an interfering form of FADD (FADDdd) is rescued by crossing with RIPK3−/− mice, although such rescue ultimately leads to lymphadenopathy. Enhanced recovery of these double-mutant T cells following stimulation demonstrates that FADD, casp8, and RIPK3 are all essential for clonal expansion, contraction, and antiviral responses. Finally, we demonstrate that caspase-mediated cleavage of RIPK1-containing necrosis inducing complexes (necrosomes) is sufficient to prevent necroptosis in the face of death receptor signaling. These studies highlight the “two-faced” nature of casp8 activity, promoting clonal expansion in some situations and apoptotic demise in others.
Following ligation of death receptors (DR), death domain-containing members of the TNF receptor superfamily recruit proteins that are essential for promoting DR-induced apoptosis (1). These include caspase-8 (casp8), a noncatalytic paralogue of casp8 called c-FLIP, and the adaptor protein FADD (Fas-associated death domain protein). Curiously, loss of any of these proteins leads to early embryonic lethality and significant defects in hematopoiesis and activated lymphocyte survival (2). Furthermore, T-cell–specific expression of an interfering form of FADD containing only the death domain of this adaptor (FADDdd) leads to defective T-cell clonal expansion and altered thymopoiesis (3–5). These findings suggest that the signaling molecules that promote apoptosis following DR function serve additional roles that are linked, but unrelated to apoptosis. Recently, it was discovered that the defective survival of T cells lacking active casp8 is associated with a hyperautophagic morphology, and that such T cells die from an alternative form of cell death mediated by receptor-interacting protein kinase-1 (RIPK1) (6, 7).
For several years, it has been known that triggering DRs in the absence of caspase activity can lead to a nonapoptotic form of cell death that resembles necrosis (8, 9) that requires the serine/threonine kinase activity of RIPK1 (10). By using a small-molecule library, Yuan and colleagues identified a family of molecules termed necrostatins that are capable of binding to RIPK1 and blocking DR-induced necrosis (11), a process defined as necroptosis. RNAi screening of genes responsible for DR-induced necroptosis validated that RIPK1 is required for this alternative form of cell death (12) by forming a complex with RIPK3 termed the necrosome (13) in the absence of casp8 function (14–16). Thus, it is now clear that both RIPK1 and RIPK3 are functionally required for the elaboration of necroptotic signaling following DR ligation in cells lacking the capacity to activate caspases. As RIPK1 and RIPK3 have both been shown to be targets for casp8 activity, it has been suggested that failure in casp8-mediated cleavage of RIPK1 and RIPK3 may lead preferentially to necroptosis (13, 17).
Although our previous work has demonstrated that FADDdd-expressing and casp8-deficient T cells succumb to RIPK1-dependent necroptosis, we wished to assess the potential involvement of RIPK3 in this process. Interestingly, although FADD is required, the classic DRs are unlikely to be involved in the demise of such mutant T cells, as antagonizing them failed to block the induction of casp8 activity following T-cell mitogenic stimulation (18). Thus, we sought to establish the in vivo impact of non–DR-induced necroptosis to T-cell–mediated immune function in the context of T cells lacking the capacity to activate casp8. Importantly, because mice bearing a germline RIPK1 deletion succumb to perinatal lethality (19), we chose instead to cross FADDdd-expressing mice (4) with RIPK3−/− mice, as the latter strain develops in an overtly normal fashion, and RIPK3−/− T cells display no obvious activation defects (20). We find that a RIPK3 deficiency acts as a second site suppressor mutation in the context of FADDdd-expressing T cells, and prevents acute necroptosis of these cells following mitogenic stimulation. This RIPK3 deficiency also restores in vivo T-cell–mediated antiviral activity observed in FADDdd transgenic mice (21), but promotes development of lymphoproliferative disease. RIPK1 and RIPK3 both appear to be cleaved following T-cell antigenic stimulation, and we demonstrate that blockade of RIPK1 processing is sufficient to promote DR-induced necroptosis.
Results
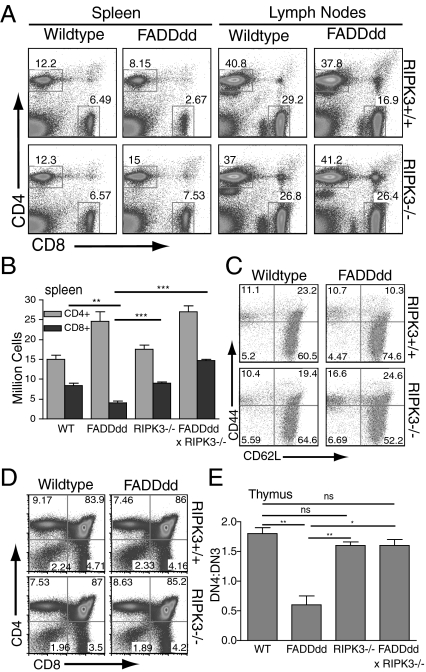
To investigate the role of RIPK3 in T-cell development and homeostasis, spleens, lymph nodes, and thymi were harvested from WT mice, mice expressing FADDdd in thymocytes and T cells (4), RIPK3−/− mice (20), and RIPK3−/− × FADDdd mice for initial comparison of CD4+ and CD8+ T-cell ratios. WT naive splenocytes typically display a 2:1 CD4:CD8 ratio, whereas FADDdd splenocytes display a 4:1 CD4:CD8 ratio as a result of defective CD8+ accumulation and homeostasis (21). Loss of RIPK3 signaling in FADDdd T cells restored the CD4:CD8 ratio to WT levels in spleen and lymph nodes (Fig. 1A), and rescued the diminished fraction of FADDdd CD44High/CD62LHigh T cells or central memory T cells (TCM; Fig. 1B). These dual mutant mice also displayed an enhanced fraction of CD44High/CD62LLow effector T cells (Fig. 1C), potentially because of defective clearance of autoreactive T cells. We observed no significant differences in the fractions of CD4, CD8, CD4/CD8 double positive, or CD4/CD8 double-negative (DN) populations among the four genotypes (Fig. 1D). FADDdd T cells display a partial block during thymopoiesis at the CD4−CD8−CD25+CD44− (DN3) stage associated with expression of the pre-Tα/T-cell receptor (TCR)-β complex (4). Failure to express the pre-Tα/TCRβ complex leads to thymocyte death, whereas successful surface expression promotes proliferation and differentiation into the CD25−/CD44− “DN4” stage (22). As expected, we observed an enhanced DN3 population and a paucity of DN4 cells in FADDdd thymocytes, whereas WT, RIPK3−/−, and FADDdd × RIPK3−/− thymocytes developed normal fractions of DN3 cells (Fig. 1E and Fig. S1).
Fig. 1.
CD8+ T-cell homeostasis restored in FADDdd × RIPK3−/− mice. (A) Spleen and lymph node cells were analyzed by flow cytometry for CD4+ vs. CD8+ populations. Numbers represent percentage of cells staining in each gate. (B) Graph displays number of CD4+ and CD8+ cells in the spleen, representative of three separate experiments. Error bars represent SEM (**P < 0.01 and ***P < 0.001 vs. WT CD8+). (C) Decreased CD8+ memory T cell population in FADDdd mice restored with RIPK3 deficiency. CD8+ gated splenocytes analyzed for CD44+CD62L− (D). Thymocyte DN population shown by anti-CD4 and anti-CD8 staining; numbers represent percentage of cells per quadrant. (E) Graph displays DN4:DN3 population in thymocytes of indicated genotypes, representative of three experiments (*P < 0.05 and **P < 0.01). Error bars indicate SEM.
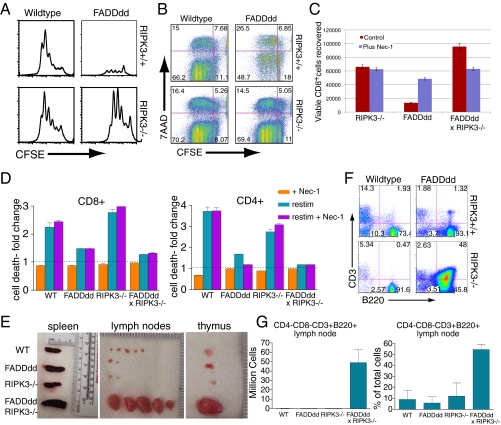
Previous studies have demonstrated that FADDdd or casp8−/− T cells display defective clonal expansion following antigen receptor stimulation (2) as a result of the induction of necroptotic cell death (6, 7). With recent evidence demonstrating that both RIPK1 and RIPK3 are involved in promoting necroptotic death following DR ligation (23), we sought to determine if RIPK3 may participate in the necroptotic death of FADDdd T cells. Splenocytes from FADDdd × RIPK3−/− mice were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured with α-CD3/-CD28 for 3 d to detect the T-cell proliferative response. As observed previously, FADDdd T cells had a diminished proliferative response, as assessed by accumulation of live CD8+/CFSELo T cells (Fig. 2A). In contrast, FADDdd × RIPK3−/− T cells displayed an enhanced proliferative response, correlating with a reduced fraction of 7-actinomycin D (7AAD) high T cells, demonstrating that the loss of RIPK3 rescued the defective clonal expansion and death of FADDdd T cells (Fig. 2B). Although treatment with the RIPK1 inhibitor Nec-1 restored FADDdd T-cell proliferation to levels comparable to RIPK3−/− T cells, the loss of both FADD and RIPK3 signaling led to an appreciable enhancement in recovery of live proliferating T cells (Fig. 2C). Further assessment of the role of FADD in peripheral tolerance revealed that, upon restimulation through the TCR, FADDdd and FADDdd × RIPK3−/− T cells are resistant to restimulation-induced cell death (RICD) (Fig. 2D). However, loss of RIPK3 signaling, alone or in the context of the FADDdd mutation, led to little enhanced recovery of live T cells upon restimulation, demonstrating that the death pathway induced during RICD is almost entirely FADD-directed, casp8-mediated apoptosis.
Fig. 2.
RIPK3 deficiency restores proliferation and survival of FADDdd CD8+ T cells. (A) CFSE analysis of CD8+ T cells treated with α-CD3 (145-2C11; 1 μg/mL) plus αCD28 (200 ng/mL). Splenocytes were stained and analyzed by cytometry after 3 d. Shown are plots for CFSE vs. cell numbers of CD8+ splenocytes. (B) Rescue of enhanced death phenotype of FADDdd CD8+ T cells by RIPK3 deficiency. Plots for CFSE vs. 7-AAD of CD8 cells; upper and lower left quadrants represent populations that have divided and are dead or alive, respectively. (C) Recovery of live CD8+ T cells in FADDdd × RIPK3−/− vs. WT cultures following 3 d stimulation. Activated splenocytes treated with or without 10 μM necrostatin-1 (Nec-1) added at start of culture. Graph represents percent viable CD8+ cells recovered ± SEM. (D) Fold change in cell death of CD4+ or CD8+ cells upon restimulation (restim) with or without Nec-1 relative to death observed in activated cells (1 μg 1 × 105 cells αCD3, 200 ng/mL αCD28) not subjected to restimulation (dotted line). Error bars represent SEM (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. WT restimulation). (E) Lymphoid tissue from aged (40 wk) mice of indicated genotypes. (F) FACS plots display percentage of double-negative population that are CD3+ B220+ in mice of indicated genotypes. (G) Graphs represent counts or percentage of total live cells (after RBC lysis) that are CD4−CD8−CD3+B220+. Error bars represent SEM; n = 3 of each genotype.
Mice and human subjects lacking functional Fas receptor (CD95) or Fas-ligand (CD95L) develop lymphoproliferative disease and autoimmune lymphoproliferative syndromes, respectively, as well as an accumulation of CD4−, CD8−, CD3+, B220+ T lymphocytes. Similarly, older FADDdd × RIPK3−/− mice displayed a slight splenomegaly with frank lymphadenopathy and enlarged thymi (Fig. 2E), coupled with a dramatic increase of CD4−, CD8−, CD3+, B220+ T lymphocytes (Fig. 2 F and G). Histological analyses revealed lymphocyte infiltrates within livers of the DKO mice (Fig. S2). These findings demonstrate that, although RICD drives a mostly apoptotic form of death, chronic loss of both DR-induced apoptosis and necroptosis leads to recapitulation of the lymphoproliferative phenotype observed in mice lacking functional Fas or FasL.
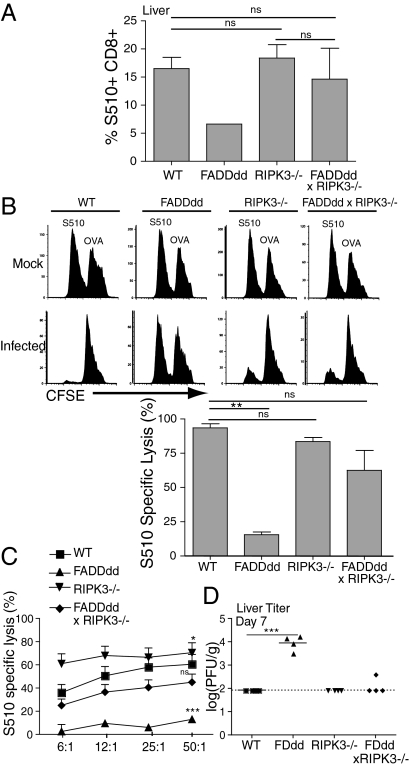
FADDdd mice are incapable of mounting an effective immune response against viral pathogens, including lymphocytic choriomeningitis virus and murine hepatitis virus (MHV) (21). To address the consequence of the loss of both RIPK3 and FADD signaling during an antiviral response, FADDdd × RIPK3−/− mice were injected intraperitoneally with 2 × 105 pfu of MHV and killed 7 d after infection. Tetramer staining of T cells in the liver showed infiltration of virus-specific T cells to the infected tissue, which was not evident in FADDdd mice as expected, but restored in FADDdd × RIPK3−/− mice (Fig. 3A). To determine whether FADD and RIPK3 play a role in cytotoxic T lymphocyte (CTL) activity, in vivo and in vitro CTL assays were performed by using target cells pulsed with nonspecific [ovalbumin (OVA)] or virus-specific immunodominant (S510) peptides. To assess in vivo killing activity, C57BL56/J splenocytes were pulsed with low and high concentrations of CFSE, followed by peptide pulsing with S510 and OVA peptides, respectively. One hour after injection of pulsed splenocytes into 7 d mock- and MHV-infected mice, spleens were harvested to assess the recovery of S510- vs. OVA-pulsed target cells. Whereas FADDdd mice failed to kill S510-pulsed target cells because of defective accumulation of effector cells, killing activity was similar among WT, RIPK3−/−, and FADDdd × RIPK3−/− mice (Fig. 3 B and C). As with restoration of virus-specific effector cells, deletion of RIPK3 in FADDdd mice rescued their defective clearance of MHV (Fig. 3D).
Fig. 3.
FADDdd × RIPK3−/− mice exhibit normal immune response to murine hepatitis virus (MHV) infection. (A) T cells from livers of infected mice were stained with MHV-specific (S510) tetramer. Graph represents percentage of S510-positive cells of total CD8+ cells. (B) Nonspecific and specific target cells were labeled with CFSEhi/low, respectively, and adoptively transferred into infected and sham mice for in vivo CTL analysis shown by FACS plots. Graph displays percent specific lysis (**P < 0.01). (C) Splenocytes from infected and sham mice were incubated with specific/nonspecific target cells for 4 h to measure in vitro CTL activity. Graph represents percent specific lysis. Considered a significant difference with respect to WT specific lysis (*P < 0.01, ***P < 0.001). (D) Livers from infected mice were harvested 7 d after infection for viral titer analysis. Error bars indicate SEM (***P < 0.001).
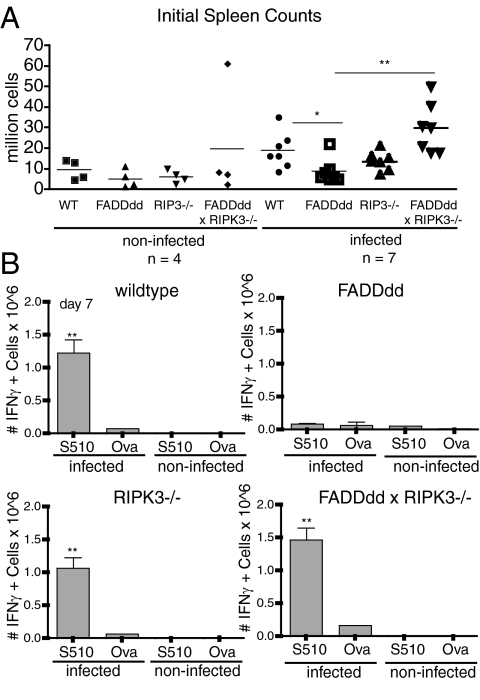
To address the T-cell–intrinsic role of both RIPK3 and FADD signaling during an antiviral response, we performed MHV infection studies in immunodeficient Rag2−/− × IL2Rγc−/− mice reconstituted with purified T cells from the four genotypes. 2.5 × 106 T cells were adoptively transferred into Rag2−/− × IL2Rγc−/− mice, and rested for 7 d before injected intraperitoneally with 2 × 105 pfu of MHV. Although overall splenocyte numbers 7 d after infection were approximately similar in uninfected mice, indicating no defect in homeostatic proliferation, FADDdd × RIPK3−/− possessed a greater number of splenocytes relative to other mice, consistent with a potential defect in lymphocyte homeostasis following viral infection (Fig. 4A). To evaluate the generation of virus-specific T cells, IFN-γ production was analyzed after a 6-h in vitro restimulation of splenocytes with the immunodominant CD8+ T-cell epitope, the MHV S510 peptide. RIPK3−/− and FADDdd × RIPK3−/− mice developed comparable numbers of IFN-γ–expressing splenic CTLs as WT mice, whereas the recovery of these virus-specific T cells was significantly diminished in FADDdd spleens (Fig. 4B). These findings demonstrate that the loss of RIPK3 rescued the expansion capacity of antiviral T cells expressing FADDdd. Consistent with this, the diminished fraction of effector/memory CD8+ (CD44High/CD62LLow) T cells observed after infection in FADDdd splenocytes was restored to WT levels in FADDdd × RIPK3−/− mice (Fig. S3).
Fig. 4.
T-cell–intrinsic FADD and RIPK3 activity required for antiviral response to murine hepatitis virus (MHV) infection. (A) Initial splenocyte counts of infected and control mice in indicated genotypes (*P < 0.05 and **P < 0.01). (B) FADDdd × RIPK3−/− adoptive transfer mice and controls were infected intraperitoneally with MHV. IFN-γ levels in splenocytes of infected and control mice were determined by intracellular IFN-γ staining 7 d after infection. Splenocytes were restimulated 6 h with S510 or OVA peptides and stained for CD8. IFN-γ+ cells were calculated by multiplying total splenocytes by percentage of IFN-γ cells (**P < 0.01 vs. infected FADDdd S510).
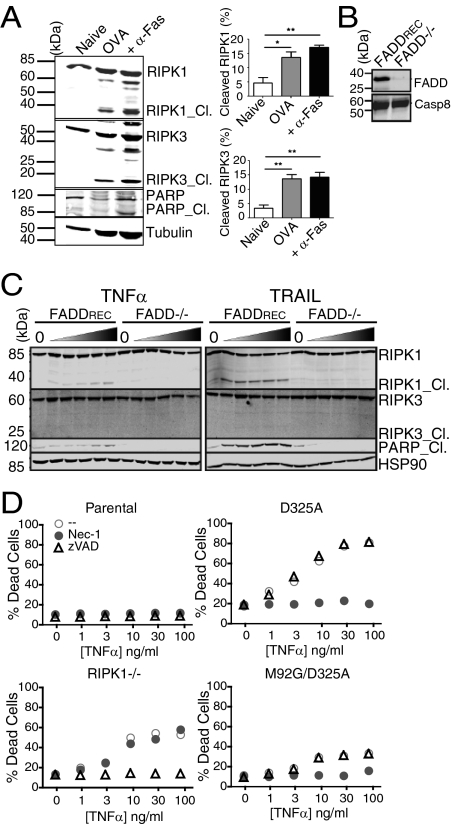
Processing of RIPK1 and/or RIPK3 by casp8, itself activated by FADD [and possibly cFLIP (24)], is thought to prevent necroptosis (13, 17). Considering that casp8 catalytic activity is required for T-cell clonal expansion (18), processing of RIPK1 or RIPK3, both of which are targets of casp8 (25, 26), was assayed following T-cell antigenic stimulation. We detected cleaved RIPK1 and RIPK3 in viable OT-I OVA-specific T cells following stimulation with OVA peptide or anti-Fas (27) (Fig. 5A). This led us to pose the broader question of whether processing of RIPK1 and/or RIPK3 may also occur during DR-induced apoptosis vs. necroptosis. We treated Jurkat T cells lacking FADD (28), which are known to be highly sensitive to DR-induced necroptosis (10) (Fig. S4), and FADD−/− Jurkat cells transfected with full length FADD (FADDREC; Fig. 5B) with TNF-α or TRAIL and detected RIPK1 (and PARP1) cleavage fragments in FADDREC cells (Fig. 5C).
Fig. 5.
RIPK1/RIPK3 cleavage following TCR vs. DR ligation. (A) OT-I T cells (27) were stimulated with OVA peptide for 72 h and stimulated without or with α-Fas or left untreated (naive); immunoblots were hybridized with α-RIPK1 and α-RIPK3 to detect processing. Graphs represent percent RIPK1/3 cleavage (*P < 0.05 and **P < 0.01). (B) Reconstitution of FADD-deficient Jurkat cells (28) (FADD−/−) with full-length FADD (FADDREC), and blots of lysates probed with α-FADD and α-casp8. (C) Western blot of RIPK1 and RIPK3 cleavage in FADD−/− and FADDREC Jurkat cells; HSP90 used as loading control. RIPK1_ Cl., PARP_ Cl., cleaved RIPK1 and PARP1, respectively. (D) Parental, RIPK1−/−; D325A RIPK1, M92G/D325A RIPK1 Jurkat cells treated with TNFα, Nec-1 (10 μM), or z-VAD-FMK (20 μM) and stained with 7AAD and annexin-V to detect death.
To assess the potential that casp8-mediated processing of RIPK1 prevents the elaboration of a necroptotic response following DR ligation, we treated RIPK1-deficient Jurkat cells (29) stably reconstituted with cleavage-resistant RIPK1_D325A or kinase dead/cleavage-resistant RIPK1_M92G/D325A [rendered catalytically inactive by a kinase “gatekeeper” mutation (30); Fig. S5] with TNF-α ± Nec-1 or zVAD-FMK (Fig. 5D). RIPK1−/− cells were sensitized to TNF-induced apoptosis, which was rescued with the addition of zVAD-FMK. In contrast, cells expressing noncleavable RIPK1_D325A were sensitized to TNF-induced necroptosis, which was rescued by Nec-1. Blocking kinase activity of cleavage-resistant RIPK1 (M92G/D325A) diminished TNF-induced necroptotic death observed in RIPK1_D325A reconstituted RIPK1−/− Jurkat cells. Ectopic expression of RIPK1_D325A in RIPK1-sufficient Jurkat cells failed to shift TNF-induced apoptosis to necroptosis (Fig. S6). Thus, uncleaved RIPK1 does not act in a dominant fashion to promote necroptosis. Presumably, only a small fraction of activated RIPK1, recruited via association with FADD or other adaptors, is necessary for a necroptotic response to TNF. These results provide evidence that direct inactivation of RIPK1 and/or RIPK3, through a FADD-dependent mechanism, differentiates between apoptosis and necroptosis, and RIPK1 activity is required to promote necroptosis.
Discussion
Our findings here demonstrate that RIPK3 acts in concert with RIPK1 in promoting the necroptotic demise of T cells lacking the nonapoptotic casp8 activity that is normally observed following mitogenic signaling (6, 7, 18). Thus, early casp8 catalytic activity within T cells acts to prevent the elaboration of signaling from an RIPK1/RIPK3 containing necrosome (13) in a manner similar to that observed following DR signaling. Although T-cell–intrinsic casp8 and FADD activity are required for antiviral T-cell responses, loss of RIPK3 restores the ability of FADDdd T cells to control MHV infection, suggesting that casp8 and RIPK3 play additional opposing roles during antiviral immune responses.
Unlike the case for DR signaling, the process that leads to RIPK1/RIPK3-containing necrosome formation is currently unclear. We previously observed RIPK1 recruitment to complexes that likely exist on autophagosomal membranes (6) and found that extracellular blockade of DR ligation fails to rescue casp8−/− or FADDdd-expressing T cells (18). This suggests that the nucleation of RIPK1/RIPK3 necrosomes occurs in a manner independent of DR signaling. Here, we demonstrate that RIPK3, like RIPK1 (6, 7), promotes necroptotic death of FADDdd T cells following their activation. Our findings contrast with a recent publication that suggested RIPK3 signaling is not involved in the necroptotic demise of T cells lacking FADD (31) based on the fact that RIPK3 failed to coimmunoprecipitate with RIPK1. Given that our results demonstrate a requirement for RIPK3 in TCR-induced necroptosis, it is possible that, whereas the upstream pathways that promote the nucleation of RIPK1/RIPK3 containing necrosomes may be unique following DR ligation vs. T-cell mitogenesis, the downstream pathways are likely similar.
Here we present data that demonstrate an essential role for DR-induced apoptotic death during reactivation-induced cell death, a process that has previously been shown to require Fas signaling (32). We emphasize that loss of RIPK3 signaling had little impact on this; FADDdd was highly effective in blocking T-cell RICD. In contrast, the loss of RIPK3 in FADDdd T cells led to a significant increase of live proliferating T cells following mitogenic stimulation. Although Nec-1 treatment also rescued the expansion of FADDdd T cells, it did not lead to embellished recovery of proliferating cells vs. WT T cells. This result suggests that RIPK3 may have independent functions in T cells that may have been previously overlooked. Alternatively, Nec-1 treatment may have off-target effects that limit T-cell expansion.
Although RIPK3−/− T cells were shown to develop and proliferate relatively normally in previous studies (20) and in our work presented here, Moquin and Chan found that RIPK3−/− mice fail to promote efficient inflammatory responses to vaccinia virus infection (17). This may be a likely consequence of the expression of the caspase inhibitory protein SPI-2, a protein related to poxvirus CrmA, and possible assembly of necrosomes in an antiviral inflammatory environment. In contrast, we note here that RIPK3−/− mice were also capable of controlling murine hepatitis virus (MHV) infection. In previous studies, FADDdd expressing mice failed to adequately respond to viral infection, and this was likely a result of defective CD8+ T-cell responses (21). Supporting this conclusion, the T-cell–intrinsic loss of RIPK3 restored the ability of FADDdd mice to efficiently clear MHV infections (Fig. 4). Thus, the primary defect in FADDdd-expressing mice to MHV infection is in the expansion/survival of MHV-specific T cells. Furthermore, as direct infection of RIPK3−/− mice led to efficient viral clearance, our results call into question a general requirement for RIPK3 signaling in antiviral immunity. Importantly, the immune defects seen in FADDdd mice were rescued with a RIPK3 deficiency, although older FADDdd × RIPK3−/− mice bear a phenotypic resemblance to Fas-deficient (lpr/lpr) mice. These data indicate casp8-dependent apoptosis and RIPK1/3-dependent necroptosis are both necessary to maintain homeostasis within the adaptive immune system.
These studies emphasize a primary role for RIPK3 in promoting TCR-induced necroptosis in T cells lacking catalytically active casp8 (18). The simplest interpretation is that RIPK1 and/or RIPK3 are inactivated by casp8 directly (13) when brought into close apposition. Supporting this, we observed cleavage of both RIPK1 and RIPK3 in antigenically stimulated primary T cells, but only RIPK1 cleavage was detected in Jurkat T cells treated with DR ligands (Fig. 5). Expression of cleavage-resistant RIPK1 was sufficient to convert TNF-induced apoptosis into necroptosis, whereas kinase-dead RIPK1 diminished TNF-induced necroptosis and apoptosis. Thus, a failure in caspase-mediated cleavage of RIPK1 alone is sufficient to promote necroptosis following DR ligation. Following the submission of our manuscript, several other laboratories have reported parallel findings in the context of T cells, supporting our studies here (33–36). Taken together, our findings show that casp8 activity is essential for controlling RIPK1/3-dependent necroptosis in activated T cells, and that RIPK1/3 processing orchestrates the switch between apoptotic vs. necrotic cell death. Furthermore, these findings demonstrate that RIPK3-induced necroptotic activity leads to the early demise of FADDdd T cells, and this blocks antiviral immunity and the development of lymphoproliferative disease in mice lacking FADD signaling in T cells.
Methods
Mice.
FADDdd transgenic mice (4) were crossed with RIPK3−/− mice (20), the latter provided by Kim Newton and Vishva Dixit at Genentech (South San Francisco, CA). Rag2−/− × γc and C57BL6/J (B6) mice were obtained from Jackson Laboratories. Mice were bred and maintained in accordance with the institutional animal use and care committee at the University of California, Irvine, vivarium.
T-Cell Assays/Infections.
T cells were activated as described previously (37). In some cases, 10 μM Nec-1 was added at the start of culture to block necroptosis (6). For transfer, T cells from mice of the indicated genotypes were isolated by depletion of B220+, CD11b+ and MHCII+ cells with magnetic beads (Miltenyi), and injected i.v. at a dose of 2.5 × 106 cells per Rag2−/− × IL-2Rγc−/− mouse. For infection, 2 × 105 pfu MHV (strain JHM-DM) was injected intraperitoneally into mice. PBS solution was injected into “mock” controls. Intracellular staining, viral titer plaque assays, and statistical analyses were performed by using methods reported previously (38).
RICD and CTL Assay.
Restimulation was by using an approach based on a previous publication (39) by using 1 × 105 cells per well and proceeded overnight before staining with 7-AAD. For the in vivo CTL assay, mice were infected, and, after 7d, CTL activity was assessed as described previously (40). B6 splenocytes were pulsed with 5 μM OVA or S510 peptide as target cells and resuspended at 20 × 106/mL for CFSE labeling. OVA/S510-pulsed cells were labeled with 2.5 and 0.5 μM CFSE, respectively, and mixed at a 1:1 ratio to obtain a cell suspension of 100 × 106/mL. Target cells (100 μL) were transferred i.p. into each infected mouse, and the assay was allowed to proceed 75 min before harvesting spleens for FACS. The specific lysis percentage was defined as 100*[1 − R(sham) / R(infected)], with R representing the ratio of CFSEhi to CFSElo. In vitro, EL4 target cells were pulsed with peptides as stated earlier. OVA-pulsed/S510-pulsed EL4 cells were labeled with 2.5 μM CFSE and 5 μM EF670, respectively (10 min., 37 °C; eBioscience), and mixed at equal volumes. To prepare effectors, splenocytes of infected mice were RBC-lysed and plated in 96-well round-bottom plates at 100 μL/well before the addition of 100 μL target cells. Plates were incubated at 37 °C, 5% CO2, for 4 h before FACS analysis. The specific lysis percentage was determined as 100*[1 − R(infected) / R(no effectors)], with R representing the ratio of EF670 to CFSE.
Retroviral Infection.
Jurkat cells were infected with retroviral supernatants by using methods reported previously (6). Two days after infection, cells were treated with Nec-1 (10 μM), zVAD-FMK (20 μM), CHX (5 μg/mL), TNF-α (20 ng/mL), and etoposide (50 μM). Fourteen hours after treatment, cells were stained with annexin V, anti-Thy1, and 7-AAD for FACS analysis.
Supplementary Material
Acknowledgments
The authors thank Kim Newton and Vishva Dixit (Genentech) for RIPK3−/− mice, David Fruman and Aimee Edinger for review of this manuscript, and Huy Q. Nguyen, Isaac H. Chu, and Alan Nguyen for technical assistance. This work was supported by National Institutes of Health Grants AI50506 (to C.M.W.), AI63419 (to C.M.W.), CA69381 (to G.S.S.), and NS41249 (to T.E.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.J.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102779108/-/DCSupplemental.
References
- 1.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 3.Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh CM, et al. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 5.Zörnig M, Hueber AO, Evan G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Curr Biol. 1998;8:467–470. doi: 10.1016/s0960-9822(98)70182-4. [DOI] [PubMed] [Google Scholar]
- 6.Bell BD, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci USA. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ch'en IL, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci USA. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 11.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 12.Hitomi J, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 15.He SW, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leverrier S, Salvesen GS, Walsh CM. Enzymatically active single chain caspase-8 maintains T-cell survival during clonal expansion. Cell Death Differ. 2010;18:90–98. doi: 10.1038/cdd.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelliher MA, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 20.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beisner DR, Chu IH, Arechiga AF, Hedrick SM, Walsh CM. The requirements for Fas-associated death domain signaling in mature T cell activation and survival. J Immunol. 2003;171:247–256. doi: 10.4049/jimmunol.171.1.247. [DOI] [PubMed] [Google Scholar]
- 22.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 23.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 24.Pop C, et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J. 2011;433:447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 28.Juo P, et al. FADD is required for multiple signaling events downstream of the receptor Fas. Cell Growth Differ. 1999;10:797–804. [PubMed] [Google Scholar]
- 29.Ting AT, Pimentel-Muiños FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 30.Shah K, Liu Y, Deirmengian C, Shokat KM. Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc Natl Acad Sci USA. 1997;94:3565–3570. doi: 10.1073/pnas.94.8.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osborn SL, et al. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci USA. 2010;107:13034–13039. doi: 10.1073/pnas.1005997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 33.Oberst A, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010;285:16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ch'en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208:633–641. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arechiga AF, et al. A Fas-associated death domain protein/caspase-8-signaling axis promotes S-phase entry and maintains S6 kinase activity in T cells responding to IL-2. J Immunol. 2007;179:5291–5300. doi: 10.4049/jimmunol.179.8.5291. [DOI] [PubMed] [Google Scholar]
- 38.Ramos SJ, Hardison JL, Stiles LN, Lane TE, Walsh CM. Anti-viral effector T cell responses and trafficking are not dependent upon DRAK2 signaling following viral infection of the central nervous system. Autoimmunity. 2007;40:54–65. doi: 10.1080/08916930600996700. [DOI] [PubMed] [Google Scholar]
- 39.Ramaswamy M, et al. Cutting edge: Rac GTPases sensitize activated T cells to die via Fas. J Immunol. 2007;179:6384–6388. doi: 10.4049/jimmunol.179.10.6384. [DOI] [PubMed] [Google Scholar]
- 40.Barber DL, Wherry EJ, Ahmed R. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.