Abstract
Substitution mutations in adjacent amino acids of the N-terminal domain of NPC1, a lysosomal membrane protein, abolish its cholesterol binding activity and impair its ability to export cholesterol from lysosomes of cultured cells lacking npc1 [Kwon HJ, et al. (2009) Cell 137:1213–1224]. Here, we show that the same two mutations (proline-202 and phenylalanine-203, both changed to alanine) reproduce the phenotype of complete NPC1 deficiency when knocked into the mouse npc1 gene by homologous recombination. Homozygous npc1pf/pf mice exhibited neurodegeneration beginning at day 49 and died at a median age of 84 d, as previously reported for mice that lack npc1. Liver and other organs of the npc1pf/pf mice accumulated excess cholesterol in lysosomes. In liver, mRNAs encoding several lysosomal proteins were elevated, including NPC1 and NPC2 and several digestive enzymes (acid lipase, β-glucuronidase, and cathepsins B and D). Weekly treatment with hydroxypropyl-β-cyclodextrin (HPCD) beginning at 7 wk reduced hepatic cholesterol accumulation and diminished the lysosomal mRNAs. We conclude that the cholesterol binding site in the N-terminal domain of NPC1 is essential for cholesterol export from lysosomes in living animals as it is in cultured cells. The HPCD-mediated reduction of excess lysosomal enzymes may contribute to the ability of this drug to delay the progression of NPC disease in mice.
Keywords: cholesterol binding domain, cyclodextrin, knockin mutation, lysosomal storage disease, Niemann-Pick disease
In Neimann-Pick C disease, a mutation in either of two lysosomal proteins, NPC1 or NPC2, causes cholesterol and other lipids to accumulate in lysosomes throughout the body, resulting in death from multiorgan failure (1). To explain this dual requirement, we proposed a handoff model in which NPC2 and NPC1 act sequentially to remove cholesterol from lysosomes (2, 3). Malfunction of either protein causes a primary accumulation of cholesterol with secondary accumulation of other lipids, and this leads to cell death, especially in liver, lung, and cerebellum.
The sequential handoff model for NPC action is supported by biochemical and ultrastructural studies. Cholesterol enters lysosomes as a result of the receptor-mediated endocytosis of LDL. Lysosomal acid lipase hydrolyzes the cholesteryl esters of LDL, and the resulting insoluble cholesterol is postulated to be scavenged by NPC2 before it has a chance to form crystals. NPC2 is a soluble protein of 132 amino acids that is localized to the lysosomal lumen. Sterol binding studies (4) and X-ray crystallography (5) have demonstrated that NPC2 binds cholesterol in a specific orientation, with the isooctyl side chain buried and the 3β-hydroxyl group exposed. The model postulates that NPC2 carries cholesterol to the lysosomal lining membrane, but it is prevented from accessing the membrane because the membrane is coated by a dense glycocalyx (2). At this point NPC2 transfers its cholesterol to NPC1, a 1,278-amino acid protein with 13 transmembrane helices that is embedded in the lysosomal membrane (6, 7). The cholesterol acceptor site on NPC1 is contained in the N-terminal domain, a sequence of 240 amino acids that is postulated to project into the lumen of the lysosome (2, 4).
The truncated N-terminal domain of NPC1, referred to as NPC1(NTD), can be expressed and purified as a soluble protein (4). Crystallographic studies show that this domain forms a stable structure that is largely α-helical (2). Cholesterol binds to this domain in an orientation opposite to its orientation in NPC2 (i.e., the 3β-hydroxyl group is buried in the protein, where it makes specific contacts with certain amino acids, whereas the isooctyl side chain projects to the surface). When NPC1(NTD) is incubated with cholesterol in solution containing ethanol and dilute detergent, the cholesterol binds to the protein very slowly at 4 °C (8). Slow binding is attributed to the presence of several α-helices that partially occlude the sterol binding pocket (2). Binding is accelerated by orders of magnitude if the cholesterol is first bound to NPC2 and then transferred to NPC1(NTD) (8). We speculate that NPC2 interacts with NPC1(NTD) so as to move aside the obstructing helices, allowing cholesterol to slide from NPC2 to NPC1 without having to enter the water phase. The sliding model is supported by crystallography showing the opposite orientation of cholesterol in the two binding sites (2, 3).
The cholesterol handoff model is supported by alanine-scanning mutagenesis of NPC2 and NPC1(NTD) (2, 3). These mutations revealed two classes of defects. Replacement of amino acids in either of the sterol binding pockets led to a loss of cholesterol binding. The other class of mutations localized to patches on the surface of both proteins that are not required for cholesterol binding but are required for cholesterol transfer from NPC2 to NPC1(NTD). In modeling studies, these patches could be aligned so as to bring the two cholesterol binding sites into close proximity, in such a way that the plane of the cholesterol would be held constant as the sterol slid from NPC2 to NPC1(NTD) (3).
The physiologic relevance of the identified amino acids required for binding and transfer was confirmed by introducing the mutations into plasmids encoding NPC2 or full-length NPC1. Proteins encoded by these mutant plasmids failed to rescue defective sterol transport in cultured cells deficient in NPC2 or NPC1, respectively (2, 3).
In the present studies, we have sought to confirm the physiologic relevance of the cholesterol binding site on NPC1(NTD) in organs of living animals. For this purpose, we chose a mutation that replaces two adjacent amino acids in the cholesterol binding pocket (proline-202 and phenylalanine-203) with alanines. In previous studies, NPC1(NTD) containing these mutations failed to bind cholesterol when tested in vitro, and full-length NPC1 containing these mutations failed to complement the defect in NPC1-deficient CHO cells (2). Here, we show that knockin mice homozygous for the P202A/F203A mutation (designated as npc1pf/pf mice) exhibit a clinical and pathologic syndrome indistinguishable from that reported for npc1nih/nih mice, the classic model for NPC1 deficiency (9). Treatment of the npc1pf/pf mice with hydroxypropyl-β-cyclodextrin (HPCD) restores tissue cholesterol homeostasis, just as it does in the npc1nih/nih mice (10–12). Our data also show that livers of the knockin mice have elevated levels of several lysosomal enzymes, including cathepsin B and D, that are restored to normal by treatment with HPCD. The latter finding may explain the extended lifespan in HPCD-treated NPC1-deficient mice.
Results
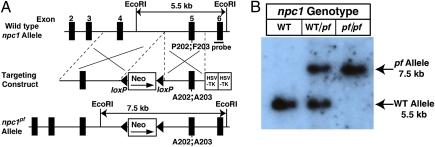
Fig. 1A shows the gene-targeting strategy that was used to generate a line of npc1 knockin (npc1pf/pf) mice in which the adjacent proline and phenylalanine residues 202 and 203 were replaced with alanines. These two residues are part of the cholesterol binding pocket in the NPC1(NTD) (2). Fig. 1B shows representative Southern blot analysis of EcoRI-digested genomic DNA extracted from the tails of mice with the indicated genotype. For WT mice, a single 5.5-kb band was observed. In mice homozygous for the knockin allele (designated npc1pf/pf), a single 7.5-kb band was detected. Both bands were present in the npc1+/pf heterozygous mice. For all experiments described here, npc1+/pf heterozygous male and female mice were crossed to obtain WT and npc1pf/pf littermates.
Fig. 1.
Generation of npc1 knockin mice harboring two point mutations in NPC1 that abolish cholesterol binding activity. (A) Schematic of the mouse WT npc1 allele and the targeting construct used to generate an npc1pf allele by introducing two point mutations (P202;F203 to A202;A203) into exon 5 of npc1. The targeting vector also contains a loxP-flanked pgkneopA cassette and two copies of the HSV-TK gene as selection markers. The probe used for the Southern blot is denoted by the horizontal filled rectangle labeled “probe.” (B) Representative Southern blot analysis of EcoRI-digested genomic DNA from tails of mice with the indicated genotypes.
Compared with those of WT littermates, livers of npc1pf/pf mice exhibited increases in NPC1 and NPC2 mRNAs (1.7- and 4.7-fold, respectively) (Fig. 2A). Hepatic NPC1 and NPC2 protein levels were also markedly increased (Fig. 2B). Fig. 2C compares the glycosylation patterns of WT NPC1 and mutant NPC1(P202A/F203A). Membrane fractions prepared from livers of WT and npc1pf/pf mice were treated with endoglycosidase H (Endo H) or peptide:N-glycosidase F (PNGase F) and then subjected to immunoblot analysis with anti-NPC1. To compensate for the increased protein level in npc1pf/pf mice, we used only half as much membrane protein from the mutants. In both WT and npc1pf/pf mice, the N-linked carbohydrate chains of NPC1 were partially resistant to Endo H, indicating that both WT and mutant NPC1 proteins had trafficked from the endoplasmic reticulum (ER) to the Golgi apparatus. PNGase F, which removes all N-linked carbohydrates, decreased the molecular weights of WT and mutant NPC1 proteins to the same degree.
Fig. 2.
NPC1 and NPC2 levels in livers of WT and npc1pf/pf mice. Nine-week-old male WT and npc1pf/pf littermates were fed an ad libitum chow diet before study. (A) mRNA analysis. Relative amounts of NPC1 and NPC2 mRNAs in livers were determined by quantitative real-time PCR with apoB as the invariant control. Values represent the amount of mRNA relative to that in WT littermates, which is arbitrarily defined as 1. Each bar represents the mean ± SEM of data from 4 mice. Asterisks denote the level of statistical significance (Student t test) between the WT and npc1pf/pf mice. **P < 0.01. The number of PCR cycles (cycle threshold, Ct) required to reach the threshold line of 0.15 is shown inside the bar graph. (B) Immunoblot analysis. For each genotype, whole-cell lysates were prepared from four mouse livers. Aliquots of the pooled lysates (40 μg protein) were subjected to 8% SDS/PAGE and immunoblot analysis. β-Tubulin was used as a loading control. (C) Glycosidase digestion. For each genotype, membrane fractions from four mouse livers were pooled. Aliquots of the pooled fractions (40 μg and 20 μg for WT and npc1pf/pf, respectively) were incubated in the absence or presence of the indicated glycosidase and subjected to SDS/PAGE and immunoblot analysis.
To confirm that the P202A/F203A mutation did not alter the subcellular localization of NPC1, we examined its location by double-labeled immunofluorescent confocal laser scanning microscopy in transfected cells (Fig. 3). Human SV-589 cells were transfected with plasmids encoding Flag-tagged WT NPC1 or the P202A/F203A mutant. The Flag-tagged NPC1 was immunolabeled with anti-FLAG plus a red fluorescent secondary antibody (Figs. 3 A and D). Lysosomal-associated membrane protein 1 (LAMP1), a known lysosome marker (13) was immunolabeled with anti-LAMP1 plus a green fluorescent secondary antibody (Figs. 3 B and E). WT NPC1 (Fig. 3C) and the mutant P202A/F203A NPC1 (Fig. 3F) colocalized with LAMP1.
Fig. 3.
Colocalization of transfected NPC1(P202A/F203A) with endogenous LAMP1. SV-589 fibroblasts were transfected with TK-driven plasmids expressing Flag-tagged versions of either WT NPC1 (A–C) or mutant NPC1(P202A/F203A) (D–F) as described in Materials and Methods. Twenty-four hours after transfection, the cells were fixed and immunostained for Flag-tagged NPC1 (red) and endogenous LAMP1 (green). Nuclei were stained blue with DAPI. Fluorescence images were viewed and processed by confocal microscopy as described in Materials and Methods. Each image represents a maximum intensity projection of a confocal z-stack of 32 images. (Scale bars, 10 μm; magnification: 190×.)
The npc1pf/pf mice were born at the expected Mendelian ratio from intercrosses of the npc1+/pf heterozygous male and female mice. At the time of weaning (3 to 4 wk of age), of a total of 314 offspring derived from 57 such intercrosses, the observed ratio of WT, npc1+/pf, and npc1pf/pf mice was 87:148:79. The WT and npc1pf/pf littermates were indistinguishable at birth, and both showed normal growth and weight gain until ≈45 d of age (Fig. 4A). Thereafter, the WT mice continued to gain weight, whereas the npc1pf/pf mice began to lose weight. By 85 d, npc1 pf/pf mice (16.1 ± 0.6 g) weighed 41% less than their WT littermates (27.4 ± 0.9 g). At ≈50 d of age, npc1pf/pf mice began to exhibit tremor and ataxia that progressed until death at an average age of 84 ± 3 d (n = 30) (Fig. 4B). The age of onset of weight loss and locomotor dysfunction in npc1pf/pf mice was comparable to that in npc1nih/nih mice, the classic mouse model of NPC disease (9). The average lifespan of the npc1pf/pf mice (84 d) also agreed with that of npc1nih/nih mice (85 d) reported by Li et al. (14) in studies of npc1nih/nih mice in the same C57BL/6 and 129Sv/Ev mixed genetic background. Heterozygous npc1+/pf mice were indistinguishable from WT littermates.
Fig. 4.
Growth and survival of WT and npc1pf/pf mice. (A) Littermate WT (n = 30, 18 male and 12 female) and npc1pf/pf (n = 30, 18 male and 12 female) mice were weighed weekly beginning at 5 wk of age. (B) The same cohort of WT (n = 30) and npc1pf/pf (n = 30) mice were followed up to 112 d of age, and the percent survival of each group was plotted as a function of time. For comparison, the reported survival curve for npc1nih/nih mice (14) was replotted in red.
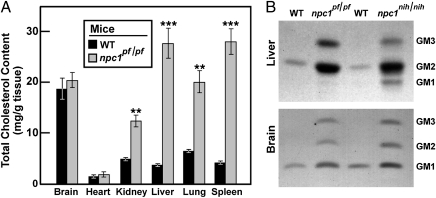
At 9 wk of age, npc1pf/pf mice showed pathological features identical to those of npc1nih/nih mice, including loss of Purkinje cells in the cerebellum (Fig. 5D) and numerous large foam cells in liver (Fig. 5E), spleen (Fig. 5F), lung, and kidney. At 9 wk of age, npc1pf/pf mice accumulated excess cholesterol in many organs (Fig. 6A). The increases ranged from 2.5-fold in kidney to 7.4-fold in liver. Compared with other organs, the cholesterol content in brain was relatively high in WT mice, and there was no increase in npc1 pf/pf mice. Similar results have been reported for npc1nih/nih mice (15).
Fig. 5.
Histology of various tissues from WT and npc1pf/pf mice. Tissues from 9-wk-old female WT (A–C) and npc1pf/pf (D–F) littermates were fixed, sectioned, and stained with H&E as described in Materials and Methods. Arrows (A) denote Purkinje cells that are present in cerebellum of WT mice but absent from npc1pf/pf mice. (Scale bars, 50 μm; magnification: 40×.)
Fig. 6.
Accumulation of cholesterol and gangliosides in organs of npc1pf/pf mice. (A) Total cholesterol content in various organs of 9-wk-old male WT and npc1pf/pf littermates was determined as described in Materials and Methods. Each bar represents mean ± SEM of data from four mice. Asterisks denote level of statistical significance (Student t test) between the WT and npc1pf/pf mice. **P < 0.01. (B) Gangliosides from tissues of 9-wk-old male npc1pf/pf, npc1nih, and WT mice were subjected to TLC and visualized by orcinol staining as described in Materials and Methods. For each genotype, equal weights of liver or brain tissue from four mice were pooled. Each lane corresponds to the ganglioside fraction isolated from 3 mg of brain or 1.5 mg of liver (wet weight). Position of migration of standards for GM1, -2, and -3 are indicated.
A noteworthy feature of NPC disease is the buildup of lipids such as gangliosides GM2 and GM3 (16). As shown in Fig. 6B, in liver and brain of the npc1pf/pf mice, levels of gangliosides GM2 and GM3 were elevated to a similar extent as in npc1nih/nih mice.
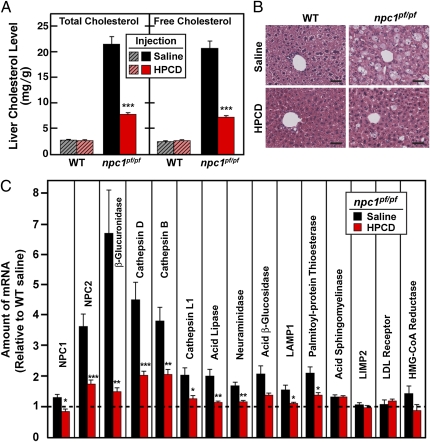
Fig. 7 shows an experiment in which WT and npc1pf/pf mice were treated with three weekly injections of saline or HPCD beginning at 7 wk of age. At 9.5 wk of age, the content of total and free cholesterol in livers of saline-injected npc1pf/pf mice was 7.4-fold and 7.5-fold higher than that in the saline-injected WT mice, respectively (Fig. 7A). HPCD treatment reduced the total and free cholesterol content in the livers of npc1pf/pf mice by 64% and 65%, respectively (Fig. 7A) and greatly reduced the number of lipid-laden foam cells (Fig. 7B). Thus, HPCD administration can overcome the cholesterol transport defect in npc1pf/pf mice, as it does in mice deficient in either npc1 or npc2 (10–12).
Fig. 7.
Cyclodextrin treatment of WT and npc1pf/pf mice. Male WT and npc1pf/pf littermates were treated with three weekly injections of saline or HPCD beginning at 7 wk of age as described in Materials and Methods. At 9.5 wk of age (3 d after the final injection), the mice were killed to obtain plasma and organs for analysis. (A) Content of total and free cholesterol in livers of WT and npc1pf/pf mice injected with saline or HPCD. Each bar represents the mean ± SEM of data from five mice. (B) Histology of representative livers from WT and npc1pf/pf mice injected with saline or HPCD. Livers were fixed, sectioned, and stained with H&E. (Scale bars, 40 μM; magnification: 40×.) (C) Relative amounts of mRNAs in livers of the indicated groups were determined by real-time PCR, with apoB as the invariant control. Values represent the amount of mRNA relative to that in WT littermates injected with saline, which is arbitrarily defined as 1 and denoted by the dotted line. Each bar represents the mean ± SEM of data from five mice. Statistical analysis (A and B) was performed with two-tailed Student t test. *P < 0.05; **P < 0.01; ***P < 0.001.
In addition to elevations in the mRNAs for NPC1 and NPC2 (Fig. 2A), the livers of npc1pf/pf mice maintained elevated levels of mRNAs encoding a variety of lysosomal enzymes, including acid lipase, cathepsin B, cathepsin D, and β-glucuronidase (Fig. 7C). A notable exception was acid sphingomyelinase, whose expression level was not changed. Up-regulation of two of these mRNAs (cathepsin D and cathepsin B) was observed previously in human fibroblasts homozygous for the I1061T NPC1 mutation (17) and in NPC1-deficient mice (18). Importantly, as shown in Fig. 7C, the increases in the cathepsin mRNAs, as well as those for NPC2, acid lipase, and β-glucuronidase, were abolished when cholesterol accumulation was reversed by HPCD treatment of the npc1pf/pf mice.
Discussion
The studies in this article demonstrate that a knockin mutation (P202A/F203A) that abolishes cholesterol binding to the N-terminal domain of NPC1 reproduces the lethal phenotype of complete NPC1 deficiency in mice. This observation provides strong evidence that the cholesterol binding site on NPC1(NTD) that was demonstrated previously to be essential for NPC1 function in cultured cells (2, 4) is also essential for the function of the protein in the whole animal.
The npc1pf/pf mice harboring the knockin P202A/F203A mutation accumulated cholesterol in various organs in a pattern that resembles that seen in NPC1-deficient npc1nih/nih mice, the standard model in the field (9). This accumulation led to organ dysfunction and death at the same age as previously observed in the npc1nih/nih mice. Like the npc1nih/nih mice (10–12, 15), the npc1pf/pf mice showed dramatic improvement when treated with the cholesterol solubilizer HPCD. Weekly injections caused a marked fall in hepatic cholesterol content (Fig. 7 A and B).
In important quantitative studies, Dietschy and coworkers observed that weekly injections of HPCD, beginning at 7 d of age, markedly prolonged the lifespan of npc1nih/nih mice (15). This improvement was associated with a marked reduction in the content of unesterified cholesterol in liver. In another study they showed that a single injection of HPCD at 49 d of age lowered tissue cholesterol to a much lesser extent (19). Our results indicate that a reduction in liver cholesterol can be attained when the initial injection is given at 7 wk of age and repeated twice at weekly intervals. We have not yet studied the npc1pf/pf mice to determine whether lifespan can be extended under these circumstances.
We considered the possibility that cholesterol accumulation in the npc1pf/pf mice was caused by impaired folding of the mutant NPC1, thereby precluding its exit from the ER and its transport to the lysosome. Two observations argued against this possibility. First, we used endoglycosidase H treatment to show that the N-linked carbohydrates of the mutant NPC1 were processed in the Golgi to the same extent as wild-type NPC1, indicating that the protein left the ER (Fig. 2C). Second, when an epitope-tagged version of the mutant NPC1 was transfected into cultured cells, the protein colocalized with the lysosome marker LAMP1 (Fig. 3).
A unique observation in the current studies was the elevation in the hepatic content of mRNAs encoding a subset of lysosomal proteins. A previous study showed that brains of npc1nih/nih mice had elevations in two lysosomal enzymes, cathepsins B and D (18). In livers of the npc1pf/pf mice, we found elevations in the mRNAs encoding these two enzymes as well as the mRNAs encoding other lysosomal enzymes, such as β-glucuronidase, cathepsin L1, lysosomal acid lipase, neuraminidase, and acid β-glycosidase. However, not all lysosomal mRNAs were elevated. Most importantly, there was no elevation in the mRNA encoding acid sphingomyelinase. Cultured cells lacking NPC1 are reported to have reduced acid sphingomyelinase activity despite normal levels of the enzyme protein (20, 21). Reduced sphingomyelinase activity is likely responsible for the elevated sphingomyelin levels in NPC1-deficient lysosomes (1, 22).
In addition to the elevations in certain lysosomal enzyme mRNAs, we also found elevations in the mRNAs encoding both NPC1 and NPC2 (Figs. 2A and 7C). The elevations in NPC1 and NPC2 protein were at least as profound as the mRNA elevations (Fig. 2B). These data raise the possibility that the accumulation of cholesterol triggers a compensatory response designed to up-regulate the proteins that might enhance cholesterol removal from lysosomes. Consistent with this hypothesis was the observation that all of the elevated lysosomal mRNAs were restored toward normal when cholesterol was depleted from the lysosomes by HPCD treatment (Fig. 7C).
Amritraj et al. (18) provided evidence that the up-regulation of cathepsins B and D in cerebellum of NPC1-deficient mice was associated with a maldistribution of the enzyme from lysosomes to the cytoplasm. They postulated that these proteolytic enzymes initiated a series of reactions leading to neuronal death. Considered together with their data, our observation that cathepsin mRNA levels are reduced by HPCD treatment raises the possibility that the improvement in lifespan in HPCD-treated mice is related to the lowering of cathepsin levels that occurs when lysosomal cholesterol is reduced. This hypothesis can be tested in future experiments in the mouse model.
Materials and Methods
Generation of npc1 Knockin Mice.
The targeting vector used to generate npc1 knockin mice was constructed in pJB1, a gene replacement vector with a loxP-flanked pgkneopA cassette and two copies of the HSV-TK gene as selection markers (23). The two regions of homology were generated by PCR using genomic DNA derived from SM-1 ES cells (derived from 129SvEv blastocysts). PCR products were cloned into pJB1. For the short-arm region containing exon 5 of npc1, the codons for a proline (CCA) and a phenylalanine (UUU) at adjacent residues 202 and 203 of NPC1 were mutated to alanine codons (GCA and GCU, respectively) by site-directed mutagenesis (Stratagene QuikChange Site-Directed Mutagenesis Kit). The integrity of all constructs was confirmed by restriction analysis and DNA sequencing.
SM-1 ES cells were cultured, transfected with the linearized targeting vector, and selected with 250 μg/mL G418 as described previously (24). Three independent ES clones that had undergone homologous recombination as determined by PCR and Southern blotting were injected into C57BL/6J blastocysts to generate chimeric mice. When crossed to C57BL/6J females, male chimeras from all three clones gave offspring that carried the npc1 knockin allele through the germ line.
Animal Studies.
All experiments were carried out with WT and npc1 knockin (npc1pf/pf) littermates obtained from intercrosses of npc1+/pf heterozygous male and female mice, all of which are hybrids of C57BL/6J and 129Sv/Ev strains. The npc1nih/nih mice used in Fig. 6 (obtained from Dr. John Dietschy, University of Texas Southwestern Medical Center, Dallas, TX) were on a pure BALB/c background (9, 11). All mice were housed in colony cages with a 12-h light/12-h dark cycle and fed ad libitum with Harlan Teklad Global Diet 2018 (Harlan Laboratories) and killed in the nonfasting state during the early phase of the light cycle. For the growth and survival study, the WT and npc1pf/pf littermates were weighed weekly, and their general appearance was monitored daily. Starting at 7 wk of age when the npc1pf/pf mice started to show tremor and ataxia, food pellets were placed directly in the cage. When a mouse exhibited visible difficulty in ambulating to reach water or take food, it was killed, and this was noted as the day of death. All animal experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at University of Texas Southwestern Medical Center at Dallas.
Cyclodextrin Treatment.
HPCD (CTD Holdings, catalog no. THPB-EC) in a 20% (wt/vol) saline solution was administered to mice at a dose of 4,000 mg/kg body weight by s.c. injection as described previously (11). Male WT and npc1pf/pf littermates were injected with HPCD or saline at 7, 8, and 9 wk of age. Three days after the final injection the mice were killed to obtain plasma and tissues for various analyses.
Quantitative Real-Time PCR Analysis.
Total RNA was prepared from mouse liver using RNA STAT-60 (Tel-Test) and subjected to quantitative real-time PCR as described previously (24). All reactions were carried out in triplicate, and the relative amounts of mRNAs were calculated using the comparative Ct method with apolipoprotein B (apoB) as the invariant control. Primers for apoB were described previously (24). Primers not previously described can be found in Table S1.
Glycosidase Treatment and Immunoblot Analysis.
Membrane proteins were prepared from frozen tissues as described previously (25). For glycosidase treatment, the 100,000 × g membrane pellet from 50 mg liver or brain was resuspended in 100 μL buffer containing 10 mM Tris·chloride at pH 6.8, 100 mM NaCl, and 0.5% (wt/vol) SDS and shaken for 30 min at room temperature. Equal amounts of solubilized membrane protein from the liver or brain tissues of four mice were then pooled. Aliquots of the pooled membrane protein were incubated in the absence or presence of 2,500 U Endo H or 2,500 U PNGase F (New England Biolabs) and subjected to 8% SDS/PAGE and immunoblot analysis as described previously (3). The primary antibodies for immunoblot analyses were 0.5 μg/mL rabbit polyclonal antibody directed against a synthetic peptide corresponding to C-terminal region of human NPC1 (catalog no. 36983, Abcam), 1 μg/mL rabbit polyclonal anti-NPC2 (3), and 1:1,000 dilution of rabbit monoclonal anti-β-tubulin (catalog no. 2128, Cell Signaling). Bound antibodies were visualized by chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate; Thermo Scientific) using a 1:3,000 dilution of anti-rabbit IgG (GE Healthcare) conjugated to horseradish peroxidase.
Histology.
For histological analysis, anesthetized mice were transcardially perfused with normal saline and then with 10% (vol/vol) formalin. After perfusion, the tissues were fixed in 10% formalin overnight at 4 °C. The fixed tissues were embedded in paraffin, sectioned at 5 μm, and stained with H&E.
Immunofluorescence.
Human SV-589 fibroblasts were grown at 37 °C in 5% CO2 in DMEM containing 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 10% FCS. On day 0, the cells were set up at 65 × 103 cells/35-mm glass-bottom dish (MatTek, catalog no. P35G-1.5-14-C). On day 1, the cells were transfected with 1 μg pTK-NPC1-His8-Flag3 or pTK-NPC1(P202A/F203A)-His8-Flag3 using FuGENE 6 transfection reagent (Roche Applied Science) as described previously (2). Twenty-four hours after transfection, the cells were fixed for 10 min at room temperature in 4% (wt/vol) paraformaldehyde, washed with PBS (three times; 1 min per wash), and treated with 0.1% (vol/vol) Triton X-100 in PBS for 90 s. After washing 3 times with PBS, the cells were incubated for 30 min in a blocking solution containing 10% (vol/vol) normal goat serum (Invitrogen, catalog no. 50-062Z). Thereafter, the cells were incubated in the same blocking solution for 16 h at 4 °C with 1 μg/mL mouse monoclonal anti-LAMP1 (Abcam, catalog no. ab25630) and 0.5 μg/mL rabbit polyclonal anti-Flag (Sigma-Aldrich, catalog no. F7425) to detect endogenous LAMP1 and the transfected Flag-tagged NPC1 proteins, respectively. After washing three times with PBS, the cells were incubated for 1 h at room temperature with 5 μg/mL Alexa Fluor 488 (green) donkey anti-mouse IgG (Invitrogen, catalog no. A-21202) and 5 μg/mL Alexa Fluor 568 (red) goat anti-rabbit IgG (Invitrogen, catalog no. A-11036) antibodies. After three washes with PBS, the cells were incubated with 300 nM DAPI (Invitrogen, catalog no. D3571) in PBS for 10 min to stain the nuclei. Fluorescence was viewed with a Leica TCS SP5 laser scanning confocal microscope using an Argon Laser with excitation wavelength of 488 nm for Alexa Fluor 488 (green) and a HeNe1 laser with excitation wavelength of 561 nm for Alexa Fluor 568 (red). DAPI (blue) fluorescence was viewed using a HeNe1 laser with excitation wavelength of 405 nm. Colocalization analysis was done using the Imaris software (version 7.2.3; www.bitplane.com).
Lipid Measurements.
The content of total and free cholesterol in tissues was measured using Wako kits (catalog no. 439-17501 and 435-35801, respectively). Extraction and analysis of gangliosides were carried out as previously described (26). Briefly, total lipids from brain and liver tissues were extracted in chloroform:methanol:PBS (1:1:0.9, vol/vol/vol) by the method of Bligh and Dyer (27). Equal amounts (based on wet tissue weight) of total lipid extracts from four mice per group were pooled, and aliquots of the pooled samples were then separated into neutral and anionic lipid fractions using DEAE Sephadex A-25 columns (GE Healthcare). The anionic lipid fractions (containing gangliosides) were desalted with PD-10 Sephadex G-25 columns (GE Healthcare). Aliquots of the ganglioside fraction corresponding to 3 mg brain or 1.5 mg liver tissue were analyzed by TLC using high-performance TLC plates (catalog no. 818140, Macherey-Nagel). The plates were developed in a choloroform:methanol:0.2% CaCl2 (60:35:8, vol/vol/vol) solution. Gangliosides were visualized by staining with 0.5% (wt/vol) orcinol dissolved in 3 M sulfuric acid. Aliquots (4 μg each) of GM1, GM2, and GM3 standards (Avanti) were loaded on the same TLC plate.
Supplementary Material
Acknowledgments
We thank Monica Mendoza and Isis Soto for invaluable help with animal studies, Liz Lummus and Robert Hammer (University of Texas Southwestern Transgenic Core) for ES cell injections, and Kate Luby-Phelps (University of Texas Southwestern Live Cell Imaging Core) and Lina Abi Mosleh for help with immunofluorescence. This work was supported by National Institutes of Health Grant HL20948 and grants from the Ara Parseghian Medical Research Foundation and Moss Heart Fund.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112751108/-/DCSupplemental.
References
- 1.Pentchev PG, Vanier MT, Suzuki K, Patterson MC. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw-Hill; 1995. pp. 2625–2639. [Google Scholar]
- 2.Kwon HJ, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ML, et al. Identification of surface residues on Niemann-Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 2010;12:166–173. doi: 10.1016/j.cmet.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infante RE, et al. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 5.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carstea ED, et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 7.Davies JP, Ioannou YA. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem. 2000;275:24367–24374. doi: 10.1074/jbc.M002184200. [DOI] [PubMed] [Google Scholar]
- 8.Infante RE, et al. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci USA. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftus SK, et al. Murine model of Niemann-Pick C disease: Mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 10.Camargo F, et al. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001;70:131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, et al. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc Natl Acad Sci USA. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson CD, et al. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskelinen E-L, Tanaka Y, Saftig P. At the acidic edge: Emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–145. doi: 10.1016/s0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Turley SD, Liu B, Repa JJ, Dietschy JM. GM2/GD2 and GM3 gangliosides have no effect on cellular cholesterol pools or turnover in normal or NPC1 mice. J Lipid Res. 2008;49:1816–1828. doi: 10.1194/jlr.M800180-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez CM, et al. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr Res. 2010;68:309–315. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sleat DE, et al. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc Natl Acad Sci USA. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy JV, Ganley IG, Pfeffer SR. Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS ONE. 2006;1:e19. doi: 10.1371/journal.pone.0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amritraj A, et al. Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice. Am J Pathol. 2009;175:2540–2556. doi: 10.2353/ajpath.2009.081096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, et al. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51:933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reagan JW, Jr, Hubbert ML, Shelness GS. Posttranslational regulation of acid sphingomyelinase in niemann-pick type C1 fibroblasts and free cholesterol-enriched chinese hamster ovary cells. J Biol Chem. 2000;275:38104–38110. doi: 10.1074/jbc.M005296200. [DOI] [PubMed] [Google Scholar]
- 21.Devlin C, et al. Improvement in lipid and protein trafficking in Niemann-Pick C1 cells by correction of a secondary enzyme defect. Traffic. 2010;11:601–615. doi: 10.1111/j.1600-0854.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulze H, Sandhoff K. Lysosomal lipid storage diseases. Cold Spring Harb Perspect Biol. 2011;3:a004804. doi: 10.1101/cshperspect.a004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelking LJ, et al. Schoenheimer effect explained—feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J Clin Invest. 2005;115:2489–2498. doi: 10.1172/JCI25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang G, et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 25.Engelking LJ, et al. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J Clin Invest. 2004;113:1168–1175. doi: 10.1172/JCI20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Echten-Deckert G. Sphingolipid extraction and analysis by thin-layer chromatography. Methods Enzymol. 2000;312:64–79. doi: 10.1016/s0076-6879(00)12900-3. [DOI] [PubMed] [Google Scholar]
- 27.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.