Abstract
Objective
Cortical spreading depression (CSD) has long been implicated in migraine attacks that begin with visual aura. Having shown that a wave of CSD can trigger long-lasting activation of meningeal nociceptors – the first-order neurons of the trigeminovascular pathway thought to underlie migraine headache – we now report that CSD can activate central trigeminovascular neurons in the spinal trigeminal nucleus (C1-2).
Methods
Stimulation of the cortex with pin prick or KCl granule was used to induce CSD. Neuronal activity was monitored in C1-2 using single-unit recording in anesthetized rats.
Results
In 25 trigeminovascular neurons activated by CSD, mean firing rate (spikes/sec) increased from 3.6 ± 1.2 before CSD (baseline) to 6.1 ± 1.8 after CSD (p < 0.0001) for a period >13 min. Neuronal activity returned to baseline level after 30.0 ± 3.1 min in 14 units, and remained elevated for 66.0 ± 8.3 (22–108) min through the entire recording period in the other 11 units. Neuronal activation began within 0.9 ± 0.4 (0–2.5) min after CSD in 7 neurons located in laminae I-II, or after a latency of 25.1 ± 4.0 (7–75) min in 9 neurons located in laminae I-II, and 9 neurons located in laminae III-V. In 27 trigeminovascular neurons not activated by CSD, mean firing rate was 2.0 ± 0.7 at baseline and 1.8 ± 0.7 after CSD.
Interpretation
We propose that CSD constitutes a nociceptive stimulus capable of activating peripheral and central trigeminovascular neurons that underlie the headache of migraine with aura.
Introduction
Approximately 30% of episodic migraine attacks are associated with one or more symptoms of transient cortical malfunction, collectively referred to as aura.1–3 Most common among those symptoms are visual aura (scintillating lights or scotoma4) sensory aura (tingling or numbness5) speech aura (expressive aphasia or dysarthria6) and motor aura (impaired coordination or paresis6). Symptoms of aura typically develop some 30–60 minutes ahead of the onset of migraine headache.7–9 Patients experiencing migraine with aura (especially women) were found to be at higher risk for impaired cognitive functions10 and prone to developing cerebral infarction11–13 and deep white-matter lesions in the cerebellum and the red nucleus.14–16
Aura has long been suggested to be caused by a biphasic electrophysiological phenomenon dubbed cortical spreading depression (CSD). Animal studies have documented the propagation of spreading depression through the cortex (especially the visual cortex) as a wave of cellular hyperexcitability (depolarization) followed by a prolonged phase of quiescence.17,18 The phenomenon has been replicated, using subdural electrocorticography, during craniotomy in humans with ischemic stroke or acute brain injury.19–21 Short of intracranial electrophysiology, neuroimaging studies during migraine with aura have documented a number of hemodynamic changes arguably reflecting CSD including local changes in cerebral blood flow, cortical hyperemia, spreading oligaemia, and hypoperfusion.22,23 A near-continuous functional MRI recording during migraine with visual aura detected an initial wave of hyperemia followed by a wave of hypoperfusion that progressed slowly through the visual cortex in lockstep with the developing symptoms of aura.23
It has been proposed that CSD can precipitate headache during migraine with aura by activating a trigeminovascular pathway that originates in meningeal nociceptors.24 Using single-unit recording in the rat trigeminal ganglion, we have provided the first direct evidence that meningeal nociceptors can be activated by a wave of spreading depression passing through the visual cortex underneath their dural receptive field. Such activation was manifested as a twofold increase in mean neuronal firing rate which began either immediately, or some 14 min after CSD, and persisted for 45 min or longer.25
Pursuant to the activation of meningeal nociceptors by CSD, we sought to examine the effects of CSD on the activity of second-order trigeminovascular neurons in spinal trigeminal nucleus. Such activation has been deduced from evidence that CSD can induce neuronal c-fos immunoreactivity in the superficial laminae of the trigeminal nucleus caudalis at the level of spinal segments C1-2.26
Materials and Methods
Surgical Preparation
Experiments were approved by the Beth Israel Deaconess Medical Center and Harvard Medical School standing committees on animal care, and in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (250–350 g) were anesthetized with urethane (1.2 g/kg i.p.), fitted with an intratracheal tube for artificial ventilation of the lungs. The rat’s head was then mounted in a stereotaxic apparatus, and core body temperature was maintained at 37°C using a heating blanket. Rats were paralyzed with gallamine triethiodide (0.5 g/kg i.p.) and ventilated. End-tidal CO2 was continuously monitored and kept within a physiological range of 3.5–4.5%.
For stimulation of the cranial dura later in the experiment, a 5×5-mm opening was carefully carved in the parietal and occipital bones in front and behind the lambda suture, directly above the left transverse sinus. The exposed dura was kept moist using a modified synthetic interstitial fluid (135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM CaCl2, 10 mM glucose and 10 mM Hepes, pH 7.2). For single-unit recording in the spinal trigeminal nucleus, a segment of the spinal cord between the obex and C2 was uncovered from overlying tissues, stripped of the dura mater, and kept moist with mineral oil as described before.27
Single-Unit Recording
A tungsten microelectrode (impedance 0.8–1.2 MΩ) was lowered repeatedly into the medullary dorsal horn in search for a trigeminovascular unit. A neuron was selected for the study if it exhibited discrete firing bouts in response to ipsilateral electrical and mechanical stimulation of the exposed cranial dura and to mechanical stimulation of the facial skin. Stimulation of the dura with electric pulses (0.1–4.0 mA, 0.5 msec, 0.5 Hz pulses), and mechanical stimulation of the dura with a calibrated von Frey monofilaments (3.63 g) were associated with a simultaneous bout of activity. At the end of each experiment, the neuron under study was also challenged with chemical stimulation of the dura (50 or 100 mM KCl), resulting in increased neuronal firing over ~30–60 sec. Stimulation of the facial skin consisted of brush, pressure and pinch, delivered sequentially (10 sec each, 10-sec inter-stimulus interval) using an artist paint brush, loose arterial clip, and forceps, respectively. Three classes of neurons were thus identified: wide-dynamic-range (WDR) neurons (incrementally responsive to brush, pressure and pinch), low-threshold (LT) neurons (equally responsive to all stimuli) and high-threshold (HT) neurons (unresponsive to brush). Real-time waveform discriminator was used to create and store a template for the action potential evoked in the neuron under study by electrical pulses on the dura; spikes of activity matching the template waveform were acquired and analyzed online and offline using Spike 2 software (CED, Cambridge, UK).
Induction and recording of CSD
Single waves of CSD were induced using mechanical and chemical stimulation of the visual cortex, about 6 mm away from the dural receptive field of the neuron under study.25 Changes in steady potential were recorded on the surface of the cortex using a glass micropipette filled with 150 mM NaCl (impedance 70–120Ω), about halfway between the dural receptive field and the site of cortical stimulation. At a propagation rate of 3–5 mm/min,28 a wave of CSD was expected to be registered by the recording electrode and enter the neuronal receptive field within 1–2 min of cortical stimulation. Mechanical stimulation (pin prick) was delivered by inserting a glass micropipette (diameter 25 ⌈m) about 1 mm into the visual cortex for 10 sec. Chemical stimulation was delivered by placing a granule of crystalline KCl on the surface of the cortex; the granule was washed away with SIF at the end of the CSD wave.
Experimental paradigm
Dural receptive field of the nociceptor under study was mapped using calibrated von-Frey monofilaments. Small incisions were made in the dura overlaying the visual cortex at the site designated for cortical stimulation and CSD recording. Ongoing activity of the nociceptor was recorded over 30 min before dural incision, and for another 30–60 min afterwards. Once neuronal activity reached a stable rate of firing, the cortex was stimulated to induce a wave of CSD, and ongoing activity was monitored for a period of 30–120 min after CSD. At the end of the experiment, a small lesion was produced at the recording site and its localization in the dorsal horn was determined postmortem using histological analysis as described before27.
Data analysis
Data were analyzed by nonparametric statistics, using two-tailed level of significance set at 〈 = 0.05. Mean firing rate (spikes/sec) before cortical stimulation (baseline) was calculated in each trial over a period of 30 min. A given neuron was considered activated when its mean firing rate after CSD exceeded its mean baseline activity by 2 standard deviations of that mean for a period >10 min, which translated to ≥33% increase in the final analysis. Latency to onset of neuronal activation and the duration of neuronal activation were compared across WDR, LH and HT neurons using Kruskal-Wallis one-way analysis of variance. Mean firing rates before and after the induction of CSD were compared using Wilcoxon matched-pairs signed-ranks test.
Results
Using single-unit recording in the medullary dorsal horn (C1-2), we identified 52 trigeminovascular neurons (1 neuron/rat) that exhibited discrete bouts of activity in response to electrical, mechanical and chemical stimulation of the cranial dura (Fig 1A–C). These units were functionally classified as HT, WDR, or LT according to the relative magnitude of their responses to ipsilateral stimulation of the facial skin with brush, pressure and pinch (Fig 1D–F). Histological localization of the neurons (Fig 2) showed that 30 units were located superficially in the dorsal horn (lamina I or II) and 22 in the deeper laminae (III, IV or V). Excluding 5 unclassified units, the proportions of the different cell types were similar (p > 0.73) between the superficial and deep laminae (HT – 43 vs. 47%; WDR – 40 vs. 29%; LT – 17 vs. 24%). Neuronal receptive fields at the dura (mostly at the transverse sinus above the visual cortex) and facial skin (mostly periorbital/ophthalmic) were consistently similar between superficial units (Fig 3A, B) and units located in deeper layers (Fig 3C, D). Baseline ongoing activity was significantly different across HT, WDR and LT units in the superficial laminae (0.3 ± 0.1, 2.2 ± 0.9 and 3.0 ± 1.7 spikes/sec, respectively; p = 0.007), but not in the deep laminae (1.9 ±1.0, 2.9 ± 2.5 and 4.4 ± 2.9 spikes/sec, respectively; p > 0.5).
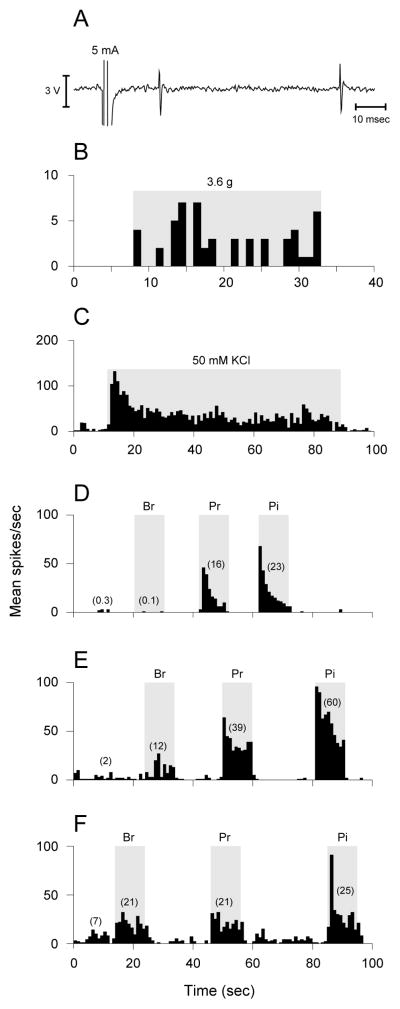
Figure 1.
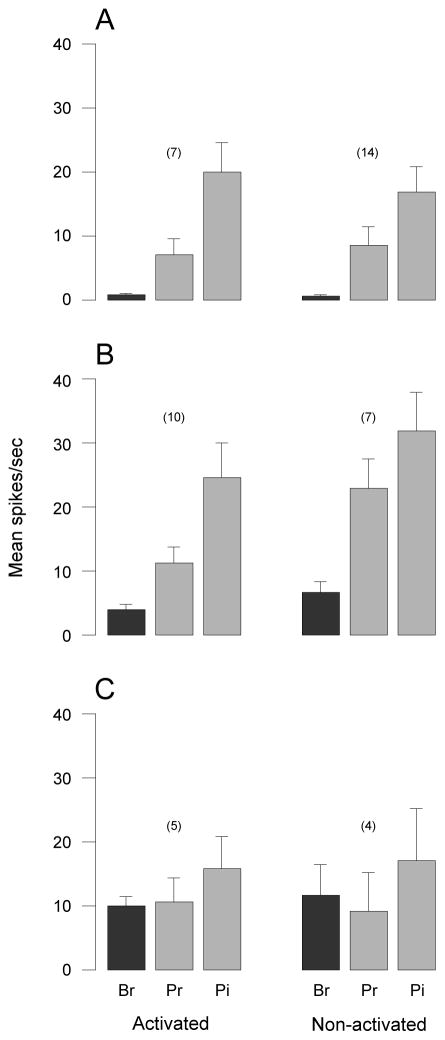
Identification and characterization of central trigeminovascular neurons. Neurons receiving input from the dura were identified based on their responses to electrical (A), mechanical (B) and chemical (C) stimulation of the dura overlying the visual cortex. Neurons thus identified were further classified as HT (D), WDR (E) or LT (F) based on their responses to innocuous and noxious stimulation of their cutaneous receptive field on the ipsilateral face. Br, brush; Pr, pressure; Pi, pinch.
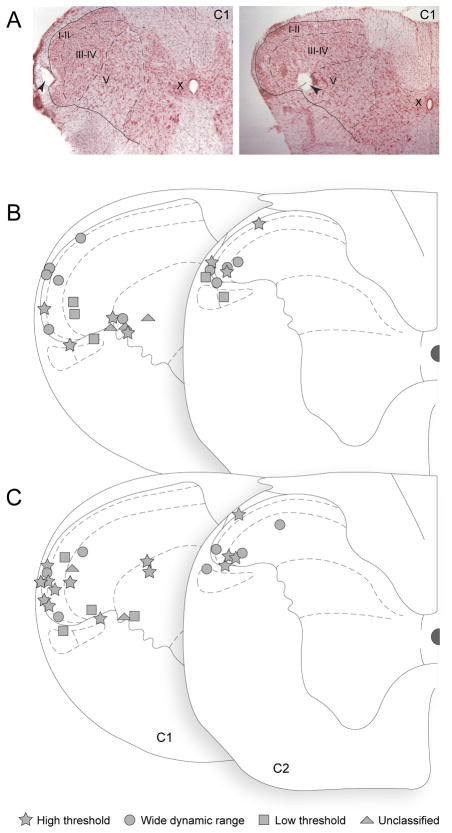
Figure 2.
Localization of lesions marking the recording sites in the dorsal horn (1 neuron per rat). (A) Photomicrographs showing lesion (pointed by arrowheads) in laminae I (left) and V (right) of the first cervical segment (C1). (B) Mapping of 25 lesions marking the locations of activated neurons. (C) Mapping of 27 lesions marking the locations of non-activated neurons. Neuronal type is indicated by the key at bottom of figure.
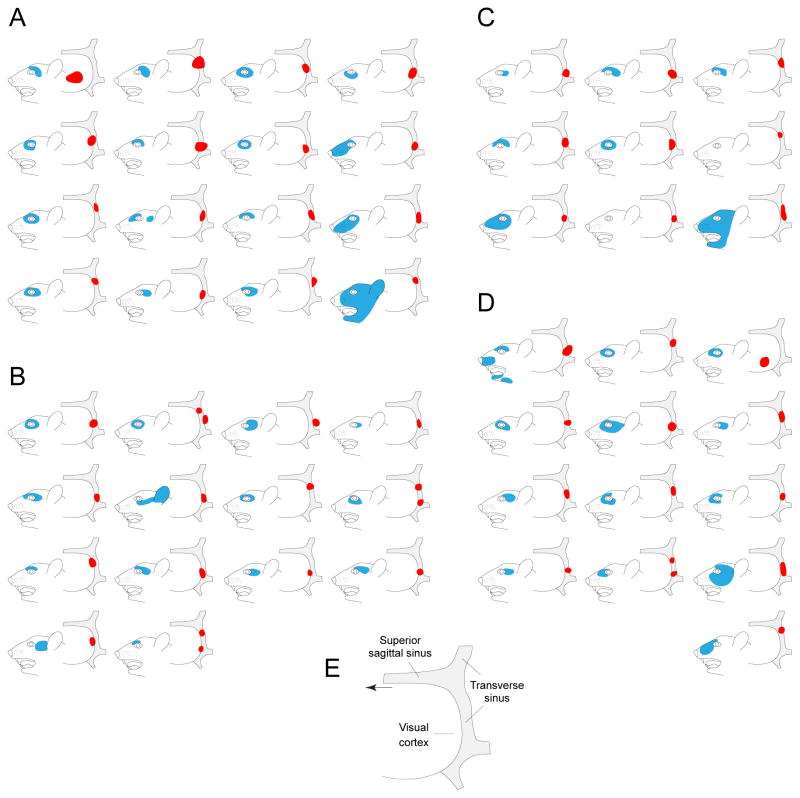
Figure 3.
Localization of dural (red) and cutaneous (blue) receptive fields. (A) Activated neurons in the superficial dorsal horn. (B) Non-activated neurons in the superficial dorsal horn. (C) Activated neurons in the deep dorsal horn. (D) Non-activated neurons in the deep dorsal horn. E, Detailed view of drawing illustrating dural receptive field. Arrow points forward.
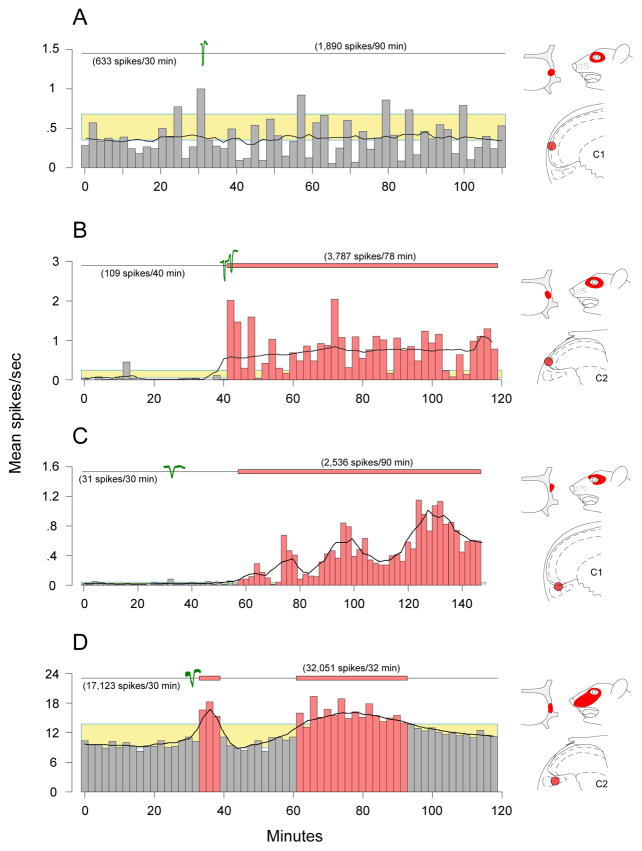
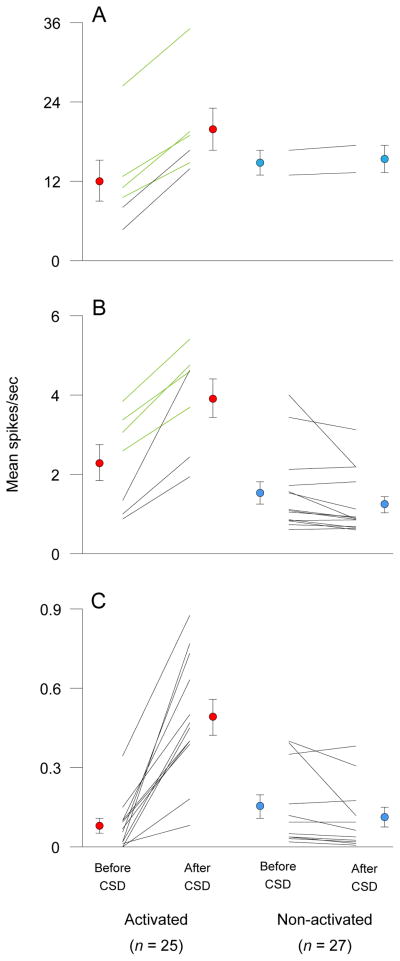
Waves of CSD lasting 72.8 ± 4.9 sec (mean ± SEM) were registered within 33.4 ± 3.0 sec of stimulating the visual cortex in 52 trials (41 with pin-prick; 7 with crystalline KCl). Mean firing rate of all 52 tested neurons increased significantly (p = 0.0009) from 2.7 ± 0.7 at baseline to 3.9 ± 1.0 after CSD. Twenty seven of those units, however, did not pass the criteria for neuronal activation (Fig 4A); their ongoing firing rate was 2.0 ± 0.7 at baseline and 1.8 ± 0.7 spikes/sec after CSD (Fig 5). The remaining 25 neurons increased their firing rate for an extended duration (≥13 min) from 3.6 ± 1.2 spikes/sec at baseline to 6.1 ± 1.8 after CSD (p < 0.0001). Firing rate increased after CSD by 33–76% in 8 activated units (Fig 4D, Fig 5A, B, green plots), and by >106% in the 17 remaining units (Fig 4B, C; Fig 5A–C, black plots). The fold increase in firing rate was most pronounced in units with mean baseline activity below 0.4 spikes/sec (Fig 5C) compared to those with baseline activity greater than 0.6 spikes/sec (Fig 5A, B). Five units (all located in the superficial laminae) became activated during CSD or immediately after (0.2 ± 0.2; range 0–1.0 min; Fig 4B). The remaining 20 units (equally divided between superficial and deep laminae) became activated as late as 22.8 ± 3.9 (2–75) min after CSD (Fig 4C, D). Neuronal activity returned back to baseline level after 30.1 ± 3.1 min in 14 units (Fig 4D), and remained elevated for 66.0 ± 8.3 (22–108) min through the end of the recording period in the other 11 units (Fig 4B, C). The pattern of neuronal activation (latency, duration, increased firing rate) was unrelated to whether CSD was induced by pin prick (n = 18) or KCl (n = 7). The mean number of spikes recorded over the period of activation in all 25 neurons was 14867 ± 4317 compared with 7592 ± 2164 calculated for a corresponding interval at baseline.
Figure 4.
Individual examples of neuronal firing before and after CSD. Waves of CSD induced by a single stimulation of the cortex are shown in green (cortical activity was monitored continuously throughout the experiment, and no other CSD waves were registered). Neuronal activation (red bars) was determined for each neuron when its firing rate after CSD exceeded its mean baseline activity (bottom of yellow box) by 2 standard deviations of that mean (top of yellow box) for a period >10 min. Black-line curves shown across the histograms describe the pattern of neuronal activity after applying a moving-average smoothing function. (A) Example of a neuron that was not activated by CSD. (B) Example of a neuron that became activated immediately after CSD and remained activated for 78 min, through the end of the recording session. (C) Example of a neurons that became activated 30 min after CSD and remained activated for 90 min through the end of the recording session. (D) Example of a neuron that became activated 30 min after CSD and remained activated for 32 min. Note the transient (6 min) surge of activation immediately after CSD. Laminar location of each neuron and its dural and facial receptive fields are illustrated on the right.
Figure 5.
Firing rate before and after CSD in activated and non-activated central trigeminovascular neurons. Individual plots and mean ± SEM values are shown for activated (left, red circles) and non-activated units (right, blue circles). A neuron was considered activated when its mean firing rate after CSD exceeded its mean baseline activity by 2 standard deviations of that mean over a period >10 min as shown in Fig. 4. (A) Neurons exhibiting high rate of baseline activity (12.09 ± 3.09; range 5–26 spikes/sec) increased their firing rate to 19.85 ± 3.19 spikes/sec (65%) after CSD. (B) Neurons exhibiting medium rate of baseline activity (2.3 ±0.46; range 0.6–4 spikes/sec) increased their firing rate to 3.92 ± 0.49 spikes/sec (75%) after CSD. (C) Neurons exhibiting low rate of baseline activity (0.08 ± 0.03; range 0–0.4 spikes/sec) increased their firing rate to 0.49 ± 0.07 spikes/sec (600%) after CSD.
The prospects for neuronal activation by CSD appeared to be unrelated to the functional classification of the neurons, their laminar position in the dorsal horn, or their cutaneous receptive field territories. The response profiles of activated HT, WDR and LT neurons were similar to the corresponding profiles of their non-activated counterparts (Fig 6). Neuronal activation occurred in 59% of WDR units (10/17), 56% of LT units (5/9), and 33% of HT units (7/21). Activated and non-activated neurons were present in similar numbers in superficial laminae (16 and 14, respectively) and deep laminae (9 and 13, respectively) of the dorsal horn (Fig. 2). Dural and cutaneous receptive fields did not appear to vary between activated (Fig 3A, C) and non-activated neurons (Fig 3B, D).
Figure 6.
Response magnitude to innocuous and noxious stimulation of the cutaneous receptive fields in activated and non-activated neurons. (A) High threshold neurons. (B) Wide dynamic range neurons. (C) Low threshold neurons. Note that the relative response magnitude to brush (Br), pressure (Pr) and pinch (Pi) were similar between activated and non-activated neurons in each category.
Discussion
This is the first study to show that induction of CSD by focal stimulation of the rat visual cortex can lead to increased ongoing activity in central trigeminovascular neurons in the spinal trigeminal nucleus. Together with our evidence for a similar effect of CSD on the activity of meningeal nociceptors,25 the findings strongly support the proposition that a wave of spreading depression in the visual cortex—the presumed mechanism of visual aura—can induce nociceptive signals in the overlaying meninges, resulting in sequential activation of peripheral (first-order) and central (second-order) neurons of the trigeminovascular pathway—one of the presumed mechanisms of migraine headache.
Our earlier evidence for the activation of meningeal nociceptors by CSD should be taken as essential—but not necessarily sufficient—for to the activation of second-order trigeminovascular neurons, because the absolute increase in the ongoing activity of the nociceptors25 was rather small (<1 spike/sec) relative to the magnitude of neuronal discharge elicited by skin stimulation.27 That a single wave of CSD induced a modest yet persistent increase in the ongoing neuronal activity in the spinal trigeminal nucleus (present study) implies that small rises in activity of the primary afferents should suffice to induce action potentials in the central neurons (rather than mere subthreshold EPSPs or IPSPs). For example, for a neuron whose activity increased from 0.06 spikes/sec at baseline to 0.73 spikes/sec after CSD (Fig 2B), the increase in the total number of spikes over the recording period of activation of this neuron was 3,575. Taken as a group, the 25 activated neurons sampled in the study generated 182,000 additional spikes along the trigeminovascular pathway. Assuming these neurons project to the thalamus, we propose that a relatively small rise in their firing rate after CSD can become over time sufficient to activate trigeminovascular neurons in the thalamus. The cumulative magnitude of such an effect provides a novel perspective on the potential power of CSD as a trigger of migraine headache.
The magnitude and duration of neuronal activation induced by CSD, as well as the latency to its onset, were quite similar between the peripheral25 and the central trigeminovascular neurons (present study), suggesting that CSD-evoked activation of meningeal nociceptors is sufficient to drive the central trigeminovascular neurons into an activity state. Both peripheral and central neuronal activation were manifested as a twofold increase in mean ongoing firing rate. Neuronal activity returned back to baseline after a mean period of 30 min in 65% of the peripheral units and 37 min in 56% of the central units, which is by far shorter than the typical duration of migraine headache. This duration appears to correlate with the duration of the second wave of altered neurovascular function shown recently to be associated with negative current shift, arterial constriction and haemoglobin desaturation.29 However, the remaining trigeminovascular units (35% peripheral; 44% central) maintained their elevated firing rate throughout the recording period (interrupted after nearly 2 hours), which would be longer than the reported duration of altered neurovascular functions following CSD.29,30 This raises the possibility that the initiation of headache during migraine with aura depends on CSD and its accompanied neurovascular changes, whereas the maintenance phase of the headache depends on long-lasting activation of the neural component of the trigeminovascular system.
Consistent with the typical delay between the onset of visual aura and the onset of migraine headache,7–9 neuronal activation started with a mean delay of 14 and 25 min after CSD in 78% and 72% of the peripheral25 and central units, respectively. A gradual increase in the firing rate of meningeal nociceptors25 may be due to a progressive recruitment of ramifying collateral branches31 during the propagation of the CSD wave underneath them. A gradual increase in the firing rate of the central neurons may reflect increased input from a growing number of nociceptors impacted by the propagating wave of CSD. Activation of the remaining units (22% peripheral; 28% central) coincided with the wave of CSD. Such immediate activation may be related to the direct current shift and the accompanied arterial constriction, desaturation of haemoglobin, and marked increase in extracellular potassium concentration recorded while the CSD wave was still propagating29. It is tempting to propose that meningeal nociceptor whose axons extended into the pia32 are involved in the immediate activation of the central neurons upon exposure to the increased extracellular level of potassium at the surface of the cortex during the wave of CSD.
In agreement with a c-fos study,26 we found that CSD can activate trigeminovascular neurons in the ventrolateral region of the superficial dorsal horn of C1-2 which constitutes a main termination zone of meningeal nociceptors.33,34 Histological examination of lesions marking the recording sites, which were performed at the end of each experiment, revealed that activated units were present not only in laminae I as previously reported using c-fos26 but, for the first time, also in laminae II, III, IV and V, adjacent (within 200 μm) to the medial tip of the ventrolateral region we targeted originally. After sampling additional units in the deeper laminae, we found that the likelihood of inducing neuronal activation by CSD was similar between the superficial and the deep laminae of C1-2 (53 and 41%, respectively). Is it possible, therefore, that nociceptive signals are processed differentially between trigeminovascular neurons of the superficial dorsal horn compared to their deeper counterparts?
Delayed neuronal activation by CSD was documented in meningeal Aδ and C nociceptors25 and in dura-sensitive neurons located in laminae I–II and laminae III–V of the dorsal horn (present study). In contrast, immediate neuronal activation occurred mostly with C nociceptors25 and selectively in trigeminovascular neurons located laminae I–II (present study). While the role of laminae I and V in pain sensation remains controversial,35–38 anatomical evidence suggests that their afferent connections are different. Lamina I receives direct input from peptidergic C nociceptors (releasing CGRP and substance P) and Aδ nociceptors,39,40 whereas lamina V receives direct input from Aδ nociceptors and low-threshold Aβ fibers, and only indirect input from C nociceptors.41 Thus, the activation of trigeminovascular neurons in the superficial dorsal horn immediately after CSD might be mediated by peptidergic meningeal nociceptors42 that project directly to lamina I. It also supports the view that CSD is capable of activating peptidergic meningeal nociceptors and consequently the proposed initiation of neurogenic inflammation they can initiate.24 CSD-induced activation of meningeal nociceptors has been proposed to involve (a) release of ATP, glutamate, K+ and H+ from cortical neurons, glia and vascular cells during the depolarization phase of CSD, and (b) diffusion of those agents to the adjacent cerebral meninges where they, presumably, reach the nociceptors.43–55
Due to the size of the lesions marking the recording sites, we were unable to determine the precise localization of the neurons between the outer and inner sub-layers of lamina II. The outer layer of lamina II receives direct input from peptidergic C-nociceptors, whereas the inner layer receives direct input from non-peptidergic C-nociceptors and from low-threshold, myelinated Aβ fibers.56 Furthermore, the outer layer contains inhibitory interneurons,56 whereas the inner layer contains excitatory interneurons implicated in the transition from acute to persistent pain.39,40,57 The activation of lamina-II neurons by CSD leads us to propose that activity along the pathway ascending from the spinal trigeminal nucleus to the brainstem and thalamus can be modulated already at the level of lamina II of the dorsal horn by inhibitory interneurons in the outer layer and facilitatory interneurons in the inner layer. Malfunction of such a local modulatory circuit (presumably due to cumulative damage from repeated migraine attacks) may contribute to a transformation from episodic migraine to chronic migraine.
Eighty percent of the activated neurons were classified as HT or WDR. The exact role HT and WDR neurons play in nociception is controversial, but they both lie at the origin of the so-called labeled pain pathways and their activation is believed to produce the complex human experience of pain. The remaining 20% of the activated neurons were LT. The activation of LT trigeminovascular neurons in the dorsal horn by CSD is puzzling because they are at the origin of tactile but not pain sensation. As their activation can only produce tactile sensation, they may be positioned to mediate non painful symptoms of migraine such as the ongoing facial tingling or numbness that can accompany attacks in some patients. This speculation may be more appropriate than the notion that the numbness and tingling are manifestation of sensory aura as auras are not believed to last for hours or days.
The relevance of CSD-induced activation of the trigeminovascular pathway to precipitation of headache during migraine with aura remains to be determined. While the endogenous triggers of CSD are largely unknown, recent data suggested altered cerebrovascular function, potentially related to vasculopathy, as the culprit.58,59 For example, induction of cerebral vasoconstriction by endothelin-1,60,61 or brief hypoxic-ischemic episodes by cerebral microembolism62, have been shown to trigger CSD.
The main conclusion drawn from our study—that CSD is a nociceptive stimulus capable of activating peripheral and central trigeminovascular neurons—appears to be at odds with studies that attempted and failed to induce activation of central trigeminovascular neurons by CSD. Those negative results were obtained in the transition zone between subnuclei caudalis and interpolaris63,64 or in laminae III and IV of C1-2,63,64 whereas our positive results were observed in other laminae (I, II and V) of C1-2. In view of these anatomical differences, we call for revising the categorical exclusion of CSD as a trigger for headache in migraine with aura,65 which has been based entirely on the aforementioned negative studies, and suggest that neurons susceptible to CSD are located specifically in layers I, II, V of the spinal trigeminal nucleus at the level of C1-2. Future studies will be necessary to document the effects of CSD on higher-order trigeminovascular neurons in the thalamus and brainstem in order to explain the full duration and fluctuating intensity of migraine headache, and its spread from one side to the other, or from the periorbital to occipital area.
Acknowledgments
This research was supported by NIH grants NS069847 and NS-035611 (RB).
References
- 1.Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885–894. doi: 10.1212/wnl.58.6.885. [DOI] [PubMed] [Google Scholar]
- 2.Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995;24:612–618. doi: 10.1093/ije/24.3.612. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen BK, Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12:221–228. doi: 10.1046/j.1468-2982.1992.1204221.x. discussion 186. [DOI] [PubMed] [Google Scholar]
- 4.Lashley KS. Patterns of cerebral integration indicated by the scotomas of migraine. Arch Neurol Psychiatry. 1941;46:259–264. [Google Scholar]
- 5.Lord GDA. Clinical characteristics of the migrainous aura. In: Amery WK, Wauquier A, editors. The prelude to the migraine attack. London: Bailliere Tindall; 1986. pp. 87–98. [Google Scholar]
- 6.Heyck H. Varieties of hemiplegic migraine. Headache. 1973;12:135–142. doi: 10.1111/j.1526-4610.1973.hed1204135.x. [DOI] [PubMed] [Google Scholar]
- 7.Olesen J, Goadsby P, Ramadan NM, et al. The Headaches. 3. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 8.Blau JN. Migraine: theories of pathogenesis. Lancet. 1992;339:1202–1207. doi: 10.1016/0140-6736(92)91140-4. [DOI] [PubMed] [Google Scholar]
- 9.Liveing E. On megrim, sick headache. Nijmegen: Arts & Boeve Publishers; 1873. [Google Scholar]
- 10.Gaist D, Pedersen L, Madsen C, et al. Long-term effects of migraine on cognitive function: a population-based study of Danish twins. Neurology. 2005;64:600–607. doi: 10.1212/01.WNL.0000151858.15482.66. [DOI] [PubMed] [Google Scholar]
- 11.Schurks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain. 2005;128:2068–2077. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 13.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–2570. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Brain stem and cerebellar hyperintense lesions in migraine. Stroke. 2006;37:1109–1112. doi: 10.1161/01.STR.0000206446.26702.e9. [DOI] [PubMed] [Google Scholar]
- 15.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- 16.Swartz RH, Kern RZ. Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol. 2004;61:1366–1368. doi: 10.1001/archneur.61.9.1366. [DOI] [PubMed] [Google Scholar]
- 17.Leao A. Spraeding depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 18.Leao AA. Spreading depression. Funct Neurol. 1986;1:363–366. [PubMed] [Google Scholar]
- 19.Fabricius M, Fuhr S, Willumsen L, et al. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin Neurophysiol. 2008;119:1973–1984. doi: 10.1016/j.clinph.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohmen C, Sakowitz OW, Fabricius M, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- 21.Strong AJ, Fabricius M, Boutelle MG, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- 22.Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion-weighted imaging defects during spontaneous migrainous aura. Annals of Neurology. 1998;43:25–31. doi: 10.1002/ana.410430108. [DOI] [PubMed] [Google Scholar]
- 23.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993;5:159–177. [PubMed] [Google Scholar]
- 25.Zhang XC, Levy D, Noseda R, et al. Activation of meningeal nociceptors by cortical spreading depression: implications to migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13:1167–1177. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. Journal of Neurophysiology. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 28.Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- 29.Chang JC, Shook LL, Biag J, et al. Brain. Vol. 133. Biphasic direct current shift, haemoglobin desaturation and neurovascular uncoupling in cortical spreading depression; pp. 996–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor TP, van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci. 1986;6:2200–2207. doi: 10.1523/JNEUROSCI.06-08-02200.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. The Journal of comparative neurology. 2009;515:331–348. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superior sagittal sinus. Neuroscience. 2004;129:431–437. doi: 10.1016/j.neuroscience.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zhang ML, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superficial temporal artery. Brain research. 2003;966:126–133. doi: 10.1016/s0006-8993(02)04222-1. [DOI] [PubMed] [Google Scholar]
- 35.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. [Review] [134 refs] Annual Review of Neuroscience. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 36.Price DD, Greenspan JD, Dubner R. Neurons involved in the exteroceptive function of pain. Pain. 2003;106:215–219. doi: 10.1016/j.pain.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Willis WD, Jr, Zhang X, Honda CN, Giesler GJ., Jr Projections from the marginal zone and deep dorsal horn to the ventrobasal nuclei of the primate thalamus. Pain. 2001;92:267–276. doi: 10.1016/s0304-3959(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 38.Willis WD, Jr, Zhang X, Honda CN, Giesler GJ., Jr A critical review of the role of the proposed VMpo nucleus in pain. J Pain. 2002;3:79–94. doi: 10.1054/jpai.2002.122949. [DOI] [PubMed] [Google Scholar]
- 39.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Snider WD, McMahon SB. Tackling pain at the source: New ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 41.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. Journal of Comparative Neurology. 1991;309:515–534. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- 43.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- 44.Mayevsky A, Doron A, Manor T, et al. Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain research. 1996;740:268–274. doi: 10.1016/s0006-8993(96)00874-8. [DOI] [PubMed] [Google Scholar]
- 45.Csiba L, Paschen W, Mies G. Regional changes in tissue pH and glucose content during cortical spreading depression in rat brain. Brain research. 1985;336:167–170. doi: 10.1016/0006-8993(85)90430-5. [DOI] [PubMed] [Google Scholar]
- 46.Marrannes R, Willems R, De Prins E, Wauquier A. Evidence for a role of the N-methyl-D-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain research. 1988;457:226–240. doi: 10.1016/0006-8993(88)90690-7. [DOI] [PubMed] [Google Scholar]
- 47.D’Andrea G, Cananzi AR, Joseph R, et al. Platelet glycine, glutamate and aspartate in primary headache. Cephalalgia. 1991;11:197–200. doi: 10.1046/j.1468-2982.1991.1104197.x. [DOI] [PubMed] [Google Scholar]
- 48.Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J Cereb Blood Flow Metab. 1992;12:223–229. doi: 10.1038/jcbfm.1992.32. [DOI] [PubMed] [Google Scholar]
- 49.Fabricius M, Jensen LH, Lauritzen M. Microdialysis of interstitial amino acids during spreading depression and anoxic depolarization in rat neocortex. Brain Res. 1993;612:61–69. doi: 10.1016/0006-8993(93)91644-8. [DOI] [PubMed] [Google Scholar]
- 50.Schock SC, Munyao N, Yakubchyk Y, et al. Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res. 2007;1168:129–138. doi: 10.1016/j.brainres.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 51.Brinley FJ, Jr, Kandel ER, Marshall WH. Potassium outflux from rabbit cortex during spreading depression. Journal of Neurophysiology. 1960;23:246–256. doi: 10.1152/jn.1960.23.3.246. [DOI] [PubMed] [Google Scholar]
- 52.James MF, Smith JM, Boniface SJ, et al. Cortical spreading depression and migraine: new insights from imaging? Trends Neurosci. 2001;24:266–271. doi: 10.1016/s0166-2236(00)01793-8. [DOI] [PubMed] [Google Scholar]
- 53.Rapoport SI, Marshall WH. Measurement of Cortical Ph in Spreading Cortical Depression. Am J Physiol. 1964;206:1177–1180. doi: 10.1152/ajplegacy.1964.206.5.1177. [DOI] [PubMed] [Google Scholar]
- 54.Charles A, Brennan K. Cortical spreading depression-new insights and persistent questions. Cephalalgia. 2009;29:1115–1124. doi: 10.1111/j.1468-2982.2009.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brennan KC, Beltran-Parrazal L, Lopez-Valdes HE, et al. Distinct vascular conduction with cortical spreading depression. J Neurophysiol. 2007;97:4143–4151. doi: 10.1152/jn.00028.2007. [DOI] [PubMed] [Google Scholar]
- 56.Neumann S, Braz JM, Skinner K, et al. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science (New York, NY) 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 58.Tietjen GE. Migraine as a systemic vasculopathy. Cephalalgia. 2009;29:987–996. doi: 10.1111/j.1468-2982.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- 59.Tietjen GE. Endothelial dysfunction in migraine. Cephalalgia. 2009;29:997–1002. doi: 10.1111/j.1468-2982.2009.01938.x. [DOI] [PubMed] [Google Scholar]
- 60.Dreier JP, Kleeberg J, Petzold G, et al. Endothelin-1 potently induces Leao’s cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura? Brain. 2002;125:102–112. doi: 10.1093/brain/awf007. [DOI] [PubMed] [Google Scholar]
- 61.Kleeberg J, Petzold GC, Major S, et al. ET-1 induces cortical spreading depression via activation of the ETA receptor/phospholipase C pathway in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1339–1346. doi: 10.1152/ajpheart.00227.2003. [DOI] [PubMed] [Google Scholar]
- 62.Nozari A, Dilekoz E, Sukhotinsky I, et al. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol. 67:221–229. doi: 10.1002/ana.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambert GA, Michalicek J, Storer RJ, Zagami AS. Effect of cortical spreading depression on activity of trigeminovascular sensory neurons. Cephalalgia. 1999;19:631–638. doi: 10.1046/j.1468-2982.1999.019007631.x. [DOI] [PubMed] [Google Scholar]
- 64.Ebersberger A, Schaible HG, Averbeck B, Richter F. Is there a correlation between spreading depression, neurogenic inflammation, and nociception that might cause migraine headache? Ann Neurol. 2001;49:7–13. [PubMed] [Google Scholar]
- 65.Goadsby PJ. Migraine, aura, and cortical spreading depression: why are we still talking about it? Ann Neurol. 2001;49:4–6. doi: 10.1002/1531-8249(200101)49:1<4::aid-ana3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]