Abstract
There has been an extraordinary recent accumulation of information concerning the neurobiology and neuropharmacology of dopamine (DA) receptors in the mammalian central nervous system. Many new DA molecular entities have been cloned, their gene, peptide sequences and structures have been identified, their anatomical distributions in the mammalian brain described, and their pharmacology characterized. Progress has been made toward developing selective ligands and drug-candidates for different DA receptors. The new discoveries have greatly stimulated preclinical and clinical studies to explore the neuropharmacology of DA receptors and their implications in the neuropathophysiology of different neuropsychiatric diseases including schizophrenia, Parkinson’s disease and attention-deficit hyperactivity disorder. Accordingly, it seems timely to review the salient aspects of this specialized area of preclinical neuropharmacology and its relevance to clinical neuropsychiatry.
Keywords: antipsychotics, ADHD, basal ganglia, dopamine receptors, Parkinson’s disease, schizophrenia
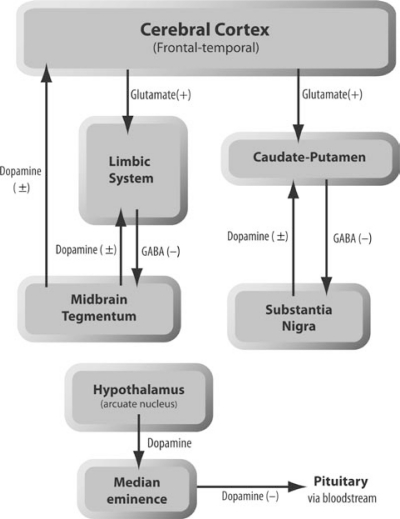
Dopamine (da) is a major neurotransmitter within the mammalian central nervous system (CNS). DA-containing neurons arise mainly from DA cell bodies in the substantia nigra and ventral tegmental area in mid-brain region, and are organized into four major subsystems [Figure 1]:1–6 (i) the nigrostriatal system involving neurons projecting from the substantia nigra pars compacta to the caudate-putamen of the basal ganglia. This is the major DA system in the brain as it accounts for about 70% of the total DA in the brain, and its degeneration makes a major contribution to the pathophysiology of Parkinson’s disease; (ii) the mesolimbic system that originates in the midbrain tegmentum and projects to the nucleus accumbens septi and lateral septal nuclei of the basal forebrain as well as the amygdala, hippocampus, and the entorhinal cortex, all of which are considered components of the limbic system and so are of particular interest for the pathophysiology of idiopathic psychiatric disorders; (iii) the mesocortical system, which also arises from neuronal cell bodies in the tegmentum which project their axons to the cerebral cortex, particularly the medial prefrontal regions; (iv) the tuberinfundibular pathway, which is a neuroendocrinological pathway arising from the arcuate and other nuclei of the hypothalamus and ending in the median eminence of the inferior hypothalamus. DA released in this system exerts regulatory effects in the anterior pituitary and inhibits the release of prolactin.
Figure 1.
Schematic organisation of the four major dopamine systems in the brain.2
DA mediates its neurocheantimical and physiological actions via membrane receptor proteins. DA receptors are found on postsynaptic neurons in brain regions that are DA-enriched. In addition, they reside presynaptically on DA neuronal cell bodies and dendrites in the midbrain as well as on their terminals in the forebrain. Stimulation of these ‘autoreceptors’ inhibits DA synthesis by blocking the activity of tyrosine hydroxylase, the rate-limiting enzymatic step in catecholamine synthesis. In addition, DA autoreceptor activation blocks DA release from presynaptic membrane-enclosed storage vesicles, and significantly attenuate the firing rate of the DA neurons.7,8 All DA receptor proteins belong to a superfamily of large peptides that are coupled to G-proteins and modified by attached carbohydrate, lipid-ester or phosphate groups. They are characterized by having seven hydrophobic transmembrane-spanning regions, as well as a functionally critical third intracytoplasmic loop that interacts with G-proteins and other effector molecules to mediate the physiological and neurochemical effects of the receptors.2–5
The DA receptors were originally differentiated into two major types.9 This was mainly based on the presence or absence of ability of DA to stimulate adenylyl cyclase and produce the second-messenger molecule cyclic-AMP (cAMP) to distinguish receptor types D1 and D2. D1 receptors were characterized initially as mediating the stimulation of cAMP production. D2 receptors, which inhibit the production of cAMP, were pharmacologically characterized based on the ability of only some DA agents to block adenylyl cyclase activity, and on the ability of catecholamines including DA to inhibit the release of prolactin in vivo and in vitro in a cAMP-independent fashion.10 Applications of recent technical advances in molecular genetics have greatly facilitated the isolation and characterization of novel DA receptors, D3, D4 and D5, with different anatomical localization from traditional D1 or D2 receptors. Based upon their pharmacological profiles, including their effects on different signal transduction cascades, these receptors are currently divided into two families: the D1-like family, which includes D1 and D5 receptors, and the D2-like family which includes D2, D3 and D4 receptors.11–13
MOLECULAR BIOLOGY OF DOPAMINE RECEPTORS
DOPAMINE D1-LIKE FAMILY
D1 receptors
The DA D1 receptor is the most abundant DA receptor in the central nervous system. The D1 receptor gene, which lacks any introns, encodes a protein that extends for 446 amino acids.14 The human gene has been localized to chromosome 5 [Table 1].15 D1 receptors show characteristic ability to stimulate adenylyl cyclase and generate inositol 1,4,5-trisphosphate (IP3) and diacylglycerol via the activation of phospholipase C.16,17 D1 receptors are highly expressed in basal ganglia followed by cerebral cortex, hypothalamus and thalamus. In striatal neurons of the basal ganglia, the mRNA for D1 receptors has been colocalized with mRNA for DARPP-32 (a DA- and cyclic-AMP-regulated phosphoprotein of molecular mass 32,000 daltons, or 32 kD), suggesting that DARPP-32 may contribute to the actions of D1 receptors.18–19
Table 1.
Properties of dopamine receptors
| Type | Amino acids | Chromosome (human) | Highest tissue sites | Selective agonists | Selective antagonists | Effectors |
|---|---|---|---|---|---|---|
| D1 like receptors | ||||||
| D1 | 446(h) 446 (r) |
5 | Basal ganglia Nucleus accumbens Cerebral cortex |
Hydroxybenzazepines A-68930 CY-208-245 Dihyrexidines |
Halobenzazepines (SCH- 23390) Thioxanthenes |
AC(+) PLC(+) |
| D5 | 477(h) 475 (r) |
4 | Hippocampus Thalamus |
Hydroxybenzazepines | Halobenzazepines | AC(+) |
| D2 like receptors | ||||||
| D2 | 443(h) | 11 | Anterior pituitary, Basal ganglia | Ergolines Hyroxyaporphines Aminotetralins |
Benzamides Butyrophenones Phenothiazines |
AC(–) PLC(–); AA(+) K+channels(+) Ca2+ channels (–) |
| D3 | 400 (h) 444 (r) |
3 | Islands of Calleja, Olfactory Tubercle Cerebellum | (+)7-OH-DPAT (+)PD-128,907 |
Nafadotride S-14297 | AC(–) (?) |
| D4 | 387(h) (& variants) 368(r) |
11 | Frontal cortex, Hippocampus, Amagdyla | CP-226,269 PD-106,077 |
L-745,870 U-101,387 RBI-257 NGD-94-1 |
AC(–) AA(+) |
Peptide length varies with species (h=human, r=rat); chromosome number are for human.
AA =arachidonic acid; A C=adenylyl cyclase; PI=phosphatidyl inositol cycle; PLC=phospholipase C; K+ channels = potassium channels; Ca2+ channels = calcium channels; (+) = activation; (–) = inhibition.
(?) indicates a tentatively proposed, or weak, association.
D5 receptors
The intronless D5 receptor gene encodes a protein that extends for 477 amino acids [Table 1].20 The protein has an overall 50% homology with the D1 receptor and 80% if only the seven transmembrane segments are considered. The gene encoding the human D5 protein is located at the short arm of chromosome 4, the same region where the Huntington disease gene has been located.21 It is unknown, however, if there is any functional interaction between the two genes. Molecular studies identified two D5-like pseudogenes that extend for 154 amino acids and show 90% homology to the D5 receptor genomic sequence. These pseudogenes, however, contain stop codons in their coding regions that prevent them from expressing functional receptors. The functions of these pseudogenes, which appear so far to be specific to humans, are not yet known.22
Expression of D5 mRNA is unique and limited to the hippocampus and parafascicular nucleus of the thalamus,23 a thalamic nucleus involved in pain perception, suggesting that D5 receptors may be involved in the thalamic processing of painful stimuli.24 D5 receptors, like D1 receptors, appear to interact with G-proteins and can stimulate adenylyl cyclase, with relatively high affinity for DA and D1-selective agonists [Table 1].20
DOPAMINE D2-LIKE FAMILY
D2 receptors
The DA D2 receptor was the first DA receptor to be cloned.25 The D2 receptor gene encodes a protein that extends for 415 amino acids [Table 1]. Similar to other G-protein coupled receptors, the D2 gene product has seven transmembrane segments, but in contrast to D1-like receptors, the third cytoplasmic domain is long and the carboxyl terminus is short. Unlike the D1-like receptor genes, the D2 receptor gene contains seven introns that are spliced out during mRNA transcription.26 The gene encoding this receptor was found to reside on q22–q23 of human chromosome 11.27 D2 receptors are involved in several signal transduction cascades, including inhibition of cAMP production,28 inhibition of phosphoinositide turnover,29 activation of potassium channels, and potentiation of arachidonic acid release [Table 1].30
D2 receptors are highly expressed in basal ganglia, nucleus accumbens septi, and ventral tegmental area.31 Molecularly, D2 receptor protein exists in two isoforms derived from the same gene by alternative RNA splicing which occurs during the maturation of the D2 receptor pre-mRNA.32 Both isoforms (known as D2L and D2S) vary within each species by the presence or less frequent absence of a 29-amino acid sequence in the third cytoplasmic domain of the D2 receptor peptide chain. Pharmacologically, both isoforms exhibit nearly similar profiles in terms of their affinities to different D2-selective agents, and both inhibit adenylyl cyclase activity. However, they display an opposite regulatory response to DA treatment: DA induces up-regulation of D2L isoform and down-regulation of D2S isoform.33
D3 receptors
The D3 receptor gene contains five introns and encodes a 446 amino acid protein.34 The gene encoding this receptor resides on chromosome 3 [Table 1].35 The D3 receptors bear close structural and pharmacological similarities to the D2R and, like the genes for D2 receptor variants, D3 mRNA also occurs in longer and shorter spliced forms generated from the same gene.36 Distribution of D3 mRNA indicated that these receptors are mainly expressed in subcortical limbic regions including islands of Calleja, nucleus accumbens septi and olfactory tubercle, with low levels of expression in the basal ganglia [Table 1].31 Surprisingly, D3R mRNA has also been found in neurons of the cerebellum, which may regulate eye-movements.37 The status of the D3 molecular entity as a functional receptor remains uncertain since it neither couples to G-proteins nor consistently transduces an effector mechanism.34,38 However, the structural similarity with D2 receptor raises the possibility that D3 receptor may also inhibit adenylyl cyclase activity in its normal cellular setting. More recent studies reported that D3 receptors might mediate positive regulatory influences of DA on production of the peptide neurotensin.39
D4 receptors
The human D4 receptor gene contains four introns and encodes a 387 amino acid protein.40 The overall homology of the D4 receptor to the D2 and D3 receptors is about 41% and 39% respectively, but this homology increases to 56% for both receptors when only the transmembrane spanning segments are considered. The gene encoding the human D4 protein is located at the tip of the short arm of chromosome 11.41 Histoprobes for its mRNA localized this gene product in non-extrapyramidal regions of human brain including hippocampus and frontal cerebral cortex.42 Like the D2 receptors, stimulation of the D4 receptors can inhibit adenylyl cyclase activity and activate release of arachidonic acid in brain neurons [Table 1].43
Human, but not primate or rodent, D4 receptors are known to occur in several genomic polymorphic variants that contain from two to eleven repeats of a 48 base-pair segment expressed in the third cytoplasmic domain.44 Two, four and seven repeats (designated as D4.2, D4.4 and D4.7) are the most common D4 alleles. These variants may contribute to the pathophysiology of certain neuropsychiatric disorders or their improved treatment.4
DISTRIBUTION OF DOPAMINE RECEPTORS IN BASAL GANGLIA
The basal ganglia consist of five interconnected subcortical nuclei including the striatum (caudate nucleus and putamen), globus pallidus, subthalamic nucleus, and substantia nigra pars compacta and pars reticulata.45–47 The medium spiny neurons, which constitute 90–95% of the neurons in the striatum, receive the bulk of the incoming excitatory input from the cerebral cortex. These neurons send their projections through two major striatal output pathways. The direct or striatonigral pathway, where striatal neurons project to the internal segment of the globus pallidus and the substantia nigra pars reticulata and the indirect or striatopallidal pathway where striatal neurons project to the external segment of the globus pallidus, then to the subthalamic nucleus and terminate in the substantia nigra pars reticulata. The later region sends projections to the ventral anterior, ventral lateral and mediodorsal thalamic nuclei, which in turn provide an excitatory input to the cerebral cortex.45–47
In the striatum, the majority of D1 receptors are expressed on striatonigral neurons, whereas D2 receptors are predominately localized to striatopallidal neurons.46,47 Some D4 receptors are co-expressed with the excitatory glutamate NMDA receptors, on terminals of glutamatergic corticostriatal projections innervating striatum, as well as on medium spiny neurons in striatum.48 Both D2 and D3 subtypes are found on terminals of dopaminergic nigrostriatal neurons projecting from substantia nigra pars compacta to striatum.49
The basal ganglia are involved in programming and initiation of movement, particularly slow movements, and in motor memory and retrieval. Abnormalities in DA neurotransmission in the basal ganglia nuclei and/or their projecting targets have been linked to attention-deficit hyperactivity disorder (ADHD) and schizophrenia.1,2,50 In addition, disorders of the basal ganglia may produce restricted and rigid movements as in Parkinson’s disease or uncontrollable and involuntary movements as in Huntington’s disease.51
DOPAMINE RECEPTORS AND NEUROPSYCHIATRIC DISEASES
DA receptors have been implicated in a variety of neuropsychiatric disorders, most notably in schizophrenia, Parkinson’s disease and attention-deficit hyperactivity disorder (ADHD). Other brain disorders in which DA receptors are involved or dopaminergic drugs have a therapeutic role are Huntington’s chorea, Tourette’s syndrome, and hyperprolactanemia.
SCHIZOPHRENIA
Schizophrenia is one of the most common neuropsychiatric diseases affecting 1% of the general population. This rate is fairly uniform throughout the world, even though the environmental and socio-economical factors vary among different countries. Additional 2–3% of the general population has schizotypal personality disorder, which is a milder form of the disease.52–55 The symptoms of schizophrenia start to develop in late adolescence or early adulthood. The ‘positive’ symptoms include thought disorder, perceptual disturbances, visual and auditory hallucinations and delusions while the ‘negative’ symptoms include loss of executive functions such as planning and working memory, neglect of hygiene, social isolation and withdrawal from interaction with other people.52–55
Genetic studies suggested that genetic factors play an important role in the pathophysiology of schizophrenia. Monozygotic twins, who have identical genome, show a concordance rate of about 40–50%, but in dizygotic twins, the rate drops to only 15%.55–57 These rates, however, indicate that genetic predisposition alone is insufficient to produce the disease, and that other neurochemical and environmental factors also contribute to the development of the disease. Injuries in the normal development of human brain including maldevelopment of the anatomical organization and connectivity of cortical afferents innervating the limbic regions may contribute to neurobiological substrates for schizophrenia.58 Disturbances in the concentrations and subsequent alterations in the neurotransmission of different neurotransmitters, including DA, serotonin and glutamate, in different cortical and limbic and extrapyramidal pathways have been also proposed to underlie the pathophysiology of schizophrenia.59–61
Treatment of Schizophrenia
Treatment of schizophrenia and other idiopathic psychotic disorders was revolutionized by the serendipitous discovery of chlorpromazine (phenothiazine derivative) and haloperidol (butyrophenone derivative) in the 1950s. This was followed by introduction of other effective antipsychotic compounds including thioxanthenes (clopentixol, flupentixol, and thiothixene), benzepines (loxapine, clothiapine and zotepine), diphenylbutylpiperidines (spiperones), indolones (molindone and oxypertine) and other heterocyclic compounds.1,2 Virtually, all of these drugs, which collectively are known as typical antipsychotic drugs, reduce DA neuronal activity, reverse the psychotic symptoms induced by psychostimulants such as amphetamine and cocaine, and block DA D2 receptors in a direct correlation with their antipsychotic efficacy.1,2,62,63
Typical antipsychotics are effective in alleviating the positive symptoms of schizophrenia. However, their effectiveness is limited and non-specific as they fail to significantly improve the cognitive deficits and negative symptoms of schizophrenia. Moreover, treatment with these medications is commonly associated with neurological extrapyramidal and endocrinological side effects, of both acute and delayed nature.1,2 Parkinsonism, a syndrome with similar symptoms to Parkinson’s disease, is the most frequent acute side effect. Other acute side effects include dystonia (sustained contraction of orofacial muscles), akathisia (motor restlessness with anxiety and agitation), and galactorrhea (excessive lactation). These side effects are the result of D2 receptor blockade in either the striatum or pituitary gland.1,2 A potentially life-threatening adverse effect of antipsychotic drug treatment is known as neuroleptic malignant syndrome.64,65 It is characterized by muscle rigidity, dystonia, unstable pulse, blood pressure, fever, and elevated serum concentrations of muscle proteins (creatine kinase, myoglobin). The syndrome has been attributed to D2 receptor blockade by typical antipsychotic agents, but its pathophysiology remains obscure, and may involve hypothalamic and brainstem dysfunction as well as extrapyramidal motor effects mediated by the basal ganglia.
Long-term treatment of schizophrenic patients with typical antipsychotic agents has been also associated with tardive dyskinesia, a delayed-onset hyperkinetic movement disorder that is often irreversible even after drug discontinuation.1,2 The most characteristic features of this syndrome are abnormal movements of the mouth, face, extremities, and trunk. DA receptor supersensitivity that results from antipsychotic-induced blockade and upregulation of D2 receptors,66,67 an imbalance in D1/D2 receptor densities in striata of medicated schizophrenic patients,10 or a disruption in γ-amino butyric acid (GABA) neurotransmission in the basal ganglia may contribute to the development of tardive dyskinesia.68,69
All these side effects prompted the search for novel drugs with less risk of the adverse effects of typical antipsychotics, but similar or even superior antipsychotic effects. This led to the introduction of several new drugs, classified as atypical antipsychotic drugs. The current prototype ‘atypical’ antipsychotic agent is clozapine (clozaril®), a dibenzodiazepine derivative. Several basic and clinical studies have provided substantial evidences that clozapine exhibit superior antipsychotic effectiveness over standard antipsychotics, especially in improving negative symptoms and cognitive deficits in schizophrenia. Clozapine is also effective in treatment-resistant schizophrenia, and other poorly responsive primary psychotic disorders, along with its very limited profile of extrapyramidal side effects or hyperprolactinemia.1,2,70,71
The pharmacological basis of the unusual clinical properties of this unique agent remains unclear. Clozapine interacts high or moderate potency at a wide range of neurotransmitter receptors including serotonergic (5-HT1A, 5-HT2A, 5-HT2C, 5-HT6, 5-HT7), acetylcholinergic (muscarinic M1–M4), adrenergic (α1, α2, β2), and histaminic (H1) receptors. In contrast, it has only moderate affinity for both DA D1 and D2 DA receptors.1,2,70–72 Clozapine has greater affinity for serotonin 5-HT2A than DA D2 receptors and this receptor-interaction pattern may contribute to its low risk of extrapyramidal side effects.73
Clozapine also displays somewhat greater affinity for D4 than other DA receptors, suggesting that these receptors may represent potential sites of action of clozapine and perhaps other antipsychotic agents.40,74–76 Post mortem brain tissue studies reported that D4 receptors are increased in the striata of medicated schizophrenic patients.77,78 In addition, laboratory studies found that repeated administration of clozapine, as well as other typical and atypical antipsychotics increased the abundance of D4 receptors in rat striatum and nucleus accumbens septi.79–82 These agents also up-regulated D2 receptors in rodent and primate prefrontal cortex but had little or no effect on D1 or D3 receptors.79–83 These findings support the view that D4 receptors in striatum and nucleus accumbens, as well as D2 receptors in prefrontal cortex are common sites where both typical and atypical antipsychotics mediate their beneficial therapeutic effects.1,2 In contrast, typical neuroleptics, but not clozapine, also increased D2 receptor binding and expression in rat and monkey striatum.79–83 This selective increase in D2 receptor labelling in the striatum may contribute to the development of neurological side effects typical of standard antipsychotics.1,2 Lack of effect of typical and atypical antipsychotic agents on D1 and D3 receptors suggest that these receptors are less likely to be involved in the mechanisms of antipsychotic drug actions.1,2
Despite its favourable characteristics, clinical use of clozapine is complicated by its high risk of potentially fatal bone marrow toxicity, agranulocytosis.1,70,84,85 Patients on clozapine are required to undergo regular monitoring of their complete blood count to ensure that the development of agranulocytosis is detected early. In addition, clozapine has other adverse effects, including dose-dependent risk of epileptic seizures, excessive sedation, significant weight-gain, and a higher incidence of hypertension and type II diabetes mellitus.1,70,84,85 These side effects collectively left the door opened for developing novel antipsychotic medications with less adverse risk than clozapine, but comparable antipsychotic effects.
Several newer agents have emerged. Among them are clozapine analogues olanzapine (Zyprexa®) and quetiapine (Seroquel®), the benzisoxazole derivative risperidone (Risperidal®) and its analogue ziprasidone (Geodon®).1,2,84,86 Like clozapine, these compounds have multiple sites of molecular interaction, and greater affinity for serotonin 5-HT2A than DA D2 receptors, which again may contribute to their benign extrapyramidal profile.1,2,84,86 These newer agents have undergone extensive pharmacological and behavioural characterization in animals, 86–88 and their therapeutic effects were assessed in many clinical trials.1,85 Despite the favourable clinical profile of most of the second generation of antipsychotic drugs, and their effectiveness in treating psychotic symptoms of schizophrenia, they are also associated with different adverse side effects. With the remarkable exception of clozapine, and perhaps quetiapine, other atypical antipsychotic agents have brought only relative avoidance of side effects on central neural control of posture and movement, urging continued searches for novel principles of developing novel antipsychotic drugs.1,85
PARKINSON’S DISEASE
Parkinson’s disease (PD) develops later in life with the average age of onset of 60 years. PD patients suffer from disturbances of movements (akinesia), increased muscle tone (rigidity), tremor (4–5 per second at rest), and postural defects, along with speech and writing problems. These symptoms progress with a gradual exacerbation along with the progress of the disease.89 Cognitive deficits and psychiatric disturbances are also common in patients with PD. The main cognitive deficits include disturbances in memory, fluency, visuospatial and construction abilities accompanied by dementia.90 The most common psychiatric disturbances include depression, anxiety, mania and psychosis. PD is observed in more than 1% of individuals over the age of 65.91 Genetics may also play a role in the aetiology of PD, but perhaps less prominent than that of schizophrenia. Recent studies have found that mutations in three different proteins (alpha-synuclein, parkin and UCHL1) can lead to autosomal dominant form of the disease.92,93
The pathological hallmark of PD is the specific degeneration of more than 80% of the nigrostriatal dopaminergic neurons and the appearance of intracellular inclusions known as Lewy bodies.94,95 This results in a profound depletion of DA in the substantia nigra pars compacta, caudate nucleus, putamen, and causes an increase in striatal D2 receptor levels. The loss of striatal DA will decrease the inhibitory activity of nigrothalamic projections, which in turn will increase the activity of the thalamocortical neurons leading to the excitation of motor cortex and spinal motor neurons. The end results will be increased contraction of both flexors and extensors at the same time causing cogwheel rigidity and movement disorder.51,89
The discovery that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can reduce the DA levels in the brain by selectively degenerating the nigrostriatal dopaminergic pathway, and producing a clinical syndrome similar to PD, helped to develop a useful animal model for PD and stimulated new approaches to investigating its pathophysiology and therapy.96,97 MPTP is converted by monoamine oxidase B to MPP+ (1-methyl-4-phenyl pyridinium) which is taken up by the DA neurons via presynaptic DA transporters. MPP+ is then accumulated in the mitochondria where it inhibits complex I of the mitochondrial electron transport chain. This blocks the process of oxidative phosphorylation and generates toxic free radicals, which in turn attacks the integrity of cell cytoskeleton and eventually leads to cell death.96–98
Treatment of Parkinson’s disease
The simplest way to replace depleted DA would be to administer DA itself. However, DA does not cross the blood brain barrier and therefore its direct administration is ineffective. Levodopa (L–3,4-dihydroxyphenyl alanine; L-DOPA) is the immediate precursor of DA which readily crosses blood brain barrier and is converted to DA by decarboxylation within the remaining few intact dopaminergic neurons.99 Administration of levodopa, at least early in the course of the disease, significantly improved tremor, rigidity and motor-impairment in PD patients.89,99 It should be noted, however, that the peripheral tissue conversion of levodopa to DA by aromatic amino acid decarboxylase permits only a small percentage of levodopa to reach the brain. Therefore, it is necessary to co-administer with levodopa, selective inhibitors of peripheral decarboxylase enzyme activity that do not cross the blood brain barrier, such as carbidopa (Sinemet®) or benserazide (Madopar®) to profoundly increase the availability of levodopa in the brain.100,101
Long-term therapy of levodopa is complicated by the fact that the beneficial effects of levodopa start to wear off and patients start to experience response fluctuations with each dose of levodopa despite maintaining the same treatment regimen.102,103 Later, patients starts to show the ‘on/off phenomenon’ in which sudden periods of tremors and rigidity alternate with periods of mobility. Increasing the dose and frequency administration of levodopa can improve this situation, but this increases the risk of dyskinesias and excessive and involuntary movements.102,103 In addition, patients may experience dopaminergic psychosis. The novel atypical antipsychotic agents, such as clozapine or quetiapine, have been shown to be effective in improving levodopa-induced psychosis.104
Inhibitors of the enzyme monoamine oxidase (MAO) represent another class of drugs for the treatment of PD. Two isoenzymes of MAO (MAO-A and MAO-B) oxidize monoamines. MAO-B is the predominant form in the striatum and is responsible for the oxidative metabolism of striatal DA.105 Deprenyl (Eldepryl®), also known as selegiline, is a selective MAO-B inhibitor that irreversibly inhibits MAO and slows the breakdown of DA in the striatum. A combination of deprenyl and levodopa is useful in prolonging the effects of levodopa and in reducing the ‘on/off’ effects.106 Currently, deprenyl is considered as one of the drugs of choice for treatment of early or mild PD.107 However, in more advanced PD patients, deprenyl may accelerate the motor and cognitive side effects of levodopa therapy. Metabolites of deprenyl include amphetamine and methamphetamine, which can cause insomnia, anxiety and mood elevation in treated PD patients.89
An alternative to levodopa or deprenyl therapy is the use of direct DA receptor agonists. These drugs are more specific in their actions and can selectively target one or more DA receptor subtype, in contrast to the non-selective effects of levodopa or deprenyl. In addition, these agonists are well absorbed orally, have longer duration of actions than levodopa and are more effective in the management of fluctuations in motor activity.108 Four DA receptor agonists are available for treatment of PD: the standard agents, bromocriptine (Parlodel®) and pergolide (Permax®), and the more recently introduced agonists, ropinirole (Requip®) and pramipexole (Mirapex®).89,108 Bromocriptine is strong D2 receptor agonist with partial antagonistic activity at D1 receptors, while pergolide is an active agonist on both DA receptor subtypes. Ropinirole and pramipexole are active agonists at D2/D3 sites with negligible activity at D1 sites.89,108
Despite the progress in PD pharmacotherapy, many medications tend to loss their beneficial effects after long-term administration. Alternative therapies try to restore DA function by means of intracerebral tissue grafts. One approach focuses on transplanting adrenal medulla tissue either into a lateral ventricle or into the striatum itself.109 Another approach is to implant fetal substantia nigra tissue with the anticipation that new DA neurons will grow, sprout and restore the lost nigrostriatal dopaminergic connectivity. This approach, however, remains controversial due to its ethical implications.110 A third approach involves the use of xenografts, that is tissue grafts obtained from other species like pigs or monkeys, although the clinical outcome of these grafts have not been well established.111 Neurosurgical intervention to selectively lesion the inner segment of globus pallidus (also known as pallidotomy) has been also utilised in treatment of PD. However, because of the risk of permanent damage to the brain, this treatment remains as the last resort.112
ATTENTION-DEFICIT HYPERACTIVITY DISORDER
ADHD is a neuropsychiatric condition characterized by inattention, impulsivity and inappropriate behavioural hyperactivity, typically associated with impaired academic and social functioning in school-aged children.50 Several studies have implicated environmental and psychosocial factors, such as pregnancy and delivery complications, marital distress, family dysfunction and low social class as predisposing risk factors for ADHD.50,113 The neuroanatomical networks involving frontal cortex and basal ganglia are proposed to be critically involved in the pathophysiology of ADHD.114,115 Neuroimaging studies found that frontal cortex, caudate and globus pallidus were smaller in children diagnosed with ADHD compared to normal controls.116,117 In addition, a functional deficit was detected in the putamen of children with ADHD relative to normal peers.118 These findings provide a compelling support for the suggested dysfunction in fronto-subcortical pathways in patients diagnosed with ADHD.119
Molecular genetic studies have identified a genetic linkage between ADHD and an allele of DA transporter using a family based association study.120,121 This association has been supported by the development of genetically altered mice that lack functional DA transporters. Such mice displayed a hyperdopaminergic state that included spontaneous hyperactivity similar to ADHD.122 In addition, an association of D4 receptor polymorphism and clinical ADHD has been also reported.75,76,123,124 This association involves increased incidence of a 7-repeat allele (receptor type D4.7) coding for a 16-amino acid sequence in the functionally critical third intracytoplasmic loop of the D4 receptor in patients diagnosed with ADHD compared to normal controls. Additional support for possible involvement of D4 receptors in ADHD is provided by recent findings that transgenic mice lacking D4 receptors show increased sensitivity to psychostimulants and increased metabolic turnover of striatal DA compared to wild type mice.125
TREATMENT OF ADHD
Psychostimulants are considered the first line of treatment for ADHD, since the pathophysiology of ADHD involves deficiency in DA neurotransmission. The most widely used compounds in this class include methylphenidate (Ritalin®), amphetamines (Adderall® and Dexedrine®) and pemoline (Cylert®).126,127 These compounds, which enhance DA neurotransmission, increase synaptic DA by inhibiting the reuptake of DA into presynaptic vesicles (methylphenidate, amphetamines and pemoline) or by releasing presynaptic DA into synaptic cleft.126–128 Although these compounds are quite effective in alleviating the symptoms of ADHD and in improving attention and academic performance ADHD patients, they are associated with different side effects. Most notable, all these medications are considered controlled substances of potential abuse. In addition, they cause insomnia, anorexia, jitteriness, and headaches. Moreover, pemoline can cause hepatitis and liver toxicity, and so monitoring liver functions is essential for patients on pemoline.126–128
Antidepressants follow psychostimulants as the second line of choice for treatment of ADHD. The tricyclic antidepressants (TCAs), such as imipramine, desipramine, venlafaxine and atomoxetine, block the reuptake of monoamine neurotransmitters, especially norepinephrine.126–129 TCAs are effective in controlling abnormal behaviours and reducing cognitive impairment in ADHD patients. They are also useful if depression or anxiety symptoms co-exit with ADHD.126–129 Antihypertensive drugs such as clonidine and guanfacine are also used for treatment of ADHD in young patients. They are particularly effective against aggressiveness and sleep disturbances. However, cardiovascular monitoring of patients on these medications is recommended.126–128
Finally, recent reports have suggested that selective DA D4 receptor antagonists may provide much-needed innovative treatments for ADHD. Motor hyperactivity observed in juvenile rats with neonatal 6-hydroxydopamine lesions, a laboratory model for ADHD, was reversed in dose-dependent manner by highly selective D4 antagonists, and worsened by selective D4 agonists.130 A direct correlation was also observed between motor hyperactivity in lesioned rats and increases in D4 receptor levels in rat caudate-putamen.130 These findings provided behavioural and pharmacological evidences for the suggested genetic association between D4 receptor alleles and ADHD.75,76,123,124 It is still premature to judge the effectiveness of D4-antagonists in treatment of ADHD. Post-mortem studies on brain tissue from patients diagnosed with ADHD are still needed to clarify the role of D4 receptors in its neuropathology or pathophysiology. In addition, selective D4-antagonists should be tested in clinical trials to determine their safety and effectiveness for treatment of ADHD.75,76
CONCLUSIONS
The new neuropharmacology of DA receptors and their effectors stimulates renewed interest in many aspects relevant to DA neurotransmission, including the molecular control of the DA synthesis and release, as well as the rational development of novel CNS drugs. The advances in molecular biology have revealed the presence of two classical DA receptors (D1 and D2) as well as novel gene products that present novel DA receptors (D3, D4, D5). clarification of the sites of expression of classical and novel DA receptor mRNAs and proteins in mammalian brain, characterization of their effector systems, and the identification of novel chemical or drug molecules selective for each receptor subtype have rapidly advanced the understanding of these novel DA receptors. Such understanding is relevant to the pathophysiology of major neuropsychiatric diseases, including schizophrenia, Parkinson’s disease and ADHD, as well as to understanding the mechanisms of action and side effects of many drugs currently used in treatment of these disorders. The exciting neuropharmacological leads reviewed here should open new avenues of research on the preclinical aspects of different DA receptor subtypes, and should lead to innovative principles guiding discovery of novel drugs for improved treatment of neuropsychiatric diseases.
Acknowledgments
Supported, in part, by National Alliance on Research on Schizophrenia and Depression (NARSAD) and Theodore and Vada Stanley Research Foundation.
REFERENCES
- 1.Baldessarini RJ, Tarazi FI. Drugs and the treatment of psychiatric disorders. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacologic Basis of Therapeutics. McGraw-Hill; New York: 2001. pp. 485–520. [Google Scholar]
- 2.Baldessarini RJ, Tarazi FI. Brain dopamine receptors: A primer on their current status, basic and clinical. Harvard Rev Psychiatry. 1996;3:301–25. doi: 10.3109/10673229609017200. [DOI] [PubMed] [Google Scholar]
- 3.Florijn WJ, Tarazi FI, Creese I. Dopamine Receptors. In: Bittar EE, Bittar N, editors. Principles of Medical Biology. JAI Press; New York: 1997. pp. 73–94. [Google Scholar]
- 4.Cooper J, Bloom F, Roth R. Dopamine. In: Cooper, Bloom, Roth, editors. The Biochemical Basis of Neuropharmacology. Oxford University Press; New York: 1991. pp. 285–337. [Google Scholar]
- 5.Carlsson A. Thirty years of dopamine research. Adv Neurology. 1993;60:1–17. [PubMed] [Google Scholar]
- 6.Moore K, Lookingland K. Dopaminergic neuronal systems in the hypothalamus. In: Bloom F, Kupfer D, editors. Psychopharmacology: Fourth Generation of Progress. Raven Press; New York: 1995. pp. 245–56. [Google Scholar]
- 7.Chiodo L, Freeman A, Bunney B. Dopamine autoreceptor signal transduction and regulation. In: Bloom F, Kupfer D, editors. Psychopharmacology: Fourth Generation of Progress. Raven Press; New York: 1995. pp. 221–26. [Google Scholar]
- 8.Roth R, Elsworth J. Biochemical pharmacology of midbrain dopamine neurons. In: Bloom F, Kupfer D, editors. Psychopharmacology: Fourth Generation of Progress. Raven Press; New York: 1995. pp. 227–44. [Google Scholar]
- 9.Kebabian J, Calne D. Multiple receptors for dopamine. Nature. 1979;277:93–6. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 10.Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980;32:229–313. [PubMed] [Google Scholar]
- 11.Civelli O, Bunzow J, Grandy D. Molecular diversity of the dopamine receptors. Ann Rev Pharmacol Toxicol. 1993;32:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 12.Sibley D, Monsma F, Shen Y. Molecular neurobiology of dopaminergic receptors. Intl Rev Neurobiol. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- 13.Sokoloff P, Schwartz J. Novel dopamine receptors half a decade later. Trends Pharmacol Sci. 1995;16:270–75. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 14.Dearry A, Gingrich J, Falardeau P, Fremeau R, Bates M, Caron MG. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990;347:72–6. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- 15.Sunahara R, Niznik H, Weiner D, Sturmann T, Brann M, Kennedy J, et al. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990;347:80–3. doi: 10.1038/347080a0. [DOI] [PubMed] [Google Scholar]
- 16.Mahan L, Burch R, Monsma F, Sibley D. Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oöcytes. Proc Natl Acad Sci USA. 1990;87:2196–2200. doi: 10.1073/pnas.87.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsma F, Mahan L, McVittie L, Gerfen C, Sibley D. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci USA. 1990;87:6723–27. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmings H, Greengard P. DARPP-32, a dopamine and 3′–5′ monophosphate-regulated phosphoprotein: regional, tissue and phylogenetic distribution. J Neurosci. 1986;6:1469–81. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmings H, Walaas S, Ouiment C, Greengard P. Dopaminergic regulation of protein phosphorylation in the striatum: DARPP-32. Trends Neurosci. 1987;10:377–83. [Google Scholar]
- 20.Sunahara RK, Guan H-C, O’Dowd BF, Seeman P, Laurier LG, George S, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:610–14. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 21.Gusella JF. Location cloning strategy for characterizing genetic defects in Huntington’s disease and Alzheimer’s disease. FASEB. 1989;3:2036–41. doi: 10.1096/fasebj.3.9.2568302. [DOI] [PubMed] [Google Scholar]
- 22.Grandy D, Zhoung Y, Bouvier C, Zhou Q, Johnson R, Allen L, et al. Multiple human D5 dopamine receptor genes: a functional receptor and two pseudogenes. Proc Natl Acad Sci USA. 1991;88:9175–9. doi: 10.1073/pnas.88.20.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meador-Woodruff JH, Mansour A, Grandy DK, Damask SP, Civelli O, Watson SJ., Jr Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett. 1992;145:209–12. doi: 10.1016/0304-3940(92)90024-2. [DOI] [PubMed] [Google Scholar]
- 24.Giesler GJ, Menetrey D, Basbaum AI. Differential origins of spinothlamic tract projections to medial and lateral thalamus in the rat. J Comp Neurol. 1979;184:107–26. doi: 10.1002/cne.901840107. [DOI] [PubMed] [Google Scholar]
- 25.Bunzow JR, Van Tol HM, Grandy DK, Albert P, Salon J, Chisre M, et al. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–87. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- 26.Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Fischer JB, et al. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci USA. 1989;86:9762–66. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, Makam H, et al. The human dopamine D2 receptor gene is located on chromosome 11 at q22–q23 and identifies a TaqI RFLP. Am J Hum Genet. 1989;45:778–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Vallar L, Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989;10:74–7. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- 29.Enjalbert A, Sladeczek F, Guillon G, Bertrand P, Shu C, Epelbaum J, et al. Angiotensin II and dopamine modulate both cAMP and inositol phosphate productions in anterior pituitary cells. J Biol Chem. 1986;261:4071–5. [PubMed] [Google Scholar]
- 30.Kanterman RY, Mahan LC, Briley EM, Monsma FJ, Jr, Sibley DR, Axelrod J, et al. Transfected D2 dopamine receptors mediate the potentiation of arachidonic acid release in chinese hamster ovary cells. Mol Pharmacol. 1991;39:364–9. [PubMed] [Google Scholar]
- 31.Bouthenent M-L, Souil E, Martres M-P, Sokoloff P, Giros B, Schwartz J-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–19. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 32.Giros B, Sokoloff P, Martres M-P, Riou J-F, Emorine LJ, Schwartz J-C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342:923–6. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Lachowicz J, Sibley D. The D2S and D2L dopamine receptor isoforms are differentially regulated in Chinese Hamster Ovary Cells. Mol Pharmacol. 1994;45:878–89. [PubMed] [Google Scholar]
- 34.Sokoloff P, Giros B, Martres M, Bouthenet M, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–51. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 35.Giros B, Sokoloff P, Martres MP, Riou JF, Emorine LJ, Schwartz J-C. Cloning of the human D3 dopaminergic receptor and chromosome identification. C R Acad Sci, Paris, Serie III. 1990;311:501–8. [PubMed] [Google Scholar]
- 36.Giros B, Martres M, Pilon C, Sokoloff P, Schwartz J-C. Shorter variants of the D3 dopamine receptor produced through various patterns of alternative splicing. Biochem Biophys Res Commun. 1991;176:1584–92. doi: 10.1016/0006-291x(91)90469-n. [DOI] [PubMed] [Google Scholar]
- 37.Lévesque D, Diaz J, Pilon C, Martres M, Giros B, Souil E, et al. Identification, characterization and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–9. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, et al. Pharmacology of human dopamine D3 receptor in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol. 1992;225:331–7. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- 39.Lévesque D, Martres M, Diaz J, Griffon N, Lammers C, Sokoloff P, et al. A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc Natl Acad Sci USA. 1995;92:1719–23. doi: 10.1073/pnas.92.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Tol HHM, Bunzow JR, Guan H-C, Sunahara RK, Seeman P, Niznik HB, et al. Cloning of a human dopamine D4 receptor gene with high affinity for the antipsychotic clozapine. Nature. 1991;350:614–9. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 41.Gelernter J, Kennedy JL, Van Tol HHM, Civelli O, Kidd KK. The D4 dopamine receptor (DRD4) maps to distal 11p close to HRAs. Genomics. 1992;13:208–10. doi: 10.1016/0888-7543(92)90222-e. [DOI] [PubMed] [Google Scholar]
- 42.Meador-Woodruff J, Grandy D, Van Tol H, Damask S, Little K, Civelli O, et al. Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10:239–48. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- 43.Chio C, Drong R, Riley D, Gill G, Slightom J, Huff R. D4 dopamine receptor-mediated signaling events determined in transfected Chinese hamster ovary cells. J Biol Chem. 1994;269:11813–9. [PubMed] [Google Scholar]
- 44.Van Tol H, Wu C, Guan H, Ohara K, Bunzow J, Civelli O, et al. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–52. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- 45.Graybiel A. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–54. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 46.Alexander G, Crutcher M. Functional architecture of basal ganglia circuits: Neuronal substrates of parallel processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 47.Gerfen C. The neostriatal mosaic: Multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–9. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 48.Tarazi FI, Baldessarini RJ. Regional localization of dopamine and glutamate receptor subtypes in striatolimbic brain regions. J Neurosci Res. 1999;55:401–10. doi: 10.1002/(SICI)1097-4547(19990215)55:4<401::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 49.Tepper JM, Sun B-C, Martin LP, Creese I. Functional roles of dopamine D2 and D3 autoreceptors on nigrostriatal neurons analyzed by antisense knockdown in vivo. J Neurosci. 1997;17:2519–30. doi: 10.1523/JNEUROSCI.17-07-02519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barkley RA. Attention Deficit Hyperactivity Disorder: A Handbook For Diagnosis And Treatment. Guilford Press; New York: 1990. [Google Scholar]
- 51.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 52.Baldessarini RJ. Schizophrenia. N Engl J Med. 1977;297:988–95. doi: 10.1056/NEJM197711032971807. [DOI] [PubMed] [Google Scholar]
- 53.Nasrallah HA, Weinberger DR. The Neurology of Schizophrenia. Elsevier Science Publishers; Amsterdam: 1986. [Google Scholar]
- 54.Tamminga CA, Schulz SC. Advances in Neuropsychiatry and Psychopharmacology: Schizophrenia Research. Raven Press; New York: 1991. [Google Scholar]
- 55.Kandel ER. Disorders of thought and volition: Schizophrenia. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. Elsevier; New York: 2000. pp. 1188–208. [Google Scholar]
- 56.Kety SS, Rosenthal D, Wender PM, Schulsinger F, Jacobsen B. Mental illness in the biological and adoptive families of adopted individuals who become schizophrenic who have become schizophrenic: A preliminary study. In: Fieve RR, Rosenthal D, Brill H, editors. Genetic Research in Psychiatry. Johns Hopkins University Press; Baltimore: 1975. pp. 147–65. [Google Scholar]
- 57.Kendler KS. The genetics of schizophrenia and related disorders: A review. In: Dunner DL, Gershon ES, Barrett JE, editors. Relative at Risk for Mental Disorder. Raven Press; New York: 1988. pp. 247–63. [Google Scholar]
- 58.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 59.Davis K, Kahn R, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiatry. 1991;148:1474–86. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 60.Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology. 1989;99:S18–27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- 61.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–77. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 62.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–3. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 63.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–9. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 64.Pearlman CA. Neuroleptic malignant syndrome: A review of the literature. J Clin Psychopharmacology. 1986;6:257–73. [PubMed] [Google Scholar]
- 65.Addonizio G, Susman VL, Roth SD. Neuroleptic malignant syndrome: Review and analysis of 115 cases. Biol Psychiatry. 1987;22:1004–20. doi: 10.1016/0006-3223(87)90010-2. [DOI] [PubMed] [Google Scholar]
- 66.Baldessarini RJ, Tarsy D. Dopamine and the pathophysiology of dyskinesia induced by antipsychotic drugs. Ann Rev Neurosci. 1980;3:23–41. doi: 10.1146/annurev.ne.03.030180.000323. [DOI] [PubMed] [Google Scholar]
- 67.Tarsy D, Baldessarini RJ. Movement disorders induced by psychotherapeutic drugs. In: Shah N, Donzid A, editors. Movement Disorders. Plenum Press; New York: 1986. pp. 365–89. [Google Scholar]
- 68.Thaker GK, Tamminga CA, Alphs LD, Lafferman J, Ferraro TN, Hare TA. Brain gamma-aminobutyric acid abnormality in tardive dyskinesia. Reduction in cerebrospinal fluid GABA levels and therapeutic response to GABA agonist treatment. Arch Gen Psychiatry. 1987;44:522–9. doi: 10.1001/archpsyc.1987.01800180032006. [DOI] [PubMed] [Google Scholar]
- 69.Thaker GK, Nguyen JA, Tamminga CA. Increased saccadic distractibility in tardive dyskinesia: functional evidence for subcortical GABA dysfunction. Biol Psychiatry. 1989;25:49–59. doi: 10.1016/0006-3223(89)90146-7. [DOI] [PubMed] [Google Scholar]
- 70.Baldessarini RJ, Frankenburg FR. Clozapine–a novel antipsychotic agent. N Engl J Med. 1991;324:746–54. doi: 10.1056/NEJM199103143241107. [DOI] [PubMed] [Google Scholar]
- 71.Brunello N, Masotto C, Steardo L, Marstein R, Racagni G. New Insights into the biology of schizophrenia through the action mechanism of clozapine. Neuropsychopharmacology. 1995;13:177–213. doi: 10.1016/0893-133X(95)00068-O. [DOI] [PubMed] [Google Scholar]
- 72.Meltzer HY. Effects of antipsychotic drugs on serotonin receptors. Pharmacol Rev. 1991;43:587–604. [PubMed] [Google Scholar]
- 73.Meltzer HY, Matsubara S, Lee J-C. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D1, D2 and 5-HT2 pKi vales. J Pharmacol Exp Ther. 1989;251:238–46. [PubMed] [Google Scholar]
- 74.Tarazi FI, Baldessarini RJ. Dopamine D4 receptors: Neuropsychiatric implications. The Economics of Neuroscience. 2000;2:54–8. [Google Scholar]
- 75.Tarazi FI, Baldessarini RJ. Brain Dopamine D4 receptors: Current basic and clinical status. Int J Neuropsychopharmacol. 1999;2:41–58. doi: 10.1017/S1461145799001352. [DOI] [PubMed] [Google Scholar]
- 76.Tarazi FI, Baldessarini RJ. Dopamine D4 receptors: Significance for molecular psychiatry at the millennium. Molecular Psychiatry. 1999;4:529–38. doi: 10.1038/sj.mp.4000674. [DOI] [PubMed] [Google Scholar]
- 77.Seeman P, Guan H-C, Van Tol HHM. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–5. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 78.Murray AM, Hyde TM, Knable MB, Herman MM, Bigelow LB, Carter JM, et al. Distribution of putative D4 dopamine receptors in postmortem striatum from patients with schizophrenia. J Neurosci. 1995;15:2186–91. doi: 10.1523/JNEUROSCI.15-03-02186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Florijn WJ, Tarazi FI, Creese I. Dopamine receptor subtypes: Differential regulation following 8 months treatment with antipsychotic drugs. J Pharmacol Exp Ther. 1997;280:561–9. [PubMed] [Google Scholar]
- 80.Tarazi FI, Florijn WJ, Creese I. Differential regulation of dopamine receptors following chronic typical and atypical antipsychotic drug treatment. Neuroscience. 1997;78:985–96. doi: 10.1016/s0306-4522(96)00631-8. [DOI] [PubMed] [Google Scholar]
- 81.Tarazi FI, Yeghiayan SK, Baldessarini RJ, Kula NS, Neumeyer JL. Long-term effects of S(+)N-n-propylnorapomorphine compared with typical and atypical antipsychotics: Differential increases of cerebrocortical D2-like and striatolimbic D4-like dopamine receptors. Neuropsychopharmacology. 1997;17:186–96. doi: 10.1016/S0893-133X(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 82.Tarazi FI, Yeghiayan SK, Neumeyer JL, Baldessarini RJ. Medial prefrontal cortical D2-like and striatolimbic D4-like dopamine receptors: Common targets for typical, atypical and experimental antipsychotics. Prog NeuroPsychopharmacol Biol Psychiatry. 1998;22:693–707. doi: 10.1016/s0278-5846(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 83.Lidow MS, Goldman-Rakic PS. Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs. neostriatum: effects of chronic treatment with typical and atypical antipsychotic drugs. J Pharmacol Exp Ther. 1997;251:238–46. [PubMed] [Google Scholar]
- 84.Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res. 1998;32:215–28. doi: 10.1016/s0022-3956(98)00023-5. [DOI] [PubMed] [Google Scholar]
- 85.Tarsy D, Baldessarini RJ, Tarazi FI. Atypical antipsychotic agents: Effects on extrapyramidal function. CNS Drugs. 2002;16:23–45. doi: 10.2165/00023210-200216010-00003. [DOI] [PubMed] [Google Scholar]
- 86.Waddington JL, Casey D. Comparative pharmacology of classical and novel (second-generation) antipsychotics. In: Waddington JL, Buckley PF, editors. Schizophrenia and Mood Disorders. Butterworth-Heinemann; Oxford: 2000. pp. 1–13. [Google Scholar]
- 87.Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 88.Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain: Implications for antipsychotic drug treatment. J Pharmacol Exp Ther. 2001;297:711–7. [PubMed] [Google Scholar]
- 89.Standaert DG, Young AB. Treatment of central nervous system degenerative disorders. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacologic Basis of Therapeutics. McGraw-Hill; New York: 2001. pp. 549–68. [Google Scholar]
- 90.Sano M, Marder K, Dooneief G. Basal ganglia diseases. In: Fogel BS, Schiffer RB, Rao SM, editors. Neuropsychiatry. Williams & Wilkins, Baltimore; 1996. pp. 805–25. [Google Scholar]
- 91.Tanner CM. Epidemiology of Parkinson’s disease. Neurol Clin. 1992;10:317–29. doi: 10.1016/S0733-8619(18)30212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duvoisin RC. Role of genetics in the cause of Parkinson’s disease. Mov Disord. 1998;13(suppl. 1):7–12. [PubMed] [Google Scholar]
- 93.Golbe LI. Alpha-synuclein and Parkinson’s disease. Mov Disord. 1999;14:6–9. doi: 10.1002/1531-8257(199901)14:1<6::aid-mds1004>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 94.Hornykiewicz O, Kish SJ. Biochemical pathology of Parkinson’s disease. Adv Neurol. 1986;45:19–34. [PubMed] [Google Scholar]
- 95.Gibb WR. Neuropathology of Parkinson’s disease and related syndromes. Neurol Clin. 1992;10:361–76. [PubMed] [Google Scholar]
- 96.Burns RS, LeWitt PA, Ebert MH, Pakkenberg H, Kopin IJ. The clinical syndrome of striatal dopamine deficiency. Parkin-sonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) N Engl J Med. 1985;312:418–21. doi: 10.1056/NEJM198505303122203. [DOI] [PubMed] [Google Scholar]
- 97.Langston JW, Irwin I. MPTP: current concepts and controversies. Clin Neuropharmacol. 1986;9:485–507. [PubMed] [Google Scholar]
- 98.Tipton KF, Singer TP. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem. 1993;61:1191–206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- 99.Mouradian MM, Heuser IJ, Baronti F, Chase TN. Modification of central dopaminergic mechanisms by continuous levodopa therapy for advanced Parkinson’s disease. Ann Neurol. 1990;27:18–23. doi: 10.1002/ana.410270105. [DOI] [PubMed] [Google Scholar]
- 100.Papavasiliou PS, Cotzias GC, Duby SE, Steck AJ, Fehling C, Bell MA. Levodopa in Parkinsonism: potentiation of central effects with a peripheral inhibitor. N Engl J Med. 1972;286:8–14. doi: 10.1056/NEJM197201062860102. [DOI] [PubMed] [Google Scholar]
- 101.Pletscher A. Levodopa treatment of Parkinson’s syndrome: past and future. Adv Neurol. 1990;53:469–73. [PubMed] [Google Scholar]
- 102.Rinne UK. Problems associated with long-term levodopa treatment of Parkinson’s disease. Acta Neurol Scand. 1983;95:19–26. doi: 10.1111/j.1600-0404.1983.tb01513.x. [DOI] [PubMed] [Google Scholar]
- 103.Chase TN, Fabbrini G, Juncos JL, Mouradian MM. Motor response complications with chronic levodopa therapy. Adv Neurol. 1990;53:377–81. [PubMed] [Google Scholar]
- 104.Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 2000;15:201–11. doi: 10.1002/1531-8257(200003)15:2<201::aid-mds1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 105.Olanow CW. Oxidation reactions in Parkinson’s disease. Neurology. 1990;40:32–7. [PubMed] [Google Scholar]
- 106.Olanow CW. MAO-B inhibitors in Parkinson’s disease. Adv Neurol. 1993;60:666–71. [PubMed] [Google Scholar]
- 107.Myllyla VV, Sotaniemi KA, Vuorinen JA, Heinonen EH. Selegiline as initial treatment in de novo parkinsonian patients. Neurology. 1992;42:339–43. doi: 10.1212/wnl.42.2.339. [DOI] [PubMed] [Google Scholar]
- 108.Goetz CG. Dopaminergic agonists in the treatment of Parkinson’s disease. Neurology. 1990;40:50–4. [PubMed] [Google Scholar]
- 109.Freed WJ, Poltorak M, Becker JB. Intracerebral adrenal medulla grafts: a review. Exp Neurol. 1990;10:139–66. doi: 10.1016/0014-4886(90)90026-o. [DOI] [PubMed] [Google Scholar]
- 110.Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989;46:615–31. doi: 10.1001/archneur.1989.00520420033021. [DOI] [PubMed] [Google Scholar]
- 111.Subramanian T. Cell transplantation for the treatment of Parkinson’s disease. Semin Neurol. 2001;21:103–15. doi: 10.1055/s-2001-13125. [DOI] [PubMed] [Google Scholar]
- 112.Bronstein JM, DeSalles A, DeLong MR. Stereotactic pallidotomy in the treatment of Parkinson disease: an expert opinion. Arch Neurol. 1999;56:64–9. doi: 10.1001/archneur.56.9.1064. [DOI] [PubMed] [Google Scholar]
- 113.Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, et al. Impact of adversity on functioning and comorbidity in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:1495–503. doi: 10.1097/00004583-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 114.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 115.Niedermeyer E, Naidu SB. Attention-deficit hyperactivity disorder (ADHD) and frontal-motor cortex disconnection. Clin Electroencephalography. 1997;28:130–6. doi: 10.1177/155005949702800303. [DOI] [PubMed] [Google Scholar]
- 116.Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatrics. 1997;36:381–93. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- 117.Castellanos FX, Giedd JN, March WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–16. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 118.Teicher MH, Anderseon CM, Polcari A, Glod CA, Maas LC, Renshaw PF. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nature Medicine. 2000;6:470–3. doi: 10.1038/74737. [DOI] [PubMed] [Google Scholar]
- 119.Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biol Psychiatry. 1998;59:628–37. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- 120.Cook EH, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993–8. [PMC free article] [PubMed] [Google Scholar]
- 121.Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry. 1997;2:311–13. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- 122.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 123.La Hoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, et al. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry. 1996;1:128–31. [PubMed] [Google Scholar]
- 124.Faraone SV, Biederman J, Weiffenbach B, Keith T, Chu MP, Weaver A, et al. Dopamine D4 gene 7-repeat allele and attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:768–70. doi: 10.1176/ajp.156.5.768. [DOI] [PubMed] [Google Scholar]
- 125.Rubinstein M, Philips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Larson JL, et al. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 126.Goldman L, Genel M, Bezman R, Slanetz P. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA. 1998;279:1100–7. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 127.Vitiello B. Long-term effects of stimulant medications on the brain: possible relevance to the treatment of attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:25–34. doi: 10.1089/104454601750143384. [DOI] [PubMed] [Google Scholar]
- 128.Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–32. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 129.Biederman J, Spencer T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol Psychiatry. 1999;46:1234–42. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- 130.Zhang K, Tarazi FI, Baldessarini RJ. Role of dopamine D4 receptors in motor hyperactivity induced by neonatal 6-hydroxydopamine lesions in rats. Neuropsychopharmacol. 2001;25:624–32. doi: 10.1016/S0893-133X(01)00262-7. [DOI] [PubMed] [Google Scholar]