Abstract
Objectives:
To determine the range of serum IgE in healthy subjects and in asthmatic patients in Oman and to assess the degree of atopy in the asthmatic patients.
Method:
Serum IgE and in vivo (the skin prick test) and in vitro (the ImmunoCAP test) allergen-specific IgE levels were measured in 44 patients with asthma. Control groups were 19 healthy subjects and 27 asymptomatic allergic subjects.
Results:
The normal range for serum IgE in the Omani population was established at ≥ 101 IU/ml. The geometric mean (and 95% confidence interval) for asthmatic patients was 468 IU/ml (323–676). Positive results for allergen-specific IgE, defined as responses to ≥ 1 allergen mix in the ImmunoCAP and to ≥ 3 allergens in the skin prick test, occurred in 26/35 (74%) and in 34/44(77%) asthmatic patients respectively. Six out of 38 patients with serum IgE ≥ 101 IU/ml and 2/6 with levels <101 IU/ml gave negative and positive results respectively in the skin prick test. Overall, the degree of reactivity in the skin prick test correlated with the level of total serum IgE (r= 0.54, p<0.001). A similar correlation could not be established with ImmunoCAP reactivity, but sIgE levels ≥ 101 IU/ml were supported by a high frequency of positive ImmunoCAP responses for the majority of allergen mixes.
Conclusions:
Total serum IgE levels should be routinely monitored in asthmatic subjects as this may give an indication of atopy where skin prick testing is not indicated. Since in a minority of patients serum IgE levels and skin prick results do not predict in the same direction, all laboratory data should be interpreted in context of clinical history.
Keywords: atopy, asthma, Oman
Bronchial asthma is one of the most common chronic disorders, both of children and of adults, worldwide. Despite regional differences in its prevalence, asthma presents considerable socio-economic burden to all societies. In Oman, studies are under way to determine the epidemiology and severity of asthma in children, through participation in the International Study of Asthma and Allergies in Childhood (ISAAC).1 As measured by the occurrence of wheezing, there is a prevalence of 9% in 13–14-year-olds.2 If these figures project through to the entire population, they suggest a high burden of disease from asthma in Oman.
The clinical expression of asthma is characterised by chronic eosinophilic inflammation, airway hyperresponsiveness to a variety of specific and non-specific stimuli and reversible airway obstruction.3,4 Whilst the pathogenesis of this inflammation has been shown to be increasingly complex, the interaction of immunoglobulin-E (IgE) with high-affinity mast cell receptors and the consequent release of inflammatory factors, are still widely accepted as the initiating/triggering events.5 Investigations of the role of the humoral immune system in the aetiology and presentation of asthma normally include the measurement both of total serum IgE (sIgE) reactivity and levels of allergen-specific IgE (asIgE). asIgE has been measured by in vivo (skin prick test: SPT) and in vitro (ImmunoCAP test) techniques.
In the present study, we have investigated the following features of atopy in asthmatic patients in Oman: (1) the relationship of total serum sIgE levels to reactivity in the skin prick test (SPT) and to levels of asIgE, (2) the range of allergen reactivity in the Omani patients and (3) the extent of concordance between the SPT and the asIgE tests.
PATIENTS AND METHODS
Subjects
Forty four patients with asthma [17, male; 27, female; 33.7 y±15.4 (mean±SD age)] seen routinely in the asthma clinic in Sultan Qaboos University Hospital, Muscat, were studied. The history of allergic asthma was established by full clinical history and examination.
The standard Bencard questionnaire was applied to all the patients included in the study, and skin tests were performed using the panel of Bencard skin test allergens. In addition, sIgE and in vitro asIgE levels were also measured.
Control groups were: (1) 27 subjects [13, male; 14, female; 33.0 y±14.1] with previous history of allergy, but currently asymptomatic, the Asymptomatic Allergic Subjects (AAS); (2) 19 [11, male; 8, female; 29.3y±8.5] Normal Control Subjects (NCS).
METHODS
1. Serum IgE
This was measured using a micro particle enzyme immunoassay6 in the IMX system (Abbot Laboratories, IL 60064, USA).
2. Allergen-specific IgE in vivo test: The skin prick test (SPT)
This was measured using the Bencard system, using a panel of 25 allergens. Positive reactions in this test were assessed as wheal-and-flare reactions greater than 3 mm in patients who also gave positive reactions on provocation with histamine (positive control).
3. Allergen-specific IgE in vitro test: The Immunocap test
AsIgE was measured using the automated ImmunoCAP system (Pharmacia and Upjohn Diagnostics, Uppsala, Sweden). Undiluted test sera and standards were incubated with allergens covalently coupled to a CNBr-activated cellulose derivative (the ImmunoCAP) for 30 minutes. β-galactosidase conjugated anti-IgE monoclonal antibodies against a range of allergens were added. The mixture was incubated for a further 150 minutes during which time a complex was formed. Unbound enzyme anti-IgE was washed away and the bound complex was incubated with a developing agent, 4-methylumbelliferyl-β-d-galactoside. After 10 minutes, the reaction was stopped and the fluorescence detected in a fluorimeter. The developed fluorescence was directly proportional to the concentration of allergen specific IgE in the serum.7 For individual allergens, standardisation permits calculation of IgE levels in allergen specific units. The system is calibrated over six ranges: <0.35 kUa/l (not atopic) to >100 kUa/l (strongly positive). For allergen mixes, only a positive/negative result is given.
Statistical analysis
The frequency of responses to SPT and ImmunoCAP allergens was compared by χ2 analysis. Correlations between sIgE levels and allergen-specific IgE data were made using Pearson’s product-moment correlation coefficient and regression analysis. The significance of the differences in log sIgE levels between subject groups was compared by t-test. Differences in quantitative reactions to ImmunoCAP allergens between different groups of subjects were made using the Mann-Whitney U test. Ranges of sIgE levels are shown as the 95% confidence interval (CI) of the geometric mean.
RESULTS
Total serum IgE levels
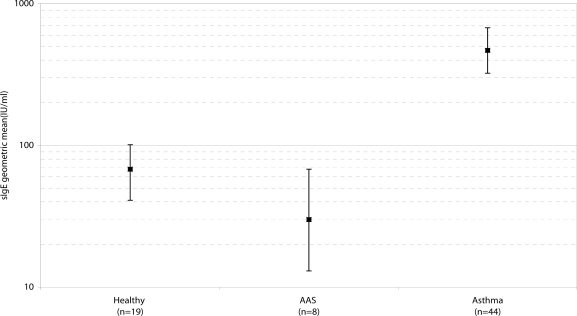
Total sIgE levels for the three groups are illustrated in Figure 1. The geometric mean and 95% CI for the asthmatic patients were 468 IU/ml and 323–676. This was significantly higher than for either the AAS—30 IU/ml (13–68) (t=5.73, p<0.001)—or the NCS—68 IU/ml (41–101) (t=5.79, p<0.001). There was no significant difference between these latter two groups. In this study therefore, the upper limit of the normal range has been set at <101 IU/ml. While 38 asthmatic patients had sIgE levels above this limit—632 IU/ml (451–887)—there were 6 patients in whom the sIgE levels were within the normal range—67 IU/ml (54–83).
Figure 1.
Ranges of sIgE levels in normal control subjects, asymptomatic allergic subjects and patients with asthma.
Bars represent geometric mean values sIgE, with 95% confidence limits
Comparison of allergens used in the in vivo and in the in vitro tests for atopy
A similar frequency of positive responses was shown for the majority of allergen pairs analysed in the in vivo and in vitro systems (p value of χ2>0.05) [Table 1a]. The only exception to this was the SPT allergen house dust mite, to which there was a 38% response. This was significantly lower than the response frequency of greater than 70% for each of the three ImmunoCAP Dermataphagoides species allergens and the ImmunoCAP House dust mix (p values of χ2<0.05) [Table 1b], but it showed a similar frequency of response to the ImmunoCAP allergens Euroglypus maynei and Hollister-Stier Laboratories dust preparation [Table 1a]. By contrast, the SPT allergen house dust, (73% response) showed a similar frequency of response to each ImmunoCAP individual and mixed preparations of the Dermataphagoides species allergens, and also to the Euroglypus maynei and the Hollister-Stier Laboratories dust preparations.
Table Ia.
SPT and ImmunoCAP allergens showing similar frequencies of responses on testing in asthmatic patients
| ImmunoCAP allergens | SPT allergens |
|---|---|
| Gx1: grass (mix) | grass, hay dust, straw, mixed threshings |
| t23: cupressus | trees |
| t72: queen palm | trees |
| Tx7: Tree (mix) | trees |
| m3: Aspergillus | A. niger, A. fumigata, hay dust,straw, mixed threshings |
| m6: Alternata | A. alternata, hay dust, straw, mixed threshings |
| Mx2: Mould (mix) | A. alternata, hay dust, mixed threshings |
| d74: E. maynei | house dust, house dust mite |
| h2: Hollister-Stier labs | house dust, house dust mite |
| Dl: D. pteronyssinus | house dust |
| d2: D. farinae | house dust |
| d3: D. microcereus | house dust |
| Hx2: House dust mix | house dust |
| el: cat | cat fur |
| e3: horse | horse hair |
| e4: cow | cow hair |
| e81: sheep | sheep wool |
| e85: chick | feathers |
| fi: egg | egg |
| f2: milk | milk, chocolate |
| f4: wheat | wheat |
| f8: maize | maize |
| f80: lobster | lobster |
| Fx2: Fish mix | cod |
| Fx3: Cereal (mix) | wheat, maize |
For each ImmunoCAP allergen shown above there was no significant difference in frequency of response (p values of χ2>0.05) by comparison with related SPT allergens.
Table 1b.
SPT and ImmunoCAP allergens showing different frequencies of response in asthmatic patients
| ImmunoCAP allergens | SPT allergen | ||
|---|---|---|---|
| Dl: D. pteronyssinus | 73% | house dust mite | 38% |
| d2: D. farinae | 73% | ||
| d3: D.microcereus | 77% | ||
| Hx2: House dust mix* | 72% |
There was a significantly higher frequency of response (p values of χ2 <0.05) to each of these ImmunoCAP allergens than to the SPT allergen “house dust mite.”
Hx2 contains dl, d2, h2 and i6 (Blatella germanica) allergens.
Skin prick tests
1. Frequency of response
The frequencies of positive responses to SPT allergens are shown in Table 2. The highest frequency was to house dust, where 73% of subjects responded, followed by cat fur (43%), house dust mite and lobster (each at 38%), mixed threshing (36%) and maize (34%). There was a less than 20% response rate for the majority of allergens.
Table 2.
Skin prick test (SPT) response to allergens in asthmatic patients in relation to total sIgE level
| Allergen | Positive response (n=44) |
Positive response in patients with sIgE |
|
|---|---|---|---|
| <101IU/ml (n=6) | ≥101IU/ml (n=38) | ||
| Cat fur | 19 (43%) | 2 (33%) | 17 (45%) |
| Cowhair | 4 (9%) | 0 (0%) | 4 (11%) |
| Feathers | 10 (23%) | 0 (0%) | 10 (26%) |
| Horse hair | 7 (16%) | 1 (17%) | 6 (16%) |
| Human hair | 6 (14%) | 0 (0%) | 6 (16%) |
| Sheep wool | 13 (29%) | 0 (0%) | 13 (34%) |
| House dust | 32 (73%) | 3 (50%) | 29 (76%) |
| Hay dust | 12 (27%) | 1 (17%) | 11 (29%) |
| House dust mite* | 17 (38%) | 0 (0%) | 17 (45%) |
| Cotton | 6 (14%) | 1 (17%) | 5 (13%) |
| A.alternata | 6 (14%) | 0 (0%) | 6 (16%) |
| A.fumigata | 5 (11%) | 0 (0%) | 5 (13%) |
| A.niger | 3 (7%) | 0 (0%) | 3 (8%) |
| Grass | 13 (30%) | 1 (17%) | 12 (32%) |
| Mixed threshings | 16 (36%) | 1 (17%) | 15 (39%) |
| Trees | 3 (7%) | 0 (0%) | 3 (8%) |
| Straw | 9 (20%) | 1 (17%) | 8 (21%) |
| Cheese | 4 (9%) | 1 (17%) | 3 (8%) |
| Chocolate | 1 (2%) | 1 (17%) | 0 (0%) |
| Cod | 0 (0%) | ||
| Egg | 2 (4%) | 0 (0%) | 2 (5%) |
| Lobster | 17 (38%) | 3 (50%) | 14 (37%) |
| Maize | 15 (34%) | 3 (50%) | 12 (32%) |
| Milk | 0 (0%) | 0 (0%) | |
| Wheat | 1 (2%) | 1 (3%) | |
| Histamine | 44 (100%) | 6 (100%) | 38 (100%) |
Using a cut off point of 101 IU/ml, sIgE levels do not differentiate frequency of SPT response to individual allergens, except, in the case of “house dust mite”, which is only responsive in the SPT at sIgE levels ≥101 IU/ml (χ2=4.37, p<0.05).
When SPT allergens were examined on an individual basis, there was no significant difference in the frequency of responses for the majority of allergens between those patients with serum IgE levels ≥ 101 and <101 IU/ml respectively. The one exception to this was the allergen house dust mite, to which there was an increased response at sIgE levels ≥ 101 (χ2= 4.37, p<0.05).
2. Association between sIgE level and positivity in SPT
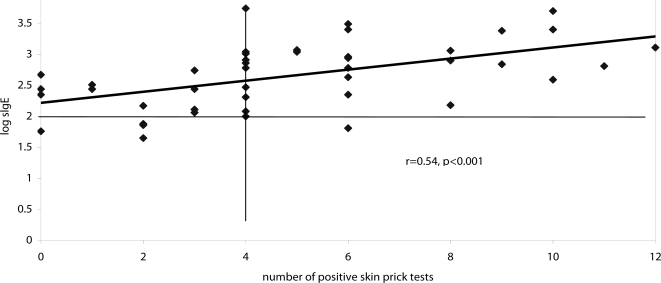
The overall positive response in the SPT was defined as reactivity to at least 3/25 of the SPT allergens in the panel, and 34/44 patients (77%) were positive and 10/44 patients (23%) were negative in this test. Of the 38 patients with sIgE ≥101 IU/ml, 32 (84%) gave a positive response in the SPT, compared with 2/6 (33%) patients with sIgE <101 IU/ml−χ2=7.64, p<0.01. Of the 10 patients with an overall negative response in the SPT, 4 had sIgE <101 IU/ml and 6 had sIgE ≥ 101 IU/ml. Four patients gave zero responses in the SPT; their sIgE levels were 467, 276, 224 and 57 IU/ml [Figure 2]. There was an overall positive correlation between sIgE level and the number of allergens reactive in the SPT (r=0.54, p<0.001) [Figure 2].
Figure 2.
Correlation between sIgE levels and degree of positivity in the skin prick test.
The horizontal line represents upper limit of normal range for IgE. Vertical line represents demarcation of overall negative and positive results in SPT.
Allergen specific IgE
1. Frequency of response
For these tests, data is available for both the AAS group and the asthmatic patients. The frequency of responses to ImmunoCAP allergen mixes is shown in Table 3. With regard to airborne allergens, 74% of asthmatic patients responded to the ImmunoCAP allergen, House dust mix, the same proportion as responded to house dust in the SPT. However, when the response to individual ImmunoCAP allergens was analysed, it became apparent that the highest proportion (73%), of subjects responded to Dermatophagoides pteronyssinus and only 44% responded to the ImmunoCAP house dust preparation, (Hollister-Stier Laboratories) i.e the reverse of the situation seen with SPT allergens [Table 2]. The response was greater than 50% for other airborne allergens. The numbers of asthmatic patients tested with the food allergen mixes was very low, so the results can only be interpreted with extreme caution.
Table 3.
Proportions of asthmatic and AAS* subjects reacting to allergen mixes using the ImmunoCAP system
| Allergens |
Patient group |
||||
|---|---|---|---|---|---|
| AAS | (%) | Asthmatic | (%) | p valueof χ2 | |
| Grass pollen mix | 2/19 | (11%) | 9/24 | (37%) | <0.05 |
| Tree pollen mix | 3/20 | (15%) | 9/15 | (60%) | <0.05 |
| Mould mix | 2/19 | (11%) | 5/12 | (42%) | <0.05 |
| House dust mix | 9/27 | (33%) | 26/35 | (74%) | <0.05 |
| Food (nut) mix | 1/16 | (6%) | 3/3 | (100%) | <0.01 |
| Food (fish) mix | 3/18 | (17%) | 3/8 | (38%) | >0.05 |
| Food (cereal) mix | 3/17 | (18%) | 7/15 | (47%) | >0.05 |
AAS: Asymptomatic allergic subjects
For all airborne allergens, and for nut mix, but not for cereal or for fish mixes, there was a significantly higher proportion of positive responses in asthmatic patients compared with the AAS group [Table 3].
2. Association between sIgE level and positivity to ImmunoCAP mixes
Due to the limited numbers there was no discrimination in the proportions of those patients with high and those with low sIgE levels reacting to allergen mixes in the ImmunoCAP test [Table 4]. However, when those patients with sIgE ≥ 101 IU/ml were considered separately, it is apparent that positive results to ImmunoCAP allergen mixes occurred in the following proportions of patients:
| Food 13/16 | (81%) | |
| House dust | 16/22 | (72%) |
| Moulds | 5/8 | (63%) |
| Tree pollen | 6/10 | (60%) |
| Grass 8/21 | (38%) |
Thus, apart from grass allergen mix, levels of sIgE ≥ 101 IU/ml are supported by reactivity to ImmunoCAP allergen mixes in a diagnosis of atopy.
Table 4.
Numbers of asthmatic patients positive to ImmunoCAP mixes compared to their total serum IgE
| Allergen mix (n)* | Response to sIgE concentration of | χ2# | p | |||
|---|---|---|---|---|---|---|
| <101 IU/ml
|
≥101 IU/ml
|
|||||
| negative | positive | negative | positive | |||
| Grass pollen (24) | 2 | 1 | 13 | 8 | 0.23 | >0.05 |
| Tree pollen (12) | 2 | 0 | 4 | 6 | 0.60 | >0.05 |
| Mould (12) | 4 | 0 | 3 | 5 | 2.10 | >0.05 |
| House dust (25) | 1 | 2 | 6 | 16 | 0.20 | >0.05 |
| Food mixes combined (18) | 1 | 1 | 3 | 13 | 0.00 | >0.05 |
Number of cases tested
Non-parametric χ2 for two independent samples
Coincidence of reactivity to SPT and to ImmunoCAP allergens in individual patients
Data was available from 31 asthmatic patients for both tests. Positivity was again defined as reactivity to ≥ 3 SPT allergens and to ≥1 ImmunoCAP allergen. Twenty three (74%), 21 (67%) and 19 (58%) patients were positive in the SPT, in the ImmunoCAP and in both tests respectively. These data, together with the mean sIgE levels for each group, are shown in Table 5. This illustrates very clearly that SPT positivity is associated with high levels of sIgE, while negativity in the SPT is associated with low levels of sIgE. A significantly higher proportion of the patients positive in SPT had a positive response in the ImmunoCAP compared with those negative in the SPT (χ2=9.014, p<0.01). That is, there is an overall tendency for the SPT and the ImmunoCAP to predict in the same direction. The levels of sIgE were similar in ImmunoCAP positive and negative subjects, both in SPT positive (t=0.28, df=21, p>0.05) and negative (t=0.23, df=6, p>0.05) patients.
Table 5.
Coincidence of reactivity in SPT and ImmunoCAP
| Test result | n | Geometric mean sIgE(IU/ml) and (95% CI) |
|---|---|---|
| Positive SPT | 23 | 776 (519–1161) |
| +ive immunocap | 19 | 796 (512–1245) |
| –ive immunocap | 4 | 684 (225–2080) |
| Positive SPT | 8 | 166 (67–302) |
| +ive immunocap | 2 | 169 (30–1146) |
| –ive immunocap | 6 | 156 (77–315) |
DISCUSSION
Total sIgE levels and allergen-specific tests, both in vivo and in vitro, are commonly used to diagnose atopic diseases. At the present time, there is no established data on either sIgE levels or the immune status of asthmatic patients in this country. Identification of triggering allergens in the Gulf region is important for the informed clinical management of allergic diseases. This is particularly so where desensitisation therapy is considered. It is equally important to establish the clinical efficiency of in vivo and in vitro diagnostic tests for allergic diseases. The present study compares methodologies used in the diagnosis of asthma in adult Omani patients attending the Asthma Clinic at Sultan Qaboos University Hospital in Oman; we report here the results of asIgE tests in the context of total sIgE levels in the study population.
The mean sIgE level in healthy Omani subjects was 68 IU/ml with a range of 41 to 101 IU/ml. These levels are comparable to those of other populations.8,9 The asIgE data in this study has been analysed in the context of a normal range for sIgE of <101 IU/ml. The mean level for the AAS group, 30 IU/ml with a range 13 to 68 IU/ml, was lower than that of the healthy Omani subjects; only one of the AAS had an sIgE level above the normal range. It is perhaps of relevance here that the entire AAS group were expatriates. Hence the differences found may illustrate a real difference between the two control groups, since variations in sIgE may occur due to environmental factors such as diet and parasite burdens related to geographical location of origin. The level of sIgE in the healthy Omani population can only be validated by increasing the size of the cohort studied. Such studies are currently in progress.
In agreement with previous findings,8 the geometric mean (469 IU/ml) and the range (323–676 IU/ml) of sIgE were increased in the Omani asthmatic patients compared to healthy control subjects. Since sIgE contributes significantly to the outcome of skin tests, the clinical significance of these sIgE levels and the interpretation of the results from the allergen-specific tests can only be assessed in context of the normal range for this population. However, in one study, skin reactivity was noted in 35% of asthmatic patients in the presence of low levels of sIgE.10 Similarly, six patients in this study had sIgE levels <101 IU/ml, and two of them (33%) showed positivity in the SPT. Where there is the coincidence of a low level of sIgE and positivity in the SPT in an individual patient, difficulties may occur in the classification of the asthma.
Positive SPT responses and allergen-specific IgE antibodies are both important markers of disorders in the upper respiratory tract. However, studies of their interactive functioning are seriously hampered by differences in the commercial antigen extracts available for use in the two tests. At the outset of this study, we established comparability of response between the two antigen systems used for a wide range of allergens. We employed the Pharmacia ImmunoCAP system for in vitro asIgE measurements and the Bencard system for in vivo SPT testing. Both systems are dependent on the quality of allergen extracts used in the tests. Quality control is difficult and in practice it can only be achieved by correlation with clinical history data by an experienced allergologist.
Lack of standardisation between allergens of different manufacturers is also a major concern.11 The introduction of recombinant allergens, whilst encouraging in this respect12 brings further problems. Due to the high specificity of B- and T-cell responses, the number of individual patients responding to a recombinant allergen will be restricted compared to the number responding to a heterogeneous allergen prepared from natural sources, because such allergens contain multiple epitopes and different isoforms.12 Hence, the use of recombinant allergens may lead to underdiagnosis of allergic patients. Studies in patients from the Gulf region should ideally be performed using allergens prepared locally. These are not available at present. In their absence, it is difficult to draw definitive conclusions on the relative diagnostic efficiencies of assay modalities from studies using different commercial allergens. Variations between them may reflect affinity and epitope recognition patterns within individual IgE responses rather than imprecision in the test. A further complication is that in vivo and in vitro tests monitor different aspects of asIgE, tissue-bound (i.e. stable over many months) in the SPT, versus serum levels (with a half-life of only 2 days) in the ImmunoCAP.
Despite these factors, we found no statistical difference in response between the majority of comparable allergens in the ImmunoCAP and the SPT, though other studies suggest a higher overall frequency of response in the SPT.14,15 This is probably a reflection of the more persistent biological life of tissue bound IgE. The only major discrepancy in this study between comparable allergens was between the house dust and house dust mite allergens. In agreement with another reported study, the ImmunoCAP was more sensitive for house dust mite allergens.16 By contrast, the SPT was more sensitive for the house dust allergen. These data are suggestive of a crossover in specificities of these particular allergens from the two different systems. There is as yet no agreement as to whether the SPT and the ImmunoCAP tests are17 or are not18 complementary to each other.
In this study, 34/44 (77%) of patients gave positive responses to the panel of allergens in the SPT, and the association of these positive responses with raised levels of sIgE9 was confirmed. Of the 10 (23%) patients negative in the SPT, 6 had sIgE levels ≥ 101 IU/ml and 4 had sIgE levels <101 IU/ml. Six out of 44 patients (14%) had sIgE levels <101 IU/ml, and 2 of these were positive in the SPT. Reactivity to individual allergens in the SPT was associated with a large range of values of sIgE levels in different patients e.g., amongst those subjects with reactivity to 4 SPT allergens, the range of sIgE levels was 99–5,521 IU/ml [Figure 2]. It has been suggested that where high levels of sIgE are found in atopic patients, they additionally reflect immune dysregulation. IgE itself may be involved in allergen uptake via the CD23 receptor (FceR11).5 Such IgE-mediated allergen presentation may lead to a continuous (over) activation of the immune system due to high levels of IgE. This could explain the deterioration in the clinical condition of children with asthma, which is characterised both by increased severity of symptoms and sensitivity to an increased number of (non related) allergens.5
Four patients in this study showed no reactivity to any SPT allergens. Three of them had sIgE levels above the normal range. This apparent anomaly may merely be a reflection of the lack of knowledge of the precise amount of the relevant allergen required to provoke an SPT reaction in each individual patient, that is, inadequate technical understanding of the fundamental aspects of the tests. It has been shown that with similar levels of allergen-specific IgE, the amount of allergen required for a positive SPT may differ by as much as a factor of 100 between patients.19 Thus, there may be a threshold amount of allergen required to provoke a response for each individual; this will be elucidated only when purified allergens become generally available.
From this study, it is obvious that raised levels of serum IgE and positivity in the SPT both aid the diagnosis of atopy, but the absence of these factors does not exclude it. Raised sIgE levels are thought to be predictive of the subsequent development of allergic disease in children,19,20 but in the adult, it does not appear that, alone, they invariably predict an allergic state. Genetic and environmental factors also play an important role in the production of clinical symptoms.
Overall, the SPT is considered to have a better diagnostic efficiency and sensitivity than in vitro methods,21,22 and it has the advantage of providing immediate information for both patient and physician. The high degree of sensitivity offered by the SPT is important when a patient is assessed for potentially life threatening allergies, for example, from penicillin or stinging insects. It is worth emphasising, however, that in a minority of patients the expected association of sIgE level with SPT reactivity does not occur. As previously reported, some patients were found with sIgE levels in the normal range, but with multiple SPT reactivity,10 and the converse, where some patients had raised sIgE levels but little or no SPT reactivity. These findings are suggestive of false positive and false negative reactions in the SPT, and they emphasise the care that must be taken in the categorisation of such patients. It is most important that a detailed clinical history is taken, as a third component of the diagnostic procedure. The subjects in the AAS group had, as expected, low levels of sIgE and reduced ImmunoCAP reactivity compared with the asthmatic patients.
CONCLUSION
We have described here a population of asthmatic patients in Oman who have a sIgE profile comparable to that found in similar studies.9 Estimation of sIgE may be helpful in the diagnosis of atopic asthma, but it has poor correlation with symptoms. In individual patients there is a good correlation between sIgE level and the degree of overall reactivity to the panel of allergens in the SPT, irrespective of ImmunoCAP reactivity. sIgE levels, in combination with the SPT, should therefore be used in the routine diagnosis of allergy in the majority of asthmatic patients who have no complications from skin conditions or medication or who are considered so sensitive by history that anaphylaxis is possible. In cases where these types of difficulties are found, use of the ImmunoCAP may be useful since its wide range of measurement permits a good discrimination of negative cases from those with atopic disease. Due to non-specific binding, high levels of sIgE may interfere with both in vivo and in vitro assays of asIgE. In view of the occasional occurences of false positive and false negative reactions in the Skin Prick Test (SPT), its results should always be interpreted in the context of a detailed clinical history.
REFERENCES
- 1.ISAAC Steering Committee Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis and atopic eczema. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 2.Al-Riyami MSB, Al-Rowas OAS, Al-Riyami AA, Jasim LG, Mohamed AJ. Prevalence of asthma symptoms in Omani schoolchildren. SQU J Sci Res: Med Sci. 2001;3:21–27. [PMC free article] [PubMed] [Google Scholar]
- 3.Kroegel C, Virchow J-C, Luttmann W, Walker C, Warner JA. Pulmonary immune cells in health and disease: the eosinophil leucocyte. Eur Resp J. 1994;7:519–543. doi: 10.1183/09031936.94.07030519. [DOI] [PubMed] [Google Scholar]
- 4.Smith L, McFadden ER. Bronchial hyperreactivity revisited. Ann Allergy, Asthma and Immunol. 1995;74:454–469. [PubMed] [Google Scholar]
- 5.Mudde GC, Bheekha R, Bruijnzeel-Koomen CAFM. Consequences of IgE/CD23 mediated allergen presentation in allergy. Immunol Today. 1995;16:380–382. doi: 10.1016/0167-5699(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 6.Yman L, Roosdorp N, Schroder H, Andrae MI. Methods for the determination of IgE and allergen-specific antibodies. International Allergy Symposium, Excerpta Medica. 1981;67:74–83. [Google Scholar]
- 7.Paganelli R, Ansotegui J, Sastre J, Lang CE, Roovers MH, de Groot H, et al. Specific IgE antibodies in the diagnosis of atopic disease. Clinical evaluation of a new in vitro test system, UNICAP TM, in Six European allergy clinics. Clin Allergy. 1988;18:581–587. [PubMed] [Google Scholar]
- 8.John AB, Lee HS, Lee Fy, Chug HH. Allergen skin test and total IgE in adults with rhinitis in Singapore. Asian Pac J Allergy Immunol. 1996;14:9–12. [PubMed] [Google Scholar]
- 9.Janson DF, Rijeken B, Schouten JP, Kraan J, Weiss ST, Timens W, et al. The relationship of skin test positivity, high serum total IgE levels, and peripheral blood eosinophilia to symptomatic and asymptomatic airway hyperresponsiveness. Am J Respir Crit Care Med. 1999;159:924–931. doi: 10.1164/ajrccm.159.3.9804024. [DOI] [PubMed] [Google Scholar]
- 10.Hoshina K, Kawasaki A, Mizushima Y, Yano S. Positivities of skin test and IgE RAST to allergens in bronchial asthma with normal serum IgE levels. Arerugi. 1991;40:16–20. [PubMed] [Google Scholar]
- 11.Dolen WK. Allergen extract standardization: reality. myth or dream. Ann Allergy Asthma Immunol. 1996;75:81–82. [PubMed] [Google Scholar]
- 12.Crameri R, Lidholm J, Gronlund H, Stuber D, Blaser K, Menz G. Automated specific IgE assay with recombinant allergens: evaluation of the recombinant Aspergillus fumigatus allergen I in the CAP system. Clin Exp Allergy. 1996;26:1411–1419. [PubMed] [Google Scholar]
- 13.Hayglass KT. Allergy: Who, why and what to do about it. Immunol Today. 1995;16:505–507. doi: 10.1016/0167-5699(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 14.Ewan PW, Coote D. Evaluation of a capsulated hydrophilic carrier polymer (the ImmunoCAP) for measurement of specific IgE antibodies. Allergy. 1990;45:22–29. doi: 10.1111/j.1398-9995.1990.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 15.Gleeson M, Cripps AW, Hensley MJ, Wlodarczyk JH, Henry RL, Clancy RL. A clinical evaluation in children of the Pharmacia ImmunoCAP system for inhalant allergens. Clin Exp Allergy. 1996;6:692–702. [PubMed] [Google Scholar]
- 16.Plebani M, Bourghesan F, Faggian D. Clinical efficiency of in vitro and in vivo tests for allergic diseases. Ann Allergy Asthma Immunol. 1995;74:23–28. [PubMed] [Google Scholar]
- 17.Droste JH, Kerhof M, de Monchy JG, Schouten JP, Rijken B. Association of skin test reactivity, specific IgE, total IgE and eosinophils with nasal symptoms in a community-based population study. The Dutch ECRHS Group. J Allergy Clin Immunol. 1996;97:922–932. doi: 10.1016/s0091-6749(96)80066-2. [DOI] [PubMed] [Google Scholar]
- 18.Witteman AM, Stapel SO, Perdok GJ, Sjamsoedin DH, Jansen HM, Aalberse RC, et al. The relationship between RAST and skin tests in patients with asthma or rhinitis: a quantitative study with purified major allergens. J Allergy Clin Immunol. 1996;97:116–125. doi: 10.1016/s0091-6749(96)70278-6. [DOI] [PubMed] [Google Scholar]
- 19.Kjellman NI. Predictive value of high IgE levels in children. Acta Paediatric Scand. 1976;65:465–471. doi: 10.1111/j.1651-2227.1976.tb04915.x. [DOI] [PubMed] [Google Scholar]
- 20.Haahtela T, Suoniemi I, Jaakonmaki I, Bjorksten F. Relationship between sIgE and the occurrence of immediate skin test reactions and allergic disorders in young people. Allergy. 1982;37:597–602. doi: 10.1111/j.1398-9995.1982.tb02346.x. [DOI] [PubMed] [Google Scholar]
- 21.Ownby DR. In vivo versus in vitro. Pediatr Clin North Am. 1988;35:995–1009. doi: 10.1016/s0031-3955(16)36544-0. [DOI] [PubMed] [Google Scholar]
- 22.Tschopp JM, Sistek D, Schindler C, Leuenberger P, Perruchoud AP, Wuthrich B, et al. Current allergic asthma and rhinitis: diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests, and Phadiatop). Results from 8329 randomized adults from the SAPALDIA Study. Swiss study on Air pollution and Lung Diseases in Adults. Allergy. 1998;53:606–613. doi: 10.1111/j.1398-9995.1998.tb03937.x. [DOI] [PubMed] [Google Scholar]