Abstract
Rationale
Neural deficits at the interface of affect and cognition may improve with pharmacotherapy in pediatric bipolar disorder (PBD).
Objectives
We examined lamotrigine treatment impact on the neural interface of working memory and affect in PBD.
Methods
Un-medicated, acutely ill, patients with mania and hypomania (n=17), and healthy controls (HC; n=13; mean age=13.36±2.55) performed an affective two-back functional magnetic resonance imaging task with blocks of angry vs neutral faces (i.e., angry face condition) or happy vs neutral faces (i.e., happy face condition) before treatment and at follow-up, after 8-week treatment with second-generation antipsychotics followed by 6 weeks of lamotrigine monotherapy.
Results
At baseline, for the angry face condition, PBD, relative to HC, showed reduced activation in the left ventrolateral prefrontal cortex (VLPFC) and right caudate; for the happy face condition, PBD showed increased activation in bilateral PFC and right amygdala and middle temporal gyrus. Post-treatment, PBD showed greater activation in right amygdala relative to HC for both conditions. Patients, relative to HC, exhibited greater changes over time in the right VLPFC and amygdala, left subgenual anterior cingulate cortex and left caudate for the angry face condition, and in right middle temporal gyrus for the happy face condition.
Conclusions
Pharmacotherapy resulted in symptom improvement and normalization of higher cortical emotional and cognitive regions in patients relative to HC, suggesting that the VLPFC dysfunction may be state-specific in PBD. Amygdala was overactive in PBD, relative to HC, regardless of reduction in manic symptoms, and may be a trait marker of PBD.
Keywords: Functional magnetic resonance imaging (fMRI), Bipolar, Lamotrigine, Working memory, Face emotion, Adolescent, Child, State, Trait, Brain imaging, Emotion
Introduction
The current study examined the effects of pharmacotherapy on the neural interface of working memory and emotion processing among un-medicated, acutely ill adolescents with pediatric bipolar disorder (PBD), types I and II, while performing an affective working memory task. It is important to study this issue in PBD since findings in PBD studies suggest that the functional interface of ventral affective systems (including the ventrolateral prefrontal cortex (VLPFC; Brodmann areas (BA) 45 and 47), the ventromedial prefrontal cortex (VMPFC; BA 10–12), the pregenual (BA 24) and subgenual anterior cingulate cortex (ACC; BA 25), and the amygdala) and dorsal cognitive systems, which are composed of the dorsolateral prefrontal cortex (DLPFC; BA 9 and 46), the dorsal ACC (BA 32/24), and temporoparietal regions (BA 21 and 40; Dolcos and McCarthy 2006; Philips et al. 2008; Pavuluri et al. 2008, 2010a, 2011; Passarotti et al. 2010a, b, c; Hart et al. 2010) are impaired to varying degrees in bipolar disorder (BD) illness or mixed samples of illness and euthymic states (Rich et al. 2006; Pavuluri et al. 2008, 2009a, 2010a, b, c). Similarly, amygdala hyperactivity is seen in patients with BD with manic symptoms (Foland et al. 2008) or in remission (Pavuluri et al. 2007, 2009a; Versace et al. 2010; Gruber et al. 2009).
In order to probe the interface of working memory and affect processing systems with a task close to real-life challenges, we adopted a two-back working memory paradigm that typically engages an extended working memory network of fronto-striato-parietal regions (Smith and Jonides 1998; Baddeley 2003; Owen et al. 2005) including lateral prefrontal regions for maintenance, manipulation, and selection processes (Petrides 1994), the dorsal ACC for stimulus and response selection, and the inferior parietal cortex and precuneus for attention allocation and working memory storage (Smith and Jonides 1998). Furthermore, to examine pharmacotherapy effects specifically on the neural interface of working memory and affect processing, which has not been done previously in PBD, we employed affective face stimuli to elicit affective responses with to-be-remembered emotional faces. This type of stimuli has been successfully used to probe amygdala response both in adults (Foland et al. 2008; Versace et al. 2010) and children (Rich et al. 2006; Pavuluri et al. 2007, 2009a; Passarotti et al. 2010c; Brotman et al. 2010) with BD, and in the context of n-back tasks in healthy adults and children (Braver and Bongiolatti 2002; Casey et al. 1995).
The brain regions engaged by this affective working memory task are also differentially implicated in BD pathology and other illnesses with mood dysregulation. Using this same paradigm, Passarotti et al. (2010c) found significant differences in activation in the ventral affective and the dorsal cognitive systems in PBD as compared with ADHD or healthy controls (HC). Specifically, for angry vs neutral faces, PBD patients exhibited greater activation than ADHD patients in subgenual ACC and orbitofrontal cortex and reduced activation in DLPFC, while, relative to HC, the PBD patients showed reduced activation at the junction of the DLPFC and VLPFC, a region at the interface of cognitive and affective processes (Petrides and Pandya 2002). Similar regions of dysfunction in PBD were implicated during encoding of emotional faces in a study by Dickstein et al. (2007). Moreover, using a similar memory task with faces, Roberson-Nay et al. (2006) found increased amygdala and hippocampal activation in youth with depression compared with youth with anxiety or healthy controls.
In the present study, we wished to examine how lamotrigine treatment may improve mood regulation and alter the impact of affective arousal on working memory circuits in PBD. The stability of these alterations over time may depend on treatments used to achieve clinical stabilization. Lamotrigine is a broad-spectrum anticonvulsant that aids mood stabilization in bipolar disorder and decreases CNS excitability (Geddes et al. 2009; Goodwin et al. 2004). Its mechanisms of action include modulation of sodium channels to improve mood regulation and glutamatergic attenuation to improve cognition (White 1999; Wang et al. 2001; Geddes et al. 2009). While there is still some disagreement on its effectiveness as a mood stabilizer (Young 2004), given the lack of a strong consensus on what is needed to consider an agent efficacious as a mood stabilizer (Bauer and Mitchner 2004), lamotrigine has been found to be safe and effective in improving mood regulation both in terms of manic and depressive symptoms (Chang et al. 2008; Pavuluri et al. 2009c; Biederman et al. 2000), and there is initial evidence that it improves function in prefrontal circuits involved in motor response inhibition (Pavuluri et al. 2010a) as well as performance during working memory tasks (Pavuluri et al. 2010b) in PBD. Therefore, this is a clinically used intervention for achieving manic remission in PBD.
Several attempts have been made to use functional magnetic resonance imaging (fMRI) to track treatment effects on functional brain systems in patients relative to HC. There are currently three published studies on the effect of lamotrigine treatment on affective and cognitive neural systems in patients with PBD. In an important initial study, Chang et al. (2008) found that, in children with bipolar depression, lamotrigine treatment led to a decrease in right amygdala activation during an emotion rating task and that there was a significant correlation between decreased right amygdala activation and reduction in depressive symptoms. However, this study did not have an HC group; therefore, it is still an open question whether the decreased amygdala activation found in the PBD patients would correspond to normalized activation of this region relative to HC, or whether the amygdala would still be hyperactive relative to HC. Furthermore, other recent studies showed that lamotrigine treatment in patients with hypo/mania led to increased activation in VMPFC during a motor response inhibition task (Pavuluri et al. 2010a) and an affective color matching task with emotional words (Pavuluri et al. 2010c). Taken together, these initial studies suggest that lamotrigine may be useful in PBD, especially to aid the VLPFC function, since this region serves as an important interface of cognition and affective systems (Passarotti et al. 2010a; Pavuluri et al. 2009a, b, 2008).
In the present study, pharmacotherapy consisted of second-generation antipsychotics (SGAs) followed by lamotrigine maintenance over 14 weeks (see details in “Methods”).
Based on previous studies with similar cognitive and affective paradigms (Pavuluri et al. 2008, 2010c; Passarotti et al. 2010a, c), we hypothesized that, at baseline (i.e., before treatment), relative to HC, the PBD group would show increased amygdala activity and decreased prefrontal activity in regions involved in working memory (DLPFC and dorsal ACC) and affect appraisal and regulation (VLPFC, VMPFC, and ventral ACC). We also predicted more severe neural dysfunction in PBD relative to HC at baseline with negative valence stimuli (i.e., angry faces) than with positive valence stimuli (i.e., happy faces), since the greater emotional challenge of negative emotions may disrupt more significantly the already present dysfunctional regulatory systems in PBD (Pavuluri et al. 2008, 2010a, b; Passarotti et al. 2010b, c). Specifically, based on previous findings (Pavuluri et al. 2008, 2010a, b; Passarotti et al. 2010b, c; Dickstein et al. 2007), we predicted that, at baseline, PBD relative to HC would show decreased activation in prefrontal regulatory regions for angry faces and increased activation in similar circuits for happy faces.
Moreover, based on findings from our previous lamotrigine fMRI studies (Pavuluri et al. 2010a, c), we predicted normalization of function in regulatory prefrontal regions in patients after treatment. However, given discordant findings in subcortical activity in remission, we also wanted to ascertain whether amygdala activation would normalize (Chang et al. 2008; Van der Schot et al. 2010) or remain overactive (Lawrence et al. 2004; Blumberg et al. 2003; Pavuluri et al. 2007; Foland et al. 2008; Pavuluri et al. 2009a) in PBD patients relative to HC.
Methods
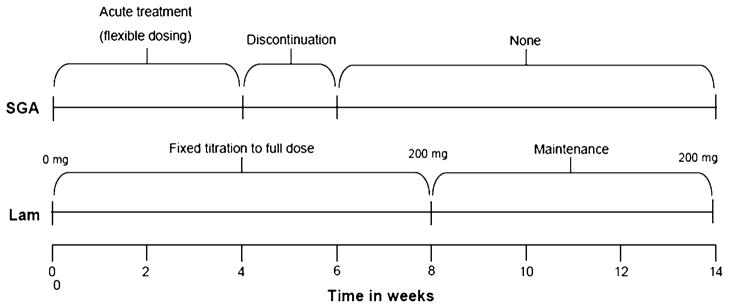
The present study was approved by the Institutional Review Board (IRB) at the University of Illinois at Chicago and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. This study was a prospective 14-week outpatient open-label trial. During the first 8 weeks, lamotrigine was prospectively titrated up alongside SGAs in PBD patients. This was followed by 6 weeks of lamotrigine monotherapy at a dose consistent with standard clinical practice (Fig. 1). The SGAs served as rescue medication during acute treatment in order to stabilize acute hypo/manic symptoms during the initial titration phase of lamotrigine given the necessity to gradually titrate up the dose of lamotrigine over 8 weeks to avoid rash (Pavuluri et al. 2009c). The fMRI affective n-back task was administered to all participants at baseline, before treatment initiation, and at follow-up. HC subjects did not receive treatment but were tested at baseline and retested at follow-up to account for potential changes in brain activation due to practice effects.
Fig. 1.
Illustration of 14-week pharmacological treatment which consisted of second-generation antipsychotics (SGAs) for acute mania followed by lamotrigine for maintaining stable mood
Participants
Participants with PBD (n=17) were recruited from the child psychiatry clinics at the University of Illinois at Chicago (UIC), and healthy controls (n=13) were recruited from the neighboring community through advertisement. Prior to inclusion in the study, we obtained an assent for children younger than age 15 years and an informed consent for adolescents aged 15 years or older. Consent from at least one parent or legal guardian was always obtained. Groups were matched for age, sex, race, socioeconomic status (SES), IQ as estimated with the Wechsler Abbreviated Scale of Intelligence (WASI 1999), and handedness as measured by a handedness questionnaire (Annett 1970).
Inclusion criteria for subjects with PBD were as follows: Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) diagnosis of BD with either a mixed, manic, or hypomanic episode (American Psychiatric Association 1994) with BD type I (n=9) or type II (n=8), 10 to 18 years of age, a baseline score greater than 12 on the Young Mania Rating Scale (YMRS) (Young et al. 1978), and consent to be scanned in a medication-free state. Patients were either medication free (not requiring a washout at study entry) or sufficiently unstable on prior medications to justify discontinuation of an ineffective treatment prior to the beginning of lamotrigine treatment with the consent of parents and assent of patients. The washout period consisted of tapering previous medications over 1 week prior to study entry, except for those who received aripiprazole for whom there was a 4-week washout period. All patients were medication free for at least 7 days prior to scanning. None of the patients were on fluoxetine that would have required a longer washout period. Close clinical supervision and monitoring was provided during drug-free periods according to the approved IRB protocol. HC participants were excluded if they met criteria for a DSM-IV Axis I disorder or had a family history of bipolar disorder, schizophrenia, depression, autism, alcohol or drug abuse, ADHD, learning disability or obsessive compulsive disorder (OCD). Inclusion criteria for HC included being 10–18 years of age and having a baseline YMRS score of <12. Exclusion criteria for all subjects included: current substance abuse/dependence, comorbid psychiatric diagnosis requiring pharmacotherapy, including ADHD, schizophrenia, autism, psychosis not otherwise specified, pervasive developmental disorder, compulsive disorder (CD), OCD, and serious medical illness. Also, patients and HC were excluded from the study if they had a history of non-febrile seizures, head trauma with loss of consciousness for more than 10 min, neurological symptoms, speech or hearing difficulties, a full-scale IQ score of less than 70, a history of substance abuse, previous exposure to lamotrigine, and any contra-indications to magnetic resonance imaging (MRI) scans (i.e., metal implants, retractors, braces, established or possible pregnancy, and claustrophobia).
Of the patients who met the inclusion criteria, one had a comorbid diagnosis of social phobia, one of generalized anxiety disorder, and one of oppositional defiant disorder (ODD).
Assessment
The subject and a parent or legal guardian were interviewed by a board-certified child psychiatrist (MNP), or an advanced practice nurse and another board-certified clinician within our program, using the Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia (Geller et al. 1998) supplemented by the episode characterization of BD from the KSADS-Present and Lifetime versions (Kaufman et al. 2000). The primary clinical measures of treatment efficacy were the YMRS and the Child Depression Rating Scale-Revised (CDRS-R; Poznanski et al. 1984). All available clinical information was reviewed to make a consensus clinical diagnosis. Live diagnostic interviews of ten cases were independently coded by two researchers to establish inter-rater scoring reliability (Cohen’s kappa=0.94).
Pharmacotherapy
The order of preference for SGAs during the lamotrigine titration phase was: risperidone, aripiprazole, quetiapine, and ziprasidone. The order was modified according to the report of previous ill effects of any SGA. For example, if a patient did not respond to risperidone and became agitated on aripiprazole, she or he received quetiapine. The SGA was slowly withdrawn over 2–4 weeks as tolerated (i.e., between the fourth- and eighth-week periods). An overall guideline for withdrawal of SGAs was followed with reduction at 0.25 mg of risperidone, 2.5–5 mg of aripiprazole, 25–50 mg of Seroquel, or 20–40 mg of ziprasidone, every other day until they were off of the SGA. Benzotropine was allowed only during the first 4-week period on an “as-needed” basis for extrapyramidal symptoms if on SGAs.
Lamotrigine dosing over 14 weeks
During week 1, the starting dose of lamotrigine was 12.5 mg (Fig. 1). This was increased at 12.5 mg/week for the first 4 weeks, 25 mg/week for the next 2 weeks, and titrated to 200 mg by 8 weeks. All patients remained on a dose of 200 mg from weeks 8 to 14, and the follow-up scan was performed on the 14th week.
Symptom remission
Based on the research study goals, at follow-up, we defined patients in terms of post-treatment remission (i.e., YMRS score<12) or non-remission (i.e., YMRS score>12) of manic symptoms. Eighty-three percent of the PBD patients (i.e., 14/17) were in remission of manic symptoms and did not meet the DSM-IV (American Psychiatric Association 1994) criteria for mania, hypomania, or depression. Therefore, 83% of patients met the criteria for euthymic state.
The affective two-back task with emotional faces
After a brief training session in a mock scanner, participants underwent an fMRI scanning session when they were administered a block design two-back working memory task with emotional faces for 7 min. Our face stimuli were 160 Gur emotional faces (Gur et al. 2002) with neutral, angry, or happy expressions that were balanced by gender, race, and facial expression. The paradigm involved two runs. The first run consisted of four alternating 30-s blocks of angry and neutral faces (i.e., angry face condition), and the second run consisted of four alternating 30-s blocks of happy and neutral faces (i.e., happy face condition). Face blocks were presented in a counter-balanced pseudo-random sequence. On each trial, a face stimulus with a certain emotion (i.e., happy or angry) or a neutral expression was presented for 3 s, and subjects responded by pressing a response key if they saw the same face as the one presented two trials earlier (Fig. 2). A two-back match trial always required a match both in face identity and face emotion. Unlike a previous emotional n-back task where participants had to ignore irrelevant emotional information presented in the background (Casey et al. 2000), in our paradigm the emotion was integral to the processing. We decided to avoid having trials with a match based on either face identity or face emotion alone, in order to avoid confounds due to selective attention processes in this working memory task that may complicate data interpretation. A 20-s fixation mark in between blocks allowed for emotional arousal to return to baseline and for a rest period during the attention demanding task (Pavuluri et al. 2007). A color high-resolution LCD projector projected visual stimuli onto a rear projection screen that was viewed via an angled double mirror system mounted on a standard GE head coil.
Fig. 2.
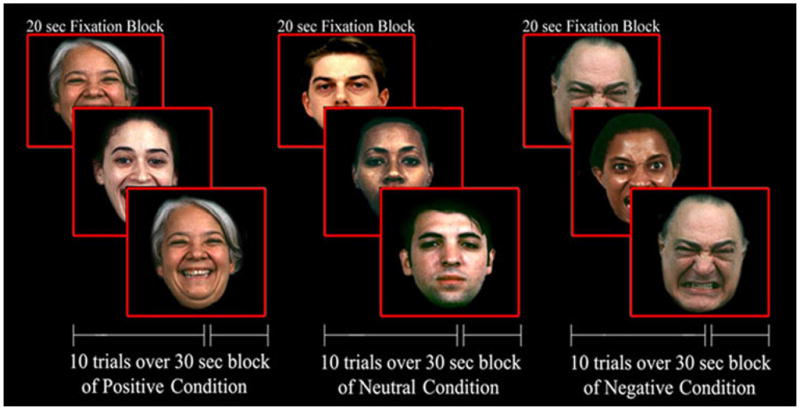
Illustration of match trials in the two-back working memory task, with happy, neutral, and angry faces
MRI protocol
Gradient-echo echo-planar functional imaging and structural acquisitions were performed with a 3.0 Tesla whole body scanner (Signa, General Electric Medical System, Milwaukee, WI). We acquired 25 slices in the axial plane (TE=25 ms; flip angle=90°; field of view=20=20cm2; acquisition matrix=64=64; TR=2.5 s; slice thickness= 5 mm with 1 mm gap). Anatomical images were also acquired in the axial plane (three-dimensional spoiled gradient recalled (SPGR), 1.5-mm thick contiguous axial slices) and were later co-registered with the functional data.
Image processing and data analysis
We used FIASCO software (Functional Imaging Analysis Software-Computational Olio; Eddy et al. 1996) to implement 3D motion estimation and motion correction, and to remove slow signal drift. Individual volumes were excluded from analyses if, relative to median head position, head displacement was greater than 1.5 mm or head rotation was greater than 0.5°. T tests revealed no significant group differences in the number of volumes retained after discarding those with motion artifact.
For each subject, voxel-wise effect size (r) maps were obtained by contrasting activation for angry versus neutral face blocks as well as for happy versus neutral face blocks, separately for baseline and follow-up. A Fisher’s z transform was also applied to normalize the effect size maps (zr) (Rosenthal 1991). Subjects’ zr maps and SPGR anatomical images were warped into Talairach space using automated procedures in AFNI (Analysis of Functional NeuroImages; Cox and AFNI 1996). We re-sampled each individual Talairach-transformed functional map (3.125× 3.125×6 mm grid) to an isotropic 3×3×3 mm grid to provide a voxel dimension similar to that of the in-plane resolution of the acquired data.
In order to examine effects of lamotrigine treatment in PBD, the primary analysis for this study was a whole-brain voxel-wise 2×2×2 analysis of variance (ANOVA), including all patients (i.e., the 14 patients who achieved euthymic state at follow-up and the three patients who did not), with group (PBD and HC) as the between-subjects factor and testing time (baseline, follow-up) and face emotion condition (i.e., angry vs neutral, happy vs neutral) as within-subjects factors, where a significant three-way interaction of group × testing time × face emotion condition was followed by pair-wise comparisons for the significant clusters emerging from the interaction, to clarify within- and between-group differences in activation for testing time and face emotion conditions. Furthermore, to correct type I error rates for multiple group comparisons, we used an adjusted voxel-wise probability threshold for significance of p<.01. Then, to correct for voxel-wise multiple comparisons in the fMRI analyses, we performed AlphaSim Monte Carlo simulations (Ward 2000) at the whole-brain level, which were restricted to in-brain voxels, to identify clusters of voxels with significant group difference using a contiguity threshold (minimum volume threshold= 351 cubic mm; minimum clustering radius=3.1 mm; and uncorrected p=.01) that ensured an experiment-wise type 1 error rate of p<0.02 (corrected p).
A secondary analysis included data from all HC subjects (n=13) but only those patients who achieved euthymic state at follow-up (i.e., 14 of 17 patients) in order to explore brain function in euthymia. Both for fMRI and behavioral data, the pattern of results did not differ significantly for the two sets of analyses. Therefore, in the “Results” and “Discussion,” we focus on the findings from our primary analysis that included all patients. Future studies will need to directly compare and contrast patterns of treatment improvement in comparable samples of euthymic and non-euthymic PBD patients.
Finally, we also performed Pearson’s correlation analyses to explore the relationship between patients’ changes in activation from baseline to follow-up in brain regions that resulted significant in the primary analysis whole-brain ANOVA (i.e., right amygdala and VLPFC) and clinical measures (YMRS and CDRS-R). Bonferroni corrections for multiple comparisons (n=4) were applied in these analyses (i.e., using a p<.0125, corresponding to a corrected p<.05). The anatomical ROIs adopted for these analyses, defined in standard Talairach space, are available at our UIC Center for Cognitive Medicine site: http://ccm.psych.uic.edu/Research/NormalBrain/ROI_rules.htm.
Results
Demographic and clinical data
Clinical and demographic data for the two groups are illustrated in Table 1. Separate ANOVAs for each demographic measure revealed that the two groups did not differ significantly for age, estimated IQ, and SES. Fisher’s p tests (two-tailed) also revealed no significant group differences for handedness, gender, and racial composition. Two separate 2×2 ANOVAs compared subjects’ scores for YMRS and CDRS at baseline and follow-up. As shown in Table 1, manic and depressive symptoms in PBD improved significantly with pharmacotherapy.
Table 1.
Demographic and clinical characteristics of patients with PBD and HC
| Variables | PBD (n=17)
|
HC (n=13)
|
Analyses
|
||
|---|---|---|---|---|---|
| Mean (SD) | N (%) | Mean (SD) | N (%) | F (p value) | |
| Age (years) | 14.29 (2.05) | 14.38 (3.57) | 0.008 (.93) | ||
| WASI-FSIQa | 97 (8.12) | 104 (10.10) | 3.98 (.06) | ||
| Socioeconomic statusb | 2.34 (.63) | 1.92 (1.12) | 1.63 (.21) | ||
| YMRS (group by time interaction) | 6.98 (.01) | ||||
| YMRS at baseline | 14.59 (7.00) | 1.08 (1.6) | 46.20 (.000001) | ||
| YMRS at follow-up | 6.47 (7.80)* | 0.77 (1.36) | 6.73 (.02) | ||
| CDRS (group by time interaction) | 20.48 (.0001) | ||||
| CDRS-R at baseline | 50.47 (12.95)* | 19.0 (1.29) | 75.60 (.000001) | ||
| CDRS-R at follow-up | 29.71 (15.41) | 18.8 (1.11) | 6.39 (.02) | ||
| Sex | (.45) | ||||
| Male | 5 (29%) | 6 (46%) | |||
| Female | 12 (71%) | 7 (54%) | |||
| Race | (.71) | ||||
| Caucasian | 12 (71%) | 8 (62%) | |||
| Other | 5 (29%) | 5 (38%) | |||
| Handedness | (1.00) | ||||
| Right-handed | 16 (94%) | 13 (100%) | |||
| Left-handed | 1 (6%) | 0 (0%) | |||
WASI IQ Wechsler Abbreviated Scale of Intelligence Intelligent Quotient, PBD pediatric bipolar disorder, HC healthy control, YMRS young mania rating scale, CDRS-R child depression rating scale-revised
p<. 000001, significant score difference at follow-up relative to baseline
Wechsler Abbreviated Scale of Intelligence Intelligent Quotient (Matrix Reasoning and Vocabulary Subtests)
Mean revised Hollingshead socioeconomic status
Behavioral performance results
Behavioral data from one PBD and two HC subjects were lost due to technical problems, and therefore, the behavioral analyses were conducted on 27 subjects. A repeated-measures 2×2×3 ANOVA with group (PBD and HC) as a between-subjects factor, and testing time (baseline, follow-up) and emotion condition (angry, happy, or neutral) as within-subjects factor, was carried out on mean RT and accuracy data. Results are summarized in Table 2. With regard to mean RT data, the main effect of group was not significant, but there was a main effect of testing time (F(1, 25)=5.03; p=.03) indicating that RT was faster at follow-up compared with baseline in both groups. A main effect of emotion condition (F(2, 50)=4.21; p=.02) revealed that, in both groups, RT for angry faces was slower than for happy faces (F(1, 25)=6.04; p=.02), but RT for angry or happy faces did not differ significantly from that for neutral faces (p=.12). For accuracy, a main effect of group (F(1, 25)= 10.23; p=.004) indicated that overall the PBD group had lower performance accuracy than HC, but this did not vary across facial emotion conditions. No other significant results were found.
Table 2.
Response time and accuracy measures for subjects with pediatric bipolar disorder (PBD) and healthy controls (HC) at baseline and follow-up
| PBD (n=17)
|
HC (n=13)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD)
|
% (SD)
|
Mean (SD)
|
% (SD)
|
|||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Response time (in ms) | ||||||||
| Angry face emotion | 1,083 (295) | 1,002 (232) | 916 (130) | 898 (207) | ||||
| Happy face emotion | 1,022 (254) | 889 (110) | 887 (140) | 806 (207) | ||||
| Neutral face emotion | 995 (230) | 945 (147) | 918 (115) | 880 (182) | ||||
| Total average | 1,033 (260) | 945 (163) | 907 (128) | 861 (199) | ||||
| Accuracya | ||||||||
| Angry face emotion | .90 (.08) | .88 (.12) | .96 (.04) | .97 (.06) | ||||
| Happy face emotion | .92 (.07) | .92 (.10) | .97 (.06) | .96 (.08) | ||||
| Neutral face emotion | .90 (.08) | .89 (.12) | .96 (.04) | .96 (.07) | ||||
| Total average | .91 (.08) | .90 (.11) | .95 (.05) | .96 (.07) | ||||
Significant group effect (p=.004) for accuracy. The PBD group exhibited lower accuracy relative to HC across face emotion conditions. There were no significant group differences for RT
fMRI results
The three-way interaction of group by testing time by emotion condition in the whole-brain, voxel-wise ANOVA, was significant ) F(1, 28)=5.57, p=0.025). Tables 3, 4, 5, and 6 illustrate clusters for which the three-way interaction was significant and the directionality of within- and between-group differences for the “angry face condition” (i.e., angry vs neutral faces) or “happy face condition” (i.e., happy vs neutral faces), at baseline and follow-up, as assessed by t tests.
Table 3.
Between-group comparisons for angry or happy face condition at baseline
| Group difference | Talairach coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values |
|---|---|---|---|---|---|
| Angry vs neutral | |||||
| HC>PBD | −46, 20, 8 | L inferior frontal gyrus | BA 45 | 1,134 | 4.57 |
| HC>PBD | −46, 33, 0 | L inferior frontal gyrus | BA 47 | 351 | 2.89 |
| HC>PBD | 15, 8, 11 | R dorsal caudate | 432 | 3.89 | |
| Happy vs neutral | |||||
| PBD>HC | 25, −1, −13 | R amygdala | 351 | 2.53 | |
| PBD>HC | 53, 35, 9 | R inferior frontal gyrus | BA 45/46 | 486 | 3.84 |
| PBD>HC | −34, 23, −7 | L inferior frontal gyrus | BA 47 | 1,539 | 2.85 |
| PBD>HC | 59, 8, 20 | R middle frontal gyrus | BA 44, 9 | 1,458 | 3.46 |
| PBD>HC | 41, 47, 11 | R middle frontal gyrus | BA 10, 46 | 1,512 | 3.89 |
| PBD>HC | −13, 59, 20 | L medial frontal gyrus | BA 10 | 837 | 4.82 |
| PBD>HC | 53, −58, −7 | R middle temporal gyrus | BA 22 | 513 | 3.16 |
Talairach coordinates and t values for clusters for which there is a significant three-way interaction (corrected p<0.020 with cluster contiguity threshold) in the whole-brain ANOVA for the angry or happy face condition. The table also lists the significant group differences obtained from post hoc decomposition of the significant interaction
HC healthy control group, PBD pediatric bipolar disorder group, BA Brodmann’s area, L left, R right
Table 4.
Between-group comparisons for angry or happy face condition at follow-up
| Group difference | Talairach coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values |
|---|---|---|---|---|---|
| Angry vs neutral | |||||
| PBD>HC | 25, −9, −9 | R amygdala | 351 | 2.73 | |
| HC>PBD | 56, −47, 12 | R superior temporal gyrus | BA 22 | 540 | 3.39 |
| HC>PBD | 38, −74, 42 | R precuneus | BA 7 | 756 | 3.46 |
| Happy vs neutral | |||||
| PBD>HC | −24, −9, −11 | L amygdala | 351 | 2.42 | |
| PBD>HC | 53, −11, −13 | R middle temporal gyrus | BA 22 | 351 | 3.8 |
Talairach coordinates and t values for clusters for which there is a significant three-way interaction (corrected p<0.020 with cluster contiguity threshold) in the whole-brain ANOVA for the angry or happy face condition. The table also lists the significant group differences obtained from post hoc decomposition of the significant interaction. Abbreviations as for Table 3
Table 5.
Within-group comparisons for angry or happy face condition at follow-up vs baseline
| Talairach coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values | |
|---|---|---|---|---|---|
| Angry vs neutral | |||||
| PBD | |||||
| Follow-up>baseline | 35, 29,−4 | R inferior frontal gyrus | BA 47 | 351 | 2.29 |
| Baseline>follow-up | 29, −5, −22 | R amygdala | 351 | 2.39 | |
| Baseline>follow-up | −52, −41, 49 | L inferior parietal lobule | BA 40 | 405 | 3.76 |
| HC | |||||
| Baseline>follow-up | 24, 29, −8 | R VMPFC | BA 47,11 | 378 | 3.91 |
| Baseline>follow-up | −0, 42, 15 | L dorsal ACC | BA 32 | 351 | 3.50 |
| Baseline>follow-up | −10, 10, 10 | L dorsal caudate | 729 | 4.85 | |
| Baseline>follow-up | −22, 16, −3 | L putamen | 1,485 | 4.18 | |
| Baseline>follow-up | −49, −67, 7 | L middle temporal gyrus | BA 21 | 783 | 3.77 |
| Happy vs neutral | |||||
| PBD | |||||
| Baseline>follow-up | 25, −1, −12 | R amygdala | 351 | 3.05 | |
| Baseline>follow-up | 2, 2, 20 | R dorsal ACC | BA 24 | 513 | 3.70 |
| HC | |||||
| Follow-up>baseline | None | None | None | None | None |
| Baseline>follow-up | None | None | None | None | None |
Talairach coordinates and t values for clusters for which there is a significant three-way interaction (corrected p<0.020 with cluster contiguity threshold) in the whole-brain ANOVA. The table also lists the significant within-group difference for the comparison of follow-up and baseline activation, obtained from post hoc decomposition of the significant interaction
ACC anterior cingulate cortex; VMPFC ventromedial prefrontal cortex (abbreviations are as for Table 3)
Table 6.
Between-group comparisons for angry or happy face condition at follow-up vs baseline
| Talairach coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values | |
|---|---|---|---|---|---|
| Angry vs neutral | |||||
| PBD>HC | 35, 29, −4 | R VLPFC | BA 47 | 459 | 3.70 |
| PBD>HC | −2, 18, −6 | L subgenual ACC | BA 24 | 432 | 2.97 |
| PBD>HC | 23, −11, −8 | R amygdala | 351 | 2.73 | |
| PBD>HC | −10, 11, 11 | L dorsal caudate | BA 24 | 459 | 3.39 |
| HC>PBD | −10, −52, 62 | L precuneus | BA 7 | 405 | 3.42 |
| Happy vs neutral | |||||
| PBD>HC | 53, −10, −13 | R middle temporal gyrus | BA 21 | 567 | 3.08 |
Group differences for the angry or happy face condition at baseline vs follow-up. Talairach coordinates and t values for clusters for which there is a significant three-way interaction (corrected p<0.020 with cluster contiguity threshold) in the whole-brain ANOVA. The table also lists the significant group differences obtained from post hoc decomposition of the significant interaction
ACC anterior cingulate cortex (abbreviations are as for Table 3)
Group differences for the angry or happy face condition at baseline
Angry face condition at baseline
Relative to HC, the PBD group exhibited no areas of increased activation and reduced activation in the left VLPFC (BA 45, 47; Fig. 3a) and right dorsal caudate (Table 3).
Fig. 3.
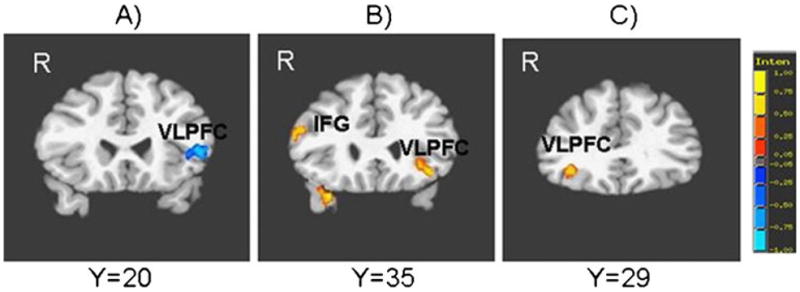
Group differences in brain activation during the affective n-back task for: a angry face condition at baseline. b Happy face condition at baseline. c Angry face condition at follow-up vs baseline. Red indicates greater activation in the pediatric bipolar disorder group (PBD) relative to healthy controls (HC). Blue indicates greater activation in HC relative to PBD. IFG inferior frontal gyrus, VLPFC ventrolateral prefrontal cortex, R right
Happy face condition at baseline
Relative to HC, PBD exhibited no areas of reduced activation, and greater activation in the right amygdala, bilateral VLPFC (BA 45 and 47) (Fig. 3b), right DLPFC (BA 44 and 9; BA 10/46) and middle temporal gyrus, and left medial frontal gyrus.
Group differences for the angry or happy face conditions at follow-up
Angry face condition at follow-up
Relative to HC, the PBD group showed increased activation in right amygdala and decreased activation in right precuneus and superior temporal gyrus (Table 4).
Happy face condition at follow-up
The PBD group showed greater activation than HC in left amygdala and middle temporal gyrus, and no areas with reduced activity relative to HC.
Within-group differences for the angry or happy face condition at follow-up vs baseline
Angry face condition at follow-up vs baseline
The PBD group, at follow-up relative to baseline, exhibited increased activation in the right VLPFC (BA 47) and reduced activation in right amygdala and inferior parietal lobule. At follow-up, relative to baseline, HC exhibited reduced activation in the right VMPFC, left dorsal ACC, dorsal caudate, putamen and middle temporal gyrus and no areas with increased activation (Table 5).
Happy face condition at follow-up vs baseline
For follow-up vs baseline, the PBD group exhibited no increases in activation and reduced activation in right amygdala and right dorsal ACC. The HC group exhibited no significant differences.
Group differences for angry or happy face condition at follow-up vs baseline
Angry face condition
For the angry face condition at follow-up vs baseline, the PBD group exhibited greater activation than HC in the right VLPFC (BA 47) (Fig. 3c), left subgenual ACC, left dorsal caudate and right amygdala, and reduced activation in the left precuneus (Table 6).
Happy face condition
For the happy face condition at follow-up vs baseline, the PBD group exhibited greater activation than HC in right middle temporal gyrus, and reduced activation in no region.
Neural correlates of symptom improvement
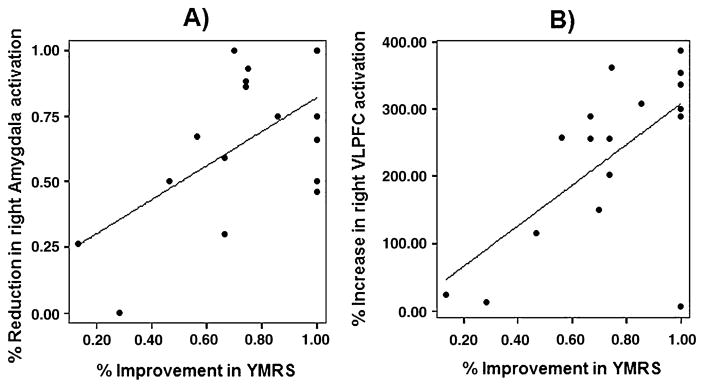
Based on our finding of improved right amygdala and VLPFC function from baseline to follow-up with treatment in PBD, which was important for our hypotheses (see Table 5), for the angry face condition we carried out Pearson’s correlation analyses between YMRS and CDRS scores and right amygdala and VLPFC activation. We report our results with Bonferroni corrections for multiple comparisons (n=4; i.e., using a p<.0125, corresponding to a corrected p<.05). With regard to YMRS, for the angry face condition there was a significant positive correlation between percent improvement in YMRS scores and percent of reduction in right amygdala activation from baseline to follow-up (r=.56, corrected p<.05) (Fig. 4a) There was also a significant positive correlation between percent improvement in YMRS scores and percent of increased activation in right VLPFC from baseline to follow-up (r=.64, corrected p<.05) (Fig. 4b). With regard to CDRS, after corrections for multiple comparisons no significant results were found.
Fig. 4.
Significant correlation, in the PBD group for the angry face condition, between percent improvement with treatment in YMRS scores and: a Percent reduction in right amygdala activity (r=.56; corrected p<.05); b percent increase in right VLPFC activity (r=.64; corrected p<.05). VLPFC ventrolateral prefrontal cortex
Discussion
The present study suggests that lamotrigine treatment may improve mood regulation and alter the impact of affective arousal on prefrontal regions and amygdala in PBD during an affective working memory task. The central findings include normalization of prefrontal function in VLPFC in PBD after pharmacotherapy, which correlated significantly with improvement in manic symptoms. With treatment, activation in right amygdala decreased in PBD, but the right amygdala was still overreactive in patients relative to HC. Amygdala dysfunction, as well as prefrontal dysfunction in VLPFC, are a prominent feature of the hypo/manic state (Foland et al. 2008; Pavuluri et al. 2009a, 2010a, b), and it has been suggested that reduced functional connectivity between VLPFC and amygdala in adult patients with BD relative to HC during emotion processing tasks may contribute to the persistent fronto-limbic dysfunction seen in this disorder (Foland et al. 2008).
With regard to behavioral performance, patients exhibited lower task accuracy relative to HC at baseline and follow-up, but there were no significant effects of treatment on group differences or within the patient group, which is possibly due to the small samples and the small number of trials in each condition in order to keep scanning time short.
Fronto-limbic dysfunction in PBD relative to HC at baseline
In line with our predictions, for the angry face condition at baseline, the PBD group, relative to HC, exhibited reduced activation in left VLPFC regions (i.e., BA 45 and 47) which are key to both regulation of behavior (Konishi et al. 1999; Aaron et al. 2003; Pavuluri et al. 2009c, 2010a, b; Passarotti et al. 2010b) and regulation of affect (Botvinick et al. 2001; Pavuluri et al. 2008; Passarotti et al. 2010a, c). Moreover, directly related to the current working memory paradigm, specific regions within the left VLPFC have been implicated in cognitive control of memory process (Petrides 1994; Badre and Wagner 2007), with the mid-VLPFC (BA 45) being involved in selection and the anterior VLPFC (BA 47) controlling access to stored memory representation. Our previous studies found VLPFC to be consistently underactive in PBD during processing of negative emotions (Pavuluri et al. 2008, 2009a, b, c; Passarotti et al. 2010b, c), and this region has been suggested as a potential neural phenotype for affect regulation deficits in mania (Frangou et al. 2005). We also found that PBD relative to HC exhibited decreased activation in the right dorsal caudate. The dorsal caudate is part of a fronto-striatal system (Alexander et al. 1986) subserving DLPFC-dependent working memory processes (Levy et al. 2005), for instance by aiding prefrontal cortex with strategic manipulation of information during working memory tasks (Lewis et al. 2004; Owen et al. 2005). For the happy face condition, at baseline the PBD group, relative to HC, exhibited increased activation in the VLPFC, DLPFC, VMPFC, and temporal regions. Moreover, we found increased amygdala activation in PBD relative to HC, in line with previous findings of persistent amygdala hyperactivity in bipolar disorder (Pavuluri et al. 2008, 2009a, b, c; Gruber et al. 2009). Our baseline findings with negative and positive valence stimuli are in line with our previous results with PBD using an affective color matching task (Pavuluri et al. 2008; Passarotti et al. 2010a). These findings are consistent across several of our studies (Pavuluri et al. 2008, 2011; Passarotti et al. 2010a, c), where PBD relative to HC exhibits greater impact of negative emotions illustrated with VLPFC under-activity, while positive emotions, which are not as challenging, lead to increased VLPFC activation in PBD relative to HC. Our repeated findings across studies and patient samples suggest that VLPFC under-activity with negative emotion is a consistent finding in PBD pathology and may be a potential bio-marker of the manic state of illness.
Normalization of prefrontal activity with lamotrigine treatment in PBD
At retest after 14-week pharmacotherapy, the PBD group illustrated ‘prefrontal normalization’ in that patients did not differ from HC anymore in terms of VLPFC, VMPFC or DLPFC activation for both the angry and happy face conditions. In fact, within the PBD group, for the angry face condition at follow-up relative to baseline, increased activation in prefrontal regulatory regions was noted, similar to other studies (Pavuluri et al. 2010a, b, c). Importantly, in line with previous findings on lamotrigine studies in PBD during an affective color matching task (Pavuluri et al. 2010c), there was a significant correlation between changes in activation in right VLPFC and improvement in YMRS score with treatment, suggesting a direct relationship between functional recovery in this emotional control region and manic symptom improvement. While more evidence is needed in this regard, our preliminary findings of a reversal in prefrontal dysfunction after pharmacotherapy suggest that prefrontal dysfunction in PBD may be related to mood state and may improve significantly alongside symptom remission in euthymic patients.
Moreover, supporting the growing evidence that neural dysfunction for emotion processing in BD differs from that of other mood disorders (Lawrence et al. 2004), in line with our previous studies (Pavuluri et al. 2008; Passarotti et al. 2010a, c), we obtained here more robust changes in brain activation with treatment for the negative valence relative to the positive valence emotion condition. Only for the negative valence condition we also find significant correlations between improvements in YMRS scores and improved function in the VLPFC and amygdala. This finding suggests that that the dysfunctional interface of prefrontal and limbic regions in PBD is dynamic in nature and modulated by the emotional valence of events in the environment. Decreased deployment of higher cortical regions in PBD relative to HC with negative emotion stimuli (e.g., angry faces) is understood to be due to greater demand on the already dysfunctional regulatory cortical systems as compared to positive emotion stimuli and this has been found both in children (Passarotti et al. 2010a, c; Pavuluri et al. 2007) and adults (Lawrence et al. 2004) with BD. Therefore, using stimuli with negative valence may better help identify markers of manic state in PBD. Furthermore, our findings may be interpreted as preliminary evidence showing specifically that lamotrigine treatment alters the influence of affective arousal on working memory systems, especially for negative emotion stimuli, in PBD. In future studies, neural response to negative emotions may be further explored and used as outcome measure, providing useful information, for example on the path to recovery during cognitive enhancement or psychosocial intervention.
Amygdala hyperactivity after lamotrigine treatment in PBD relative to HC
Our findings showed that lamotrigine treatment showed systems-level changes in fronto-striatal and amygdala function. When examining limbic activity within the PBD group and in PBD relative to HC at baseline and after treatment, our main finding was that of decreased amygdala activity within patients after treatment, while there was still greater amygdala activity in patients relative to HC after treatment. More specifically, our study found a significant correlation between improvement in YMRS scores and a decrease in right amygdala activity with treatment for negative valence stimuli in our manic/mixed and hypomanic patients, suggesting direct relationship between severity of manic symptoms and amygdalar dysfunction. Chang et al. (2008) found a correlation between improvement in CDRS score for depression and decrease in amygdala activity in depressed children with PBD. These findings, while preliminary, suggest regulatory effects of lamotrigine treatment on amygdala function that relate to improvement in manic or depressive symptoms in PBD patients, depending on their relative mood state.
However, it is important that future studies better parse out and differentiate between amygdala dysfunction in depressed or manic bipolar patients, and between “state” vs “trait” aspects of PBD dysfunction. Furthermore, it will be crucial to gain a better understanding of how pharmacotherapy may affect the illness and shape euthymic state. A recent study by Versace et al. (2010) in adult BD suggests that patterns of functional connectivity between amygdala and ventral prefrontal regions during emotion processing differ for state vs trait in bipolar depression, but we still do not know whether children with BD would show the same patterns.
In the present study, activation in right amygdala decreased significantly with treatment in PBD for both task conditions, indicating that the adopted lamotrigine treatment alongside improving prefrontal function also aided in regulating amygdala activation in response to both negative and positive emotions. Nevertheless, despite the normalization of VLPFC function, amygdala activation remained greater in PBD relative to HC for both face emotion conditions after 14 weeks of pharmacotherapy, even though the majority of patients were in euthymic state. Our present findings are in line with previous studies (Gruber et al. 2009; Pavuluri et al. 2009a, b, c) that suggest that amygdala over-reactivity may be a trait in bipolar diathesis, which persists even in stable patients despite positive clinical outcome. Alternatively, it is possible that lamotrigine treatment and/or the present treatment duration may only partially resolve amygdala dysfunction. Future studies with larger samples and targeted tasks that challenge affective circuitry will need to better investigate and confirm whether amygdala dysfunction persists as a trait of PBD in euthymic patients with longer treatment duration or with different medications.
Impact of lamotrigine treatment on brain function from baseline to follow-up in PBD relative to HC
When comparing the two groups for changes in brain activation over time, for the angry face condition, PBD relative to HC showed greater changes over time in right amygdala, right VLPFC, left subgenual ACC and left caudate. The VLPFC along with VMPFC is involved in emotional control (Aaron et al. 2003; Konishi et al. 1999; Pavuluri et al. 2008; Rubia et al. 2010) and evaluation of emotions (Botvinick et al. 2001; Pavuluri et al. 2009a, b, c; Passarotti et al. 2009) and has been found to be functionally (Drevets et al. 2008; Pavuluri et al. 2008, 2009a, b, c; Passarotti et al. 2010a, b; Frangou et al. 2005; Leibenluft et al. 2007) and structurally (Bora et al. 2010; Adler et al. 2004; Pavuluri et al. 2009a, b, c) abnormal in bipolar pathology. The subgenual ACC is involved in automatic regulation of emotional behavior and there is evidence that it exhibits reduced BOLD signal (Drevets et al. 2008) as well as gray matter reduction (Bora et al. 2010) in BD. Moreover, reduced functional connectivity between VLPFC (Foland et al. 2008) or perigenual ACC (Wang et al. 2009) and amygdala in BD during emotion processing suggest functional abnormalities in this fronto-limbic network. In conclusion, our present fMRI findings, while preliminary, suggest that lamotrigine treatment improves significantly not only clinical symptoms in PBD but also neural dysfunction in regions that are crucial for emotion regulation during a working memory task with emotional challenge.
The present block design study is based on a relatively small sample of patients and HC, and has some limitations that suggest caution with regard to our data interpretation. Firstly, while a block design offers greater statistical power and BOLD signal stability relative to an event-related design, which is advantageous with clinical population studies, it cannot differentiate between brain activation for correct and incorrect trials. We think it is likely that our results reflect mainly brain activation for correct trials, given that both groups had high accuracy rates. However, it is beyond the scope of this block design fMRI study to address what neural alterations result from correct or incorrect responses, which would require an event-related design. Moreover, blocked presentations of angry and happy faces may favor habituation in amygdala response to emotions or prefrontal regions involved in emotion evaluation, while an event-related design may have greater ecological validity when studying brain response to emotional stimuli in patients with affect dysregulation. Secondly, while our main goal was to examine neural function at the interface of affect and working memory, we were unable to examine pharmacotherapy effects on working memory function in the absence of potentially emotional information. In fact, even though in each group we did find significant differences in brain activation between emotional and neutral faces, we did not necessarily have a truly non-emotional control condition for our PBD patients, given the evidence that even neutral faces, because of their potential social saliency, might be perceived as ‘threatening’ by children with PBD (Rich et al. 2006). Future studies may need to include a non-face condition to assess baseline working memory function independent of emotion processing. Third, our study could not address the question of whether the beneficial effects of lamotrigine on working memory circuits are secondary to mood stabilization, or whether lamotrigine directly improves functioning in working memory regions. Preliminary evidence suggests that while lamotrigine has a general inhibitory effect on cortical neuronal excitability in motor regions, it also presents a more complex effect on frontal and subcortical regions (Li et al. 2004; Tergau et al. 2003), which are functionally compromised in bipolar disorder (Pavuluri et al. 2010a). Nevertheless, this question has not been addressed in child or adult patients with BD. Therefore future studies will need to further clarify lamotrigine effects on cognition as well as the interface of cognition and affect in PBD and other mood disorders.
Finally, our preliminary findings with 14 weeks of SGAs and lamotrigine monotherapy provide some preliminary evidence that in PBD prefrontal dysfunction may be state-specific, whereas amygdala over-reactivity may be a trait marker. Nevertheless, it is possible that longer or different pharmacotherapy treatments may yield different results. Future studies will need to disentangle state-versus trait-related dysfunction in PBD, possible differences in treatment response between BD Type I or II, the impact of comorbidities such as anxiety disorders, CD or ODD, as well as the effects of duration and type of pharmacological treatment, towards more effective intervention based on mood dysregulation profiles.
Acknowledgments
We wish to thank the children and families for their participation and for making this study possible. This work is supported by NIH K23 RR18638-01, the Dana Foundation, and NARSAD. The present study complies with the current laws of the USA.
Footnotes
Conflicts of interest Dr. Passarotti has no financial relationships to disclose. Dr. Pavuluri’s work, unrelated to this manuscript, is supported by NARSAD Independent Investigator Award, NIMH, NICHD, DANA foundation, and American Foundation for Suicide Prevention. Dr. Sweeney, also unrelated to this work, has received support from NIH, Janssen and Eli Lilly.
Contributor Information
Alessandra M. Passarotti, Email: apassarotti@psych.uic.edu, Pediatric Brain Research and Intervention Center, Institute for Juvenile Research, University of Illinois Medical Center at Chicago, 1747 West Roosevelt Road, Chicago, IL 60608, USA. Center for Cognitive Medicine, University of Illinois Medical Center at Chicago, Chicago, IL, USA
John A. Sweeney, Center for Cognitive Medicine, University of Illinois Medical Center at Chicago, Chicago, IL, USA
Mani N. Pavuluri, Pediatric Brain Research and Intervention Center, Institute for Juvenile Research, University of Illinois Medical Center at Chicago, 1747 West Roosevelt Road, Chicago, IL 60608, USA. Center for Cognitive Medicine, University of Illinois Medical Center at Chicago, Chicago, IL, USA
References
- Aaron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Adler C, Holland S, Schmithorse V, Wilke M, Weiss K, Pan H, Strakowski S. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–338. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders IV. 4. American Psychiatric Association; Washington: 1994. [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Mitchner L. What is a “mood stabilizer”? An evidence-based response. Am J Psychiatry. 2004;161(1):3–18. doi: 10.1176/appi.ajp.161.1.3. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Spencer T, Wilens TE, Wozniak J. Pediatric mania: a developmental subtype of bipolar disorder? Biol Psychiatry. 2000;48:458–466. doi: 10.1016/s0006-3223(00)00911-2. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie C, et al. A functional magnetic resonance imaging study of bipolar disorder: state and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:599–607. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67(11):1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108 (3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsfoird JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chang KD, Wagner C, Garrett A, Howe M, Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Cox RW, AFNI Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, Leibenluft E. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord. 2007;9 (7):679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in computational statistics. Physica-Verlag; Heidelberg: 1996. pp. 39–49. [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficitent modulation of amygdale response by prefrontal cortex in bipolar mania. Psychiatry Res Neuroimaging. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol Psychiatry. 2005;58(10):838–839. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194:4–9. doi: 10.1192/bjp.bp.107.048504. [DOI] [PubMed] [Google Scholar]
- Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51(2):93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Bowden CL, Calabrese JR, Grunze H, Kasper S, White R, et al. A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiatry. 2004;65:432–441. doi: 10.4088/jcp.v65n0321. [DOI] [PubMed] [Google Scholar]
- Gruber O, Tost H, Henseler I, Schmael C, Scherk H, Ende G, Ruf M, Falkai P, Rietschel M. Pathological amygdala activation during working memory performance: evidence for a pathophysiological trait marker in bipolar affective disorder. Hum Brain Mapp. 2009;31(1):115–125. doi: 10.1002/hbm.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Meth. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Hart SJ, Green SR, Casp M, Belger A. Emotional priming during Stroop task performance. Neuroimage. 2010;49:2662–2670. doi: 10.1016/j.neuroimage.2009.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips M. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci. 1997;17(10):3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Li X, Teneback CC, Nahas Z, Kozel FA, Large C, Cohn J, et al. Interleaved transcranial magnetic stimulation/functional MRI confirms that lamotrigine inhibits cortical excitability in healthy young men. Neuropsychopharmacology. 2004;29:1395–1407. doi: 10.1038/sj.npp.1300452. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional Neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Developmental differences between adolescents and adults during directed and incidental processing of emotional facial expressions. Social Cognitive and Affective Neuroscience. 2009;4:387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2010a;16(1):106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition deficits in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res Neuro-imaging. 2010b;181(1):36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010c;9(10):1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O’ Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162 (3):244–245. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M, Passarotti A, Harral E, Sweeney J. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009a;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M, West A, Hill S, Jindal K, Sweeney J. Neuro-cognitive function in pediatric bipolar disorder: 3-year follow-ups show cognitive development lagging behind health youth. J Am Acad Child Adolesc Psychiatry. 2009b;48:235–236. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Henry DB, Moss M, Mohammed T, Carbray JA, Sweeney JA. Effectiveness of lamotrigine in maintaining symptom control in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2009c;19(1):75–82. doi: 10.1089/cap.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry. 2010a;71:1–9. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Mohammed T, Carbray J, Sweeney JA. Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disord. 2010b;12(2):213–220. doi: 10.1111/j.1399-5618.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Parnes SA, Fitzgerald JM, Sweeney JA. A Pharmacological fMRI study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010c;20(5):395–406. doi: 10.1089/cap.2009.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Curr Opin Neurobiol. 1994;4 (2):207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Philips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Grossman J, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Adolesc Psychiatry. 1984;23(2):191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Brace & Company; San Antonio: 1999. [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyper-activation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA. 2006;103 (23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an FMRI study. Biol Psychiatry. 2006;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Sage; Newbury Park: 1991. [Google Scholar]
- Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer M. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of working memory. PNAS. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergau F, Wischer S, Somal HS, Nitsche MA, Joe Mercer A, Paulus W, et al. Relationship between lamotrigine oral dose, serum level and its inhibitory effect on CNS: insights from transcranial magnetic stimulation. Epilepsy Res. 2003;56:67–77. doi: 10.1016/j.eplepsyres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Van der Schot A, Kahn R, Ramsey N, Nolen W, Vink M. Trait and state dependent functional impairments in bipolar disorder. Psychiatry Res Neuroimaging. 2010;184:135–142. doi: 10.1016/j.pscychresns.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JRC, Hassel S, Klein CR, Kupfer DJ, Phillips ML. Abnormal left and right amygdale-orbitofrintal cortical functional connectivity to emotional faces: state vs trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Sihra TS, Gean PW. Lamotrigine inhibition of glutamate release from isolated cerebrocortical nerve terminals (synaptosomes) by suppression of voltage-activated calcium channel activity. NeuroReport. 2001;12:2255–2258. doi: 10.1097/00001756-200107200-00042. [DOI] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66(5):516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. ALPHASIM. Natl. Inst. Of Health; Bethesda: 2000. Available at: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. [Google Scholar]
- White HS. Comparative anticonvulsant and mechanistic profile of the established and newer antiepileptic drugs. Epilepsia. 1999;40 (Suppl 5):S2–S10. doi: 10.1111/j.1528-1157.1999.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Young LT. What exactly is a mood stabilizer? J Psychiatry Neurosci. 2004;29(2):87–88. [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]