Abstract
Introduction
Recent studies demonstrated the importance of ADAM9 in prostate cancer relapse upon therapy. In this study, we determined the role of ADAM9 in the therapeutic resistance to radiation and chemotherapy.
Material and Methods
ADAM9 was either transiently or stably knocked down in C4-2 prostate cancer cells. The sensitivity of ADAM9 knockdown cells toward radiation and chemotherapeutic agents were determined. Additionally, the effects of ADAM9 knockdown on prostate cancer cell morphology, biochemical and functional alterations were accessed.
Results
Both transient and stable knockdown of ADAM9 resulted in increased apoptosis and increased sensitivity to radiation. ADAM9 knockdown also increased prostate cancer sensitivity to several chemotherapeutic drugs. ADAM9 knockdown resulted in increased E-cadherin and altered integrin expression and underwent phenotypic epithelial transition. These were reflected by the morphological, biochemical and functional alterations in the ADAM9 knockdown cells.
Conclusions
ADAM9 plays a crucial role in prostate cancer progression and therapeutic resistance in part by altering E-cadherin and integrin expression. ADAM9 is an important target for the consideration of treating prostate cancer patients who developed therapeutic resistance and disease relapse.
Keywords: ADAM9, radiation, prostate cancer, E-cadherin
Introduction
The A Disintegrin and Metalloprotease (ADAM) family is a group of transmembrane and secreted proteins. These proteins have been shown to regulate cell phenotype and behavior (1). ADAM9 has been shown to be important in prostate cancer development (2–4). ADAM9 mRNA and protein have been shown to be upregulated in prostate cancer specimens and is significantly associated with shortened prostate specific antigen (PSA) relapse free survival in patients who received androgen deprivation therapy (4). ADAM9 overexpression has also been correlated with poor survival in breast (5), colon (6), lung (7) and pancreatic cancers (8).
ADAM proteins modulate cell phenotype by their effects on cell adhesion, migration, proteolysis and signaling (1). ADAMs are zinc metalloproteases, which have diverse functional roles. They are involved in ectodomian shedding of growth factors, adhesion molecules, cytokines and receptors and thus affect cell signaling. ADAM9 is shown to have proteolytic activity and its substrates include pro-HB-EGF (9), kit ligand (10), IGFBP-1 (11), ADAM10 (12), collagen XVII (13), laminin (6), pro-EGF, FGF receptor 2 iiib (3). ADAM9 has been shown to be expressed in mesenchymal stem cells, placenta, pancreas, adult stem cells and adipose tissue (1). The expression of ADAM9 has been previously shown to be upregulated in cancer cells by reactive oxygen species, androgens, hypoxia and overcrowding in prostate cancer cells (2,14). Therefore they may be important players in therapeutic resistance. Since ADAM9 has been recently thought to be a cancer stem cell marker, it is important to understand its mechanism of action. In this study we inhibited ADAM9 in aggressive prostate cancer cells and determined its role in therapeutic resistance. Previous studies demonstrate that ADAM10 can induce epithelial to mesenchymal transition partly by its proteolytic activity on E-cadherin. In this study we demonstrate that like ADAM10, ADAM9 knockdown results in increased E-cadherin and integrins and modulates epithelial phenotype and functional characteristics of prostate cancer cells with increased sensitivity to therapeutic agents.
Material and Methods
Cell culture
C4-2 androgen independent metastatic prostate cancer cells were cultured in T-medium (GibcoBRL, Grand Island, NY) supplemented with 5 % heat inactivated fetal bovine serum (FBS) (Bio-Whittaker, Walkersville, MD), 50 IU/ml penicillin and 50 µg/ml streptomycin (GibcoBRL) and maintained in 5 % CO2 at 37°C.
3-D studies
Two million C4-2 cells were suspended in rotary wall vessels (USA Synthecon Inc., TX) with 1 mm piece of gelfoam® (Pfizer, NY). Cells were maintained in complete medium and 48 h later, cell organoids were isolated and radiated. Cells were separated by pipetting. Colony survival assay was performed 4 h after radiation treatment.
Cell viability assays: Clongenic assay
Cells were radiated and plated in low densities in 6-well plates and incubated until they formed colonies having at least more than 50 cells. Seventeen days later colonies were counted. The colonies were rinsed with PBS and stained with methanol/crystal violet dye. The surviving fraction was calculated as a ratio of the number of colonies formed divided by the total number of cells plated, times the plating efficiency. The surviving fraction was further plotted in log scale. Cell viability assay: The cell viability was determined with sulforhodamine B assay (SRB assay, Sigma) or with CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI); these two assays yield comparable results. Cells were plated in 96-well plates. After 24 h of incubation, media were replaced by serum-free media. Control and ADAM9 knockdown cells were treated with various chemotherapeutic drugs, for 48 h and absorbance was measured.
Flow cytometric analysis using annexin V and integrin staining
After ADAM9 siRNA transfection and radiation treatment, cells were collected by trypsinization and fixed with 70 % ethanol. The fixed cells were incubated with 100 µg/ml RNase A (Sigma, St. Louis, MO) for 30 min. and stained with 25 µg/ml propidium iodide (Chemicon, Temecula, CA) for 30 min. Cell cycle was analyzed with a FACScan flow cytometer and CellQuest software (Becton Dickinson Labware, Lincoln Park, NJ). Stable ADAM9 kd and control cells were detached with 7mM EDTA solution at pH 7.4 and washed with PBS and stained for 20 min. with the following integrin antibodies (PE anti-CD29, PE anti-integrin β5, FITC anti-CD51, FITC anti-CD49a and FITC anti-CD49b, BioLegend, CA). Cells were washed and measured by flow cytometric analysis.
Live/Death analysis
Cells were transfected with ADAM9 siRNA, and 4 h later radiated. Twenty four hours after radiation, live and dead cells were detected using Live/Death Viability/Cytotoxicity assay kit (Molecular Probes, Eugene, OR) where fluorescence was observed and pictures were taken at 4X magnifications.
Immunoblot analysis
Protein was extracted from cell pellets and western analysis performed as previously described (15). The membranes were incubated with mouse monoclonal antibody against anti-ADAM9 antibody (R&D Systems), E-cadherin (1:1000 dilution; BD Biosciences), anti-β-actin antibody (1:5000 dilution; Sigma) respectively, at 4 °C overnight. Immunoreactive bands were visualized with ECL (Amersham Pharmacia Biotech, Little Chalfont, UK). All westerns were run on 4%–12% Bis-Tris gels (Invitrogen), with the exception of ADAM9 protein which was detected on native Tris-Glycine gels (Invitrogen) where samples were not denatured at 100°C for 4 min.
Radiation studies
External beam radiation treatment was delivered on a 600 Varian linear accelerator with a 6MV photon beam. 1) ADAM9 siRNA transient transfection: Cells were irradiated 4 h after siRNA transfection, and subjected to Flow Cytometry or Live/Death assays 24 hrs later. 2) Time-course studies: C4-2 cells were plated and 24 hrs later irradiated. Cells were exposed to 2 Gy of radiation and ADAM9 protein expression was measured at 24, 48, and 72 hrs after radiation exposure. 3) Dose-response studies: Cells were plated and 24 hrs later irradiated with doses of 2 Gy, 6 Gy, and 10 Gy. At 72 hrs, cells were harvested, protein concentrations were determined, normalized, and western blots were performed in the manner as described above. 4) Repeated dose-exposure studies: 2 Gy were given consecutively for 3 days. 5) ADAM9 stable knockdown cells and control C4-2 cells were plated for 24 hrs, radiated with 6 Gy and 24 hrs later, and the cells were lysed and assayed for ADAM9 protein by western blot analysis.
Reactive oxygen species studies
Cells were treated with radiation, and after appropriate amounts of time, were trypsinized. Cells were centrifuged and suspended in 5% FBS in HBSS. Hydrogen peroxide was detected using dichlorofluorescin diacetate (DCF, 2 µM) (Molecular Probes, Eugene, OR). Superoxide was detected using dihydroethidium (DHE, 10 µM) (Molecular Probes, Eugene, OR). Samples were incubated for 40 min at room temperature in the dark on a rotator. Analysis of DCF and DHE fluorescence was performed on a FACS Calibur from Becton Dickinson according to manufacture’s instruction.
ADAM9 siRNA knockdown studies
To efficiently knockdown ADAM9 expression, dicer reaction of 500 bp ADAM9 PCR amplicons were used to create multiple small fragments of RNA. PCR was first performed with primers, ADAM9-445(+): 5’-ATTGAACCCCTGCAGAACAG-3’ and ADAM9-965(−): 5’-CACACTGTTCCCACAAATGC-3’, to generate 521 bp PCR amplicon for dicer reaction. To perform dicer reaction, BLOCK-IT Dicer RNA Kit (Invitrogen) was used to create RNAi from ADAM9 amplicons and manufactures recommendation were followed. In brief, T7 linker was added to PCR amplicon with TA linking reaction. T7 primer with ADAM9-965(−) or T7 primer with ADAM9-445(+) were paired to create PCR amplicons with T7 linker at 5` or 3` end as sense or antisense template for RNA transcription. The RNA transcription reaction was performed to generate sense and antisense single stranded RNA using T7 primer and reverse transcription. The sense and antisense RNA transcripts were annealed to produce dsRNA after purification. Dicing reaction was than performed to create 21 to 23 nucleotide RNA fragments and purified. Once the level of ADAM9 gene expression was determined, 20 pM of siRNA was used to transfect these cells using a liposome vehicle. The level of gene knockdown was confirmed with western blot 48 hrs after transfection. C4-2 with or without transfection with negative siRNA (Ambion, Austin, TX) were used as control. ADAM9 shRNA (Open Biosystems, Huntsville, AL) and Control shRNA were retrovirally transduced into C4-2 cells and stable clone was generated.
Invasion and migration assays
Cancer cell invasion was assayed using Companion 24-well plates (Becton Dickinson Labware) with 8 µm porosity polycarbonate filter membrane and migration assay was performed using QCM™ 24-well colorimetric cell migration assay (Chemicon International). The assays were performed as per instructions, with cell numbers counted for quantification of percent of cell invasion and migration.
Statistical analysis
Analysis was performed using the Student's t-test. Values of p<0.05 were considered to be statistically significant.
Results
ADAM9 knockdown increases apoptosis and sensitizes prostate cancer cells to radiation treatment
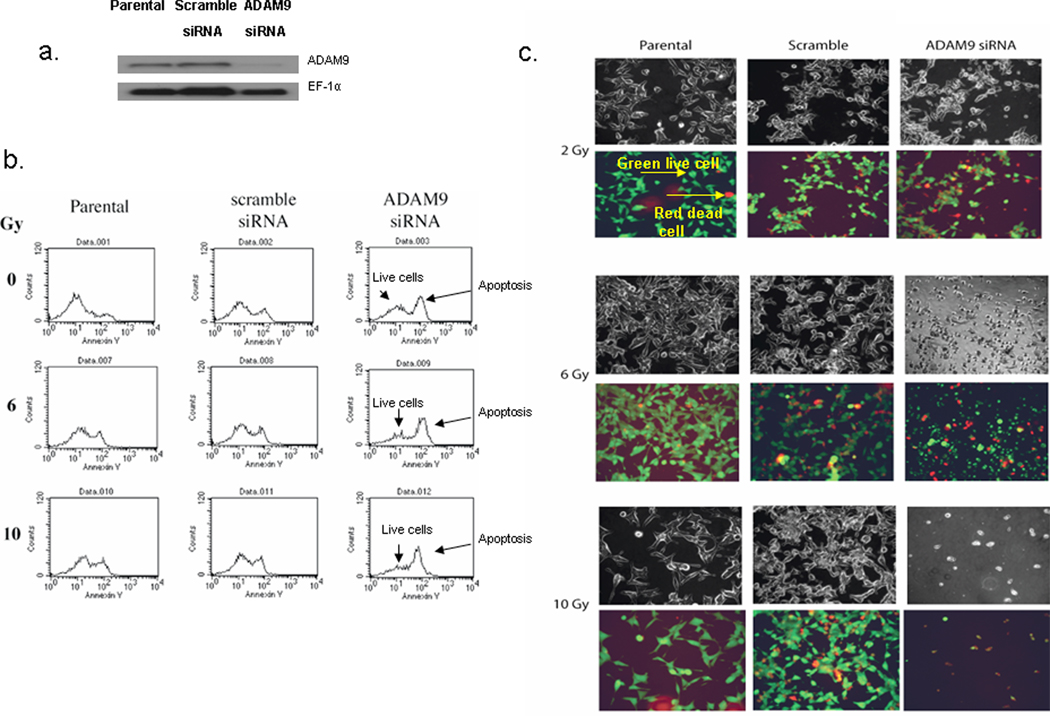
ADAM9 expression was knocked down transiently using ADAM9 siRNA in C4-2 prostate cancer cells. ADAM9 protein expression was decreased in ADAM9 siRNA treated cells compared to controls (Figure 1a). To determine radiation sensitivity of ADAM9 siRNA treated cells, cells transfected with either ADAM9 siRNA or scramble siRNA were irradiated and assayed for apoptosis and cell live/death 24 hrs later. ADAM9 siRNA treated cells had increased annexin V staining. A further increase in apoptosis peak was observed in ADAM9 siRNA knockdown cells plus radiation when compared to scramble siRNA-treated group at 6 Gy and 10 Gy (Figure 1b). We further confirmed this enhanced apoptosis with results from cell live/death assay. The latter assay stains live cells green fluorescence and dead cells with red fluorescence dye. ADAM9 siRNA knockdown cells, treated with radiation, had significantly more dead cells when compared to control scramble siRNA-treated cells subjected to radiation treatment. For example, we observed that at 2 Gy-exposure, parental non-transfected C4-2 cells had 5% dead cells whereas scramble control or ADAM9 siRNA transfected C4-2 cells yielded respectively 20% and 60% dead cells per field (Figure 1c). These results suggest that ADAM9 knockdown significantly sensitizes prostate cancer cells to low dose of radiation. Taken together, ADAM9 inhibition sensitizes prostate cancer cells to apoptosis treated with radiation therapy.
Figure 1.
ADAM9 knockdown C4-2 prostate cancer cells have increased apoptosis in response to radiation. a. Western analysis of ADAM9 expression in C4-2 cells transiently transfected with control and ADAM9 siRNA. b. Cell death was quantified using in ADAM9 siRNA treated cells and control cells in response to radiation. Cells were radiated 4 h after transfection and 24 h later cell death was assayed. Apoptosis assay using annexin V staining using flow cytometry. c. Live/Death viability/cytotoxicity kit assay to detect live (green) and dead (red) cells using fluorescence microscopy (4X magnification).
Radiation treatment induces superoxide levels but not ADAM9 expression
Previous studies demonstrate that the steady-state levels of ADAM9 expression in cancer cells is responsive to hydrogen peroxide and stress-inducing factors (2,16). Since radiation increases reactive oxygen species, we determined the time-course and dose-dependent induction of increased hydrogen peroxide (H2O2) and superoxide levels and ADAM9 expression in prostate cancer cells in response to radiation. H2O2 levels did not change significantly in response to increasing dose of radiation form 2 Gy to 10 Gy (Suppl. 1a), or after repeated dose of 2 Gy for 3 days (Suppl.1d). Superoxide levels, however, were significantly increased in cells exposed to increasing radiation dose from 2 Gy to 10 Gy (Suppl. 1b) and for 2 Gy over 3 days (Suppl.1e). ADAM9 expression levels were also determined under these similar treatment protocols. We found that with increasing radiation doses (Suppl. 1c) or at 2 Gy for 24 h and 72 h; and 2 Gy given daily for 3 days (Suppl. 1f) did not change the levels of ADAM9 expression in response to radiation in both cases. These results in aggregate suggest that in response to radiation treatment there is an increased steady-state level of superoxide (but not hydrogen peroxide) without affecting concomitantly the levels of expression of ADAM9 protein.
ADAM9 stable knockdown sensitizes prostate cancer cells to ionizing irradiation and chemotherapeutic agents
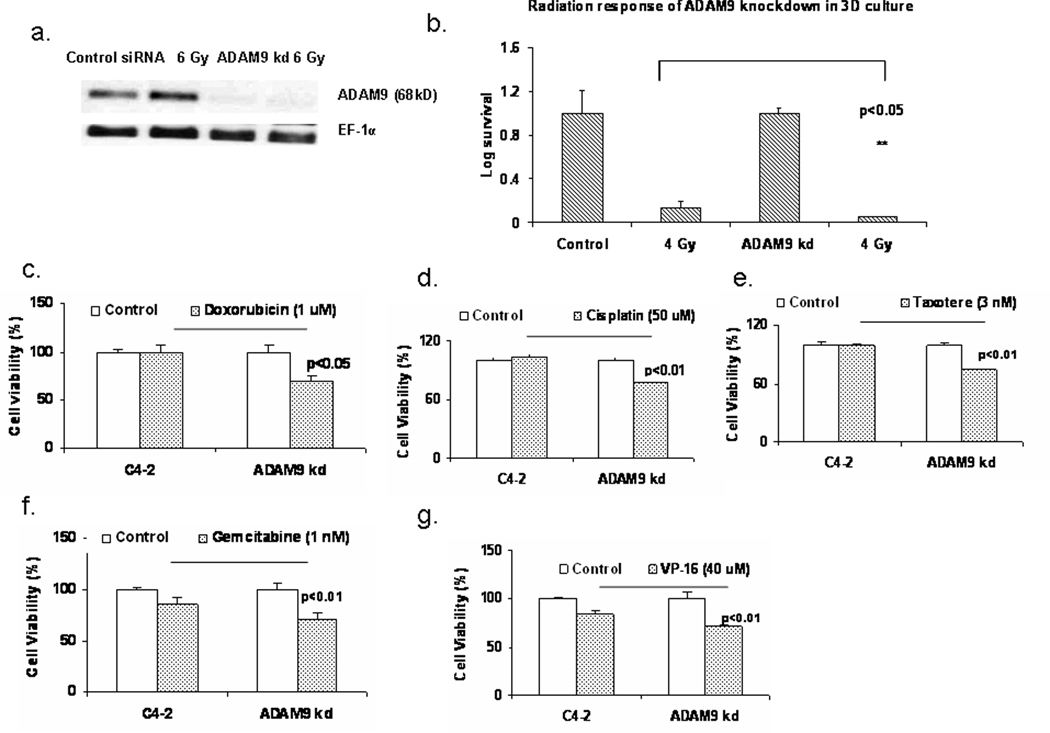
Stable ADAM9 knockdown cells were used to study the function of ADAM9 in C4-2 prostate caner cells. Retroviral transduction using ADAM9 shRNA were used to knockdown ADAM9 protein. Using western analysis we determined the levels of ADAM9 in control transduced and ADAM9 shRNA transduced cells and in response to irradiation treatment. ADAM9 levels were significantly lower in ADAM9 knockdown (ADAM9 kd) cells compared to control transduced C4-2 cells (Figure 2a). ADAM9 kd cells underwent increased cell death. ADAM9 kd cells had undetectable levels of ADAM9 expression in radiation treated cells. Three dimensional (3D) culture conditions were used to determine the radiation sensitivity of ADAM9 kd cells. Both control C4-2 and ADAM9 kd cells formed organoids under 3D culture conditions. The organoids were irradiated with 4 Gy after 48 h after formation and clongenic assay was performed. Radiation decreased the surviving population in both control and ADAM9 knockdown cells, but ADAM9 knockdown had significantly lower number of colonies compared to control irradiated (Figure 2b). These results are consistent with previous data which demonstrate that inhibition of ADAM9 sensitizes prostate cancer cells to radiation. We further tested the sensitivity of ADAM9 kd cells to chemotherapeutic agents such as doxorubicin, cisplatin, taxotere, gemcitabine and VP-16. ADAM9 kd cells were more sensitive to all of these chemotherapeutic drugs compared to control C4-2 prostate cancer cells (Figure 2c–2g). ADAM9 kd were more sensitive (25–30%) to Doxorubicin (1 µM), Cisplatin (50 µM), Taxotere (3 nM) and moderately sensitive (15%) to Gemcitabine (1 µM) and VP-16 (40 µM). These results suggest that ADAM9 inhibition significantly sensitizes prostate cancer cells to ionizing irradiation and chemotherapy.
Figure 2.
Inhibition of ADAM9 in C4-2 prostate cancer cells sensitizes them to radiation and chemotherapeutic agents. a. Protein analysis of ADAM9 expression in control transfected and ADAM9 knockdown (ADAM9 kd) retrovirally stably transfected C4-2 prostate cancer cells in response to radiation treatment. b. Clongenic assay of control and ADAM9 kd C4-2 prostate cancer cells using cancer cell organoids generated by 3D culture. c–g. Cell viability assay in response to doxorubicin, cisplatin, taxotere, gemcitabine and VP-16 in control and ADAM9 kd C4-2 prostate cancer cells.
ADAM9 knockdown induces E-cadherin and integrin expression and drives C4-2 prostate cancer cells to a epithelial phenotype
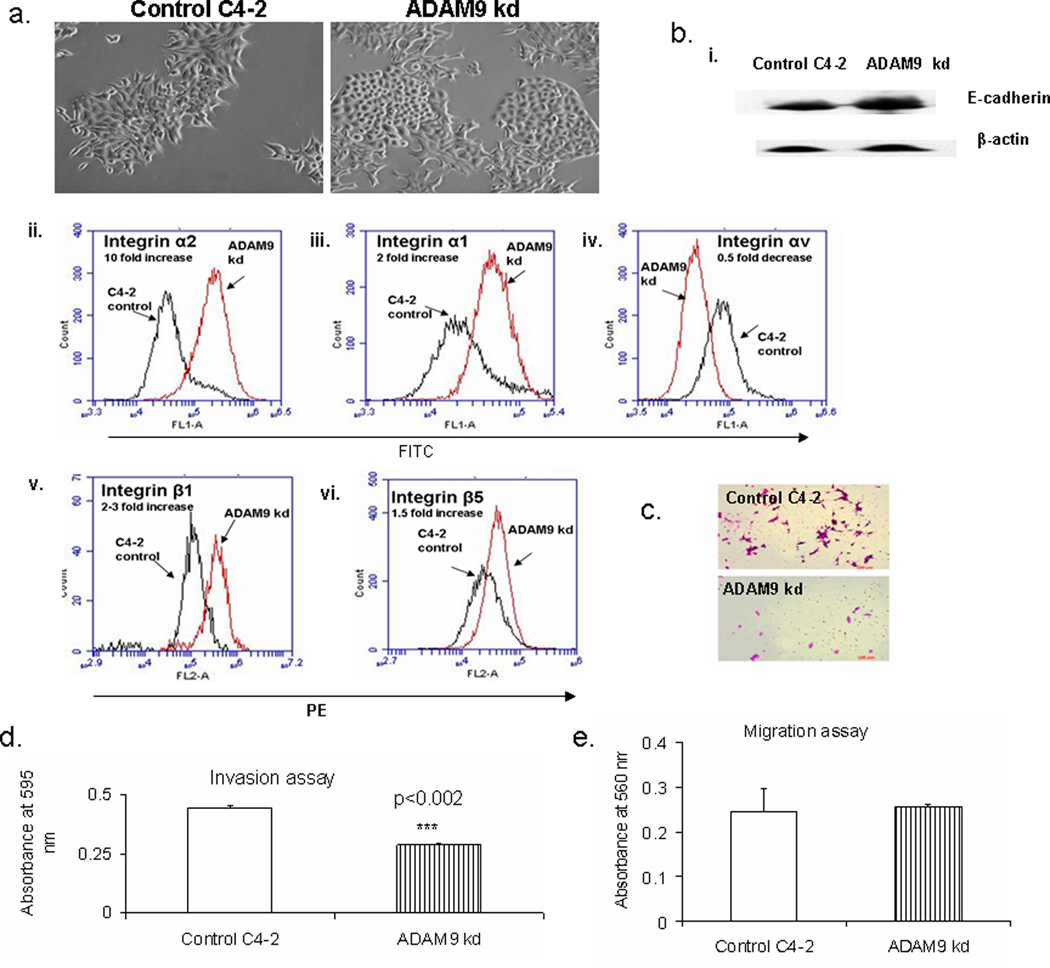
ADAM9 kd cells initially underwent increased cell death, and after several passages they gradually underwent phenotypic alterations to a cobblestone morphology (Figure 3a). This morphologic transition did not appear in the control vector-treated C4-2 cells. Since cobblestone morphology is a characteristic of epithelial cells, we determined the epithelial marker, E-cadherin and several integrins which interact with ADAM9. Interestingly, E-cadherin expression levels were higher in ADAM9 kd cells compared to control C4-2 prostate cancer cells (Figure 3bi). However, E-cadherin levels were even higher in ADAM9 kd cells prior to the appearance of cobblestone morphology. We tested for the following integrins in control and knockdown ADAM9 cells- CD49a (α1), CD49b (α2), CD51 (αv), CD29 (β1) and integrin β5. Interestingly, several integrins such as α1, α2, β1 and β5 were increased, and αv was decreased in ADAM9 kd cells compared to control C4-2 prostate cancer cells (Figure 3bii–vi). Integrin α2 had the highest increase with 10 fold difference in response to ADAM9 knockdown. Integrins α1, β1 and β5 were also increased 1.5-3 fold in ADAM9 knockdown and αv decreased by 0.5 fold in ADAM9 knockdown cells compared to control C4-2 cells. Since E-cadherin and integrins controls multiple aspects of cell function, we determined if alteration in ADAM9 would affect the invasive and migratory ability of C4-2 prostate cancer cells. ADAM9 kd cells had decreased invasive ability, however, the migratory ability had not changed (Figure 3c, 3d, 3e).
Figure 3.
ADAM9 knockdown induces epithelial phenotypic, biochemical and functional changes in prostate cancer cells. a. Morphological alterations in control and ADAM9 kd C4-2 prostate cancer cells. b. i. E-cadherin expression by western analysis in control and stable ADAM9 kd C4-2 prostate cancer cells. Flow cytometric analysis of membrane expression of ii. integrin α2 (CD49b), iii. integrin α1 (CD49a), iv. integrin αv (CD51), v. integrin β1 (CD29) and vi. integrin β5 in control and stable ADAM9 kd C4-2 prostate cancer cells. c. Pictures of invasion assay form control and stable ADAM9 kd C4-2 prostate cancer cells. d. Invasion assay of control and stable ADAM9 kd C4-2 prostate cancer cells. e. Migration assay of control and stable ADAM9 kd C4-2 prostate cancer cells.
Discussion
Our study demonstrates that the loss of ADAM9 triggers the morphologic, biochemical and behavioral transitions in human prostate cancer cells toward epithelial phenotype and reverses therapeutic resistance. We demonstrate that inhibition of ADAM9 alters the cancer cell phenotype to a cobblestone epithelial morphology, with increased E-cadherin and integrin expression and decreased invasive ability. Our results suggest that blocking ADAM9 induced E-cadherin expression, and this precedes the morphological transition. This also suggests that E-cadherin maybe a substrate for ADAM9 ectodomain shedding activity and in the absence of ADAM9 there is increased E-cadherin protein. ADAM9 kd cells also had increased integrin membrane expression, such as α1, α2, β1, β5 and decreased levels of αv. Integrin α2 had a 10 fold increase in ADAM9 knockdown cells compared to control C4-2 prostate cancer cells. Interestingly, in breast carcinoma, α2β1 expression in decreased in adenocarcinoma and re-expression of α2β1 results in reversion of the malignant phenotype to a more differentiated epithelial phenotype (17). These results are consistent with our observation where increase in α2β1 occurs in response to ADAM9 knockdown in C4-2 prostate cancer cells and results in a reversion to a more differentiated epithelial phenotype. These results further emphasize the role of ADAM9 in EMT in prostate cancer. ADAM9 is crucial for survival of C4-2 prostate cancer cells and knockdown of ADAM9 in prostate cancer cells results in increased cell death, increased E-cadherin and integrins such as α2β1 and induced epithelial phenotype and decreased invasive ability. These results demonstrate for the first time that ADAM9 plays crucial role in prostate cancer cell survival, in modulating epithelial phenotypic characteristics and its sensitivity to various cancer treatments. ADAM9 overexpression demonstrated in aggressive prostate cancer specimens maybe an important biomarker for prostate cancer metastasis.
Additionally, increased epithelial phenotype also sensitizes the cells to various cancer treatments such as radiation, doxorubicin, cisplatin, taxotere, gemcitabine and VP-16. Several studies demonstrate the role of ADAM9 in prostate carcinogenesis and metastasis. ADAM9 has been shown to be overexpressed in prostate cancer patients and increased in patients with disease recurrence (4). In patients who had received androgen ablation therapy (4), ADAM9 levels were significantly associated with shortened PSA relapse free survival. Consistent with these data several studies demonstrate the importance of ADAM9 in poorly differentiated tumors and metastasis. In W10 prostate cancer mouse model, the glandular epithelial cells have, SV40 large T antigen under the control of probasin promoter. W10/Adam9−/− mice had, no poorly differentiated tumors and the tumors which developed were mostly well-differentiated to moderately differentiated compared to control mice which had 50% poorly differentiated tumors (3). These results suggest that ADAM9 maybe necessary for the development of poorly differentiated tumors (3). These are consistent with our studies where, inhibition of ADAM9 in prostate cancer cells resulted in a differentiated epithelial phenotype with increased E-cadherin and α2 integrin. Additionally, ADAM9 is known to be a marker for cancer stem cells. Overexpression of ADAM9 in a transgenic model was sufficient to induce PIN and benign hyperplasia in the mouse prostate. ADAM9 transgenic mice had increased ectodomian shedding of epidermal growth factor (EGF) and fibroblast growth factor receptor 2iiib (3). EGF has been shown to be involved in epithelial to mesenchymal transition of human prostate cancer cells (18,19). E-cadherin maybe a substrate for ADAM9 ectodomain shedding activity and in the absence of ADAM9, as in ADAM9 kd cells (Fig. 3) there is increased E-cadherin protein which promotes the morphologic (cobblestone morphology) and behavioral (increased invasion) transition. ADAM9 has been previously associated with metastasis. ADAM9 overexpression in non-small cell lung carcinoma cell resulted in increased metastasis to brain (7). Increased ADAM9 expression in lung cancer cells resulted in increased responsiveness to nerve growth factor and increased adhesion to brain tissue. Additionally, there was an increase in the expression of integrin α3 and β1 subunits (7). These results demonstrate that different integrin partners are altered in response to ADAM9 overexpression and knockdown and mediate downstream effects. The mechanism by which ADAM9 modulates E-cadherin levels is still controversial. It is possible that ADAM9 directly interacts with E-cadherin and induces ectoderm shedding. Previous studies demonstrate that ADAM9 physically interacts with E-cadherin in colon cancer cell. However in these cells they detected very little ectoderm shedding (20). Further studies need to be perfomed to define the exact relationship between ADAM9 and E-cadherin in modulation of EMT.
Cancer cells that are resistant to radiation and chemotherapy treatment are the ones that metastasize. We determined the sensitivity of ADAM9 knockdown to these treatments. As expected, ADAM9 knockdown had increased sensitivity to radiation (Fig.1 and 2). Both transient and stable transfection of ADAM9 induced increased apoptosis and cell death. Apoptotic cell numbers were significantly higher when ADAM9 siRNA treated cells were exposed to various radiation doses. This suggests that ADAM9 is essential for the survival of C4-2 prostate cancer cells. Recent studies from our laboratory demonstrate that ADAM9 is an important stress response protein and its expression is activated in response to hydrogen peroxide, overcrowding and hypoxia (2). Thus we determined if radiation induced reactive oxygen species would modulate ADAM9 expression. Radiation increased superoxide levels significantly but did not increase hydrogen peroxide. ADAM9 expression was not altered in response to increases in superoxide, thus suggesting differential effect on its expression by hydrogen peroxide and superoxide. Additionally, basal levels of ADAM9 may infer radiation resistance.
Conclusion
In this study we demonstrate that inhibition of ADAM9 in prostate cancer cells promote epithelial phenotypic and functional changes and sensitize prostate cancer cells to radiation and chemotherapeutic treatments. ADAM9 is an attractive new therapeutic target since ADAM9 overexpression in human prostate cancer may confer increased distant metastasis and therapeutic resistance.
Supplementary Material
Radiation treatment induces superoxide levels but does not induce ADAM9 expression. In response to increasing radiation dose (2 Gy, 6 Gy and 10 Gy) in C4-2 prostate cancer cells we detected a. H2O2 detection using DCF dye and flow cytometry. b. Superoxide detection using DHE dye and flow cytometry. c. ADAM9 protein expression by western analysis. In response to a single dose of 2 Gy in C4-2 prostate cancer cell, the following parameters were looked for at 24 h, 48 h and 72 h and at 2 Gy consecutively for 3 days every 24 h, d. H2O2 detection using DCF dye and flow cytometry. e. Superoxide detection using DHE dye and flow cytometry. f. ADAM9 protein expression by western analysis.
Acknowledgements
This work is supported in part by 2P01CA098912 and 1RO1CA122602 and NSC 96-2320-B-039-033-MY3.
We would like to thank Gregory H. Corn for doing the radiation treatments.
Abbreviations
- ADAM9
A Disintegrin and Metalloprotease 9
- ADAM10
A Disintegrin and Metalloprotease 10
- DHE
dihydroethidium
- DCF
dichlorofluorescin diacetate
- EMT
epithelial to mesenchymal transition
- FGF
fibroblast growth factor
- Gy
Gray
- IGFBP-1
Insulin-like growth factor binding protein 1
- H2O2
hydrogen peroxide
- Kd
knockdown
- PSA
prostate specific antigen
- Pro-HB-EFG
pro-heparin binding epidermal growth factor
- 3D
three dimensional
References
- 1.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Molecular aspects of medicine. 2008;29(5):258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung SY, Kubo H, Shigemura K, Arnold RS, Logani S, Wang R, Konaka H, Nakagawa M, Mousses S, Amin M, Anderson C, Johnstone P, Petros JA, Marshall FF, Zhau HE, Chung LW. Oxidative stress induces ADAM9 protein expression in human prostate cancer cells. Cancer research. 2006;66(19):9519–9526. doi: 10.1158/0008-5472.CAN-05-4375. [DOI] [PubMed] [Google Scholar]
- 3.Peduto L, Reuter VE, Shaffer DR, Scher HI, Blobel CP. Critical function for ADAM9 in mouse prostate cancer. Cancer research. 2005;65(20):9312–9319. doi: 10.1158/0008-5472.CAN-05-1063. [DOI] [PubMed] [Google Scholar]
- 4.Fritzsche FR, Jung M, Tolle A, Wild P, Hartmann A, Wassermann K, Rabien A, Lein M, Dietel M, Pilarsky C, Calvano D, Grutzmann R, Jung K, Kristiansen G. ADAM9 expression is a significant and independent prognostic marker of PSA relapse in prostate cancer. European urology. 2008;54(5):1097–1106. doi: 10.1016/j.eururo.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 5.O'Shea C, McKie N, Buggy Y, Duggan C, Hill AD, McDermott E, O'Higgins N, Duffy MJ. Expression of ADAM-9 mRNA and protein in human breast cancer. International journal of cancer. 2003;105(6):754–761. doi: 10.1002/ijc.11161. [DOI] [PubMed] [Google Scholar]
- 6.Mazzocca A, Coppari R, De Franco R, Cho JY, Libermann TA, Pinzani M, Toker A. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer research. 2005;65(11):4728–4738. doi: 10.1158/0008-5472.CAN-04-4449. [DOI] [PubMed] [Google Scholar]
- 7.Shintani Y, Higashiyama S, Ohta M, Hirabayashi H, Yamamoto S, Yoshimasu T, Matsuda H, Matsuura N. Overexpression of ADAM9 in non-small cell lung cancer correlates with brain metastasis. Cancer research. 2004;64(12):4190–4196. doi: 10.1158/0008-5472.CAN-03-3235. [DOI] [PubMed] [Google Scholar]
- 8.Grutzmann R, Luttges J, Sipos B, Ammerpohl O, Dobrowolski F, Alldinger I, Kersting S, Ockert D, Koch R, Kalthoff H, Schackert HK, Saeger HD, Kloppel G, Pilarsky C. ADAM9 expression in pancreatic cancer is associated with tumour type and is a prognostic factor in ductal adenocarcinoma. British journal of cancer. 2004;90(5):1053–1058. doi: 10.1038/sj.bjc.6601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S, Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. The EMBO journal. 1998;17(24):7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike H, Tomioka S, Sorimachi H, Saido TC, Maruyama K, Okuyama A, Fujisawa-Sehara A, Ohno S, Suzuki K, Ishiura S. Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. The Biochemical journal. 1999;343 Pt 2:371–375. [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41(51):15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- 12.Cisse MA, Sunyach C, Lefranc-Jullien S, Postina R, Vincent B, Checler F. The disintegrin ADAM9 indirectly contributes to the physiological processing of cellular prion by modulating ADAM10 activity. The Journal of biological chemistry. 2005;280(49):40624–40631. doi: 10.1074/jbc.M506069200. [DOI] [PubMed] [Google Scholar]
- 13.Franzke CW, Tasanen K, Borradori L, Huotari V, Bruckner-Tuderman L. Shedding of collagen XVII/BP180: structural motifs influence cleavage from cell surface. The Journal of biological chemistry. 2004;279(23):24521–24529. doi: 10.1074/jbc.M308835200. [DOI] [PubMed] [Google Scholar]
- 14.Shigemura K, Sung SY, Kubo H, Arnold RS, Fujisawa M, Gotoh A, Zhau HE, Chung LW. Reactive oxygen species mediate androgen receptor- and serum starvation-elicited downstream signaling of ADAM9 expression in human prostate cancer cells. The Prostate. 2007;67(7):722–731. doi: 10.1002/pros.20565. [DOI] [PubMed] [Google Scholar]
- 15.Nomura T, Huang WC, Zhau HE, Wu D, Xie Z, Mimata H, Zayzafoon M, Young AN, Marshall FF, Weitzmann MN, Chung LW. Beta2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12(24):7294–7305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 16.Fischer OM, Hart S, Gschwind A, Prenzel N, Ullrich A. Oxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factor. Molecular and cellular biology. 2004;24(12):5172–5183. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zutter MM, Sun H, Santoro SA. Altered integrin expression and the malignant phenotype: the contribution of multiple integrated integrin receptors. J Mammary Gland Biol Neoplasia. 1998;3(2):191–200. doi: 10.1023/a:1018798907544. [DOI] [PubMed] [Google Scholar]
- 18.Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, Marshall FF, Zhau HE, Chung LW. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008;18(8):858–870. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 19.Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, Liu ZR, Zhou BP, Huang WC, Chung LW. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25(6):601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirao T, Nanba D, Tanaka M, Ishiguro H, Kinugasa Y, Doki Y, Yano M, Matsuura N, Monden M, Higashiyama S. Overexpression of ADAM9 enhances growth factor-mediated recycling of E-cadherin in human colon cancer cell line HT29 cells. Experimental cell research. 2006;312(3):331–339. doi: 10.1016/j.yexcr.2005.10.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Radiation treatment induces superoxide levels but does not induce ADAM9 expression. In response to increasing radiation dose (2 Gy, 6 Gy and 10 Gy) in C4-2 prostate cancer cells we detected a. H2O2 detection using DCF dye and flow cytometry. b. Superoxide detection using DHE dye and flow cytometry. c. ADAM9 protein expression by western analysis. In response to a single dose of 2 Gy in C4-2 prostate cancer cell, the following parameters were looked for at 24 h, 48 h and 72 h and at 2 Gy consecutively for 3 days every 24 h, d. H2O2 detection using DCF dye and flow cytometry. e. Superoxide detection using DHE dye and flow cytometry. f. ADAM9 protein expression by western analysis.