Abstract
Objective
Intra-amniotic infection/inflammation (IAI) is one of the most important mechanisms of disease in preterm birth. Resistin is an adipocytokine that has been linked to insulin resistance, diabetes, obesity and inflammation. The objective of this study was to determine if resistin is present in amniotic fluid (AF) and if its concentration changes with gestational age, in the presence of labor, and in IAI in patients with spontaneous preterm labor (PTL) and intact membranes, preterm prelabor rupture of membranes (PPROM), and clinical chorioamnionitis.
Study design
This cross-sectional study included 648 patients in the following groups: 1) women in the mid-trimester of pregnancy (14-18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=61); 2) normal pregnant women at term with (n=49) and without (n=50) spontaneous labor; 3) patients with an episode of PTL and intact membranes who were classified into: a) PTL who delivered at term (n=153); b) PTL who delivered preterm (<37 weeks gestation) without IAI (n=108); and c) PTL with IAI (n=84); 4) women with PPROM with (n=47) and without (n=44) IAI; and 5) patients with clinical chorioamnionitis at term with (n=22) and without (n=30) microbial invasion of the amniotic cavity. Resistin concentration in AF was determined by enzyme-linked immunoassay. Non-parametric statistics were used for analyses.
Results
1) Resistin was detected in all AF samples; 2) the median AF resistin concentration at term was significantly higher than in the mid-trimester (23.6 ng/mL vs. 10 ng/mL; p<0.001); 3) among patients with PTL, the median AF resistin concentration was significantly higher in patients with IAI than in those without IAI (144.9 ng/mL vs. 18.7 ng/mL; p<0.001) and those with PTL and intact membranes who delivered at term (144.9 ng/mL vs. 16.3 ng/mL; p<0.001); 4) patients with PPROM with IAI had a significantly higher median AF resistin concentration than those without IAI (132.6 ng/mL vs. 13 ng/mL; p<0.001); 5) no significant differences were observed in the median AF resistin concentration between patients with spontaneous labor at term and those at term not in labor (28.7 ng/mL vs. 23.6 ng/mL; p=0.07); and 6) amniotic fluid resistin concentration ≥37 ng/mL (derived from a ROC curve) had a sensitivity of 85.4% and a specificity of 94.3% for the diagnosis of intra-amniotic inflammation.
Conclusions
Resistin is a physiologic constituent of the amniotic fluid, and its concentrations in amniotic fluid: 1) is significantly elevated in the presence of intra-amniotic infection/inflammation; 2) increases with advancing gestation; and 3) does not change in the presence of spontaneous labor at term. We propose that resistin may play a role in the innate immune response against intra-amniotic infection.
Keywords: preterm labor, preterm delivery, preterm prelabor rupture of membranes, PPROM, pregnancy, amniocentesis, microbial invasion of the amniotic cavity, MIAC, adipokines, cytokines, chorioamnionitis
INTRODUCTION
Intrauterine infection is one of the most important mechanisms of disease in preterm birth and is the single pathologic process for which a causal association with prematurity has been recognized[1-11]. Microbial invasion of the amniotic cavity (MIAC), elevated pro-inflammatory cytokines in the amniotic fluid (AF) and histologic chorioamnionitis are part of the “inflammatory cluster” related to patients with preterm parturition[12]. Intra-amniotic infection and/or inflammation (IAI) is present in approximately one third of the patients with spontaneous preterm labor[10,13,14], is associated with development of fetal inflammatory response syndrome[15,16], and it is considered a risk factor for fetal injury[17-28]. Moreover, the outcome of patients with spontaneous preterm labor with intra-amniotic inflammation and a negative amniotic fluid culture is similar to that of patients with microbiologically proven intra-amniotic infection[14].
A solid body of evidence supports a role for pro-inflammatory cytokines in the mechanisms of preterm parturition: 1) interleukin (IL)-1β and tumor necrosis factor (TNF)-α can induce preterm parturition when administered systemically to pregnant animals[29-31]; 2) IL-1β and TNF-α stimulate prostaglandin production by amnion, decidua and myometrium[4,32]; 3) human decidua can produce IL-1β and TNF-α in response to bacterial products[33-35]; and 4) amniotic fluid IL-1β and TNF-α concentrations and bioactivity are elevated in women with preterm labor and intraamniotic infection[36,37]. Other cytokines, such as IL-6[38-42], IL-16[43], IL-18[44] and IL-10[45] have also been implicated in preterm parturition.
Adipocytokines are soluble mediators mainly produced by adipose tissue and include adiponectin, leptin, visfatin, resistin, as well as other cytokines such as TNF-α, IL-6, and IL-1[46]. Resistin belongs to the resistin–like molecule (RELM) family of cysteine-rich proteins. In mice, resistin is exclusively expressed and secreted by adipocytes, its serum concentration is increased in both diet-induced and genetic obesity models, and administration of resistin impairs glucose tolerance and insulin action[47]. These findings led to the suggestion that resistin may play an important role in glucose homeostasis and obesity-induced insulin resistance[48,49]. In contrast to adiponectin and leptin, which are produced abundantly by adipocytes[46], resistin is mainly expressed in humans by circulating monocytes[50,51] and macrophages[52]. Consistent with these findings, compelling evidence support a role for resistin in inflammation: 1) resistin mRNA expression is higher in human peripheral blood mononuclear cells (PBMC) after exposure to IL-1β, IL-6, TNF-α, and lipopolysaccharide (LPS) [53]; 2) resistin stimulation of PBMC leads to increased expression of TNF-α, IL-1β, and IL-6 mRNA, and the concentrations of cytokines released after stimulation with the highest resistin concentration are similar to those induced by LPS[54]; and 3) resistin induces nuclear factor (NF)-κB activity and its translocation from the cytoplasm to the nucleus[54]. In addition, serum resistin concentration has been reported to be significantly elevated in patients with severe sepsis or septic shock when compared to healthy controls[55], as well as in chronic inflammatory processes such as alcoholic liver disease, hepatitis C virus-induced chronic hepatitis[56], rheumatoid arthritis and osteoarthritis[54,57,58], and chronic kidney disease[59].
In normal pregnancy, the maternal plasma resistin concentration is significantly higher than in the non-pregnant state[60-63], its concentration increase with gestational age[61], and contradictory results have been reported regarding the maternal plasma/serum resistin concentrations in patients with preeclampsia[62,64-66] and in those with gestational diabetes[60,67,68]. Of note, current data concerning resistin in pregnancy is restricted to maternal circulating concentrations of this adipocytokine and there is a paucity in the literature about its concentrations in amniotic fluid. The objective of this study was to determine if resistin is present in amniotic fluid, if its concentration changes with advancing gestation, spontaneous labor at term, and in the presence of intra-amniotic infection/inflammation in patients with spontaneous preterm labor (PTL) and intact membranes, preterm prelabor rupture of membranes (PPROM), and clinical chorioamnionitis.
MATERIALS AND METHODS
Study design and population
A cross-sectional study was designed by searching our clinical database and bank of biological samples and included 648 patients in the following groups: 1) women in the mid-trimester of pregnancy (14-18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=61); 2) normal pregnant women at term with (n=49) and without (n=50) spontaneous labor; 3) patients with an episode of PTL and intact membranes who were classified into: a) PTL who delivered at term (n=153); b) PTL who delivered preterm (<37 weeks gestation) without IAI (n=108); and c) PTL with IAI (n=84); 4) women with PPROM with (n=47) and without (n=44) IAI; and 5) patients with clinical chorioamnionitis at term with (n=22) and without (n=30) MIAC.
All women provided written informed consent prior to the collection of amniotic fluid. The collection and utilization of amniotic fluid for research purposes was approved by the Institutional Review Boards of the participant institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been previously used to study the biology of inflammation, hemostasis, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Definitions
Patients were considered to have a normal pregnancy outcome if they did not have any medical, obstetrical, or surgical complication, and delivered a term neonate (≥37 weeks) of appropriate birth weight for gestational age [69,70] without complications. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical change before 37 completed weeks of gestation that required hospitalization. Preterm PROM was diagnosed by sterile speculum examination confirming pooling of amniotic fluid in the vagina in association with nitrazine and ferning tests when necessary, before 37 weeks of gestation and in the absence of labor. Clinical chorioamnionitis was diagnosed according to the criteria proposed by Gibbs et al[71]. including maternal temperature of ≥37.8°C and two or more of the following criteria: uterine tenderness, malodorous vaginal discharge, maternal leukocytosis (≥15000 cells/mm3), maternal tachycardia (>100 beats/min) and fetal tachycardia (>160 beats/min). Microbial invasion of the amniotic cavity was defined as a positive amniotic fluid culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an amniotic fluid interleukin (IL)-6 concentration ≥2.6 ng/mL[14]. Histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes. Acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly using the criteria previously described[72].
Sample collection
Amniotic fluid samples were obtained from transabdominal amniocentesis performed for genetic indication, evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity in patients approaching term. Women at term in labor consisted of women who were admitted for suspected preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering that these patients were at term in labor was derived retrospectively, if the following criteria were met: 1) spontaneous labor; 2) delivery within 24 hours from amniocentesis; 3) analysis of amniotic fluid consistent with maturity; 4) birthweight >2500 grams; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) physical examination of the newborn by pediatricians consistent with a term neonate. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection as previously described[73-75]. The results of these tests were used for clinical management. Amniotic fluid IL-6 concentrations were used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C and the supernatant was aliquoted and stored at –70°C until analysis. Among patients with spontaneous preterm labor with intact membranes who delivered within 72 hours of amniocentesis, placenta, umbilical cord, and chorioamniotic membranes were collected and the presence or absence of histologic chorioamnionitis and/or funisitis was assessed. The 72 hours interval was chosen to preserve a meaningful temporal relationship between amniotic fluid resistin concentration and placental histopathologic findings.
Determination of human resistin concentration in amniotic fluid
Specific and sensitive enzyme-linked immunoassays were used to determine concentrations of resistin in human amniotic fluid. Immunoassays for human resistin were purchased from Linco Research (St. Charles, MO, USA). Resistin assays were validated for use in human amniotic fluid in our laboratory prior to their use in this study. The calculated inter-assay and intra-assay coefficients of variation for resistin in our laboratory were 3.4% and 4.5% respectively. The sensitivity was 0.05 ng/ml.
Statistical analysis
The normality of the data was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Because amniotic fluid resistin concentrations were not normally distributed, non-parametric tests were used for analyses. Comparisons between proportions were performed with the Chi-square test. Kruskal-Wallis with post-hoc analysis and Mann-Whitney U tests were used for continuous variables. Adjustment for multiple comparisons was performed using the Bonferroni method[76]. Analysis of covariance (ANCOVA) was used to investigate the association between the preterm labor and PPROM subgroups, amniotic fluid resistin concentration and storage time. Spearman rank correlation was utilized to assess correlations between amniotic fluid concentration of resistin, WBC count and IL-6. Among patients with preterm labor and intact membranes, receiver-operating characteristic (ROC) curve analyses were performed to determine amniotic fluid resistin concentration cutoffs for the identification of patients who had MIAC and intra-amniotic inflammation. A Kaplan-Meier survival analysis was conducted to assess the amniocentesis-to-delivery interval, using an amniotic fluid resistin concentration cutoff derived from the ROC curve for the presence of intra-amniotic inflammation. Spontaneous labor was entered in the analysis as the event of interest, and patients who delivered due to fetal or maternal indications were treated as censored observations with a censoring time equal to the amniocentesis-to-delivery interval. Cox proportional hazard modeling was performed to examine the differences in amniocentesis-to-delivery interval, according to resistin concentration in amniotic fluid while controlling for other confounding factors [maternal body mass index (BMI), amniotic fluid culture result, cervical dilatation and gestational age at amniocentesis]. A p-value of <0.05 was considered statistically significant. The statistical package used was SPSS v.15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics of the study population
Table I presents the demographic and clinical characteristics of patients in the midtrimester, term not in labor and term in labor groups. Tables II and III display the demographic and clinical characteristics of patients with spontaneous preterm labor and intact membranes and those with PPROM, respectively. Among patients with PTL with intact membranes, those with IAI had a significantly lower median gestational age at amniocentesis than those without IAI who delivered preterm and those who delivered at term (Table I). Similar results were observed between patients with PPROM with IAI than in those without IAI (Table II).
Table I.
Demographic and clinical characteristics of patients in the midtrimester and those at term with and without spontaneous labor
| Midtrimester (n=61) | pa | Term No labor (n=50) | Term In labor (n=49) | pb | |
|---|---|---|---|---|---|
| Maternal age (years) | 36 (35-38) | <0.01 | 27 (21-32) | 22 (19-27) | <0.01 |
| Gestational age at amniocentesis (weeks) | 16 (16-17) | <0.01 | 39 (38-39) | 38.5 (37.7-39.3) | NS |
| Gestational age at delivery (weeks) | 39 (38-40) | NS | 39 (38-39) | 38.5 (37.7-39.3) | NS |
| Birthweight (grams) | 3,344 (3,145-3,627) | NS | 3,260 (3,080-3,630) | 3,360 (3,085-3,550) | NS |
Values are expressed as percentage (number) or median (interquartile range).
NS: not significant.
pa: comparison between patients in the mid-trimester and those at term not in labor
pb: comparison between patients at term not in labor and those at term in labor
Table II.
Demographic and clinical characteristics of patients presenting with spontaneous preterm labor with intact membranes
| PTL without IAI Term delivery (n=153) | p | PTL without IAI Preterm delivery (n=108) | pa | PTL with IAI Preterm delivery (n=84) | pb | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 22 (19-29.8) | NS | 22 (19-30) | NS | 23 (20-28) | NS |
| Smoking | 18.4 (28/152) | NS | 10.4 (11/106) | <0.01 | 30 (24/80) | 0.04 |
| GA at amniocentesis (weeks) | 31.9 (29.4-33.3) | NS | 31.9 (29.8-33.1) | <0.01 | 28.7 (25.1-33) | <0.01 |
| GA at delivery (weeks) | 38.7 (38-39.7) | <0.01 | 34.6 (33.3-35.6) | <0.01 | 29.8 (25.6-33.3) | <0.01 |
| Birthweight (grams) | 3,170 (2,900-3,515) | <0.01 | 2,330 (1,940-2,678) | <0.01 | 1,310 (735-2,118) | <0.01 |
Values expressed as percentage (number) or median (interquartile range)
p: comparison between PTL who delivered at term and PTL without IAI
pa: comparison between PTL who delivered preterm without IAI and PTL with IAI
pb: comparison between PTL who delivered at term and PTL with IAI
PTL: preterm labor; GA: gestational age; BMI: body mass index; IAI: intra-amniotic infection/inflammation NS: not significant
Table III.
Demographic and clinical characteristics of patients presenting with preterm prelabor rupture of membranes
| Preterm PROM without IAI (n=44) | Preterm PROM with IAI (n=47) | p | |
|---|---|---|---|
| Maternal age (years) | 24.5 (20-32.8) | 30 (23.8-37.5) | 0.008 |
| Smoking | 18.2 (8/44) | 17.8 (8/45) | NS |
| GA at amniocentesis (weeks) | 32.6 (29.4-33.8) | 30.4 (28-32.4) | 0.02 |
| GA at delivery (weeks) | 33.2 (31.4-34.4) | 30.7 (28.7-33) | <0.001 |
| Birthweight (grams) | 2,020 (1,678-2,323) | 1,660 (1,390-2,270) | 0.02 |
Values expressed as percentage (number) or median (interquartile range)
PROM: prelabor rupture of membranes; GA: gestational age; BMI: body mass index; IAI: intra-amniotic infection/inflammation NS: not significant.
Resistin in amniotic fluid of normal pregnancies
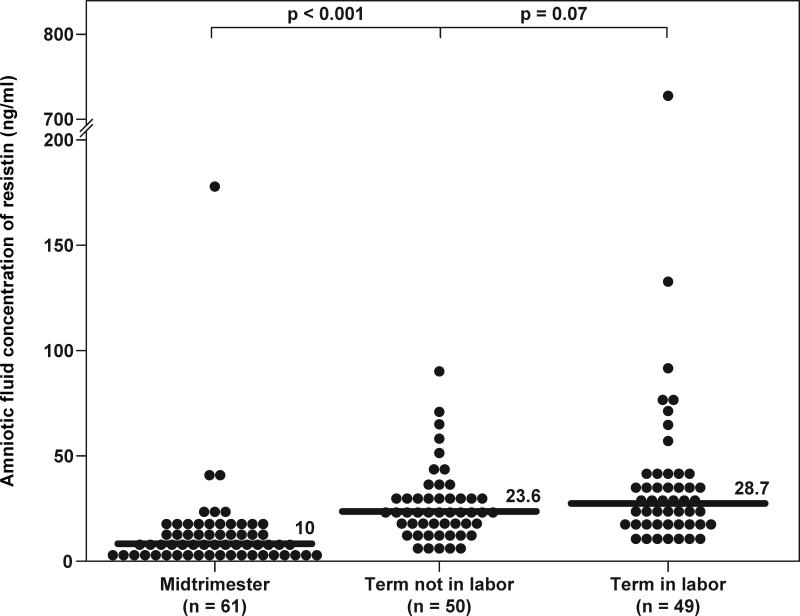
Resistin was detected in all amniotic fluid samples. Women with a normal pregnancy at term not in labor had a significantly higher median resistin concentration in amniotic fluid than women in the mid-trimester [term not in labor: 23.6 ng/mL, interquartile range (IQR) 16.3-31.1 vs. mid-trimester: 10 ng/mL, IQR 6.2-15.3; p<0.001] (Figure 1). In contrast, no significant differences were observed in the median amniotic fluid resistin concentration between patients with spontaneous labor at term and those at term not in labor (term in labor: 28.7 ng/mL, IQR 19.3-39.4 vs. term not in labor: 23.6 ng/mL, IQR 16.3-31.1; p=0.07) (Figure 1).
Figure 1. Amniotic fluid concentrations of resistin in normal pregnancies at mid-trimester and in those at term with and without labor.
The median amniotic fluid concentration of resistin was significantly higher in pregnancies at term not in labor than in those in the mid-trimester (23.6 ng/mL, IQR 16.3-31.1 vs. 10 ng/mL, IQR 6.2-15.3; p<0.001). In contrast, no significant differences were observed in the median amniotic fluid resistin concentration between women with spontaneous labor at term and those at term not in labor (28.7 ng/mL, IQR 19.3-39.4 vs. 23.6 ng/mL, IQR 16.3-31.1; p=0.07).
Amniotic fluid resistin concentrations in spontaneous preterm labor and intact membranes
Among patients with PTL, those with IAI had a significantly higher median amniotic fluid concentration of resistin than those who delivered preterm without IAI (PTL with IAI: 144.9 ng/mL, IQR 44.6–623.2 vs. PTL without IAI: 18.7 ng/mL, IQR 12.1–25.8; p<0.001) and than those who delivered at term (PTL delivered at term: 16.3 ng/mL, IQR 12.5–22.3; p<0.001) (Figure 2a). There were no differences in the median amniotic fluid resistin concentration between patients with PTL without IAI who delivered preterm and those who delivered at term (Figure 2a). These results did not change after adjusting for storage time (ANCOVA, p<0.001). When the analysis was restricted to patients with PTL without IAI who delivered within 48 hours from the amniocentesis, this subgroup had a significantly higher median amniotic fluid concentration of resistin than those with PTL and intact membranes who delivered at term (PTL without IAI: 23.2 ng/mL, IQR 18.4–28.3 vs. PTL delivered at term: 16.3 ng/mL, IQR 12.5–22.3; p=0.02) (Figure 2b). Similar results were found in patients with PTL and intact membranes who delivered within 7 days (p=0.007).
Figure 2. Amniotic fluid concentrations of resistin among women with spontaneous preterm labor (PTL) and intact membranes.
(a) The median amniotic fluid concentration of resistin was significantly higher in patients with intra-amniotic infection/inflammation (IAI) than in women without IAI (144.9 ng/mL, IQR 44.6–623.2 vs. 18.7 ng/mL, IQR 12.1–25.8; p<0.001) as well as in those who delivered at term (144.9 ng/mL, IQR 44.6–623.2 vs. 16.3 ng/mL, IQR 12.5–22.3; p<0.001). There was no significant difference in the median amniotic fluid concentration of resistin between those who delivered preterm and those who delivered at term. (b) Patients with spontaneous preterm labor without IAI delivered within 48 hours from the amniocentesis had a significantly higher median amniotic fluid concentration of resistin than those who delivered at term (23.2 ng/mL, IQR 18.4–28.3 vs. 16.3 ng/mL, IQR 12.5–22.3; p=0.02).
Amniotic fluid resistin concentrations in preterm PROM
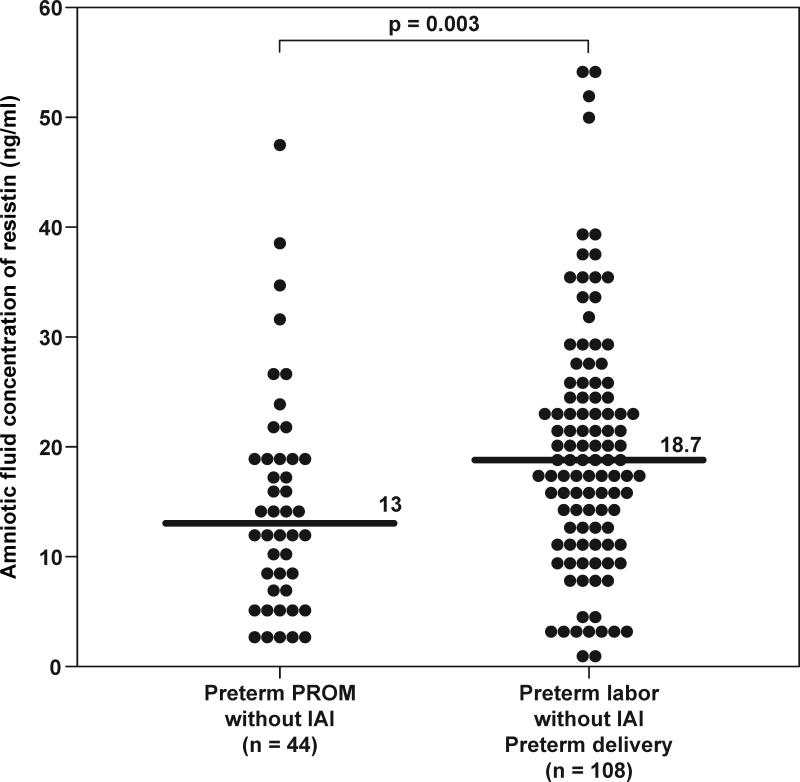
Patients with PPROM and IAI had a significantly higher median amniotic fluid resistin concentration than women with PPROM without IAI (PPROM with IAI: 132.6 ng/mL, IQR 32.3-869.7 vs. PPROM without IAI: 13 ng/mL, IQR 6.9–19.4; p<0.001) (Figure 3a). These results did not change after adjusting for storage time (ANCOVA, p<0.001). In the absence of IAI, patients with PTL who delivered preterm had a significantly higher median amniotic fluid resistin concentration than those with PPROM (PTL without IAI: 18.7 ng/mL, IQR 12.1–25.8 vs. PPROM without IAI: 13 ng/mL, IQR 6.9–19.4; p=0.003) (Figure 3b).
Figure 3. Amniotic fluid concentrations of resistin in women with preterm prelabor rupture of the membranes (PPROM).
(a) The median amniotic fluid concentration of resistin was significantly higher in patients with intra-amniotic infection/inflammation (IAI) than in those without IAI (132.6 ng/mL, IQR 32.3-869.7 vs. 13 ng/mL, IQR 6.9–19.4; p=0.02). (b) In the absence of IAI, patients with PTL who deliver preterm had a significantly higher median amniotic fluid resistin concentration than those with PPROM (18.7 ng/mL, IQR 12.1–25.8 vs. 13 ng/mL, IQR 6.9–19.4; p=0.003).
Amniotic fluid resistin concentrations in clinical chorioamnionitis at term
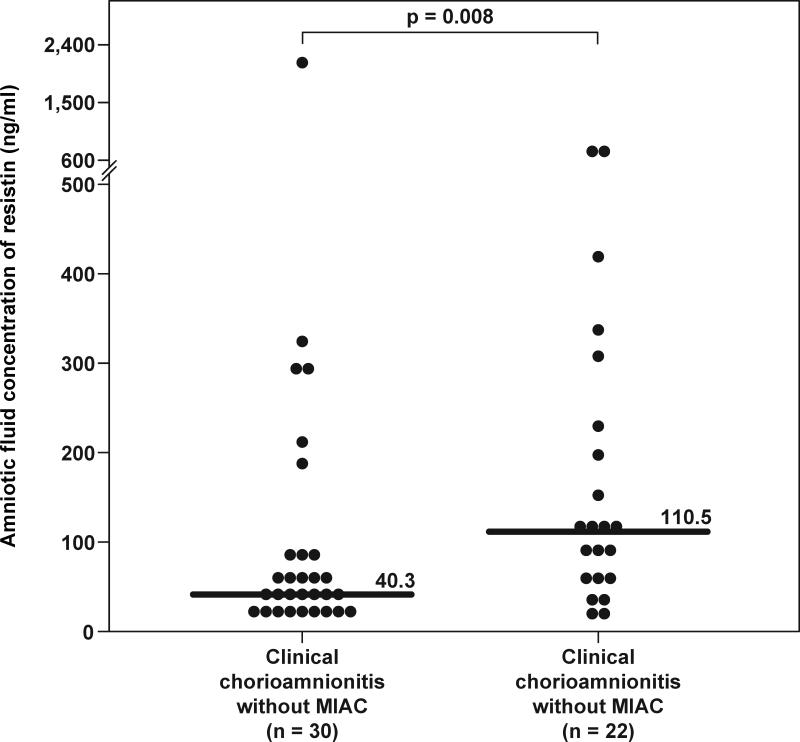
Among patients with clinical chorioamnionitis at term, those with positive amniotic fluid cultures for microorganisms had a significantly higher median amniotic fluid concentration of resistin than those without (110.5 ng/mL, IQR 52.4–243.4 vs. 40.3 ng/mL, IQR 26.2–85.9, respectively; p=0.008) (Figure 4).
Figure 4. Amniotic fluid concentrations of resistin in patients with clinical chorioamnionitis at term.
The median amniotic fluid concentration of resistin was significantly higher in patients with microbial invasion of the amniotic cavity (MIAC) than in those without MIAC (110.5 ng/mL, IQR 52.4–243.4 vs. 40.3 ng/mL, IQR 26.2–85.9; p=0.008).
Amniotic fluid resistin concentrations in patients with histologic chorioamnionitis
Placental histopathologic diagnoses were available in 80% (63/79) of patients with spontaneous preterm labor who delivered within 72 hours of amniocentesis. Of those, 51% (32/63) had evidence of placental inflammation. Patients with histologic chorioamnionitis and/or funisitis had a significantly higher median resistin concentration in amniotic fluid than those without histologic inflammation (495.1 ng/mL, IQR 15.7–3554.1 vs. 29.1 ng/mL, IQR 3–846.4, respectively; p<0.001) (Figure 5).
Figure 5. Amniotic fluid concentrations of resistin in patients with spontaneous preterm labor with and without histologic chorioamnionitis who delivered within 72 hours from amniocentesis.
Patients with histologic chorioamnionitis and/or funisitis had a significantly higher median resistin concentration in amniotic fluid than those without histologic inflammation (495.1 ng/mL, IQR 15.7–3554.1 vs. 29.1 ng/mL, IQR 3–846.4, respectively; p<0.001).
Amniotic fluid resistin concentrations and intra-amniotic inflammation
A significant correlation was observed between amniotic fluid concentrations of resistin and IL-6, glucose, and WBC count in patients with spontaneous preterm labor and those with preterm PROM (Spearman rho coefficient: IL-6: 0.64, p<0.001; glucose: -0.24, p<0.001; and WBC count: 0.4; p<0.001).
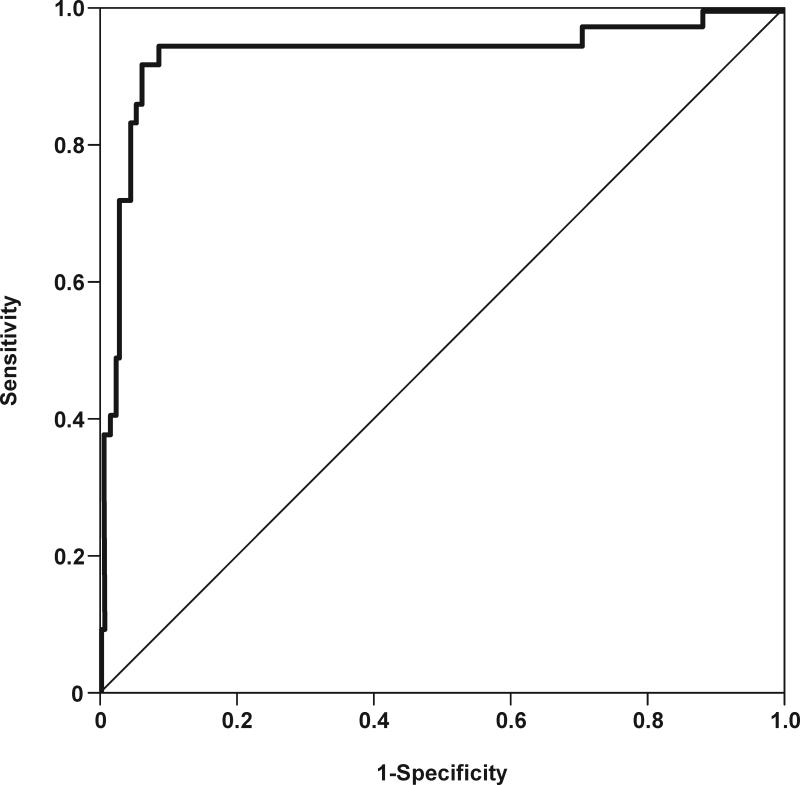
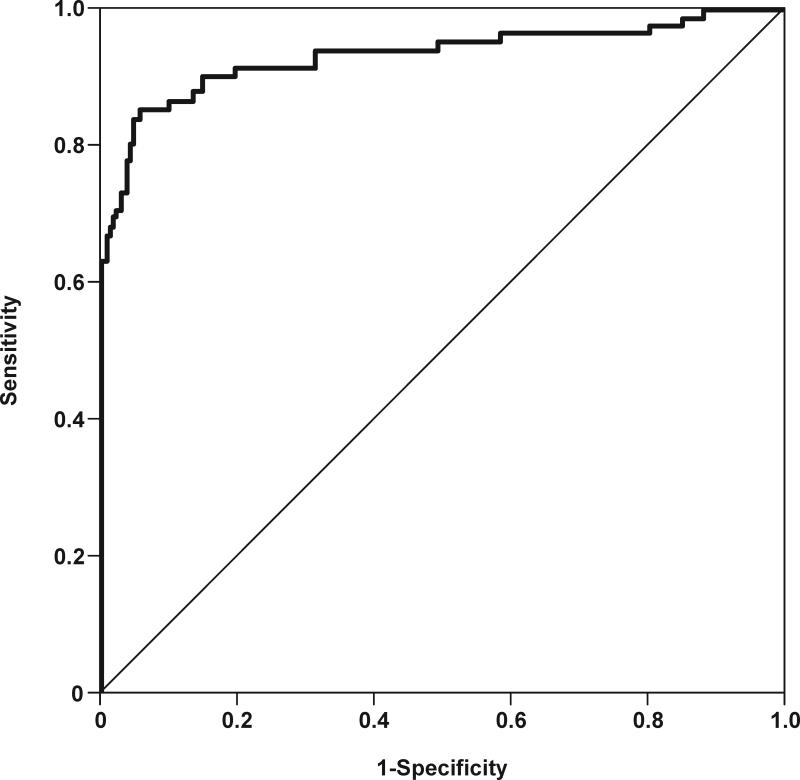
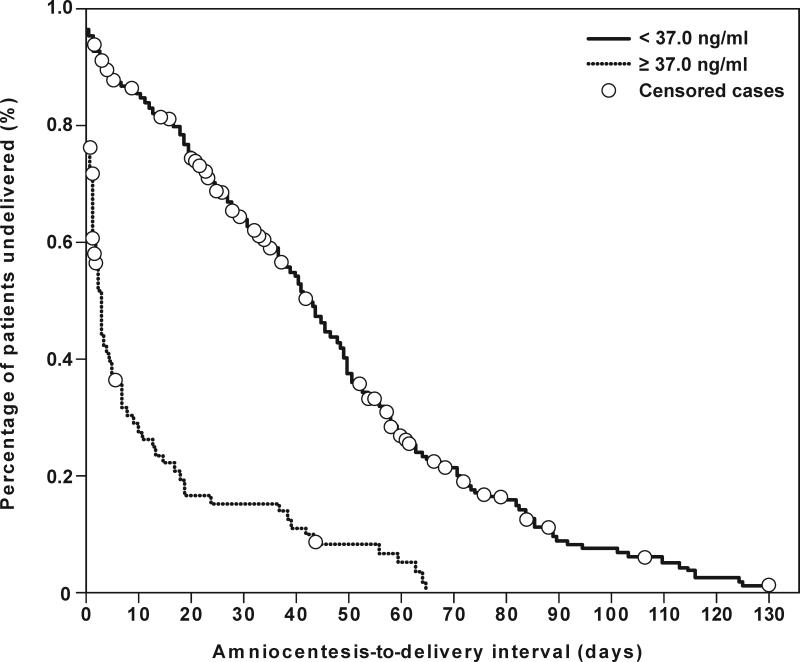
Figures 6a and 6b depict receiver operating characteristic (ROC) curves for the identification of MIAC and intra-amniotic inflammation, respectively, in patients with spontaneous preterm labor and intact membranes. The cutoff values for resistin concentration in amniotic fluid were derived from these ROC curves, and their diagnostic performance for the identification of MIAC and intra-amniotic inflammation are displayed in Table IV. Table V shows the diagnostic indices and the likelihood ratios of amniotic fluid resistin concentration ≥37 ng/mL for the identification of patients who delivered within 48hrs, 7 days, 14 days and at <32 and <34 weeks of gestation. Using an amniotic fluid resistin concentration cutoff ≥37 ng/mL, a survival analysis was conducted to determine the relationship between intra-amniotic inflammation and the duration of the amniocentesis-to-delivery interval. Patients delivered due to fetal or maternal indications were censored. The median amniocentesis-to-delivery interval was significantly shorter in patients with an amniotic fluid resistin concentration ≥37 ng/mL compared to those with a concentration <37 ng/mL [median amniocentesis-to-delivery interval: 3 days (95% CI 2-4) vs. 43 days (95% CI 39-47), respectively; p<0.001] (Figure 7). These results remained significant after adjusting for the results of the amniotic fluid culture, BMI, storage time, and cervical dilatation and gestational age at amniocentesis, (Cox proportional-hazards regression: 3.3, 95% CI 2.3-4.7; p<0.001).
Figure 6. Receiver operating characteristic (ROC) curves of amniotic fluid resistin concentration in patients with spontaneous preterm labor and intact membranes.
(a) ROC curve for the identification of positive amniotic fluid culture for microorganisms [area under the curve (AUC) for amniotic fluid resistin: 0.932; p<0.001]. (b) ROC curve for the identification of intra-amniotic inflammation, defined as amniotic fluid IL-6 concentration ≥2.6 ng/mL (AUC for amniotic fluid resistin: 0.933; p<0.001).
Table IV.
Diagnostic indices and likelihood ratios of amniotic fluid resistin concentration for the detection of intra-amniotic infection and intra-amniotic inflammation in patients presenting with spontaneous preterm labor with intact membranes
| Prevalence* (%) | Cutoff (ng/mL) | Area under the ROC curve | Sensitivity* (%) | Specificity* (%) | Efficiency* (%) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Intra-amniotic infection | 10.1 (35/345) | ≥ 56.1 | 93.2 | 94.3 (33/35) | 91.6 (284/310) | 91.9 (317/345) | 11.2 (8.5-12.4) | 0.06 (0.02-0.2) |
| Intra-amniotic inflammation | 23.8 (82/345) | ≥ 37 | 93.3 | 85.4 (70/82) | 94.3 (248/263) | 92.2 (318/345) | 15 (10.1-21.7) | 0.16 (0.1-0.2) |
ROC: receiver-operating characteristic; CI: confidence interval
Intra-amniotic infection: positive amniotic fluid culture for microorganisms
Intra-amniotic inflammation: amniotic fluid IL-6 concentration ≥ 2.6 ng/mL
Numbers in parentheses represent proportions
Table V.
Diagnostic indices and likelihood ratios of amniotic fluid resistin concentration ≥ 37 ng/mL for the identification of patients with spontaneous preterm delivery within 48 hours, 7days, 14 days and at <32 and <34 weeks of gestation
| Prevalence* (%) | Sensitivity* (%) | Specificity* (%) | Efficiency* (%) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Delivery within 48 hours | 21.2 (60/283) | 66.7 (40/60) | 83.4 (186/223) | 79.9 (226/283) | 4 (2.9-5.4) | 0.4 (0.3-0.5) |
| Delivery within 7 days | 31.4 (89/283) | 61.8 (55/89) | 88.7 (172/194) | 80.2 (227/283) | 5.4 (3.7-8.1) | 0.4 (0.3-0.5) |
| Delivery within 14 days | 38.2 (108/283) | 56.5 (61/108) | 90.9 (159/175) | 77.7 (220/283) | 6.2 (3.9-10) | 0.5 (0.4-0.6) |
| Delivery at <32 weeks | 38.4 (58/151) | 72.4 (45/58) | 90.3 (84/93) | 83.4 (126/151) | 7.5 (4.3-13.5) | 0.3 (0.2-0.4) |
| Delivery at <34 weeks | 38.7 (98/253) | 64.3 (63/98) | 93.5 (145/155) | 82.2 (208/253) | 9.9 (5.7-18.1) | 0.4 (0.3-0.5) |
CI: confidence interval
Numbers in parenthesis represents proportions
Figure 7. Survival analysis of the amniocentesis-to-delivery interval (days) according to amniotic fluid resistin concentration cutoff ≥37 ng/mL in patients with spontaneous preterm labor and intact membranes.
Patients with an amniotic fluid resistin concentration ≥37 ng/mL (dotted line) had a shorter median amniocentesis-to-delivery interval than those with an amniotic fluid resistin concentration <37 ng/mL (solid line) (3 days, 95% CI 2-4 vs. 43 days, 95% CI 39-47, respectively; p<0.001, log rank test).
DISCUSSION
Principal findings of the study
1) Resistin is a physiologic constituent of amniotic fluid; 2) its concentration in amniotic fluid is significantly elevated in the presence of intra-amniotic infection/inflammation in patients with spontaneous preterm labor with intact membranes, preterm PROM, and those with clinical chorioamnionitis; 3) patients with histologic chorioamnionitis and/or funisitis have higher median amniotic fluid resistin concentrations than those without; 4) amniotic fluid resistin concentrations are significantly correlated with indirect amniotic fluid markers for intra-amniotic infection/inflammation (WBC count and IL-6 concentrations); and 5) amniotic fluid resistin concentration increases with advancing gestation, and does not change in the presence of labor at term.
What is resistin?
Resistin is a 12.5 kDa polypeptide that belongs to the RELM family of cysteine-rich proteins, including RELM-α, RELM-β and RELM-γ, which is similar to the “found in inflammatory zone” (FIZZ) family[77,78]. In serum, resistin circulates in two different isoforms: a high molecular-weight hexamer and a significantly increased bioactivity low molecular-weight form[79]. Resistin was originally identified as FIZZ3 in mice by Holcomb et al. [80], where FIZZ3 mRNA was expressed in white adipose tissue in association to secretion of a protein (FIZZ1) found in bronchoalveolar lavage fluid after experimental induction of an allergic inflammatory reaction in mice lungs, suggesting that FIZZ3 may play a role in inflammation. Subsequently, two independent groups[47,81] identified resistin as a potential link between obesity and insulin resistance. Steppan et al.[47] discovered a protein that was called resistin after screening for genes induced during adipocyte differentiation but downregulated in mature adipocytes exposed to thiazolidinediones (insulin sensitizing agents). The authors found in mice that: 1) the RETN gene expression is induced during adipocyte differentiation, and is expressed and secreted by adipocytes; 2) its gene expression and protein secretion is reduced after exposure to thiazolidinediones; 3) resistin circulates in serum, and its concentration is increased in both diet-induced and genetic obesity; 4) administration of anti-resistin IgG improves blood glucose and insulin action; and 5) administration of resistin impairs glucose tolerance and insulin action in normal mice. At the same time, Kim et al.[81] reported the mRNA expression of an adipocyte-specific secretory factor (ADSF) only in murine white adipose tissue. The mRNA expression was very low or non-detectable in adipose tissue of starved or diabetic animals, but increased approximately 25-fold upon feeding or insulin administration.
These findings led to the suggestion that resistin may play an important role in adipogenesis and metabolism by impairing glucose tolerance and insulin sensitivity in mice[48,49]. However, recent studies have shown contradictory results: 1) resistin mRNA expression was unchanged in white adipose tissue of adrenalectomized leptin-deficient ob/ob mice compared to wild-type mice, or reduced by obesity-inducing diet and fasting[82]; and 2) down-regulation of resistin mRNA was observed in adipose tissue in ob/ob and db/db murine models of type 2 diabetes as compared with wild-type controls[83]. In humans, using real-time RT-PCR, resistin gene expression was found neither in human muscle nor in most human isolated fat cells from overweight individuals and who had normal insulin sensitivity or were insulin-resistant or type 2 diabetic[84]. In contrast, McTernan et al.[85] found that resistin mRNA expression is similar in both omental and subcutaneous abdominal fat, but is significantly increased in abdominal depots when compared to fat from the thigh or breast. This could explain the increased risk of type 2 diabetes in patients with central obesity. Similarly, a significantly higher resistin mRNA expression in adipose tissue was found in patients with Prader-Willi syndrome (metabolically characterized by insulin resistance) than in both healthy lean controls and obese patients[86], and no association was found between serum resistin and insulin concentrations. The authors concluded that these results support a link between circulating resistin and obesity in humans but not a role for resistin in human insulin resistance, although a significant correlation has been observed between plasma concentration of resistin and insulin resistance determined by the homeostasis model assessment ratio (HOMA-R) formula[87]. Collectively, these data suggest that the role of resistin in glucose homeostasis and insulin resistance in human has not been clarified.
Resistin and inflammation
Adipocytokines are soluble mediators mainly produced by adipose tissue and include adiponectin, leptin, visfatin, and resistin as well as other cytokines such as TNF-α, IL-6, IL-1, and monocyte chemotactic protein (MCP)-1[46]. Although adiponectin and leptin are the most abundantly expressed adipocytokines in adipose tissue, resistin is primarily expressed in circulating monocytes[50,51] and macrophages[52]. Indeed, Anderson et al.[88] demonstrated in humans that resistin mRNA expression in adipose tissue is much lower than that of adiponectin and leptin and, while whole blood and monocyte resistin mRNA expression was high, adipose resistin mRNA expression was low or undetectable. In addition, after LPS-induced endotoxemia, the adipose resistin mRNA expression was modest compared to the marked resistin mRNA expression observed in blood.
Although resistin has been linked to insulin resistance, diabetes and obesity[47], compelling evidence of in vitro and in vivo studies support a role for resistin in inflammation. Kaser et al.[53] reported that resistin mRNA expression is increased in human PBMC exposed to IL-1β, IL-6, TNF-α, and LPS. In addition, Bokarewa et al.[54] demonstrated that: 1) resistin stimulation of PBMC led to increased expression of TNF-α, IL-1β, and IL-6 mRNA, as well as up-regulation of resistin mRNA itself. This was also accompanied by increased release of TNF-α, IL-1β, and IL-6 into the medium in a concentration-dependent manner; 2) the concentrations of cytokines released after stimulation with the highest resistin concentration were similar to those induced by LPS; and 3) resistin induced NF-κB activity and its translocation from the cytoplasm to the nucleus, and blockage of NF-κB with parthenolide produced a marked suppression of IL-6 activity in supernatants of PBMC. Silswal et al.[89] reported that recombinant human resistin induced secretion of TNF-α and IL-12 from murine and human macrophages similar to that obtained using LPS, and also induced nuclear translocation of NF-κB. Furthermore, Lehrke et al.[90] reported: 1) a dramatic increase in RETN gene expression and protein secretion when primary human macrophages were treated with LPS and when primary human macrophages were stimulated with TNF-α; 2) blockage of TNF-α with anti-TNF-α antibodies reduced the increase in resistin gene expression; 3) rosiglitazone has a marked anti-inflammatory effect on macrophages via PPARγ, and down-regulates resistin gene expression and protein secretion in LPS-stimulated macrophages; and 4) aspirin and rosiglitazone inhibit NF-κB, which is required for LPS induction of resistin in macrophages.
Resistin has been associated with acute infection. Serum resistin concentration is significantly higher in patients with severe sepsis or septic shock than in healthy controls[55]. The authors found a positive correlation between resistin and IL-6, IL-8, IL-10 and TNF-α serum concentration at 24 hours. Interestingly, the elevation of serum resistin concentration persisted at 96 hours, whereas IL-6, IL-8, IL-10 and TNF-α showed a significant decrease. In addition, resistin has been associated with chronic inflammation. Resistin mRNA expression is present in the liver and is up-regulated in patients with end-stage disease, alcoholic liver disease and hepatitis C virus-induced chronic hepatitis[56]. In addition, resistin is also present in the synovial fluid and tissue from patients with both rheumatoid arthritis and osteoarthritis[54,57,58]. Resistin concentration in synovial fluid is significantly higher in patients with rheumatoid arthritis than in those with osteoarthritis[54,57,58], its concentration in synovial fluid is higher than in serum[54,58], and it correlates significantly with synovial WBC count and IL-6 concentration in patients with rheumatoid arthritis[54]. Moreover, intra-articular injection of resistin in the knee joints of healthy mice caused arthritis, and histological examination revealed leukocyte infiltration of synovial tissue[54].
Collectively, there is compelling evidence suggesting that resistin has features of a pro-inflammatory cytokine and plays an important role in inflammation by eliciting cytokine production and NF-κB activation.
Resistin in human pregnancy
A limited number of studies have investigated resistin in maternal circulation. Recently, Nien et al.[61] found higher plasma resistin concentrations in pregnant women than in non-pregnant subjects, which is consistent with previous reports by Yura et al. [60], Cortelazzi et al. [62], and Palik et al.[63] In contrast, Chen et al.[64] reported that differences in serum resistin concentrations between non-pregnant and pregnant women are significant only in the third trimester. In addition, plasma resistin concentrations in normal pregnancy increase with gestational age[61]. This is in contrast with a previous report[62]; however, differences in study design and sample size may contribute to the differences between studies. Cortelazzi et al.[62] determined plasma resistin concentrations in patients with pregnancy complications and found no significant differences between normal pregnant women and patients with gestational diabetes, chronic hypertension and gestational hypertension. In contrast, patients with preeclampsia had a significantly lower plasma resistin concentration than women with normal pregnancies[62]. While this is consistent with a previous study[64], it is not with others reporting no differences between patients with mild and severe preeclampsia and those with a normal pregnancy[65], or higher plasma resistin concentrations in patients with preeclampsia compared to controls[66]. In addition, other studies have reported that the maternal serum concentration of resistin is significantly higher[67] or lower[68] in patients with gestational diabetes compared to those without.
Resistin has also been measured in umbilical cord blood. Cho et al.[91] found that the mean umbilical cord serum resistin concentration is significantly higher than that of the maternal circulation, and that no differences were observed between male and female neonates. Similar findings were reported in another study[62] which also found no differences in umbilical cord blood resistin concentration between either the umbilical artery and vein or between neonates from mothers with gestational diabetes and those from women with normal pregnancies. Moreover, Ng et al.[92] found that neonates born from mothers with preexisting or gestational diabetes who required exogenous insulin for glycemic control during pregnancy had an umbilical cord plasma resistin concentration significantly lower than neonates born from mothers with normal pregnancies or neonates born to patients with gestational diabetes that required only dietary treatment. In a different study, the same authors reported that the umbilical cord plasma resistin concentration is significantly higher in term than preterm neonates, as well as in neonates delivered vaginally compared to those by cesarean delivery[93]. Finally, in a study including monochorionic twins with twin-to-twin transfusion syndrome, umbilical cord serum resistin concentration correlated positively with birthweight. Furthermore, only approximately 40% of the small-for-gestational age twins had a resistin concentration lower than that of the normal co-twin[94].
Resistin in amniotic fluid
This study reports, for the first time, the identification of resistin in amniotic fluid. Resistin was detected in all amniotic fluid samples included in this study, suggesting that it is a physiologic constituent of the amniotic fluid. In addition, amniotic fluid resistin concentration increases with gestational age, which is in parallel to the results of previous studies reporting that the maternal plasma/serum resistin concentrations increase as gestation progresses. Moreover, previous studies[60,95,96] have found that resistin is expressed in the placenta and chorioamniotic membranes, and its mRNA expression is significantly higher in term placentas than in the chorionic villi in the first trimester, suggesting that the placenta may be the source of resistin in amniotic fluid. Sagawa et al.[95] and Yura et al.[60] identified resistin mRNA expression in the syncytiotrophoblast as well as in the decidua. In patients with elective cesarean section at term, resistin mRNA expression was higher in the placenta than in the subcutaneous fat from the abdominal wall[60]. Lappas et al.[96] determined resistin release from human placenta and fetal membranes, maternal subcutaneous adipose tissue and skeletal muscle from patients with gestational diabetes and those with normal pregnancies at the time of elective cesarean section at term. Tissue explants were incubated with and without LPS, TNF-α, IL-6, IL-8, phorbol myristate acetate (PMA, a potent activator of protein kinase C), glucose, insulin, dexamethasone, progesterone and estrogen. The authors demonstrated that, in basal conditions, all tissues secreted resistin and that placenta and choriodecidua secreted significantly more resistin than the amnion. In addition, PMA significantly increased the release of resistin from placenta and adipose tissue; insulin increased placental resistin release, whereas dexamethasone, progesterone and estrogen significantly decreased placental resistin release. The observation that resistin is expressed in the chorioamniotic membranes suggests that these tissues may contribute to the increased amniotic fluid concentration of resistin with advancing gestation and in patients with intra-amniotic infection/inflammation.
Resistin in intra-amniotic infection and inflammation
A novel observation of this study is that the median amniotic fluid resistin concentration was increased in patients with intra-amniotic infection/inflammation regardless of the membrane status, as well as in patients with clinical and histologic chorioamnionitis. Indeed, in patients with spontaneous preterm labor and intact membranes and those with PPROM who had intra-amniotic infection/inflammation, the median amniotic fluid resistin concentration was approximately 10-fold higher than in those without intra-amniotic infection/inflammation. In addition, among patients with spontaneous preterm labor who delivered within 72 hours from the amniocentesis, the median amniotic fluid resistin concentration was 17-fold higher in patients with histologic chorioamnionitis than in those without. Among patients with spontaneous preterm labor with intact membranes, those with IAI had a significantly higher median amniotic fluid concentration of resistin than those without IAI who delivered either preterm or at term, and no differences were found between the last two groups. Interestingly, the analysis including patients with spontaneous preterm labor without IAI who delivered within 48 hours and 7 days from the amniocentesis demonstrated that these subgroups had a significantly higher median amniotic fluid concentration of resistin than those who delivered at term, suggesting that resistin may play a role in the process of preterm parturition in the absence of intra-amniotic infection and inflammation. Alternatively, some patients with spontaneous preterm labor classified as without IAI could have subclinical infection and/or inflammation that may be responsible for initiating the parturition pathway.
In patients with spontaneous preterm labor as well as in those with PPROM, amniotic fluid resistin concentration significantly correlated with IL-6 concentration and WBC count, which are indices of intra-amniotic infection and inflammation[38,97-99]. It is possible that amniotic fluid WBC may be the source of resistin in the amniotic cavity. Since it has been proposed that amniotic fluid WBC are of fetal origin[100], and because the chorioamniotic membranes are considered to be fetal tissue, it is likely that the fetus may contribute to the increased concentration of resistin in the amniotic fluid. Other possible sources of resistin in amniotic fluid may be maternal, placenta, and decidua.
We tested the diagnostic performance of resistin concentration in amniotic fluid to identify a positive amniotic fluid culture for microorganisms and intra-amniotic inflammation (defined as an IL-6 amniotic fluid concentration >2.6 ng/mL[14]) in patients with preterm labor and intact membranes. There was no difference in the identification of microbial invasion of the amniotic cavity and intra-amniotic inflammation, since the area under the ROC curve was almost identical for both (93.2% and 93.3%, respectively; see Table IV). We focus on the diagnostic performance of resistin for identification of intra-amniotic inflammation because the prevalence of intra-amniotic inflammation is approximately twice of that of intra-amniotic infection in patients with spontaneous preterm labor and intact membranes. In addition, the outcome of patients with microbiologically proven intra-amniotic infection is similar to that of patients with intra-amniotic inflammation and a negative amniotic fluid culture[14]. Amniotic fluid resistin concentration had a sensitivity of 85.4% and a specificity of 94.3% for the diagnosis of intra-amniotic inflammation using a cutoff of ≥37 ng/mL derived from a ROC curve. Using this cutoff in patients with spontaneous preterm labor, the median amniocentesis-to-delivery interval was significantly shorter in patients with an amniotic fluid resistin concentration ≥37 ng/mL than in those with <37 ng/mL (3 days vs. 43 days; p<0.001). Similarly, amniotic fluid resistin concentration was related to the detection of histological evidence of chorioamnionitis in patients with spontaneous preterm labor who delivered within 72 hours of amniocentesis. Among patients who had elevated resistin in amniotic fluid (≥37 ng/mL) and delivered within 72 hours, 71% of them (30/42) had histologic chorioamnionitis and/or funisitis.
The findings reported herein support the view that resistin is a pro-inflammatory cytokine[46,49,54,89], and suggest that resistin participates in the host response to intrauterine infection or is involved in the inflammatory response observed in patients with clinical and histologic chorioamnionitis. These results are in agreement with those reported for visfatin in amniotic fluid, another adipocytokine with pro-inflammatory characteristics[101].
Conclusions
This is the first study describing the presence of resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. Collectively, these results suggest that resistin plays a role in normal gestation, preterm parturition and in the inflammatory process that occurs during intra-amniotic infection/inflammation. Moreover, the concentration of resistin in amniotic fluid relates to the risk for impending preterm delivery and histological chorioamnionitis.
Acknowledgment
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin.Obstet.Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 2.Minkoff H. Prematurity: infection as an etiologic factor. Obstet.Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 3.Romero R, Mazor M. Infection and preterm labor. Clin.Obstet.Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin.Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 5.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 6.Ledger WJ. Infection and premature labor. Am.J.Perinatol. 1989;6:234–236. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am.J.Obstet.Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 8.Brocklehurst P. Infection and preterm delivery. BMJ. 1999;318:548–549. doi: 10.1136/bmj.318.7183.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N.Engl.J.Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 10.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment.Retard.Dev.Disabil.Res.Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc.Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, Bracken MB. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am.J.Obstet.Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 14.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 15.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am.J.Obstet.Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 16.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am.J.Obstet.Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 17.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am.J.Obstet.Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 18.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr.Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am.J.Obstet.Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am.J.Obstet.Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 21.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, Kuban K, Van Marter LJ, Pagano M, Hegyi T, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr.Res. 1999;46:566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am.J.Obstet.Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 23.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr.Opin.Pediatr. 2000;12:99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am.J.Obstet.Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 25.Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann.Periodontol. 2001;6:153–163. doi: 10.1902/annals.2001.6.1.153. [DOI] [PubMed] [Google Scholar]
- 26.Patrick LA, Smith GN. Proinflammatory cytokines: a link between chorioamnionitis and fetal brain injury. J.Obstet.Gynaecol.Can. 2002;24:705–709. doi: 10.1016/s1701-2163(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 27.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 28.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat.Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J.Obstet.Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 30.Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol.Toxicol.Methods. 1998;39:147–154. doi: 10.1016/s1056-8719(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am.J.Obstet.Gynecol. 2006;194:1334–1340. doi: 10.1016/j.ajog.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 33.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet.Gynecol. 1989;73:31–34. [PubMed] [Google Scholar]
- 34.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin.Invest. 1989;83:430–436. doi: 10.1172/JCI113901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur.J.Obstet.Gynecol.Reprod.Biol. 1991;41:123–127. doi: 10.1016/0028-2243(91)90089-4. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. American Journal of Obstetrics and Gynecology. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J.Clin.Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta, -1 alpha, and -6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J.Clin.Endocrinol.Metab. 1993;77:805–815. doi: 10.1210/jcem.77.3.8370702. [DOI] [PubMed] [Google Scholar]
- 40.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet.Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 41.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod.Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 42.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–612. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 43.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, Edwin SS. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–141. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 44.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intraamniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 45.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, Vaisbuch E, Than NG, Mazaki-Tovi S, Chaiworapongsa T, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation and spontaneous parturition at term: A role for Interleukin-10. J Matern.Fetal Neonatal Med. 2008 doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat.Rev.Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 47.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 48.Steppan CM, Lazar MA. The current biology of resistin. J.Intern.Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 49.McTernan PG, Kusminski CM, Kumar S. Resistin. Curr.Opin.Lipidol. 2006;17:170–175. doi: 10.1097/01.mol.0000217899.59820.9a. [DOI] [PubMed] [Google Scholar]
- 50.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 51.Lu SC, Shieh WY, Chen CY, Hsu SC, Chen HL. Lipopolysaccharide increases resistin gene expression in vivo and in vitro. FEBS Lett. 2002;530:158–162. doi: 10.1016/s0014-5793(02)03450-6. [DOI] [PubMed] [Google Scholar]
- 52.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem.Biophys.Res.Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 53.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem.Biophys.Res.Commun. 2003;309:286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J.Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 55.Sunden-Cullberg J, Nystrom T, Lee ML, Mullins GE, Tokics L, Andersson J, Norrby-Teglund A, Treutiger CJ. Pronounced elevation of resistin correlates with severity of disease in severe sepsis and septic shock. Crit Care Med. 2007;35:1536–1542. doi: 10.1097/01.CCM.0000266536.14736.03. [DOI] [PubMed] [Google Scholar]
- 56.Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Gines P, et al. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am.J.Pathol. 2006;169:2042–2053. doi: 10.2353/ajpath.2006.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J, Muller-Ladner U, Gay S. Adipocytokines in synovial fluid. JAMA. 2003;290:1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 58.Senolt L, Housa D, Vernerova Z, Jirasek T, Svobodova R, Veigl D, Anderlova K, Muller-Ladner U, Pavelka K, Haluzik M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann.Rheum.Dis. 2007;66:458–463. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Axelsson J, Bergsten A, Qureshi AR, Heimburger O, Barany P, Lonnqvist F, Lindholm B, Nordfors L, Alvestrand A, Stenvinkel P. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006;69:596–604. doi: 10.1038/sj.ki.5000089. [DOI] [PubMed] [Google Scholar]
- 60.Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, Korita D, Takemura M, Fujii S. Resistin is expressed in the human placenta. J.Clin.Endocrinol.Metab. 2003;88:1394–1397. doi: 10.1210/jc.2002-011926. [DOI] [PubMed] [Google Scholar]
- 61.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Pineles BL, Friel LA, Espinoza J, Goncalves L, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J.Perinat.Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, Cetin I, Cortelazzi R, Beck-Peccoz P, Spada A. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin.Endocrinol.(Oxf) 2007;66:447–453. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 63.Palik E, Baranyi E, Melczer Z, Audikovszky M, Szocs A, Winkler G, Cseh K. Elevated serum acylated (biologically active) ghrelin and resistin levels associate with pregnancy-induced weight gain and insulin resistance. Diabetes Res.Clin.Pract. 2007;76:351–357. doi: 10.1016/j.diabres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Chen D, Dong M, Fang Q, He J, Wang Z, Yang X. Alterations of serum resistin in normal pregnancy and pre-eclampsia. Clin.Sci.(Lond) 2005;108:81–84. doi: 10.1042/CS20040225. [DOI] [PubMed] [Google Scholar]
- 65.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, Cotton DB. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am.J.Obstet.Gynecol. 2005;193:979–983. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 66.Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am.J.Physiol Endocrinol.Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 67.Chen D, Fang Q, Chai Y, Wang H, Huang H, Dong M. Serum resistin in gestational diabetes mellitus and early postpartum. Clin.Endocrinol.(Oxf) 2007;67:208–211. doi: 10.1111/j.1365-2265.2007.02862.x. [DOI] [PubMed] [Google Scholar]
- 68.Megia A, Vendrell J, Gutierrez C, Sabate M, Broch M, Fernandez-Real JM, Simon I. Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition. Eur.J.Endocrinol. 2008;158:173–178. doi: 10.1530/EJE-07-0671. [DOI] [PubMed] [Google Scholar]
- 69.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet.Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000]. Rev.Med.Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 71.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J.Infect.Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 72.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J.Matern.Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am.J.Obstet.Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am.J.Obstet.Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 75.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am.J.Obstet.Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 76.Bonferroni C. Il calcolo delle assicurazioni su gruppi di teste. 1935:13–60. [Google Scholar]
- 77.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, et al. A family of tissue-specific resistin-like molecules. Proc.Natl.Acad.Sci.U.S.A. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerstmayer B, Kusters D, Gebel S, Muller T, Van Miert E, Hofmann K, Bosio A. Identification of RELMgamma, a novel resistin-like molecule with a distinct expression pattern. Genomics. 2003;81:588–595. doi: 10.1016/s0888-7543(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 79.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 80.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J.Biol.Chem. 2001;276:11252–11256. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 82.Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am.J.Physiol Endocrinol.Metab. 2002;283:E1266–E1271. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- 83.Rajala MW, Lin Y, Ranalletta M, Yang XM, Qian H, Gingerich R, Barzilai N, Scherer PE. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol.Endocrinol. 2002;16:1920–1930. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- 84.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem.Biophys.Res.Commun. 2001;285:561–564. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 85.McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–47. doi: 10.1016/s0140-6736(02)07281-1. [DOI] [PubMed] [Google Scholar]
- 86.Pagano C, Marin O, Calcagno A, Schiappelli P, Pilon C, Milan G, Bertelli M, Fanin E, Andrighetto G, Federspil G, et al. Increased serum resistin in adults with prader-willi syndrome is related to obesity and not to insulin resistance. J.Clin.Endocrinol.Metab. 2005;90:4335–4340. doi: 10.1210/jc.2005-0293. [DOI] [PubMed] [Google Scholar]
- 87.Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur.J.Endocrinol. 2003;149:331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 88.Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, Tabita-Martinez J, Sellers KF, Rickels MR, Ahima RS, et al. Innate immunity modulates adipokines in humans. J.Clin.Endocrinol.Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 89.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem.Biophys.Res.Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 90.Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS.Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho GJ, Yoo SW, Hong SC, Oh MJ, Kim T, Kim HJ, Lee KW, Kim SH. Correlations between umbilical and maternal serum resistin levels and neonatal birth weight. Acta Obstet.Gynecol.Scand. 2006;85:1051–1056. doi: 10.1080/00016340500470150. [DOI] [PubMed] [Google Scholar]
- 92.Ng PC, Lee CH, Lam CW, Wong E, Chan IH, Fok TF. Plasma ghrelin and resistin concentrations are suppressed in infants of insulin-dependent diabetic mothers. J.Clin.Endocrinol.Metab. 2004;89:5563–5568. doi: 10.1210/jc.2004-0736. [DOI] [PubMed] [Google Scholar]
- 93.Ng PC, Lee CH, Lam CW, Chan IH, Wong E, Fok TF. Resistin in preterm and term newborns: relation to anthropometry, leptin, and insulin. Pediatr.Res. 2005;58:725–730. doi: 10.1203/01.PDR.0000180556.76864.9A. [DOI] [PubMed] [Google Scholar]
- 94.Gohlke BC, Bartmann P, Fimmers R, Huber A, Hecher K, Roth CL. Fetal adiponectin and resistin in correlation with birth weight difference in monozygotic twins with discordant growth. Horm.Res. 2008;69:37–44. doi: 10.1159/000111794. [DOI] [PubMed] [Google Scholar]
- 95.Sagawa N, Yura S, Itoh H, Mise H, Kakui K, Korita D, Takemura M, Nuamah MA, Ogawa Y, Masuzaki H, et al. Role of leptin in pregnancy--a review. Placenta. 2002;23(Suppl A):S80–S86. doi: 10.1053/plac.2002.0814. [DOI] [PubMed] [Google Scholar]
- 96.Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J.Endocrinol. 2005;186:457–465. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 97.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found.Symp. 1992;167:205–220. doi: 10.1002/9780470514269.ch13. [DOI] [PubMed] [Google Scholar]
- 98.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am.J.Reprod.Immunol. 1993;30:167–183. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 99.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 100.Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, Jack RM. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am.J.Obstet.Gynecol. 1997;176:77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 101.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, Nhan-Chang C, Hamill N, Vaisbuch E, et al. Visfatin/Pre-B Cell Colony-Enhancing Factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat.Med. 2008 doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]