Abstract
Nucleotide-binding oligomerization domain 2 (NOD2) is involved in innate immune responses to peptidoglycan degradation products. Peptidoglycans are important mediators of organic dust-induced airway diseases in exposed agriculture workers; however, the role of NOD2 in response to complex organic dust is unknown. Monocytes/macrophages were exposed to swine facility organic dust extract (ODE), whereupon NOD2 expression was evaluated by real-time PCR and Western blot. ODE induced significant NOD2 mRNA and protein expression at 24 and 48 h, respectively, which was mediated via a NF-κB signaling pathway as opposed to a TNF-α autocrine/paracrine mechanism. Specifically, NF-κB translocation increased rapidly following ODE stimulation as demonstrated by EMSA, and inhibition of the NF-κB pathway significantly reduced ODE-induced NOD2 expression. However, there was no significant reduction in ODE-induced NOD2 gene expression when TNF-α was inhibited or absent. Next, it was determined whether NOD2 regulated ODE-induced inflammatory cytokine production. Knockdown of NOD2 expression by small interfering RNA resulted in increased CXCL8 and IL-6, but not TNF-α production in response to ODE. Similarly, primary lung macrophages from NOD2 knockout mice demonstrated increased IL-6, CXCL1, and CXCL1, but not TNF-α, expression. Lastly, a higher degree of airway inflammation occurred in the absence of NOD2 following acute (single) and repetitive (3 wk) ODE exposure in an established in vivo murine model. In summary, ODE-induced NOD2 expression is directly dependent on NF-κB signaling, and NOD2 is a negative regulator of complex, organic dust-induced inflammatory cytokine/chemokine production in mononuclear phagocytes.
Keywords: cytokines, chemokines, monocyte/macrophage, swine/hog/pig, gram-positive components, inflammation, pattern recognition receptor, pathogen-associated molecular patterns, siRNA, airway, organic dust extract
chronic inhalation of organic dust in agricultural environments, particularly from animal farming facilities, results in significant airway diseases including chronic bronchitis, occupational asthma, and obstructive lung disease (9). Organic dust is recognized to be a complex mixture containing microbial-rich components, also referred to as pathogen-associated molecular patterns (PAMPs), that can activate host defense responses via recognition by pattern recognition receptors (PRRs). Lipopolysaccharide (LPS), a major component of the outer cell wall of gram-negative bacteria, is a PAMP recognized by the PRR Toll-like receptor 4 (TLR4). Although important, LPS levels are not consistently associated with inflammatory outcomes in swine confinement workers (6, 35, 43), and mice deficient in TLR4 signaling are not completely protected against swine barn air-induced airway inflammation (5). Recent molecular biology techniques show that bioaerosols in animal environments are dominated by an incredibly diverse population of gram-positive bacteria (10, 25, 26, 28). Moreover, levels of muramic acid, a marker of peptidoglycan (PGN) derived predominately from gram-positive bacteria (i.e., ∼85% of cell wall), but also gram-negative bacteria (i.e., ∼5% of cell wall), are associated with airway inflammatory outcomes in humans following swine barn exposure (48). We have also shown that large-animal farming confinement environments (e.g., swine and dairy) contain high levels of muramic acid, and, furthermore, depletion of LPS from these dusts results in significant retention of innate immune inflammatory responses in vitro (30, 31, 34). Thus targeting PRRs recognized by bacterial PGN breakdown products might reveal potential novel therapeutic pathways that could ultimately be modulated to reduce airway inflammatory outcomes in exposed workers.
Nucleotide oligomerization domain 2 (NOD2), a member of the NACHT-LRR protein family, is a potential novel target because it senses muramyl dipeptide, a component of virtually all types of bacterial-derived PGN (39). NOD2 is constitutively expressed intracellularly by mononuclear phagocytes, which are key innate immune cells that are rapidly activated by exposure to inhaled toxins such as organic dust to induce pyrexia, neutrophil recruitment, cytokine/chemokine release, and activation of airway epithelial cells (1, 21, 33, 39). Interest in NOD2 has also risen because mutations in the receptor are associated with several inflammatory diseases such as Crohn's disease, sarcoidosis, Blau syndrome, graft-vs.-host disease, and tuberculosis (2, 4, 22, 38, 42). NOD2 activation results in production of proinflammatory mediators through activation of NF-κB and mitogen-activated protein kinase (MAPK) pathways (39). However, the role of NOD2 in mediating disease is complicated in that, depending on the type and/or route of insult, NOD2 can act as either a positive or negative regulator of innate immune responses (12, 15, 46).
In this study, we hypothesized that NOD2 is important in the innate immune response to a complex, PGN-enriched, swine confinement facility organic dust extract (ODE) in mononuclear phagocytes. Using a combinational approach, we first show that ODE exposure increases mononuclear phagocyte NOD2 expression via NF-κB activation. In contrast to previous studies demonstrating that TNF-α is responsible for NOD2 expression in intestinal and bronchial epithelial cells (11, 20, 37), autocrine/paracrine TNF-α elicited following ODE exposure was not responsible for augmenting NOD2 expression in mononuclear phagocytes. Next, we demonstrate a functional role for NOD2 in mediating ODE-induced proinflammatory parameters. These studies found that NOD2 loss augments the expression of select inflammatory mediators following ODE stimulation in vitro and in vivo. Overall, these findings suggest that the NOD2 pathway could be manipulated to attenuate the excessive inflammation that is characteristic of individuals repeatedly exposed to organic dust in swine confinement facilities.
METHODS
Organic dust extract.
Organic dust extract (ODE) was prepared as previously described (30, 31, 36). Briefly, settled surface dust samples were obtained from local swine confinement animal feeding operation facilities, placed into solution, and centrifuged, and final supernatant was filter (0.22 μm) sterilized (a process that also removes coarse particles) and frozen in aliquots at −20°C. The aqueous extract (ODE) was diluted to a final concentration of 1% (vol/vol) in growth medium for in vitro experiments. The diluted 1% ODE, which has previously been determined to elicit optimal proinflammatory mediator release from mononuclear phagocytes, contains 20–40 μg/ml of total protein and is not cytotoxic for the time periods tested (1–72 h; Ref. 30). Endotoxin levels in the 1% ODE utilized in these experimental studies ranged from 1.8 to 3.9 EU/ml as assayed by using the limulus amebocyte lysate assay according to manufacturer's instruction (Sigma, St. Louis, MO), and these levels have consistently been shown not to be solely responsible for ODE-induced proinflammatory responses (30, 31, 34, 36). Previously published gas chromatography-tandem mass spectrometry analysis revealed high muramic acid [mean: 203.5 ng/mg (ODE) vs. 25 ng/mg (domestic home comparison); muramic acid is a marker of PGN] (30). These findings of high PGN/muramic acid in swine confinement facility dust extracts were confirmed in a subsequent independent study (31).
Mouse strains.
C57BL/6 wild-type (WT), NOD2 knockout (KO; C57BL/6 background; strain: B6.129S1-Nod2tm1Flv/J), and TNF-α KO (C57BL/6 background; strain: B6.129S-Tnftm1Gkl/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were used for studies approved by the Institutional Animal Care and Use Committee of the Omaha Veterans Affairs Medical Center and the University of Nebraska Medical Center according to National Institutes of Health (NIH) guidelines for the use of rodents.
Cell populations and cell culture assays.
The human promonocytic THP-1 and the murine alveolar macrophage (MH-S) cell line (American Type Culture Collection, Manassas, VA) were utilized. Cells were maintained in complete l-glutamine-RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA), 2-mercaptoethanol (5 × 10−5 M), and 50 μg/ml of penicillin/streptomycin (Invitrogen). For small interfering RNA (siRNA)-mediated knockdown experiments, antibiotic-free and/or serum-free medium was used. Mononuclear phagocyte cell lines were utilized since large numbers of cells were required for several of the experimental assays that could not be adequately obtained from animal or human species. Importantly, key experiments were performed with primary macrophages to confirm findings obtained with these cell lines.
Mouse primary lung macrophages were obtained after animals were euthanized and the right ventricle was perfused with sterile PBS (pH 7.4) to remove circulating blood cells from the pulmonary vasculature. Whole lungs were isolated, minced, and digested in a solution containing collagenase type I (324 U/ml; Fisher, Pittsburgh, PA), bovine DNase (75 U/ml), porcine heparin (25 U/ml), and PBS with Ca2+ and Mg2+ and rocked for 30 min. Lung tissues were dissociated in an automated fashion by using the gentleMAC Dissociator instrument according to manufacturer's instructions (Miltenyi Biotec, Auburn, CA). After passing lung solution through nylon mesh (40 μm; Fisher) to remove any large fragments and lysing red blood cells, single cell suspensions were underlaid with lymphocyte separation solution (Fisher) and centrifuged at 400 g for 20 min to collect mononuclear cells. The mononuclear cells were incubated for 2 h in culture medium and enriched for lung macrophages by removal of nonadherent cells. Lung macrophage yield was >92% as determined by Giemsa staining. Lung macrophages were then immediately utilized in experimental assays.
At the completion of all cell culture incubation intervals, cell-free supernatants were collected and frozen at −20°C for later cytokine/chemokine analysis, and an aliquot of cells was counted by hematocytometer and assessed for viability by the Trypan blue exclusion method. Cell pellets were either stored in RNAlater buffer (Applied Biosystems) at 4°C for 24 h before being stored at −20°C until RNA extraction or immediately lysed and utilized for Western blot studies.
Real-time quantitative RT-PCR.
RNA was extracted from cell pellets by using the Magmax 96 kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. RNA concentration and purity was determined by NanoDrop spectrophotometer and samples had A260-to-A280 ratio of 1.9–2.0. cDNA was synthesized by using 100 ng of template RNA and a TaqMan reverse transcription kit (Applied Biosystems) as previously described (3). Real-time PCR reactions were prepared in triplicate using 1× TaqMan Master Mix (Applied Biosystems) and primers and probes for NOD2 (Applied Biosystems; human NOD2: Hs00223394_ml; murine NOD2: Mm00467543_m1). Ribosomal (18s) RNA was used as an endogenous control. PCR was performed by using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Threshold values were normalized to the expression of ribosomal RNA. Real-time PCR results are expressed either as the percent fold increase in induction (normalized copy number of stimulated cells divided by normalized copy number of unstimulated cells × 100) or as values normalized to expression of ribosomal RNA. For siRNA-mediated knockdown studies, the percentage of relative gene expression was calculated as the amount of NOD2 mRNA in cultures transfected with NOD2 and stimulated for 24 h with 1% ODE compared with that of cells transfected with the nontargeting control siRNA and stimulated with 1% ODE, which was set to 100%.
Western blot analysis.
As previously described (3), THP-1 monocytes (5 × 106/well) were lysed and protein concentrations were determined by a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). Each well was loaded with 30 μg of total protein before PAGE and electroblotting to nitrocellulose. A mouse anti-human NOD2 (CARD15) monoclonal antibody (2D9; Abcam, Cambridge, MA: ab31488) was used at a 2 μg/ml dilution and was detected with a hamster anti-mouse horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, Boston, MA) at a 1:1,000 dilution. Blots were imaged by use of SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific Pierce, Rockford, IL) and exposed to X-ray film. β-Actin loading controls were performed to ensure loading of equal amounts of protein. The blots were scanned and densitometry was performed by use of NIH ImageJ software (http://rsb.info.nih.gov/ij/).
NF-κB EMSA.
THP-1 monocytes (1 × 107) were incubated for 0, 15, 30, 60, and 90 min with 1% ODE, whereupon nuclear lysates were harvested according to Panomics (Fremont, CA) nuclear extraction protocol to detect NF-κB nuclear translocation. Protein concentrations were quantified by spectrophotometry. Next, 5 μg of nuclear extract was utilized to determine NF-κB p65 binding by EMSA (Panomics, EMSA kit), according to manufacturer's instructions.
RNA interference.
The HiPerfect transfection reagent kit (Qiagen, Valencia, CA) for transfection of nontargeting and targeting siRNAs was used. ON-TARGETplus SMARTpool siRNAs targeting human NOD2 transcripts were obtained from Dharmacon (Chicago, IL). To induce adherent cells required for transfection, THP-1 cells (2 × 104 cells/well) were pretreated in 24-well plates with 0.05 μM 1α,25-dihydroxyvitamin D3 (Calbiochem-Nova Biochem International, La Jolla, CA) for 48 h prior to transfection (7). Although vitamin D has been reported to increase NOD2 expression (45), prior studies with an alternative agent to induce adherence for optimal transfection conditions, phorbol myristic acid, resulted in unacceptable cellular activation and cellular toxicity (data not shown). On the day of transfection, media and 25-dihydroxyvitamin D3 were removed and replaced with 100 μl of antibiotic-free, serum-free medium. Single transfection complexes (100 μl) were generated by vortexing and incubating 100 nM siRNA with 3 μl of HiPerfect transfection reagent at room temperature for 10 min in serum-free, antibiotic-free medium. A volume of 100 μl of the transfection complex was added to each well containing 100 μl of cells. The plates were incubated at 37°C for 6 h, after which 400 μl of fresh antibiotic-free medium with serum was added to each well. Cells were incubated for an additional 18 h, whereupon conditioned medium was removed and replaced with fresh antibiotic-free medium containing serum immediately prior to ODE stimulation. Next, NOD2 and nontargeting siRNA-transfected cells were stimulated for 24 h with 1% ODE or vehicle control media. As assessed by Trypan blue exclusion at all time points, transfection complexes were not cytotoxic to THP-1 cells (>96% viability after transfection).
Cytokine/chemokine assays.
Human TNF-α, IL-6, and CXCL8/IL-8 proteins were assayed by sandwich ELISA as previously published with lower limit of detection at 15.6 pg/ml, 15.6 pg/ml, and 156.3 pg/ml, respectively (30, 36). Murine TNF-α, IL-6, keratinocyte chemoattractant (CXCL1), and macrophage inflammatory protein-2 (CXCL2) concentrations were determined according to manufacturer's instructions by using commercially available ELISA kits with lower limit of detection at 15.6, 7.8, 7.8, and 7.8 pg/ml, respectively (R&D Systems, Minneapolis, MN). Cytokine secretion was reported as concentration (pg/ml).
Mouse model of ODE-induced airway inflammation.
Mice received saline or 12.5% ODE (optimal concentration for eliciting lung inflammation) by an intranasal exposure method as previously established (33). Bronchoalveolar lavage fluid was collected as previously described (33). Total cell number of pooled lavages (3 × 1 ml lavages) was enumerated, and differential cell counts were determined on cytospin-prepared slides (Cytopro Cytocentrifuge, Wescor, Logan, UT) stained with DiffQuick (Dade Behring, Newark, DE). Cell-free supernatant from the first lavage was collected and frozen at −20°C until later cytokine/chemokine analysis. Whole lungs were excised after lavage and inflated to 10 cmH2O pressure with 10% formalin (Sigma) solution to preserve pulmonary architecture. Lungs were embedded in paraffin, and sections (4–5 μM) were cut, stained with hematoxylin and eosin, microscopically reviewed, and semiquantitatively assessed for the degree of inflammation as well as the distribution of the inflammation by a reviewer (pathologist, W. W. West) blinded to the treatment conditions utilizing a previously published scoring system (33).
Reagents.
Staphylococcus aureus PGN (10 μg/ml is comparable to the protein concentration in 1% ODE [∼2-fold less]) and Escherichia coli LPS O55:B5 (1 μg/ml is ∼20-fold more than the LPS concentration detected in 1% ODE) were purchased from Sigma. For LPS-independent ODE assays, polymyxin B (50 mg/ml; Sigma) plus ODE was utilized. Anti-human TNF-α neutralizing and isotype control antibodies were purchased from R&D Systems. The pharmaceutical fusion protein TNF-α inhibitor etanercept was obtained from Amgen/Pfizer (Thousand Oaks, CA). To inhibit the NF-κB pathway, caffeic acid phenethyl ester (CAPE; Ref. 27) and BAY 11-7082 (29) were purchased from Calbiochem (San Diego, CA). All other reagents not specified were purchased from Sigma.
Statistical analysis.
All data are presented as means ± SE. One-way ANOVA with Tukey multiple-comparison posttest was employed to compare NOD2 expression or NF-κB activation to different concentrations of cytokines and inhibitors + ODE across all time points examined. To detect significant changes between two groups, statistics were performed by two-tailed, nonpaired, or paired t-tests as appropriate by use of GraphPad Prism (version 5.0) software. In all analyses, P values less than 0.05 were considered statistically significant.
RESULTS
Organic dust exposure increases mononuclear phagocyte NOD2 expression.
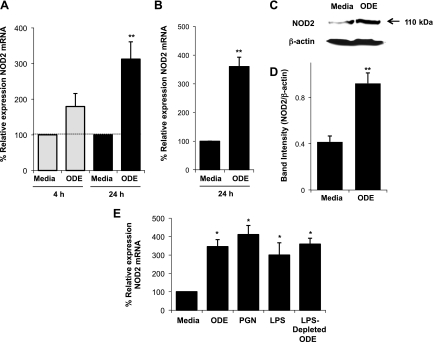
To determine whether organic dust would augment expression of cytosolic NOD2, mononuclear phagocytes were stimulated with 1% ODE, whereupon NOD2 mRNA and protein levels were assessed by quantitative RT-PCR (qRT-PCR) and Western blotting, respectively. NOD2 mRNA expression was significantly increased in human THP-1 monocytes at 24 h in response to ODE treatment (Fig. 1A; n = 4 independent studies; P = 0.003). NOD2 mRNA expression was also significantly increased at 24 h in MH-S alveolar macrophages following 1% ODE stimulation (Fig. 1B; n = 4 independent studies; P = 0.004). At 48 h, NOD2 protein levels were significantly increased following ODE stimulation compared with media control in THP-1 monocytes (representative blot in Fig. 1C; Fig. 1D depicts densitometry of n = 6 independent experiments; P = 0.009). In addition, ODE upregulation of NOD2 was independent of endotoxin given that there was a significant increase in NOD2 gene expression following stimulation of cells with 1% ODE pretreated with polymyxin B (Fig. 1E), a process that removes endotoxin (34). The PAMPs PGN (10 μg/ml) and LPS (1 μg/ml) also significantly increased NOD2 mRNA expression compared with media control (Fig. 1E). Collectively, these studies demonstrate that ODE and its respective microbial wall components increase mononuclear phagocyte NOD2 expression.
Fig. 1.
Organic dust extract (ODE) stimulated nucleotide-binding oligomerization domain 2 (NOD2) expression. ODE (1%) increased NOD2 mRNA at 24 h in human THP-1 monocytes (A) and MH-S murine alveolar macrophages (B). Data are expressed as percent fold change in NOD2 compared with media control ± SE (n = 4 independent experiments). NOD2 protein levels are increased following 1% ODE stimulation at 48 h by Western blotting in THP-1 monocytes. Results are presented as the raw gel data (C) and quantitative analysis of NOD2 expression normalized against β-actin loading control by densitometry (band intensity of NOD2/β-actin; mean ± SE of 6 independent experiments) (D). Peptidoglycan (PGN, 10 μg/ml), lipopolysaccharide (LPS, 1 μg/ml), and LPS-depleted 1% ODE also significantly increased NOD2 mRNA in THP monocytes (E; n = 4 independent experiments). Statistical significance: *P < 0.05 and **P < 0.01 compared with media alone.
Organic dust-induced NOD2 expression is not explained by autocrine/paracrine action of TNF-α.
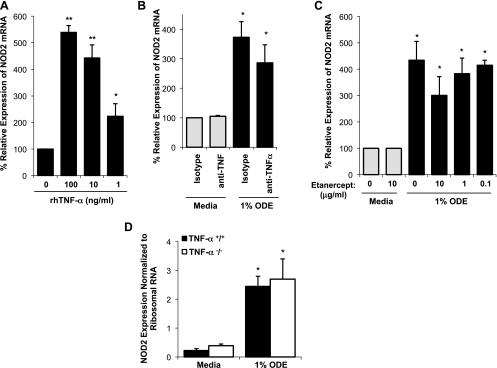
Because others have demonstrated that TNF-α is responsible for NOD2 expression in intestinal and bronchial epithelial cells (11, 20, 37), and ODE is well recognized to induce TNF-α in human airway diseases and mononuclear phagocytes (34, 44), we next investigated the role of TNF-α in mediating ODE-induced NOD2 expression by an autocrine/paracrine mechanism. Although human recombinant TNF-α alone induced a significant increase in NOD2 expression in THP-1 monocytes at 24 h (Fig. 2A; n = 3; P = 0.005), neutralization or deletion of TNF-α activity did not dramatically reduce ODE-induced NOD2 expression. Pretreatment with a neutralizing antibody to human TNF-α (1 μg/ml) resulted in a modest, nonsignificant reduction in ODE-induced NOD2 gene expression (Fig. 2B; n = 3; P > 0.05). Likewise, inhibition of TNF-α by the fusion protein etanercept had no significant effect on NOD2 expression (Fig. 2C; n = 3; P > 0.05). There was also no difference in NOD2 mRNA levels in primary lung macrophages from TNF-α KO (TNF-α−/−) mice stimulated with ODE ex vivo compared with lung macrophages from WT (TNF-α+/+) mice stimulated with ODE in side-by-side experiments (Fig. 2D; n = 4 mice/group; P > 0.05). NOD2 expression following PGN or LPS stimulation in TNF-α KO lung macrophages was also comparable to WT lung macrophages (data not shown). Of note, we did not find a role for IL-6 or CXCL8/IL-8 in regulating NOD2 mRNA expression alone or in negating ODE-induced NOD2 expression (data not shown). Collectively, these studies demonstrate that upregulation of mononuclear phagocyte NOD2 expression by organic dust is not entirely dependent on a TNF-α autocrine/paracrine mechanism and highlight key differences in NOD2 regulation between phagocytes and epithelial cells.
Fig. 2.
ODE-induced NOD2 gene expression in mononuclear phagocytes is not exclusively mediated by TNF-α. Although various concentrations of human recombinant TNF-α (rhTNF-α) resulted in increased NOD2 gene expression at 24 h (A), blocking TNF-α by an anti-TNF-α (1 μg/ml) monoclonal antibody (B) or by etanercept (fusion protein) did not significantly reduce NOD2 expression (C). Data are expressed as percent fold change in NOD2 compared with media control (mean ± SE; n = 3 independent experiments). There was also no difference in ODE-induced NOD2 mRNA as normalized to ribosomal 18s in isolated TNF-α −/− lung macrophages compared with wild-type (TNF-α+/+) lung macrophages (D). Statistical significance: *P < 0.05 and **P < 0.01 compared with media control (no ODE).
NF-κB-dependent signaling is pivotal for ODE-induced NOD2 expression.
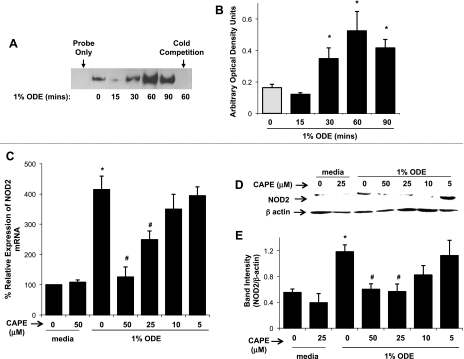
The finding that ODE-induced NOD2 expression was not prevented by TNF-α neutralization suggested that alternative mediator(s) participate in the ODE-dependent increase in NOD2 expression. Because the NOD2 promoter contains a putative NF-κB binding site (14), we next investigated whether NF-κB could be activated in cells following ODE treatment. Indeed, NF-κB p65 translocation was increased at 30–90 min following 1% ODE stimulation as analyzed by EMSA (representative blot in Fig. 3A; Fig. 3B depicts arbitrary density optical units of n = 6 independent experiments; P = 0.0016). Next, THP-1 monocytes were pretreated with various concentrations of the NF-κB inhibitor CAPE (24) for 1 h and subsequently stimulated with 1% ODE for 24 h to determine effects on NOD2 expression. Inhibition of NF-κB signaling led to a dose-dependent inhibition of NOD2 gene expression as determined by qRT-PCR (Fig. 3C; n = 4 independent experiments). This finding was confirmed at the protein level at 48 h following ODE stimulation by Western blotting (Fig. 3D depicts a representative blot of 1 of 4 independent experiments and Fig. 3E depicts densitometry of n = 4 independent experiments). Inhibition of NF-κB signaling by use of another inhibitor, BAY 11-7082, which blocks IκBα phosphorylation (29), also led to a significant inhibition of ODE-induced NOD2 mRNA (10 μM BAY 11-7082 reduced 1% ODE-induced NOD2 expression by 63% and 7.5 μM BAY 11-7082 reduced ODE-induced NOD2 expression by 45% compared with ODE-induced NOD2 expression without the inhibitor, n = 4 independent experiments, P < 0.05). Cell viability by Trypan blue staining revealed that CAPE and BAY 11-7082 were not cytotoxic (>98% viability) at any of the concentrations examined, which indicates that the effects observed were not due to cell death. Furthermore, inhibition of NF-κB by CAPE also inhibited PGN-induced NOD2 gene expression (data not shown). Together, these data demonstrate that ODE rapidly activates NF-κB and that NF-κB-dependent signaling is central to ODE-induced NOD2 expression in mononuclear phagocytes.
Fig. 3.
ODE-induced NOD2 expression is mediated via NF-κB activation. NF-κB translocation is increased at 30–90 min following 1% ODE stimulation in THP-1 monocytes. Representative EMSA blot of NF-κB p65 binding activity (A) and arbitrary density optical units of n = 6 independent experiments are shown (B). The NF-κB inhibitor caffeic acid phenethyl ester (CAPE) reduced NOD2 expression by ODE stimulated monocytes. Cells were pretreated for 1 h with indicated concentrations of CAPE (5–50 μM) followed by stimulation with 1% ODE for 24 h. There is a dose-dependent decrease in the percent relative expression of NOD2 mRNA in CAPE + ODE-treated cells compared with ODE-alone-stimulated cells (C, n = 4 independent experiments). NOD2 protein levels are also decreased in CAPE + ODE-treated cells after 48 h of 1% ODE stimulation by Western blotting (D, representative raw gel of 1 of 4 independent experiments; E, quantitative densitometry analysis of NOD2 expression normalized against β-acting loading control, n = 4 with mean ± SE depicted). Statistical significance: *P < 0.05 from media control (no ODE) compared with ODE stimulation; #P < 0.05 ODE-stimulated NOD2 expression in the absence of inhibitor compared with ODE-stimulated NOD2 expression with inhibitor.
siRNA-mediated knockdown of NOD2 in mononuclear phagocytes enhances select inflammatory mediator responses to ODE stimulation.
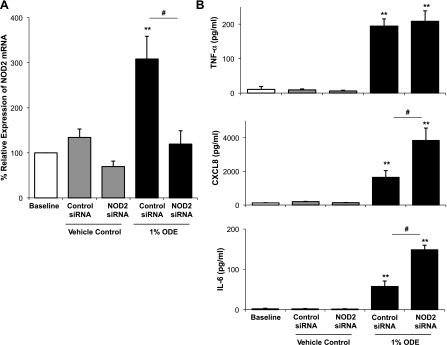
To assess the direct involvement of NOD2 in mediating the production of inflammatory cytokines/chemokines induced by ODE, we used RNA interference (RNAi) to attenuate the expression of NOD2 in human THP-1 monocytes. Transfection of adherent THP-1 cells with NOD2-specific siRNA, but not by a control scrambled siRNA, significantly reduced the expression of the NOD2 gene transcript that was induced in these cells by ODE (Fig. 4A; n = 4 independent experiments, P = 0.003). The functional consequence of siRNA-mediated knockdown of NOD2 was a significant enhancement of CXCL8/IL-8 and IL-6 production following ODE stimulation (Fig. 4B; n = 4 independent experiments). The apparent negative regulatory role for NOD2 in ODE-dependent monocyte activation was not universal since TNF-α production induced by ODE remained unaffected by siRNA-mediated knockdown of NOD2 (Fig. 4B).
Fig. 4.
Small interfering RNA (siRNA)-mediated knockdown of NOD2 selectively enhances ODE-induced proinflammatory cytokine/chemokine production in mononuclear phagocytes. THP-1 monocytes were transfected with nontargeting (scrambled) control or NOD2 specific siRNA and subsequently stimulated with 1% ODE or vehicle control media for 24 h. There is a decrease in percent relative expression of NOD2 mRNA in NOD2 siRNA-transfected cells stimulated with ODE compared with nontargeting siRNA-transfected cells + ODE (A). CXCL8 and IL-6, but not TNF-α, production is significantly augmented in NOD2 siRNA-transfected cells + ODE compared with nontargeting control siRNA-transfected cells + ODE. Means ± SE of 5 independent studies are shown. **P < 0.01, statistically significant between appropriate control media alone stimulation vs. ODE stimulation. #P < 0.05, statistically significant between control siRNA + ODE and NOD2 siRNA + ODE.
Organic dust selectively increases inflammatory mediator production in macrophages via a NOD2-dependent mechanism.
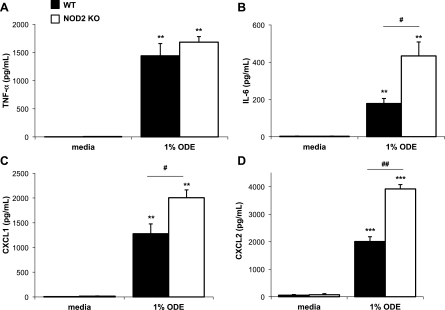
To confirm our observations with siRNA-mediated knockdown of NOD2 in THP-1 monocytes, responses of primary lung macrophages isolated from NOD2 KO (NOD2−/−) and WT (NOD2+/+) mice to ODE were examined. NOD2 KO and WT lung macrophages were stimulated ex vivo with 1% ODE for 24 h, whereupon cell-free culture supernatants were quantified for CXCL1 and CXCL2 (murine neutrophil chemoattractants; functional homologs of human IL-8/CXCL8), TNF-α, and IL-6 by ELISA. Similar to what was observed following siRNA-mediated knockdown of NOD2, CXCL1, CXCL2, and IL-6 production were increased in NOD2 KO lung macrophages following ODE treatment, whereas TNF-α was not affected (Fig. 5; n = 4 mice per group). There were no differences in cell number or viability between NOD2 KO and WT macrophages, thus allowing these changes to be directly attributable to NOD2 loss. These data confirm our siRNA NOD2 transfection results and highlight a role for NOD2 as a negative regulator of select ODE-induced proinflammatory mediators in lung macrophages.
Fig. 5.
Lung macrophages from NOD2 knockout (KO, NOD2−/−) mice demonstrated increased IL-6, CXCL2, CXCL1, but not TNF-α production following 1% ODE ex vivo stimulation at 24 h compared with lung macrophages from ODE-stimulated lung macrophages from wild-type (WT, NOD2+/+) mice. Mean results are presented as pg/ml per 200,000 cells ± SE; n = 4 mice per 1% ODE group and n = 3 per media control group. **P < 0.01, ***P < 0.001, statistically significant between saline and ODE-stimulated lung macrophages. #P < 0.05 and ##P < 0.01, statistically significant between WT and NOD2 KO lung macrophages stimulated with ODE.
NOD2 is a negative regulator of ODE-induced airway inflammatory responses in vivo.
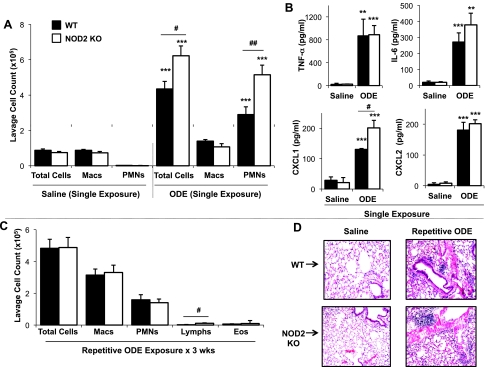
To determine whether there were any functional consequences of NOD2 in mediating ODE-induced airway inflammatory responses in vivo, NOD2 KO and WT mice were intranasally challenged with saline or ODE (12.5%) once (single exposure) or once daily for 3 wk (repetitive exposure) according to an established protocol (33). Interestingly, only airway neutrophils (Fig. 6A) and lavage fluid CXCL1 levels (Fig. 6B) were significantly increased in NOD2 KO mice following a one-time exposure to ODE compared with WT mice (n = 10–12 mice per ODE-treatment groups and n = 3–4 mice per saline treatment groups; P < 0.05). In contrast, there were no significant differences in TNF-α, IL-6, and CXCL2 levels between the two groups (Fig. 6B).
Fig. 6.
There is an effect of NOD2 in regulating ODE-induced airway inflammation in vivo. Lung lavage fluid mean ± SE concentration of total cells and cell differential (A) and cytokine/chemokine release (B) 5 h after single intranasal inhalation of ODE (12.5%) or saline in WT (solid bars) vs. NOD2 KO (open bars) mice (n = 3 mice per saline treatment group and 10–12 mice per ODE treatment group) are shown. PMN, polymorphonuclear neutrophils. WT and NOD2 KO mice also received repetitive, daily instillations of ODE for 3 wk and were subsequently euthanized 24 h after final challenge (C and D). Lavage fluid mean ± SE of total cells and cell differential from 6 mice per group is depicted (C). D: a representative 4- to 5-μm-thick section following 3-wk repetitive exposure to ODE vs. saline at ×10 magnification is shown. **P < 0.01, ***P < 0.001, statistically significant between saline and ODE-treated mice. #P < 0.05 and ##P < 0.005, statistically significant between WT and NOD2 KO-treated mice.
After 3 wk of repetitive, daily exposure to ODE, there was no difference in total cells, macrophages, neutrophils, or eosinophils in the lavage fluid between the NOD2 KO mice compared with WT mice (Fig. 6C; n = 6 mice/group). In addition, no differences in lavage fluid TNF-α, IL-6, CXCL1, and CXCL2 levels were observed between NOD2 KO and WT groups (data not shown). However, there was a subtle, but statistically significant increase in lymphocyte influx observed in the NOD2 KO mice compared with WT animals [lymphocyte percent (NOD2 KO): 2.23% vs. (WT): 0.99%; P = 0.041]. Interestingly, there were also modest lung parenchymal differences between NOD2 KO and WT mice following repetitive challenge with ODE (representative lung section of one of five mice demonstrated in Fig. 6D). Utilizing an established lung pathology inflammatory scoring system (inflammatory score range of 0 to 3) (33), the ranges of ODE-induced histopathological changes were semiquantitatively assessed by a pathologist (W. W. West) blinded to treatment conditions. There was a significant increase in alveolar compartment inflammation (mean inflammatory score ± SE, WT: 1.00 ± 0.12 vs. NOD2 KO: 1.61 ± 0.13, P = 0.01, n = 5 mice/group) and bronchiolar compartment inflammation (mean inflammatory score ± SE, WT: 1.20 ± 0.16 vs. NOD2 KO: 1.75 ± 0.14, P = 0.01, n = 5 mice/group) in NOD2 KO compared with WT mice. Previously, we have demonstrated that ODE-induces mononuclear cellular aggregates (33), and in this present study, no differences in distribution or scoring of ODE-induced mononuclear cellular aggregates between WT and NOD2 KO mice were observed. These studies support a temporal role for NOD2 as a negative regulator of ODE-induced inflammation in vivo.
DISCUSSION
In this study, we found that swine confinement facility organic dust enhances NOD2 gene and protein expression in mononuclear phagocytes and that this response is dependent on ODE-induced NF-κB activity and not by a TNF-α autocrine/paracrine mechanism. Importantly, the latter observation highlights a distinction between NOD2 regulatory pathways operative in phagocytes vs. epithelial cells, which may be able to be exploited for future cell-specific therapeutic targeting. In vitro studies also revealed a functional consequence for NOD2 in mononuclear phagocytes as demonstrated by the augmentation of select cytokines/chemokines with NOD2 inhibition or deletion following organic dust stimulation. Furthermore, consistent with the cell culture experiments, studies demonstrated a higher degree of airway inflammation in the absence of NOD2 following organic dust exposure in a time-dependent manner in vivo. Our results are in accordance with previous studies that suggest a potential negative (as opposed to positive) regulatory role for NOD2 (41, 46). To our knowledge, this is the first report to demonstrate the functional importance of NOD2 in mediating mononuclear phagocyte and airway responses to a complex, environmental organic dust exposure.
We initially showed a time-dependent increase in NOD2 expression in mononuclear phagocytes following organic dust exposure using human THP-1 monocytes as well as murine MH-S alveolar macrophages. To date, previous studies with various myelomonocytic cells have focused on expressional changes of NOD2 mRNAs demonstrating biphasic responses occurring early at ∼2 h and later at 8–24 h following either LPS or TNF-α stimulation (13, 40, 41). Although we found that NOD2 mRNA increases were detected as early as 4 h post-ODE exposure, marked upregulation of NOD2 mRNA occurred at 24 h, consistent with reports by Gutierrez et al. (13). We also confirmed that LPS, PGN, and TNF-α alone increased mononuclear phagocyte NOD2 mRNA expression and that in the case of complex ODE, ODE-induced NOD2 expression was not dependent on LPS. Currently, there is no means to specifically negate PGN activity from the dust extract; therefore, the impact of PGN on NOD2 activation is implied but cannot be directly demonstrated. Other studies have demonstrated that an early rise (∼2 h) in NOD2 mRNA expression is dependent on NF-κB activation via a TLR4-mediated pathway (13, 40) and suggested that the second rise (∼8–24 h) in NOD2 mRNA expression is secondary to TNF-α production through an amplification of NF-κB signaling since LPS induces TNF-α production and in turn, TNF-α activates NF-κB (40). However, there is relatively little information regarding regulation of NOD2 protein expression in mononuclear phagocytes. Although ODE is well recognized to rapidly increase mononuclear phagocyte TNF-α production (30, 32, 34), we did not find here convincing evidence that a TNF-α autocrine/paracrine mechanism was responsible for organic dust-induced NOD2 expression since inhibitors of TNF-α at best only slightly reduced NOD2 mRNA expression and there was no effect on ODE-induced NOD2 mRNA expression when TNF-α was absent.
Our studies demonstrated that NF-κB activation is instrumental in mediating ODE-induced NOD2 expression in mononuclear phagocytes. NF-κB resides in the cytoplasm in an inactive form; in response to bacterial products or cytokines, IκBα degradation occurs, leading to liberation of the NF-κB complex and its nuclear translocation where it initiates the transcriptional activation of target genes (47). Importantly, a putative NF-κB binding site is located in the promoter region of NOD2 (14). The present study found that NF-κB translocation occurs rapidly following ODE stimulation in mononuclear phagocytes and that inhibition of NF-κB signaling reduced ODE-induced NOD2 gene and protein expression. However, a potential limitation of our data is that chemical NF-κB inhibitors can have off-target effects, and further studies with p50−/− and p65+/− animals and dominant-negative constructs could be considered to further address the role of NF-κB regulation of NOD2 expression. Nonetheless, our findings support a direct role for ODE-induced NF-κB transcriptional activation to augment NOD2 as opposed to ODE inducing TNF-α, which, in turn, activates NF-κB. Our data would also support the notion that NOD2 can upregulate itself because it is known that NOD2 activates NF-κB following ligand stimulation (39).
We found that NOD2 negatively regulates the expression of select organic dust-induced proinflammatory cytokines/chemokines in mononuclear phagocytes and found evidence to support its role as a potential negative regulator of airway inflammation in vivo. With both RNAi and murine KO strategies, a reduction or loss in NOD2 resulted in increased expression of neutrophil chemoattractants (human CXCL8, murine CXCL1 and CXCL2) and IL-6 following dust extract stimulation. However, TNF-α levels were not affected, indicating that the inhibitory effects of NOD2-dependent signaling in response to ODE are not a global phenomenon. This observation was extended to an established murine model where, in the absence of NOD2, organic dust-induced airway inflammatory indexes were modestly increased following a one-time exposure, and following repetitive dust extract exposure there was an enhancement of lung parenchymal pathology changes. However, compared with the in vitro mononuclear phagocytic studies, the enhancement of inflammatory parameters in the context of NOD2 deficiency was less pronounced in vivo, suggesting that the effect of NOD2 loss differs or may be less important in nonmyelomonocytic lung cells. Thus this observation suggests a specific, rather than a generalized, cellular effect. However, our studies also suggest a temporal role for NOD2 in that the negative regulatory role for NOD2 appears more important following acute ODE exposure as opposed to repetitive ODE exposures. This may be explained by the induction of a compensatory response over time that negates the increases in several inflammatory parameters that is seen following a single ODE exposure.
Whereas our initial expectation was that targeting NOD2 would result in a dampening of organic dust-induced inflammation, our findings are consistent with other studies that demonstrate a paradoxical role for NOD2. For example, mice deficient in NOD2 are more susceptible to oral, but not intraperitoneal, administration of Listeria monocytogenes and are more sensitive to intraperitoneal infection with Staphylococcus aureus (8, 19). It was first proposed by Watanabe et al. (46) that NOD2 is a negative regulator of TLR2 signaling by reporting that NOD2-deficient splenic macrophages produced markedly more IL-12, but not TNF-α, when stimulated with PGN. Moreover, a recent report found evidence to suggest that NOD2 might also play a negative regulatory role in TLR4 signaling because production of several proinflammatory mediators were significantly enhanced following LPS exposure following NOD2 knockdown (41). In contrast, several other studies have not found increased production of cytokines in NOD2-deficient bone marrow-derived macrophages after stimulation with TLR agonists (17, 18). Organic dust from large animal farming confinement environments represents a complex, “real-life” biological exposure to an admixture of gram-positive-enriched but also gram-negative-enriched PAMPs, and this present study supports that NOD2 appears to be a negative regulator of select proinflammatory responses in vitro and in vivo following ODE exposure.
The mechanism by which NOD2 loss results in selective exaggerated inflammatory responses is not entirely clear. One possibility as proposed by Strober et al. (39) focuses on the effect of NOD2 on the nuclear translocation of the NF-κB subunit RelA in that increased RelA activation occurs in the absence of NOD2 following PGN stimulation. Tsai et al. (41) recently described that many signaling cascades including IKK, MAPK, STAT1, and NF-κB are all increased following LPS stimulation under conditions in which NOD2 expression is reduced or absent. We also found that inhibition of MAPK and NF-κB pathways reduced ODE-induced proinflammatory cytokine expression in lung macrophages in the absence or presence of NOD2 (data not shown). However, since these are not direct inhibitors for NOD2 signaling pathway, it could represent the expected attenuated direct response to ODE rather than a specific response.
It is also possible that an upregulation of NOD2 via PAMPs may be one potential cellular mechanism utilized to control or inhibit further inflammatory responses. Consistent with this speculative role for NOD2 in a tolerance response, mononuclear phagocytes overexpressing NOD2 have been shown by others to suppress LPS-induced NF-κB activity and MAPK phosphorylation (41). Since the exact mechanisms to explain these collective findings still remain unclear, future studies should determine whether NOD2-interacting proteins such as RIP2 (CARD3, RICK) or the mitogen-activated protein kinase kinase kinase family member TAK1 play an important role in organic dust-induced responses (16, 27). Whereas we have not found significant differences in TLR2 and TLR4 gene expression between WT and NOD2 KO lung macrophages with or without ODE (data not shown), and cell surface TLR2 and TLR4 expression is unchanged in ODE-treated monocyte-derived macrophages (30), it remains possible that NOD2 could impact the biological response to ODE by regulating internalization of TLR2. Muller et al. (23) recently suggested that both TLR4 and NOD2 regulate internalization of TLR2 in keratinocytes, and thus it is possible to speculate that attenuation of TLR2 surface expression through a NOD2 pathway could be important in regulating organic dust-induced airway inflammatory responses.
In summary, the present study demonstrates that an important, complex biological agonist from the workplace, namely swine confinement facility ODE, augmented NOD2 mRNA and protein expression in mononuclear phagocytes, which was found to be dependent on NF-κB activity as opposed to an autocrine/paracrine TNF-α. In addition, a functional role for NOD2 in organic dust-induced proinflammatory responses was demonstrated. NOD2 appears to be a negative regulator of organic dust-induced proinflammatory cytokine/chemokine production in a selective manner in cultured macrophages. A functional role for NOD2 can be extended to potential in vivo consequence because in the absence of NOD2 there was evidence of increased airway inflammatory parameters following organic dust exposure in mice. Overall, these studies suggest that the NOD2 pathway could play an important immunomodulating role in inflammatory outcomes in individuals exposed to organic dust in swine confinement facilities. Finally, it may also be warranted in future studies to examine whether known polymorphisms of NOD2 gene contribute to airway responses in exposed workers.
GRANTS
This study was supported by grants from the National Institute of Environmental Health Sciences [K08: ES015522-01 and (ARRA) ES015522-03S1 and R01: ES019325; J. A. Poole], National Institute of Occupational Safety Health (1R01OH008539-01; D. J. Romberger), and National Institute on Alcohol Abuse and Alcoholism (R01 AA017993; T. A. Wyatt).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Conrad Parks and Chris Bauer for technical assistance, Thomas Jerrells, PhD, for assistance with digital microscopy images prepared for manuscript, and Lisa Chudomelka for the assistance with manuscript preparation.
REFERENCES
- 1. Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 117: 1396–1403, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Austin CM, Ma X, Graviss EA. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J Infect Dis 197: 1713–1716, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ. Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium. Am J Physiol Lung Cell Mol Physiol 294: L1049–L1054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chamaillard M, Philpott D, Girardin SE, Zouali H, Lesage S, Chareyre F, Bui TH, Giovannini M, Zaehringer U, Penard-Lacronique V, Sansonetti PJ, Hugot JP, Thomas G. Gene-environment interaction modulated by allelic heterogeneity in inflammatory diseases. Proc Natl Acad Sci USA 100: 3455–3460, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 34: 19–35, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Cormier Y, Israel-Assayag E, Racine G, Duchaine C. Farming practices and the respiratory health risks of swine confinement buildings. Eur Respir J 15: 560–565, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Dennis VA, Dixit S, O'Brien SM, Alvarez X, Pahar B, Philipp MT. Live Borrelia burgdorferi spirochetes elicit inflammatory mediators from human monocytes via the Toll-like receptor signaling pathway. Infect Immun 77: 1238–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deshmukh HS, Hamburger JB, Ahn SH, McCafferty DG, Yang SR, Fowler VG., Jr Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun 77: 1376–1382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest 136: 716–725, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Fallschissel K, Klug K, Kampfer P, Jackel U. Detection of airborne bacteria in a German turkey house by cultivation-based and molecular methods. Ann Occup Hyg 54: 934–943, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Farkas L, Stoelcker B, Jentsch N, Heitzer S, Pfeifer M, Schulz C. Muramyldipeptide modulates CXCL-8 release of BEAS-2B cells via NOD2. Scand J Immunol 68: 315–322, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev 227: 106–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, Nunez G, Fernandez-Luna JL. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem 277: 41701–41705, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hu C, Sun L, Hu Y, Lu D, Wang H, Tang S. Functional characterization of the NF-kappaB binding site in the human NOD2 promoter. Cell Mol Immunol 7: 288–295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem 278: 5509–5512, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Kim JY, Omori E, Matsumoto K, Nunez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem 283: 137–144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim YG, Park JH, Daignault S, Fukase K, Nunez G. Cross-tolerization between Nod1 and Nod2 signaling results in reduced refractoriness to bacterial infection in Nod2-deficient macrophages. J Immunol 181: 4340–4346, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity 28: 246–257, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731–734, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Koslowski MJ, Beisner J, Stange EF, Wehkamp J. Innate antimicrobial host defense in small intestinal Crohn's disease. Int J Med Microbiol 300: 34–40, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, Hazucha MJ, Devlin RB, Peden DB. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol 120: 719–722, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Lesage S, Zouali H, Cezard JP, Colombel JF, Belaiche J, Almer S, Tysk C, O'Morain C, Gassull M, Binder V, Finkel Y, Modigliani R, Gower-Rousseau C, Macry J, Merlin F, Chamaillard M, Jannot AS, Thomas G, Hugot JP. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet 70: 845–857, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muller P, Muller-Anstett M, Wagener J, Gao Q, Kaesler S, Schaller M, Biedermann T, Gotz F. The Staphylococcus aureus lipoprotein SitC colocalizes with Toll-like receptor 2 (TLR2) in murine keratinocytes and elicits intracellular TLR2 accumulation. Infect Immun 78: 4243–4250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA 93: 9090–9095, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nehme B, Gilbert Y, Letourneau V, Forster RJ, Veillette M, Villemur R, Duchaine C. Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols. Appl Environ Microbiol 75: 5445–5450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nehme B, Letourneau V, Forster RJ, Veillette M, Duchaine C. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ Microbiol 10: 665–675, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Nembrini C, Kisielow J, Shamshiev AT, Tortola L, Coyle AJ, Kopf M, Marsland BJ. The kinase activity of Rip2 determines its stability and consequently Nod1- and Nod2-mediated immune responses. J Biol Chem 284: 19183–19188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nonnenmann MW, Bextine B, Dowd SE, Gilmore K, Levin JL. Culture-independent characterization of bacteria and fungi in a poultry bioaerosol using pyrosequencing: a new approach. J Occup Environ Hyg 7: 693–699, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem 272: 21096–21103, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, Larsson L, Allen-Gipson D, Von Essen SG, Romberger DJ. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol 122: 375–382, 382.e1–e4, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health 73: 684–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poole JA, Thiele GM, Alexis NE, Burrell AM, Parks C, Romberger DJ. Organic dust exposure alters monocyte-derived dendritic cell differentiation and maturation. Am J Physiol Lung Cell Mol Physiol 297: L767–L776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 296: L1085–L1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, Romberger DJ. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol 120: 366–373, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Rask-Andersen A, Malmberg P, Lundholm M. Endotoxin levels in farming: absence of symptoms despite high exposure levels. Br J Ind Med 46: 412–416, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 93: 289–296, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, Schreiber S. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 124: 1001–1009, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Sato H, Williams HR, Spagnolo P, Abdallah A, Ahmad T, Orchard TR, Copley SJ, Desai SR, Wells AU, du Bois RM, Welsh KI. CARD15/NOD2 polymorphisms are associated with severe pulmonary sarcoidosis. Eur Respir J 35: 324–330, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 6: 9–20, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Takahashi Y, Isuzugawa K, Murase Y, Imai M, Yamamoto S, Iizuka M, Akira S, Bahr GM, Momotani E, Hori M, Ozaki H, Imakawa K. Up-regulation of NOD1 and NOD2 through TLR4 and TNF-alpha in LPS-treated murine macrophages. J Vet Med Sci 68: 471–478, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Tsai WH, Huang DY, Yu YH, Chen CY, Lin WW. Dual roles of NOD2 in TLR4-mediated signal transduction and -induced inflammatory gene expression in macrophages. Cell Microbiol 13: 717–730, 2011 [DOI] [PubMed] [Google Scholar]
- 42. van der Velden WJ, Blijlevens NM, Maas FM, Schaap NP, Jansen JH, van der Reijden BA, Feuth T, Dolstra H, Donnelly JP. NOD2 polymorphisms predict severe acute graft-versus-host and treatment-related mortality in T-cell-depleted haematopoietic stem cell transplantation. Bone Marrow Transplant 44: 243–248, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Vogelzang PF, van der Gulden JW, Folgering H, Heederik D, Tielen MJ, van Schayck CP. Longitudinal changes in bronchial responsiveness associated with swine confinement dust exposure. Chest 117: 1488–1495, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 9: 185–196, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem 285: 2227–2231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 5: 800–808, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto Y, Gaynor RB. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci 29: 72–79, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Zhiping W, Malmberg P, Larsson BM, Larsson K, Larsson L, Saraf A. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am J Respir Crit Care Med 154: 1261–1266, 1996 [DOI] [PubMed] [Google Scholar]