Abstract
Airway smooth muscle phenotype may be modulated in response to external stimuli under physiological and pathophysiological conditions. The effect of mechanical forces on airway smooth muscle phenotype were evaluated in vitro by suspending weights of 0.5 or 1 g from the ends of canine tracheal smooth muscle tissues, incubating the weighted tissues for 6 h, and then measuring the expression of the phenotypic marker protein, smooth muscle myosin heavy chain (SmMHC). Incubation of the tissues at a high load significantly increased expression of SmMHC compared with incubation at low load. Incubation of the tissues at a high load also decreased activation of PKB/Akt, as indicated by its phosphorylation at Ser 473. Inhibition of Akt or phosphatidylinositol-3,4,5 triphosphate-kinase increased SmMHC expression in tissues at low load but did not affect SmMHC expression at high load. IL-13 induced a significant increase in Akt activation and suppressed the expression of SmMHC protein at both low and high loads. The role of integrin signaling in mechanotransduction was evaluated by expressing a PINCH (LIM1–2) fragment in the muscle tissues that prevents the membrane localization of the integrin-binding IPP complex (ILK/PINCH/α-parvin), and also by expressing an inactive integrin-linked kinase mutant (ILK S343A) that inhibits endogenous ILK activity. Both mutants inhibited Akt activation and increased expression of SmMHC protein at low load but had no effect at high load. These results suggest that mechanical stress and IL-13 both act through an integrin-mediated signaling pathway to oppositely regulate the expression of phenotypic marker proteins in intact airway smooth muscle tissues. The stimulatory effects of mechanical stress on contractile protein expression oppose the suppression of contractile protein expression mediated by IL-13; thus the imposition of mechanical strain may inhibit changes in airway smooth muscle phenotype induced by inflammatory mediators.
Keywords: PKB/Akt, IPP complex, PINCH, ILK, differentiation
many cell types are sensitive to mechanical forces and modulate their phenotype and function in response to changes in their local mechanical environment (26, 50). Airway smooth muscle is subjected to large changes in mechanical forces that modulate airway smooth muscle function under physiological conditions (21, 23, 45, 46, 56). Pathophysiological conditions such as airway inflammation and asthma may cause local changes in mechanical forces on airway cells that modulate signaling pathways that regulate the contractility, differentiation, proliferation, or secretory status of airway muscle cells and tissues (9, 50, 56). These mechanical stresses may occur locally due to many possible factors: e.g., local inflammation that results in edema, constriction of some airways that exert tension on surrounding airways, ventilation inhomogeneities, disruption of matrix structure, airway remodeling, or alterations in surface tension. The imposition of positive end-expiratory pressure or changes in breathing pattern that alter functional residual capacity can result in more global changes in loads on the airways. However, the mechanisms by which mechanical signals are sensed and transduced to regulate airway smooth muscle cell function remain to be elucidated.
Integrin receptors have been implicated as mediators of mechanotransduction and are important in regulating both smooth and skeletal muscle differentiation (6, 26, 33, 43, 69). Integrins interact with the actin cytoskeleton via macromolecular protein complexes that bind to the cytoplasmic domains of integrin proteins and to actin cytoskeletal filaments. Integrin-linked kinase (ILK), a multidomain protein that binds directly to the cytoplasmic domain of β1-integrins, serves as a scaffolding protein that links integrin proteins and the actin cytoskeleton (17). ILK forms a heterotrimeric complex with PINCH (Particularly interesting new cysteine-histidine rich protein), an adaptor protein that consists of 5 LIM domains, and α-parvin, also known as actopaxin (4, 57, 67, 72). The ILK/PINCH/α-parvin (IPP) complex is an important constituent of β1- and β3-containing adhesion sites and is known to play a central role in regulating multiple cellular processes (4, 32). ILK has also been characterized as a serine-threonine kinase, although its kinase activity has been questioned (63). However, ILK is an effector for the phosphatidylinositol-3,4,5 triphosphate kinase-dependent (PI3-kinase-dependent), extracellular matrix-mediated activation of PKB/Akt (10, 39).
ILK and the IPP complex are important regulators of airway smooth muscle phenotype and contractility (68, 71). The localization of the IPP complex to integrin adhesion junctions is critical for normal airway smooth muscle contractility (71). The depletion of ILK protein from airway smooth muscle tissues decreases the activation of Akt and increases the transcription and expression of smooth muscle phenotype-specific marker proteins (68). In airway smooth muscle tissues, Akt activation inhibits activity of the transcriptional regulator, serum response factor (SRF), and thereby suppresses the expression of smooth muscle-specific marker proteins (68).
In the present study, we investigated the role of ILK and the IPP complex in transducing mechanical stimuli to signaling pathways that regulate the expression of smooth muscle phenotype-specific protein, smooth muscle myosin heavy chain (SmMHC), in airway smooth muscle tissues. We also evaluated the effects of IL-13, a key cytokine associated with airway inflammation and the pathogenesis of asthma (18, 59, 65, 66) on the mechanosensitive expression of SmMHC in airway smooth muscle.
Our findings demonstrate that the expression of SmMHC increases in airway smooth muscle tissues in response to chronic mechanical loading. Furthermore, we find that the activation of Akt is inversely regulated in response to mechanical load: the imposition of mechanical load on airway smooth muscle suppresses the activation of Akt and induces the expression of the smooth muscle phenotype-specific marker protein, SmMHC. Our results indicate that the localization of the IPP complex to integrin adhesion sites is critical for the transduction of mechanical signals to downstream pathways that regulate load-sensitive changes in airway smooth muscle protein expression and Akt activity, which suggests that integrin proteins are the primary sensors for mechanical signals. Our results demonstrate that IL-13 stimulates the activation of Akt and suppresses the expression of SmMHC and thus opposes the effects of high mechanical loads. These results suggest that mechanical loading is important in maintaining the differentiated state of airway smooth muscle and that inflammation may suppress the effects of load-sensitive signals that regulate the expression of smooth muscle phenotype-specific contractile proteins.
MATERIALS AND METHODS
Preparation of smooth muscle tissues.
Mongrel dogs (30–35 kg) were anesthetized with pentobarbital sodium (30 mg/kg, intravenously) and euthanized in accordance with procedures approved by the Institutional Animal Care and Use Committee, Indiana University School of Medicine. A segment of the trachea was immediately removed and immersed in physiological saline solution (PSS) (composition in mM: 110 NaCl, 3.4 KCl, 2.4 CaCl2, 0.8 MgSO4, 25.8 NaHCO3, 1.2 KH2PO4, and 5.6 glucose). Smooth muscle strips (1.0 × 0.2–0.5 × 15 mm) were dissected free of connective and epithelial tissues. For the measurement of contractile force, muscle tissues were attached to force transducers and maintained within a tissue bath in PSS at 37°C. Muscle length was then progressively increased and the muscle was subjected to repeated contractions until the force of active contraction in response to acetylcholine reached a maximum.
Protocol for mechanical loading of tissues.
Following the initial equilibration procedure, weights were suspended from the ends of the tissue strips to stress the tissues with low load (0.5 g) or high load (1.0 g). The 1-g load resulted in a level of strain that results in maximal force generation, and the 0.5-g load causes stretches of the muscle to ∼50–60% of its length of maximal active force and generally results in submaximal levels of isometric force. These loads are within the range normally experienced by airway smooth muscle during breathing under physiological conditions in vivo (20, 22). Strips were incubated in serum-free DMEM for 6 h at 37°C. In some experiments, inhibitors of Akt (50 μM Akt inhibitor VIII in DMSO), inhibitors of PI3-kinase (50 μM LY294002 in DMSO), and/or canine IL-13 (50 ng/ml) were added to the DMEM for the duration of the 6-h incubation period. In preliminary experiments, a dose-response curve was performed to test the effects of IL-13 on STAT6 activation in these tissues. A 50 ng/ml concentration was required to achieve consistently elevated STAT6 activation in response to exposure to IL-13. A 50 ng/ml concentration is consistent with that used to stimulate airway smooth muscle by a number of other laboratories (11, 30, 31, 35). Because of limitations posed by diffusion in intact tissues, it is often necessary to use higher concentrations of reagents than for cultured cells.
Control muscles in experiments using Akt or PI3-kinase inhibitors were incubated in serum-free DMEM containing serum-free in 0.1% DMSO. Some tissues were transfected with plasmids and incubated for 2 days for the expression of recombinant proteins prior to mechanical loading. At the completion of the 6-h incubation period, tissues were quickly frozen using liquid N2-cooled tongs and pulverized under liquid N2 for biochemical analysis of tissue extracts.
Transfection of smooth muscle tissues and expression of recombinant proteins.
Plasmids encoding the PINCH LIM 1–2 domain (73), ILK S343A (29), and mouse kinase-inactive Akt [HA(Hemagglutinin)-Akt1-AAA] (point mutations to substitute K179, T308, and S473 to alanine) (3, 14) were used in this study. The DNA encoding the mutant forms of human PINCH and ILK were cloned into the pEGFP-C2 expression vector whereas the DNA encoding the HA-tagged kinase-inactive Akt was cloned into the pcDNA3.1 expression vector.
Plasmids were introduced into tracheal smooth muscle strips by the method of reversible permeabilization (38, 53, 68, 71). After tissue equilibration and establishment of a muscle length for the generation of maximal isometric force, muscle strips were attached to metal mounts to maintain their length and incubated successively in each of the following solutions: Solution 1 (4°C for 120 min) containing (in mM) 10 EGTA, 5 Na2ATP, 120 KCl, 2 MgCl2, and 20 TES; solution 2 (4°C overnight) containing (in mM) 0.1 EGTA, 5 Na2ATP, 120 KCl, 2 MgCl2, 20 TES, and 10 μg/ml plasmids; solution 3 (4°C for 30 min) containing (in mM) 0.1 EGTA, 5 Na2ATP, 120 KCl, 10 MgCl2, 20 TES; and solution 4 (22°C for 60 min) containing (in mM) 110 NaCl, 3.4 KCl, 0.8 MgSO4, 25.8 NaHCO3, 1.2 KH2PO4, and 5.6 dextrose. Solutions 1-3 were maintained at pH 7.1 and aerated with 100% O2. Solution 4 was maintained at pH 7.4 and aerated with 95% O2-5% CO2. After 30 min in solution 4, CaCl2 was added gradually to reach a final concentration of 2.4 mM. The strips were then incubated in a CO2 incubator at 37°C for 2 days in serum-free DMEM medium containing 5 mM Na2ATP, 100 units/ml penicillin, 100 μg/ml streptomycin, and 20 μg/ml plasmids. Sham-treated tissues were studied in parallel in all protocols and were subjected to identical protocols except that plasmids were omitted from the solutions during the reversible permeabilization procedures. After 2 days, the muscle tissues were placed in PSS at 37°C in a 25-ml organ bath and attached to a Grass force transducer for measurement of contractile force. Tissues were then subjected to mechanical loading as described above.
Protein extraction and analysis by immunoblot.
Pulverized muscle tissues were mixed with extraction buffer containing 2% Triton X-100, 2 mM EDTA, pH 8.3, 20 mM Tris·HCl, pH 7.6, 0.4% SDS, phosphatase inhibitors (in mM: 2 sodium orthovanadate, 2 molybdate, and 2 sodium pyrophosphate), and protease inhibitors (in mM: 2 benzamidine, 0.5 aprotinin, and 1 PMSF). Each sample was centrifuged for the collection of supernatant and boiled in 4× Laemmli sample buffer. Proteins were separated by 7 or 8% SDS-PAGE, transferred to nitrocellulose membranes, and immunoprobed for proteins of interest with specific primary antibodies at 4°C overnight followed by secondary antibodies for 1 h at room temperature. Proteins were visualized by enhanced chemiluminescence and quantified by scanning densitometry.
Reagents and antibodies.
Sources of antibodies were as follows: mouse α-actinin (clone BM-75.2) and mouse SmMHC (clone hSM-V), Sigma Chemical; polyclonal phospho-Akt (Ser 473, clone 14–6), Invitrogen; polyclonal Akt, Cell Signaling; polyclonal EGFP, Abcam; monoclonal HA (clone 12CA5) conjugated with peroxidase, Roche. Secondary monoclonal and polyclonal antibodies were from Amersham.
Sources of other reagents were as follows: Recombinant canine IL-13, R&D Systems; LY294002, Cell Signaling; Akt inhibitor VIII (Isozyme-Selective, Akt-1/2), Calbiochem; DMEM and antibiotics, Invitrogen. All other reagents used for preparation of tissue extraction buffer were from Sigma whereas reagents used for preparation of PSS were from Fisher Scientific.
Statistical analysis.
Data are expressed as means ± SE; n represents the number of experiments. Differences between treatment groups were determined by using GraphPad software and two-way repeated-measures ANOVA with Bonferroni's posttest. Differences were considered statistically significant when P < 0.05.
RESULTS
SmMHC expression and Akt activation are inversely modulated by mechanical stress in tracheal smooth muscle tissues.
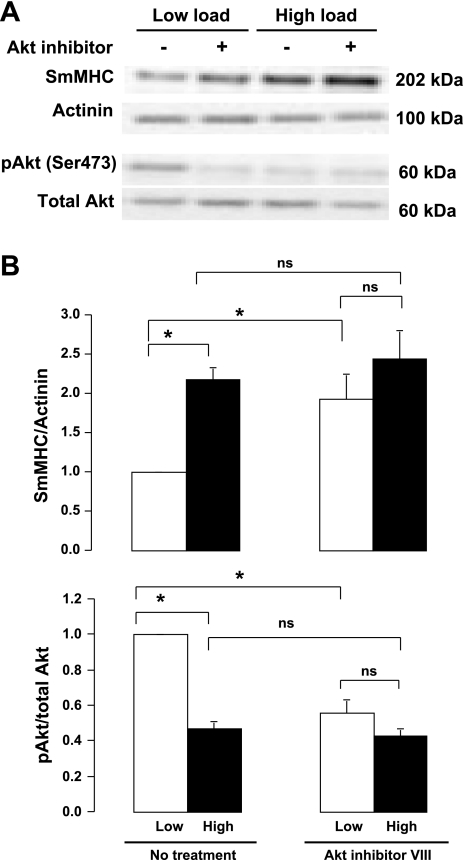
The effects of mechanical stress on the expression of SmMHC and the activation of Akt were evaluated by subjecting tracheal muscle tissues to low (0.5 g) or high loads (1 g) for 6 h (Fig. 1). Following mechanical loading, the expression of SmMHC and the phosphorylation of Akt on Ser 473, an indicator of its activation, were analyzed in extracts from each muscle tissue. Expression of SmMHC protein was significantly higher and Akt phosphorylation on Ser 473 was significantly lower in muscles subjected to a high load relative to muscles subjected to low load after 6-h incubation. These data indicate that chronic mechanical stress inhibits Akt activation and induces the expression of SmMHC protein in intact tracheal smooth muscle tissues.
Fig. 1.
Mechanical loading inhibits Akt activation and induces expression of smooth muscle myosin heavy chain (SmMHC) protein in smooth muscle tissues. A: immunoblot of extracts from 4 individual muscle tissues subjected to low load or high load for 6 h in the presence or absence of Akt inhibitor VIII. B: mean values for effects of mechanical load and Akt inhibitor VIII on Akt phosphorylation and SmMHC expression. A and B: in muscles without Akt inhibitor (No treatment), high load increased SmMHC expression and decreased Akt phosphorylation at Ser 473. Akt inhibitor VIII significantly inhibited Akt activation and significantly increased SmMHC expression in low load muscles but did not significantly affect Akt phosphorylation or SmMHC expression in high load muscles. Values are means ± SE (n = 7). *Paired values are significantly different, P < 0.05. ns, Pairs of values that are not significantly different.
Akt and PI3-kinase regulate changes in the expression of SmMHC induced by chronic mechanical loading.
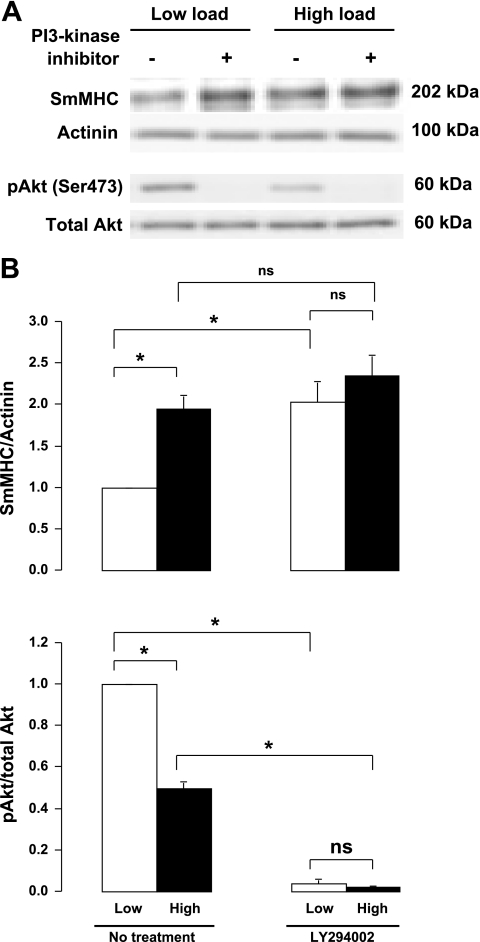
The roles of Akt and PI3-kinase in the mechanosensitive regulation of SmMHC expression were evaluated by treating the tissues with either Akt inhibitor VIII (Fig. 1) or the PI3-kinase inhibitor LY294002 (Fig. 2) for 6 h while they were subjected to a high load or low load. Akt inhibitor depressed the activation of Akt in muscles subjected to low loads to the level observed in muscles subjected to high loads and eliminated the load-dependent differences in SmMHC expression (Fig. 1). Treatment of the muscles with PI3-kinase inhibitor completely suppressed Akt activity and increased SmMHC expression in muscles incubated under low load to the level found in muscle incubated with a high load (Fig. 2). These observations suggest that PI3-kinase and Akt are critical mediators of mechanosensitive signals that regulate the expression of SmMHC protein in airway smooth muscle tissues.
Fig. 2.
Inhibition of phosphatidylinositol-3,4,5 triphosphate (PI3)-kinase activity increases expression of SmMHC protein in smooth muscle tissues subjected to low mechanical load. A: immunoblot of extracts from 4 individual muscle tissues subjected to low load or high load for 6 h in the presence or absence of the PI3-kinase inhibitor LY294002. B: mean values for effects of mechanical load and PI3-kinase inhibitor on Akt phosphorylation and SmMHC expression. A and B: in muscles without PI3-kinase inhibitor (No treatment), high load increased SmMHC expression and decreased Akt phosphorylation at Ser 473. PI3-kinase inhibitor significantly inhibited Akt activation and significantly increased SmMHC expression in low load muscles, but it did not significantly affect SmMHC expression in high load muscles, although it caused further inhibition of Akt. Values are means ± SE (n = 7). *Paired values are significantly different, P < 0.05.
Mechanosensitive SmMHC expression and Akt activity are mediated by the IPP complex and require ILK activation.
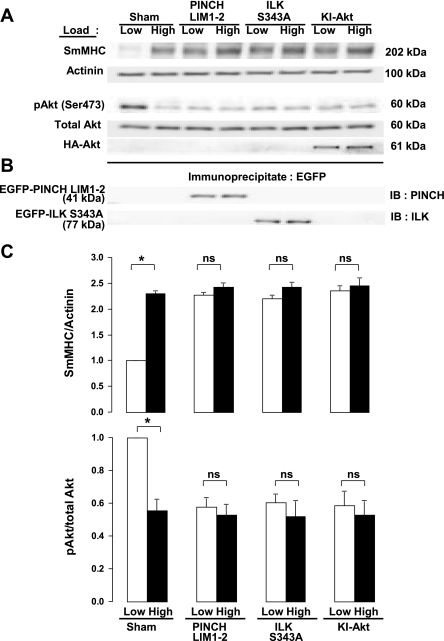
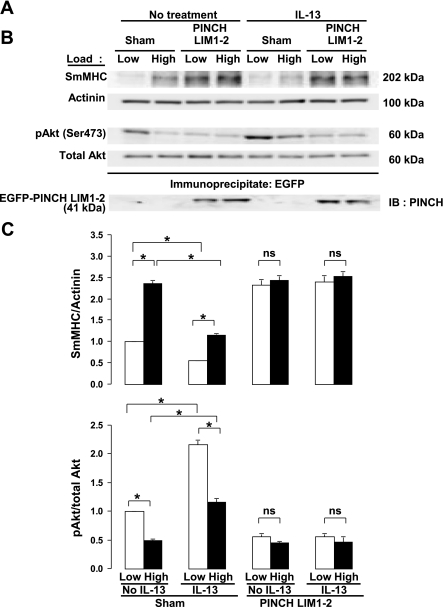
A truncated PINCH molecule containing only the first two LIM domains (EGFP-PINCH LIM1–2) was expressed in tracheal tissue strips to evaluate the role of the heterotrimeric IPP complex containing ILK, PINCH, and α-parvin complex in the transduction of mechanical signals that regulate Akt activity and SmMHC protein expression. The IPP complex is recruited to membrane adhesion complexes in airway smooth muscle, where ILK binds to the cytoplasmic domains of β-integrin proteins (68). ILK binds to the NH2-terminal LIM1 domain of PINCH (73). EGFP-PINCH LIM1–2 competes with endogenous PINCH for binding to ILK and thereby disrupts the binding of ILK to PINCH and its recruitment to integrin adhesion sites (73). The expression of the EGFP-PINCH LIM1–2 fragment in tracheal smooth muscle tissues inhibits the recruitment of the IPP complex to the membrane in response to ACh stimulation and prevents the association of ILK with β-integrins (71). In addition, the kinase-inactive ILK mutant, ILK S343A, was expressed in airway smooth muscle tissues to evaluate the role of ILK activation in regulating the mechanosensitive expression of SmMHC. The amino acid residue serine 343 within the ILK activation loop is required for ILK activity and for PKB/Akt phosphorylation (40, 55).
The effects of these mutants on the tissues were compared with that of the kinase-inactive Akt mutant, HA-Akt1-AAA (K179A, T308A, S473A) (3, 14). Muscle tissues transfected with plasmids encoding PINCH LIM 1–2, ILK S343A, HA-Akt1-AAA, and sham-treated muscles were incubated for 2 days to allow for expression of the recombinant proteins and then subjected to high load or low load for 6 h. The expression of EGFP-PINCH LIM1–2 and EGFP-ILK S343A were confirmed by immunoprecipitating recombinant proteins by use of anti-EGFP antibody and then immunoblotting immunoprecipitates for PINCH and ILK (Fig. 3B). HA-Akt1-AAA expression was confirmed by immunoblotting extracts from muscle tissues by use of anti-HA antibody. Expression of PINCH LIM 1–2, ILK S343A, or kinase-inactive HA-Akt1-AAA all significantly suppressed the activation of Akt and significantly potentiated the expression of SmMHC in muscles subjected to low load (Fig. 3). However, there was no significant effect of these treatments on muscles subjected to a high load. These results suggest that transduction of mechanical signals that regulate Akt activation and the expression of SmMHC protein in airway smooth muscle tissues is mediated by the IPP complex and requires ILK activity.
Fig. 3.
Expression of PINCH LIM1–2 fragment, kinase-inactive integrin-linked kinase (ILK) S343A, or kinase-inactive Akt1 T308A S473A suppresses Akt activation and increases expression of SmMHC protein at low mechanical load in smooth muscle tissues. A: immunoblots from extracts of tissues treated with ILK S343A, PINCH LIM1–2, kinase-inactive Akt1 T308A S473A (KI-Akt) plasmids or sham treated. B: immunoblots (IB) of anti-green fluorescent protein (GFP) immunoprecipitates from muscle tissues expressing mutant constructs demonstrating expression of recombinant PINCH and ILK proteins. C: mean results for the effects of PINCH, ILK S343A, and Akt1 T308A S473A on mechanosensitive Akt activity and SmMHC expression. Recombinant proteins significantly potentiated SmMHC expression and inhibited Akt phosphorylation at low mechanical load, but did not significantly affect SmMHC expression or Akt phosphorylation at high mechanical load. Values are means ± SE (n = 5). *Paired values are significantly different, P < 0.05.
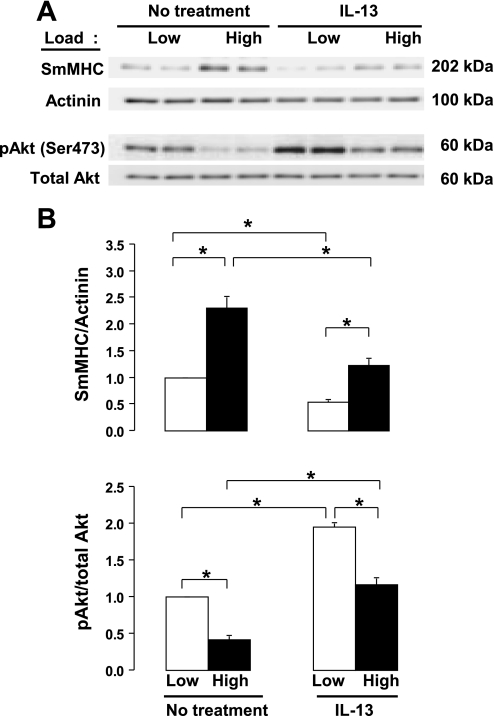
Stimulation of tracheal smooth muscle tissues with IL-13 decreases mechanosensitive SmMHC expression by activating Akt.
The effect of the inflammatory cytokine, IL-13, on the mechanosensitive expression of SmMHC protein was evaluated in tracheal smooth muscle tissues by stimulating tissues with IL-13 for 6 h while subjecting them to low and high mechanical loads (Fig. 4). Treatment of muscle tissues with IL-13 significantly inhibited expression of SmMHC protein and significantly increased Akt activation in tissues subjected to both low and high loads (Fig. 4). These results suggest that IL-13 inhibits the mechanosensitive expression of SmMHC protein in airway smooth muscle tissues.
Fig. 4.
IL-13 potentiates activation of Akt and suppresses expression of SmMHC protein. A: immunoblot illustrating suppressive effects of IL-13 on mechanosensitive expression of SmMHC protein and Akt phosphorylation at Ser 473. B: IL-13 significantly suppressed SmMHC expression-potentiated Akt activation at both low and high mechanical loads. Values are means ± SE (n = 7). *Paired values are significantly different, P < 0.05.
The IPP complex is required for IL-13-induced suppression of mechanosensitive SmMHC expression.
The role of the IPP complex and ILK in the regulation of the IL-13 induced suppression of SmMHC was evaluated by expressing the PINCH LIM1–2 peptide in airway smooth muscle tissues to inhibit IPP and ILK localization at integrin adhesion junctions. (Fig. 5). Expression of the PINCH LIM1–2 protein significantly inhibited the mechanosensitivity of IL-13 induced Akt activation and suppression of SmMHC protein expression in tracheal smooth muscle tissues (Fig. 5). These results suggest that the activation Akt and suppression of SmMHC induced by IL-13 stimulation are also mediated by the IPP complex and ILK and suggest that IL-13 stimulation induces signals that modulate activation of the mechanosensitive ILK/PI3-kinase/Akt signaling pathway.
Fig. 5.
Expression of PINCH LIM1–2 inhibits mechanosensitivity of IL-13-induced Akt activation and suppression of SmMHC expression. A: immunoblots from extracts of 8 tracheal muscle tissues treated with PINCH LIM1–2 or sham treated, stimulated with IL-13 or not stimulated, and subjected to low or high mechanical load. B: immunoblots of anti-GFP immunoprecipitates from muscle tissues expressing EGFP-PINCH LIM 1–2 protein demonstrating expression of recombinant PINCH proteins. C: mean results for the effects of IL-13 and IL-13 plus PINCH LIM 1–2 on mechanosensitive Akt activity and SmMHC expression (n = 6). IL-13 stimulation did not suppress the mechanosensitivity of Akt phosphorylation or SmMHC expression, but PINCH LIM 1–2 expression abolished the mechanosensitivity of both SmMHC expression and Akt phosphorylation. Values are means ± SE. *Paired values are significantly different, P < 0.05.
DISCUSSION
The results of this study demonstrate that mechanical loading of intact airway smooth muscle tissues is necessary to stimulate the expression of the smooth muscle contractile protein, SmMHC. This suggests that mechanical loading enhances expression of the contractile phenotype. We further demonstrate that the transduction of mechanical stimuli to signals that regulate smooth muscle phenotypic protein expression is mediated by adhesion junction proteins that localize to membrane integrin complexes in smooth muscle cells, suggesting that the mechanical signals that regulate airway smooth muscle phenotype are sensed primarily by integrin proteins. We also observed that the stimulation of airway smooth muscle with the inflammatory mediator, IL-13, suppresses the mechanically induced expression of SmMHC. These results suggest that airway inflammation may interfere directly with signals from cell adhesion contacts that maintain the differentiated state of the airway muscle tissue and that this may be a mechanism by which the phenotype of the muscle can be altered under pathophysiological conditions.
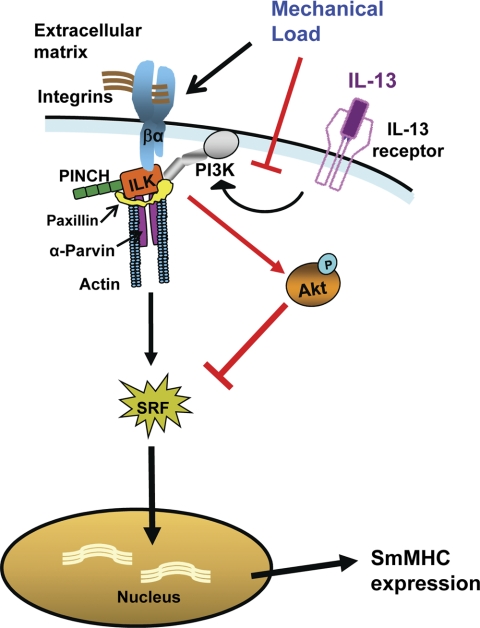
We found that the membrane localization of the IPP complex, a heterotrimeric complex of ILK, PINCH, and α-parvin that binds to the cytoplasmic domains of β-integrins, is essential for the transduction of mechanical signals to downstream pathways that regulate SmMHC expression. Mechanical signals transduced by the IPP complex inversely regulate the activity of PI3-kinase-dependent Akt: the imposition of mechanical load inhibits Akt activation, resulting in an increase in SmMHC expression, whereas a reduction in imposed load increases Akt activation, causing the inhibition of SmMHC expression. The inflammatory mediator IL-13 stimulates the activation of Akt; this suppresses the expression of SmMHC in muscles subjected to higher mechanical loads because it overcomes the suppression of Akt activity induced by mechanical loading. We previously showed that Akt activity inhibits the translocation of the transcriptional regulator, SRF, to the nucleus, which is required for the transcription of SmMHC and other smooth muscle phenotype-specific proteins (68) (Fig. 6).
Fig. 6.
Schematic diagram of the pathway for the transduction of mechanical signals that regulate SmMHC expression and the interaction of this pathway with the IL-13-activated signaling pathway. The IPP complex (ILK/PINCH/α-parvin) localizes to integrin adhesion junctions and mediates the PI3-kinase-dependent activation of Akt. Activated Akt inhibits the translocation of serum response factor (SRF) to the nucleus (red bar) and prevents the expression of smooth muscle myosin heavy chain (SmMHC). Mechanical load imposed on the smooth muscle inhibits Akt activation (red bar) and promotes the nuclear localization of SRF and the expression of SmMHC. Activation of the IL-13 receptor stimulates PI3-kinase and Akt activation, which inhibits SmMHC expression and opposes the effects of mechanical load.
The IPP complex resides both in the cytoplasm and at the membrane of mammalian cell types and is recruited to β-integrin-containing adhesion junctions in response to a variety of external stimuli (63). At these sites, it binds to the cytoplasmic domain of β-integrin proteins and to actin filaments and thereby links integrin proteins to downstream pathways that regulate multiple cell functions. The expression of a PINCH fragment that contains the ILK binding site inhibits PINCH-ILK and thereby disrupts the recruitment of the IPP complex to cell matrix contact sites (73). We previously demonstrated that the expression of PINCH LIM 1–2 peptides in airway smooth muscle inhibits the recruitment of the IPP complex to membrane adhesion junctions in airway smooth muscle during contractile stimulation, and we found that this inhibits tension development (71). Our present observations indicate that the membrane localization of the IPP complex is necessary for the signal transduction pathway that regulates mechanosensitive protein transcription. These observations provide strong evidence that β-integrins and the signaling proteins that associate with them are the primary transducers of mechanical signals that regulate phenotypic protein expression in airway smooth muscle.
We previously reported that the activation of Akt inhibits the nuclear translocation of the transcriptional regulator SRF in airway smooth muscle and thereby inhibits the expression of SmMHC, calponin, and Sm22α (68). SRF binds to CARG boxes in the promoter regions of a number of smooth muscle specific genes to regulate their transcription (34). Thus our results describe a signaling pathway that provides a direct link between integrin receptors and nuclear signaling pathways that regulate the transcription of genes critical for maintaining the differentiation state of smooth muscle cells and tissues. The activation of the cytoskeletal signaling proteins paxillin and focal adhesion kinase are also sensitive to mechanical forces imposed on airway smooth muscle (51, 52). These proteins are critical in the regulation of cytoskeletal organization, actin dynamics, and contractility in airway smooth muscle (24, 70). Because paxillin binds directly to ILK, it is likely that the IPP complex also regulates the mechanosensitivity of these proteins, suggesting that this complex may play a primary role in the transduction of extracellular mechanical stimuli to multiple downstream pathways that regulate the contractile function as well as the phenotype of airway smooth muscle.
Previous studies have shown that PINCH and ILK are required for optimal activation of Akt (15, 16, 40, 55). ILK has been shown to directly phosphorylate Akt on Ser 473, which is required for full Akt activation (40). In canine tracheal muscle tissues, the depletion of ILK protein decreases activation of Akt and increases the expression of SmMHC, whereas the overexpression of ILK protein inhibits the expression of SmMHC (68). To evaluate the specific role of ILK kinase activity in the mechanotransduction process, we expressed a kinase-inactive mutant of ILK, ILK S343A (40, 55) in the tracheal muscle tissues and compared its effects to that of the PINCH LIM 1–2 mutant and to a kinase-inactive HA-Akt1-AAA mutant. The expression of ILK S343A inhibited the activation of Akt at low mechanical loads and prevented the mechanosensitive modulation of Akt activity and SmMHC expression. These effects were similar to those of the PINCH LIM 1–2 mutant and the kinase-inactive HA-Akt1-AAA mutant. This suggests that ILK kinase activity is important for the regulation of the transduction of mechanical stimuli in airway smooth muscle.
There are conflicting reports regarding the effects of mechanical strain on smooth muscle differentiation. In several studies of cultured vascular and airway smooth muscle cells, the imposition of cyclic mechanical strain has been reported to stimulate the expression of phenotype-specific markers proteins (41, 49, 54). Mechanical strain has also been reported to induce the differentiation of murine embryonic stem cells to vascular smooth muscle cells (47). However, Wang et al. (61) reported that 48 h of cyclical strain imposed on canine tracheal myocytes inhibited the transcriptional activity of Sm22 and SmMHC promoters and that PLC-dependent signaling pathways played a role in the signaling pathways. A few studies have also evaluated effects of mechanical forces on the expression of phenotype-specific marker proteins in differentiated smooth muscle tissues. Exposure of rat mesenteric microvessels to elevated pressure and circumferential wall strain results in enhanced coverage by mature, fully differentiated smooth muscle cells and increased expression of α-SMA and SmMHC (58). Stretch of intact rat portal veins increased the rate of synthesis of SM22α, calponin, and α-actin through a Rho-associated kinase (ROCK) pathway (2). Contractile stimulation of tracheal muscle tissues with carbachol-induced transcription of the SmMHC A isoform in tissues maintained at a slack length (60). This effect did not occur in muscles subjected to sinusoidal length oscillation, which may have competed with or disrupted the mechanical stimulus induced by contraction with carbachol. These studies are consistent with the concept that signaling pathways elicited by mechanical tension or strain are fundamental for the differentiation of smooth muscle tissues and for maintenance of the differentiated state.
Akt has been implicated in the regulation of the transcription and translation of phenotypic marker proteins in both airway and vascular smooth muscle cells and tissues and in the regulation of rat aortic smooth muscle cell phenotype (7, 25, 28). We previously found that the activation of Akt inhibits SRF localization to the nucleus and prevents gene transcription in tracheal smooth muscle tissues (68), which is consistent with previous observations in rat aortic smooth muscle cells (28). Akt activation has also been reported to increase the expression of SmMHC in airway smooth muscle by stimulating ribosomal translation through its downstream targets, mammalian target of rapamycin (mTOR), and p70 ribosomal S6 kinase (5, 25). Our results suggest that the inhibitory effect of mechanical strain on integrin signaling to Akt and the consequent activation of SmMHC gene transcription is the predominant effect in airway smooth muscle tissues.
We found that IL-13 activated the PI3-kinase substrate, Akt in airway smooth muscle tissues. Il-13 stimulates the activation of PI3-kinase via the PI3-kinase-binding proteins, insulin receptor substrate (IRS)-1 and IRS-2, which are recruited the IL-4α chain of the IL-13 receptor heterodimers in response to IL-13 stimulation (27). The PI3-kinase-dependent activation of Akt by IL-13 has been previously reported in airway smooth muscle (13) as well as in other cell types, including intestinal goblet cells (62) and dermal fibroblasts (36). We also found that IL-13 stimulation suppressed the expression of SmMHC. This effect of IL-13 is predictable from our findings that Akt activation inhibits the transcription and expression of SmMHC and other smooth muscle phenotype-specific proteins (68).
The incubation of airway smooth muscle cells or tissues with IL-13 increases the airway smooth muscle responsiveness to contractile agonists and has been suggested as a mediator of airway hyperresponsiveness in asthma (1, 8, 12, 19, 37, 48, 64). IL-13 has direct effects on the signaling pathways that regulate airway smooth muscle contractility (37, 44); however, it is not known whether these effects are responsible for the IL-13-induced augmented contractility. Although the suppression of expression of the contractile phenotype-specific proteins by IL-13 would seem to be counterintuitive to its known effects on airway muscle responsiveness, the functional effects of such alterations in protein expression on airway smooth muscle contractility are unknown. The effects of IL-13 on cellular signaling pathways that regulate contractility and/or on airway smooth muscle cell proliferation (42) may enhance airway smooth muscle contractility even while contractile protein expression is suppressed.
In conclusion, our results suggest an important role for integrin receptors in transducing effects of mechanical signals to downstream pathways that regulate the phenotype of airway smooth muscle tissues. We find that the integrin-associated IPP complex, and specifically the integrin-binding protein ILK, plays a central role in the mechanotransduction process. Inflammatory mediators such as IL-13 suppress signaling pathways that stimulate the differentiated state of airway smooth muscle and may thereby alter the functions of the muscle under pathophysiological conditions. As signals activated by mechanical stress and IL-13 converge on the same signaling pathway to oppositely regulate the expression of phenotypic marker proteins in intact airway smooth muscle tissues; mechanical strain may suppress phenotypic changes in smooth muscle function stimulated by inflammation. Our findings provide insights into the mechanisms contributing to the regulation of the differentiated state of airway smooth muscle tissue by mechanical forces and suggest a mechanism for the alterations of airway smooth muscle phenotype and function in the pathogenesis of inflammatory airway diseases such as asthma.
GRANTS
This work was supported by National Institutes of Health Grants HL-29289, HL-074099, and HL-048522.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 282: G226–G232, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Albinsson S, Nordstrom I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem 279: 34849–34855, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 4. Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell 14: 4813–4825, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc 5: 89–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res 296: 183–190, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Chen CN, Li YS, Yeh YT, Lee PL, Usami S, Chien S, Chiu JJ. Synergistic roles of platelet-derived growth factor-BB and interleukin-1beta in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci USA 103: 2665–2670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M. Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol 40: 159–167, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Choe MM, Sporn PHS, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol 35: 306–313, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 95: 11211–11216, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol 31: 36–42, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Eum SY, Maghni K, Tolloczko B, Eidelman DH, Martin JG. IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288: L576–L584, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Farghaly HS, Blagbrough IS, Medina-Tato DA, Watson ML. Interleukin 13 increases contractility of murine tracheal smooth muscle by a phosphoinositide 3-kinase p110delta-dependent mechanism. Mol Pharmacol 73: 1530–1537, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Fujio Y, Guo K, Mano T, Mitsuuchi Y, Testa JR, Walsh K. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol 19: 5073–5082, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukuda T, Chen K, Shi XH, Wu CY. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem 278: 51324–51333, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Fukuda T, Guo L, Shi XH, Wu CY. CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J Cell Biol 160: 1001–1008, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grashoff C, Thievessen I, Lorenz K, Ussar S, Fassler R. Integrin-linked kinase: integrin's mysterious partner. Curr Opin Cell Biol 16: 565–571, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Moh M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282: 2261–2263, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grunstein MM, Hakonarson H, Leiter J, Chen M, Whelan R, Grunstein JS, Chuang S. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 282: L520–L528, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Gunst SJ, Lai-Fook SJ. Effect of inflation on trachealis muscle tone in canine tracheal segments in vitro. J Appl Physiol 54: 906–913, 1983 [DOI] [PubMed] [Google Scholar]
- 21. Gunst SJ, Shen X, Ramchandani R, Tepper RS. Bronchoprotective and bronchodilatory effects of deep inspiration in rabbits subjected to bronchial challenge. J Appl Physiol 91: 2511–2516, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Gunst SJ, Stropp JQ. Pressure-volume and length-stress relationships in canine bronchi in vitro. J Appl Physiol 64: 2522–2531, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Gunst SJ, Tang DD, Opazo SA. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol 137: 151–168, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576–C587, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, Forsythe SM, Hershenson MB, Solway J. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70(S6K) regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol 31: 266–275, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Janmey PA, Weitz DA. Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem Sci 29: 364–370, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol 105: 1063–1070, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Kaplan-Albuquerque N, Garat C, Desseva C, Jones PL, Nemenoff RA. Platelet-derived growth factor-BB-mediated activation of akt suppresses smooth muscle-specific gene expression through inhibition of mitogen-activated protein kinase and redistribution of serum response factor. J Biol Chem 278: 39830–39838, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Khyrul WAKM, LaLonde DP, Brown MC, Levinson H, Turner CE. The integrin-linked kinase regulates cell morphology and motility in a Rho-associated kinase-dependent manner. J Biol Chem 279: 54131–54139, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA, Jr, Kinet JP, Shore SA. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med 164: 141–148, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, Erle DJ, Sheppard D. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol 25: 474–485, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Legate KR, Montañez E, Kudlacek O, Fässler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7: 20–31, 2006 [DOI] [PubMed] [Google Scholar]
- 33. McDonald KA, Lakonishok M, Horwitz AF. Alpha v and alpha 3 integrin subunits are associated with myofibrils during myofibrillogenesis. J Cell Sci 108: 2573–2581, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292: C70–C81, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Moore PE, Church TL, Chism DD, Panettieri RA, Jr, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol 282: L847–L853, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Moriya C, Jinnin M, Yamane K, Maruo K, Muchemwa FC, Igata T, Makino T, Fukushima S, Ihn H. Expression of matrix metalloproteinase-13 is controlled by IL-13 via PI3K/Akt3 and PKC- in normal human dermal fibroblasts. J Invest Dermatol 131: 655–661, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Moynihan B, Tolloczko B, Michoud MC, Tamaoka M, Ferraro P, Martin JG. MAP kinases mediate interleukin-13 effects on calcium signaling in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 295: L171–L177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Opazo Saez A, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol 286: C433–C447, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA 97: 3207–3212, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276: 27462–27469, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Reusch P, Wagdy H, Reusch R, Wilson E, Ives HE. Mechanical strain increases smooth muscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circ Res 79: 1046–1053, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST, Davies DE. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol 25: 385–391, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol 133: 169–184, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L26–L34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen X, Gunst SJ, Tepper RS. Effect of tidal volume and frequency on airway responsiveness in mechanically ventilated rabbits. J Appl Physiol 83: 1202–1208, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Shen X, Wu MF, Tepper RS, Gunst SJ. Mechanisms for the mechanical response of airway smooth muscle to length oscillation. J Appl Physiol 83: 731–738, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Shimizu N, Yamamoto K, Obi S, Kumagaya S, Masumura T, Shimano Y, Naruse K, Yamashita JK, Igarashi T, Ando J. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor β. J Appl Physiol 104: 766–772, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin Exp Pharmacol Physiol 29: 859–866, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Smith PG, Moreno R, Ikebe M. Strain increases airway smooth muscle contractile and cytoskeletal proteins in vitro. Am J Physiol Lung Cell Mol Physiol 272: L20–L27, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci USA 98: 6180–6185, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang DD, Gunst SJ. Roles of focal adhesion kinase and paxillin in the mechanosensitive regulation of myosin phosphorylation in smooth muscle. J Appl Physiol 91: 1452–1459, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Tang DD, Mehta D, Gunst SJ. Mechanosensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Am J Physiol Cell Physiol 276: C250–C258, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Tang DD, Wu MF, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol 542: 501–513, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tock J, Van Putten V, Stenmark KR, Nemenoff RA. Induction of SM-α-actin expression by mechanical strain in adult vascular smooth muscle cells is mediated through activation of JNK and p38 MAP kinase. Biochem Biophys Res Commun 301: 1116–1121, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Troussard AA, Mawji NM, Ong C, Mui A, Arnaud R, Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem 278: 22374–22378, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Tschumperlin DJ, Drazen JM. Chronic effects of mechanical force on airways. Annu Rev Physiol 68: 563–583, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 19: 2425–2434, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res 92: 929–936, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Venkayya R, Lam M, Willkom M, Grunig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol 26: 202–208, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Wahl M, Eddinger TJ, Hai CM. Sinusoidal length oscillation- and receptor-mediated mRNA expression of myosin isoforms and α-SM actin in airway smooth muscle. Am J Physiol Cell Physiol 287: C1697–C1708, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Wang L, Liu HW, McNeill KD, Stelmack G, Scott JE, Halayko AJ. Mechanical strain inhibits airway smooth muscle gene transcription via protein kinase C signaling. Am J Respir Cell Mol Biol 31: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Wang ML, Keilbaugh SA, Cash-Mason T, He XC, Li L, Wu GD. Immune-mediated signaling in intestinal goblet cells via PI3-kinase- and AKT-dependent pathways. Am J Physiol Gastrointest Liver Physiol 295: G1122–G1130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 29: 281–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17: 255–281, 1999 [DOI] [PubMed] [Google Scholar]
- 65. Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev 202: 175–190, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 282: 2258–2261, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 155: 505–510, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu Y, Huang Y, Herring BP, Gunst SJ. Integrin-linked kinase regulates smooth muscle differentiation marker gene expression in airway tissue. Am J Physiol Lung Cell Mol Physiol 295: L988–L997, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zargham R, Thibault G. Alpha 8 integrin expression is required for maintenance of the smooth muscle cell differentiated phenotype. Cardiovasc Res 71: 170–178, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Zhang W, Gunst SJ. Interactions of airway smooth muscle cells with their tissue matrix: implications for contraction. Proc Am Thorac Soc 5: 32–39, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang W, Wu Y, Wu C, Gunst SJ. Integrin-linked kinase (ILK) regulates N-WASp-mediated actin polymerization and tension development in tracheal smooth muscle. J Biol Chem 282: 34568–34580, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Zhang Y, Chen K, Guo L, Wu C. Characterization of PINCH-2, a new focal adhesion protein that regulates the PINCH-1-ILK interaction, cell spreading, and migration. J Biol Chem 277: 38328–38338, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Zhang YJ, Guo LD, Chen K, Wu CY. A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J Biol Chem 277: 318–326, 2002 [DOI] [PubMed] [Google Scholar]