Abstract
Human immunodeficiency virus (HIV)-associated pulmonary arterial hypertension (PAH) is a serious noninfectious disease involving an aberrant increase in pressure in the blood vessels of the lung, which leads to right ventricular (RV) heart failure and can eventually result in death. A lack of viable animal models of HIV-PAH has limited the identification of signaling pathways involved in HIV-mediated onset and progression of PAH. To determine whether the HIV-1 transgenic (HIV Tg) rat displays pathophysiological end points associated with PAH, we evaluated peak RV systolic pressure (RVSP), RV hypertrophy, pulmonary vessel remodeling, and alterations in gene expression by real-time PCR and microarray. RVSP was measured by RV catheterization via the right jugular vein in 3- and 9-mo-old HIV Tg and age-matched Fischer 344 (control) male rats while under 2% isoflurane anesthesia. RVSP was elevated in the HIV Tg rats (34.2 ± 2.5 mmHg) compared with the F344 controls (21.2 ± 2.5 mmHg), with more significant elevations in the 9-mo-old HIV Tg rats (42.5 ± 3.7 mmHg). We observed significant increases in RV wall thickness in HIV Tg rats compared with controls, both histologically and by echocardiograph measurement. HIV Tg rats also show increased thickening of the pulmonary artery and remodeling of small pulmonary arteries, as well as altered expression of gene pathways associated with PAH. These data represent the first analysis of PAH in HIV Tg rats and suggest that this model will be useful for investigating pathways and identifying potential therapies for HIV-PAH.
Keywords: human immunodeficiency virus-associated pulmonary arterial hypertension, echocardiograph, right ventricular systolic pressure, microarray
pulmonary arterial hypertension (PAH) is a progressive, nearly always fatal condition that is characterized by a sustained elevation in right ventricular (RV) pressure and pulmonary arterial pressure (PAP), leading to vascular remodeling of the pulmonary circulation and the RV of the heart. Incidence of PAH is significantly higher, as much as 2,500-fold (29, 36), in the human immunodeficiency virus (HIV)-infected than the general population, and PAH considerably lowers the median survival of HIV-infected patients (29). In North America and Western Europe, where ∼3.8 million people are living with HIV, there are ∼10,000 cases of HIV-related PAH (57); however, recent reports suggest that the incidence of PAH-associated HIV infection (PAH-HIV) may be even higher than previously reported. A recent study found elevated PAP in ∼35.2% of HIV-infected outpatients (14). Mortality in patients with PAH-HIV is generally attributed to pulmonary vascular disease, rather than complications from the HIV infection; thus PAH is considered to be an independent predictor of death (23, 28). Early data suggest that highly active antiretroviral therapy (HAART) markedly improves the prognosis of patients with PAH-HIV; however, more recent findings suggest that HAART does not prevent the development of PAH-HIV and confirm that PAH develops in patients with well-controlled HIV infection status (9, 30).
PAH is defined as an elevated mean PAP ≥25 mmHg at rest (50). The increased demand on the RV of the heart leads to hypertrophy and can eventually progress to heart failure. PAH results from chronic obstruction of small pulmonary arteries, possible contributors of which include increased vasoconstriction and/or remodeling of the vessel wall and narrowing of the lumen diameter of the vessels. As HIV does not directly infect endothelial cells (24) or vascular smooth muscle cells (VSMCs), the virus is likely signaling through receptors on these cell types. Vascular cell proliferation and extracellular matrix reorganization pathways upregulated by HIV include transforming growth factor (TGF-β), VEGF, and PDGF-BB, among others (15). Additionally, HIV infection has been associated with increased expression of vasoconstrictor peptides such as endothelin-1 (ET-1) and ANG II (10, 18, 31).
While several pathways appear to be involved in progression of PAH, including those associated with increased contraction of VSMCs (1) and deregulated proliferation of vascular endothelial cells and VSMCs (16), the etiology of HIV-PAH has not been fully elucidated. Therefore, it is important to identify factors that contribute to this increased incidence of PAH with HIV infection, as well as gain a further understanding of the mechanistic pathways involved. Of importance, HIV viral proteins have been implicated in the development of HIV-PAH. While there are transgenic animal models of HIV-infection (41), as well as simian HIV (SHIV)-infected nonhuman primate models (21), a limited number of these models display pulmonary effects of HIV similar to those observed in humans. This may be due to the lack of the presence of multiple (or all) HIV viral proteins concurrently or the location of HIV protein tissue expression in these models. Therefore, in this study, we investigated whether the HIV transgenic (HIV Tg) rat, which has seven of the nine HIV viral proteins (a functional deletion of Gag and Pol within the HIV-1 provirus to renders the animals noninfectious), displays altered RV pressures or RV wall or pulmonary vascular remodeling. Additionally, using a microarray analysis of lung tissue from HIV Tg rats, we determined gene pathways that are significantly different in expression from those in lung tissue from F344 control rats; also, using RT-PCR, we examined the expression profile of genes implicated in the pathology of PAH, such as bone morphogenetic protein (a member of the TGF-β superfamily) type II receptor (BMPR2), the voltage-gated K+ channel Kv1.5, ET-1, endothelial nitric oxide (NO) synthase (eNOS), PDGF-BB, PDGF receptor β (PDGFR-β), VEGF, VEGF receptor 2 (VEGFR2), and hypoxia-inducible factor-1α (HIF-1α).
METHODS
Animals.
We used 3-mo-old (n = 9) and 9-mo-old (n = 3) HIV Tg male rats [Hsd:HIV-1 (F344) Harlan] and 3-mo-old (n = 9) and 9-mo-old (n = 3) Fischer 344 (F344) male rats (as age- and background-matched controls). HIV Tg rats express viral genes in the lymph nodes, spleen, thymus, and blood, suggesting that rat cyclin T is functional with Tat; however, the animals are noninfectious because of the functional deletion of Gag and Pol within the HIV-1 provirus (and, thus, carry only 7 of the 9 HIV genes) (42). Rats were housed in pairs in microisolator cages within a rodent housing facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International for the entirety of the study. Constant temperature was maintained at 20–24°C and relative humidity at 30–60%, and rats had access to chow and water ad libitum throughout the study period. All procedures were approved by the Lovelace Respiratory Research Institute's Animal Care and Use Committee and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Immediately after peak RV systolic pressure (RVSP) measurements, deep anesthesia was induced (5% isoflurane by mask), and animals were euthanized by exsanguination. Heart, lung, aorta, and brain tissues were collected, weighed (Table 1), immediately snap-frozen [a portion of the heart was embedded in Tissue Tek optimal cutting temperature (OCT) compound (VWR Scientific, West Chester, PA) for histology and frozen on dry ice], and stored at −80°C until used for analysis. Blood was collected with heparin-treated syringes, and plasma was immediately separated by centrifugation at 3,000 g for 10 min at 4°C.
Table 1.
Body and tissue weights of HIV Tg and age-matched F344 rats
| Body Weight, g | Heart Weight, g | Brain Weight, g | |
|---|---|---|---|
| Male 3-mo-old | |||
| F344 | 326 ± 16 | 0.946 ± 0.079 | 1.944 ± 0.115 |
| HIV Tg | 270 ± 10* | 0.776 ± 0.038* | 1.709 ± 0.068* |
| Male 9-mo-old | |||
| F344 | 493 ± 26 | 1.229 ± 0.216 | 2.222 ± 0.082 |
| HIV Tg | 331 ± 24† | 0.898 ± 0.082‡ | 1.796 ± 0.078† |
Values are means ± SD. F344, Fischer 344 control; HIV Tg, human immunodeficiency virus transgenic.
P ≤ 0.050 vs. 3-mo-old F344.
P ≤ 0.050 vs. 9-mo-old F344.
P = 0.060 vs. 9-mo-old F344.
RV pressure measurements.
RV pressures were measured by catheterization. Anesthesia was induced with 5% isoflurane and maintained with 2–3% isoflurane. The fur from the neck region was shaved, and the skin was scrubbed with ethanol. A cutaneous incision was made at the right of the midline to expose the right external jugular vein. Once it was exposed and isolated from surrounding tissue, a small incision was made in the vein, and a small “J-shaped” fluid-filled catheter was passed into the jugular vein. The catheter, connected to a disposable pressure transducer (DTX/Plus, Becton Dickinson, Franklin Lakes, NJ) fed into an amplifier (model 6600, Gould Instrument Systems, Valley View, OH), was slowly advanced into the heart, where changes in blood pressure signal were monitored to determine correct placement of the catheter tip. Once the trace indicated that the catheter was placed in the RV, the pressures were recorded for 2–5 min. An average RVSP was obtained for each animal over the time of recording.
Echocardiography.
RV chamber size in systole (RVs) and diastole (RVd) was measured using echocardiography, and RV wall thickness (RVWT) was determined using M-mode technology, just prior to pulmonary pressure catheterization under isoflurane anesthesia. The echocardiogram was obtained by a transthoracic, linear-array probe placed on the chest wall using an Acuson 512 Sequoia Doppler Ultrasound with a 15-MHz linear-array transducer.
Vascular morphometry.
Immediately after PAP measurement, animals were exsanguinated, and the lung block (trachea with right and left lungs) was removed. The right lung was tied off at the main bronchus, and the lobes were dissected and immediately embedded in OCT, frozen on dry ice, and stored at −80°C until they were used for histology. The left lung was suspended via the trachea, perfused with 10% neutral buffered formalin and nitroprusside (1 mg/ ml; Sigma Aldrich, St. Louis, MO) for 2 h, and then soaked for 48 h in 10% neutral buffered formalin to complete fixation. After fixation, the left lung was dissected and embedded in paraffin for sectioning. Lung sections (5 μm) were stained with hematoxylin-eosin (main pulmonary artery) or Masson's trichrome with Verhoeff elastic stain (small pulmonary arteries) and examined on an Everest Digitals Imaging Microscopy System at ×40 or ×60. For Masson's trichrome-Verhoeff staining, slides were deparaffinzed, rinsed in double-distilled H2O, soaked in Bouin's solution for 1 h at 56°C, and rinsed again. Slides were then processed through Verhoeff stain for 45 min, rinsed, dipped in 2% ferric chloride, rinsed, dipped in 5% Hypo solution, rinsed, exposed to Biebrich scarlet stain for 5 min, rinsed, exposed to 0.5% acetic acid for 30 s, and then placed into 100% xylene for application of coverslips. Pulmonary artery wall thickness was measured by calculating the difference between the perimeter of the vessel (inside the tunica externa) and the perimeter of the lumen (inside the tunica intima). Images were analyzed using ImageJ (NIH). Lung histology was completed in three to four animals per group and four to six vessels per lung.
Real-time RT-PCR.
Total RNA was isolated from the right superior lobe using an AllPrep DNA/RNA/protein kit (Qiagen, Valencia, CA). cDNA was synthesized from total RNA in a 20-μl final reaction volume according to the manufacturer's instructions (iScript Select, Bio-Rad, Hercules, CA). The cDNA mixture was heated at 42°C for 1 h and then cooled to 4°C for the RT reaction. Real-time PCR was performed with gene-specific primers (Table 2) in the ABI 7900 instrument (Applied Biosystems, Carlsbad, CA). Control reactions without reverse transcriptase and those without RNA were run to verify the absence of contaminated DNA and primer dimerization, respectively. PCR amplification was carried out as previously described (19). A melt curve was also obtained for each sample using the following parameters: 84 cycles starting at 54°C and increasing 0.5°C every 5 s. Quantification of mRNA was evaluated using Applied Biosystems software. Samples were run in triplicate, and results for each run were averaged. Change in cycle threshold (ΔCT) was calculated by subtracting the CT of the GAPDH control gene from the CT of the gene of interest, and mean normalized gene expression was calculated as previously described (19).
Table 2.
Real-time PCR primer sequences
| Sequence | |
|---|---|
| ET-1 | |
| Forward | 5′-TGGACATCATCTGGGTCAACA-3′ |
| Reverse | 5′-GCTTAGACCTAGAAGGGCTTCCTAGT-3′ |
| BMPR2 | |
| Forward | 5′-GGGAAATTCTGCAGTGGAATG-3′ |
| Reverse | 5′-CCGGATTACAATGCAGTTTGAA-3′ |
| eNOS | |
| Forward | 5′-GACTTTTAAGGAAGTAGCCAATGCA-3′ |
| Reverse | 5′-CCATACAGGATAGTCGCCTTCAC-3′ |
| Kv1.5 | |
| Forward | 5′-TTCGACCCCTTGAGAAATGAA-3′ |
| Reverse | 5′-AGTAGTACAAAATGCCATCGAAGCT-3′ |
| VEGF | |
| Forward | 5′-GGGCTGCTGCAATGATGAA-3′ |
| Reverse | 5′-TCCGCATGATCTGCATAGTGA-3′ |
| VEGFR2 | |
| Forward | 5′-GCTGCGGGTCTCACTACCA-3′ |
| Reverse | 5′-GGGTATGGGTACTTCTTGGAGGAT-3′ |
| HIF-1α | |
| Forward | 5′-CCCAGCTGTTCACTAAAGTGGAA-3′ |
| Reverse | 5′-GCATCGGGCTCTTTCTTAAGC-3′ |
| PDGF-BB | |
| Forward | 5′-CCTGCAAGTGTGAGACAGTAGTGA-3′ |
| Reverse | 5′-AGGTGTCTTGGCTCGATGCT-3′ |
| PDGFR-β | |
| Forward | 5′-TGTTCACATCCTCCTCCTGTGTAGCA-3′ |
| Reverse | 5′-GCTTCAGTTTTGTCGGTTTGG-3′ |
| GAPDH | |
| Forward | 5′-TGGCCTCCAAGGAGTAAGAAAC-3′ |
| Reverse | 5′-GGCCTCTCTCTTGCTCTCAGTATC-3′ |
ET-1, endothelin-1; BMPR2, bone morphogenic protein receptor (member of TGF-β superfamily) type 2; eNOS, endothelial nitric oxide synthase; Kv1.5, voltage-gated K+ channel; VEGFR2, VEGF receptor type 2; HIF-1α, hypoxia inducible factor-1α; PDGFR-β, PDGF receptor β.
Microarray.
RNA was isolated from 25 mg of tissue from the right superior lobe of the lung as described above (see Real-time PCR). The quality of the RNA was confirmed by spectrophotometry (260 nm-to-280 nm ratio) and in the Bioanalyzer (Agilent Technologies, Santa Clara, CA). Total RNA was then processed through RT to synthesize first-strand cDNA using the GeneChip 3′ IVT Express Kit (catalog no. 901228, Affymetrix, Santa Clara, CA). cDNA was then converted to a double-stranded DNA template for transcription. In vitro transcription synthesized amplified RNA (aRNA, also known as cRNA) and incorporated a biotin-conjugated nucleotide. Then aRNA was purified to remove unincorporated NTPs, salts, enzymes, and inorganic phosphate. Fragmentation of the biotin-labeled aRNA prepared the sample for hybridization onto GeneChip 3′ expression arrays (catalog no. 900506, Affymetrix Rat 230 2.0 Arrays). The genes that were differentially expressed were identified from microarray study in a HIV vs. control experiment. Those genes, together with their relative fold changes, were used as input for MetaCore (https://portal.genego.com/) to identify the associated processes. The false discovery rate was set to 0.05. In total, 13 processes were discovered based on the above criterion, and their P values were calculated based on hypergeometric distribution (Table 3). All raw microarray data have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO accession no. GSE27393) repository for public access.
Table 3.
Microarray results of cell signaling transcript pathways altered in lung tissue from HIV Tg vs. F344 rats
| Cell Pathways | P Value |
|---|---|
| Inflammation kallikrein-kinin system | 2.848e−7 |
| Chemotaxis | 1.598e−5 |
| Blood coagulation | 7.844e−5 |
| Inflammation innate inflammatory response | 4.784e−4 |
| Inflammation complement system | 8.013e−4 |
| Inflammation histamine signaling | 1.076e−3 |
| Apoptosis/antiapoptosis mediated by external signals via NF-κB | 1.396e−3 |
| Cell adhesion leukocyte chemotaxis | 1.720e−3 |
| Development of blood vessel morphogenesis | 2.039e−3 |
| Cell adhesion, platelet-endothelium-leukocyte interactions | 2.631e−3 |
| Inflammation NK cell cytotoxicity | 4.008e−3 |
| Inflammation neutrophil activation | 4.016e−3 |
| Development, regulation of angiogenesis | 4.016e−3 |
Statistical analysis.
Real-time PCR data are expressed as means ± SE; RVSP, PAP, vessel dimension measurements, tissue weights, and echocardiograph parameters are expressed as means ± SD. Student's t-test was used to statistically compare control with HIV Tg end points in each age group (3 mo or 9 mo). P < 0.05 was considered statistically significant. For description of microarray analysis, see above (Microarray).
RESULTS
HIV Tg rats exhibit significantly elevated RVSP.
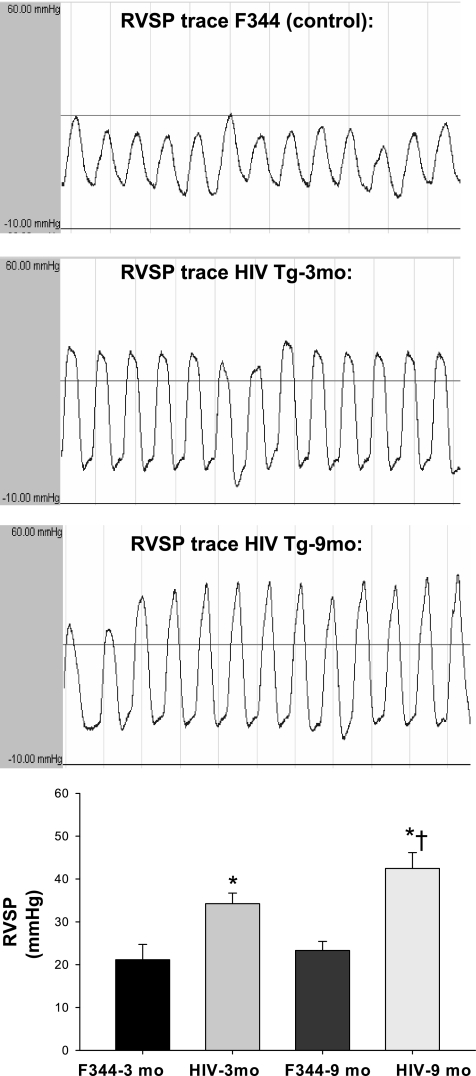
To determine whether male HIV Tg rats display elevated RV pressures, we used indwelling catheters to assess RV pressure measurements. We measured a significant increase in RVSP in 3-mo-old male HIV Tg rats compared with F344 controls (Fig. 1). The increase in RVSP measurements was greater in the 9-mo-old HIV Tg rats than the age-matched F344 controls (Fig. 1). Such findings indicate that male HIV Tg rats display elevations in RVSP consistent with that observed in pulmonary hypertension, which progresses with age of the rat.
Fig. 1.
Top: representative traces of peak right ventricular (RV) systolic pressure (RVSP) traces in Fischer 344 (F344) control and human immunodeficiency virus transgenic (HIV Tg) rats as measured by catheterization via the right jugular vein and recorded via transducer and Gould amplifier (traces not shown for F344 controls at 9 mo of age). Scale of y-axis is −10 to 60 mmHg. Bottom: graphic representation of data from traces (n = 9 per 3-mo-old group, n = 3 per 9-mo-old group). *P < 0.050 vs. age-matched F344 control. †P < 0.050 vs. 3-mo-old HIV Tg.
Increased RV hypertrophy in HIV Tg rat hearts.
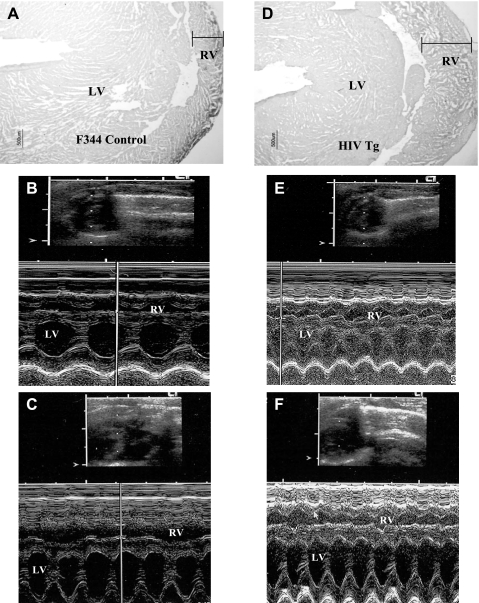
As PAP increases, due to an increase in pulmonary vascular resistance, the RV begins to hypertrophy to accommodate the increased afterload. Thus we measured RVWT by echocardiography and histologically in the HIV Tg rat. HIV Tg rats display significant increases in RVWT (Fig. 2, D–F) compared with age-matched F344 controls (Fig. 2, A–C). In 3-mo-old HIV Tg rat hearts, RV chamber size in diastole (RVd) was 0.206 ± 0.018 cm, RV chamber size in systole (RVs) was 0.098 ± 0.021 cm, and RVWT was 0.089 ± 0.012 cm; in F344 controls, RVd was 0.269 ± 0.057 cm (P ≤ 0.001 vs. HIV RVd), RVs was 0.131 ± 0.030 cm (P ≤ 0.001 vs. HIV RVd), and RVWT was 0.060 ± 0.009 cm (P ≤ 0.050 vs. HIV RVWT). Further increases in RVWT were observed in the 9-mo-old HIV Tg rat hearts (Fig. 2F): 0.119 ± 0.013 cm compared with 0.091 ± 0.011 cm in age-matched F344 controls (Fig. 2C). Additionally, there was a larger increase in RVs proportionate to heart weight in the 9-mo-old HIV Tg rat heart (0.120 ± 0.009 cm) than in the F344 heart (0.152 ± 0.010), suggesting alterations in RV performance in the HIV Tg rat. RVd was 0.240 ± 0.017 cm for 9-mo-old HIV Tg rats and 0.326 ± 0.016 cm for 9-mo-old F344 controls, respectively. The echocardiograph finding of increased RVWT is in agreement with histological assessment of these hearts. It is important to note that overall heart size was smaller in HIV Tg rats (Table 1), as was body size. While not directly measured in our study, HIV Tg rats have been reported to display atrophy of cardiac and skeletal muscle, the latter of which is associated with increased protein degradation (42).
Fig. 2.
A and D: RV wall thickness. Representative sections of male 3-mo-old F344 control and HIV Tg rats were stained with hematoxylin and eosin. Magnification ×4. B, C, E, and F: M-mode echocardiography of left ventricle (LV) and RV of 3-mo-old F344 control (B), 9-mo-old F344 control (C), 3-mo-old HIV Tg (E), and 9-mo-old HIV Tg (F) rat. Images are representative of sections from 9 rats in each 3-mo-old group and 3 rats in each 9-mo-old group.
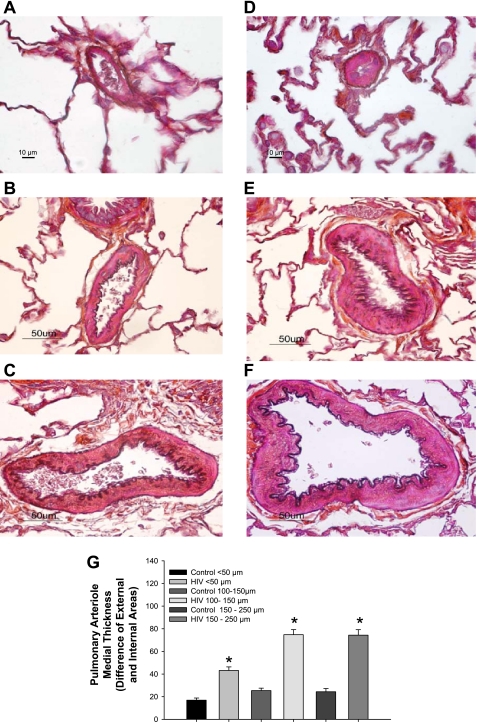
HIV Tg rats display significant alterations in pulmonary vessel remodeling.
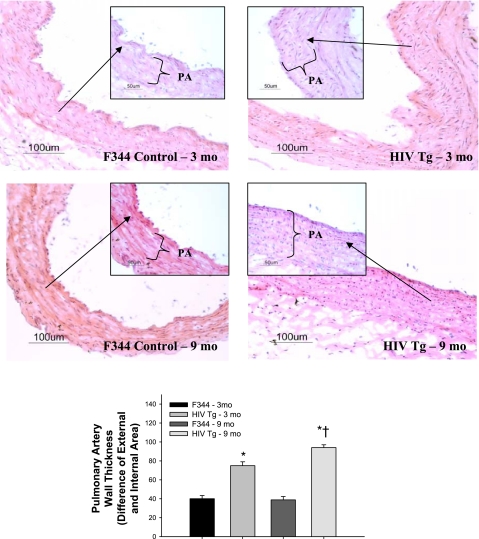
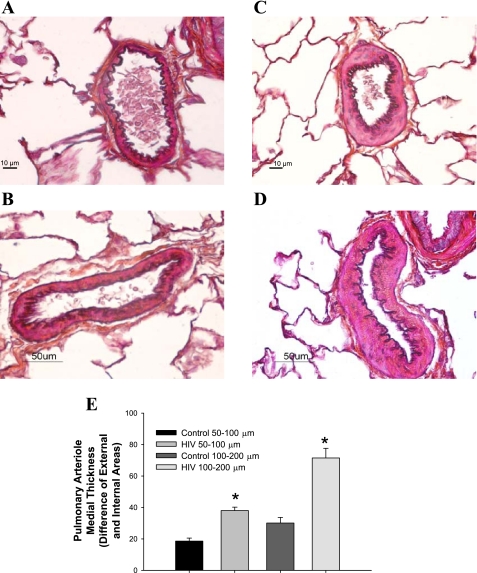
Structural remodeling of the walls of pulmonary vessels is a hallmark feature of PAH and HIV-PAH (4), which may lead to a decreased luminal radius and a resulting increase in pulmonary vascular resistance. HIV Tg rat pulmonary vessels (pulmonary artery and small muscular arteries; Figs. 3, C and D, and Fig. 4, C and D) histologically display increased medial thickening compared with age-matched F344 controls (Figs. 3, A and B, and Fig. 4, A and B), which is observed to progress with age (Figs. 3 and 5). Given the fixation protocol used, the contribution of vasoconstriction relative to proliferation cannot be determined in this study. Further characterization of the pulmonary vasculature in HIV Tg rats is needed to determine whether there is increased smooth muscle and/or endothelial cell proliferation, as well as whether there is an imbalance in locally produced vasodilators or vasoconstrictors, that may also account for elevated pulmonary vascular resistance in the HIV Tg rat.
Fig. 3.
Top: wall thickness of pulmonary artery (PA) sections from 3- and 9-mo-old male F344 control and HIV Tg rats. Representative sections (5 μm) were stained with hematoxylin and eosin. Images are representative of 4–5 sections from 3-mo-old and 3 sections from 9-mo-old rats. Magnification ×20 (main panel) and ×40 (inset). Bottom: wall thickness determined as difference between external and internal area of PA. *P < 0.050 vs. age-matched F344 control. †P < 0.050 vs. 3-mo-old HIV Tg.
Fig. 4.
A–D: representative Masson's trichrome-Verhoeff-stained lung sections (5 μm) containing small pulmonary arteries (50–100 and 100–200 μm diameter) from 3-mo-old male F344 control (A and B) and HIV Tg (C and D) rats. Magnification ×60 (A and C) and ×40 (B and D). E: graphic representation of wall thickness determined as difference between external and internal area of pulmonary vessels (n = 3–4 animals per group, 4–6 vessels per lung). *P < 0.050 vs. age-matched F344 control.
Fig. 5.
A–F: representative Masson's trichrome-Verhoeff-stained lung sections (5 μm) containing small pulmonary arteries (50–100 and 100–200 μm diameter) from 9-mo-old male F344 (A–C) and HIV Tg (D–F) rats. G: graphic representation of wall thickness determined as difference between external and internal area of the pulmonary vessels (n = 3 animals per group, 4–6 vessels per lung). Magnification ×60 (A and D) and ×40 (B, C, E, and F). *P < 0.050 vs. age-matched F344 control.
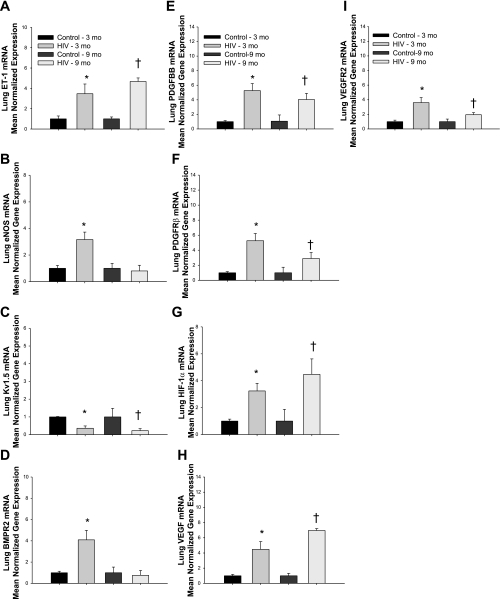
Gene expression profile in HIV Tg rats is consistent with that observed in other models of PAH.
To determine if pulmonary hypertension in HIV Tg rats is characterized by gene expression pathways and profiles similar to those observed in other types of PAH (e.g., idiopathic and familial), we conducted microarray and real-time PCR analysis of lung tissue. Transcription of ET-1 (Fig. 6A), eNOS (Fig. 6B), BMPR2 (Fig. 6D), PDGF-BB (Fig. 6E), and PDGFR-β (Fig. 6F) is significantly increased in lungs of 3-mo-old HIV Tg rats compared with F344 controls. Expression of members of hypoxia-induced pathways, including HIF-1α (Fig. 6G), VEGF (Fig. 6H), and VEGFR2 (Fig. 6I) was also elevated in 3-mo-old HIV Tg lungs. We measured a further increase in expression of ET-1 (Fig. 6A), HIF-1α (Fig. 6G), and VEGF (Fig. 6H) mRNA in lungs from 9-mo-old HIV Tg rats compared with 3-mo-old rats. Interestingly, eNOS (Fig. 6B) and BMPR2 (Fig. 6D) lung mRNA were significantly decreased in the 9-mo-old HIV rats compared with age-matched F344 controls. Expression of PDGF-BB (Fig. 6E), PDGFR-β (Fig. 6F), and VEGFR2 (Fig. 6I) was upregulated in 9-mo-old HIV Tg lungs, but at statistical levels similar to that seen in 3-mo-old HIV Tg rat lungs. Consistent with previous reports that describe a decrease in expression of Kv1.5 in various models of PAH (1a, 2, 12, 26, 62), Kv1.5 was significantly downregulated in pulmonary tissue in 3- and 9-mo-old HIV Tg rats compared with age-matched F344 controls.
Fig. 6.
Real-time PCR quantification from pulmonary tissue of endothelin-1 (ET-1, A), endothelial nitric oxide synthase (eNOS, B), voltage-gated K+ channel 1.5 (Kv1.5, C), bone morphogenetic protein receptor 2 (BMPR2, D), PDGF-BB (E), PDGF receptor-β (PDGFR-β, F), hypoxia-inducible factor-1α (HIF-1α, G), VEGF (H), and VEGF receptor-2 (VEGFR-2, I) in 3-mo-old (n = 9) and 9-mo-old (n = 3) HIV Tg and F344 control rats. *P < 0.050 vs. age-matched F344 control. †P < 0.050 vs. HIV-3 mo.
In an effort to identify key signaling pathways that may contribute to PAH in HIV Tg rats, we performed microarray analysis of pulmonary tissue. While 666 genes showed alterations in expression (≥1.5-fold change in expression) between HIV Tg and F344 rat lung tissue, more in-depth analysis revealed that 12 key signaling pathways were determined to be significantly different (Table 3, all individual gene data in NCBI GEO repository), including those involved in the kallikrein-kinin pathway (e.g., plasma kallikrein, bradykinin, and kallidin), inflammation and chemotaxis (e.g. tissue factor, thrombomodulin, discoidin domain receptor 1, IL-18, PDGFR-β, inhibitory κB, CCL5, and CD68), coagulation (e.g., tissue factor, thrombomodulin, heparin cofactor II, keratin 1, and plasminogen activator urokinase receptor), and angiogenesis (e.g., VEGFR-1, CXCR4, IL-8 receptor-α, IL-8 receptor-β, plasminogen activator urokinase receptor, p21, and connexin 43).
DISCUSSION
PAH, a hemodynamic state characterized by elevations in mean PAP and pulmonary vascular resistance due to remodeling and vasoconstriction within the pulmonary vasculature, can lead to RV failure and premature death. PAH is generally classified by etiology that includes idiopathic PAH (unknown etiology), genetic-based or familial PAH, and PAH resulting secondarily from infections (including HIV), drugs, or other toxins (1a). While the connection between HIV infection and the development of PAH is well documented (29, 50, 51, 52), the underlying pathobiology has not been determined. Furthermore, as the prognosis of HIV infection has been vastly improved by available pharmacotherapies (HAART), prevalence of HIV-PAH is increasing (51). Recent findings reported elevated PAP in >35% of HIV-infected outpatients in an echocardiography study, indicating that prevalence may be even higher than previously thought (14). There are limited animal models available to investigate the underlying mechanisms involved in HIV-PAH; thus we examined whether the HIV Tg rat displays PAH and PAH-associated remodeling of the RV and pulmonary vasculature. To our knowledge, this is the first report that the HIV Tg rat displays elevated RV pressures, RV hypertrophy, increased wall thickness of pulmonary arteries, and gene expression associated with PAH. Taken together, these findings suggest that this may be a useful model to gain a further understanding of the pathogenesis of the development and early stages of HIV-PAH without the confounding implications of antiretroviral therapies.
While the pulmonary vasculature of HIV Tg rats shows increased wall thickness of small and large pulmonary arteries, the cell types involved and mechanisms responsible have not been determined. Increased proliferation of pulmonary arterial smooth muscle cells is generally the key mechanism responsible for increased PA medial thickness (38, 53), although it is feasible that fibroblast proliferation may also be involved in this model of hyperplasia. Further characterization of RV hypertrophy in the HIV Tg rat is also required to determine if there is direct viral protein-endocardium interaction, in addition to increased afterload and induction of mitogenic protein expression, which may also contribute to the induction of hypertrophic cell signaling pathways in the myocardium (3). Interestingly, in a study of SHIV-Nef-infected macaques, Marecki et al. (21) observed no evidence of RV hypertrophy (pulmonary arterial pressures were not assessed); however, they report medial and intimal hypertrophy of the pulmonary artery and complex pulmonary vascular lesions that are specifically associated with the presence of the HIV-1 Nef protein, as simian immunodeficiency virus infection alone does result in plexiform lesions. Proliferating endothelial cells have been identified as the predominant component of plexiform lesions (7, 8), and while the HIV Tg rat does not exhibit endothelial cell proliferation and/or plexiform lesions at 9 mo of age, it is possible that they may develop at a later time, as we observe progression of pathological end points associated with PAH in the 9-mo-old HIV Tg rats compared with the 3-mo-old HIV Tg rats. Further age-related studies are required to determine whether the pathology in the lung and pulmonary vasculature is further exacerbated in older HIV Tg animals.
Many experimental data support the involvement of key signaling and mechanistic pathways in development and/or progression of PAH. At the core of the pathogenesis of PAH is evidence of endothelial cell dysfunction, characterized by elevated vasoconstrictor synthesis coupled with decreased vasodilator production and enhanced thrombosis (45, 46, 58). For example, ET-1, a potent vasoconstrictor and mitogenic peptide, has been reported to be upregulated in endothelial cells stimulated with the HIV viral glycoprotein 120, as well as in humans with HIV infection (10, 17, 34). In agreement with these findings, we also observed that ET-1 mRNA expression was significantly upregulated in pulmonary tissue from HIV Tg rats. There are conflicting reports regarding the role of eNOS, which is responsible for producing the vasodilator NO in endothelial cells, in PAH progression. Recent studies have shown that eNOS downregulation may be associated with the pathogenesis of PAH, as chronic hypoxia exposures in eNOS transgenic mice did not result in increased RVSP, pulmonary vascular remodeling, or RV hypertrophy (32, 61). Conversely, increased eNOS expression and persistent activation have also been associated with PAH (and elevated PAP) in animal models and human tissues (44, 62). Interestingly, we observed a significant elevation of eNOS expression in pulmonary tissue of 3-mo-old HIV Tg rats but a significant decrease in 9-mo-old HIV Tg rats. Such findings suggest that there may be a differential role for NO pathways in the pathology of more advanced HIV-PAH compared with earlier stages.
In VSMCs, KV channels contribute to resting membrane potential and have also been implicated in pulmonary artery-selective hypoxic vasoconstriction. Decreased expression and activity of Kv1.5 is reported in various forms of PAH in human and animal models (2, 12, 25, 37, 60). Further evidence for a significant role of Kv1.5 expression in PAH has been shown by the ability to attenuate PAH progression through restoration of Kv1.5 expression (37). In the HIV Tg model of PAH, we also observed a significant downregulation of Kv1.5 expression in the lungs of 3-mo-old HIV Tg rats, with an even further decrease in the 9-mo-old HIV Tg rats. The mechanism for the downregulation of Kv1.5 expression in PAH has not been elucidated, and there is limited information available on the role of this signaling pathway in HIV-PAH.
The occurrence of PAH has been associated with a genetic predisposition resulting from a mutation in BMPR2 (27, 55) that is believed to contribute to abnormal proliferation of VSMCs. BMPR2 has been shown to suppress proliferation of pulmonary artery smooth muscle cells from healthy individuals but not from patients with PAH (26), suggesting that there may be a differential role in signaling in normal vs. diseased cells. The HIV-1 Tat protein has been shown to inhibit expression of BMPR2 in cultured monocyte cells (5); however, we measured an increase in expression of BMPR2 mRNA in pulmonary tissue from HIV Tg rats. The rationale for this is likely multifactoral. 1) HIV Tg rats express seven of the nine HIV viral proteins (including Tat), each of which may initiate different signaling pathways in pulmonary endothelial cells and VSMCs. 2) Since we are quantifying BMPR2 mRNA from whole lung homogenate, it is plausible that we are measuring more BMPR2 expression from pulmonary endothelial cells.
PDGF is known to be an important mediator of the pulmonary vascular remodeling process (47). Active PDGF comprises homo- or heterodimers of PDGF-A or PDGF-B polypeptides or homodimers of PDGF-C or PDGF-D polypeptides, which stimulate the α- or β-isoform of the PDGF receptor (PDGFR-α or PDGFR-β) located on the surface of the cell (13, 43). PDGF has previously been reported to be a key mediator in vascular remodeling associated with PAH (35). While PDGF-A, PDGF-B, PDGFR-α, and PDGF-β have been reported to be upregulated in endothelial and smooth muscle cells of small pulmonary arteries in patients with idiopathic PAH, PDGFR-β expression was specifically shown to be increased in proliferating pulmonary vascular lesions, with PDGF-BB mediating increased proliferation and migration of pulmonary artery smooth muscle cells (35). In agreement with these findings, we also observed a significant increase in PDGFR-β and PDGF-BB expression in pulmonary tissue from HIV Tg rats compared with age-matched F344 controls.
HIF-1α is a transcriptional factor that is involved in regulation of several redox-sensitive genes, including PDGF, VEGF, and ET-1. Importantly, HIF-1 has been shown to contribute to the pathogenesis of PAH in vivo, as deficiency of HIF-1α reduces the muscularization of small pulmonary arteries in rodent models of chronic hypoxia (59). A role for HIF in pulmonary hypertension has been further substantiated by studies showing that HIF-1α regulates pulmonary artery myocyte electrophysiology (49) and promotes the proliferation of VSMCs (48). In the HIV Tg model of pulmonary hypertension, we observed a significant elevation in HIF-1α expression in pulmonary tissue from HIV Tg rats compared with F344 controls. Additionally, expression of VEGF and its receptor VEGFR2 is significantly upregulated in HIV Tg rats. In the lung, expression of VEGF in epithelial cells that are in close proximity to endothelial cells may be responsible for regulation of endothelial cell turnover and differentiation and may also mediate vascular permeability (54). Our findings of VEGF expression are in disagreement with other PAH models, where lung VEGF mRNA expression has been reported to be unchanged or decreased (33). However, several studies report an increase in VEGF expression in lung tissue in various hypoxia-exposed animal models of pulmonary hypertension that appears to be temporally regulated and is coupled with increased vascular permeability (6, 56).
Several of the pathways found to be significantly altered by microarray in lung tissue of HIV Tg rats compared with age-matched F344 rats may be of importance in the pathogenesis of pulmonary hypertension. The kallikrein-kinin system is an endogenous metabolic cascade, the activation of which results in the release of vasoactive kinins, or bradykinin-related peptides. Kinins regulate processes such as vasodilatation, increased vascular permeability, and smooth muscle contraction via receptor activation on these cell types (see Ref. 20 for review). Other cellular pathways identified as statistically different in lung tissue of HIV Tg rats compared with F344 controls include inflammation, apoptosis, and angiogenesis, each of which warrants further investigation to gain an understanding of their role in development of pulmonary hypertension.
While the findings reported here show significant alterations in pulmonary vascular morphometry and physiology, some limitations to these studies require further in-depth, age-related experiments. 1) The 9-mo-old HIV Tg rats show no evidence of occlusive plexiform lesions, which are a hallmark of pulmonary hypertension in humans and have been observed in other models of HIV-PAH, such as SHIV-Nef-infected nonhuman primates (21). Since the HIV-Tg rats in this study were not followed past the age of 9 mo, it is possible that as the disease progresses, they may eventually develop plexiform lesions; however, further studies in older animals are required to determine whether this pathology develops. Aging studies in these animals may also be able to address whether the PAH in these animals eventually leads to death, although such conclusions may be challenging to discern, as HIV Tg rats also display skeletal muscle (i.e., wasting) and cardiovascular pathologies (42). 2) We did not specifically assess whether vasoconstrictive pathways are directly involved in the PAH reported in the HIV Tg rats. While we did measure ET-1 mRNA levels, we did not investigate the physiological role of this vasoconstrictor in PAH. 3) Future studies are needed to identify the role of other known factors and pathways that are targets for current therapies, which are known to contribute to PAH pathogenesis, such as Rho kinase (for review see Ref. 22), angiotensin (31), and the NO and prostacyclin pathways (for review see Ref. 11).
In conclusion, our findings show that HIV Tg rats display elevated RVSP, RV hypertrophy, pulmonary artery remodeling, and altered expression of key factors and pathways associated with pulmonary hypertension. This animal model may thus serve as a novel in vivo model to further elucidate signaling pathways involved in the early stages of HIV-PAH, as well as allow for determination of potential drug targets for therapies in patients with HIV.
GRANTS
The preparation of this manuscript by A. K. Lund was funded in part by National Institute of Environmental Health Sciences Grant R00 ES-016586. All experimental studies were funded by internal funding sources at Lovelace Respiratory Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Jamie Padilla and Gavin Pickett (Keck-University of New Mexico Genomics Resource facility at the University of New Mexico Medical School) for microarray work and initial data analysis.
Present address of J. S. Naik: Department of Cell Biology and Physiology, University of New Mexico, Albuquerque, NM 87131.
REFERENCES
- 1. Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, Grossman W, De Marco T, Yeghiazarians Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med 177: 1268–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 1a. Anonymous. Proceedings of the 3rd World Symposium on Pulmonary Arterial Hypertension. J Am Coll Cardiol 43: 1S–90S, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Archer SL, Souil E, Dinh-Xuan AT, Shremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 101: 2319–2330, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker JV, Lundgren JD. Cardiovascular implications from untreated human immunodeficiency virus infection. Eur Heart J 32: 945–951, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benisty JI. Pulmonary hypertension. Circulation 106: E192–E194, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Caldwell RL, Gadipatti R, Lane KB, Shepherd VL. HIV-1 TAT represses transcription of the bone morphogenic protein receptor-2 in U937 monocytic cells. J Leukoc Biol 79: 192–201, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 18: 768–776, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol 28: 434–442, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Degano B, Guillaume M, Savale L, Montani D, Jais X, Yaici A, Pavec JL, Humbert M, Simonneau G, Sitbon O. HIV-associated pulmonary hypertension: survival and prognostic factors in the modern therapeutic era. AIDS 24: 67–75, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Ehrenreich H, Rieckmann P, Sinowatz F, Weih KA, Arthur LO, Goebel FD, Burd PR, Coligan JE, Clouse KA. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J Immunol 150: 4601–4609, 1993 [PubMed] [Google Scholar]
- 11. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 34: 1219–1263, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79: 1283–1316, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Hsue PY, Farah HH, Palav S, Ahmed SY, Schnell A, Ellman AB, Huang L, Dollard SC, Martin JN. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS 22: 825–383, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther 92: 1–20, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 45: 173–202, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun 333: 1107–1115, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kimmel PL, Mishkin GJ, Umana WO. Captopril and renal survival in patients with human immunodeficiency virus nephropathy. Am J Kidney Dis 28: 202–208, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol 29: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madeddu P, Emanueli C, El-Dahr S. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol 3: 208–221, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, Carville A, Shannon RP, Cota-Gomez A, Tuder RM, Voelkel NF, Flores SC. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-Nef-infected macaques. Am J Respir Crit Care Med 174: 437–445, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMurtry IF, Abe K, Ota H, Fagan KA, Oka M. Rho kinase-mediated vasoconstriction in pulmonary hypertension. Adv Exp Med Biol 661: 299–308, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest 188: 1133–1141, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Mette S, Palevsky H, Pietra G. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis 145: 1196–1200, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105: 244–250, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β1 and bone morphogenetic proteins. Circulation 104: 790–795, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, 3rd, Knowles JA, Janssen B, Eickelberg O, Eddahibi S, Herve P, Nichols WC, Elliott G. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med 345: 319–324, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, Le Gall C, Parent F, Garcia G, Hervé P, Barst RJ, Simonneau G. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 167: 1433–1439, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Opravil M, Pechere M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, Hirschel B, Lüthy R. HIV-associated primary pulmonary hypertension. A case control study Swiss HIV Cohort Study. Am J Respir Crit Care Med 155: 990–995, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Opravil M, Sereni D. Natural history of HIV-associated pulmonary hypertension: trends in HAART era. AIDS 22: 35–40, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Orte C, Polak JM, Haworth SG, Haworth SG, Yacoub MH, Morrell NW. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary pulmonary hypertension. J Pathol 192: 379–384, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Ozaki M, Kawashima S, Yamashita T, Ohashi Y, Rikitake Y, Inoue N, Hirata KI, Hayashi Y, Itoh H, Yokoyama M. Reduced hypoxic pulmonary vascular remodeling by nitric oxide from the endothelium. Hypertension 37: 322–327, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, Raffestin B, Frelin C. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 275: H1948–H1956, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Pellicelli AM, Palmieri F, Cicalini S, Petrosillo N. Pathogenesis of HIV-related pulmonary hypertension. Ann NY Acad Sci 946: 82–94, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Perros F, Montani D, Dorfmüller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Hervé P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 81–88, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Petrosillo N, Chinello P, Cicalini S. Pulmonary hypertension in individuals with HIV infection. AIDS 20: 2128–2129, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JRB, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 107: 2037–2044, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Prabha M, Jin HF, Tian Y, Tang CS, Du JB. Mechanisms responsible for pulmonary hypertension. Chin Med J (Engl) 121: 2604–2609, 2008 [PubMed] [Google Scholar]
- 40. Pruznak AM, Hong-Brown L, Lantry R, She P, Frost RA, Vary TC, Lang CH. Skeletal and cardiac myopathy in HIV-1 transgenic rats. Am J Physiol Endocrinol Metab 295: E964–E973, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rahim MM, Chrobak P, Hu C, Hanna Z, Jolicoeur P. Adult AIDS-like disease in a novel inducible human immunodeficiency virus type 1 Nef transgenic mouse model: CD4+ T-cell activation is Nef dependent and can occur in the absence of lymphopenia. J Virol 83: 11830–11846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fous T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC. An HIV-1 transgenic rat that develops HIV-related pathology an immunologic dysfunction. Proc Natl Acad Sci USA 98: 9271–9276, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reigstad LF, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J 272: 5723–5741, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Resta TC, Chicoine LG, Omdahl JL, Walker BR. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol Heart Circ Physiol 276: H699–H708, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Rich S, Brundage B. Pulmonary hypertension: a cellular basis for understanding the pathophysiology and treatment. J Am Coll Cardiol 14: 545–550, 1989 [DOI] [PubMed] [Google Scholar]
- 46. Rubin LJ. Pathology and pathophysiology of primary pulmonary hypertension. Am J Cardiol 75: 51A–54A, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissman N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor-1α promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290: H2528–H2534, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 281: L202–L208, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Sitbond O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 177: 108–113, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 100: 1268–1271, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Tavares de Lima W, Kwasniewski FH, Sirois P, Janear S. Studies on the mechanism of PAF-induced vasopermeability in rat lungs. Prostaglandins Leukocyte Essent Fatty Acids 52: 245–249, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliot GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE, Trembath RC, Nichols WC. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J Med Genet 37: 741–745, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tuder RM, Flook BE, Voelkel NF. Increased expression of VEGF and VEGF receptors KDR/Flk and Flt in lungs exposed to acute or chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest 95: 1798–1807, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Voelkel NF, Flores S, Petrosillo N. PVRI Task Force Annual Reports—2008: Pulmonary Hypertension Associated With HIV Task Force. Ann Rep PVRI Task Forces 1: 51–53, 2009 [Google Scholar]
- 58. Voelkel NF, Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J 8: 2129–2138, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JSK, Wiener CM, Sylvester JT, Semeza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest 103: 691–696, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 351: 726–727, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96: 442–450, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HSK, Vogel SM, Brovkovych V, Yuan JXJ, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119: 2009–2018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]