Abstract
Inducible nitric oxide synthase (NOS2) expression is increased in the airway epithelium in acute inflammatory disorders although the physiological impact remains unclear. We have previously shown that NOS2 inhibits NF-κB (p50-p65) activation in respiratory epithelial cells by inducing S-nitrosylation of the p65 monomer (SNO-p65). In addition, we have demonstrated that mouse lung SNO-p65 levels are acutely depleted in a lipopolysaccharide (LPS) model of lung injury and that augmenting SNO-p65 levels before LPS treatment results in decreased airway epithelial NF-κB activation, airway inflammation, and lung injury. We now show that aerosolized LPS induces NOS2 expression in the respiratory epithelium concomitant with an increase in lung SNO-p65 levels and a decrease in airway NF-κB activity. Genetic deletion of NOS2 results in an absence of SNO-p65 formation, persistent NF-κB activity in the respiratory epithelium, and prolonged airway inflammation. These results indicate that a primary function of LPS-induced NOS2 expression in the respiratory epithelium is to modulate the inflammatory response through deactivation of NF-κB via S-nitrosylation of p65, thereby counteracting the initial stimulus-coupled denitrosylation.
Keywords: S-nitrosothiol, nitric oxide synthase, nuclear factor-κB, acute lung injury
nitric oxide (NO) is known to be a critical factor in the pathogenesis of inflammatory lung diseases, including acute lung injury (ALI) (27). All three nitric oxide synthase (NOS) isoforms are expressed in the lung (20), although the majority of NO production in the inflammatory state derives from inducible NOS (NOS2) (27). In animal models of ALI, a variety of insults (e.g., hyperoxia, sepsis, mechanical stretch injury) elicit an increase in airway NOS2 expression, suggesting that NOS2 serves a common role in multiple types of lung injury (19, 21, 32). A number of studies have suggested that NOS2 is predominantly proinflammatory in ALI, ostensibly because of indiscriminate generation of deleterious reactive nitrogen species (RNS) (8, 21, 32). However, the anti-inflammatory effects of NOS2 are increasingly recognized (19, 38) and likely offset any impact of RNS-mediated injury.
Protein S-nitrosylation has emerged as the predominant molecular mechanism underlying NOS2-dependent inhibition of immune response pathways (6, 31). This paradigm is best exemplified by the NF-κB transcription factor pathway, within which a number of proteins modified by S-nitrosylation have been identified (17, 25, 33). We have previously shown that cytokine-induced NOS2 expression in the respiratory epithelium and macrophages inhibits NF-κB activity via S-nitrosylation of the p65 (SNO-p65) subunit of the p50-p65 heterodimer (17). S-nitrosylation of p65 inhibits p50-p65 DNA binding, leading to the reduced expression of κB-dependent inflammatory mediators, including NOS2 itself. However, evidence that NOS2 inhibits NF-κB via S-nitrosylation in animal models of inflammatory lung disease is lacking.
Recently, we established that S-nitrosothiols (SNOs) are acutely depleted in both airway fluid and lung tissue in a lipopolysaccharide (LPS) mouse model of ALI (24). We further demonstrated that the decrease in SNOs leads to denitrosylation of NF-κB p65, activation of the NF-κB in the respiratory epithelium, and initiation of the pulmonary inflammatory response. Augmentation of airway SNOs and lung tissue SNO-p65 levels by treatment with ethyl nitrite (ENO), before LPS administration, attenuates the inflammatory response and protects from the development of lung injury (24), highlighting the importance of airway SNO homeostasis and NF-κB protein denitrosylation in the pathogenesis of ALI. However, the endogenous mechanism(s) that restore lung SNO-p65 levels after LPS-induced denitrosylation have not been elucidated. In the present study, we show that LPS-induced airway epithelial NOS2 expression functions to resolve lung inflammation by mediating S-nitrosylation of NF-κB p65, thereby attenuating NF-κB activity and inhibiting continued κB-dependent proinflammatory gene expression. These findings support an anti-inflammatory role for NOS2 in the lung and provide further evidence that SNOs and protein S-nitrosylation serve to inhibit lung inflammation.
MATERIALS AND METHODS
Animal exposure.
Six- to eight-week old, male C57BL/6J and NOS2 null (Nos2−/−; B6.129P2-Nos2tm1Lau) mice were purchased from Jackson Laboratories (Bar Harbor, ME). All procedures were approved by the Duke University institutional animal care and use committee. Animals were exposed to aerosolized LPS (0111:B4 Escherichia coli LPS, 4 μg/m3 × 2.5 h) or PBS as previously described (14). Mice were killed by CO2 narcosis followed by thoracotomy at 1 or 6 h after completion of the aerosol treatment.
Lung samples.
Whole lung lavage and lung tissue extraction were performed as previously described (24). After being harvested, lungs were frozen in liquid N2 for later preparation of tissue homogenates. Cell counting of the pooled bronchoalveolar lavage fluid (BALF) was performed with a hemocytometer, and cell differentials were determined on stained cytospin preparations. The BALF was centrifuged at 1,500 g for 10 min to collect cells, and the protein concentration of the BALF supernatant was determined by BCA assay (Pierce Biotechnology, Rockford, IL). BALF supernatant was stored at −80°C for cytokine analysis.
Mouse lung extracts (MLE) were prepared by mechanical homogenization of lung tissue with a mortar and pestle in liquid N2 followed by resuspension of the powdered tissue in two volumes of hypotonic buffer [10 mM HEPES, pH 7.9, 15 mM KCl, 2 mM MgCl2, 0.5 mM EDTA, 0.6% Nonidet P-40, and 0.5 mM phenylmethylsulfonyl fluoride (PMSF)] and incubation on ice for 10 min. Nuclei were collected by centrifugation at 3,500 g for 10 min, and the supernatant (cytoplasmic extract) was removed. The nuclear pellet was resuspended in hypertonic lysis buffer (20 mM HEPES, pH 7.9, 420 mM NaCl, 25% gycerol, 2 mM MgCl2, 0.5 mM EDTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM benzamadine, and 0.5 mM PMSF) and placed on ice for 20 min to complete lysis. Nuclear debris was removed by centrifugation (15,000 g for 20 min), and the supernatant (nuclear extract) was collected. Protein concentration in the extracts was determined by BCA.
Cytokine analysis.
A fluorescent bead immunoassay (Bio-Plex; Bio-Rad Laboratories, Hercules, CA) was used to quantify the concentrations of murine tumor necrosis factor (TNF)-α, KC, interleukin (IL)-1β, IL-6, macrophage inflammatory protein (MIP)-1β, and granulocyte macrophage colony-stimulating factor (GM-CSF) in BALF. Samples were measured in duplicate (n = 5 animals/treatment group).
Western blotting.
Equivalent protein concentration of MLE was separated by SDS-PAGE and transferred to nitrocellulose, and blots were probed with 1:50,000 of a rabbit polyclonal antibody to NOS2 (Millipore, Billerica, MA). After probing with a horseradish peroxidase-conjugated 2° antibody (sc-2054; Santa Cruz Biotechnology, Santa Cruz, CA), immunoreactivity was detected by enhanced chemiluminescence.
Detection of S-nitrosylated NF-κB p65.
A biotin switch assay was performed on freshly prepared MLE as previously described (24). Protein lysate (750 μg) was diluted in HEN buffer (250 mM HEPES-NaOH, pH 7.7, 1 mM EDTA, and 0.1 mM neocuproine), and free thiols were blocked by the addition of 3 mM MMTS and 1% SDS, followed by incubation at 50°C for 30 min. After acetone precipitation and resuspension in HENS buffer (HEN + 1% SDS), SNO proteins were labeled by 1 h incubation with 50 mM sodium ascorbate and 50 μg/ml biotin-HPDP (Pierce Biotechnology). Negative controls omitted ascorbate. Biotinylated proteins were precipitated by overnight incubation (4°C) with Neutravidin agarose (Pierce Biotechnology). After extensive washing, proteins were eluted from the beads by heating to 95°C in Lamelli buffer. Protein eluate and input lysate were separated by SDS-PAGE followed by Western blotting for NF-κB p65 (sc-372, rabbit polyclonal antibody; Santa Cruz Biotechnology). SNO-p65/total p65 ratios were determined by densitometry.
NF-κB activity assay.
Nuclear protein binding to a consensus NF-κB oligonucleotide was determined using an ELISA-based assay (TransAm p65; Active Motif, Carlsbad, CA). Absorbance was read at 450 nm with samples appropriately blanked. Results were standardized to a positive control (cytokine-stimulated Jurkat cell lysate) that was provided by Active Motif.
Immunohistochemistry.
Lungs were inflation fixed in situ at 25 cm H2O with 4% paraformaldehyde and embedded in paraffin, and 5-μm-thick sections were prepared. After antigen retrieval, sections were blocked in 5% goat sera and incubated overnight with primary antibodies [1:500 NF-κB p65 (Santa Cruz Biotechnology) or 1:100 NOS2 (Millipore)]. Localization of NOS2 was detected by immunoperoxidase staining (DAB Peroxidase substrate kit; Vector Laboratories, Burlingame, CA). For NF-κB p65 detection, goat anti-rabbit Alexa Fluor 594 antibodies (Molecular Probes, Invitrogen, Carlsbad, CA) and a DAPI counterstain were used for fluorescent detection as previously described (24). Sections were scanned with a confocal microscope (Zeiss LSM 510) with 594-nm HeNe and 405-nm diode lasers for detection of fluorescently labeled antibodies.
Data analysis.
Data are expressed as means ± SE. Significant differences between groups were identified by Student's t-test.
RESULTS
Aerosolized LPS induces NOS2 expression in the lung airway.
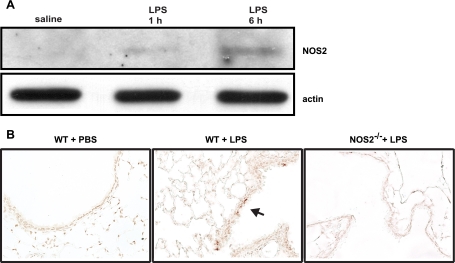
Both systemic and direct airway administration of LPS are known to augment NOS2 expression in the lung (4, 21). To ascertain the temporal association between LPS exposure and lung NOS2 expression in our ALI model, NOS2 levels were probed in MLE from animals treated with aerosolized PBS or LPS for 2.5 h and analyzed at 1 or 6 h postexposure. NOS2 protein was not observed in the lungs of PBS controls and was minimally detectable in LPS-treated mice at 1 h postexposure (Fig. 1A). However, prominent NOS2 induction was observed in the lung at 6 h post-LPS exposure, which was consistent with the timing of NOS2 expression seen in other LPS lung injury models (21, 38).
Fig. 1.
Lung expression of inducible nitric oxide synthase (NOS2) in mice exposed to aerosolized lipopolysaccharide (LPS). Mice (n = 5/group) were treated with aerosolized saline or LPS for 2.5 h, and tissues were harvested at 1 or 6 h posttreatment. A: NOS2 expression in mouse lung extracts (MLE) was assayed by Western blotting. B: immunoperoxidase staining for NOS2 was performed on lung sections prepared from wild-type (WT) (C57BL/6) or NOS2−/− mice analyzed 6 h after saline or LPS treatment. Arrow indicates peroxidase-positive cells in the airway epithelium.
To determine the specific lung cell type(s) responsible for the increase in NOS2 expression in response to LPS, we performed immunochemical staining for NOS2 in mouse lung sections. NOS2 immunoreactivity was not observed in lung sections from the saline controls, consistent with low basal NOS2 expression (Fig. 1B). However, at 6 h post-LPS exposure, NOS2 expression was evident in the airway epithelium but not in inflammatory cells or endothelium. These findings are consistent with the timing of expression in cultured respiratory epithelial cells after cytokine stimulation (17) and indicate that the respiratory epithelium is the primary source of lung NOS2 activity in our aerosolized LPS model of ALI.
Airway inflammation is enhanced in NOS2−/− mice.
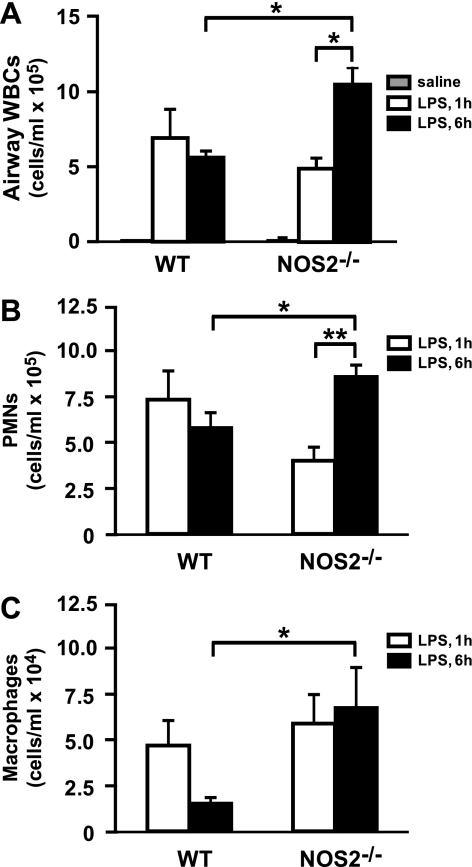
Previous studies utilizing either NOS inhibitors or NOS2−/− mice have indicated that NOS2 can have either a pro- or anti-inflammatory role in ALI (29, 38). To determine the effect of NOS2 activity on the inflammatory response in our ALI model, cell counts and differentials were performed on BALF recovered from wild-type (WT) and NOS2 −/− mice at 1 and 6 h after LPS exposure. A rapid increase in airway macrophages and neutrophils was seen in response to LPS in both WT and NOS2−/− mice at 1 h postexposure (Fig. 2). Although no difference in airway WBC counts was seen in the NOS2−/− vs. WT mice at 1 h postexposure, airway white blood cells (WBCs) remained significantly elevated in the NOS2−/− compared with WT mouse at 6 h post-LPS (Fig. 2). In addition, whereas airway inflammation appeared to have peaked in the WT mice between 1 and 6 h postexposure, airway WBC counts in the NOS2−/− mice were higher at 6 vs. 1 h postexposure, indicative of a more severe and/or sustained inflammatory state in the absence of NOS2 expression.
Fig. 2.
LPS-induced airway inflammation in WT and NOS2−/− mice. A: total white blood cell (WBC) in bronchoalveolar lavage fluid (BALF) were measured by cell counting. *P < 0.005. B: airway neutrophils in BALF were measured by cytostaining. PMN, polymorphonuclear neutrophils. *P < 0.01 and **P < 0.001. C: airway macrophages in BALF were measured by cytostaining. *P < 0.05. Data in A-C are means ± SE (n = 5/group).
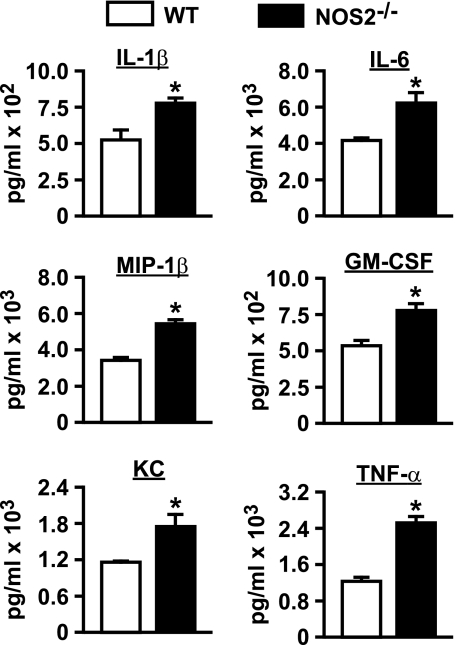
Aerosolized LPS is known to increase BALF levels of TNF-α, IL-1β, IL-6, KC, GM-CSF, and MIP-1β, all of which are expressed by the respiratory epithelium and function in inflammatory cell recruitment to the lung airway (24). To determine if NOS2 activity modulates the expression of these cytokines/chemokines, we quantified them in BALF from WT and NOS2−/− mice at 6 h post-LPS exposure. We found that airway levels of all of these mediators were significantly higher in the NOS2−/− mice compared with WT controls (Fig. 3). Cumulatively, the observed persistent influx of airway inflammatory cells and increased expression of proinflammatory cytokines/chemokines indicate that NOS2 attenuates inflammation in this model of LPS-induced lung injury.
Fig. 3.
BALF cytokine/chemokine levels in WT and NOS2−/− mice. BALF cytokines and chemokines were measured by fluorescence-based immunoassay. IL, interleukin; MIP, macrophage inflammatory protein; GM-CSF, granulocyte macrophage colony-stimulating factor; TNF, tumor necrosis factor. *P < 0.01. Data are means ± SE (n = 5/group).
Prolonged NF-κB activation in NOS2−/− mice.
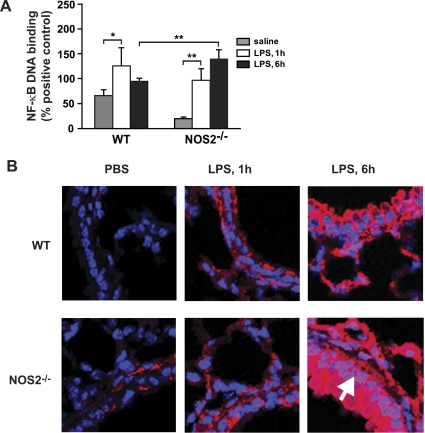
The transcription factor NF-κB controls the expression of numerous inflammatory mediators that function in the lung inflammatory response, including the LPS- and NOS2-responsive cytokines/chemokines TNF-α, IL-1β, IL-6, KC, GM-CSF, and MIP-1β (Fig. 3). These data, coupled with our previous finding that NOS2 modulates NF-κB activity in cultured respiratory epithelial cells and macrophages (7, 17), suggest that NF-κB might similarly be regulated by NOS2 in mouse lung. Thus we quantified NF-κB DNA binding in nuclear lysates prepared from MLE of LPS-exposed WT and NOS2−/− mice. Interestingly, basal NF-κB appeared to be lower in lungs from NOS2−/− vs. WT mice (Fig. 4A). At 1 h post-LPS exposure, both WT and NOS2−/− mouse lungs showed a significant increase in NF-κB activation compared with PBS controls (Fig. 4A), although NF-κB activity was not different between WT and NOS2−/− at this time point. At 6 vs. 1 h post-LPS, NF-κB activity appeared to be waning in the WT mice but was higher in the NOS2−/− mice; NF-κB expression was also higher in NOS2−/− vs. WT mice at 6 h post-LPS. These data suggest that airway NOS2 is a negative regulator of stimulus-coupled NF-κB activity in the lung; the onset of this effect correlates with the lag in NOS2 expression post-LPS exposure.
Fig. 4.
LPS-induced lung nuclear factor-κB (NF-κB) activity in WT and NOS2−/− mice. A: NF-κB p65 DNA-binding activity was measured in nuclear fractions isolated from lung homogenate by TransAm assay. *P < 0.05 and **P < 0.01. B: NF-κB p65 localization in the lung airways was measured by immunofluorescence on sections from WT and NOS2−/− mice post-LPS exposure (1 and 6 h). Red fluorophore, anti-p65; blue, DAPI (nucleus). White arrow denotes nuclear p65 translocation in airway epithelium. Data in A are means ± SE (n = 5/group). Images in B are representative of n = 5 analyses.
An increase in NF-κB activity can be the result of enhanced expression of the p65 transactivating monomer and/or nuclear translocation of p50-p65. We have previously shown that the respiratory epithelium is the predominant site of lung NF-κB activity in our LPS model of ALI (24). To determine if airway epithelial NF-κB activity is altered in NOS2−/− mice, immunofluorescent staining for NF-κB p65 was performed on fixed lung sections of WT and NOS2−/− mice 1 and 6 h post-LPS. At 1 h post-LPS, we observed a similar increase in total NF-κB p65 expression in the respiratory epithelium of both WT and NOS2−/− mice and a similar degree of NF-κB p65 nuclear translocation (Fig. 4B), consistent with the similar degree of NF-κB activation (Fig. 4A). However, NOS2−/− mice show significantly higher expression and nuclear NF-κB p65 content in the respiratory epithelium at 6 h post-LPS compared with WT mice, indicating that NOS2 modulates both NF-κB p65 expression and nuclear translocation in the respiratory epithelium in vivo.
Decreased S-nitrosylation of NF-κB in NOS2−/− mice.
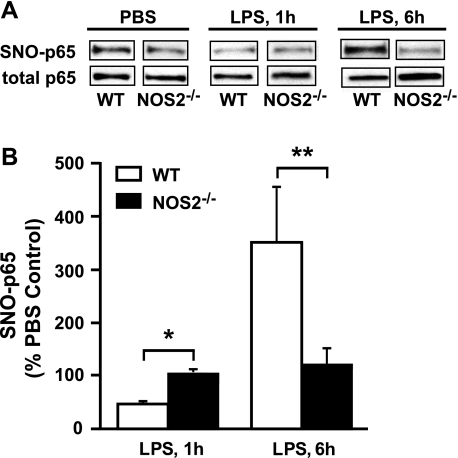
We have previously shown that NOS2-mediated S-nitrosylation of NF-κB p65 inhibits NF-κB transcriptional activity in respiratory epithelial cells and that aerosolized LPS exposure acutely decreases inhibitory S-nitrosylation of p65 in vivo (17, 24). To determine the contribution of NOS2 to lung levels of SNO-p65, we performed biotin switch assays on MLE from PBS- and LPS-treated WT and NOS2−/− mice. WT mice were characterized by a marked denitrosylation of lung NF-κB p65 at 1 h post-LPS treatment, but SNO-p65 levels were substantially increased at 6 h postexposure (Fig. 5A), consistent with the increase in airway epithelial NOS2 expression (Fig. 1). In comparison, basal lung SNO-p65 levels were low in NOS2−/− mice, and no significant change in SNO-p65 was observed in these mice at 1 or 6 h postexposure. Collectively, these results indicate that NOS2 is a principal mediator of NF-κB p65 S-nitrosylation in mouse lung and that NOS2 mediates renitrosylation of p65 after LPS-induced lung injury.
Fig. 5.
Effect of aerosolized LPS on S-nitrosylation of NF-κB p65 (SNO-p65) in the lung. A: SNO-p65 levels were determined by biotin switch assay performed on MLE from WT and NOS2−/− mice 1 and 6 h post-LPS. B: ratios of SNO-p65 to total p65 at 1 and 6 h post-LPS were determined by densitometry and compared with PBS controls. *P < 0.05 and **P < 0.001. Images in A are representative of n = 5 analyses. Data in B are means ± SE (n = 5).
DISCUSSION
The results of this study demonstrate that NOS2 functions to attenuate the inflammatory response in LPS-induced lung injury and provide a molecular mechanism for the effects of NOS2, namely S-nitrosylation of NF-κB p65 and deactivation of NF-κB in the respiratory epithelium. Our findings thus suggest that the LPS-induced increase in airway epithelial NOS2 expression serves to restore lung SNOs to homeostatic levels after their initial depletion in ALI (24).
Protein S-nitrosylation is now recognized to be integral in coordinating the immune response by regulating numerous proteins in a multitude of signaling pathways. The effect of S-nitrosylation is primarily inhibitory, resulting in the deactivation of proinflammatory pathways. For example, S-nitrosylation inhibits c-Jun NH2-terminal kinase 1 and the Toll-like receptor 4 signaling protein MyD88 (6, 31), both of which are integral in the lung response to LPS-induced injury (2, 14). Furthermore, S-nitrosylation likely serves to resolve airway inflammation, with SNO modification of β-actin and the calcium-binding protein S100A8 altering leukocyte-endothelial interaction (23, 39) and S-nitrosylation of aminophospholipid translocase enhancing macrophage clearance of neutrophils (40).
It is the NF-κB transcription factor pathway, however, that appears to be most sensitive and critical to SNO regulation of the pulmonary inflammatory response. Protein S-nitrosylation inhibits NF-κB activation both at the cytoplasmic [i.e., IκBα kinase β (IKK2)] and nuclear (i.e., p50-p65) (17, 25, 33) level. In the nucleus, both subunits of the p50-p65 heterodimer are targeted for S-nitrosylation at a conserved cysteine in the Rel DNA-binding domain with modification of either monomer disrupting DNA binding and inhibiting κB-dependent transcription (17, 25). In the case of the cytoplasmic protein IKK2, S-nitrosylation basally inhibits its activity in the respiratory epithelium (33). Upon cytokine stimulation, IKK2 is rapidly denitrosylated, resulting in the phosphorylation and degradation of the inhibitory IκBα protein, nuclear translocation of the p50-p65 heterodimer, and initiation of NF-κB-dependent gene transcription. Similar to IKK2, we observe cytokine-induced denitrosylation of p65 in respiratory epithelial cells and LPS-treated mouse lung (24), implicating a common mechanism by which inhibitory protein S-nitrosylation is reversed in the NF-κB pathway. Given that NF-κB activation in the respiratory epithelium is essential in initiating the inflammatory response to LPS-induced lung injury (34), denitrosylation of NF-κB likely serves as the trigger for this response.
NOS2 expression is induced in the respiratory epithelium in inflammatory lung disease (22, 35, 37), and NOS2 is known to regulate the activity of a number of different inflammatory mediators via S-nitrosylation (13, 18, 31, 42). We have previously shown that cytokine-induced NOS2 expression in respiratory epithelial cells induces S-nitrosylation and inhibition of NF-κB p65 (17). The results of the present study extend these observations to a mouse model of lung injury supporting an anti-inflammatory role for NOS2 in the lung via inhibition of NF-κB. Of note, NOS2 deficiency was recently demonstrated to increase NF-κB activity and modestly augment allergic airway inflammation in a mouse model of asthma, although mechanistic correlation to S-nitrosylation of p65 was equivocal (30).
While our findings indicate that airway NOS2 activity inhibits inflammation in lung injury, other studies have found NOS2 to instead be proinflammatory. This discrepancy may be explained by different methods of injury, dosing, and analysis time points postinsult. For example, NOS2 deficiency appears to protect from lung injury when LPS is administered systemically as opposed to direct airway instillation (21, 38). These results may be due, in part, to cell- and site-specific NOS2 expression, since bone marrow reconstitution of NOS2-deficient mice with WT cells restores sensitivity to sepsis-induced lung injury (9). In this regard, we saw NOS2 expression only in the respiratory epithelium at 6 h post-LPS with no significant contribution from lung inflammatory cells (Fig. 1B). It is possible that, with airway-administered LPS, induction of NOS2 expression occurs initially in the respiratory epithelium followed later by inflammatory cells, whereas the reverse is true with systemic LPS.
The predominant mechanism cited for NO and, specifically, NOS2 exacerbation of lung injury is increased cell damage due to RNS production (28). However, protein nitration in ALI has been shown to be primarily dependent upon myeloperoxidase (MPO) activity and not NOS2 (5, 19). In fact, NOS2 expression is augmented in MPO−/− mouse lung with the increase in NOS2 activity shown to be protective from sepsis-induced lung injury (5). Whether this observed anti-inflammatory effect of NOS2 is mediated via lung SNOs is unclear. However, S-nitrosylation is known to inhibit NADPH oxidase (36), and we have demonstrated that augmentation of lung SNOs (via inhalation of ENO) decreases MPO activity and protein nitration in two distinct models of lung injury (3, 24). Based on these data, it is likely then that the balance of proinflammatory RNS production vs. anti-inflammatory SNOs in the lung establishes the physiological response to NOS2-derived NO.
Lung SNO depletion has been implicated in the pathogenesis of a number of different inflammatory lung diseases (11, 12), and we recently demonstrated the acute depletion of lung SNOs in our LPS model of ALI (24); our present results provide further confirmation of this phenomenon. Augmentation of airway SNOs and corresponding lung tissue SNO-p65 levels by inhalation of ENO gas before LPS exposure prevents NF-κB activation and diminishes the inflammatory response, thus establishing the anti-inflammatory role of SNOs in the lung (24). We now show NOS2 to be the endogenous mediator that restores inhibitory protein S-nitrosylation of NF-κB after LPS-induced denitrosylation. In addition, it appears that NOS2 may also function constitutively to maintain basal lung SNO-protein levels, since NOS2−/− mice exhibited substantially lower SNO-p65 levels at baseline compared with WT controls (Fig. 5), which is consistent with recent findings by Olson et al. (30). In this regard, we are currently attempting to elucidate the mechanisms responsible for basal airway/lung SNO maintenance and LPS-induced denitrosylation.
Although studies in animal models are abundant, little clinical data exist regarding the role of NOS2 in patients with ALI. NOS2 expression is known to be increased in the respiratory epithelium and alveolar macrophages of ALI patients (1, 37) similar to what is observed in other acute inflammatory lung diseases. BALF nitrite/nitrate concentration, a marker of NOS2 activity, is also increased in ALI and other inflammatory lung disease although the predictive value in clinical outcomes is unproven (10, 37). However, higher urinary nitrite/nitrate levels have been shown to correlate with improved clinical outcomes in ALI, suggesting that an increase in systemic NOS2 expression may be protective (26). While not studied in ALI, polymorphisms in the NOS2 gene have been linked to susceptibility to asthma, viral bronchiolitis, and tuberculosis (15, 16, 41), providing further evidence that NOS2 is an important factor in the pathogenesis of inflammatory lung disease.
In summary, our findings establish a link between airway epithelial NOS2 expression, S-nitrosylation and deactivation of NF-κB, and the resolution of airway inflammation in LPS-induced lung injury. These data thus corroborate the physiological significance of our prior observations that NOS2-mediated S-nitrosylation inhibits NF-κB in the respiratory epithelium (17) and establish an anti-inflammatory role for NOS2 in the lung airway. Furthermore, these findings provide additional evidence that airway SNO metabolism is an important factor in the regulation of airway inflammation and the pathogenesis of ALI.
GRANTS
This research was supported by the March of Dimes (R. L. Auten) and National Institutes of Health Grants ES-016347 (W. M. Foster) and HL-092994 (H. E. Marshall).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Albertine KH, Wang ZM, Michael JR. Expression of endothelial nitric oxide synthase, inducible nitric oxide synthase, and endothelin-1 in lungs of subjects who died With ARDS. Chest 116: 101S–102S, 1999 [PubMed] [Google Scholar]
- 2. Arndt PG, Young SK, Worthen GS. Regulation of lipopolysaccharide-induced lung inflammation by plasminogen activator inhibitor-1 through a JNK-mediated pathway. J Immunol 175: 4049–4059, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med 176: 291–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baron RM, Carvajal IM, Fredenburgh LE, Liu X, Porrata Y, Cullivan ML, Haley KJ, Sonna LA, De Sanctis GT, Ingenito EP, Perrella MA. Nitric oxide synthase-2 down-regulates surfactant protein-B expression and enhances endotoxin-induced lung injury in mice. FASEB J 04–15: 18fje, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Brovkovych V, Gao XP, Ong E, Brovkovych S, Brennan ML, Su X, Hazen SL, Malik AB, Skidgel RA. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol 295: L96–L103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carvalho-Filho MA, Ueno M, Carvalheira JBC, Velloso LA, Saad MJA. Targeted disruption of iNOS prevents LPS-induced S-nitrosation of IRbeta/IRS-1 and Akt and insulin resistance in muscle of mice. Am J Physiol Endocrinol Metab 291: E476–E482, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cheng Ds, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, Blackwell TS. Airway epithelium controls lung inflammation and injury through the NF-κB pathway. J Immunol 178: 6504–6513, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Fakhrzadeh L, Laskin JD, Laskin DL. Deficiency in inducible nitric oxide synthase protects mice from ozone-induced lung inflammation and tissue injury. Am J Respir Cell Mol Biol 26: 413–419, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Farley KS, Wang LF, Razavi HM, Law C, Rohan M, McCormack DG, Mehta S. Effects of macrophage inducible nitric oxide synthase in murine septic lung injury. Am J Physiol Lung Cell Mol Physiol 290: L1164–L1172, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fitzpatrick AM, Brown LAS, Holguin F, Teague WG. Levels of nitric oxide oxidation products are increased in the epithelial lining fluid of children with persistent asthma. J Allergy Clin Immunol 124: 990–996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaston B, Sears S, Woods J, Hunt J, Ponaman M, McMahon T, Stamler JS. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet 351: 1317–1319, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J Pediatr 135: 770–772, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7: 665–674, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Hollingsworth JW, II, Cook DN, Brass DM, Walker JKL, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med 170: 126–132, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Islam T, Breton C, Salam MT, McConnell R, Wenten M, Gauderman WJ, Conti D, Van Den Berg D, Peters JM, Gilliland FD. Role of inducible nitric oxide synthase in asthma risk and lung function growth during adolescence. Thorax 65: 139–145, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Janssen R, Bont L, Siezen Christine LE, Hodemaekers HM, Ermers MJ, Doornbos G, Slot R, Wijmenga C, Goeman JJ, Kimpen JL, van Houwelingen HC, Kimman TG, Hoebee B. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis 196: 826–834, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-κB by S-nitrosylation of p65. J Biol Chem 282: 30667–30672, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310: 1966–1970, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi H, Hataishi R, Mitsufuji H, Tanaka M, Jacobson M, Tomita T, Zapol WM, Jones RC. Antiinflammatory properties of inducible nitric oxide synthase in acute hyperoxic lung injury. Am J Respir Cell Mol Biol 24: 390–397, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol 9: 371–377, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Kristof Arnold S, Goldberg P, Laubach V, Hussain Sabah N. A role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med 158: 1883–1889, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Lakari E, Soini Y, Säily M, Koistinen P, Pääkkö P, Kinnula VL. Inducible nitric oxide synthase, but not xanthine oxidase, is highly expressed in interstitial pneumonias and granulomatous diseases of human lung. Am J Clin Pathol 117: 132–142, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Lim SY, Raftery M, Cai H, Hsu K, Yan WX, Hseih HL, Watts RN, Richardson D, Thomas S, Perry M, Geczy CL. S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol 181: 5627–5636, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Marshall HE, Potts EN, Kelleher ZT, Stamler JS, Foster WM, Auten RL. Protection from lipopolysaccharide-induced lung injury by augmentation of airway S-nitrosothiols. Am J Respir Crit Care Med 180: 11–18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 25. Marshall HE, Stamler JS. Inhibition of NF-κB by S-nitrosylation. Biochemistry 40: 1688–1693, 2001 [DOI] [PubMed] [Google Scholar]
- 26. McClintock DE, Ware LB, Eisner MD, Wickersham N, Thompson BT, Matthay MA, the National Heart Lung and Blood Institute ARDS Network Higher urine nitric oxide is associated with improved outcomes in patients with acute lung injury Am J Respir Crit Care Med 175: 256–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehta S. The effects of nitric oxide in acute lung injury. Vascul Pharmacol 43: 390–403, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Nys M, Preiser JC, Deby-Dupont G, Habraken Y, Mathy-Hartert M, Damas P, Lamy M. Nitric oxide-related products and myeloperoxidase in bronchoalveolar lavage fluids from patients with ALI activate NF-kappa B in alveolar cells and monocytes. Vascul Pharmacol 43: 425–433, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Okamoto T, Gohil K, Finkelstein EI, Bove P, Akaike T, van der Vliet A. Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol 286: L198–L209, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Olson N, Kasahara DI, Hristova M, Bernstein R, Janssen-Heininger Y, van der Vliet A. Modulation of NF-κB and HIF-1 by S-nitrosoglutathione does not alter allergic airway inflammation in mice. Am J Respir Cell Mol Biol 2010–0035OC, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci USA 97: 14382–14387, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peng X, Abdulnour REE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JGN, Hassoun PM. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med 172: 470–479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EFM, van der Vliet A, Janssen-Heininger YMW. Nitric oxide represses inhibitory κB kinase through S-nitrosylation. Proc Natl Acad Sci USA 101: 8945–8950, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, Cheng Ds, Yull FE, Christman JW, Blackwell TS. Targeted immunomodulation of the NF-κB pathway in airway epithelium impacts host defense against pseudomonas aeruginosa. J Immunol 176: 4923–4930, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J 12: 929–937, 1998 [PubMed] [Google Scholar]
- 36. Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovas Res 75: 349–358, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 503–510, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC, Murphy ME, Murphy HS, Ward PA. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol 163: 2319–2328, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thom SR, Bhopale VM, Mancini DJ, Milovanova TN. Actin S-nitrosylation inhibits neutrophil beta-2 integrin function. J Biol Chem 283: 10822–10834, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Tyurina YY, Basova LV, Konduru NV, Tyurin VA, Potapovich AI, Cai P, Bayir Hl Stoyanovsky D, Pitt BR, Shvedova AA, Fadeel B, Kagan VE. Nitrosative stress inhibits the aminophospholipid translocase resulting in phosphatidylserine externalization and macrophage engulfment. J Biol Chem 282: 8498–8509, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Velez D, Hulme W, Myers J, Weinberg J, Levesque M, Stryjewski M, Abbate E, Estevan R, Patillo S, Gilbert J, Hamilton C, Scott W. NOS2A, TLR4, and IFNGR1 interactions influence pulmonary tuberculosis susceptibility in African-Americans. Hum Genet 126: 643–653, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu Z, Kuncewicz T, Dubinsky WP, Kone BC. Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene. J Biol Chem 281: 9101–9109, 2006 [DOI] [PubMed] [Google Scholar]