Abstract
Exhaled NO (eNO) is a potential noninvasive biomarker of inflammation in asthma. The significant intersubject variability of eNO within clinically similar patients has contributed to its limited clinical application. Arginase and NO synthase (NOS) utilize the same substrate (l-arginine) and contribute to the fibrotic and inflammatory features of asthma, respectively. Interestingly, TGF-β2 can increase the expression of arginase, stimulates fibrosis, and is overexpressed in asthma. We hypothesized that TGF-β2-enhanced arginase activity would decrease gas phase NO release from lung epithelial cells by limiting l-arginine availability for NOS. Our results show that TGF-β2 (5 ng/ml) significantly enhances total arginase activity up to two- to threefold in both primary small airway epithelial cells (SAECs) and the A549 cell line. Preincubation with TGF-β2 prior to cytokine (IL-1β, TNF-α, and IFN-γ, 10 ng/ml each) stimulation decreases gas phase NO release to baseline levels (from 1.66 ± 0.52 to 0.30 ± 0.12 pl·s−1·cm−2 and from 0.27 ± 0.03 pl·s−1·cm−2 to near zero in SAEC and A549 cells, respectively). Addition of arginase inhibitor (Nω-hydroxy-nor-l-arginine) or small interfering RNA only partly reverses the reduction. In contrast, Rho-kinase (ROCK) pathway inhibitor (Y-27632) completely recovers the cytokine-induced NO flux in the present of TGF-β2. Inducible NO synthase (iNOS) mRNA and protein levels change in a similar trend as NO release from the cells. We conclude that TGF-β2 impacts cytokine-induced NO production in airway epithelial cells by reducing iNOS mRNA and protein levels through a ROCK-dependent pathway.
Keywords: arginase, cytokine, inducible nitric oxide synthase, A549, small airway epithelial cells
asthma can be characterized as an inflammatory disease of the airways. Elevated levels of NO in the exhaled breath (eNO) of asthmatic patients (17) are regarded as a potential biomarker of inflammation. However, the clinical application of eNO remains limited owing, in part, to significant intersubject variability within clinically similar individuals (7, 29, 30). This suggests that the determinants of eNO have not been fully elucidated.
NO is synthesized from l-arginine by NO synthase (NOS) isoforms. There are reports of increased inducible NO synthase (iNOS) expression in airway epithelium in biopsy specimens from asthmatic subjects (13) and confirmation of an increase in both iNOS mRNA and protein expression by using in situ hybridization and quantitative immunohistochemistry in untreated asthma (33). Studies have shown that NO release from airway epithelial cells in response to proinflammatory stimuli and cytokines such as IL-13 and cytomix (IL-1β, TNF-α, and IFN-γ) is due to iNOS expression (9, 15, 36) and the majority of eNO is due to diffusion of NO from airway epithelial cells (35). This observation is consistent with the dramatic reduction in eNO following anti-inflammatory therapy with inhaled corticosteroids (17).
Asthma is also characterized as a fibrotic disease. l-arginine can be converted by arginase to urea and l-ornithine. l-Ornithine is a precursor for polyamines and l-proline, which are involved in cell proliferation and collagen synthesis, respectively (23). Airway remodeling generally refers to structural changes in the airway wall and is a prominent, albeit variable, feature of subjects with asthma including cell proliferation (e.g., smooth muscles cells) and collagen synthesis (i.e., subepithelial fibrosis). TGF-β2 is overexpressed in the airways of asthmatic subjects (1) and is secreted in response to epithelial injury (39). Furthermore, TGF-β2 stimulates collagen synthesis in fibroblasts and is thought to be an important mediator of subepithelial fibrosis.
Two different arginase isozymes (arginase I and arginase II) have been identified; both are constitutively expressed in the airways, particularly in the epithelium, fibroblast, and alveolar macrophages (9, 31). Previous studies have demonstrated that enhanced arginase expression and/or activity may influence airway hyperresponsiveness by inducing bronchodilating constitutive NOS (cNOS)-derived NO deficiency (24). TGF-β has been shown to increase arginase activity and expression in macrophages (5) and fibroblasts (19, 43). The influence of TGF-β on arginase in airway epithelial cells has not been described.
Given the fact that NOS and arginase share a common substrate, inhibition or enhancement of either enzyme may potentially affect the relative activity of the other. For example, arginase inhibition increases NO production in bovine pulmonary arterial endothelial cells (10) and macrophages (8). The underlying mechanism for this observation may be competition for intracellular l-arginine. The impact of increased arginase activity on NO production from airway epithelial cells has not yet been reported.
We hypothesized that enhanced arginase activity by TGF-β2 impacts cytokine-induced gas phase NO release from airway epithelial cells by limiting the availability of l-arginine for NOS. Our results demonstrate that TGF-β2 does indeed increase arginase protein levels and activity in airway epithelial cells and inhibits cytokine-induced gas phase NO release. However, the inhibition is primarily due to a decrease in iNOS mRNA through a Rho-associated kinase (ROCK)-dependent signaling pathway. This result suggests that TGF-β2 expression in the airways impacts NO synthesis and release from airway epithelial cells and may contribute to the variability of eNO among clinically similar asthmatic patients.
MATERIALS AND METHODS
Cell culture.
Cryopreserved passage 1 small airway epithelial cells (SAECs) from two different donors (donor 1: 4F0715, donor 2: 0000079556) were purchased from Lonza (Walkersville, MD) and grown as previously described (15). In brief, the cells were first grown on T-75-cm2 flasks in small airway epithelial basal medium supplemented with SingleQuot kit (Lonza, Walkersville, MD) and were trypsinized and seeded onto Costar Transwells inserts at passage 3. Medium was applied both apically and basally until the cells reached confluence, at which time an air-liquid interface (ALI) was established for 7 days to achieve mucociliary differentiation. A549 cells, a human alveolar epithelial cell line, were obtained from the American Type Culture Collection (Rockville, MD), and ALI was established on Transwell inserts as previously described (15).
Exposure to cytokines and chemical inhibitors.
Culture medium was changed 24 h prior to each experiment. A549 cells were cultured with serum-free F-12K medium. Previous studies have demonstrated that arginase is induced more slowly than iNOS (38). To have both arginase activity and iNOS expression elevated maximally at approximately the same time, cells exposed to the cytomix (TNF-α, IL-1β, IFN-γ, 10 ng/ml each, R&D Systems, Minneapolis, MN) + TGF-β2 (5 ng/ml) group were preincubated with 5 ng/ml TGF-β2 (R&D Systems) for 24 h (t = −24 h). We also tried 1 ng/ml and 10 ng/ml of TGF-β2. The maximal response without affecting cell viability was observed at 5 ng/ml (data not shown). On the day of the experiment (t = 0 h), TGF-β2 alone, cytomix alone, or cytomix + TGF-β2 was added to fresh culture medium; 100 μM of Nor-NOHA (a reversible arginase activity inhibitor, Cayman Chemical, Ann Arbor, MI) or 10 μM of Y-27632 [an extensively used selective inhibitor of Rho-associated kinases (21, 42), Cayman Chemical] was added to some experimental groups prior to addition of the cytomix and/or TGF-β2. Each experiment ended 48 h after cytomix exposure.
Transfection protocol.
Some A549 cells were transfected with ARG1 small interfering RNA (siGenome smart pool Human ARG1, NM_00045 5 nmol, Dharmacon, Lafayette, CO) using DharmaFect 1 (catalog no. T-2001, Dharmacon) per manufacturer's instructions.
Arginase activity measurement.
Arginase activity was measured as described previously (12). Briefly, epithelial cells were lysed in RIPA buffer and incubated in 10 mM MnCl2 at 56°C to activate arginase. The activated lysate was then incubated with 0.5 M l-arginine at 37°C for 60 min. The reaction was stopped by addition of an acidic mixture (H2SO4, H3PO4, and H2O; 1:3:7 vol/vol/vol). Urea production by arginase was measured by optical density at 540 nm after addition of 9% isonitrosopropiophenone (dissolved in 100% ethanol) and heating at 100°C for 60 min. Arginase activity is expressed as micrograms urea produced per milligram total protein. Urea concentration was calculated according to a urea standard curve.
Gas-phase NO measurement and NO flux calculation.
Gas-phase NO was measured at t = 0, 8, 24, 32, and 48 h, and NO flux was calculated as previously described (15, 36). In brief, 12-well Transwell plates were fitted with modified lids with two holes on the top and edges were sealed to form a gas tight enclosure. One of the holes was connected to the inlet of a chemiluminescent NO analyzer (NOA 280, Sievers, Boulder, CO) at a constant flow of 40 ml/min. Real-time NO signal reaches a plateau value (in ppb) representing the steady-state NO release into the gas phase after the washout of accumulated NO from the headspace. The steady-state NO concentration was determined by fitting an exponential form to the smoothed transient response, and the NO flux was calculated on the basis of the surface area of the Transwell membranes and flow of the gas stream.
Total nitrate assay.
Total nitrate in culture medium was measured by a Griess assay kit (Cayman Chemical) according to the manufacturer's instructions. Nitrate in the sample medium was converted to nitrite by nitrate reductase and Griess reagent was added to the 96-well plate. Absorbance was determined at 540 nm. The concentration of total nitrate was calculated according to a standard curve of known nitrate concentrations.
Western blotting.
At each time point after NO gas phase measurement, protein was extracted by use of RIPA buffer and quantified via the Bradford assay (Bio-Rad, Hercules, CA). Samples (40 μg equal protein) were subjected to SDS-PAGE and transferred electrophorically to a polyvinylidene fluoride-nitrocellulose membrane (Millipore, Bedford, MA). The blots were probed with monoclonal mouse anti-iNOS antibody (1:1,000, Research and Development Antibodies, Las Vegas, NV) and anti-arginase I and anti-arginase II antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA) and subsequently incubated with horseradish peroxidase-conjugated secondary antibodies (1:10,000, Santa Cruz Biotechnology). The proteins were visualized by use of an enhanced chemiluminescence system (Bio-Rad imaging system, Bio-Rad). The blots were also probed with mouse monoclonal anti-β-actin (Abcam, Cambridge, MA) as a loading control.
Reverse transcription and quantitative PCR.
RNA was also collected at each time point after NO gas phase measurement. Total RNA was isolated using NucleoSpin RNA II kit (Macherey-Nagel, PA) and quantified by Quant-iT RiboGreen RNA assay kit (Invitrogen, Carlsbad, CA). Reverse transcription was carried out by use of a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). PCR reactions were performed in a CFX96 real-time quantitative PCR machine (Bio-Rad) using SsoFast EvaGreen supermix (Bio-Rad). The primers for real-time PCR were as follows: 5′-TCATCCGCTATGCTGGCTAC-3′ (iNOS Forward), 5′-CTCAGGGTCACGGCCATTG-3′ (iNOS Reverse); 5′-TTGGCAATTGGAAGCATCTCTGG-3′ (arginase I Forward), 5′-TCCACTTGTGGTTGTCAGTGGAGT-3′ (arginase I Reverse); 5′-ATGCCCATGCTGACATCAACACAC -3′ (arginase II Forward), 5′-AAATGTTCAGGAGGGTCCACGTCT-3′ (arginase II Reverse); 5′-TCGACAGTCAGCCGCATCTTCTTT-3′ (GAPDH Forward), 5′-ACCAAATCCGTTGACTCCGACCTT-3′ (GAPDH Reverse).
Real-time PCR data were quantified by the comparative threshold cycle (Ct) method (34). In brief, the Ct values of both the control and the samples of interest are normalized to GAPDH and then the Ct values of the samples of interest are further normalized by the control.
Intracellular l-arginine concentration measurement.
Cells were washed twice with PBS and lysed with RIPA buffer without Tris. The analysis of l-arginine concentration was performed by the Protein Chemistry Lab (Texas A&M University, College Station, TX) using precolumn derivatization with o-phthalaldehyde and 9-fluorenylmethyl chloroformate and separation on a C18 HPLC column.
Statistics.
Data are presented as means ± SD. Statistical significance was tested by performing a one-way ANOVA, followed by a Student-Newman-Keuls test. P values less than 0.05 were considered significant.
RESULTS
Arginase expression and activity.
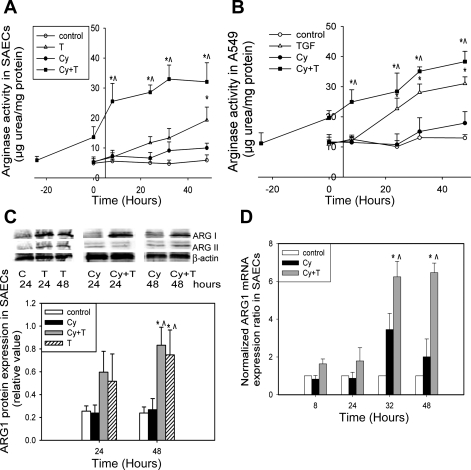
The basal level of arginase activity is 5.90 ± 1.79 μg urea/mg protein in SAECs (Fig. 1A) and 13.0 ± 1.98 μg urea/mg protein in A549 cells (Fig. 1B). Cytomix alone does not elevate arginase activity. Total arginase activity was significantly enhanced by TGF-β2, reaching a peak of 19.3 ± 4.34 and 31.0 ± 2.22 μg urea/mg protein in SAECs and A549 cells, respectively (Fig. 1, A and B). Addition of TGF-β2 to the cytomix group significantly enhances total arginase activity, and the increased activity is sustained through the duration of the experiment in SAECs and A549 cells (32.1 ± 6.37 and 38.3 ± 3.42 μg urea/mg protein, respectively). Arginase I protein expression (Fig. 1C) and mRNA (Fig. 1D) are increased 48 and 32 h, respectively, after TGF-β2 stimulation. In contrast, arginase II expression does not change significantly with TGF-β2 or cytomix stimulation (Fig. 1C).
Fig. 1.
TGF-β2 upregulates arginase activity and arginase I expression in lung epithelial cells. C, control; T, TGF-β2; Cy, cytomix; Cy + T, cytomix + TGF-β2. A: TGF-β2 enhances arginase activity in small airway epithelial cells (SAECs) 24 h after stimulation. Preincubation and addition of TGF-β2 to cytomix-treated SAECs increases total arginase activity throughout the experiment (n = 8–10). B: TGF-β2 enhances arginase activity in A549 cells with the same trend as in SAECs (n = 8–10). C: representative Western blots and densitometry analysis (normalized by β-actin) indicates that TGF-β2 increases arginase 1 (ARG1) protein expression in SAECs (n = 3). Spaces between lane T48 and lane Cy24 and between lane Cy + T24 and lane Cy48 indicate that the data are from 1 Western blot experiment, but not run in the same order as shown in the figure. D: quantitative PCR results demonstrate that TGF-β2 increases arginase 1 mRNA expression in SAECs. Data are shown as means ± SD. *P < 0.05 compared with control group; ^P < 0.05 compared with cytomix group.
NOS expression and NO production.
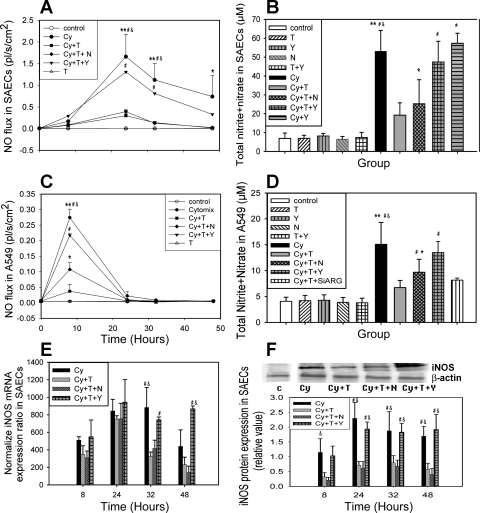
NO gas phase concentration and NO flux from the surface in both SAECs and A549 cells was measured at t = 0, 8, 24, 32, and 48 h. TGF-β2 did not induce NO flux. Significant NO release was observed 6 h after cytomix treatment and reached a peak at 24 h in SAECs and 8 h in A549 cells (1.66 ± 0.52 and 0.27 ± 0.03 pl·s−1·cm−2, respectively) (Fig. 2, A and C). Preincubation and addition of TGF-β2 significantly decreased the gas phase NO release in each cell type (e.g., from 1.66 ± 0.52 to 0.30 ± 0.12 pl·s−1·cm−2 in SAECs at t = 24 h and from 0.27 ± 0.03 pl·s−1·cm−2 to negligible baseline level in A549 cells at t = 8 h). Addition of the arginase inhibitor Nor-NOHA (Fig. 2, A–D) partly reversed the reduction in A549 cells (30% of initial reduction), and a similar trend was observed in SAECs (20% of initial reduction), but the latter observation was not statistically significant. A higher concentration of Nor-NOHA had no additional effect (data not shown). ARG1 silencing in A549 cells only partly reversed the reduction, as well (Fig. 2D). However, NO flux returned to cytomix-alone levels after addition of the ROCK inhibitor Y-27632 (e.g., from 0.30 ± 0.12 to 1.31 pl·s−1·cm−2 in SAECs at t = 24 h and from negligible baseline level to 0.22 ± 0.01 pl·s−1·cm−2 in A549 at t = 8 h) (Fig. 2, A–D). The amount of total nitrite + nitrate in the medium mirrors the trend of real-time NO flux (Fig. 2, B and D). Y-27632 or Nor-NOHA alone did not impact NO production in control or cytomix-treated conditions (Fig. 2B).
Fig. 2.
Preincubation of TGF-β2 decreases gas phase NO release in cytomix-treated lung epithelial cells. Y, control + Y-27632; N, control + Nor-NOHA; Cy + T, cytomix + TGF-β2; Cy + T + N, cytomix + TGF-β2 + Nω-hydroxy-nor-l-arginine (nor-NOHA); Cy + T + Y, cytomix + TGF-β2 + Y-27632; Cy + Y, cytomix + Y-27632; T + Y, TGF-β2 + Y-27632; Cy + T + siARG, cytomix + TGF-β2 + small interfering RNA for arginase I. Cells were preincubated with TGF-β2 for 24 h (t = 24 h) in Cy + T, Cy + T + N, and Cy + T + Y groups. At t = 0, fresh medium was supplied, and cytokines and inhibitors were added as the group names indicated. A: TGF-β2 decreases cytomix-induced gas phase NO release from SAECs (n = 3). Nor-NOHA partly and Y-27632 completely reverses the reduction. B: total nitrate content in SAECs culture medium after 48-h exposure (n = 10). C: TGF-β2 decreases cytomix-induced gas phase NO release from A549 cells. Nor-NOHA partly reverses the reduction, and Y-27632 significantly reverses the effect (n = 4). D: total nitrate content in A549 culture medium after 48 h exposure (n = 12, n = 3 for Cy + T + siARG). E: normalized inducible nitric oxide synthase (iNOS) mRNA expression in SAECs. TGF-β2 decreases iNOS mRNA level, and addition of Y-27632 completely reverses the reduction in SAECs. F: representative Western blotting and densitometry analysis of iNOS protein expression normalized by β-actin in SAECs (n = 3). TGF-β2 inhibits iNOS protein expression and Y-27632 completely reverses the inhibition. The space between control blot and the experimental conditions indicates that they were from 1 Western blot experiment, but not run in the same order as shown in the figure. Control blot is separately from treated conditions. Data are shown as means ± SD. *P < 0.05 compared with control group; **P < 0.01 compared with control group; #P < 0.05 compared with Cy + T group; &P < 0.05 compared with Cy + T + N group.
Quantitative PCR and Western blotting demonstrate that TGF-β2 reduces iNOS mRNA (Fig. 2E) and protein expression (Fig. 2F) in SAECs, respectively. Nor-NOHA did not affect iNOS mRNA or protein expression levels. In contrast, Y-27632 significantly reversed the TGF-β2 induced iNOS reduction of mRNA and protein (Fig. 2, E and F). The same trend was seen in A549 cells (data not shown).
Intracellular l-arginine concentration in SAECs.
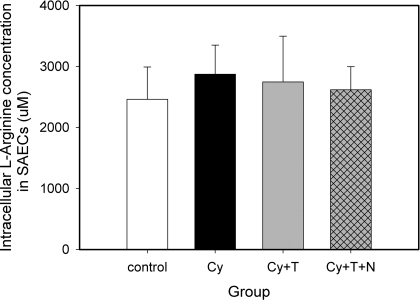
To further investigate the effect of enhanced arginase activity on NO production, intracellular l-arginine concentration was measured in SAECs. The average of intracellular l-arginine concentration in SAECs control group was 2,460 ± 530 μM. Cytomix alone or in combination with TGF-β2, or in combination with TGF-β2 and Nω-hydroxy-nor-l-arginine (nor-NOHA), did not significantly change the intracellular l-arginine concentration in SAECs (Fig. 3). Intracellular l-arginine concentration in A549 cells was in the same range as in SAECs, and no significant difference was observed between the same combination of cytokines and inhibitors as well (data not shown).
Fig. 3.
Intracellular l-arginine concentrations in small airway epithelial cells. The average concentration of intracellular l-arginine in SAECs is 2.68 mM. No significant difference was observed following different combinations of cytokine stimulation (n = 3). Data are shown as means ± SD.
DISCUSSION
In this study, we investigated the impact of TGF-β2 on cytokine-induced gas phase NO release from small airway epithelial cells and A549 cells. Our results demonstrate that TGF-β2 significantly enhanced arginase expression and activity and decreased cytokine-induced gas phase NO production. Although inhibiting arginase activity partly recovered NO production and release, the dominant effect of TGF-β2 was ROCK-dependent inhibition of iNOS mRNA expression.
TGF-β, secreted by inflammatory cells as well as structural cells, induces multiple cellular responses including differentiation, apoptosis, survival, proliferation, and extracellular matrix synthesis (22). Three isoforms of TGF-β are found in mammals, two of which (TGF-β1 and TGF-β2) appear to have critical roles in bronchial asthma. Levels of TGF-β1 in asthmatic airways are elevated as shown by bronchoalveolar lavage (2, 32) and direct biopsy (4). It has also been reported that the level of TGF-β2 is greater than that of TGF-β1 in asthmatic and normal airways (11), and epithelial-derived TGF-β2 can drive collagen synthesis in subepithelial fibroblasts (39, 41). Although TGF-β has been intensively studied in fibrosis, its specific role in airway epithelial cell biology remains largely unclear. In this study, we confirmed that both arginase I and II are present in airway epithelial cells. However, TGF-β2 increases total arginase activity by increasing arginase I (not arginase II) mRNA and protein expression 24 h after stimulation. Elevated levels of TGF-β2 in asthma thus may contribute to enhanced arginase I expression in human asthmatic patients (28, 44) and ovalbumin-challenged animal models (16, 44).
Several studies have suggested that interactions between arginase and NOS may affect the allergic response, as well as the variable levels of NO seen in asthma (3, 44). Considering the temporal relationship of arginine as a substrate for iNOS/NO pathway (early response to acute inflammatory stimulation) and the arginase/ornithine pathway (latent repair phase), it is perhaps not surprising that regulation of these pathways might overlap and be impacted by inflammatory cytokines and mediators. Previous studies have demonstrated that arginase inhibition increases NO production in bovine pulmonary arterial endothelial cells (10) and macrophages (8). In addition, one report demonstrated that increased expression of arginase I in airway epithelial cells might lead to low eNO in smoking asthmatic subjects (3). These studies attributed the interaction between NOS and arginase to competition for l-arginine. In other words, increased arginase expression and activity would consume more l-arginine, thus potentially limiting the availability for iNOS. Our results demonstrate that arginase inhibition (by silencing or chemical inhibition) only partly reverses the reduction in NO production by TGF-β2. Previous studies have demonstrated that nor-NOHA is a potent inhibitor for arginase (6, 38, 40). Although we cannot rule out that arginase activity was not fully inhibited in our study, higher doses of nor-NOHA had no additional impact.
The most compelling explanation for why substrate limitation is not a significant factor in the interaction between NOS and arginase is the observation that the affinity of NOS for l-arginine (Km = 2–20 μM) is nearly three orders of magnitude larger than its affinity for arginase (Km = 2–20 mM) (45), and the average intracellular concentration of l-arginine exceeds 2 mM, even when arginase expression is increased (Fig. 3). Thus the average concentration of l-arginine is nearly three orders of magnitude larger than the Km of NOS, essentially ensuring that NOS is saturated with substrate under all conditions investigated in this study. One explanation for the partial recovery of NO production by nor-NOHA is that intracellular l-arginine is not equally distributed, and the l-arginine that iNOS accesses is distinct from (albeit related to) the bulk cytosolic l-arginine (14). Given the fact that our measured intracellular concentration of l-arginine is near the Km of arginase (2–20 mM), enhanced arginase activity would lead to more substrate consumption and may partially deplete the l-arginine source for local iNOS.
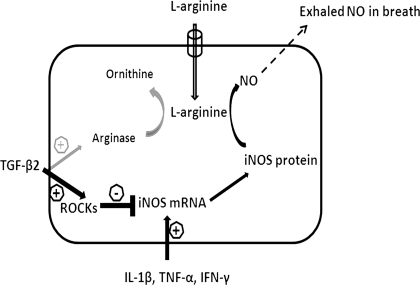
Previous studies demonstrated that TGF-β1 suppresses IFN-γ-induced NO production in macrophages by suppressing STAT1 activation, accelerating iNOS protein degradation (37), and regulating iNOS gene expression posttranslationally (26). Surprisingly, our results in lung epithelial cells showed that addition of Y-27632, a selective ROCK inhibitor, could significantly prevent the TGF-β2-induced NO reduction by recovering iNOS mRNA and protein expression. This result suggests that TGF-β2 inhibits iNOS mRNA expression through a ROCK-dependent pathway in lung epithelial cells. ROCK is a downstream target of RhoA, and its major physiological functions include cell contraction, migration, and proliferation. RhoA/ROCK is involved in airway hyperresponsiveness and is regarded as a novel target molecule for the treatment of asthma (20). Although previous studies have shown that RhoA/ROCK is a key downstream mediator of TGF-β (18) and inversely regulates endothelial NO synthase (eNOS) expression through alteration in eNOS mRNA stability (25, 27), our study is the first to show an effect of ROCK on iNOS. Figure 4 summarizes the pathways stimulated by TGF-β2 and cytomix leading to altered expression of arginase and iNOS, and ultimately NO release to the gas phase in lung epithelial cells.
Fig. 4.
Schematic for regulation of iNOS expression and NO release in airway epithelial cells. Cytomix stimulation of epithelial cells leads to an increase in iNOS mRNA increase at transcriptional level. TGF-β2 decreases NO production by predominantly reducing iNOS mRNA expression or stability through a Rho-kinase (ROCK)-dependent pathway. TGF-β2 enhanced arginase activity can reduce NO production, but to a small degree.
In summary, our results demonstrate that TGF-β2 enhances arginase activity and limits cytomix-induced gas phase NO release in lung epithelial cells mainly by inhibiting iNOS mRNA expression through a ROCK-dependent signaling pathway. We speculate that the balance between fibrosis mediated by TGF-β and inflammation mediated by cytokines could impact eNO from epithelial cells and could thus potentially contribute to the significant variability of eNO in clinically similar subjects with asthma.
GRANTS
This work was supported by grants from the National Institutes of Health (R01 HL067954 and R01 HL070645).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Balzar S, Chu HW, Silkoff P, Cundall M, Trudeau JB, Strand M, Wenzel S. Increased TGF-beta2 in severe asthma with eosinophilia. J Allergy Clin Immunol 115: 110–117, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Batra V, Musani AI, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy 34: 437–444, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bergeron C, Boulet LP, Page N, Laviolette M, Zimmermann N, Rothenberg ME, Hamid Q. Influence of cigarette smoke on the arginine pathway in asthmatic airways: increased expression of arginase I. J Allergy Clin Immunol 119: 391–397, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bosse Y, Stankova J, Rola-Pleszczynski M. Transforming growth factor-beta1 in asthmatic airway smooth muscle enlargement: is fibroblast growth factor-2 required? Clin Exp Allergy 40: 710–724, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Boutard V, Havouis R, Fouqueray B, Philippe C, Moulinoux JP, Baud L. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J Immunol 155: 2077–2084, 1995 [PubMed] [Google Scholar]
- 6. Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol 234: 273–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol 115: 1130–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol Heart Circ Physiol 274: H342–H348, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Chibana K, Trudeau JB, Mustovich AT, Hu H, Zhao J, Balzar S, Chu HW, Wenzel SE. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy 38: 936–946, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 287: L60–L68, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Chu HW, Balzar S, Seedorf GJ, Westcott JY, Trudeau JB, Silkoff P, Wenzel SE. Transforming growth factor-beta2 induces bronchial epithelial mucin expression in asthma. Am J Pathol 165: 1097–1106, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 174: 231–235, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. Induction of nitric oxide synthase in asthma. Lancet 342: 1510–1513, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Hardy TA, May JM. Coordinate regulation of L-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med 32: 122–131, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Jiang J, Malavia N, Suresh V, George SC. Nitric oxide gas phase release in human small airway epithelial cells. Respir Res 10: 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenyon NJ, Bratt JM, Linderholm AL, Last MS, Last JA. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicol Appl Pharmacol 230: 269–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 343: 133–135, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Kita T, Hata Y, Arita R, Kawahara S, Miura M, Nakao S, Mochizuki Y, Enaida H, Goto Y, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proc Natl Acad Sci USA 105: 17504–17509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitowska K, Zakrzewicz D, Konigshoff M, Chrobak I, Grimminger F, Seeger W, Bulau P, Eickelberg O. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L34–L45, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kume H. RhoA/Rho-kinase as a therapeutic target in asthma. Curr Med Chem 15: 2876–2885, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol 85: 348–356, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol Sci 24: 450–455, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Meurs H, McKay S, Maarsingh H, Hamer MA, Macic L, Molendijk N, Zaagsma J. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. Br J Pharmacol 136: 391–398, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol 22: 8467–8477, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitani T, Terashima M, Yoshimura H, Nariai Y, Tanigawa Y. TGF-beta1 enhances degradation of IFN-gamma-induced iNOS protein via proteasomes in RAW 264.7 cells. Nitric Oxide 13: 78–87, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 290: C661–C668, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol 296: L911–L920, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Olin AC, Bake B, Toren K. Fraction of exhaled nitric oxide at 50 ml/s: reference values for adult lifelong never-smokers. Chest 131: 1852–1856, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest 130: 1319–1325, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Que LG, Kantrow SP, Jenkinson CP, Piantadosi CA, Huang YC. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol Lung Cell Mol Physiol 275: L96–L102, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, Howarth PH. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med 156: 642–647, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Redington AE, Meng QH, Springall DR, Evans TJ, Creminon C, Maclouf J, Holgate ST, Howarth PH, Polak JM. Increased expression of inducible nitric oxide synthase and cyclo-oxygenase-2 in the airway epithelium of asthmatic subjects and regulation by corticosteroid treatment. Thorax 56: 351–357, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Shin HY, George SC. Microscopic modeling of NO and S-nitrosoglutathione kinetics and transport in human airways. J Appl Physiol 90: 777–788, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Suresh V, Mih JD, George SC. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol 37: 97–104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takaki H, Minoda Y, Koga K, Takaesu G, Yoshimura A, Kobayashi T. TGF-beta1 suppresses IFN-gamma-induced NO production in macrophages by suppressing STAT1 activation and accelerating iNOS protein degradation. Genes Cells 11: 871–882, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Tenu JP, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher JL. Effects of the new arginase inhibitor N(omega)-hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide 3: 427–438, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Thompson HG, Mih JD, Krasieva TB, Tromberg BJ, George SC. Epithelial-derived TGF-beta2 modulates basal and wound-healing subepithelial matrix homeostasis. Am J Physiol Lung Cell Mol Physiol 291: L1277–L1285, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J Pharmacol Exp Ther 318: 1368–1374, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol 28: 142–149, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25: 681–686, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Witte MB, Barbul A, Schick MA, Vogt N, Becker HD. Upregulation of arginase expression in wound-derived fibroblasts. J Surg Res 105: 35–42, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest 111: 1863–1874, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zimmermann N, Rothenberg ME. The arginine-arginase balance in asthma and lung inflammation. Eur J Pharmacol 533: 253–262, 2006 [DOI] [PubMed] [Google Scholar]