Abstract
The mechanical properties of endothelial glycocalyx were studied using atomic force microscopy with a silica bead (diameter ∼18 μm) serving as an indenter. Even at indentations of several hundred nanometers, the bead exerted very low compressive pressures on the bovine lung microvascular endothelial cell (BLMVEC) glycocalyx and allowed for an averaging of stiffness in the bead-cell contact area. The elastic modulus of BLMVEC glycocalyx was determined as a pointwise function of the indentation depth before and after enzymatic degradation of specific glycocalyx components. The modulus-indentation depth profiles showed the cells becoming progressively stiffer with increased indentation. Three different enzymes were used: heparinases III and I and hyaluronidase. The main effects of heparinase III and hyaluronidase enzymes were that the elastic modulus in the cell junction regions increased more rapidly with the indentation than in BLMVEC controls, and that the effective thickness of glycocalyx was reduced. Cytochalasin D abolished the modulus increase with the indentation. The confocal profiling of heparan sulfate and hyaluronan with atomic force microscopy indentation data demonstrated marked heterogeneity of the glycocalyx composition between cell junctions and nuclear regions.
Keywords: atomic force microscopy, bovine lung microvascular endothelial cell, heparan sulfate, hyaluronan
the endothelial glycocalyx is a polysaccharide-protein coating on the luminal surface of the vascular endothelium and forms a negatively charged, complex meshwork. The primary glycosaminoglycan (GAG) constituents of glycocalyx are heparan sulfates (HS), chondroitin sulfates, and hyaluronan (HA). The syndecan family of transmembrane proteoglycans and membrane-bound glypicans both carry HS and chondroitin sulfate side chains (29, 33), while HA is a nonsulfated GAG that is secreted into the pericellular space and is associated with other components of glycocalyx. In vivo, the glycocalyx is known to associate with blood proteins, such as fibrinogen (21) and albumin (2), that contribute to the permeability barrier of the vessel wall. Several important functions are assigned to the in vivo glycocalyx (37): 1) a molecular sieve and hydrodynamic barrier for transvascular exchange of macromolecules; 2) an exclusion layer preventing interactions of blood proteins and cells with the endothelial membrane, per se; 3) a modulator of leukocyte binding and rolling; and 4) a transducer of mechanical impulses to the intercellular cytoskeleton and associated signaling pathways.
Glycocalyx shedding and degradation, in inflammation models, lead to impaired endothelial mechanotransduction of fluid shear stress (13, 25, 34), adhesion of platelets (36), and leukocytes (7, 17, 22). It also leads to the leakage of plasma proteins and fluid from the vascular space (1, 17). The activation of inflammatory pathways (17), edema (19), loss of capillary density (38), and deregulation of organ blood flow (16) can all be related to the loss of glycocalyx function; however, the mechanism(s) that trigger glycocalyx shedding has yet to be established.
The highly complex, multifunctional and multicomponent structure of the endothelial glycocalyx poses a question: which approach is the most appropriate to study its multifaceted features? A reductionist approach used by many groups, including ours, is to elucidate the roles and functions of its constituent parts using enzyme digestions (9, 16, 23, 27, 35). We have used specific enzymes to degrade glycocalyx HS and HA components and measure diffusion and the dynamics of albumin association within the glycocalyx expressed by bovine lung microvascular endothelial cells (BLMVECs) in vitro (31). Here, atomic force microscopy (AFM) was used to quantify the elastic properties of BLMVEC glycocalyx before and after enzymatic degradations of these components.
AFM has been a method of choice to measure mechanical stiffness of endothelial cells using indentation techniques (8, 24, 26, 28, 30). Typical AFM indentation experiments involve the use of a sharp tip that indents the cell membrane and exerts pressure on the membrane and cytoskeleton. Using AFM, Mathur et al. (24) have found fivefold differences in elastic moduli measured over the nucleus vs. the peripheral cell body of human umbilical vein endothelial cells. Ohashi et al. (26) have used AFM and finite-element analysis to show the increase in elastic moduli for bovine endothelial cells exposed to shear stress. Costa et al. (8) used non-Hertzian pointwise approach to analyze how the elastic moduli of human aortic endothelial cells changes with indentation depth (δ).
We approached the measurement of glycocalyx stiffness using the same pointwise approach with one major difference: because the sharp AFM tip (typical r = 10 nm) could easily exert high local pressure on glycocalyx elements, or poke through the glycocalyx layer with little resistance, we substituted this sharp AFM tip with a larger silica bead (diameter ∼18 μm). Thus we traded the high resolution of sharp tip AFM for low compressive pressures and spatial averaging of mechanical properties in the bead-cell contact region. These lower compressive pressures and spatial averaging in the bead-cell contact area allowed us to determine the elastic modulus of BLMVEC in a pointwise fashion, as a function of the δ, before and after enzymatic degradation of specific glycocalyx components. Three enzymes were used: heparinase III (HSase III) or heparinase I (HSase I), both at the concentrations of 15 mU/ml, or hyaluronidase (HAase) at 1.2, 12, and 50 U/ml concentrations. In addition, cytochalasin D was used to disrupt the cell cytoskeleton and differentiate between the elastic contributions of glycocalyx and underlying cellular structures.
METHODS
Cell culture.
BLMVECs (Vec Technologies, Rensselaer, NY) were cultured onto glass coverslips (1 in. round, 0.17 mm thick, Fisher Scientific, Pittsburgh, PA) precoated with 0.4% bovine gelatin (SigmaAldrich, St. Louis, MO) for 1 h, followed by 100 μg/ml bovine fibronectin (Sigma-Aldrich, St. Louis, MO) for 1 h at 37°C and 5% CO2. BLMVECs were plated at a density of 2.5 × 105 cells/cm2 and cultured for 7–10 days.
Enzymatic degradation of the glycocalyx.
Glycocalyx components were selectively digested by incubating cells with HAase (from strep. hyalurolyticus, Sigma-Aldrich, St. Louis, MO; EC 4.2.2.1; concentrations 1.2, 12, or 50 U/ml), HSase I (Sigma-Aldrich; EC 4.2.2.7; 15 mU/ml), or HSase III (Sigma-Aldrich; EC 4.2.2.8; 15 mU/ml) in MCDB-131 medium supplemented with 25 mM HEPES, pH 7.4, 0.01% penicillin/streptomycin, and 1% BSA (Fraction V, Sigma) at 37°C and 5% CO2 for 1 h. Cells were then rinsed with the same medium and used in AFM experiments.
Cytochalasin disruption of cytoskeleton.
The effect of cytoskeleton disruption on elastic modulus was determined by incubating confluent monolayers of BLMVECs with 100 nM cytochalasin D (Sigma-Aldrich; EC 244–804-1) for 30 min at 37°C and performing subsequent AFM indentation as described below. Cytochalasin D was initially solubilized in DMSO, then diluted in cell medium to 100 nM (∼300 μM DMSO or 0.02%). Identical cytochalasin treatment was conducted with BLMVEC monolayers pretreated for 1 h with 50 U/ml HAase.
AFM indentation.
A borosilicate glass microsphere (diameter = 17.3 ± 1.4 μm; catalog no. 9020, Duke Scientific, Palo Alto, CA) was glued to the tip of a rectangular AFM cantilever (nominal spring constant = 0.03 N/m) and mounted to the z-piezo on an Explorer AFM head (Topometrix, Santa Clara, CA). The AFM scanner was placed above the BLMVEC monolayer covered with medium, and the bead was brought into contact with cells. The loading force was minimized to prevent any cell damage. In the indentation measurement, the cantilever deflection was measured by a position-sensitive diode (PSD) as a function of z-piezo displacement producing raw AFM data. The measurements were taken at multiple (n > 80) locations on the BLMVEC monolayer surface. The loading rate was 10 μm/s, and the maximal loading force varied between 5 and 10 nN. The typical δ was up to ∼500 nm. After the indentation measurements were completed, the sample topography was mapped by scanning the same AFM bead over the BLMVEC monolayer over a (100 μm)2 region. From the topological scans, the specific loci of cell-cell junctions and cell nuclei were judiciously assigned to each indentation run (Fig. 1). The indentation runs that did not localize exactly to either of the two locations were excluded from the analysis. The contribution of individual glycocalyx components to the overall stiffness was assessed by the enzymatic digestions, followed by subsequent AFM indentation measurements.
Fig. 1.
Atomic force microscopy (AFM) topography scan of a 100 × 100 μm2 area of the bovine lung microvascular endothelial cell (BLMVEC) monolayer using a spherical bead as an AFM tip. The numbers indicate the locations at which force-indentation measurements were taken. These were assigned to cell junction or cell nucleus locations.
Data analysis.
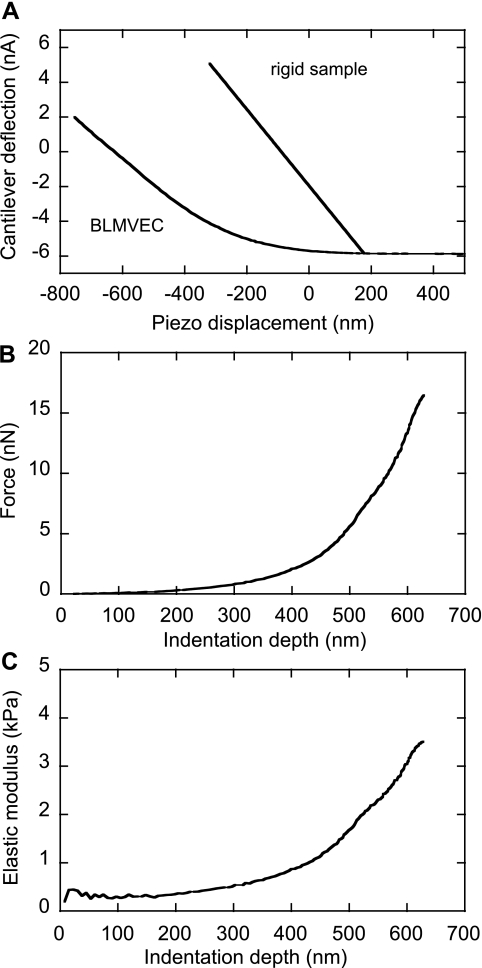
The AFM indentation data were analyzed by finding the point of contact between the bead and the cell surface layer in raw AFM data and then performing a pointwise analysis to determine the stiffness of the glycocalyx as a function of the δ. The stiffness of the cantilever (in N/m) was calibrated using the AFM instrument built-in software function, which also provided the conversion factor between the cantilever deflection signal (in nA) and cantilever force (in nN). Once the stiffness of the cantilever was known, a hypothetical rigid substrate deflection-displacement line, with the slope equal to the negative cantilever stiffness, was plotted through the contact point, as shown in Fig. 2A. The indentation into the glycocalyx, δ, was found by subtracting the displacement value of each data point from the displacement value on the rigid substrate line at the identical force, F. This procedure produced the force-indentation F(δ) curve shown in Fig. 2B. To account for spherical geometry of the AFM probe, the indenter geometry function for a sphere of radius R was used (3):
| (1) |
where R is that of the bead indenter. This indenter geometry function was then used to find the pointwise elastic modulus, E, for every data point on the F(δ) curve (8):
| (2) |
The E of a cell (in kPa), plotted as a function of indentation, δ (Fig. 2C), showed that the E of control BLMVECs glycocalyx was constant up to the δ values of ∼200 nm. The depth of indentation, where the E(δ) curve shows an inflection, was used as an estimate of the effective thickness of the glycocalyx, δg. Statistical analysis of BLMVEC moduli recorded before and after enzymatic treatments was performed using a Wilcoxon-Mann-Whitney rank-sum test.
Fig. 2.
Conversion of raw AFM data into the pointwise elastic modulus E. A: typical raw AFM data for untreated BLMVEC and rigid samples. B: BLMVEC force vs. indentation data. C: the pointwise E calculated from the force-indentation data plotted as a function of indentation depth δ.
Confocal imaging.
BLMVEC monolayers were treated with enzymes, washed with phosphate-buffered saline, and fixed with 4% paraformaldehyde at room temperature. HS was immunostained with anti-HS (HepSS-1, US Biologicals, Swampscott, MA) and incubated with Alexa Fluor 596 labeled anti-IgM-k (BD Biosciences, San Jose, CA). HA was localized using biotinylated HA-binding protein (US Biologicals; H7980–35) and then labeled with avidin-Alexa Fluor 488 conjugate (Invitrogen, Carlsbad, CA; A-21370). A FV1000-XY, Olympus IX81 confocal microscope and a 60 × NA 1.45 oil immersion lens were utilized for imaging (Core Facilities, University of Utah). Images of (200 μm)2 area were taken with vertical separation distance of 0.2 μm. The fluorescence intensity profiles through the confocal images stack were extracted using ImageJ software (W. Rasband, National Institutes of Health) at 6 μm2 areas at junction and nucleus locations.
RESULTS
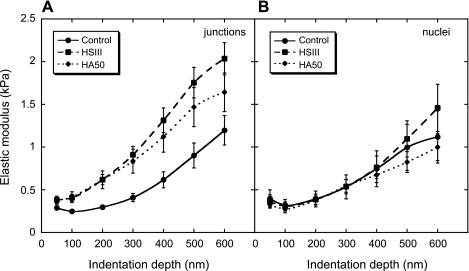
Analysis of AFM spherical bead indentation experiments yielded two dependent variables: E and δ. Figure 3 shows how average modulus changes with the δ, <E>(δ) at cell junctions (Fig. 3A) and nuclei (Fig. 3B), before and after enzymatic digestions with HSase III (15 mU/ml) and HAase (50 U/ml). The difference between BLMVEC controls and the enzyme-treated cells was remarkable; in the case of the controls, the E initially rose slowly with indentation and reached ∼1 kPa at δ = 600 nm at the junctions. After the enzyme digestion, the modulus increased more rapidly at smaller indentations for both enzymes and also showed signs of leveling at large indentations. The modulus of control BLMVECs had a low value of ∼0.25 kPa and was approximately constant up to the δ values of ∼200 nm. Upon further indentation, the modulus increased, which indicated that the loading forces compressing the glycocalyx were progressively transmitted to the less compliant cell membrane and underlying cytoskeleton. The finding that the enzymatic treatment increased stiffness in the region between 0 < δ < 600 nm relative to controls indicated that the glycocalyx, which resided in this region, was being degraded. For nuclear locations, however, the difference between <E>(δ) data for controls and enzyme-treated BLMVECs was indistinguishable. The average modulus diverged only at larger δ values, and HSase III action made the cells stiffer, while HAase made them a bit softer (Fig. 3B).
Fig. 3.
Average E shown as a function of the δ, E(δ), at cell junction (A) and nuclear (B) locations for untreated BLMVECs (junctions: n = 43, nuclei: n = 11), and after enzymatic digestions of glycocalyx with heparinase (HSase) III (HS III; 15 mU/ml) (junctions: n = 43, nuclei: n = 11), and hyaluronidase (HAase) (50 U/ml; HA50) (junctions: n = 33, nuclei: n = 9). The vertical bars represent the SE of the mean.
One way to analyze the <E>(δ) data is by using two-layer composite compliance model (10):
| (3) |
where Eglycocalyx and Ecell are the mean elastic moduli of the glycocalyx and the cell, respectively, and α is a parameter defining the mechanical interlayer interactions. The compliance of the spherical bead indenter was assumed to be much smaller compared with the other two right-hand side terms and was omitted from analysis. The model accounts for the transfer of mechanical deformation between the two layers, i.e., the glycocalyx and the cell, by the term exp(−αδ/δg) (20). This transfer function is dependent on the δ, the δg, and the extent of interlayer interactions, which are dependent on the local composition of glycocalyx. A fit to the two-layer composite compliance model for BLMVECs treated by HSase III and HAase yielded the best fitted parameters as follows: Ecell = 2.93 ± 0.38 kPa and Eglycocalyx = 0.26 ± 0.03 for HSase III, and Ecell = 2.35 ± 0.31 kPa and Eglycocalyx = 0.28 ± 0.03 kPa for HAase (50 U/ml). The δg was estimated to be 420 nm (HSase III) and 450 nm (HSase 50). The fitted α parameter was ∼2.2; however, because it appears in the exponent ratio α/δg, its effect on the fit was strongly affected by estimate of the δg. The <E>(δ) results for untreated BLMVECs did not display a sigmoidal shape, so the fitted results were inconclusive.
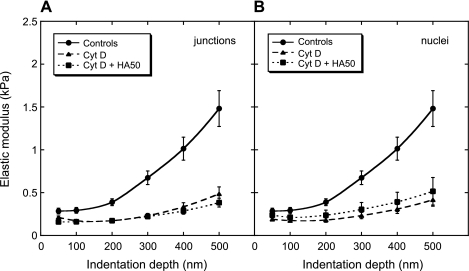
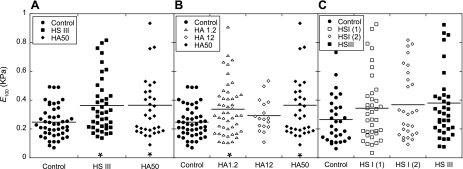
The biomechanical role of cellular structures below the glycocalyx was investigated by treating the cells with cytochalasin D. Cytochalasin D is known to inhibit actin polymerization within the cell, thus causing softening of the cell (18). Figure 4 compares the <E>(δ) data at the cell junctions for untreated BLMVECs, cells treated with cytochalasin D, and cells treated first with enzyme HAase (50 U/ml) and then with cytochalasin D. The E of cytochalasin D-treated BLMVECs remained <0.5 kPa for the whole range of δ values (δ < 500 nm). The pretreatment of cells with enzyme HAase followed by cytochalasin D treatment resulted in an E profile that was almost indistinguishable from the cell treatment with cytochalasin D alone. A similar trend of cytochalasin D cell softening was found at the nuclear locations (data not shown). The results of the cytochalasin D experiments confirmed that the observed increases in elastic moduli at intermediate indentations (100 < δ < 500 nm), for control and enzyme-treated BLMVECs (Figs. 3 and 4), were due to the progressive transmission of the compressive loads from the glycocalyx to the underlying cytoskeletal structure. Consequently, the effective stiffness of glycocalyx was represented by the modulus at 100-nm δ, E100. Figure 5 shows the actual E100 data for each indentation run and for all enzyme treatments measured at cell junctions. Figure 5A compares the digestion of BLMVEC glycocalyx with HSase III (15 mU/ml) or HAase (50 U/ml) to the control. For both enzymes, the mean elastic moduli <E100> (shown by the horizontal lines), as well as the spread of E100 data, increased upon the enzyme digestion (HSase III, P = 0.003, HAase, P = 0.035, each compared with the controls). Clearly, the enzymatic digestion made the BLMVECs appear a bit stiffer compared with controls. The majority of the E100 data, however, remained <0.4 kPa, indicating the enzymes might not have completely digested the glycocalyx. Figure 5B compares the effect of HAase concentrations on E100. Similar findings, such as larger spread of moduli and increases in <E100>, were observed for BLMVECs treated with HAase at low (1.2 U/ml, P = 0.017) and high (50 U/ml, P = 0.035) concentration compared with controls. Figure 5C compares two enzymes that are known to degrade HS: HSase I and HSase III. While the repeated treatment of BLMVECs with HSase I showed very similar <E100>, only the treatment with HSase III showed significant difference from the controls [HSase I (first run) P = 0.192, HSase I (second run) P = 0.100, HSase III, P = 0.050].
Fig. 4.
E(δ) for the cell junctions of untreated BLMVECs (n = 27), cells treated with cytochalasin D (Cyt D; n = 32), and cells treated first with HA50 and then with Cyt D (n = 23). The vertical bars represent the SE of the mean.
Fig. 5.
Elastic moduli at the 100-nm δ (E100) before and after enzymatic digestions. A: the comparison between the BLMVEC controls and the cells treated with enzymes [HS III, P = 0.003, HA50, P = 0.035]. B: the effect of the HAase concentrations [HAase 1.2 U/ml (HA 1.2), P = 0.017, HAase 12 U/ml (HA 12), P = 0.128, HA50, P = 0.035]. C: the comparison between BLMVEC controls and duplicate run of HSase I (HS I) [(1) P = 0.192, (2) P = 0.100] and HS III (P = 0.051). The mean modulus for each data set is shown by horizontal line. Each data set is compared with untreated BLMVEC controls (*P < 0.05).
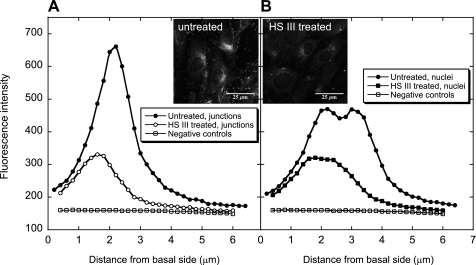
Confocal imaging of enzyme-treated and control BLMVECs was used to create the concentration profiles for HS (Fig. 6) and HA (Fig. 7) at both cell junction and nuclear locations. For untreated BLMVECs, a higher concentration of HS was found at the cell junctions compared with nuclear locations (Fig. 6, A vs. B). The action of HSase III (15 mU/ml) reduced the integrated fluorescence intensity at both locations (junctions: a 65% decrease, nuclei: a 54% decrease) and shifted the fluorescence maxima to smaller distances from the basal side of the cells. This indicated the enzyme was more effective at the upper cell surface, as expected.
Fig. 6.
Confocal vertical profiles of HS before and after enzyme digestion with HS III (15 mU/ml) (n = 3). A: cell junction location. B: nuclear location. Negative controls were not treated with the enzyme or anti-HS, but only stained with the secondary anti-IgM-k antibody. The insets show confocal images of untreated and HS III-treated BLMVECs.
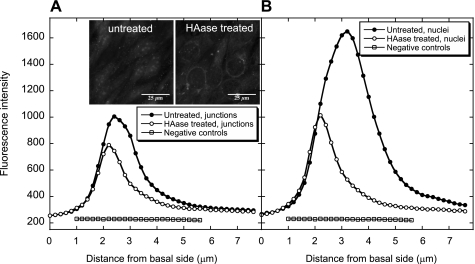
Fig. 7.
Confocal vertical profiles of HA before and after enzyme digestion with HA50 (n = 3). A: cell junction location. B: nuclear location. Negative controls were not treated with the enzyme or HA binding protein, but only stained with avidin-Alexa Fluor 488 conjugate. The insets show confocal images of untreated and HAase-treated BLMVECs.
The confocal imaging also revealed that the spatial distribution of HA was different from HS; the fluorescence intensity was much larger above the nuclei than at the cell junctions (Fig. 7, A vs. B). The HAase (50 U/ml) digestion of HA was more effective at nuclear locations, reducing the integrated fluorescence intensity by 58%, than at the cell junctions (a mere 32% reduction). Like in the case of HS digestion, the maxima of the HA fluorescence intensity shifted to smaller distances from the basal side of the cells upon the treatment with HAase.
DISCUSSION
The goal of the present study was to characterize the effect of glycocalyx components on stiffness of BLMVEC glycocalyx and to elucidate the contributions of HS and HA. The technique selected was spherical probe AFM, because the probe exerted smaller loading forces over larger contact area than in the case of typical indentation with a sharp AFM tip. For example, the contact area of a spherical bead (d = 18 μm) at 50 nm indentation was ∼2.8 μm2, or a circular area with r = 0.94 μm, and the bead exerted only 180-Pa pressure at 0.5 nN load. The same spherical bead used to indent the cells was used to scan the topography of BLMVEC monolayers and to develop coordinates that allowed precise assignment of indentation locations at cell junctions, which are believed to be the loci of mechanotransduction (37). For comparison, indentation measurements were also carried out at the nuclear locations.
The force-indentation data, F(δ), were used in a pointwise calculation of the E as a function of the δ, E(δ) (8). A large number of E(δ) curves (typical n > 30) were averaged to analyze the difference between BLMVEC controls and cells treated with enzymes. In the case of controls, the <E>(δ) measured at the cell junctions was essentially flat up to 200-nm indentation and then reverted to an increasing function up to an indentation of 600 nm. After enzymatic digestion with HSase III (15 mU/ml) or HAase (50 U/ml), the average modulus <E>(δ) increased more rapidly at smaller indentations at ∼100-nm δ (Fig. 3A). The effective δg was estimated from the inflection of <E>(δ) curves where possible, or from the model described by Eq. 3. As previously reported, the δg determined by in vivo fluorescence imaging of mesenteric vessels (or hamster cheek pouches) (11), by in vitro measurements on BLMVECs utilizing fluorescence correlation spectroscopy, and by confocal immunohistochemistry (31) was found to be larger than 500 nm. This thickness agrees with the estimates from individual E(δ) curves (for example, see Fig. 2C). The average <E>(δ) data after HSase III or HAase digestion showed that the enzymes decreased this value to ∼420–450 nm. The two-layer composite compliance model (10) was used to assess the biomechanical properties of glycocalyx and underlying cell membrane and cytoskeleton. The fit to the model yielded reasonable E estimates for glycocalyx and underlying cellular structures, but was less sensitive to the δg and the parameter α. Such simple models do not represent the physical properties of cellular structures well; neither glycocalyx nor underlying cytoskeleton is a uniform homogeneous layer, but is instead a mesh of interconnected stiffer and softer elements (4). Hence, the usefulness of such model might be rather limited. In addition, not all <E>(δ) data could be fitted well with the model, because some E(δ) curves did not display sigmoidal shape, which was a prerequisite for a good fit. This was especially noticeable for BLMVEC controls that required larger δ values to show the leveling of the E (Fig. 3). One may infer in such cases that the δg was larger than the δ used. The estimates for the δg from the inflection of <E>(δ) confirm such an inference. The change of the glycocalyx composition, for example, by enzymatic digestion, has the potential to affect the way by which loading forces are transmitted to the underlying membrane and cytoskeleton. In terms of the two-layer composite compliance model (Eq. 3), the mechanical coupling effect was described by the empirical term exp(−αδ/δg). However, no firm conclusion about the coupling factor α could be made, as its influence on the fit was heavily affected by the δg.
The <E>(δ) results indicated that increase of loading transmits the compressive forces to the underlying cellular structures (Figs. 3 and 4). This agrees with the accepted physical picture in which mechanical perturbations of the glycocalyx are transduced to a chemical signal within the cell cytoplasm (37). The matching of mechanical compliances is expected for proper mechanochemical transduction; a very soft glycocalyx would not be sensitive enough to small perturbations due to circumferential stretch and pressure changes within the vasculature. Similarly, a rigid glycocalyx imbedded in a compliant matrix could be too sensitive to weak force perturbations. Disrupting the underlying cytoskeleton with cytochalasin D largely eliminated the increase of <E>(δ) in the range of 0 < δ < 500 nm (Fig. 4), confirming that modulus increases observed in BLMVECs were due to progressive compression of underlying cytoskeleton. It was not possible to estimate how much of the <E>(δ) increase was due to the bending stiffness of the cell membrane; however, membrane contribution was expected to be small (4).
Based on the appearance of <E>(δ) curves, the pointwise modulus at 100-nm indentation was assigned as an effective measure of glycocalyx stiffness. At a smaller δ of 50 nm, the modulus was determined to be too noisy due to AFM raw data noise and the accuracy of determining the contact zero indentation point (Fig. 2). Larger variations of E at very small indentations have been reported in the literature due to the sharp AFM tip used when human aortic endothelial cells (8), osteoblasts (32), bovine pulmonary artery endothelial cells (28), and human umbilical vein endothelial cells (24) were indented. The distribution of E100 was narrow for BLMVEC controls (0.1 < E100 < 0.5 kPa), but the range increased after enzymatic digestion of the glycocalyx (0.1 < E100 < 1.0 kPa, Fig. 5A). This demonstrated structural heterogeneity of the glycocalyx layer. Lectin-binding studies have confirmed that there is both macro- and microheterogeneity of the glycocalyx structure over the length scale of a single cell (5, 15). Our own confocal images of BLMVEC glycocalyx also demonstrated significant heterogeneity (14, 31). Experimentally, it was possible that, at some of the cell junctions, the spherical bead probe indented a larger area than predicted due to the concave shape of these regions.
Insight into heterogeneous distribution of glycocalyx components is provided by the confocal depth profiles of HA and HS before and after enzymatic digestion (Figs. 6 and 7). There was a significant difference between <E>(δ) at cell junctions and at nuclear locations. One can speculate that the mechanical coupling between glycocalyx and the underlying cytoskeleton at the cell junctions is different than at the nuclear locations. Confocal profiling showed that nuclear locations of BLMVECs were predominantly decorated with HA polymer chains (Fig. 7, A vs. B), known to be noncovalently attached to cell membrane receptors and to HS proteoglycans. In contrast, the cell junctions appeared to be richer in HS than the nuclear regions (Fig. 6, A vs. B). Admittedly, confocal vertical profiling does not have the same resolution as lateral confocal imaging. However, it was possible to resolve the vertical distribution of HS above the nuclei where the stain showed two peaks, indicating that HS is present at both the basal and upper cell surfaces (Fig. 6B). No such resolution was possible for the HA stain. In general, the fluorescence was not eliminated by the enzyme treatments; the enzymes predominantly digested HA and HS polymers from the upper cell surface, which was exposed to enzyme solution. We conclude that the enzymes did not completely digest each glycocalyx component, as indicated by depth profiles (Figs. 6 and 7).
Two forms of HSases (HSase I and HSase III) have been tested for their effects on glycocalyx stiffness. HSase I cleaves disaccharide substrates that have a higher sulfate content, whereas HSase III cleaves unsulfated disaccharides. It has been reported that endothelial HS participate in both flow (13, 27) and pressure-induced (12) mechanotransduction that subsequently activates nitric oxide synthase. Increased levels of nitric oxide are associated with barrier dysfunction, as assessed by increased hydraulic conductivity (6, 12, 13). It has been reported that selective removal of cell-surface HS with HSase III abolished pressure and flow-mediated nitric oxide production and the associated barrier dysfunction, establishing a direct link between the glycocalyx and barrier-dependent mechanotransduction (12, 13, 27). According to the present results, only HSase III had a significant effect on increasing the glycocalyx modulus (Fig. 5C, P = 0.050), thus supporting the previous findings. HAase degradation of the glycocalyx at higher enzyme concentrations also resulted in a significant increase in E100 compared with untreated cells (Figs. 5, A and B, P = 0.035). It is, therefore, possible that HA acted as a softer, multiattachment cross-linker within the glycocalyx structure, and that its removal exposed stiffer elements of the glycocalyx.
One major conclusion from the present study is that enzymatic digestion of a single GAG in the glycocalyx leaves the other components in place so that they are able to maintain a similar stiffness in the probe-cell contact area. For example, it is likely that digestion of HA, which was found more concentrated above nuclear regions, leaves HS and associated transmembrane proteins, such as syndecan core protein, to transmit the compressive forces exerted by the AFM probe. Similarly, digestion of HS, which was found more concentrated in the cell junction areas as well, leaves syndecans in place and possibly also associated HA polymer. Yet these spatial differences in the composition of vascular glycocalyx must exist for functional reasons, which have yet to be fully elucidated.
Summary.
AFM was used to assess the mechanical properties of BLMVEC glycocalyx: its modulus and thickness. The AFM indenter was a silica bead (diameter ∼18 μm) used instead of sharp AFM cantilever tip. This resulted in low compressive pressures on the glycocalyx, allowing determination of the E in a pointwise fashion as a function of the δ. The modulus-δ profiles showed cells becoming progressively stiffer with the indentation. Three different enzymes were used to digest glycocalyx components: HSases III and I and HAase. For HSase and HAase treatments, the E in the cell junction increased more rapidly at lower indentations than in controls. These enzymes also reduced the δg. Cytochalasin D abolished the modulus increases with the indentation. It was found that the digestion of a single glycocalyx component leaves the other components in place so that they were able to maintain a similar stiffness in the probe-cell contact area. More importantly, the combined confocal profiling and AFM results demonstrated marked heterogeneity of the glycocalyx spatial composition between cell junctions and nuclear regions.
GRANTS
The support from National Heart, Lung, and Blood Institute Grant (5RO1HL085255) is gratefully acknowledged. R. O'Callaghan acknowledges the support from the Undergraduate Research Opportunity Program at the University of Utah.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1. Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol 428: 1–13, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol 557: 889–907, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beatty MF, Usmani SA. On the indentation of a highly elastic half-space. Q J Mech Appl Math 28: 47–62, 1975 [Google Scholar]
- 4. Boal D. Mechanics of the Cell. Cambridge, UK: Cambridge University Press, 2002 [Google Scholar]
- 5. Brouland JP, Gilbert MA, Bonneau M, Pignaud G, Bal Dit Solier C, Drouet L. Macro and microheterogeneity in normal endothelial cells: differential composition of luminal glycocalyx and functional implications. Endothelium 6: 251–262, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Chang YS, Munn LL, Hillsley MV, Dull RO, Yuan J, Lakshminarayanan S, Gardner TW, Jain RK, Tarbell JM. Effect of vascular endothelial growth factor on cultured endothelial cell monolayer transport properties. Microvasc Res 59: 265–277, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Constantinescu AA, Vink H, Spaan JAE. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 23: 1541–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Costa KD, Sim AJ, Yin FCP. Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. J Biomech Eng 128: 176–184, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol Heart Circ Physiol 258: H647–H654, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Doerner MF, Nix WD. A method for interpreting the data from depth-sensing indentation instruments. J Mater Res 1: 601–609, 1986 [Google Scholar]
- 11. Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res 26: 163–170, 1970 [DOI] [PubMed] [Google Scholar]
- 12. Dull RO, Mecham I, McJames S. Heparan sulfates mediate pressure-induced increase in lung endothelial hydraulic conductivity via nitric oxide/reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 292: L1452–L1458, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Giantsos KM, Kopeckova P, Dull RO. The use of an endothelium-targeted cationic copolymer to enhance the barrier function of lung capillary endothelial monolayers. Biomaterials 30: 5885–5891, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Haldenby KA, Chappell DC, Winlove CP, Parker KH, Firth JA. Focal and regional variations in the composition of the glycocalyx of large vessel endothelium. J Vasc Res 31: 2–9, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol Heart Circ Physiol 277: H508–H514, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 279: H2815–H2823, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Ito S, Suki B, Kume H, Numaguchi Y, Ishii M, Iwaki M, Kondo M, Naruse K, Hasegawa Y, Sokabe M. Actin cytoskeleton regulates stretch-activated Ca2+ influx in human pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol 43: 26–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacob M, Bruegger D, Rehm M, Stoeckelhuber M, Welsch U, Conzen P, Becker BF. The endothelial glycocalyx affords compatibility of Starling's principle and high cardiac interstitial albumin levels. Cardiovasc Res 73: 575–586, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kovalev A, Shulha H, Lemieux M, Myshkin N, Tsukruk VV. Nanomechanical probing of layered nanoscale polymer films with atomic force microscopy. J Mater Res 19: 716–728, 2004 [Google Scholar]
- 21. LeBoeuf RD, Raja RH, Fuller GM, Weigel PH. Human fibrinogen specifically binds hyaluronic acid. J Biol Chem 261: 12586–12592, 1986 [PubMed] [Google Scholar]
- 22. Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation 12: 5–15, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lopez-Quintero SV, Amaya R, Pahakis M, Tarbell JM. The endothelial glycocalyx mediates shear-induced changes in hydraulic conductivity. Am J Physiol Heart Circ Physiol 296: H1451–H1456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathur AB, Truskey GA, Reichert WM. Atomic force and total internal reflection fluorescence microscopy for the study of force transmission in endothelial cells. Biophys J 78: 1725–1735, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JAE, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285: H722–H726, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Ohashi T, Ishii Y, Ishikawa Y, Matsumoto T, Sato M. Experimental and numerical analyses of local mechanical properties measured by atomic force microscopy for sheared endothelial cells. Biomed Mater Eng 12: 319–327, 2002 [PubMed] [Google Scholar]
- 27. Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 355: 228–233, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pesen D, Hoh JH. Micromechanical architecture of the endothelial cell cortex. Biophys J 88: 670–679, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflügers Arch 440: 653–666, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Sato H, Kataoka N, Kajiya F, Katano M, Takigawa T, Masuda T. Kinetic study on the elastic change of vascular endothelial cells on collagen matrices by atomic force microscopy. Colloids Surf B Biointerfaces 34: 141–146, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am J Physiol Lung Cell Mol Physiol 293: L328–L335, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takai E, Costa KD, Shaheen A, Hung CT, Guo XE. Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Ann Biomed Eng 33: 963–971, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med 259: 339–350, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A 101: 16483–16488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. VanTeeffelen JWGE, Brands J, Jansen C, Spaan JAE, Vink H. Heparin impairs glycocalyx barrier properties and attenuates shear dependent vasodilation in mice. Hypertension 50: 261–267, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation 101: 1500–1502, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol 99: 1471–1476, 2005 [DOI] [PubMed] [Google Scholar]