Abstract
In patients with acute ischemic stroke, diabetes and hyperglycemia are associated with increased infarct size, more profound neurologic deficits and higher mortality. Notwithstanding extensive clinical and experimental data, treatment of stroke-associated hyperglycemia with insulin is controversial. In addition to hyperglycemia, diabetes and even early prediabetic insulin resistance are associated with increased levels of amino acids, including the neurotoxic glutamate, in the circulation. The pleiotropic metabolic effects of insulin include a reduction in the concentration of amino acids in the circulation. In this article, we show that in diabetic rats exposed to transient middle cerebral artery occlusion, a decrease of plasma glutamate by insulin or glucagon reduces CSF glutamate, improves brain histology, and preserves neurologic function. The neuroprotective effect of insulin and glucagon was similar, notwithstanding their opposite effects on blood glucose. The therapeutic window of both hormones overlapped with the short duration (∼30 min) of elevated brain glutamate following brain trauma in rodents. Similar neuroprotective effects were found after administration of the glutamate scavenger oxaloacetate, which does not affect glucose metabolism. These data indicate that insulin and glucagon exert a neuroprotective effect within a very brief therapeutic window that correlates with their capacity to reduce glutamate, rather than by modifying glucose levels.
Keywords: stroke, brain damage, glutamate
in ∼30–40% of patients with acute ischemic stroke, diabetes is found at presentation (5, 24), ∼70% have elevated blood glucose levels (27), and about 25% develop persistent hyperglycemia (21). Hyperglycemia is associated with increased infarct size (5, 9, 12, 21, 36, 44), as well as with a ∼3-fold higher risk of death (15), and survivors have more profound neurologic deficits and disability (26). Hyperglycemia is also associated with aggravated postischemic brain damage in animal models. In cats, acute hyperglycemia is associated with a ∼3-fold increase in the volume of hemispheric infarcts induced by cerebrovascular occlusion (19), and in dogs, even moderate hyperglycemia has been shown to increase brain damage and mortality induced by ischemia (33). Consistent with these findings, treatment of hyperglycemia with insulin in these models, improves the outcome (10, 29, 45). Notwithstanding extensive clinical and experimental data indicating that hyperglycemia exacerbates poststroke brain damage and evidence from animal models that reversal of hyperglycemia with insulin attenuates injury (10, 29, 45), in clinical practice, this approach is controversial (8, 23, 25, 31, 35, 37). This disconcerting lack of clinical success is not entirely surprising, since insulin has pleiotropic effects on cell metabolism that extend beyond the lowering of blood glucose.
We hypothesize that in the acute setting of stroke, hyperglycemia, as such, is not neurotoxic and that the deleterious effects are mediated through the associated increase in circulating amino acids, including the neurotoxic amino acid glutamate. In all stages of diabetes, including early prediabetic insulin resistance (IR), increased levels of amino acids, such as alanine, proline, valine, leucine/isoleucine, phenylalanine, tyrosine, glutamate/glutamine, and ornithine, have been observed (41); furthermore, in addition to its effect on glucose levels, insulin reduces the concentrations of these amino acids in the circulation (11, 30).
Two important corollaries of this concept are 1) following brain injury or ischemia, neurotoxic amino acids are elevated for only ∼30 min in rodents (42) and ∼6 h in humans (13), and thus, it can be predicted that insulin given after that time would be ineffective; and 2) reduction of CNS glutamate alone may improve the outcome without necessarily affecting blood glucose levels. On the basis of these postulates, we suggest that the predominant effect of insulin in poststroke management is off-target, i.e., insulin primarily lowers blood glucose rather than glutamate levels, and in the clinical setting, its brief therapeutic window has not been taken into consideration.
Recently, we reported that glucagon improves the outcome of traumatic brain injury (TBI) in mice, although it increases the concentration of glucose (1). In the present communication in a rat model, we report that insulin, glucagon, and the glutamate scavenger, oxaloacetate, improve poststroke outcome in animal models by decreasing glutamate in the circulation and in the cerebrospinal fluid (CSF) (14, 32) The neuroprotective effect of glucagon, insulin, and oxaloacetate did not correlate with glucose levels, which were affected in opposite directions, if at all.
MATERIALS AND METHODS
Animals.
All experimental protocols involving the use of vertebrate animals were approved by the Israeli Board for Animal Experiments. Adult male, 8- to 10-wk-old Sprague-Dawley rats (average weight 250–280 g) (Harlan Laboratories, Jerusalem, Israel) were anesthetized with an intraperitoneal injection of ketamine (75 mg/ml) and xylazine (5 mg/ml; Kepro Barneveld, Holland) before the experiments.
Transient occlusion of the middle cerebral artery.
Transient occlusion of the middle cerebral artery (MCAO) was performed exactly as described previously (2, 4). Briefly, the left common carotid artery (CCA) was exposed and permanently ligated. An incision was made in the CCA to insert a 4–0 monofilament nylon suture. The different treatments were given before or after the induction of ischemia, as described below. The monofilament was inserted through the CCA into the lumen of the internal carotid artery and was advanced into the circle of Willis, effectively occluding the middle carotid artery. Two hours after MCA occlusion, the monofilament was removed, the surgical wound was closed, and the animals were returned to their cages to recover. The mortality rate was about 15% and was similar in the different groups. Almost all of the deaths happened during or immediately after the induction of MCAO, and all of these rats were excluded.
Injection of insulin and glucagon.
Glucagon and insulin were given intraperitoneally 10 min before or 10, 30 or 60 min after the induction of MCAO. Insulin doses of 2 U/kg to wild-type (WT) and 3 U/kg given to diabetic rats were based on the literature (20, 40), as well as on a preliminary test that we performed to decrease plasma glutamate with insulin to the same levels achieved with glucagon. The higher dose of insulin in diabetic rats was due to the insulin resistance typical of type II diabetes.
Injection of oxaloacetate, glutamate, and glucose.
Immediately after insulin or glucagon injection and as indicated, rats were given saline alone (30 μl·min−1·100 g−1), or saline containing oxaloacetate (30 μmol·min−1·100 g−1) or oxaloacetate and sodium glutamate (Sigma, St. Louis, MO) (30 μmol·min−1·100 g−1 each) (47) into the tail vein over 30 min. Glucose was injected intraperitoneally (20% in 0.75 ml of water for injection) immediately after the injection of insulin.
Neuroscoring and exclusion criteria.
The neurological scoring was performed at 24 h after induction of stroke. The neuroscores were determined twice by independent observers who were blinded to the experimental protocol. The scoring of the neurological deficits was a composite of motor, sensory, reflex, and balance tests, as described in detail by Chen et al. (18) and others (6, 17). The severity of injury was graded as follows: Points were awarded for the inability to perform the required test or for the absence of a reflex tested. Thus, the higher the score, the more severe was the injury. Neurological function was graded on a scale of 0 to 18 (normal score, 0; maximal deficit, 18 points). Scores of 13–18 were considered to reflect severe injury; scores of 7–12 reflected moderate injury and scores of 1–6 indicated mild injury.
Measurement of infarct size.
Infarct size was determined as previously reported (2, 4). The infarcted area in each section was traced manually and measured using an image analysis system. The total infarct volume was determined by integrating the areas from all sections, and the results were expressed as a percentage of the total volume of the ipsilateral hemisphere, as described previously (2, 4, 43).
Blood tests.
For the measurement of blood glucose, blood samples were taken during the experiments from the tail of anesthetized rats to measure basal glucose levels, using a manual glucometer. For the measurement of plasma glutamate, rats were anesthetized as above. Blood samples were taken from the jugular vein acceded by venesection. In some rats, blood was obtained by cardiac puncture just prior to euthanizing the animals. The blood was collected into commercially available tubes (Vacutainer, Plymouth, UK) containing EDTA as anticoagulant. Plasma was separated immediately in a refrigerated centrifuge and deproteinized with sulfosalicylic acid. Amino acid concentrations were determined using HPLC (1, 6) on a Bio-Chrom 20 amino acid analyzer (Pharmacia Biotech, Heidelberg, Germany). When jugular blood was available, blood glucose levels were reconfirmed by measuring serum glucose on a Kodak analyzer, as described previously (1).
CSF glutamate.
Several groups of rats were used for CSF sampling only. CSF samples were taken from the cisterna magna of sham or post MCAO anesthetized rats, treated or not treated with insulin or glucagon (as indicated) (7). About 70 μl of CSF were obtained from each rat. After CSF draining, the animals were killed. Pooled CSF from two or three animals was frozen at −70°C. Amino acid concentrations were determined on a Bio-Chrom 20-amino acid analyzer, as above.
Diabetic rats.
Adult male Sprague-Dawley rats were randomly placed for 14 wk on a normal fat (NF) or high fat (HF) diet (Harlan Teklad, Madison, WI) corresponding to 10% or 55% of the calories from fat and provided with water ad libitum (n = 30 in each group). Animals were maintained in a temperature-controlled barrier facility, with a 12-h alternating light-dark cycle. Food intake monitored throughout the study was almost identical in the two groups. In addition to body weight, fasting blood glucose was monitored weekly, using an Elite Glucometer (Bayer, Mishawaka, IN). At the end of 14 wk, the group fed the HF diet had significantly higher fasting blood glucose levels than the controls (4.88 ± 2.31 vs. 8.9 ± 3.11 mmol/l, respectively, P = 0.02). Glucose tolerance tests were performed by tail vein injection of glucose (1 g/kg) after an overnight fast, as described previously (38). HF rats displayed the expected glucose intolerance (data not shown), compared with the control NF group.
Statistical analysis.
All data are presented as means ± SE. Differences were analyzed using the Student's t-test or one-way ANOVA with the Newman-Keuls post hoc test, as indicated in results. In the case of neuroscoring data, the “between-group” comparisons were performed by one-way ANOVA with the Newman-Keuls test and by the Kruskal-Wallis rank test; in addition to the means ± SE, the median values are also shown in Figs. 2–4. Statistical significance was set at P < 0.05.
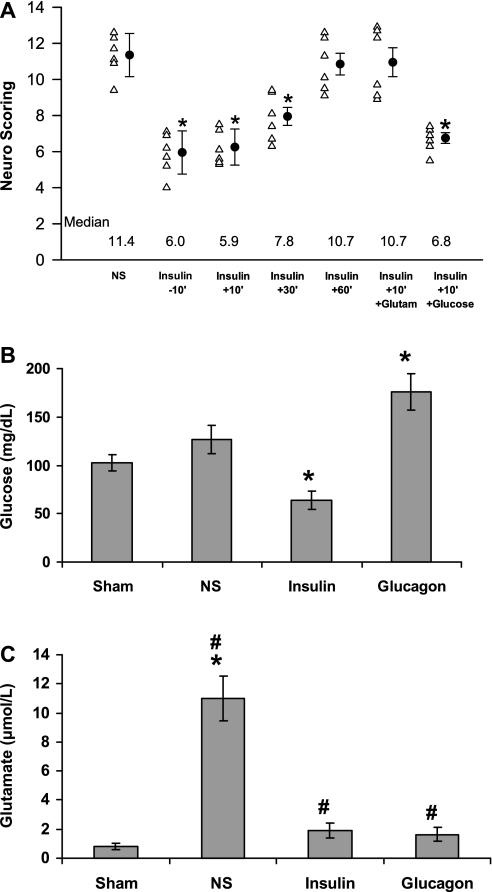
Fig. 2.
Time-dependent neuroprotective effect of insulin on postischemic neurological recovery. The experiments were performed as in Fig. 1. Twenty-four hours after middle cerebral artery occlusion (MCAO), the neurological scores were determined. A: scores for each rat besides the mean ± SE for each group. The median value for each group is presented at the bottom of the graph. B: asterisks above the bars denote a significant difference from controls receiving only NS. Blood and cerebrospinal fluid (CSF) samples taken 15 min after insulin injection were used to measure glucose (B) and glutamate (C) in the plasma and CSF, respectively. The mean ± SE is shown. C: significant difference from controls receiving only NS (#P > 0.004). Significant difference compared with sham operated animals (*P > 0.001).
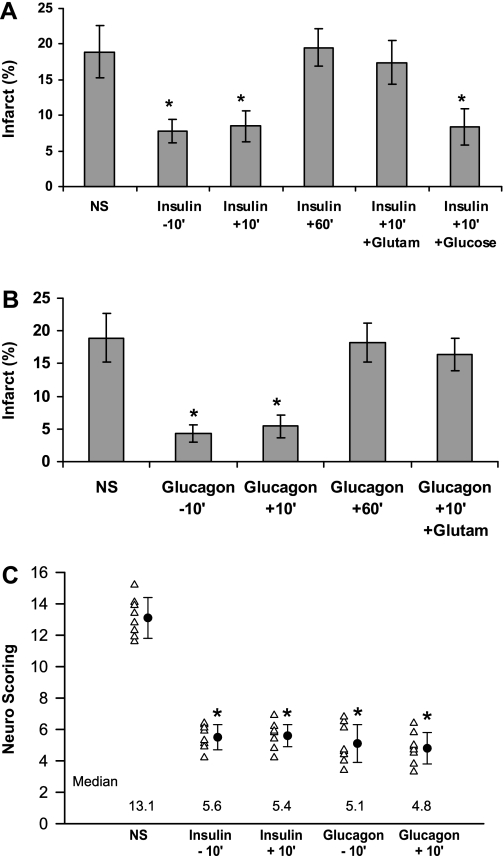
Fig. 4.
The neuroprotective effect of insulin and glucagon in diabetic rats. NS or NS-containing insulin (3 U/kg) (A) or glucagon (5 μg) (B) was given intraperitoneally to diabetic rats 10 min before (−10′) or 10 or 60 min after (+10′ or +60′) the occlusion of the MCA, as indicated. Twenty-four hours later, the brain infarcts were measured (A and B) after determining the neurological score (C). In some groups, 10 min after occlusion of the MCA, the administration of insulin or glucagon was followed by intravenous injection of glutamate (Glutam) or glucose (Glucose) (20%, 0.75 ml). The mean ± SE of data from 8–14 animals/group is shown. *Significant difference from controls receiving only NS: A, *P < 0.005; B, *P = 0.009.
RESULTS
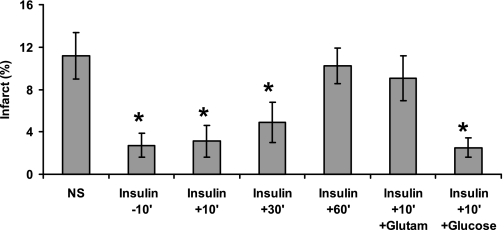
To examine the role of insulin in the development of poststroke brain damage, normal rats were treated with 2 IU/kg of insulin or normal saline (NS) at 10 min prior to the induction of transient middle cerebral artery occlusion (tMCAO). Injection of insulin decreased the infarct size by about 65% (P < 0.002, one-way ANOVA with Newman-Keuls post hoc test) (Fig. 1) and improved the neuroscoring (Fig. 2A) significantly (P > 0.0002, one-way ANOVA with Newman-Keuls post hoc test and by Kruskal-Wallis rank test). Fifteen minutes after insulin injection, the concentrations of glucose in the blood were 63 ± 9.3 mg/dl in animals injected with insulin compared with 117 ± 12.19 mg/dl in animals given saline alone (P = 0.001, Student's t-test) (Fig. 2B). The decrease of glucose levels was accompanied by decreased glutamate levels in the circulation and the CSF of the treated animals. Fifteen minutes after insulin injection, the concentrations of glutamate in the CSF were 1.89 ± 0.21 μmol/l in animals injected with insulin compared with 11 ± 1.6 μmol/l in animals given saline alone (P = 0.017, Student's t-test) (Fig. 2C); insulin also significantly decreased the plasma concentrations of glutamate from 201.3 ± 29 to 87.3 ± 19 μmol/l (P = 0.004, Student's t-test). To examine the therapeutic window of the insulin treatment, animals were treated at 10 min before or 10, 30, or 60 min after the induction of tMCAO. Figure 1 shows that the beneficial effect of insulin on brain damage, as reflected by decreased infarct size and improved neuroscoring (Fig. 2A), was observed in pretreated rats or rats treated up to 30 min after initiation of tMCAO; animals treated with insulin 60 min after the initiation of tMCAO did not differ significantly from controls (Figs. 1 and 2A). Neuroprotection afforded by lower doses of insulin (0.5–1.5 U/kg) induced dose-dependent neuroprotection that correlated with its effects on blood and CSF levels of glutamate (data not shown).
Fig. 1.
Time-dependent neuroprotective effect of insulin on postischemic brain damage The middle cerebral artery (MCA) of rats was occluded with an intraluminal filament. The thread was withdrawn 2 h later. Ten minutes before (−10′) or 10, 30, or 60 min after (+10′ to +60′) the occlusion of the MCA, normal saline (NS) or saline containing insulin (Insulin) (2 U/kg ip) was injected. In two of the groups, 10 min after occlusion of the MCA, the administration of insulin was followed by intravenous injection of glutamate (Glutam) or glucose (Glucose) (20% in 0.75 ml). The mean ± SE of data from 8–14 animals/group at each time point is shown. *Significant difference from controls receiving only NS (*P < 0.002).
We next examined the effect of the administration of glucose or glutamate together with insulin. Intravenous injection of glutamate (47) prevented the decrease of glutamate in the circulation by insulin [187.7 ± 37 μmol/l in rats given glutamate and insulin compared with 71 ± 14 μmol/l in animals given insulin alone (P = 0.001, Student's t-test)] and prevented its neuroprotective effect (Figs. 1 and 2A). We next examined the effect of preventing the reduction of glucose levels following administration of insulin. Injection of glucose (20% in 0.75 ml of water for injection) intraperitoneally immediately after insulin prevented the hypoglycemic effect of insulin; 15 min after injection of insulin, plasma glucose concentrations were 132 ± 27 mg/dl in rats given insulin and glucose compared with 59 ± 12 mg/dl (P = 0.005, Student's t-test) in animals given insulin alone. However, prevention of the decrease in glucose levels did not affect insulin-induced neuroprotection (Figs. 1 and 2A). To evaluate the potential long-term neuroprotection by insulin, we examined neurobehavioral functions at 1, 7, 14, and 28 days after tMCAO. Similar to the prolonged neuroprotective effect of glucagon (1), we found that tMCAO mice treated with insulin showed significantly improved neuroscoring at all time points studied, compared with the controls (data not shown).
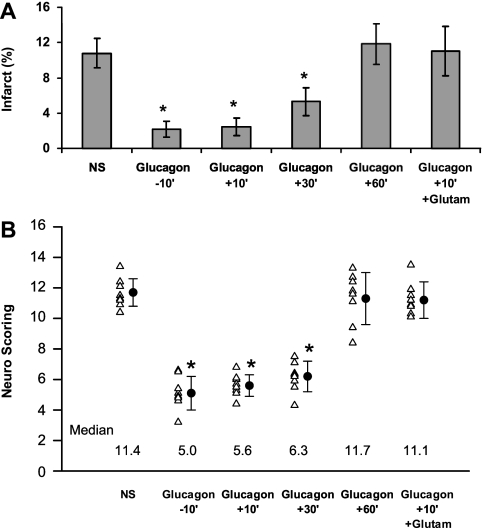
To further substantiate our findings on the irrelevance of glucose concentration to the outcome of brain injury, we injected glucagon instead of insulin. Glucagon is known to induce gluconeogenesis, leading to increased blood glucose levels and a decrease of glucogenic amino acid concentrations, such as glutamate (11, 34, 46). Injection of glucagon 10 min before or 10 min after induction of stroke decreased infarct size by ∼70% (P = 0.001, one-way ANOVA with Newman-Keuls post hoc test) (Fig. 3A) and led to an improvement in the neuroscore (P = 0.01) (Fig. 2B); injection of glucagon 1 h after induction of stroke had no protective effect (Fig. 3, A and B). The protective effect of glucagon was accompanied by a decrease in blood and CSF glutamate and increased glucose concentrations. Fifteen minutes after the injection of NS or glucagon (5 μg IP), blood glucose in animals treated with glucagon was 176 ± 6.4 compared with 121 ± 5.18 mg/dl (P < 0.001, Student's t-test) after NS. When the concentration of glutamate was maintained by giving intravenous glutamate along with glucagon (47) [glutamate concentrations were 173.7 ± 28 μmol/l in rats treated with glucagon and glutamate, compared with 65 ± 22 μmol/l (P = 0.03, Student's t-test) in animals treated with glucagon alone], and neuroprotection was abolished (Fig. 3, A and B).
Fig. 3.
Time-dependent neuroprotective effect of glucagon on postischemic neurological recovery. The experiments were performed as in Fig. 1. NS or NS-containing glucagon (Glucagon) (5 μg ip) was injected 10 min before (−10′) or 10, 30, or 60 min after (+10′ to +60′) the occlusion of the MCA. As in Fig. 1, one group received intraperitoneal glucagon followed by intravenous glutamate (Glutam). Twenty-four hours later, the brain infarct size was measured (A) after determining the neurological score (B). The individual data and the mean ± SE from 8–14 animals/group at each time point is shown. *Significant difference from controls receiving only NS: A: *P = 0.001; B: *P = 0.01.
In contrast to the widely accepted concept that diabetes (26) and hyperglycemia (12, 15, 36, 44) are risk factors for exacerbation of ischemic brain injury, our data suggest that neurotoxicity is mediated through increased glutamate concentrations, rather than by poststroke hyperglycemia. To directly examine this hypothesis in the setting of diabetes, we looked at the effect of insulin and glucagon on the outcome of stroke in animals with basal hyperglycemia. Intraperitoneal injection of saline containing 5 μg of glucagon or 3 U/kg of insulin 10 min before or after tMCAO improved the outcome of ischemic stroke in diabetic rats, compared with those given saline alone (Fig. 4, A–C). Both hormones provided comparable neuroprotection (Fig. 4, A–C) with similar therapeutic windows (Fig. 4, A and B). The improvement in neurological recovery did not correlate with glucose levels, which were affected by insulin and glucagon in opposite directions, i.e., injection of insulin decreased blood glucose in the diabetic rats from 186 ± 27.9 to 71 ± 7.41 mg/dl (P < 0.005, Student's t-test), whereas glucagon increased blood glucose from 174 ± 22.14 to 236 ± 25 mg/dl (P = 0.009, one-way ANOVA with Newman-Keuls post hoc test). As in the case of nondiabetic rats (Figs. 1–3), injection of glutamate abolished the neuroprotective effect of both hormones (Fig. 4, A and B), whereas elevated glucose concentrations of diabetic rats treated with insulin did not attenuate neuroprotection (Fig. 4A).
To further exclude the possibility that the effect of insulin and glucagon on glucose levels or any other hormone receptor mediates neuroprotection, we examined the effect of the glutamate scavenger oxaloacetate (47), which has also been shown to provide neuroprotection in a TBI model in nondiabetic animals. In this case, the action of glutamate-oxaloacetate transaminase in the circulation, transforms glutamate into 2-ketoglutarate in the presence of oxaloacetate (47). Oxaloacetate provided neuroprotection in a tMCAO model in diabetic rats and had the same therapeutic window as insulin and glucagon (Fig. 5; P > 0.004 one-way ANOVA with Newman-Keuls post hoc test), without affecting the blood levels of glucose (data not shown). As in nondiabetic animals (47), restoration of plasma glutamate levels abolished the neuroprotective effect of oxaloacetate (Fig. 5).
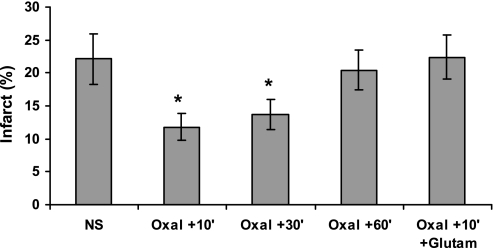
Fig. 5.
The neuroprotective effect of oxaloacetate in diabetic rats. NS or NS containing 1 mmol oxaloacetate/100 g rat weight (Oxal) was given intravenously to diabetic rats 10, 30, or 60 min after (+10′ to +60′) the occlusion of the MCA, as indicated. Twenty-four hours later, the brain infarct sizes were measured. As indicated, in some groups, 10 min after occlusion of the MCA, the administration of oxaloacetate was followed by intravenous injection of glutamate (Glutam). The mean ± SE of data from 7 to 12 animals/group is shown. *Significant difference from controls receiving only NS (P > 0.004).
DISCUSSION
The finding that insulin, glucagon, and oxaloacetate have a similar neuroprotective effect and share a therapeutic window that overlaps with the postischemia increase in brain glutamate, strongly suggests that the neuroprotective effect of the three agents is due to their capacity to reduce the concentration of this excitatory amino acid in the CNS, as reflected by the changes we measured in the CSF.
Our data are in line with those of others showing a close correlation between glutamate concentrations in the CSF and plasma (1, 16, 22, 47) and that decreasing plasma glutamate leads to decreased concentrations in the CSF (1, 22, 47), notwithstanding the large differences in the actual concentrations in the two compartments.
Furthermore, our results help to explain the observed dichotomy between the beneficial effects of insulin in various animal models, when given prior to induction of stroke (29, 45) and its controversial efficacy in humans when given after the insult (8, 23, 31, 35, 37). We posit that the difference is due to the early and brief increase in glutamate in the brain after stroke; the corollary of this concept is the very brief therapeutic window that has to be taken into consideration in any therapeutic approach.
The fact that glucagon given prior to brain injury is neuroprotective, suggests that gluconeogenesis may contribute to the preconditioning response to cerebral injury. The beneficial effect of glucagon or insulin given prior to traumatic brain injury suggests that they may also be useful before certain neurosurgical or cardiac interventions in which the incidence of perioperative stroke approaches 10% (39).
In any case, a better understanding of the correlation between CSF and plasma glutamate concentrations and the mechanism of the generation and transport of neurotoxic products is needed to optimize the timing, duration and intensity of the glutamate-reducing effect of either hormone.
Perspectives and Significance
These data reveal a previously undescribed mechanism for insulin and glucagon as neuroprotective agents in an experimental model of stroke. The lack of association between blood glucose and neuroprotection mitigates concerns about the deleterious effects of insulin-induced hypoglycemia, which for many years was the major reason for avoiding its administration in stroke patients with hyperglycemia (3, 28). Nevertheless, the efficacy and safety profile of glucagon stress its potential as part of the initial management of stroke.
GRANTS
This work was supported by Grants HL077760 and HL82545 from the National Institutes of Health and Grant 930/04 from the Israeli Science Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
A. A. Hijazi designed the research, R. Abu Fanne., T. Nassar, and N. Hijazi performed the research and collected the data. A. A. Higazi, R. Abu Fanne, T. Nassar, N. Hijazi, and S. N. Heyman analyzed the data. A. A. Higazi wrote the paper.
REFERENCES
- 1. Abu Fanne R, Nassar T, Mazuz A, Waked W, Heyman N, Goelman G, Higazi A. Neuroprotection by glucagon: Role of gluconeogenesis. J Neurosurg 114: 85–91, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Abu Fanne R, Nassar T, Yarovoi S, Rayan A, Lamensdorf I, Karakoveski M, Vadim P, Jammal M, Cines D, Higazi A. Blood-brain barrier permeability and tPA-mediated neurotoxicity. Neuropharmacology 58: 972–980, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams H, Del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF, American Heart Association, American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups Guidelines for the early management of adults with ischemic stroke. Stroke 38: 1655–1711, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, Higazi A. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci 9: 1150–1155, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 34: 2208–2214, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bederson J, Pitts L, Tsuji M, Nishimura M, Davis R, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17: 472–476, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Bendotti C, Tortarolo M, Suchak KS, Calvaresi N, Carvelli L, Bastone A, Rizzi M, Rattray MTM. Transgenic SOD1 G93A mice develop reduced GLT-1 in spinal cord without alterations in cerebrospinal fluid glutamate levels. J Neurochem 79: 737–746, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Bilotta F, Caramia R, Cernak I, Paoloni F, Doronzio A, Cuzzone V, Santoro A, Rosa G. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit Care 9: 159–166, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Blanca Fuentes B, Castillo J, San Jose B, Leira R, Serena J, Vivancos J, Dávalos A, Gil Nuñez A, Egido J, Díez-Tejedor E. The prognostic value of capillary glucose levels in acute stroke: the GLycemia in Acute Stroke (GLIAS) study. Stroke 40: 562–568, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Bômont L, MacKenzie E. Neuroprotection after focal cerebral ischaemia in hyperglycaemic and diabetic rats. Neurosci Lett 197: 53–56, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Brockman RP, Bergman EN, Joo Pk, Ganns JG. Effects of glucagon and insulin on net hepatic metabolism of glucose precursors in sheep. Am J Physiol 229: 1344–1350, 1975 [DOI] [PubMed] [Google Scholar]
- 12. Bruno A, Levine S, Frankel M, Brott T, Lin Y, Tilley B, Lyden P, Broderick J, Kwiatkowski T, Fineberg S. Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology 59: 669–674, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Bullock R, Zauner A, Woodward J, Young H. Massive persistent release of excitatory amino acids following human occlusive stroke. Stroke 26: 2187–2189, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Campos F, Sobrino T, Ramos-Cabrer P, Argibay B, Agulla J, Perez-Mato M, Rodryguez-Gonzalez R, Brea D, Castillo J. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J Cereb Blood Flow Metab 26: 1–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Capes S, Hunt D, Malmberg K, Pathak P, Gerstein H. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 34: 2426–2432, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Castillo J, Dávalos A, Noya M. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet 349: 79–83, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Chandra S, White R, Everding D, Feuerstein G, Coatney R, Sarkar S, Barone F. Use of diffusion-weighted MRI and neurological deficit scores to demonstrate beneficial effects of isradipine in a rat model of focal ischemia. Pharmacology 58: 292–299, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32: 2682–2688, 2001 [DOI] [PubMed] [Google Scholar]
- 19. de Courten-Myers G, Myers RE, Schoolfield L. Hyperglycemia enlarges infarct size in cerebrovascular occlusion in cats. Stroke 19: 623–630, 1988 [DOI] [PubMed] [Google Scholar]
- 20. Durham H, Truett G. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol 290: R652–R658, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Fuentes B, Ortega-Casarrubios M, San Jose B, Castillo J, Leira R, Serena J, Dávalos J, Gil-Nuñz A, Egido J, Díez-Tejedor E. Persistent hyperglycemia >155 mg/dl in acute ischemic stroke patients: How well are we correcting it? Implications for outcome. Stroke 41: 2362–2365, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Gottlieb M, Wang Y, Teichberg V. Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem 87: 119–126, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KM. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol 6: 397–406, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol 6: 397–406, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Haratz S, Tanne D. Diabetes, hyperglycemia and the management of cerebrovascular disease. Curr Opin Neurol 24: 81–88, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Kaarisalo M, Raiha I, Sivenius J, Immonen-Raiha P, Lehtonen A, Sarti C, Mahonen M, Torppa J, Tuomilehto J, Salomaa V. Diabetes worsens the outcome of acute ischemic stroke. Diabetes Res Clin Pract 69: 293–298, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kernan W, Inzucchi S. Type 2 diabetes mellitus and insulin resistance: Stroke prevention and management. Curr Treat Options Neurol 6: 443–450, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Lees KR, Walters MR. Acute stroke and diabetes. Cerebrovasc Dis 20 Suppl 1: 9–14, 2005 [DOI] [PubMed] [Google Scholar]
- 29. LeMay D, Gehua L, Zelenock G, D'Alecy L. Insulin administration protects neurologic function in cerebral ischemia in rats. Stroke 19: 1411–1419, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Luck JM, Morrison G, Wilbur LF. The effect of insulin on the amino acid continent of blood. JBC: 151–156, 1928 [Google Scholar]
- 31. McCormick M, Hadley D, McLean J, Macfarlane J, Condon B, Muir K. Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol 67: 570–578, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Nagy D, Marosi M, Kis Z, Farkas T, Rakos G, Vecsei L, Teichberg V, Toldi J. Oxaloacetate decreases the infarct size and attenuates the reduction in evoked responses after photothrombotic focal ischemia in the rat cortex. Cell Mol Neurobiol 29: 827–835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Natale JE, Stante SM, D'Alecy LG. Elevated brain lactate accumulation and increased neurologic deficit are associated with modest hyperglycemia in global brain ischemia. Resuscitation 19: 271–289, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Nelson D, Cox M. Gluconeogenesis. In: Lehninger: Principles of Biochemistry, 4th ed., New York: Freeman, chap. 14, 2005, pp. 543–549 [Google Scholar]
- 35. Oddo M, Schmidt J, Carrera E, Badjatia N, Connolly E, Presciutti M, Ostapkovich N, Levine J, Le Roux P, Mayer A. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: A microdialysis study. Crit Care Med 36: 3233–3238, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Parsons M, Barber P, Desmond P, Baird T, Darby D, Byrnes G, Tress B, Davis S. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol 58: 20–28, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Quinn T, Dawson J, Walters M. Sugar and stroke: Cerebrovascular disease and blood glucose control. Cardiovasc Ther In press [DOI] [PubMed] [Google Scholar]
- 38. Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes 54: 2314–2319, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Selim M. Perioperative stroke. N Engl J Med 356: 706–713, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Shafrir E. Animal Models of Diabetes, 2nd ed. CRC Press: London, 328 pp.. 2007 [Google Scholar]
- 41. Tai E, Tan M, Stevens R, Low Y, Muehlbauer M, Goh D, Ilkayeva R, Wenner B, Bain J, Lee J, Lim CS, Khoo C, Shah S, Newgard C. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53: 757–767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takagi K, Ginsberg MD, Globus MY, Dietrich WDEM, Kraydieh S, Busto R. Changes in amino acid neurotransmitters and cerebral blood flow in the ischemic penumbral region following middle cerebral artery occlusion in the rat: correlation with histopathology. J Cereb Blood Flow Metab 13: 575–585, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Tejima E, Katayama Y, Suzuki Y, Kano T EHL. Hemorrhagic transformation after fibrinolysis with tissue plasminogen activator: evaluation of role of hypertension with rat thromboembolic stroke model. Stroke 32: 1336–1340, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Toni D, De Michele M, Fiorelli M, Bastianello S, Camerlingo M, Sacchetti M, Argentino C, Fieschi C. Influence of hyperglycaemia on infarct size and clinical outcome of acute ischemic stroke patients with intracranial arterial occlusion. J Neurol Sci 123: 129–133, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Warner D, Gionet T, Todd M, McAllister A. Insulin-induced normoglycemia improves ischemic outcome in hyperglycemic rats. Stroke 23: 1775–1789, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Wasserman D, Spalding J, Brooks L, Colburn C, Goldstein R, Cherrington A. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol Endocrinol Metab 257: E108–E117, 1989 [DOI] [PubMed] [Google Scholar]
- 47. Zlotnik A, Gurevich B, Tkachov S, Maoz I, Shapira Y, Teichberg VI. Brain neuroprotection by scavenging blood glutamate. Exp Neurol 203: 213–220, 2007 [DOI] [PubMed] [Google Scholar]