Abstract
The neural control of feeding involves many neuromodulators, including the endogenous opioids that bind μ-opioid receptors (MORs). Injections of the MOR agonist, Damgo, into limbic and hypothalamic forebrain sites increase intake, particularly of palatable foods. Indeed, forebrain Damgo injections increase sucrose-elicited licking but reduce aversive responding (gaping) to quinine, suggesting that MOR activation may enhance taste palatability. A μ-opioid influence on taste reactivity has not been assessed in the brain stem. However, MORs are present in the first-order taste relay, the rostral nucleus of the solitary tract (rNST), and in the immediately subjacent reticular formation (RF), a region known to be essential for consummatory responses. Thus, to evaluate the consequences of rNST/dorsal RF Damgo in this region, we implanted rats with intraoral cannulas, electromyographic electrodes, and brain cannulas aimed at the ventral border of the rNST. Licking and gaping elicited with sucrose, water, and quinine were assessed before and after intramedullary Damgo and saline infusions. Damgo slowed the rate, increased the amplitude, and decreased the size of fluid-induced lick and gape bouts. In addition, the neutral stimulus water, which typically elicits licks, began to evoke gapes. Thus, the current results demonstrate that μ-opioid activation in the rNST/dorsal RF exerts complex effects on oromotor responding that contrast with forebrain effects and are more indicative of a suppressive, rather than a facilitatory effect on ingestion.
Keywords: licking, gaping, feeding, nucleus of the solitary tract
considerable evidence suggests that the endogenous opioid system modulates feeding and ingestive behavior. Until recently, the focus had been primarily on opioid effects at forebrain sites or after systemic injections. Systemic injections of morphine increase feeding, whereas the general opioid antagonist, naltrexone, attenuates intake (38, 39, 43). These effects are greatest when the animals are presented with a diet high in sugar or fat, suggesting that opioids can facilitate feeding by enhancing palatability. Experiments using the taste reactivity test support this conclusion. Morphine lengthens (11), and naltrexone shortens (52), the length of licking bouts elicited by sucrose, a measure positively correlated with stimulus palatability (15, 68), whereas systemic morphine reduces the number of aversive reactions (i.e., gapes) to the bitter stimulus, quinine (52).
Experiments conducted using central injections have begun to establish which brain sites underlie these effects. Similar to systemic administration, infusions of the μ-opioid receptor (MOR) agonist d-Ala2, N-Me-Phe4, and Gly-ol5-enkephalin (Damgo) into various hypothalamic and limbic regions, including the nucleus accumbens, increase food intake, and alter taste reactivity by elevating the number of licks to sucrose and reducing gaping to quinine (2, 3, 16, 17, 32, 53, 54, 61, 62, 72, 74, 75).
The effects of brain stem μ-opioids on feeding and ingestive behavior have not been as extensively characterized, but an important modulatory role is emerging. Damgo infused into the pontine parabrachial nucleus (73) facilitates intake of standard chow, whereas an irreversible MOR antagonist attenuates feeding (71). MOR modulation of ingestive behavior also seems likely at the level of the medulla; in particular, in the first gustatory relay, the rostral nucleus of the solitary tract (rNST), and the adjacent parvocellular and intermediate zones of the reticular formation (RF). The rNST and RF are anatomically connected and together exert a major influence on taste processing and oromotor integration (5, 6). Both the rNST and RF contain MORs (40, 47), and their endogenous ligands, the enkephalins and endomorphins (9, 28, 44, 48, 56). Indeed, experiments examining the consequences of manipulating MORs near the rNST/RF have demonstrated increases in chow intake (16, 33, 35, 36). However, facilitatory effects of Damgo were not observed until the 2nd h of testing, and thus, the immediate consequences of MOR manipulation in this region are still unclear. Moreover, the presence of intertwined gustatory and oromotor circuitry in this area of the brain begs the questions of whether there are taste stimulus-specific effects and whether consummatory movements themselves are affected by MOR manipulation. To address these issues, we made small infusions of Damgo targeted for the ventral rNST/dorsal RF and used electromyographic (EMG) recording paired with the taste reactivity paradigm to assess the immediate effects of MOR activation on sucrose and quinine-driven licking and gaping. Because previous Damgo injections in this vicinity had orexigenic effects (16, 33, 35, 36), we initially hypothesized that Damgo in the rNST/RF would have similar effects on taste reactivity as orexigenic forebrain infusions, i.e., that it would increase the size of lick bouts elicited by sucrose and decrease gaping elicited by quinine. Instead, the results presented below show that Damgo infusions into the rNST/RF have strikingly different consequences than those arising from forebrain or systemic injections. In fact, the immediate effects of Damgo are to alter the rate, bout size and amplitude of the oromotor behaviors in a fashion consistent with suppression rather than facilitation of consummatory behavior.
MATERIALS AND METHODS
Animals.
All procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Fourteen male Sprague-Dawley rats (250–400 g initial body wt) were used for this study. Animals were housed individually, given ad libitum access to rat chow and water, and maintained on a 12:12-h light-dark cycle. All behavioral testing took place during the light phase.
Surgical procedures.
Following overnight food deprivation, animals were deeply anesthetized with pentobarbital sodium (Nembutal; 50 mg/kg ip) and given supplements when necessary to maintain a state of areflexia. Body temperature was maintained at 37°C using a heating pad. After an incision was made overlying the skull, intraoral (IO) cannulas were implanted lateral to the first maxillary molar to allow for controlled delivery of tastants directly into the oral cavity (19). Subsequently, bipolar EMG electrodes (Teflon-coated stainless-steel 7-strand wire, A-M Systems) were inserted bilaterally into the anterior digastric (jaw opener) muscles, and the other ends were guided subcutaneously to the top of the head and attached to an Amphenol connector. The rat was placed in a stereotaxic device, two small holes were drilled in the parietal bone posterior to lambda, and the dura was removed to reveal the cerebellum directly above the left and right gustatory rNST. Standard extracellular recording techniques were used to guide the implant sites. The rostral pole of the rNST (initial coordinates 4.5 caudal and 1.8 mm lateral to lambda) was first identified on the basis of multiunit responses evoked by application of 0.1 M NaCl to the anterior tongue. Once identified, the electrode was moved 0.4 mm posterior and 0.3 mm medial to a site responsive to stroking of the circumvallate and/or foliate papillae, which is approximately the center of the gustatory NST. Subsequently, guide cannulas (20-mm length; 26-gauge stainless-steel tubing) were positioned either bilaterally above this location or at the same anteroposterior coordinates at the midline for control placements. To facilitate accurate positioning of the guide cannulas along the dorsal-ventral axis, an electrode/cannula assembly was constructed by inserting a thin, tungsten electrode through the cannula to extend 0.5–1.0 mm beyond the end of the guide cannula. Once the correct location was confirmed electrophysiologically, the guide cannulas, IO cannulas, and Amphenol strip connector were secured to the skull with dental acrylic, and then the tungsten electrodes were removed from the guide cannulas and a 33-gauge stainless-steel tube was used as a stylet. Following surgery, the incision was closed with wound clips, a topical antibiotic was applied, and injections of Ampicillin (135 mg/kg sc), and an anti-inflammatory/analgesic (carprofen, 5 mg/kg sc) administered. Ampicillin injections were continued for two additional days. Rats were provided with a diet of powdered rat chow and pure vegetable oil (Crisco) to encourage weight gain for the week following surgery.
Behavioral adaptation and stimulation.
Throughout recovery, the animals were adapted to the Plexiglas testing chamber for ∼1 h for a minimum of 3 days. During initial adaptation sessions, 50 μl of distilled water was infused 3 times through each IO cannula, and EMG activity was evaluated to ensure that the electrodes were securely placed in the anterior digastric muscles. On the last day of adaptation and on subsequent test days, rats received a minimum of nine blocks of IO taste stimulation. Each block consisted of 50-μl infusions of 0.5 M sucrose, water, and 0.001 M quinine in that order. This stimulus volume is identical to that used in the classic studies of Grill and Norgren (19, 20), as well as previous studies from our laboratory and produces reliable, distinct oromotor responses to different tastants (5, 6, 34, 66–68). We used these small-volume stimulations, in preference to another common paradigm, 1-min infusions (e.g., Ref. 21), to facilitate repeated testing before and after drug delivery. One minute following each stimulus, two consecutive water rinses were delivered. Each block lasted 5–6 min, and blocks were separated by a 5-min break. However, when testing was extended beyond 9 blocks to allow for recovery, blocks were then separated by 10 min. For each block, fluid-evoked oromotor movements were videotaped, and EMG activity from the AD was amplified (AM systems, model no. 1700), digitized, processed, and stored (Power 1401, Spike 2; Cambridge Electronics Design, Cambridge, UK).
Drug infusion.
All rats received two intramedullary infusions, Damgo and sterile saline, counterbalanced across animals, and separated by two rest days. Rats in the experimental group received bilateral infusions (target dose: 60 nl/30 pmol/side) of Damgo (Sigma, St. Louis, MO) and saline (0.9%) aimed for the ventral border of the rNST. These relatively low volumes and doses were chosen to optimize our ability to observe anatomically discrete but reliable effects. To limit drug diffusion, we used the smallest volume practical for making consistent infusions in awake, behaving animals, one slightly lower than that used in similar experiments by J. Travers and colleagues (5, 6, 67). The dose was similar to the lower range employed in earlier investigations of behavioral effects of intracranial Damgo infusions made in a variety of brain regions [50 pmol unilaterally in raphe magnus, pain-elicited behavior (51); 60 pmol unilaterally in rostral ventromedial medulla, pain-elicited behavior (63); 97 pmol, nucleus accumbens, sucrose-licking (64); 97 pmol, nucleus accumbens, taste reactivity (61)]. Interestingly, pilot studies suggested that just modestly higher doses (80–150 pmol/side) than the one that we ultimately chose could produce complete suppression of all oromotor behaviors, which hampered our ability to study the more nuanced effects reported below.
One rat in the anatomical control group received bilateral injections in the vestibular nucleus, and the remaining six received midline infusions at the same rostrocaudal and dorsoventral level as the NST/RF group. In some failed bilateral cases (not presented here), we observed that unilateral injections into the rNST/RF could cause similar effects as bilateral injections. Thus, for five out of six midline controls, we used a unilateral volume of Damgo (60 nl total). However, one midline control received a volume comparable to the bilateral total. Data from the midline control receiving the bilateral volume and another rat that received a bilateral volume but that was not included in the final analysis were indistinguishable from midline controls receiving unilateral volumes (see results). The vestibular control case was also comparable. Thus, these seven rats were combined into a single control group.
Intramedullary infusions always took place after the third block of testing. Therefore, the first three blocks served as baseline, and the remaining blocks were used to assess drug effects on taste-evoked consummatory responses. During drug infusions, the animals were gently held by a second researcher, and stylets were removed from the guide cannulas. Preloaded infusors filled with either saline or Damgo were used to make infusions into the brain. Infusors were composed of 33-gauge stainless-steel tubing (extending 0.5–1.0 mm beyond the guide cannulas) connected to polyethylene (PE)-10 tubing, which was attached to 10-μl glass syringes driven by a microinfusion pump. All saline and Damgo infusions were administered at a rate of 100 nl/min. Rather than relying solely on the pump settings, the actual amount delivered was estimated by measuring the movement of a small air bubble deliberately inserted into the fluid column in the PE tubing. Mean-estimated volumes for Damgo infusions into the rNST/RF were 65 nl/32.5 pmol unilaterally (130 ± 15.9 nl, bilateral), and mean bilateral saline volumes were 145 ± 34.6 nl. Volumes for the control injections of Damgo were 83 ± 10.1 nl/41.5 pmol and 84 ± 7 nl for saline. Following the injections, infusors were left in place for a minimum of 2 min; then they were removed, stylets were replaced, and behavioral testing resumed. Animals were tested for a minimum of 6 blocks following intracranial injections or until drug effects had at least partially recovered.
Histology.
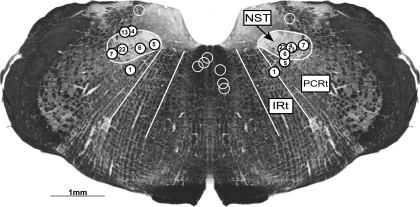
After the completion of testing, 2% Fluorogold was infused through each cannula to mark injection sites. Within 10 min after Fluorogold infusion, the animal was injected with a lethal dose of Nembutal (150 mg/kg) and perfused transcardially with 0.9% saline followed by 10% formalin. The brain was removed, and the brain stem was sectioned (52 μm) on a freezing microtome into two series and mounted on chromium potassium sulfate/gelatin-coated slides. One series was stained with cresyl violet, and the other was coverslipped with a water-based mountant to preserve fluorescence and to enhance the borders of brain structures viewed under dark-field microscopy. The centers of the injection sites were verified under a fluorescent microscope and were plotted on a summary diagram of coronal rNST sections (Fig. 1). Because the target for the infusions was the ventral border of rNST, Fluorogold typically extended into both the rNST and dorsal RF. The spread of Fluorogold was similar for several placements that were (unintentionally) implanted a few hundred micrometers more dorsally (in the rNST) or ventrally (in the dorsal RF). Therefore, bilateral placements in the rNST and/or RF were included together in the experimental group.
Fig. 1.
Representative coronal section illustrating the rostral nucleus of the solitary tract/reticular formation (rNST/RF) and control injection sites. Circles mark the ventral extent of each cannula placement. White, numbered circles represent the location of the seven bilateral rNST/RF placements, whereas open circles mark the cannula placements for the medial (n = 6) and dorsal (n = 1) controls. All animals in the experimental group had bilateral cannulas in either the rNST, RF, or both. Midline control injections were 700–1,300 μm medial to the rNST, whereas the vestibular control was 200 μm dorsal. The rNST and parvocellular (PCRt) and intermediate (IRt) zones of the RF are outlined in white and labeled.
Behavioral analysis.
Both the video and EMG records were used to analyze taste-elicited oromotor behaviors. First, video clips were evaluated frame by frame (20 frames/s), and the times of occurrence of gapes, grooming, and chin rubs were marked on the EMG records. Subsequently, raw EMG records were rectified, integrated (time constant = 20 ms), and then analyzed using custom software in CED Spike 2 (5, 6). The amplitude, rate, and number of discrete rhythmic anterior digastric bursts of activity (i.e., jaw openings) were calculated for the 1st bout of behavior; thus, this measure included both licking and gaping (but not grooming and chin rubbing). The end of the bout was defined as a pause in oromotor responding ≥0.5 s. These measures allowed a detailed assessment of the motor characteristics of the responses without the necessity of categorizing them. Subsequently a more specific analysis of responses representing ingestion (positive taste reactivity) and rejection (negative taste reactivity) was performed by consulting the marked video records in conjunction with the rate and amplitude measures afforded by the EMG; i.e., licks are associated with low-amplitude, 6–7 Hz jaw openings; gapes with larger-amplitude, slower (3–4 Hz) triangular jaw openings as previously described (19, 68). In these analyses, we focused on lick bout size (the number of licks), gape bout size (number of gapes), and a relative measure of these two behaviors (duration of gaping/total duration of first bout; G/T), to better assess any sensory-related effects. Licks are typically elicited by neutral (e.g., water) or palatable (e.g., sucrose) stimuli, gapes by unpalatable, bitter compounds, and in both cases, bout size is a positive function of stimulus concentration (15, 19, 68). For each rat and test session, EMG amplitude was normalized to the amplitude of the water-evoked response during the second baseline block. To simplify analyses, statistics were conducted on the basis of mean data calculated for three sets of blocks: those preceding drug delivery (blocks 1–3, “baseline”), those immediately after drug delivery (blocks 4–6, “postdrug 1”), and the last 3 blocks (blocks 7–9, “postdrug 2”).
Statistical analysis.
All statistical analyses were performed using Systat (versions 12 and 13). Statistical significance was defined as P ≤ 0.05. ANOVAs were first conducted for rate, amplitude, and number of jaw openings (bout size) without regard to whether they were licks or gapes. Subsequent analyses focused on the numbers of licks and gapes (lick bout size, gape bout size) and proportion of time spent gaping (G/T). Initial AVOVAs included location (rNST/RF vs. control) as a between-groups factor and stimulus (sucrose, water, quinine), block (1–3: baseline; 4–6 postdrug 1; 7–9: postdrug 2), and condition (Damgo vs. saline) as repeated-measures factors. When appropriate main effects and/or interactions were obtained, subsequent ANOVAs analyzed NST/RF and control groups separately, and then ANOVAs for individual stimuli were carried out. Finally, when block × drug interactions were obtained for individual stimuli, paired Student's t-tests compared saline and Damgo conditions for a given location, stimulus, and block. To simplify the text, only the significant drug or significant interactions of the drug with other variables are routinely presented.
RESULTS
Overview of placements.
Fourteen animals were included in the main analysis examining the effects of Damgo in the rNST/RF on fluid-evoked licking and gaping: seven with bilateral placements in the rNST/RF and seven with control injections either medial (n = 6; unilateral) or above (n = 1; bilateral) the rNST (Fig. 1). However, two rats in the rNST/RF group had no or unstable EMG electrodes and had to be excluded from the amplitude analyses. For all rNST/RF sites, the ventral tip of the infusor cannula was in the rNST, dorsal RF, or at the border of these two structures. Medial control locations were 1,012 ± 81 μm (range: 700–1300 μm) medial to the medial border of rNST. For the bilateral control placement, injections were made in the vestibular nuclei ∼200 μm dorsal to the rNST. All placements were at or rostral to the level where the rNST leaves the fourth ventricle, with the majority of placements in the center of the rNST (x̄ = 0.5 ± 0.06; with 0.0 defined as the caudal extent of the rNST and 1.0 the rostral pole of the rNST). They are collapsed on a single section representing the middle of the distribution in Fig. 1.
Characteristics of oromotor behaviors to three taste stimuli.
Under baseline conditions, intraoral sucrose evoked an ingestive sequence comprising midline tongue protrusions, lateral tongue protrusions, and small mouth movements. These behaviors were accompanied by rhythmic (6–7 Hz) bursts of EMG activity in the anterior digastric muscle that correlated with alternating patterns of jaw opening and closing and thus were indicative of licking. Infusion of the neutral stimulus, water, primarily evoked ingestive behavior as well. However, lick bout size was larger for sucrose than water (40 vs. 25 licks; paired Student's t-test, P < 0.01), consistent with previous work demonstrating a monotonic relationship between bout size and sucrose concentration (15, 19, 68). In addition to licking, quinine elicited rejection behaviors, including gapes, chin rubs, and paw flails, consistent with many previous reports of bitter-evoked oromotor responses (19).
Bilateral Damgo effects.
Bilateral Damgo infusions into the rNST/RF significantly altered fluid-evoked licking and gaping compared with saline injections. Midline or vestibular control injections of Damgo or saline had attenuated or no effects (Fig. 2–5). Overall, contrary to previous reports demonstrating that activating MORs in the NST facilitates ingestion (16, 35), our results primarily show that rNST/RF Damgo had a suppressive effect on taste-driven oromotor behaviors. ANOVAs for each measure revealed a significant interaction between block, drug, and location (rate: P = 0.027, normalized amplitude: P = 0.026, bout size: P = 0.003, lick bout size: P = 0.002; proportion of gapes: P = 0.01) or between block, drug, location, and stimulus (number of gapes: P = 0.006), supporting the contention that effects were spatially and pharmacologically specific.
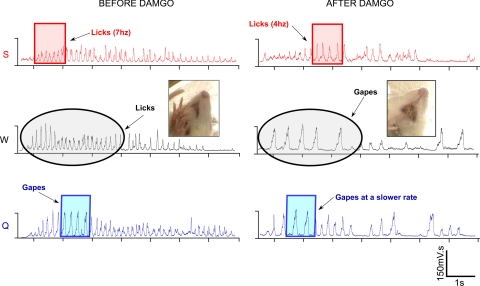
Fig. 2.
Integrated electromyographic records from an individual rat, illustrating oromotor responses to taste stimuli before and after Damgo. The three taste stimuli are color coded: sucrose = red, water = black, and quinine = blue. The colored boxes highlight 1 s of oromotor activity both before and after Damgo, demonstrating that the rate of responding was attenuated for all three stimuli. For example, before Damgo, rats licked to sucrose at a rate of ∼7 Hz (7 licks/s), whereas after Damgo, lick rate was reduced to 4 Hz. In addition to the Damgo-evoked rate change, a switch from licking to gaping in response to water also occurred. Black circles denote this switch. A single frame picture from the video records before and after Damgo corroborates the change from licking to gaping.
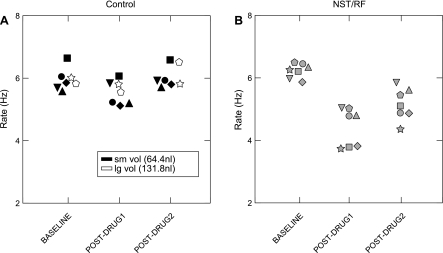
Fig. 5.
Scatterplots illustrating the effects of Damgo on the sucrose-elicited lick rate for individual midline control (A) and rNST/RF (B) animals. Differently shaped symbols represent different animals. A: data from five small volume (black symbols) vs. two larger volume (white) controls. Regardless of injection size, subjects in the control group had attenuated behavioral effects after Damgo compared with those in the rNST/RF group (B). The lack of a difference between the smaller and larger volume controls was consistent for all behavioral measures and taste stimuli.
Rate.
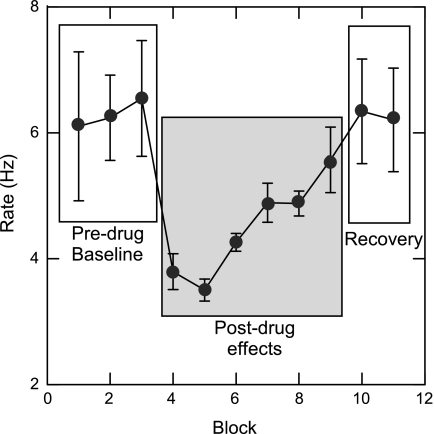
rNST/RF Damgo significantly slowed rhythmic oromotor movements (i.e., licking and gaping combined) (Figs. 2 and 3A). A repeated-measures ANOVA for the rNST/RF group yielded significant main effects for drug (P < 0.0005), and all interactions with drug were significant (P ≤ 0.006). During baseline blocks (1–3), sucrose-elicited jaw openings occurred at 6.2 ± 0.09 (Damgo days) − 6.4 ± 0.24 (saline days)/s. Immediately after Damgo (blocks 4–6, postdrug 1), the rate dropped to 4.4 ± 0.23/s, a 29% decrease, but after saline, it was similar to baseline (6.6 ± 0.26/s). An ANOVA for sucrose confirmed a drug × block interaction, (P < 0.01). Similar preinfusion and postinfusion rates were observed for water (ANOVA, water, drug × block: P < 0.001; Fig. 2A). Quinine normally elicits both licks and gapes, and this was reflected in a lower rate of ororhythmic movements in the baseline blocks (5.2 ± 0.15/s), which fell to 3.9 ± 0.2 just after Damgo infusion. The relative drop in rate (25%) was somewhat smaller than for sucrose or water, and an ANOVA yielded just a marginally insignificant interaction between block and drug (P = 0.073). Paired Student's t-tests confirmed that Damgo significantly slowed ororhythmic activity for sucrose and water, an effect that persisted to the second postdrug period for the 3-block averages (∼1 h). However, when individual trials were inspected, more recovery was evident by the last block. In addition, in some cases, we ran extra blocks to observe more complete recovery. Fig. 4 shows rate data (averaged across stimuli) for individual blocks for a rat with prolonged testing. In this animal, full recovery was evident by the end of the 10th block of testing (7 blocks post-Damgo).
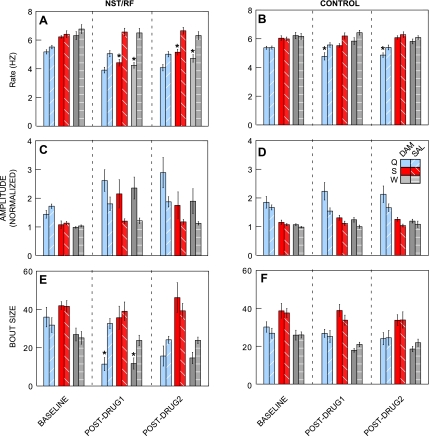
Fig. 3.
Bar graphs depicting effects on rate, amplitude, and bout size before and after Damgo or saline injections into the rNST/RF (n = 7) and control locations (n = 7). The three taste stimuli are color coded: quinine = blue; sucrose = red; water = gray. Results from the Damgo condition are represented with solid bars, whereas data from the saline condition are represented in the bars with hatch marks. Baseline refers to blocks 1–3; postdrug 1 to blocks 4–6, and postdrug 2 to blocks 7–9. *Significant difference between the Damgo and saline condition for a given taste stimulus. Damgo in the rNST/RF significantly reduced rate of responding to sucrose and water (A) and decreased bout size to water and quinine (E) compared with saline injections. Damgo at control locations had attenuated effects compared with rNST/RF sites; however, Damgo at control locations produced a smaller, but significant suppression of rate in response to quinine (B).
Fig. 4.
Line graph representing full recovery of a Damgo-evoked rate effect for an individual animal. The single line represents averaged lick and gape rate across all three taste stimuli-sucrose, water, and quinine. Before Damgo, the rate of responding for all stimuli averaged ∼6 Hz. After Damgo (block 4), rate dropped below 4 Hz. By block 10 (∼70 min postinfusion), the rate effect fully recovered to 6 Hz. Notice that before Damgo (predrug baseline) and the recovery phase, standard error bars are large, indicative of the normal rate variation associated with licks vs. gapes evoked by the different taste stimuli (sucrose and water: 6–7 Hz; quinine: 3–4 Hz). However, immediately after Damgo, the rate of responding to all three stimuli was similar at 3–4 Hz.
Damgo infused at control sites medial and dorsal to rNST likewise produced rate decrements, but these were greatly blunted compared with rNST/RF infusions (Fig. 3, A and B; ANOVA: drug, P = 0.016; drug × block, P = 0.002). Fig. 3B shows consistent nominal decreases for each stimulus, but there was a significant block × drug interaction only in the case of quinine (P = 0.001). Moreover, the decrement was much smaller than for the rNST/RF group. Before Damgo, quinine-elicited jaw openings occurred at 5.4/s and during the postdrug 1 period, the rate dropped to 4.8/s, just an 11% decrease. Indeed, infusions made into the midline had minor effects even with larger volumes of the drug. Rate effects were compared for midline control animals receiving unilateral (n = 5; x̄ = 64.4 nl) vs. bilateral (n = 2, x̄ = 131.8 nl) volumes, including an additional animal excluded from the main analysis because of missing saline data. Fig. 5 shows that the two rats receiving larger injections had similarly blunted rate effects as the small volume controls.
Normalized amplitude.
rNST/RF Damgo markedly increased the amplitude of fluid-induced mouth movements (Fig. 3C; ANOVA: drug × block, P = 0.032). In response to sucrose, water, and quinine, Damgo increased anterior digastric EMG amplitudes by 100%, 140%, and 86% in the postdrug 1 period, compared with baseline blocks. Subsequent ANOVAs revealed significant or nearly significant drug × block interactions for each stimulus: sucrose (P = 0.051), water (P = 0.033), and quinine (P = 0.049), but post hoc Student's t-tests failed to confirm differences between saline and Damgo for either postdrug period, perhaps because of the smaller sample for the amplitude data (n = 5 vs. 7 for the other measures). There was also an increased amplitude after Damgo infusions at control sites (Fig. 3D; ANOVA, drug × block interaction, P = 0.042). However, relative to baseline, increments were much less pronounced, ranging from 8% for sucrose to 18% for quinine in the postdrug 1 period.
Burst number/bout size.
In addition to slowing the rate and increasing the amplitude, rNST/RF Damgo infusions decreased the numbers of fluid-elicited bursts of anterior digastric activity occurring during the first bout of ororhythmic activity. This effect was stimulus specific, occurring only for quinine and water (Fig. 3E; ANOVA, block × drug, P = 0.003; stimulus × drug, P = 0.022; drug × block × stimulus, P = 0.053). Individual ANOVAs confirmed significant interactions between drug and block for quinine (P = 0.003) and water (P = 0.027) but not sucrose (P = 0.291). ANOVAs for the control group yielded neither an effect of drug nor any interaction of drug with the other variables (P > 0.1; Fig. 3F).
Licks and gapes.
Differences in the rate and amplitude of ororhythmic movements are major characteristics that differentiate licks from gapes, distinctive oral movements signifying ingestion vs. rejection. Thus, the slower and larger mouth movements that occurred after Damgo infusion could suggest an overall switch from ingestion to active rejection. However, in addition to rate and amplitude, gapes are characterized by a unique triangularly shaped mouth opening (19) and a different synergy between the tongue and jaws (68). Thus, using the additional information from the video records, we explicitly analyzed the numbers of licks and gapes and the proportion of time spent gaping (Fig. 6).
Fig. 6.
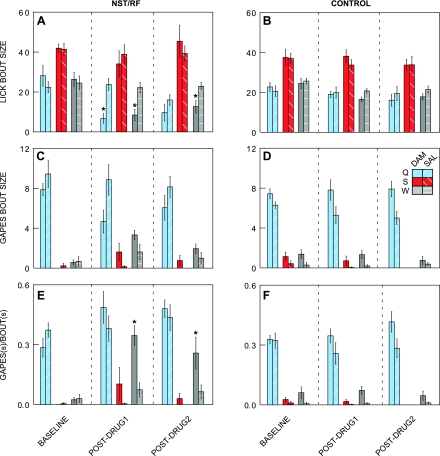
Bar graphs depicting lick bout size, gape bout size, and the proportion of time spent gaping before and after Damgo or saline infusions into the rNST/RF or control sites. The three taste stimuli are color coded: quinine = blue; sucrose = red; and water = gray. Results from the Damgo condition are represented with solid bars, whereas data from the saline condition are represented by hatch marks. *Significant differences between the Damgo and saline condition in response to a particular taste stimulus. Damgo in the rNST/RF significantly reduced the size of lick bouts to quinine and water compared with saline (A). Additionally, Damgo in the rNST/RF increased the proportion of time spent gaping relative to the total bout duration in response to water (E). No significant differences emerged between the Damgo and saline injections for control sites medial to or above the rNST (B, D, F).
Figure 6A depicts effects of activating rNST/RF MORs on licks. Damgo had a stimulus-specific effect, decreasing lick bout size in response to quinine and water but not sucrose (ANOVA: drug × block, P = 0.001; drug × stimulus, P = 0.01). Subsequent ANOVAs showed significant block × drug interactions for water (P = 0.004) and quinine (P = 0.004) but not sucrose (P = 0.671). Post hoc Student's t-tests verified significant decrements in the postdrug 1 period for water and quinine that extended to the postdrug 2 period for water. Damgo injections at control locations (Fig. 6B) had no effects on licks (ANOVA: drug and interaction of drug with other variables, P > 0.4).
Figure 6C illustrates the influence of rNST/RF Damgo infusions on gapes. Damgo tended to decrease gapes elicited by quinine but to increase the few gapes normally elicited by sucrose and water (ANOVA: drug × stimulus, P = 0.016; drug × stimulus × block, P = 0.019). ANOVAs for quinine and water, however, failed to show significant drug × block interactions. Moreover, there was a significant block × drug interaction (P = 0.046) for sucrose, but t-tests for individual blocks failed to confirm significant differences between saline and Damgo for sucrose during either postdrug period (P > 0.05). Figure 6D shows comparable data for gape bout size following Damgo injections into control locations. Inexplicably, there was a tendency for more gapes on the Damgo than the saline day, but this tendency was evident prior to intracranial infusions (ANOVA: drug, P = 0.017; drug × other variables, P > 0.05).
In summary, effects of rNST/RF Damgo on lick bout size were robust, but effects on gaping were less clear. However, because Damgo appeared to influence these behaviors in opposite, stimulus-dependent directions, we attempted to clarify the effects by combining the behaviors in a measure that reflected the proportion of time spent gaping relative to licking (G/T). With this measure, Damgo had clear effects (ANOVA: drug, P = 0.031, drug × block, P = 0.002; drug × stimulus, P = 0.01), significantly increasing the proportion of time the rat engaged in gaping in response to water. This change from licking to gaping was dramatic when viewing raw video and EMG records (Fig. 2). Before Damgo, the proportion was just 0.02, but following Damgo, it increased more than ∼10-fold, rivaling quinine during baseline blocks (Fig. 6E; ANOVA for water: drug, P < 0.0005; drug × block, P < 0.001). Post hoc Student's t-tests confirmed that the proportion of time spent gaping was higher for Damgo than saline during both the postdrug 1 and postdrug 2 periods. Similar trends were evident for quinine and sucrose but were not statistically significant (P > 0.1). Damgo infused into control sites did not enhance the proportion of time spent gaping (Fig. 6F; ANOVA: drug × block × stimulus, P = 0.05 but no drug × block interactions for any stimulus, P > 0.1).
DISCUSSION
Small, bilateral infusions of Damgo (x̄ = 130 nl total) into the medullary rNST/RF significantly altered taste-evoked licking and gaping compared with injections into the midline or saline injections. Damgo slowed the rate and increased the amplitude of oromotor behaviors. In addition, the sizes of water- and quinine-elicited lick bouts were significantly reduced, and rats began gaping in response to water. This outcome appears to be a complex combination of sensory and motor effects, most likely resulting from μ-opioid modulation of gustatory and oral somatosensory neurons in the rNST and the oromotor circuitry in the immediately subjacent RF. These effects are distinct from the well-studied consequences of Damgo injections into forebrain structures. Not surprisingly, injections of Damgo into the limbic forebrain have not been associated with obvious motor effects. Instead, Damgo infusions into these structures, in particular, into the nucleus accumbens, cause well-documented increases in the numbers of sucrose-elicited licks and decreases in quinine-evoked gapes, indicative of an increase in stimulus palatablility (53, 61, 62). Although we also observed changes in water and quinine-elicited lick bout size after rNST/RF infusions, these effects consisted of decreases, more consistent with a decline in palatablility. Moreover, there was no change in sucrose-elicited lick bout size. Thus, unlike accumbens injections, rNST Damgo infusions do not modulate the sensory signal in a way consistent with an increase in stimulus palatability. Indeed, overall, our results suggest that infusing the MOR agonist Damgo into the rNST/RF suppresses, rather than facilitates, consummatory responses. Furthermore, the Damgo-induced increase in gaping to water suggests that MORs may play an important role in the switch from licking to gaping. To our knowledge, this is the first study that has examined the consequences of manipulating MORs in the rNST/RF on taste-driven licking and gaping, and the first area of the brain where μ-opioid modulation has attenuated ingestive behaviors.
Suppression vs. facilitation of ingestion.
Our results demonstrating that Damgo in the rNST/RF suppresses ingestion contrast with previous work showing that systemic and forebrain injections of MOR ligands facilitate positive taste reactivity responses and intake (2, 3, 32, 37, 43, 53, 74, 75). They also seem at odds with the facilitatory effects on feeding when Damgo is injected into the parabrachial nucleus (73). In fact, a series of experiments examining the consequences of manipulating MORs in the vicinity of the rNST likewise suggest that μ-opioids in the medulla can increase food intake (16, 35, 36). However, although these previous studies injected Damgo into a similar location as the current study, the volumes and doses were several-fold greater—the threshold dose for eliciting a feeding effect was 2 nmol (unilaterally), a dose about 30× as large as the current bilateral dose (16, 35) and was injected in a volume of 1 μl, a volume expected to occupy a sphere with a radius over twice as large as our 65-nl infusions. Considered together with the observation that orexigenic effects were not observed until the second hour after injection, it seems possible that they arose from more distant brain stem structures that also contain MORs, perhaps the caudal, visceral NST. Indeed, a more recent paper by the same group investigated the consequences of blocking MORs using smaller (300 nl) injections of naltrexone into different NST regions (36) and found suppressive effects on intake only at more caudal NST regions, specifically at the level where the nucleus approaches the 4th ventricle. Interestingly, in vitro patch-clamp experiments have shown that Damgo suppresses excitation produced by CCK in the caudal NST, suggesting that this drug can act to reduce satiety signaling (1). Thus, a reasonable hypothesis is that our experiments unveiled the immediate consequences of manipulating MORs in the more rostral rNST and immediately subjacent RF, whereas the delayed orexigenic effects observed earlier (16, 35, 36) were the result of drug diffusion a few hundred micrometers caudally. Additional experiments are needed to specifically address this hypothesis.
Anatomical substrate of effects.
μ-Opiate receptors are distributed in the both the NST (7, 40, 42, 47) and subjacent RF (42, 46), and the behavioral effects observed in our experiment are likely the result of activating receptors in either site, or more likely both sites. Control injections above the rNST in the vestibular nuclei or medial in gigantocellularis nucleus of the RF, however, produced minimal effects on licking and gaping. The lack of a gigantocellularis opioid influence is similar to the lack of an effect of GABAA agonists on oromotor behavior when infused into this region compared with their profound effects in the more lateral RF (6). In the present study, injections for the experimental group were originally targeted for the ventral border of the rNST, but the final location of some placements was farther dorsally in the rNST, and a few were actually in the dorsal RF. In any event, Fluorogold infusions suggested that there was some diffusion into both the rNST and most dorsal RF regardless of the exact placement of the cannula. Moreover, we could not discern any consistent differences in Damgo effects that correlated with the relatively subtle differences in cannula placement. Nevertheless, it is important to note that, in addition to their close proximity, the rNST and RF are anatomically connected. The rNST projects to the underlying RF (10, 23), and these regions interact to produce and maintain rhythmic oromotor behaviors in response to taste stimuli (5, 6). Thus, although the present injections were not confined within classical neuroanatomical boundaries, they represent effects on a functionally unified substrate (69). Future experiments that systematically target the rNST vs. the dorsal RF with smaller doses of Damgo or more precise molecular techniques will be required to disentangle the exact origin of the various effects that we observed.
μ-Opioid mechanisms.
At a cellular level, μ opioids are inhibitory. They exert their inhibitory effects by blocking calcium currents and activating potassium (K+) conductances (58, 59) and can either act postsynaptically to directly inhibit neurons or presynaptically to suppress incoming signals (27, 40, 42, 47, 55, 57). Indeed, in vivo neurophysiological experiments have shown that met-enkephalin, a mixed δ/μ agonist, exerts an inhibitory influence on spontaneous activity and gustatory responses in a subset (∼25%) of rNST taste neurons (40). Furthermore, studies utilizing in vitro slice preparations showed that both μ- and δ-opioid receptor ligands reduce excitatory currents in rNST neurons that are elicited by solitary tract stimulation (4, 76). Such effects could underlie some of the suppressive effects of Damgo on oromotor behaviors, especially the decreases in lick bout size. Interestingly, bout size was affected for quinine and water, but not sucrose, suggesting that Damgo may differentially modulate different types of rNST neurons. Speculatively, it is possible that rNST neurons, robustly responsive to sucrose, are less likely to express MORs or to receive input from afferent fibers with these receptors.
Likewise, it seems plausible that the robust slowing of ororhythmic behaviors after Damgo results from inhibition of RF neurons or their inputs. Pilot work in our laboratory has demonstrated suppressive effects of Damgo on reticular neurons just subjacent to the rNST that are excited by activating muscle spindles in the jaw-closing muscles. Moreover, a similar suppressive effect on rhythmogenesis has been well characterized for medullary respiratory neurons in other reticular regions (13, 18, 22, 29, 45, 50). In vitro studies have demonstrated that microinjecting Damgo into the pre-Botzinger complex significantly slows respiratory frequency assessed by recording from hypoglossal rootlets (13, 18, 29). Additionally, in vivo experiments in several mammalian species have revealed that intravenous infusions of μ opioids can suppress the respiratory rate in intact or decerebrate animals (22, 45, 49), although rate increases or decreases can occur with localized infusions into different brain stem sites (25, 41, 49). Together with the current study, these results suggest that μ opioids act in the brain stem to modulate the frequency of multiple types of rhythmic behaviors.
Besides inhibiting neurons, opioids can work via disinhibition to indirectly excite neurons by suppressing their inhibitory inputs. Disinhibition by opioids has been well characterized in functional studies throughout the central nervous system. For example, as part of the descending pain pathway, opioids evoke their analgesic effects, in part, by inhibiting GABAergic neurons in the rostral ventral medial medulla and periaqueductal gray (8, 14, 24). In addition to playing a prominent role in the pain pathway, disinhibition by μ-opioid ligands has been demonstrated in other regions, including the caudal NST. Herman et al. (26) demonstrated that μ opioids inhibit gastric motility via suppression of GABAergic activity, allowing vagal afferent terminals to release glutamate and excite rNST second-order neurons. Anatomical studies also reveal interactions between GABA and opioids. Throughout the brain, including several brain stem regions, GABA and MORs are colocalized in the same neurons (12, 30, 31). It seems likely that MOR ligands could modulate GABA release in the rNST/RF as well. GABA neurons and enkephalin/endomorphin fibers are densely distributed in the rNST and RF and have overlapping distributions (44, 48, 56, 65, 70). Moreover, a recent electron microscopic study suggested that some enkephalin terminals may actually synapse onto GABAergic neurons in the rNST (28).
Although lacking a direct demonstration, several lines of evidence suggest that μ-opioid ligands could work through disinhibition in the rNST/RF to produce some of the effects observed here. Both the rNST and RF are under tonic GABAergic inhibition (6, 60) and antagonizing GABAA transmission in the RF with bicuculline causes the small-amplitude, sucrose-elicited licking movements to change to the wider, slower movements characteristic of gaping. Interestingly, in the current study, a similar switch was observed for water following Damgo infusions. Taken together, these results imply that μ opioids in the rNST/RF may suppress GABAergic transmission and that one mechanism underlying the change from ingestion to rejection may be disinhibition.
Conclusions and perspectives.
The present study is the first to demonstrate that rNST/RF μ opioids can suppress ingestive behavior. These effects are opposite to the facilitatory effects reported after Damgo injection into various forebrain and other brain stem sites, suggesting μ opioids can differentially modulate ingestion depending on their central site of action. The complex effects reported here are likely the result of modulating MORs in the rNST and RF. However, the source(s) of the endogenous ligand for rNST/RF MORs is unclear. Cell bodies immunoreactive for enkephalin and endomorphin are present in the NST (9, 44, 48, 56), suggesting that one of the major inputs to rNST/RF MORs may be from NST interneurons. Additionally, previous studies have provided evidence for a multisynaptic opioid connection between the rNST and limbic and homeostatic feeding centers, such as the central nucleus of the amygdala and hypothalamic nuclei (16, 36). Thus, it is possible that activation of forebrain regions trigger the release of μ opioids in the rNST/RF during altered homeostatic states or after gustatory-visceral learning.
GRANTS
This research was supported by National Institutes of Health Grant RO1 DC00416 (to S. P. Travers) and T32 DE-0014320 (to N. R. Kinzeler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Special thanks are due to Dr. Joe Travers, Dr. Laurie Geran, and Alison Boxwell for helpful comments when reviewing this manuscript. We also thank Ken Herman and Joshua Lamb for technical assistance.
REFERENCES
- 1. Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther 265: 1253–1260, 1993 [PubMed] [Google Scholar]
- 3. Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides 25: 697–725, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Boxwell AY, Y, Travers SP, Travers JB. Activation of mu opiate receptors presynaptically suppresses afferent input to rNST neurons. 2011, http://www.achems.org/files/2011%20Meeting%20Files/FINALABSTRACTS.pdf
- 5. Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am J Physiol Regul Integr Comp Physiol 285: R68–R83, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen Z, Travers SP, Travers JB. Muscimol infusions in the brain stem reticular formation reversibly block ingestion in the awake rat. Am J Physiol Regul Integr Comp Physiol 280: R1085–R1094, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Cheng PY, Liu-Chen LY, Chen C, Pickel VM. Immunolabeling of Mu opioid receptors in the rat nucleus of the solitary tract: extrasynaptic plasmalemmal localization and association with Leu5-enkephalin. J Comp Neurol 371: 522–536, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Chieng B, Christie MJ. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol 113: 303–309, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis BJ, Kream RM. Distribution of tachykinin- and opioid-expressing neurons in the hamster solitary nucleus: an immuno- and in situ hybridization histochemical study. Brain Res 616: 6–16, 1993 [DOI] [PubMed] [Google Scholar]
- 10. DiNardo LA, Travers JB. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. J Neurosci 17: 3826–3839, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav 46: 745–749, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res 849: 203–215, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 14: 219–245, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Flynn FW, Grill HJ. Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci 102: 934–941, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Giraudo SQ, Kotz CM, Billington CJ, Levine AS. Association between the amygdala and nucleus of the solitary tract in mu-opioid induced feeding in the rat. Brain Res 802: 184–188, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides 33: 360–368, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286: 1566–1568, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143: 263–279, 1978 [DOI] [PubMed] [Google Scholar]
- 20. Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res 143: 281–297, 1978 [DOI] [PubMed] [Google Scholar]
- 21. Grill HJ, Schwartz GJ, Travers JB. The contribution of gustatory nerve input to oral motor behavior and intake-based preference. I. Effects of chorda tympani or glossopharyngeal nerve section in the rat. Brain Res 573: 95–104, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Haji A, Takeda R, Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther 86: 277–304, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 72: 185–197, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience 63: 279–288, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Hellman KM, Mendelson SJ, Mendez-Duarte MA, Russell JL, Mason P. Opioid microinjection into raphe magnus modulates cardiorespiratory function in mice and rats. Am J Physiol Regul Integr Comp Physiol 297: R1400–R1408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herman MA, Alayan A, Sahibzada N, Bayer B, Verbalis J, Dretchen KL, Gillis RA. micro-Opioid receptor stimulation in the medial subnucleus of the tractus solitarius inhibits gastric tone and motility by reducing local GABA activity. Am J Physiol Gastrointest Liver Physiol 299: G494–G506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang J, Wang H, Pickel VM. Rostrocaudal variation in targeting of N-methyl-d-aspartate and mu-opioid receptors in the rat medial nucleus of the solitary tract. J Comp Neurol 421: 400–411, 2000 [PubMed] [Google Scholar]
- 28. Huang W, Chen JX, Li YM, Lu YC, Wu XJ. Structures and connections of enkephalin- and gamma-aminobutyric acid-immunoreactive profiles in the gustatory region of the nucleus tractus solitarius: a light and electron microscopic study. Neurol Sci 32: 53–58, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J Appl Physiol 80: 2120–2133, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Kalyuzhny AE, Dooyema J, Wessendorf MW. Opioid- and GABA(A)-receptors are co-expressed by neurons in rat brain. Neuroreport 11: 2625–2628, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Kalyuzhny AE, Wessendorf MW. Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol 392: 528–547, 1998 [PubMed] [Google Scholar]
- 32. Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76: 365–377, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Kim EM, Quinn JG, Spanswick D, O'Hare E. Feeding association between the nucleus of the solitary tract and the ventral tegmental area. Appetite 53: 457–460, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Kinzeler NR, Travers SP. Licking and gaping elicited by microstimulation of the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R436–R448, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol Regul Integr Comp Physiol 272: R1028–R1032, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Kotz CM, Glass MJ, Levine AS, Billington CJ. Regional effect of naltrexone in the nucleus of the solitary tract in blockade of NPY-induced feeding. Am J Physiol Regul Integr Comp Physiol 278: R499–R503, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Levine AS, Atkinson RL. Opioids in the regulation of food intake and energy expenditure. Fed Proc 46: 159–162, 1987 [PubMed] [Google Scholar]
- 38. Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav 82: 57–61, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol Regul Integr Comp Physiol 268: R248–R252, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Li CS, Davis BJ, Smith DV. Opioid modulation of taste responses in the nucleus of the solitary tract. Brain Res 965: 21–34, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respir Physiol Neurobiol 138: 165–178, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350: 412–438, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Marks-Kaufman R. Increased fat consumption induced by morphine administration in rats. Pharmacol Biochem Behav 16: 949–955, 1982 [DOI] [PubMed] [Google Scholar]
- 44. Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405: 450–471, 1999 [PubMed] [Google Scholar]
- 45. Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37: 821–826, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Monteillet-Agius G, Fein J, Anton B, Evans CJ. ORL-1 and mu opioid receptor antisera label different fibers in areas involved in pain processing. J Comp Neurol 399: 373–383, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Moriwaki A, Wang JB, Svingos A, van Bockstaele E, Cheng P, Pickel V, Uhl GR. mu Opiate receptor immunoreactivity in rat central nervous system. Neurochem Res 21: 1315–1331, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Murakami S, Okamura H, Pelletier G, Ibata Y. Differential colocalization of neuropeptide Y- and methionine-enkephalin-Arg6-Gly7-Leu8-like immunoreactivity in catecholaminergic neurons in the rat brain stem. J Comp Neurol 281: 532–544, 1989 [DOI] [PubMed] [Google Scholar]
- 49. Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Botzinger complex region. J Neurophysiol 103: 409–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mutolo D, Bongianni F, Einum J, Dubuc R, Pantaleo T. Opioid-induced depression in the lamprey respiratory network. Neuroscience 150: 720–729, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci 26: 1190–1198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci 106: 999–1010, 1992 [DOI] [PubMed] [Google Scholar]
- 53. Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist 12: 500–511, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Pickel VM, Colago EE. Presence of mu-opioid receptors in targets of efferent projections from the central nucleus of the amygdala to the nucleus of the solitary tract. Synapse 33: 141–152, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Pierce TL, Wessendorf MW. Immunocytochemical mapping of endomorphin-2-immunoreactivity in rat brain. J Chem Neuroanat 18: 181–207, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Poole SL, Deuchars J, Lewis DI, Deuchars SA. Subdivision-specific responses of neurons in the nucleus of the tractus solitarius to activation of mu-opioid receptors in the rat. J Neurophysiol 98: 3060–3071, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Rhim H, Miller RJ. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J Neurosci 14: 7608–7615, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sanders RD, Brian D, Maze M. G-protein-coupled receptors. Handb Exp Pharmacol 93–117, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Smith DV, Li CS. Tonic GABAergic inhibition of taste-responsive neurons in the nucleus of the solitary tract. Chem Senses 23: 159–169, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27: 1594–1605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci 25: 8637–8649, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sykes KT, White SR, Hurley RW, Mizoguchi H, Tseng LF, Hammond DL. Mechanisms responsible for the enhanced antinociceptive effects of micro-opioid receptor agonists in the rostral ventromedial medulla of male rats with persistent inflammatory pain. J Pharmacol Exp Ther 322: 813–821, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience 161: 718–733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467: 60–79, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: an electromyographic analysis in the rat. Behav Brain Res 25: 233–246, 1987 [DOI] [PubMed] [Google Scholar]
- 67. Travers JB, Herman K, Travers SP. Suppression of third ventricular NPY-elicited feeding following medullary reticular formation infusions of muscimol. Behav Neurosci 124: 225–233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci 100: 544–555, 1986 [DOI] [PubMed] [Google Scholar]
- 69. Travers JB, Travers SP. Microcircuitry of the rostral nucleus of the solitary tract. In: Handbook of Brain Microcircuits, p. 284–289, 2010 [Google Scholar]
- 70. Wang M, Bradley RM. Properties of GABAergic neurons in the rostral solitary tract nucleus in mice. J Neurophysiol 103: 3205–3218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ward HG, Simansky KJ. Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 187: 435–446, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23: 2882–2888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 285: R1055–R1065, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285: 908–914, 1998 [PubMed] [Google Scholar]
- 75. Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 132: 350–360, 1997 [DOI] [PubMed] [Google Scholar]
- 76. Zhu M, Cho YK, Li CS. Activation of delta-opioid receptors reduces excitatory input to putative gustatory cells within the nucleus of the solitary tract. J Neurophysiol 101: 258–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]