Abstract
Reduced mitochondrial oxidative phosphorylation, via activation of adenylate kinase and the resulting exponential rise in the cellular AMP/ATP ratio, appears to be a critical factor underlying O2 sensing in many chemoreceptive tissues in mammals. The elevated AMP/ATP ratio, in turn, activates key enzymes that are involved in physiologic adjustments that tend to balance ATP supply and demand. An example is the conversion of AMP to adenosine via 5′-nucleotidase and the resulting activation of adenosine A2A receptors, which are involved in acute oxygen sensing by both carotid bodies and the brain. In fetal sheep, A2A receptors associated with carotid bodies trigger hypoxic cardiovascular chemoreflexes, while central A2A receptors mediate hypoxic inhibition of breathing and rapid eye movements. A2A receptors are also involved in hypoxic regulation of fetal endocrine systems, metabolism, and vascular tone. In developing lambs, A2A receptors play virtually no role in O2 sensing by the carotid bodies, but brain A2A receptors remain critically involved in the roll-off ventilatory response to hypoxia. In adult mammals, A2A receptors have been implicated in O2 sensing by carotid glomus cells, while central A2A receptors likely blunt hypoxic hyperventilation. In conclusion, A2A receptors are crucially involved in the transduction mechanisms of O2 sensing in fetal carotid bodies and brains. Postnatally, central A2A receptors remain key mediators of hypoxic respiratory depression, but they are less critical for O2 sensing in carotid chemoreceptors, particularly in developing lambs.
Keywords: chemoreception, fetus, hypoxia, newborn
mammalian life critically depends upon oxygen, which provides cellular energy [adenosine triphosphate (ATP)] by accepting electrons at complex IV of the mitochondrial respiratory chain (Fig. 1). A continuous production of ATP is crucial for sustaining electrochemical gradients of cellular membranes, cell metabolism, organ function, and growth. Restrictions in mitochondrial oxygen supply, which can occur continuously or intermittently, trigger adaptive responses to help restore mitochondrial oxygen sufficiency and ATP synthesis.
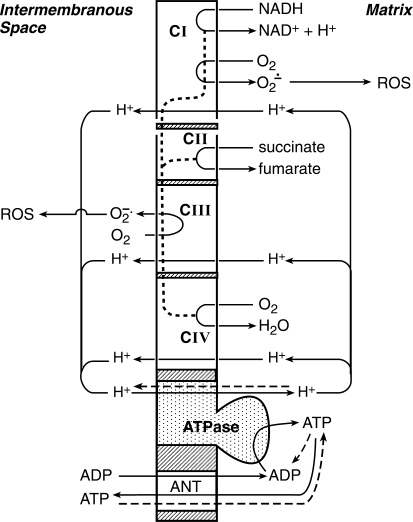
Fig. 1.
Schematic diagram of electron and proton flow along the respiratory chain complexes (CI-CIV) of the inner mitochondrial membrane. Electrons (dotted line) from NADH (CI) and FADH2 (CII) flow along the respiratory chain to O2 (CIV), which is linked to a proton flux from the mitochondrial matrix to the intermembranous space. Passage of protons back to the matrix through the ATPase complex drives ATP synthesis. ATP passes through the inner and outer mitochondrial membranes via the adenine nucleotide translocator (ANT) into cytosol. Under normal conditions, 1–2% of electron flow through the respiratory chain reduces O2 to superoxide anion radical (O2·−) at complexes I and III. Hypoxia diverts more of the electron flow to O2·− production, with O2·−'s generated at complex III a major source of mitochondria-derived reactive oxygen species (ROS) in cytosol. The dashed lines show the reversal of membrane transport for protons and ATP in O2 deficiency. ATP generated by cytosolic glycolysis is carried into mitochondria, where it is hydrolyzed by ATPase. This supplies energy for the extrusion of protons from the mitochondrial matrix to the intermembranous space to maintain the mitochondrial membrane potential. [Adapted from Solaini et al. (317).]
Fetal hypoxia can arise from numerous maternal, placental, and fetal pathophysiologic states that reduce placental O2 transport (Table 1). Normal fetuses maintain O2 consumption, and thus placental O2 transfer, with acute reductions up to ∼50% in uteroplacental blood flow, umbilical blood flow, or oxygen-carrying capacity of fetal or maternal blood. Under these conditions, oxygen sufficiency is sustained by an increase in O2 extraction by fetal tissues and other mechanisms, although longer-term reductions can result in reduced fetal O2 consumption and growth (42, 110, 157, 231). Clinically, insufficient fetal O2 delivery can lead to significant perinatal morbidity and mortality (Table 2).
Table 1.
Causes of fetal hypoxia
| Mother |
| ↓ Arterial O2 content |
| High altitude |
| Asthma |
| Pneumonia |
| Cystic fibrosis |
| Cyanotic heart disease |
| Severe anemia |
| Malaria and sickle cell crisis |
| Seizures |
| Sleep apnea |
| ↓ Uteroplacental blood flow |
| Hypotension* |
| Congenital or acquired heart disease |
| Chronic hypertension |
| Preeclampsia |
| Diabetes mellitus |
| Thrombosis |
| Long-term high altitude |
| Uterine contractions |
| Smoking |
| Illicit stimulants |
| Vasoconstrictors |
| Antiphospholipid antibody disorders |
| Placenta |
| ↑ Intervillous fibrin deposition |
| ↓ Villous capillary size and/or number |
| Villous edema |
| Thrombosis/infarction† |
| Abruptio placentae |
| Umbilical Circulation |
| Vasoconstrictors |
| Preeclampsia |
| Umbilical cord compression |
| Umbilical cord hypercoiling/knots |
| Polyhydramnios |
| Meconium |
| Fetus |
| ↓ Systemic arterial pressure |
| Acute hemorrhage |
| Cardiac failure |
| ↓ Arterial O2 content |
| Anemia |
| Smoking (carbon monoxide) |
Due to septic shock, acute hemorrhage, supine hypotension, antihypertensive agents, regional anesthesia;
includes malaria and sickle cell crisis.
Table 2.
Consequences of fetal hypoxia
| Intrauterine growth restriction |
| Prematurity |
| Hypoxic-ischemic encephalopathy |
| Multiorgan failure |
| Stillbirth |
| Neonatal demise |
| Meconium aspiration syndrome |
| Seizures |
| Cerebral palsy |
| Mental retardation |
A major cause of neonatal O2 deprivation includes the failure to establish effective pulmonary gas exchange at birth due to respiratory distress syndrome, meconium aspiration, pulmonary hypoplasia, fetal hydrops, diaphragmatic hernia, congenital cystic adenomatoid malformation of the lung, birth asphyxia, and central nervous system depressants. Postnatal hypoxia also occurs in pneumonia, apnea of prematurity, and airway obstruction in sleep.
Compensatory responses to these hypoxic challenges are essential to maintain metabolic homeostasis in the fetus and newborn. A crucial first-line defense involves O2 sensors that respond almost instantaneously to reductions in PaO2 through mechanisms independent of metabolic compromise or gene transcription. Upon activation, these chemoreceptors initiate acute physiologic compensations that support oxygen homeostasis for critical organs. Examples include carotid bodies, chromaffin cells of the adrenal medulla in the fetus or neonate, neuroepithelial bodies in the airways, the central nervous system, and the smooth muscle of pulmonary and systemic vasculature.
Various triggers have been proposed to account for the hypoxia-induced chemosensory cascade (44, 116, 123, 163, 262, 349, 353). These include an O2-limitation in mitochondrial oxidative phosphorylation at complex IV (Fig. 1), increased production of reactive oxygen species (ROS) at mitochondrial complex III and/or cell membrane NADPH oxidase, and inhibition of cell membrane potassium channels that are inhibited directly or indirectly by falls in Po2. The latter include conformational changes in associated plasma membrane hemoproteins (e.g., NADPH oxidase) and enzymatic release of CO via hemeoxygenase-2. Hypoxia-inducible factor-1, a major regulator of oxygen-dependent gene expression instantaneously stabilized by hypoxia, activates hypoxic signaling pathways within the first 2 min of a fall in tissue Po2, which inactivates anabolism, stimulates anaerobic glycolysis, and inhibits mitochondrial aerobic metabolism (151, 164, 310, 317, 325, 351). Although the relative roles of specific mechanisms are disputed, it is generally accepted that the transduction cascade in O2 sensors involves inhibition of potassium currents, depolarization of cell membranes, and elevation in intracellular [Ca2+].
Progress has been made surrounding controversies of O2 chemoreception and establishing the relative role of O2 sensing processes in development. This information has important implications for acute respiratory, cardiovascular, metabolic, and endocrine responses of the fetus and newborn to hypoxia as well as for the emergence of chronic pathophysiologic disorders in children and adults. Thus, advances in this field have relevance to fetal, newborn, and adult medicine.
This essay reviews putative regulation by the mitochondrial respiratory chain of adenosine formation in development and the resulting autocrine, paracrine, and endocrine compensations that buffer against acute O2 deprivation. A particular focus is the role of adenosine A2A receptors in O2 sensory transduction in the carotid body and the brain, as determined by cardiorespiratory responses to acute hypoxia. Potential A2A receptor involvement in other major O2 sensing tissues in perinatal life is also summarized.
ADENOSINE: AN OVERVIEW
Adenosine Production and Metabolism
Adenosine, a purine nucleoside, is primarily derived in normoxia from intracellular S-adenosyl homocysteine (Fig. 2). Cytosolic adenosine is phosphorylated via adenosine kinase to AMP, but conversion to inosine by adenosine deaminse can predominate at high levels of adenosine. Intracellular adenosine diffuses down a concentration gradient into the extracellular space via equilibrative membrane transporters. Adenosine can also be produced extracellularly from rapid hydrolysis (via membrane-bound ecto-5′-nucleotidase) of AMP that is derived from ATP or cAMP. Adenosine is not stored in vesicles, although synaptic exocytosis has been proposed for discrete brain sectors (347). Extracellular adenosine can be transported intracellularly via equilibration, as well as by sodium-dependent, concentrating transporters, which lower extracellular concentrations (93).
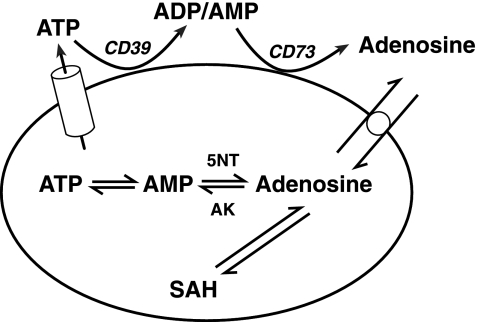
Fig. 2.
Cellular production of adenosine. CD39, nucleoside triphosphate diphosphohydrolase; CD73, ecto-5′-nucleotidase; 5NT, endo-5′-nucleotidase; AK, adenosine kinase; SAH, S-adenosyl homocysteine; cylinder, exocytosis exportation; circle, equilibrative membrane transporter. SAH hydrolase reversibly catalyses the degradation of SAH to adenosine and homocysteine. [Modified from Fredholm et al. (93).]
Hypoxic reductions in mitochondrial oxidative phosphorylation have been proposed to raise the cytosolic ADP/ATP ratio, which, via activation of adenylate kinase, converts two molecules of ADP into one molecule each of ATP and AMP (10, 85, 86, 262, 360). This reaction has two effects: 1) it blunts the fall in ATP, and 2) relative to the rise in the ADP/ATP ratio, it exponentially increases the cytosolic AMP/ATP ratio. The latter elicits adaptations that tend to restore and stabilize cellular ATP levels. Thus, negligible changes in cytosolic ATP can sufficiently increase the AMP/ATP ratio to activate AMP-activated protein kinase (AMPK), 5′-nucleotidase and other enzymes involved in chemoreceptor-signaling pathways (85, 86, 130, 142, 202, 262. 360). Compared with other tissues, the enhanced O2 sensitivity of chemoreceptors has been attributed to higher levels of key enzymes linking changes in cellular AMP/ATP ratio to membrane depolarization, greater ATP dependency on mitochondrial oxidative phosphorylation, and lower resting energy state (i.e., ↑ADP/ATP ratio).
The rise in cytosolic AMP/ATP ratio in acute hypoxia elevates adenosine levels by stimulating cytosolic and extracellular 5′-nucleotidases (131, 142, 185, 202), although other enzymes (e.g., ↓ adenosine kinase activity) can be involved (69). Because AMP levels regulate adenosine formation, the relative rates of ATP synthesis and degradation largely determine net adenosine production, which, in turn, is controlled by the ATP utilization rate and the availability of substrate (e.g., glucose, O2) that is involved in ATP synthesis. Adenosine formation from extracellular AMP normally predominates under these conditions (115), but its relative contribution likely depends on the tissue and the extent of O2 deprivation. Increased metabolic rate alone can reduce the adenylate energy state and activate 5′-nucleotidase; thus, adenosine levels can increase without a restriction in O2 supply per se (22, 129, 305).
Adenosine levels can be measured in interstitial fluid dialysate or plasma by high-performance liquid chromatography (182, 185, 191, 306), and real-time changes in adenosine concentration can be tracked with enzyme-based probes (347). Circulating levels of adenosine in fetal sheep, which are 2–4 times those in adult ewes, derive from the low fetal PaO2 and possibly from specialized features of the umbilical circulation (182, 306).
Adenosine Receptors
Adenosine binds to four extracellular G protein liganded receptor subtypes (A1, A2A, A2B, and A3), which are distinguished by differential affinities for adenosine analogs and methylxanthanine antagonists, tissue distribution, and effector-coupling mechanisms (94, 146). Adenosine has the greatest affinity for A1 receptors with less attraction for A2A, A2B, and A3 receptors. Both the A1 and A3 receptors inhibit adenylate cyclase via pertussis-sensitive G proteins (Gi/o), which can stimulate K+ and KATP currents and inhibit Q-, P-, N-type Ca2+ channels. The importance of A1 receptors in fetuses and newborns has been reviewed (290, 355).
A2A and A2B receptors primarily couple to Gs (Golf for A2A in striatum), which stimulates adenylyl cyclase, increases cAMP concentrations, and activates cAMP-dependent protein kinase A (PKA). PKA stimulates L-type calcium channels, as well as a number of other signaling pathways (Table 3). A2A receptors have been implicated in inositol phosphate formation, activation of protein kinase C, and elevation of intracellular Ca2+ levels that result in contraction of smooth muscle or neurotransmitter release. A2A receptor signaling also can occur independently of G proteins (93, 146, 382). A2B receptors can couple to Gq, which activates phospholipase C. Specific tissue responses are determined by the number and relative expression of adenosine receptor subtypes, interaction with other receptors (including heteromeres), and extracellular concentrations of adenosine (93).
Table 3.
Adenosine A2A signal transduction involving cAMP-dependent kinase*
| ↑ L-type Ca2+ channel |
| ↑ aPKC |
| ↑ CREB→↓NFkB |
| ↑ DARPP-32 |
| ↑ Rac1/Cdc42 → →→p38 |
| ↑ Src → Ras →Raf-1→MEK → ERK1/2 |
Via G protein activation of adenylyl cyclase and increase in intracellular cAMP.
aPKC, atypical protein kinase C; CREB, cAMP responsive element binding protein; NFkB, nuclear factor-кB; DARPP-32, dopamine- and cAMP-regulated neuronal phosphoprotein; p38, p38 mitogen-activated protein kinase; ERK1/2, extracellular signal-regulated kinases 1 and 2. [lsqb]Modified from Fredholm et al. (93).[rsqb]
A2A receptors, which have been localized to chromosome 22q11.23 in humans and chromosome 10 in mice (364), are widely expressed in tissues, including the immune system, platelets, myocardium, lungs, vasculature, carotid body, and central nervous system (93, 94, 146, 350). Thus, A2A receptors are involved in diverse physiologic processes, such as respiration, sleep, vascular tone, angiogenesis, inflammation, and defenses against oxidative stress. The following summarizes the developmental role of these receptors in O2 sensory transduction in the carotid body and the brain as well as the involvement of these receptors in metabolic, endocrine, and vascular responses to acute O2 deprivation.
ADENOSINE AND O2 SENSING
Carotid Body
In newborns and adults, hypoxic excitation of the carotid bodies triggers arousal from sleep, hyperventilation, and sympathetically driven cardiovascular responses that rapidly defend against acute oxygen deprivation. A detector of Po2 in arterial blood supplying the brain, the carotid body also independently senses arterial Pco2, pH, temperature, osmolality, and glucose (198).
General O2 sensory mechanisms.
Oxygen chemoreception by the carotid body likely involves the functional interaction of multiple sensors with differing thresholds of Po2 activation that enable chemosensory responses over a wide range of O2 tensions (1, 117, 201, 274). The generally accepted paradigm of O2 sensing by the carotid body involves depolarization of glomus cell membranes by hypoxic inhibition of K+ channels, a rise in intracellular Ca2+ levels, and subsequent release of neurotransmitters that stimulates adjacent fibers of the carotid sinus nerve.
While the mechanism by which hypoxia depresses K+ currents is not fully understood, it most likely involves an imbalance in the localized rates of ATP production/consumption within glomus cells (85, 86, 87, 360). The resulting elevation in the ADP/ATP ratio triggers, via adenylate kinase, a rise in the AMP/ATP ratio, which activates AMPK by allosteric stimulation of phosphorylation and by inhibition of dephosphorylation (127, 303). Activated AMPK, in turn, inhibits large-conductance, calcium-activated potassium channels (BKCa) and background potassium channels (e.g., TASK-like potassium currents, TREK channels), resulting in membrane depolarization, voltage-gated Ca2+ entry, and the release of an array of neurotransmitters and neuromodulators, including acetylcholine, catecholamines, peptides, amino acids, ATP, and adenosine (32, 85, 117, 168, 197, 201, 211, 262). ATP, acetylcholine, adenosine, and other transmitters/modulators have been implicated in chemoexcitation of the carotid sinus nerve (104, 200, 274, 288, 289, 380). This general schema of carotid O2 sensing appears generally applicable to humans, because human carotid body expresses TASK and BKCa channels, as well as receptors for γ-aminobutryic acid, acetylcholine, and dopamine (88).
The duration and frequency of hypoxia modulate O2 chemoreception. For example, chronic, intermittent hypoxia enhances carotid body sensitivity under basal and acute hypoxic conditions, a chemosensory plasticity that critically depends on ROS generation by mitochondria and NADPH oxidase (215, 275, 378).
A2A receptors and O2 sensing by adult carotid bodies.
Adenosine release by the carotid bodies is extremely sensitive to small reductions in PaO2, with the rise in interstitial levels due to catabolism of intracellular and extracellular ATP (38, 52). Exogenous adenosine, via A2 receptors, increases chemosensory discharges in the carotid sinus nerve in rats and cats and stimulates respiration in normoxia and hypoxia (221, 222, 229, 308); thus, increased extracellular levels of adenosine have physiological relevance. Low doses of an A2A receptor agonist (CGS-21680) stimulate respiration in rats, suggesting that these receptors mediate excitatory effects of adenosine (53, 308). Endogenously produced adenosine has been implicated in the stimulation of the carotid chemoreceptors in anesthetized rats (222, 230) and in hypoxic excitation of the carotid bodies in unanesthetized rhesus monkeys and human subjects (76, 138, 366).
A2A and A2B are the primary adenosine receptor subtypes in carotid glomus cells. Activation of A2 receptors stimulates these O2 sensors in vitro and in vivo (55, 90, 91, 172, 210, 229, 300, 308, 342, 350). Proposed transduction effects of adenosine A2A receptor stimulation include activation of adenylate cyclase and PKA pathways to inhibit TASK-1 K+ channels, which results in membrane depolarization, Ca2+ entry through voltage-gated calcium channels, and release of neurotransmitters (47, 90, 91, 211, 342, 362), as depicted in Fig. 3. But the post-A2A receptor cascade is likely to be more complex (51, 54, 172), and it may vary according to species, strains within species, and stage of development (11, 53, 55, 90, 172, 308).
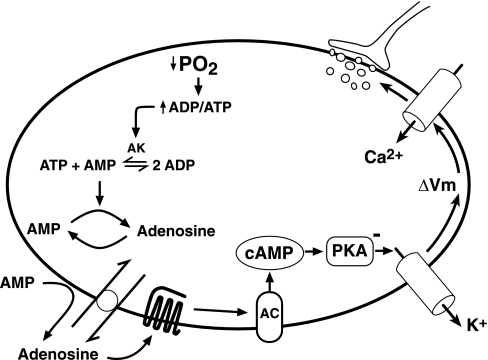
Fig. 3.
Adenosine A2A receptor transduction cascade proposed for carotid body activation. O2-limitation of oxidative phosphorylation in mitochondria activates adenylate kinase (AK), which converts two molecules of ADP into ATP and AMP. The resulting increase in the AMP/ATP ratio promotes adenosine formation from AMP in cytosol and extracellular space. Intracellular adenosine is transported extracellularly via an equilibrative nucleoside transporter. Activation of extracellular A2A receptors stimulates cAMP formation by adenylyl cyclase (AC), which, in turn, promotes protein kinase A (PKA) inhibition of potassium currents. The resulting fall in membrane potential (ΔVm) activates voltage-gated Ca2+ channels, and the rise in intracellular Ca2+ leads to secretion of neurotransmitters that stimulate the carotid sinus nerve.
O2 sensing by fetal carotid bodies.
In the sheep fetus, carotid chemoreceptors respond to changes in Po2 and Pco2 from at least 0.6 of term (24). The extremely low fetal PaO2 (25–30 Torr) greatly reduces the Po2 setpoint of carotid glomus cells. As in the adult, the fetal carotid bodies respond selectively to changes in arterial Po2 rather than in O2 content (196). The carotid bodies of fetal rats express adenosine A2A receptor mRNA (Table 4), which is consistent with a role for this receptor subtype in O2 sensing in early development. It is reasonable to assume that fetal expression of transduction elements in carotid glomus cells differ from those in adults due to the much lower PaO2 as well as developmental immaturity. The responsiveness of the fetal carotid body to other stimuli (e.g., H+, hypoglycemia, hyperkalemia, and hyperosmolality) has not been specifically tested, although sensitivity to acidosis and glucose appears likely (35, 336).
Table 4.
Semiquantitative adenosine A2A receptor gene expression in rat development revealed by in situ hybridization
| G16 | G20 | P0 | P7 | Adult | |
|---|---|---|---|---|---|
| Caudate-putamen | 2–3 | 3 | 2–3 | 3 | 3 |
| Accumbens, n | 1–2 | 3 | 2–3 | 3 | 3 |
| Olfactory tubercle | 2 | 3 | 2–3 | 3 | 3 |
| Cortex | 0–1 | 0–2 | 0 | 0 | 0 |
| Parafascicular, n | 0 | 0 | 2 | 0 | |
| Area postrema | 0 | 0–2 | 0–2 | 0–2 | 0 |
| Carotid body | 2–3 | 2–3 | 2 | ||
| Pituitary | |||||
| Intermediate lobe | 1–2 | 2–3 | 3 | 1–2 | 2 |
| Anterior lobe | 1–2 | 1 | 0 | 0 | 0 |
G, gestational days; P, postnatal days. [Data from Weaver (350).]
O2 CHEMOREFLEXES.
In fetal sheep (>0.8 term), hypoxic excitation of the carotid bodies elicits autonomic reflexes that lower heart rate through increased vagal activity and constrict blood vessels via increased sympathetic tone (15, 111, 119, 125, 145, 170, 233). Sympathetic vasoconstriction, along with other factors, lower vascular conductance to nonessential tissues (e.g., kidneys, gastrointestinal tract, liver, and lungs), which support or raise systemic arterial pressure in the face of increased vascular conductance to the myocardium, brain, and adrenal glands (263). Similar cardiovascular adaptations have been identified in human fetuses (8, 16, 17, 39, 120, 132, 352). Thus, arterial O2 chemoreflexes are involved in the redistribution of cardiac output and O2 delivery to critical organs in O2 deficient states. An important distinction in the fetus is that hypoxic excitation of the sinus nerve does not induce arousal or hyperpnea, as occurs postnatally, and fetal breathing differs in other respects as well (176).
Interestingly, acute and chronic hypoglycemia alters organ distribution of fetal combined ventricular output in a similar manner to that caused by hypoxia (34, 225, 322). Nutrient-deprived ovine fetuses have reduced liver blood flow and growth, which are abrogated by bilateral denervation of fetal carotid bodies (35). Thus, fetal carotid chemoreceptors have a role in redistribution of fetal cardiac output in both hypoxia and hypoglycemia, which can contribute to altered organ growth in oxygen- and nutrient-deprived fetuses (35, 110, 136).
The fetal O2 deficiency of anemia differs in at least three ways from that of hypoxia: 1) lowered oxygen carrying capacity (i.e., hemoglobin concentration), 2) increased spacing of red cells in capillaries (a significant cause of O2 deprivation in tissues), and 3) a minimal fall in PaO2. Because of the latter, fetal anemia has little effect on carotid O2 chemoreflexes (196). Hemodynamic differences of anemia include elevated cardiac output and blood flow to most tissues (66).
A2A RECEPTORS AND O2 SENSING.
As in the adult, excitation of the fetal carotid bodies can be elicited by metabolic consequences of reduced ATP production in mitochondria. When central inhibition of breathing has been abolished by disruptions of the brain stem in fetal sheep, intra-arterial administration of oligomycin (a mitochondrial ATPase inhibitor, see Fig. 1), adenosine, or an adenosine A2A receptor agonist rapidly increases the rate and amplitude of breathing (178, 179). This respiratory stimulation is eliminated by peripheral chemodenervation, which is consistent with a crucial role for A2A receptors in exciting fetal carotid chemoreceptors.
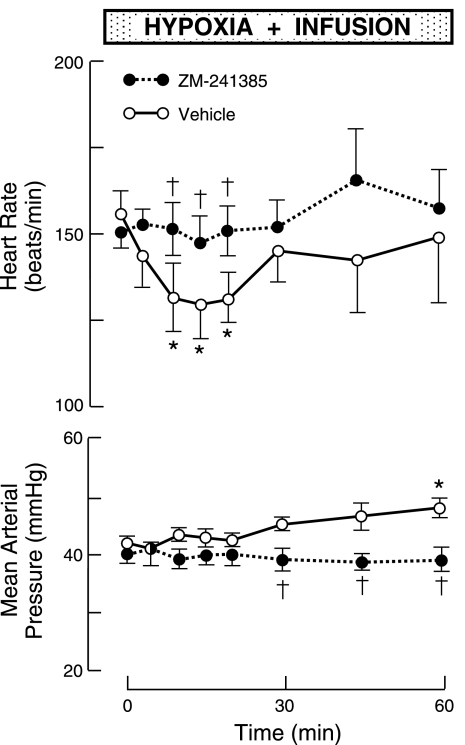
Intravascular infusion of a potent, nonselective adenosine receptor antagonist with actions restricted to peripheral tissues [8-(p-sulphophenyl)-theophylline] abolishes the bradycardia and hypertension induced by hypoxic excitation of carotid chemoreflexes (108, 181), an effect that is replicated by highly selective A2A receptor blockade (186), as shown in Fig. 4. These observations are consistent with the hypothesis that adenosine A2A receptors associated with carotid bodies are critically involved in triggering hypoxic chemoreflexes that reduce heart rate (and thus myocardial O2 consumption) and support O2 delivery to critical organs. That adenosine A2A receptors mediate the full expression of these cardiovascular chemoreflexes suggests that O2 sensing in carotid bodies is critically linked to activation of 5′-nucleotidase.
Fig. 4.
Heart rate and mean arterial pressure responses in fetal sheep to isocapnic hypoxia during intravascular infusion of an adenosine A2A receptor antagonist (ZM-241385) or vehicle. Vertical bars depict ± 1 SE. Horizontal bar shows time of hypoxia and infusion. *P < 0.05 compared with control at time 0; †P < 0.05 compared with vehicle at same time. During hypoxia, mean fetal PaO2 was ∼15 Torr (ΔPaO2, about −9 Torr compared with normoxia). [Modified from Koos and Maeda (186).]
O2 sensing by carotid bodies in postnatal development.
The control of breathing changes significantly at birth when respiratory gas exchange shifts from the placenta to the lungs.
CHANGES AT BIRTH.
The rise in PaO2 (from ∼25 to 50–70 Torr) at birth in sheep silences spontaneous carotid sinus nerve activity in the first 2–3 postnatal days, with the emerging discharge rate increasing in frequency in older lambs (24, 25, 79, 321). Although basal sinus nerve activity is depressed, isocapnic hypoxia stimulates respiration in newborn lambs and other neonates, an augmentation that is eliminated by carotid body denervation (23, 330). Thus, excitatory afferent discharges in the carotid sinus nerve are now functionally integrated into respiratory drive. The respiratory response becomes progressively more robust with advancing age, with the discharge rate increasing for a given PaO2 as well as for a change PaO2 (24, 126). This postnatal resetting of the carotid chemoreceptors, which can take days or weeks, potentially involves increases in the number of type I cells as well as changes in expression of receptors and ion channels, synaptic interactions, and afferent responsiveness (41, 74, 100, 330, 348). This adaptation does not include changes in carotid body perfusion (232).
PROLONGED OR RECURRENT PERINATAL HYPOXIA.
Chronic in utero exposure to sustained or intermittent hypoxia (112, 118, 267, 268) can result postnatally in resting hyperventilation with either a depressed or augmented ventilatory response to acute hypoxia in rats up to 3 wk of age (112, 267, 268). Naturally occurring growth restriction in fetal sheep (presumably chronically hypoxic) arrests the progressive increase in the hypoxic ventilatory response in lambs that normally occurs after the first week of age (239).
In several mammalian species, prolonged hypoxia initiated at birth induces long-term perturbations in respiratory control that include normoxic hyperventilation and/or blunting of the acute hypoxic respiratory response (79, 126, 251, 281, 282, 283, 314). The latter has been attributed to impairment of carotid body O2 sensing by low Po2 (79, 126, 321, 361).
Chronic intermittent hypoxia in the postnatal period in rats augments acute hypoxic respiratory hypernea, which persists even in adults (18, 153, 259, 260, 265, 275, 283). The exaggeration of acute carotid body O2 sensing by chronic intermittent hypoxia, which is mediated largely through the release of ROS, is greater in neonatal than adult rats and is associated with glomus cell hyperplasia in only neonates (260, 265, 266, 275, 276). Intermittent hypoxia increases ROS levels by activating NADPH oxidases, inhibiting mitochondrial complex III activity (Fig. 1), and downregulating anti-oxidant enzymes via hypoxia-inducible factors 1 and 2 (275).
In mature mammals, respiratory acclimatization to low PaO2 reverses rapidly upon the restoration of normoxia (18). Thus, the development of persistent changes in respiratory control that occur in the fetus and newborn are largely restricted to the perinatal period.
HYPEROXIA.
Sustained postnatal hyperoxia also modulates respiratory control (78). For example, prolonged hyperoxia blunts early stimulatory effects of acute hypoxia on ventilation in adult rats, apparently by altering carotid body development (19, 20).
A2A RECEPTORS AND POSTNATAL O2 SENSING.
Postnatal changes in carotid body O2 sensitivity, which are qualitatively similar in rats and sheep, may involve altered expression of A2A receptors (101, 103, 350). In rats, A2A receptor mRNA expression in the carotid body declines postnatally (103, 350); nevertheless, A2A receptors still account for ∼50% of the hypoxia-induced enhancement of sinus nerve activity in adults (55).
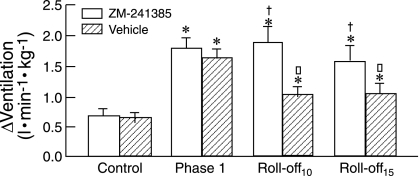
Hypoxic hyperventilation in newborn lambs is almost entirely mediated by stimulation of the carotid chemoreceptors, with negligible contribution from the aortic bodies (357). In developing lambs (1–2 wk of age), hypoxia rapidly increases the rate and depth of respiration, with maximum ventilation achieved within 1–2 min. This hyperpnea coincides with a sharp rise in arterial pressure and heart rate. Contrary to the response in the fetus, systemic antagonism of adenosine A2A receptors in developing lambs does not blunt the hypertension or the maximum ventilatory response (Fig. 5). Thus, adenosine A2A receptors have little involvement in carotid O2 chemoreflexes of the developing lamb (184). The effects of antagonism of the A2A receptors on hypoxic chemoreflexes in older sheep require investigation.
Fig. 5.
Respiratory responses to isocapnic hypoxia in lambs at 7–16 days of age. Depicted are the effects of ZM-241385 or vehicle on maximum hypoxic stimulation (Phase 1) and on ventilation 10 min (Roll-off10) and 15 min (Roll-off15) after the start of hypoxia compared with normoxia (control). *P < 0.05 compared with control; †P < 0.05 compared with vehicle; □P < 0.05 compared with phase 1. During hypoxia, mean PaO2 was ∼31 Torr (ΔPaO2, about −52 Torr compared with normoxia). [From Koos et al. (184).]
RESPIRATORY EFFECTS ON POSTNATAL HEART RATE.
The onset of pulmonary ventilation at birth profoundly affects the modulation of heart rate by the autonomic nervous system. As previously noted, acute hypoxia decreases fetal heart rate as a result of a carotid chemoreflex that increases vagal tone. In contrast, hypoxic stimulation of the carotid bodies in the newborn or adult elicits tachycardia. Postnatally, hypoxic hyperpnea increases heart rate via central autonomic effects of increased afferent traffic from pulmonary stretch receptors (60). In addition to being episodic, fetal breathing differs in that: 1) inspiration is associated with minimal changes in lung volume, which are insufficient to activate pulmonary stretch receptors, and 2) hypoxia inhibits breathing activity (176); hence the differences in heart rate response to hypoxia in fetal and postnatal life.
Brain
O2 deprivation generally increases potassium conductance and thereby hyperpolarizes neurons in the central nervous system. But in some brain sectors, acute hypoxia depolarizes neurons with effects that are consistent with those of an O2 sensor (330).
The rostral ventrolateral medulla, nucleus tractus solitarius, pre-Bötzinger complex, and posterior hypothalamus have neurons intrinsically sensitive to hypoxia that, when activated, stimulate sympathetic and/or respiratory activity in adult mammals (245, 271, 330). With respect to respiration, hypoxic excitation of the posterior hypothalamus induces tachypnea in awake cats with carotid chemodenervation (330). The neuronal O2 sensing mechanisms likely involve O2-sensitive ion channels, hemeoxygenase-2, ROS, and erythropoietin as well as other transduction pathways (2, 59, 99, 106, 271, 330). ROS activation of TASK-2 channels in cells of the retrotrapezoid region of the ventral medulla has been implicated in hypoxic respiratory depression (106).
Present in glia and presynaptic and postsynaptic neurons, adenosine A2A receptors are concentrated in striatum, nucleus accumbens, olfactory tubercle, and pineal gland with lower levels widely distributed more generally in gray matter (30, 89, 309, 367, 379). Adenosine A2A receptors can affect neuronal activity directly as well as indirectly by interacting with A1 receptors and receptors for other neuromodulators or neurotransmitters (308, 309). A2A receptors modulate the release of several neurotransmitters, including acetylcholine, GABA, and dopamine. A classic example is the striatum, where stimulation of A2A receptors reduces the affinity of dopamine D2 receptors.
Brain A2A receptors modulate sleep, temperature, and many other central functions, including tonic depression of motor activity (308, 369). Acute intermittent hypoxia induces long-term facilitation of phrenic motor activity by ROS generated by NADPH oxidase in the spinal cord, which probably involves activation of A2A receptors (133, 215).
Adenosine and fetal behavior.
A2A receptor mRNA is expressed widely in embryonic/fetal rat brains, including the striatum, cortex, hippocampus, and thalamus (Table 4). In near-term fetal sheep, a high density of A2A receptors is present in striatum and the pineal gland, with moderate levels in the thalamus, including the parafascicular nuclear complex (Pf) (367).
In the ovine fetus (>0.8 term), virtually all behavior is consistent with quiet sleep (high-voltage electrocortical activity, absence of rapid eye movements, and breathing) alternating with rapid eye movement sleep (low-voltage electrocortical activity coincident with rapid eye movements and breathing activity). Blockade of central adenosine A2A receptors increases the incidence of rapid eye movement states, indicating that endogenous adenosine levels are involved in the regulation of sleep in normoxic fetuses (178, 187).
BRAIN HYPOXIA AND FETAL BREATHING AND RAPID EYE MOVEMENTS.
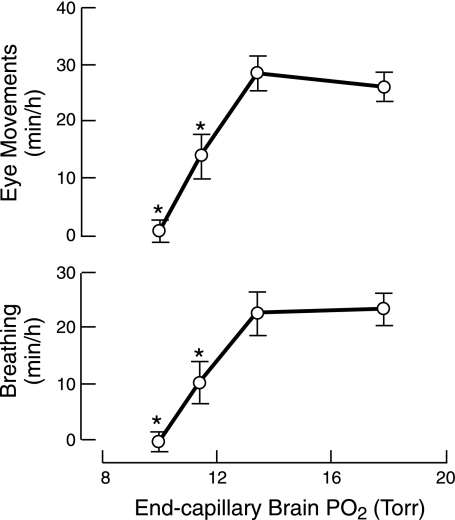
Hypoxia inhibits fetal movements through direct effects on the central nervous system (68, 193). With respect to breathing and rapid eye activity, isocapnic hypoxic depression is dose-dependent (195), beginning when fetal PaO2 acutely falls more than ∼6 Torr [Δarterial O2 content (CaO2) about −2.00 ml/dl; control CaO2 = 7.18 ml/dl], with virtual absence of these movements when PaO2 decreases by 9–10 Torr (ΔCaO2, about −3.81 ml/dl), as shown in Fig. 6. The amplitude and frequency of residual breathing are unaltered by isocapnic hypoxia (176).
Fig. 6.
Incidence of breathing and rapid eye movements in fetal sheep is shown in relation to the calculated mean end-capillary Po2 of the fetal brain. The incidence of breathing and rapid eye movements declines in dose-dependent manner once the estimated end-capillary Po2 falls below ∼14 Torr. The incidence of low-voltage electrocortical activity was not significantly reduced over the studied range of fetal PaO2. *P < 0.05 compared with normoxia with calculated mean end-capillary Po2 ∼18 Torr. The corresponding measurements of mean fetal PaO2 were ∼25 Torr for normoxia and ∼20, 18, 17 Torr, respectively, for hypoxia. [Modified from Koos et al. (195).]
Denervation of the carotid and aortic chemoreceptors delays the onset, but not the extent, of the inhibition (193). The depressant effects of moderate hypoxia (without a continued decline in pHa) are transient with the incidence of breathing and rapid eye movements increasing toward normal within 3–4 h of sustained O2 deficiency (176). This return of activity is consistent with actions of a central O2 sensor that rapidly adapts to changes in PaO2.
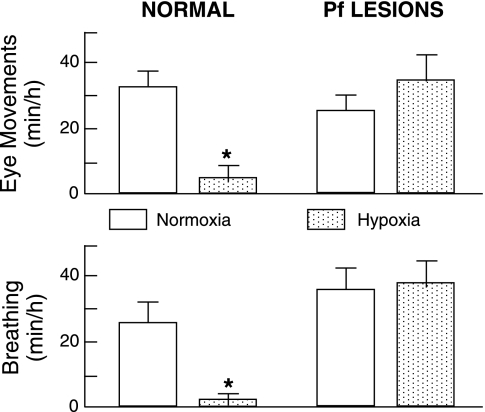
Supramedullary disruptions of the brainstem abolish hypoxic inhibition of breathing; thus, this respiratory arrest does not arise from the intrinsic effects of hypoxia on medullary respiratory motoneurons (68, 114). More discrete neuronal lesions have identified a sector of the posteromedial thalamus encompassing the Pf as the most rostral locus of the neuronal network mediating hypoxic inhibition of breathing and rapid eye movements (179), as shown in Fig. 7. This conclusion is supported by the induction of apneas by electrical stimulation within or immediately adjacent to this locus (183). The crucial role of Pf, which integrates sensorimotor function and regulates behavioral state, supports the notion that a hypoxia-induced change in behavioral state (e.g., reduction in rapid eye movement sleep) underlies the depressant effects on fetal breathing (179, 195).
Fig. 7.
Effects of normoxia and isocapnic hypoxia on the incidence of breathing and rapid eye movements in normal fetal sheep and in fetuses with neuronal lesions that encompassed the parafascicular nuclear complex (Pf) of the posteromedial thalamus. In both groups, isocapnic hypoxia did not alter the amplitude or frequency of breathing. *P < 0.05 compared with normoxia. During hypoxia, PaO2 was ∼14 Torr (ΔPaO2, about −10 Torr compared with normoxia). [Modified from Koos et al. (179).]
A2A RECEPTORS AND FETAL BRAIN O2 SENSING.
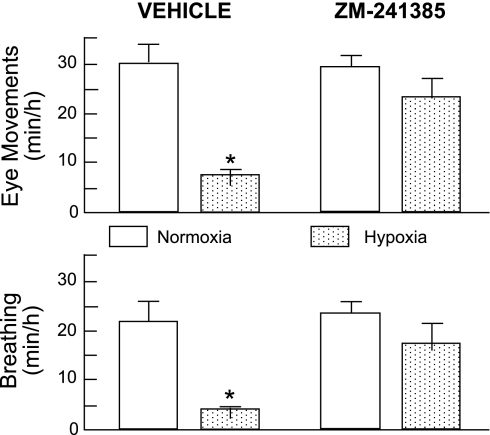
As in the carotid body (178, 192, 241) reduced mitochondrial ATP production has been implicated in the central O2 sensing mechanism that triggers hypoxic inhibition of fetal breathing and rapid eye movements (194). This metabolic hypothesis of behavioral regulation has been bolstered by observations that 1) hypoxia increases fetal levels of adenosine in plasma and the brain (182, 191); 2) intravascular infusion of exogenous adenosine mimics the inhibitory effects of hypoxia on fetal breathing and rapid eye movements (192); and 3) hypoxic depression is abolished by blockade of central adenosine A2A receptors (188), as depicted in Fig. 8. Thus, A2A receptors are critically involved in central O2-sensing mechanisms in the fetus.
Fig. 8.
Incidence of rapid eye movements and breathing during isocapnic hypoxia in normal fetal sheep with intravascular infusion of vehicle or ZM-241385. ZM-241385 blocked the inhibitory effects of hypoxia on breathing and rapid eye movements. During hypoxia, mean PaO2 was ∼14 Torr (ΔPaO2, about −10 Torr compared with normoxia). *P < 0.05 compared with normoxia. [Modified from Koos et al. (188).]
Intracarotid infusion of a potent and highly selective A2A receptor agonist (CGS-21680) has apposing effects on fetal breathing: stimulation (via carotid chemoreflexes) followed by inhibition due to activation of central A2A receptors as the agonist accumulates in the brain (178). These breathing responses in the fetus are remarkably similar qualitatively to the biphasic respiratory response to hypoxia that is observed postnatally (see next section), and they are consistent with a crucial dual role of A2A receptors in exciting fetal carotid chemoreflexes and triggering central hypoxic inhibition of fetal breathing.
Neuronal lesions in the diencephalon abolish inhibitory effects of adenosine on fetal breathing (180, 367). The locus of A2A receptors that arrest breathing has yet to be established, but the immediate proximity of thalamic A2A receptors to the rostral neuronal substrate (Pf) involved in hypoxic inhibition suggests a functional relationship.
Blockade of central α2-adrenergic receptors increases the incidence, rate, and amplitude of breathing in normoxic fetal lambs. In hypoxic fetuses, breathing persists, although with lower incidence, rate, and amplitude compared with normoxic responses (13, 109). These studies implicate α2-adrenergic receptors in a dopaminergic pathway that inhibits breathing in hypoxia.
GABA does not have a demonstrable role in hypoxic depression of breathing in unanesthetized fetal sheep (176). Thus, A2A-GABA receptor interactions do not significantly contribute to hypoxic inhibition in the fetus.
Adenosine and brain O2 sensing in postnatal development.
Adenosine is involved in central O2 sensing as illustrated by acute respiratory responses to hypoxia in newborn and developing mammals.
ROLL-OFF RESPIRATORY RESPONSE TO HYPOXIA.
The hypoxic ventilatory response in many mammals is biphasic: an initial stimulation followed by a decline or roll-off. The second phase fall in ventilation, which is more prominent in immature newborns, has been attributed to several factors, including reduced metabolic rate, central effects of hypoxia on the respiratory neuronal network, and reduced carotid sinus nerve activity and/or its central integration into respiratory drive (23, 330). The roll-off becomes dampened with age as the phase-I stimulatory response becomes more robust.
A number of other factors influence the ventilatory decline, including sex, arousal state, anesthesia, PaO2, and O2 ventilatory plasticity (330). For example, prenatal hypoxia exposure increases basal respiration in normoxia in neonatal rat pups, although this does not persist beyond postnatal day (P) 30 (112, 267, 268). In young male rats, continuous intrauterine hypoxia (embryonic days, E5–E20) abolishes the phase-2 ventilatory decline in hypoxia (268), while chronic intermittent hypoxia in utero accentuates the phase-2 depression in rat pups postnatally (118). Interestingly, perinatal exposure to alcohol changes the effects of episodic hypoxia in young rats from respiratory facilitation to depression (165), which is another example of in utero exposures disrupting postnatal respiratory regulation.
BRAIN SECTORS AND THE RESPIRATORY ROLL-OFF.
The inhibitory neuronal network involved in the roll-off includes neuronal substrate within or rostral to the pons in unanethetized young rabbits, as in fetal sheep (68, 217). Subsequent localization studies have demonstrated sectors proximate to the red nucleus in the midbrain of anesthetized young rabbits (3, 345) and to the locus coeruleus in the pons of anesthetized neonatal sheep (29, 234). Whether Pf of the posteromedial thalamus is critically involved in the ventilatory decline in developing lambs has yet to be established; thus, the developmental aspects of central hypoxic inhibition deserve further scrutiny.
A2A RECEPTORS AND THE ROLL-OFF.
The roll-off respiratory response to hypoxia in newborns and adults is attenuated by nonselective adenosine receptor blockade with methylxanthines (63, 105, 208, 210). Studies in lambs with potent and highly selective antagonists have shown that the ventilatory depression critically depends on activation of adenosine A2A receptors (184), as shown in Fig. 5. Thus, adenosine A2A receptors mediate the depressant effects of hypoxia on respiration in both fetal sheep and developing lambs, indicating that a central adenosinergic inhibitory pathway persists in postnatal life. As in fetal sheep, the thalamus has a moderate density of A2A in lambs and adult sheep, but it remains to be determined whether this locus of receptors is involved in hypoxic ventilatory depression.
Whether activation of adenosine A2A receptors is indispensible for hypoxic respiratory depression has not been established. So far, respiratory responses to hypoxia have not been reported in neonatal mice with knockouts of the A2A receptor. Other neurotranmitters or modulators [e.g., opioids, nitric oxide, substance P, serotonin, dopamine, GABA (23)] may assume more prominent roles in central respiratory depression in the absence of adenosine A2A receptor activation.
Stimulation of medullary A2A receptors activates inhibitory GABAergic inputs onto brainstem respiratory neurons in immature rats and piglets (219). These findings implicate A2A receptors in GABAergic influences on respiratory timing and respiratory drive that are involved in apnea of prematurity (219, 358).
Endocrine Systems
Adenosine modulates hormone secretion by a number of tissues, including adrenal glands, pituitary, and myocardium.
A2A receptors and fetal adrenal glands.
Adenosine is potentially involved in hormone release by the adrenal medulla and cortex.
ADRENAL MEDULLA.
In immature mammals, adrenomedullary chromaffin cells are directly sensitive to hypoxia, and the resulting rise in plasma catecholamine concentrations can be critical for survival (315). For example, in the fetus and newborn, elevated plasma catecholamine levels support cardiac contractility (via α-adrenergic receptors) and heart rate (via β1-adrenergic receptors), constrict peripheral vasculature (via α-adrenergic receptors), and signal metabolic adaptations to acute oxygen deficiency. Fetal hypoxia or asphyxia in labor can contribute to the catecholamine surge at birth, which is involved in absorption of lung fluid and secretion of surfactant by stimulating pulmonary β2-adenergic receptors.
The O2 chemosensory cascade of chromaffin cells involves inhibition of K+ channels, membrane depolarization, and calcium entry via T-type voltage-gated Ca2+ channels (27, 160, 161, 205). Mitochondrial regulation of intracellular redox levels appears to modulate O2-sensitive K+ currents in mature ovine adrenal medullary cells (160), which is consistent with other studies implicating mitochondria-derived ROS in hypoxic sensitivity in these chromaffin cells (248). Gene deficiency studies in mice have shown that NADPH oxidase (324) and hemeoxygenase-2 (253) are not essential components of the O2-sensing mechanism.
Adrenomedullary chromaffin cells generally lose their direct O2-sensing ability when splanchnic nerve innervation is completed, which occurs at approximately P10 in rats. In fetal sheep, sympathetic innervation of ovine adrenal occurs by 130 days of gestation (∼0.9 term), although ovine chromaffin cells in vitro retain ability to secrete catecholamines by a nonneurogenic mechanism (5, 48). Changes in the expression of T-type Ca2+ channels in chromaffin cells contribute to maturity-related loss of intrinsic O2 sensitivity (161, 205).
In adult and neonatal rodents, intermittent hypoxia augments the response of adrenomedullary chromaffin cells to acute hypoxia via ROS generated by NADPH oxidase and mitochondria, as well as by downregulation of endogenous antioxidants. Both HIF-1α and HIF-2α are involved in the augmentation of ROS activity under these conditions (275). While reversible in adult rats, facilitation of catecholamine secretion from chromaffin cells by intermittent hypoxia in rat pups (P0-P5) persists after >30 days of subsequent normoxia. In contrast, continuous hypobaric hypoxia (P0-P5) blunts catecholamine secretion (305).
Adenosine A2A and A2B receptors have been identified on rat pheochromocytoma cells (PC12), while only A2B receptors have been detected on plasma membranes of bovine adrenal chromaffin cells (43, 173). Whether A2A and/or A2B receptors are expressed in sheep adrenomedullary chromaffin cells has not been reported; however, the predominant plasma norepinephrine response to systemic administration of an A2A receptor agonist (CGS21680) suggests that A2A receptor activation is not directly involved in catecholamine release by the adrenal medulla in near-term fetal sheep (189). Epinephrine and norepinephrine responses have not been studied in younger fetuses when chromaffin responses to hypoxia more clearly result from a direct, nonneurogenic mechanism.
ADRENAL CORTEX.
Adenosine modulates cortisol production by the adrenal cortex. In fetal sheep, adenosine dampens the cortisol response to hypoxia by blunting corticotrophin release and by direct effects on the adrenal cortex (45). Depressant effects on adrenal secretion of cortisol are mediate by A1 receptors (150).
A2A receptors and fetal pituitary.
In the ovine fetus, acute hypoxia (preductal PaO2 ∼15 Torr) elicits a rapid rise in plasma arginine vasopressin (AVP) concentrations, with peak levels ∼24 times normoxic values (190). This AVP response to hypoxia is mimicked by intravascular administration of a selective A2A receptor agonist (CGS21680) in normoxic fetuses, which is unaltered by bilateral denervation of the carotid bodies and section of the cervical vagi. Nonselective blockade of adenosine receptors abolishes ∼75% of the hypoxia-induced rise in AVP (190). These results are consistent with a critical role of A2A receptor activation in the fetal AVP response to acute O2 deficiency.
A2A receptors and fetal myocardium.
Acute hypoxia (ΔPaO2, about −10 Torr) reduces fetal plasma volume by ∼9% through mechanisms that include increased sympathetic activity and a rise in plasma concentrations of atrial natriuretic peptide (49). About 50% of the hypoxia-induced elevations in plasma atrial natriuretic peptide likely involve A2A receptor enhancement of sympathetic activity and AVP release as well as direct stimulation of atrial A1 receptors (250).
Metabolism
Adenosine and AMPK are potentially involved in hypoxic metabolic perturbations, either directly through effects on the fetus or indirectly through actions on the placenta.
A2A receptors and fetal glucose metabolism.
Hypoxia increases fetal plasma glucose levels by reducing glucose consumption and increasing circulating catecholamine levels, which inhibit insulin release and promote hepatic glycogenolysis. Selective blockade of either adenosine A1 or A2A receptors blunts the hyperglycemia, indicating that both receptor subtypes are involved in these metabolic perturbations (216).
Oxygen deprivation shifts ATP production from mitochondria to anaerobic glycolysis in cytosol, a metabolic adaptation that is mediated in adult tissues by HIF-1 (310). Although stimulation of A2A receptors has been linked to HIF-1 induction (7, 71), this adenosine receptor subtype does not modulate the rise in plasma lactate levels in acutely hypoxic, near-term fetal sheep (216).
AMPK and fetal metabolism.
Besides redistributing fetal cardiac output, hypoxia and/or nutritional deficiency may impair growth by upregulating AMPK activity (via activation of adenosyl kinase and the ↑AMP/ATP ratio) in fetal tissues. In adult mammals, AMPK activation inhibits synthesis of fatty-acids, glycogen, and protein, while promoting glycolysis, glucose uptake, and other catabolic effects (127, 340). At least part of these effects of AMPK is mediated through inhibition of mammalian target of rapamycin, a kinase that promotes the efficiency of translation initiation (312). AMPK activity is inversely related to adipogenesis in fetal sheep muscle (339), which is consistent with a role in fetal metabolic homeostasis. Adverse intrauterine conditions may result in dysregulation of AMPK activity postnatally, predisposing in later life to obesity, diabetes, and the metabolic syndrome (297).
Placenta and fetal metabolism.
Oxygen defficiency, via HIF-1, may also reduce fetal growth by restricting placental transfer of substrates to the fetus. One proposed mechanism involves HIF-1-mediated fall in placental mitochondrial O2 consumption and increase in anaerobic glucose consumption (140). This metabolic reprogramming of the placenta would increase fetal O2 delivery but at the expense of lowering glucose transfer and thus fetal growth. Other alterations in placental transport and metabolism can be involved in fetal undernutrition (57).
Vasculature
In the fetus and newborn, inherent vascular responses to changes in arterial Po2 are particularly notable for the brain, heart, and lungs, and the ductus arteriosus. These direct effects of O2 are involved primarily in circulatory adjustments to hypoxia in the fetus (148, 263) as well as to the rise in PaO2 at birth. Adenosine A2A receptors, which are widely distributed in vasculature, are potentially involved in autocrine/paracrine and hormonal vascular responses to hypoxia, including hypoxia-induced angiogenesis (4, 324).
A2A receptors and the brain.
Hypoxia increases cerebral vascular conductance in the ovine fetus, a response that is augmented with gestational age and is generally independent of endothelium (128, 261). Stimulation of A2 receptors accounts for ∼50% of the cerebrovascular response to acute hypoxia in older fetuses (>0.8 term). As in adult rats (56), the A2A receptor subtype likely mediates the vasodilatation, although a contribution by A2B receptors cannot be excluded (26, 199). Besides direct vascular actions, the relaxant effects of adenosine on the cerebral vasculature may involve indirect effects via A2A-mediated release of arginine vasopressin, a vasodilator of the fetal cerebral circulation (190).
A2A receptors and the heart.
Adenosine is a potent dilator of coronary arteries in fetal sheep (336). A2A receptors likely mediate the vasodilation, as in most mature mammals (242, 341), although this action has not been established. The maximum coronary flow induced by exogenous adenosine in the fetus is less than the response to hypoxia, indicating that nitric oxide and other factors are also involved in hypoxia-induced coronary vasodilatation (128, 336).
A2A receptors and the lungs.
The fetal pulmonary circulation has a very high vascular resistance due to lack of gaseous alveolar expansion as well as the low fetal PaO2 (67, 107). Local factors primarily regulate pulmonary vascular resistance, although carotid body chemoreflexes enhance pulmonary vasosconstriction in acute hypoxia (233). As in postnatal mammals, the direct constrictor effects of hypoxia on pulmonary artery smooth muscle cells is endothelium independent and likely involves ROS generated principally from mitochondria, inhibition of O2-sensitive K+ channels, and a rise in [Ca2+]i (9, 353, 354).
A2A RECEPTORS AND FETAL PULMONARY ARTERY.
In fetal sheep, exogenous adenosine increases pulmonary vascular conductance through activation of A2A receptors and release of nitric oxide (174, 175, 323). The effects of selective A2A receptor blockade on pulmonary artery conductance have not been reported; therefore, the extent to which endogenous adenosine modulates pulmonary vascular resistance in the fetus remains to be established. Postnatally, A2A receptors have a key role in modulating pulmonary vascular resistance because genetic inactivation of murine A2A receptors confers pulmonary hypertension (363).
A2A RECEPTORS AND POSTNATAL PULMONARY ARTERY.
Gaseous expansion of the lungs and the abrupt rise in PaO2 at birth substantially reduce pulmonary vascular resistance, which is associated with ∼10-fold increase in pulmonary artery blood flow (329). Whether A2A receptors have a significant role in modulating pulmonary vascular conductance in the newborn has yet to be determined. Recruitment and distention in the pulmonary vasculature as well as arterial remodeling further reduce pulmonary vascular resistance over the following 2 wk in human newborns (107). As a result of the fall in pulmonary vascular resistance, ductal flow is reversed (aorta to pulmonary artery) within 60 min after birth. In contrast, right-to-left shunting of blood persists in pulmonary hypertension of the newborn (107).
A2A receptors and the ductus arteriosus.
The high vascular resistance in the fetal pulmonary circulation necessarily results in 80–90% of right ventricular output being shunted, via the ductus arteriosus, to the descending aorta (224, 298).
A2A RECEPTORS AND DUCTAL PATENCY.
In sheep and baboons, prostaglandin E2 (via EP2, EP3, EP4 receptors) and other vasoactive modulators maintain the patency of the ductus arteriosus at the normally low fetal PaO2 via activation of adenylyl cyclase, KATP channels, and probably protein kinase A-regulated pathways (211). In the ovine fetus, cytosolic Ca2+ efflux, via the forward mode of Na+/Ca2+ exchanger, also appears to be involved in maintaining reduced [Ca2+]i in smooth muscle cells of the ductus arteriosus (134).
Adenosine, which stimulates cAMP-dependent pathways via A2A receptors, has been implicated in patency of the ductus arteriosus in utero (223). But a significant role for adenosine is unlikely because caffeine lacks in vitro effects on ductal tension (50), and intravascular administration of a potent, nonselective adenosine receptor antagonist (8-phenyltheophylline) does not reduce ductal blood flow in vivo in normoxic fetal sheep (B. J. Koos, unpublished observations).
MODULATORS OF DUCTAL CLOSURE.
The acute rise in PaO2 at birth is associated with constriction of ductal smooth muscle, a functional closure that involves inhibition of O2-sensitive K+ channels, activation of L-type Ca2+ channels, release of Ca2+ from sarcoplasmic reticulum, and increased Ca2+ sensitization of actin-myosin filaments (135, 156). The constricted lumen subsequently undergoes morphological remodeling and anatomical closure.
Administration of potent nonsteroid anti-inflammatory agents to pregnant women is a particular concern because these inhibitors of prostaglandin synthesis can constrict the ductus arteriosus, particularly when administered after 32 wk of gestation. Postnatally, these drugs are therapeutically useful in premature newborns with persistent ductal patency that results from immaturity of the oxygen-dependent constriction cascade (156). On the other hand, prostaglandin E therapy can be critical to maintain ductal patency in neonates with congenital heart disease and ductal-dependent pulmonary blood flow.
A2A receptors and fetoplacental vasculature.
Clear fluid fills the intervillous space until the intervillous circulation becomes established by ∼11 wk of gestation when trophoblastic occlusion of the spiral arteries regresses (37, 256). As a result, early human development occurs in a hypoxic environment. A critical regulator of cell proliferation, angiogenesis, and probably extravillous trophoblast invasion, this low Po2 modulates organogenesis in the embryo and may protect against ROS-induced teratogenesis (36, 256, 278, 310, 312). The onset of uteroplacental blood flow greatly increases oxygen delivery, supporting increased placental metabolism and fetal growth. All four adenosine receptor subtypes have been identified in syncytiotrophoblast, endothelial cells, and myo/fibroblasts of the human placenta, which implicates adenosine in the regulation of placental angiogenesis, vascular tone, and transfer of nutrients and oxygen (84, 286, 307, 316, 343).
HYPOXIA AND UMBILICAL ARTERY CONDUCTANCE.
The umbilical vasculature, which lacks autonomic innervation, is regulated by hormonal and autocrine/paracrine factors (243, 270). In fetal lambs, the umbilical arteries and placental vasculature contribute ∼80% of the total resistance to umbilical blood flow (257). Acute hypoxia (postductal PaO2, ∼13 Torr) transiently reduces umbilicoplacental vascular conductance, but greater O2 deficiency augments conductance (98, 257, 332). In both cases, umbilical blood flow increases primarily from the rise in perfusion pressure, with greater involvement of local and/or circulating vasodilators at lower O2 tensions. Increased villous capillary blood flow in hypoxia would be expected to enhance placental O2 transfer under these conditions (209).
A2A RECEPTORS AND UMBILICAL CONDUCTANCE.
In sheep, the fetoplacental vasculature exhibits a biphasic response to systemic infusion of adenosine/transient vasoconstriction followed by prolonged vasodilatation (285). In vitro studies have implicated adenosine A2B receptors in vasoconstriction (75) and A2 receptors in vasodilatation (280) of human chorionic vessels. Although not specifically studied, the vasodilatory response is likely mediated by A2A receptors. Thus, adenosine (via A2A receptors), atrial natriuretic peptide, calcitonin gene-related peptide, prostaglandins, and probably other factors appear to be involved in the enhancement of umbilical vascular conductance in hypoxia (98, 250, 280, 285, 332).
In human fetuses, the placental fraction of the combined ventricular output is less in fetuses with chronic O2 deficiency and growth restriction from placental dysfunction, even though the combined ventricular output per kilogram estimated weight remains unchanged. Of particular interest is that the reduced umbilical blood flow, which is evident by 20–24 wk of gestation, precedes the detected impairment of fetal growth and abnormal Doppler velocimetry of the umbilical artery (292). Whether the elevated circulating adenosine concentrations in growth-restricted fetuses (377) modulate fetoplacental blood flow via A2A receptors remains to be determined.
A2A RECEPTORS AND PLACENTAL BLOOD-FLOW DISTRIBUTION.
Hypoxia more uniformly distributes fetal blood flow (and maternal flow) in the ovine placenta, improving the efficiency of respiratory gas exchange (273). Several mechanisms potentially contribute to the effects of hypoxia on placental blood flow distribution, and one of which is the direct vascular effect of Po2. In human placental cotyledons in vitro, hypoxia reversibly contracts previllous arterioles in human cotyledons via inhibition of O2-sensitive voltage-gated potassium channels and activation of voltage-gated calcium channels (124, 167). The role A2A receptors in this in vitro vascular response warrants investigation. The significance of O2-modulation of precapillary arteriolar resistance in the placenta in vivo remains controversial (98, 257, 332).
A2A RECEPTORS AND UMBILICAL VENOUS BLOOD-FLOW DISTRIBUTION.
In sheep and humans, hypoxia increases the proportion of umbilical venous flow that passes through the ductus venosus at the expense of the liver. This redistribution enhances the O2 content of blood flowing through the foramen ovale and subsequently to the heart and brain, while reducing O2 delivery to the right lobe of the liver (21, 77, 166, 169, 258, 286, 326, 327, 328). The diversion of hepatic venous blood to the ductus venosus results, at least in part, from the vasoconstrictive effects of circulating catecholamines on hepatic veins (258); and thus activation of A2A receptors could be involved indirectly via stimulation of the sympathetic nervous system.
A2A RECEPTORS AND PREECLAMPSIA.
Placental hypoxia and/or abnormalities of O2 sensing have been linked to preeclampsia (141, 279, 293, 318), and ROS from hypoxia-reoxygenation have been implicated in placental damage (139). Stable (31) or fluctuating elevations in spiral artery vascular resistance as well as increased heterogeneity of blood flow in the intervillous space are potentially involved in the placental pathophysiology of preeclampsia.
In preeclampsia, adenosine levels in the umbilical vein are elevated threefold in fetuses with abnormally high impedances in the umbilical artery with elevated adenosine concentrations present even in normoxic fetuses (374). Preeclampsia is also associated with elevated maternal plasma adenosine concentrations, which supports a more general systemic disruption in adenosine production, uptake, and/or metabolism (83, 154, 158, 311, 370–373, 375, 376).
In preeclampsia, A2A and other adenosine receptor subtypes have enhanced expression in microvascular endothelium of the placenta. Although hypoxia increases A2A receptor expression in normal term placenta in vitro, low Po2 in fetuses with growth restriction without preeclampsia does not affect placental adenosine receptor expression. Thus, factors other than O2 deficiency per se are likely involved in the preeclampsia-associated alteration of placental adenosine receptor expression.
Limitations
Studies of unanesthetized, chronically catheterized sheep have many advantages, including measuring cardiorespiratory responses to hypoxia that are free from pharmacologic analgesia and surgical stress and that reflect integrated physiologic mechanisms. The involvement of peripheral and central O2 chemoreceptors was inferred from the time course of cardiorespiratory responses, effects of polar agents that poorly cross the blood-brain barrier, peripheral arterial chemodenervation, and localized neuronal lesions. With respect to the latter, the relatively large size of the fetal sheep brain provides sufficient spatial resolution to identify posteromedial thalamic sectors involved in hypoxic inhibition. However, this approach has limitations in studying neuronal circuits or communication within or between cells, which can be more easily carried out in reduction preparations.
Sheep fetuses differ from human fetuses in several respects, including epitheliochorial placentation, lower hemoglobin concentrations, lack of a fetal zone in adrenal glands, reduced brain size relative to body weight, and greater neurologic maturity at birth. Nevertheless, ultrasound imaging and Doppler velocimetry studies in human fetuses have confirmed qualitatively the major cardiorespiratory measurements that have been observed in normoxic and hypoxic sheep fetuses.
The physiologic role of adenosine A2A receptors in fetal sheep and developing lambs derives from studies with potent, highly specific agonists and antagonists to A1 and A2A receptors. While these pharmacologic agents have sufficient selectivity to distinguish between A1, A2A, and A3 receptor activation in sheep, they cannot exclude the involvement of A2B receptors (178, 184, 186, 187, 188).
Among mammals, the brain has similarities in the general sequence of brain growth and brain composition, although the timing of the brain-growth spurt relative to birth varies greatly among species (73). Thus, stages of brain development, rather than prenatal or postnatal ages, are often more useful for interspecies comparisons of central physiologic mechanisms. Based on brain growth spurts, the newborn rat, rabbit, or mouse roughly corresponds to an 18-wk human fetus, the neonatal piglet approximates a term human fetus, and the newborn lamb and rhesus monkey resemble a 15-mo-old human infant (73). Other models have been proposed to predict the timing of developmental events in metatherian and eutherian mammals (62).
Referencing maturation to the pattern of brain growth should be used cautiously because not all physiologic mechanisms develop in a parallel manner. For example, those that initiate labor are normally associated with pulmonary maturity, suckling, and gastrointestinal function even though brain development is delayed, as in altricial species (e.g., mice, rats, rabbits). The transition to postnatal life also involves an increase in PaO2 and the establishment of pulmonary ventilation, which change the function and central integration of O2 sensing by the carotid bodies. Thus comparisons among species should be made in light of functional maturity of the involved physiologic mechanism as well as age. Although the neonatal sheep brain is relatively mature, the physiologic responses of the carotid body and brain to hypoxia are qualitatively similar to those of less neurologically mature newborn mammals.
SUMMARY
In fetal sheep, adenosine A2A receptors are critically involved in acute O2 sensing by both the carotid bodies and brain (Table 5) as well as in hypoxic regulation of endocrine systems, metabolism, and vascular conductance. Activation of A2A receptors accounts for the complete expression of hypoxic carotid chemoreflexes that are involved in reducing myocardial oxygen consumption by lowering heart rate and in redistributing cardiac output (and O2 delivery) to essential organs by supporting or increasing perfusion pressure. Stimulation of central A2A receptors contributes to a regulated reduction in fetal O2 consumption by inhibiting rapid eye movement sleep and breathing. Postnatally, A2A receptors do not have a significant role in transduction processes of carotid O2 sensing in developing lambs, whereas A2A and A2B receptors contribute to about half of hypoxic hyperventilation in adult rats. Activation of brain A2A receptors mediates the second-phase ventilatory fall in hypoxia in developing lambs and likely other species as well. In the fetus, adenosine A2A receptors in carotid bodies and the brain are crucially involved in triggering cardiobehavioral adaptations to acute hypoxia that support mitochondrial energy supply to critical organs. Postnatally, the importance of adenosine A2A receptors in hypoxic carotid chemoreflexes likely depends on age as well as species, while activation of central A2A receptors remains critically involved in hypoxic respiratory depression.
Table 5.
O2 sensing in sheep
| Carotid Body | Brain* | |
|---|---|---|
| Fetus (>0.8 term) | ||
| Modulator | Adenosine | Adenosine |
| Receptor | A2A | A2A |
| Action | ↓ Heart rate | ↓ Breathing |
| ↑ Arterial pressure | ↓ REM | |
| Lamb (1–2 wk of age) | ||
| Modulator | Not established | Adenosine |
| Receptor | Not established | A2A |
| Action | ↑ Ventilation (phase 1) | ↓ Ventilation (phase 2) |
| ↑ Heart rate** | ||
| ↑ Arterial pressure | ||
| Arousal |
REM, rapid-eye movements.
Functional brain O2 sensor that inhibits breathing and REM;
secondary to increased ventilation.
CLINICAL PERSPECTIVE
The mitochondrial respiratory chain is involved in many physiologic adaptations and pathophysiologic disorders in development through mechanisms that include release of superoxide anion radicals and adenosine.
A2A Receptors and Perinatal Hypoxia
Activation of A2A receptors has particular relevance to hypoxia in the fetus and newborn.
Fetal assessment.
The clinical identification of hypoxic fetuses depends critically upon activation of adenosine A2A receptors in the fetal carotid bodies and brain.
FETAL HEART RATE.
Fetal heart rate monitoring is commonly performed to detect fetal heart rate decelerations, which are chemoreflexes triggered by hypoxic activation of carotid body A2A receptors. Acute reductions in fetal O2 delivery associated with repetitive late or severe variable fetal heart rate decelerations release catecholamines, which progressively increase baseline heart rate. Adenosine likely contributes to the rise in heart rate by stimulating sympathetic activity via A2A receptors and by activating A2A receptors in myocardium (189). The decline in fetal heart rate that eventually develops in prolonged, severe hypoxia represents preterminal myocardial decompensation.
FETAL BREATHING.
Fetal breathing movements are one of the most sensitive components of the biophysical profile in the detection of fetal hypoxia. Falls in brain Po2 inhibit breathing through activation of central A2A receptors. The relevance of central A2A receptors to limb and body movements has not been investigated.
MIDDLE CEREBRAL ARTERY DOPPLER VELOCIMETRY.
Significant reductions in middle cerebral artery (MCA) impedance, which reflect increased vascular conductance, are used clinically to detect fetal hypoxia (70). A2A receptors appear to have a major role in hypoxic cerebrovasodilation. In contrast, the hemodynamics of reduced blood viscosity, rather than oxygen deprivation per se, largely account for the elevated Doppler peak systolic velocities of fetal anemia (66, 277, 344).
UMBILICAL ARTERY DOPPLER VELOCIMETRY.
Doppler studies of the umbilical artery are used to assess the well being of growth-restricted fetuses, with elevated impedances generally reflecting reduced conductance in the umbilical circulation (6, 95, 218, 235). Absent or reversal of diastolic flow signifies further decompensation. Elevated impedance of the umbilical artery largely derives from remodeling of previllous resistance vessels due most likely to the release of oxygen radicals, nitric oxide derivatives, and other factors (92, 124, 147, 167, 281). Further work should be performed on the potential role of A2A receptors in modulating fetoplacental vascular conductance in normal and pathologic states.
Fetuses can be hypoxic even though Doppler velocimetry of the umbilical artery is normal (46, 58, 313). In such cases, the placenta presumably lacks the typical vasculopathy of chronic hypoxia (170, 235–237, 240, 284, 326). Thus, the pulsatility indices of the MCA are generally a better indicator of the sufficiency of fetal oxygenation than those of the umbilical artery (291).
Hypoxic brain injury.
Activation of vascular A2A receptors likely contributes substantially to the hypoxia-induced increase in cerebrovascular conductance, brain blood flow, and O2 delivery thus buffering against neurologic injury due to fetal O2 deficiency. ROS generated by NADPH oxidase contributes to vasodilatation, but excessive ROS production (via NADPH oxidase, xanthine oxidase or mitochondrial respiratory chain) in severe hypoxia or ischemia causes vasoconstriction. This fall in conductance further impairs brain O2 delivery and exacerbates neuronal injury (via ROS, glutamate neurotoxicity, metalloproteinase activity, and inflammation), leading to hypoxic-ischemic encephalopathy, seizures, stroke, and long-term disabilities (121, 203, 249, 294, 331, 338).
Despite the rise in cerebral blood flow, O2 delivery can be insufficient to maintain brain integrity over time even in moderate hypoxia. For example, reduced MCA impedance or a rise in brain-tissue perfusion in growth-restricted fetuses correlates with postasphyxial encephalopathy and impaired neurodevelopment (8, 58, 81, 152, 320). Loss of variable tone and a fall in conductance in the middle cerebral artery indicate cerebrovascular dysregulation and brain injury (301).
Hypoxic vasodilatation of the coronary arteries via A2A receptors likely has an important role in maintaining fetal myocardial O2 delivery and function. In severe hypoxia or ischemia, the vasoconstrictive effects of excessive ROS release counters hypoxic coronary vasodilatation, reducing myocardial O2 delivery, ATP production, and contractility (97). Cardiac function is further impaired by increased afterload from systemic arterial vasoconstriction. These conditions create a feed-forward mechanism of myocardial decompensation, which, in turn, further compromises brain blood flow and oxygen delivery and exacerbates neurologic injury.
Placental dysfunction.
Abnormalities of placental growth and vascular function have been attributed, in part, to enhanced release of ROS and/or reduced production of enzymatic and nonenzymatic antioxidants. Such increased oxidative stress has been implicated in the pathophysiology of preterm birth, intrauterine growth restriction, and preeclampsia (28, 65, 158, 159, 370). Clinical trials lack consensus on benefits of antioxidant therapy in preeclampsia (122).
With respect to adenosine, it remains to be determined whether the preeclampsia-associated perturbations in circulating adenosine concentrations and in adenosine receptor expression in placental microvasculature arise as compensations to vascular dysregulation or as part of the pathophysiology of the disorder. It is also unknown whether adenosine is a modulating factor in the increased risk of stroke in adult offspring of preeclamptic pregnancies (155).
Fetal growth restriction.
Umbilical venous adenosine concentrations are elevated in human fetuses with growth restriction, with adenosine levels inversely proportional to Po2 (374, 377). The increased adenosine levels likely contribute to metabolic and circulatory adaptations that protect the fetus against chronic O2 deficiency, and the extent of A2A receptor involvement in these compensations warrants further exploration.
Chronic hypoxia impairs fetal growth through several mechanisms that potentially include upregulation of fetal AMPK activity, increased placental anaerobic glucose metabolism, and other placental adaptations (57, 127, 312, 340). Elevated adenosine concentrations (via A1 and A2A receptors) may also modulate fetal metabolism and growth under these conditions, and this possibility should be explored. Besides perinatal complications (Table 2), poor fetal growth is associated with epigenetic changes in postnatal physiological regulation that predispose to major adult diseases, as summarized in Table 6 (14, 36, 80, 83, 220, 244, 278, 293, 335).
Table 6.
Adult diseases predisposed by poor fetal growth
| Diabetes, type II |
| Hypertension |
| Stroke |
| Heart disease |
| Osteoporosis |
Disorders of prematurity.
Premature infants, who have reduced antioxidant production, are predisposed to ROS injury due to the rise in postnatal PaO2 and the resulting increase in superoxide anion radical formation by the mitochondrial respiratory chain. Excessive oxidative stress has been implicated in several diseases of prematurity, including bronchopulmonary dysplasia, periventricular leukomalacia, and retinopathy (65).
Pulmonary hypertension.
In the human neonate, pulmonary hypertension most commonly arises from respiratory failure due to pneumonia, respiratory distress syndrome, meconium aspiration syndrome, or pulmonary hypoplasia (107). Pulmonary hypertension can occasionally be a significant cause of hypoxemia in growth-restricted, premature infants (61). Impairment of A2A receptor function in the pulmonary vasculature may be involved in the development of pulmonary hypertension (363).
Disorders of cardiorespiratory control.
Given the prolonged, if not permanent, dampening of acute hypoxic respiratory responses reported in mammals by sustained hypoxic exposure in the perinatal period, there is concern that such exposures in early human development, as in growth-restricted fetuses, might blunt postnatal protective arousal and cardiorespiratory responses to obstructive sleep apneas or other forms of hypoxic stress.
Intermittent hypoxia in the perinatal period can result from repetitive compressions of the umbilical cord in the fetus and from recurrent central or obstructive sleep apneas in preterm infants. In addition to ROS, fluctuations in adenosine levels, caused by episodic arterial O2 desaturations, potentially contribute to the enhancement of carotid body O2 sensitivity by activating A2A receptors that modulate gene transcription and translation (71, 76, 266, 295). Augmented carotid O2 sensitivity can lead to respiratory instability, sympathetic overactivity, hypertension, and brain lesions (102, 113, 137, 144, 212, 213, 247). Furthermore, hypoxia-induced rises in brain adenosine levels, via activation of central A2A receptors, potentially blunt hypoxic arousal and hyperventilation induced by afferent chemosensory discharges. Thus, sustained functional disruptions in peripheral and/or central O2 sensors, or the central integration thereof, in fetuses or premature infants may depress arousal and respiratory responses to hypoxia and thus predispose susceptible sleeping infants and adults to sudden death (96, 102, 137, 365).
A2A Receptors and Perinatal Caffeine Exposure
Long-term stimulant effects of caffeine appear to involve blockade of A1 and A2A receptors (368); and thus the question naturally arises as to whether maternal consumption of caffeine has adverse perinatal consequences.
Development disorders.
Exposure of pregnant mice to the equivalent of two cups of coffee per day from E8.5 to E10.5 substantially inhibits hypoxia-induced HIF-1α protein expression and cardiac ventricular development in embryos, resulting in long-term impairment of cardiac function in adult offspring (356). Modest maternal subcutaneous injections of caffeine (daily, E9.5 to E18.5) adversely affects embryonic cardiovascular function and growth, possibly through A2A receptor blockade (226). Encouragingly, clinical studies have not found that caffeine increases the risk of developmental abnormalities, and they lack consensus on increased risk for spontaneous abortion.
Impairment of fetal hypoxic responses.
Another concern to be considered is whether blockade of A2A receptors by caffeine would alter fetal breathing and heart rate responses, thereby rendering clinical assessment less reliable. In normal human fetuses, maternal coffee ingestion increases breathing incidence, presumably through antagonism of central A2A receptors that modulate fetal behavioral state (187, 302). By antagonizing A2A receptors, caffeine potentially blunts falls in heart rate and breathing incidence that would normally occur in hypoxic fetuses, but this is not likely a concern for normal fetuses whose mothers drink two or fewer cups of coffee (<200 mg caffeine) per day.
In sheep, caffeine enhances fetal oxygen consumption (via A1 receptor blockade) and lowers brain Po2 (337). Excessive maternal caffeine consumption in human pregnancy has been implicated in fetal growth restriction (40). These observations support the need for limited caffeine intake in pregnancy, with avoidance in gravidas with compromised fetuses.
Long-term regulatory changes.
In rats, perinatal exposure to caffeine induces long-term changes in sleep and cardiorespiratory regulation, which likely involve changes in the expression and/or interactions of A2A receptors in the carotid body and brain (12, 227, 228). Whether this occurs in humans is unknown, but this possibility is particularly relevant given the widespread use of caffeine in treating apnea of prematurity.
GRANTS
This study was supported in part by National Institute of Child Health and Human Development Grant HD-18478.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Acker H. The oxygen sensing signal cascade under the influence of reactive oxygen species. Phil Trans R Soc B 360: 2201–2210, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Acker T, Acker H. Cellular oxygen sensing need in CNS function: physiological and pathophysiological implications. J Exp Biol 207: 3171–3188, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Ackland GL, Noble R, Hanson MA. Red nucleus inhibits breathing during hypoxia in neonates. Respir Physiol 110: 251–260, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol 289: R283–R296, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Adams MB, Simonetta G, McMillen IC. The non-neurogenic catecholamine response of the fetal adrenal to hypoxia is dependent on activation of voltage sensitive Ca2+ channels. Dev Brain Res 94: 182–189, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Adamson SL, Morrow RJ, Langille BL, Bull SB, Ritchie JWK. Site-dependent effects of increases in placental vascular resistance on the umbilical arterial velocity waveform in fetal sheep. Ultrasound Med Biol 16: 19–27, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Alchera E, Tacchini L, Imarisio C, Dal Ponte C, De Ponti C, Gammella E, Cairo G, Albano E, Carini R. Adenosine-dependent activation of hypoxia-inducible factor-1 induces late preconditioning in liver cells. Hepatology 48: 230–239, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Arbeille P. Fetal arterial Doppler-IUGR and hypoxia. Eur J Obstet Gynecol Reprod Biol 75: 51–53, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Archer SL, Michelakis ED, Théband B, Bonnet S, Moudgril R, Wu XC, Weir EK. A central role for oxygen-sensitive K+ channels and mitochondria in the specialized oxygen-sensing system. Novartis Found Symp 272: 157–71, 2006 [PubMed] [Google Scholar]
- 10. Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 7: 4030–4034, 1968 [DOI] [PubMed] [Google Scholar]
- 11. Bairam A, Carroll JL. Neurotransmitters in carotid body development. Resp Physiol Neurobiol 149: 217–232, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Bairam A, Joseph V, Lajeunesse Y, Kinkead R. Altered expression of adenosine A1 and A2A receptors in the carotid body and nucleus solitarius of adult male and female rats following neonatal caffeine treatment. Brain Res 1287: 74–83, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Bamford O, Hawkins RL. Central effects of an α2-adrenergic antagonist on fetal lambs: a possible mechanism for hypoxic apnea. J Dev Physiol 13: 353–358, 1990 [PubMed] [Google Scholar]
- 14. Barker DJP, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31: 1235–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Bartelds BF, van Bel F, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res 34: 51–55, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Baschat AA. Fetal responses to placental insuffiency: an update. BJOG 111: 1031–1041, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Baschat AA. Fetal growth restriction-from observation to intervention. J Perinat Med 38: 239–246, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol 104: 1220–1229, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Bavis RW, Olson EB, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic response in rats. J Appl Physiol 95: 1550–1559, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Bavis RW, Young KM, Barry KJ, Boller MR, Kim E, Klein PM, Ovrutsky AR, Rampersad DA. Chronic hyperoxia alters the early and late phases of the hypoxic ventilatory response in neonatal rats. J Appl Physiol 109: 796–803, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bellotti M, Pennati G, De Gasperi C, Bozzo M, Battaglia FC, Ferrazzi E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. Am J Obstet Gynecol 190: 1347–1358, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Bian X, Fu M, Mallet RT, Bünger R, Downey HF. Myocardial oxygen consumption modulates adenosine formation by canine right ventricle in absence of hypoxia. J Mol Cell Cardiol 32: 345–354, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol 278: R1391–R1400, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Blanco CE, Dawes GS, Hanson MA, McCooke MB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol 351: 25–37, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blanco CE, Hanson MA, McCooke HB. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. J Dev Physiol 10: 167–174, 1988 [PubMed] [Google Scholar]
- 26. Blood AB, Hunter CJ, Power GG. Adenosine mediates decreased cerebral metabolic rate and increased cerebral blood flow during acute moderate hypoxia in the near-term fetal sheep. J Physiol 553: 935–45, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bournaud R, Hilalgo J, Yu H, Girard E, Shimahara T. Catecholamine secretion from rat foetal adrenal chromaffin cells and hypoxic sensitization. Pflügers Arch Eur J Physiol 454: 83–92, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Braekke K, Harsem NK, Staff AC. Oxidative stress and antioxidant status in fetal circulation in preeclampsia. Pediatr Res 60: 560–564, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Breen S, Rees S, Walker D. Identification of brainstem neurons responding to hypoxia in fetal and newborn sheep. Brain Res 748: 107–121, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Brooks DJ, Doder M, Osman S, Luthra SK, Kirani E, Hume S, Kase H, Kilborn J, Martindill S, Mori A. Positron emission tomography analysis of [11C]KW-6002 binding to human and rat adenosine A2A receptors in the brain. Synapse 62: 671–681, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Browne VA, Toledo-Jaldin L, Davila RD, Lopez LP, Yamashiro H, Cioffi-Ragan D, Julian CG, Wilson MJ, Bigham AW, Shriver MD, Honigman B, Vargas E, Roach R, Moore LG. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol 300: R1221–R1229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol 157: 55–64, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Bureau MA, Lamarche J, Foulson P, Dalle D. The ventilatory response to hypoxia in the newborn lamb after carotid body denervation. Respir Physiol 60: 109–119, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Burrage DM, Braddick L, Cleal JK, Costello P, Noakes DE, Hanson MA, Green LR. The late gestation fetal cardiovascular response to hypoglycaemia is modified by prior peri-implantation undernutrition in sheep. J Physiol 587: 611–624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burrage D, Green LR, Moss TJM, Sloboda DM, Nitsos I, Newnham JP, Hanson MA. The carotid bodies influence growth responses to moderate maternal undernutrition in late-gestation fetal sheep. BJOG 115: 261–268, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215: 27–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol 54: 303–311, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Comm 322: 82–87, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Campbell S, Vyas S, Nicolaides KH. Doppler investigation of the fetal circulation. J Perinat Med 19: 21–26, 1991 [DOI] [PubMed] [Google Scholar]
- 40. CARE Study Group Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ 337: a2332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carroll JL, Kim I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir Physiol Neurobiol 149: 201–215, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Carter AM. Factors affecting gas transfer across the placenta and the oxygen supply to the fetus. J Dev Physiol 12: 305–322, 1989 [PubMed] [Google Scholar]
- 43. Casadó V, Casillas T, Mallol J, Canela EI, Lluis C, Franco R. The adenosine receptors present on the plasma membrane of chromaffin cells are of the A2b subtype. J Neurochem 59: 425–4321, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Chandel NS. Mitochondrial complex III: An essential component of universal oxygen sensing machinery? Respir Physiol Neurobiol 174: 175–181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chau A, Rose JC, Koos BJ. Adenosine modulates corticotropin and cortisol release during hypoxia in fetal sheep. Am J Obstet Gynecol 180: 1272–1277, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Cheema R, Dubiel M, Gudmundsson S. Fetal brain sparing is strongly related to the degree of increased placental vascular impedance. J Perinat Med 34: 318–322, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Chen J, Dinger B, Fidone SJ. cAMP production in rabbit carotid body: role of adenosine. J Appl Physiol 82: 1771–1775, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Cheung CY. Fetal adrenal medulla catecholamine response to hypoxia-direct and neural components. Am J Physiol Regul Integr Comp Physiol 258: R1340–R1346, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Cheung CY. Autonomic and arginine vasopressin modulation of the hypoxia-induced atrial natriuretic factor release in immature and mature ovine fetuses. Am J Obstet Gynecol 167: 1443–1453, 1992 [DOI] [PubMed] [Google Scholar]
- 50. Clyman RI, Roman C. The effects of caffeine on the preterm sheep ductus arteriosus. Pediatr Res 62: 167–169, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Conde SV, Gonzalez C, Batuca JR, Monteiro EC, Obeso A. An antagonistic interaction between A2B adenosine and D2 dopamine receptors modulates the function of the rat carotid body chemoreceptor cells. J Neurochem 107: 1369–1381, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Conde SV, Monteiro EC. Hypoxia induces adenosine release from the rat carotid body. J Neurochem 89: 1148–1156, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Conde SV, Monteiro EC, Obeso A, Gonzalez C. Adenosine in peripheral chemoreception: new insights into a historically overlooked molecule. Adv Exp Med Biol 648: 145–159, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Conde SV, Obeso A, Monteiro EC, Gonzalez C. The A2B-D2 receptor interaction that controls carotid body catecholamine release locates between the last two steps of hypoxic transduction cascade. Adv Exp Med Biol 648: 161–168, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Conde SV, Obeso A, Vicario I, Rigual R, Rocher A, Gonzalez C. Caffeine inhibition of rat carotid body chemoreceptors is mediated by A2A and A2B adenosine receptors. J Neurochem 98: 616–628, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Coney AM, Marshall JM. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of rat by systemic hypoxia. J Physiol 509: 507–518, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cretin I, Alvino G. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta, Suppl A: S77–S82, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Cruz-Martinez R, Figueras F, Hernandez-Andrade E, Puerto B, Gratacos E. Longitudinal brain perfusion changes in near-term small-for-gestational-age fetuses as measured by spectral Doppler indices or by fraccional moving blood volumen. Am J Obstet Gynecol 203: 42–46, 2010 [DOI] [PubMed] [Google Scholar]
- 59. D'Agostino D, Mazza E, Neubauer JA. Heme oxygenase is necessary for the excitatory response of cultured neonatal rat rostral ventrolateral medulla neurons to hypoxia. Am J Physiol Regul Integr Comp Physiol 296: R102–R118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daly de BM, Scott MJ. An analysis of the primary cardiovascular reflex effects of stimulation of the carotid body chemoreceptors in the dog. J Physiol 162: 555–573, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Danhaive O, Margossian R, Geva T, Kourembanas S. Pulmonary hypertension and right ventricular dysfunction in growth-restricted, extremely low birth weight neonates. J Perinatol 25: 495–499, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Darlington RB, Dunlop SA, Finlay BL. Neural development in metatherian and eutherian mammals: variation and constraint. J Comp Neurol 411: 359–368, 1999 [PubMed] [Google Scholar]
- 63. Darnal RA. Aminophylline reduces hypoxic ventilatory depression: possible role of adenosine. Pediatr Res 19: 706–710, 1985 [DOI] [PubMed] [Google Scholar]
- 64. Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest 123: 530–538, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med 15: 191–195, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Davis LE, Hohimer AR. Hemodynamic and organ blood flow in fetal sheep subjected to chronic anemia. Am J Physiol Regul Integr Comp Physiol 261: R1542–R1548, 1991 [DOI] [PubMed] [Google Scholar]
- 67. Dawes GS. Foetal and Neonatal Physiology. Chicago: Year Book Medical, 1968, pp 79–90 [Google Scholar]
- 68. Dawes GS. The central control of fetal breathing and skeletal muscle movements. J Physiol 346: 1–18, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Decking UKM, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res 81: 154–164, 1997 [DOI] [PubMed] [Google Scholar]
- 70. Degani S. Fetal cerebrovascular circulation: a review of prenatal ultrasound assessment. Gynecol Obstet Invest 66: 184–196, 2008 [DOI] [PubMed] [Google Scholar]
- 71. De Ponti C, Carini R, Alchera E, Nitti MP, Locati M, Albano E, Cairo G, Tacchini L. Adenosine A2a receptor mediated, normoxic induction of HIF-1 through PKC and PI-3K-dependent pathways in macrophages. J Leukoc Biol 82: 392–402, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol 157: 45–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dobbing J. The later development of the brain and its vulnerability. In: Scientific Foundations of Paediatrics, edited by Davis JA, Dobbing J. Baltimore: University Park Press, 1982, vol. 39, p. 744–759 [Google Scholar]
- 74. Donnelly DF. Developmental aspects of oxygen sensing by the carotid body. J Appl Physiol 88: 2296–2301, 2000 [DOI] [PubMed] [Google Scholar]
- 75. Donoso MV, Lopez R, Miranda R, Briones R, Huidobro-Toro JP. A2B adenosine receptors mediate human chorionic vasoconstriction and signals through arachidonic acid cascade. Am J Physiol Heart Circ Physiol 288: H2439–H2449, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Drumm D, Hoefer M, Juhász J, Huszár É, Sybrecht GW. Plasma adenosine during investigation of hypoxic ventilatory response. Sleep Breath 8: 31–41, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Edelstone DI. Regulation of blood flow through the ductus venosus. J Dev Physiol 2: 219–238, 1980 [PubMed] [Google Scholar]
- 78. Eden GJ, Hanson MA. Effect of hyperoxia from birth on the carotid chemoreceptor and ventilatory response to acute hypoxia (Abstract). J Physiol 374: 24P, 1986 [Google Scholar]
- 79. Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol 392: 11–19, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG 113: 580–589, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Eixarch E, Meler E, Iraola A, Illa M, Crispi F, Hernandez-Andrade E, Gratacos E, Figuera F. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol 32: 894–899, 2008 [DOI] [PubMed] [Google Scholar]
- 82. El-Kashef H, Elmazar MM, Al-Shabanah OA, Al-Bekairi AM. Effect of adenosine on pulmonary circulation of rabbits. Gen Pharmacol 32: 307–313, 1999 [DOI] [PubMed] [Google Scholar]
- 83. Escudero C, Casanello P, Sobrevia L. Human equilibrative nucleoside transporters 1 and 2 may be differentially modulated by A2B adenosine receptors in placenta microvascular endothelial cells from pre-eclampsia. Placenta 29: 816–825, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Escudero C, Puebla C, Westermeier F, Sobrevia L. Potential cell signalling mechanisms envolved in differential placental angiogenesis in mild and severe pre-eclampsia. Curr Vasc Pharmacol 7: 475–485, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Evans AM. AMP-activated protein kinase and the regulation of Ca2+ signaling in O2-sensing cells. J Physiol 574: 113–123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Evans AM, Hardie DG, Peers C, Wyatt CN, Viollet B, Kumar P, Dallas ML, Ross F, Ikematsu N, Jordan HL, Barr BL, Rafferty JN, Ogunbayo O. Ion channel regulation by AMPK. The route of hypoxia-response coupling in the carotid body and pulmonary artery. Ann NY Acad Sci 1177: 89–100, 2009 [DOI] [PubMed] [Google Scholar]
- 87. Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem 280: 41504–41511, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Fagerlund MJ, Kåhlin J, Ebberyd A, Schulte G, Mkrtchian S, Eriksson LI. The human carotid body. Expression of oxygen sensing and signaling genes of relevance for anesthesia. Anesthesiology 113: 1270–9, 2010 [DOI] [PubMed] [Google Scholar]
- 89. Ferré S, Fredholme BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20: 482–487, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Fitzgerald RS, Shirahata M, Chang I. The impact of adenosine and an A2A adenosine receptor agonist on the ACh-induced increase in intracellular calcium of the glomus cells of the cat carotid body. Brain Res 1301: 20–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fitzgerald RS, Shirahata M, Wang HYJ, Balbir A, Chang I. The impact of adenosine on the release of acetylcholine, dopamine, and norepinephrine from the cat carotid body. Neurosci Lett 367: 304–308, 2004 [DOI] [PubMed] [Google Scholar]
- 92. Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflügers Arch 459: 923–939, 2010 [DOI] [PubMed] [Google Scholar]
- 93. Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol 83: 263–276, 2007 [DOI] [PubMed] [Google Scholar]
- 94. Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001 53: 527–552, 2001 [PMC free article] [PubMed] [Google Scholar]
- 95. Galan HL, Anthony RV, Rigano S, Parker TA, de Vrijer B, Ferrazzi E, Wilkening RB, Regnault TRH. Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 192: 272–279, 2005 [DOI] [PubMed] [Google Scholar]
- 96. Gami A, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 352: 1206–1214, 2005 [DOI] [PubMed] [Google Scholar]
- 97. Gao G, Zhao X, Ahmad M, Wolin MS. Mitochondrial-derived hydrogen peroxide inhibits relaxation of bovine coronary arterial smooth muscle to hypoxia through stimulation of ERK MAP kinase. Am J Physiol Heart Circ Physiol 297: H2262–H2269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gardner DS, Poulson AS, Giussani DA. An in vivo nitric oxide clamp to investigate the influence of nitric oxide on continuous umbilical blood blood flow during acute hypoxaemia in the fetal sheep. J Physiol 537: 587–596, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gassmann M, van Patot MT, Soliz J. The neuronal control of hypoxic ventilation. Erythropoietin and sexual dimorphism. Ann NY Acad Sci 1177: 151–161, 2009 [DOI] [PubMed] [Google Scholar]
- 100. Gauda EB, Carroll JL, Donnelly DF. Developmental maturation of chemosensitivity to hypoxia of peripheral arterial chemoreceptors. Adv Exp Med Biol 648: 243–255, 2009 [DOI] [PubMed] [Google Scholar]
- 101. Gauda EB, Cooper RZ, Donnelly DF, Mason A, McLemure GL. The effect of development on the pattern of A1 and A2a-adenosine receptor gene and protein expression in rat peripheral arterial chemoreceptors. Adv Exp Med Biol 580: 121–129, 2006 [DOI] [PubMed] [Google Scholar]
- 102. Gauda EB, Cristofalo E, Nunez J. Peripheral arterial chemoreceptors and sudden infant death syndrome. Respir Physiol Neurobiol 157: 162–170, 2007 [DOI] [PubMed] [Google Scholar]
- 103. Gauda EB, Northington FJ, Linden J, Rosin DL. Differential expression of A2a, A1-adenosine and D2-dopamine receptor genes in rat peripheral arterial chemoreceptors during postnatal development. Brain Res 872: 1–10, 2000 [DOI] [PubMed] [Google Scholar]
- 104. Gaurine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol 568: 715–724, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Georgopoulos D, Holtby SG, Berezanski D, Anthonisen NR. Aminophylline effects on ventilatory response to hypoxia and hyperoxia in normal adults. J Appl Physiol 67: 1150–1156, 1989 [DOI] [PubMed] [Google Scholar]
- 106. Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci 107: 2325–2330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ghanayem NS, Gordon JB. Modulation of pulmonary vasomotor tone in the fetus and neonate. Respir Res 2: 139–144, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Giussani DA, Gardner DS, Cox DT, Fletcher AJ. Purinogenic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 280: R678–R685, 2001 [DOI] [PubMed] [Google Scholar]
- 109. Giussani DA, Moore PJ, Bennet L, Spencer JAD, Hanson MA. α1- And α2-adrenoreceptor actions of phentolamine and prazosin on breathing movements in fetal sheep in utero. J Physiol 486: 249–255, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Giussani DA, Salinas CE, Villena M, Blanco CE. The role of oxygen in prenatal growth: studies in the chick embryo. J Physiol 585: 911–917, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Giussani DA, Spencer JAD, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461: 431–449, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gleed RD, Mortola JP. Ventilation in newborn rats after gestation at simulated high altitude. J Appl Physiol 70: 1146–1151, 1991 [DOI] [PubMed] [Google Scholar]
- 113. Gleeson K, Zwillich CW. Adenosine infusion and periodic breathing during sleep. J Appl Physiol 72: 1004–1009, 1992 [DOI] [PubMed] [Google Scholar]
- 114. Gluckman PD, Johnston BM. Lesions in the upper lateral pons abolish hypoxic depression of breathing in anesthetized fetal lambs. J Physiol 382: 373–383, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gödecke A. cAMP: fuel for extracellular adenosine formation? Br J Pharmacol 153: 1087–1089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gonzalez C, Agapito MT, Rocher A, Gomez-Niño A, Rigual R, Castañeda J, Conde SV, Obeso A. A revisit to O2 sensing and transduction in the carotid body chemoreceptors in the context of reactive oxygen species biology. Respir Physiol Neurobiol 174: 317–330, 2010 [DOI] [PubMed] [Google Scholar]
- 117. Gonzalez CL, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994 [DOI] [PubMed] [Google Scholar]
- 118. Gozal D, Reeves SR, Row BW, Neville JJ, Go SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med 167: 1540–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 119. Green LR, Bennet L, Robson S, Hanson MA. The role of carotid chemoreceptors in the effects of hypoxia on renal blood flow in the late gestational sheep fetus. Exper Physiol 82: 183–192, 1997 [DOI] [PubMed] [Google Scholar]
- 120. Gudmundsson S, Dubiel M. Doppler velocimetry in the evaluation of fetal hypoxia. J Perinat Med 29: 399–407, 2001 [DOI] [PubMed] [Google Scholar]
- 121. Guo R, Hou W, Dong Y, Yu Z, Stites J, Weiner CP. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod Sci 17: 540–548, 2010 [DOI] [PubMed] [Google Scholar]
- 122. Gupta S, Agarwal A, Sharma RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv 60: 807–816, 2005 [DOI] [PubMed] [Google Scholar]
- 123. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408 [DOI] [PubMed] [Google Scholar]
- 124. Hampl V, Jakoubek V. Regulation of fetoplacental vascular bed by hypoxia. Physiol Res 58, Suppl 2: S87–S93, 2009 [DOI] [PubMed] [Google Scholar]
- 125. Hanson MA. The importance of baro- and chemoreflexes in the control of the fetal cardiovascular system. J Dev Physiol 10: 491–511, 1988 [PubMed] [Google Scholar]
- 126. Hanson MA, Kumar P, Williams BA. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in newborn kittens. J Physiol 411: 563–574, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cell energy. Nat Rev Mol Cell Biol 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 128. Harris AP, Helou S, Gleason CA, Traystman RJ, Koehler RC. Fetal cerebral and peripheral circulatory responses to hypoxia after nitric oxide synthase inhibition. Am J Physiol Regul Integr Comp Physiol 281: R381–R390, 2001 [DOI] [PubMed] [Google Scholar]
- 129. Headrick JP, Emerson CS, Berr SS, Berne RM, Matherne GP. Interstitial adenosine and cellular metabolism during β-adrenergic stimulation of the in situ rabbit heart. Cardiovasc Res 31: 699–710, 1996 [PubMed] [Google Scholar]
- 130. Headrick JP, Matherne GP, Berne RM. Myocardial adenosine formation during hypoxia: effects of ecto-5′-nucleotidase inhibition. J Mol Cell Cardiol 24: 295–304, 1992 [DOI] [PubMed] [Google Scholar]
- 131. Headrick JP, Willis RJ. Adenosine formation and energy metabolism: a 31P-NMR study in isolated rat heart. Am J Physiol Heart Circ Physiol 258: H617–H624, 1990 [DOI] [PubMed] [Google Scholar]
- 132. Hecher K, Campbell S, Doyl P, Harrington K, Nicolaides K. Assessment of fetal compromise by Doppler ultrasound investigation of the fetal circulation. Arterial, intracardiac, and venous flow velocity studies. Circulation 91: 129–138, 1995 [DOI] [PubMed] [Google Scholar]
- 133. Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2A receptor inhibition enhances phrenic long-term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hong H, Chen H, Gao W, Cai X, Sun Y, Yin M, Liu J. Hypoxia-induced cytosolic calcium decrease is mediated primarily by the forward mode of Na+/Ca2+ exchanger in smooth muscle cells of fetal ductus arteriosus. Pediatr Cardiol 30: 958–64, 2009 [DOI] [PubMed] [Google Scholar]
- 135. Hong Z, Hong F, Olschewski A, Cabrera JA, Varghese A, Nelson DP, Weir EK. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arteriosus. Circulation 114: 1372–1379, 2006 [DOI] [PubMed] [Google Scholar]
- 136. Hooper SB, Bocking AD, White S, Challis JR, Han VK. DNA synthesis is reduced in selected fetal tissues during prolonged hypoxemia. Am J Physiol Regul Integr Comp Physiol 261: R508–R514, 1991 [DOI] [PubMed] [Google Scholar]
- 137. Horne RSC, Parslow PM, Harding R. Postnatal development of ventilatory and arousal responses to hypoxia in human infants. Respir Physiol Neurobiol 149: 257–271, 2005 [DOI] [PubMed] [Google Scholar]
- 138. Howell LL, Landrum AM. Attenuation of hypoxia-induced increases in ventilation by adenosine antagonists in rhesus monkeys. Life Sci 57: 773–783, 1995 [DOI] [PubMed] [Google Scholar]
- 139. Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 90: 1274–1281, 2002 [DOI] [PubMed] [Google Scholar]
- 140. Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol 54: 409–419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Imperatore A, Rolfo A, Petraglia F, Challis JR, Caniggia I. Hypoxia and preeclampsia: increased expression of urocortin 2 and urocortin 3. Reprod Sci 17: 833–843 2010 [DOI] [PubMed] [Google Scholar]
- 142. Itoh R, Jun OKA, Ozasa H. Regulation of rat heart cytosol 5′-nucleotidase by adenylate energy charge. Biochem J 235: 847–851, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Itskovitz J, LaGamma EF, Bristow J, Rudolph AM. Cardiovascular responses to hypoxemia in sinoaortic-denervated fetal sheep. Pediatr Res 30: 381–385, 1991 [DOI] [PubMed] [Google Scholar]
- 144. Iturriaga R, Moya EA, Del Rio R. Carotid body potentiation induced by intermittent hypoxia: implications for cardiorespiratory changes induced by apnoea. Clin Exper Pharmacol Physiol 36: 1197–1204, 2009 [DOI] [PubMed] [Google Scholar]
- 145. Iwamoto HS, Rudolph AM, Mirkin BL, Keil LC. Circulatory and humoral responses of sympathectomized fetal sheep to hypoxemia. Am J Physiol Heart Circ Physiol 245: H767–H772, 1983 [DOI] [PubMed] [Google Scholar]
- 146. Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Disc 5: 247–264, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jakoubek V, Bibova J, Herget J, Hampl V. Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am J Physiol Heart Circ Physiol 294: H1638–H1644, 2008 [DOI] [PubMed] [Google Scholar]
- 148. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairments in very low birth weight infants. J Perinatal 24: 763–768, 2004 [DOI] [PubMed] [Google Scholar]
- 149. Jensen A, Garnier Y, Berger R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. Eur J Obstet Gynecol Reprod Biol 84: 155–172, 1999 [DOI] [PubMed] [Google Scholar]
- 150. Jensen EC, Bennet L, Fraser M, Power GG, Hunter CJ, Gunn AJ. Adenosine A1 receptor mediated suppression of adrenal activity in near-term fetal sheep. Am J Physiol Regul Integr Comp Physiol 298: R700–R706, 2010 [DOI] [PubMed] [Google Scholar]
- 151. Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassman M. Induction of HIF-1α in response to hypoxia is instantaneous. FASEB J 15: 1312–1314, 2001 [PubMed] [Google Scholar]
- 152. Jugovic D, Tumbri J, Medic M, Jukic MK, Kurjak A, Arbeille P, Salihagic-Kadic A. New Doppler index for prediction of perinatal brain damage in growth-restricted and hypoxic fetuses. Ultrasound Obstet Gynecol 30: 303–311, 2007 [DOI] [PubMed] [Google Scholar]
- 153. Julien C, Bairam A, Joseph V. Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. Am J Physiol Regul Integr Comp Physiol 294: R1356–R1366, 2008 [DOI] [PubMed] [Google Scholar]
- 154. Kafkasli A, Karabulut AB, Atmaca R, Laurini R. Clinical correlation between adenosine deaminaase activity and pre-eclampsia severity. J Int Med Res 34: 247–255, 2006 [DOI] [PubMed] [Google Scholar]
- 155. Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 40: 1176–1180, 2009 [DOI] [PubMed] [Google Scholar]
- 156. Kajimoto H, Hashimoto K, Bonnet SN, Haromy A, Harry G, Moudgil R, Nakanishi T, Rebeyka I, Théband B, Michelakis ED, Archer SL. Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species: a newly recognized mechanism for sustaining ductal constriction. Circulation 115: 1777–1788, 2007 [DOI] [PubMed] [Google Scholar]
- 157. Kamei H, Ding Y, Kajimura S, Wells M, Chiang P, Duan C. Role of IGF signaling in catch-up growth and accelerated temporal development in zebrafish embryos in response to oxygen availability. Development 138: 777–786, 2011 [DOI] [PubMed] [Google Scholar]
- 158. Karabulut AB, Kafkasli A, Burak F, Gozukara EM. Maternal and fetal plasma adenosine deaminase, xanthine oxidase and malondialehyde levels in pre-eclampsia. Cell Biochem Funct 23: 279–283, 2005 [DOI] [PubMed] [Google Scholar]
- 159. Kato H, Yoneyama Y, Araki T. Fetal plasma lipid peroxide levels in pregnancies complicated by preeclampsia. Gynecol Obstet Invest 43: 158–161, 1997 [DOI] [PubMed] [Google Scholar]
- 160. Keating DJ, Rychkov GY, Giacomin P, Roberts ML. Oxygen-sensing pathway for SK channels in the ovine adrenal medulla. Clin Exper Pharmacol Physiol 32: 882–887, 2005 [DOI] [PubMed] [Google Scholar]
- 161. Keating DJ, Rychkov GY, Roberts ML. The contribution of voltage-gated Ca2+ currents to K+ channel activation during ovine adrenal chromaffin cell development. Int J Dev Neurosci 27: 357–313, 2009 [DOI] [PubMed] [Google Scholar]
- 162. Kemp PJ. Hemeoxgenase-2 as an O2 sensor in K+ channel-dependent chemotransduction. Biochem Biophys Res Comm 338: 648–652, 2005 [DOI] [PubMed] [Google Scholar]
- 163. Kemp PJ, Telezhkin V, Wilkinson WJ, Mears R, Hanmer SB, Gadeberg HC, Müller CT, Riccardi D, Brazier SP. Enzyme-linked oxygen sensing by potassium channels. Ann NY Acad Sci 1177: 112–118, 2009 [DOI] [PubMed] [Google Scholar]
- 164. Kenneth NS, Rocha S. Regulation of gene transcription by hypoxia. Biochem J 414: 19–29, 2008 [DOI] [PubMed] [Google Scholar]
- 165. Kervern M, Dubois C, Nassila M, Daoust M, Pierrefiche O. Perinatal alcohol exposure in rat induces long-term depression of respiration after episodic hypoxia. Am J Crit Care Med 179: 608–614, 2009 [DOI] [PubMed] [Google Scholar]
- 166. Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T. Fetal growth restriction is associated with prioritization of umbilical blood flow to the left hepatic lobe at the expense of the right lobe. Pediatr Res 66: 113–117, 2009 [DOI] [PubMed] [Google Scholar]
- 167. Kiernan MF, Barrie A, Szkolar J, Mills TA, Wareing M. Functional evidence for oxygen-sensitive voltage-gated potassium channels in human placental vasculature. Placenta 31: 553–555, 2010 [DOI] [PubMed] [Google Scholar]
- 168. Kim D, Cavanagh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol 587: 2963–2975, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol 28: 126–136, 2006 [DOI] [PubMed] [Google Scholar]
- 170. Kiserud T, Jauniaux E, West D, Ozturk O, Hanson MA. Circulatory responses to maternal hyperoxaemia and hypoxaemia assessed non-invasively in fetal sheep at 0.3–05 gestation in acute experiments. Br J Obstet Gynecol 108: 359–364, 2003 [DOI] [PubMed] [Google Scholar]
- 171. Kiserud T, Kessler J, Ebbing C, Rasmussen S. Ductus venosus shunting in growth-restricted fetuses and the effect of umibilical circulatory compromise. Ultrasound Obstet Gynecol 28: 143–149, 2006 [DOI] [PubMed] [Google Scholar]
- 172. Kobayashi S, Conforti L, Millhorn DE. Gene expression and function of adenosine A2A receptor in the rat carotid body. Am J Physiol Lung Cell Mol Physiol 279: L273–L282, 2000 [DOI] [PubMed] [Google Scholar]
- 173. Kobayashi S, Conforti L, Pun RY, Millhorn DE. Adenosine modulates hypoxia-induced responses in rat PC12 cells via A2A receptor. J Physiol 508: 95–107, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Konduri GG, Forman K, Mital S. Characterization of purine receptors in fetal lamb pulmonary circulation. Pediatr Res 47: 114–120, 2000 [DOI] [PubMed] [Google Scholar]
- 175. Konduri GG, Mital S. Adenosine and ATP cause nitric oxide-dependent pulmonary vasodilatation in fetal lambs. Biol Neonate 78: 220–229, 2000 [DOI] [PubMed] [Google Scholar]
- 176. Koos BJ. Breathing and sleep states in the fetus and at birth. In: Sleep and Breathing in Children, Second Edition. Developmental Changes in Breathing During Sleep, edited by Marcus CL, Carroll JL, Donnelly DF, Loughlin GM. New York: Informa Healthcare, 2008, p. 1–17 [Google Scholar]
- 177. Koos BJ, Chao A, Doany W. Adenosine stimulates breathing in fetal sheep with brain stem section. J Appl Physiol 72: 94–99, 1992 [DOI] [PubMed] [Google Scholar]
- 178. Koos BJ, Chau A. Fetal cardiovascular and breathing responses to an adenosine A2a receptor agonist in sheep. Am J Physiol Regul Integr Comp Physiol 274: R152–R159, 1998 [DOI] [PubMed] [Google Scholar]
- 179. Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. A thalamic locus mediates hypoxic inhibition of breathing in fetal sheep. J Neurophysiol 79: 2383–2393, 1998 [DOI] [PubMed] [Google Scholar]
- 180. Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. Thalamic lesions dissociate breathing inhibition by adenosine and hypoxia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 278: R831–R837, 2000 [DOI] [PubMed] [Google Scholar]
- 181. Koos BJ, Chau A, Ogunyemi D. Adenosine mediates metabolic and cardiovascular responses to hypoxia in fetal sheep. J Physiol 488: 761–766, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Koos BJ, Doany W. Role of plasma adenosine in responses to hypoxia in fetal sheep. J Dev Physiol 16: 81–85, 1991 [PubMed] [Google Scholar]
- 183. Koos BJ, Kawasaki Y, Hari A, Bohorquez F, Jan C, Roostaeian J, Wilson CL, Kruger L. Electrical stimulation of the posteromedial thalamus modulates breathing in unanesthetized fetal sheep. J Appl Physiol 96: 115–123, 2004 [DOI] [PubMed] [Google Scholar]
- 184. Koos BJ, Kawasaki Y, Kim YH, Bohorquez F. Adenosine A2A receptor blockade abolishes the roll-off respiratory response to hypoxia in awake lambs. Am J Physiol Regul Integr Comp Physiol 288: R1185–R1194, 2005 [DOI] [PubMed] [Google Scholar]
- 185. Koos BJ, Kruger L, Murray TF. Source of extracellular brain adenosine during hypoxia in fetal sheep. Brain Res 778: 439–442, 1997 [DOI] [PubMed] [Google Scholar]
- 186. Koos BJ, Maeda T. Adenosine A2A receptors mediate cardiovascular responses to hypoxia in fetal sheep. Am J Physiol Heart Circ Physiol 280: H83–H89, 2001 [DOI] [PubMed] [Google Scholar]
- 187. Koos BJ, Maeda T, Jan C. Adenosine A1 and A2A receptors modulate sleep state and breathing in fetal sheep. J Appl Physiol 91: 343–350, 2001 [DOI] [PubMed] [Google Scholar]
- 188. Koos BJ, Maeda T, Lopez G. Adenosine A2A receptors mediate hypoxic inhibition of fetal breathing in sheep. Am J Obstet Gynecol 186: 663–668, 2002 [DOI] [PubMed] [Google Scholar]
- 189. Koos BJ, Mason BA, Ducsay CA. Cardiovascular responses to adenosine in fetal sheep: autonomic blockade. Am J Physiol Heart Circ Physiol 264: H526–H532, 1993 [DOI] [PubMed] [Google Scholar]
- 190. Koos BJ, Mason BA, Ervin MG. Adenosine mediates hypoxic release of arginine vasopressin in fetal sheep. Am J Physiol Regul Integr Comp Physiol 266: R215–R220, 1994 [DOI] [PubMed] [Google Scholar]
- 191. Koos BJ, Mason B, Ogunyemi D, Punla O, Adinolfi A. Hypoxic inhibition of breathing in fetal sheep: relation to brain adenosine concentrations. J Appl Physiol 77: 2734–2739, 1994 [DOI] [PubMed] [Google Scholar]
- 192. Koos BJ, Matsuda K. Fetal breathing, sleep state, and cardiovascular responses to adenosine in sheep. J Appl Physiol 68: 489–495, 1990 [DOI] [PubMed] [Google Scholar]
- 193. Koos BJ, Sameshima H. Effects of hypoxemia and hypercapnia on breathing movements and sleep state in sinoaortic-denervated fetal sheep. J Dev Physiol 10: 19–27, 1988 [PubMed] [Google Scholar]
- 194. Koos BJ, Sameshima H, Power GG. Fetal breathing movement, sleep state and cardiovascular responses to an inhibitor of mitochondrial ATPase in sheep. J Dev Physiol 8: 67–75, 1986 [PubMed] [Google Scholar]
- 195. Koos BJ, Sameshima H, Power GG. Fetal breathing movement, sleep state and cardiovascular responses to graded hypoxia in sheep. J Appl Physiol 62: 1033–1039, 1987 [DOI] [PubMed] [Google Scholar]
- 196. Koos BJ, Sameshima H, Power GG. Fetal breathing, sleep state, and cardiovascular responses to graded anemia in sheep. J Appl Physiol 63: 1463–1468, 1987 [DOI] [PubMed] [Google Scholar]
- 197. Kréneisz O, Benoit JP, Bayliss DA, Mulkey DK. AMP-activated protein kinase inhibits TREK channels. J Physiol 587: 5819–5830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kumar P, Bin-Jaliah I. Adequate stimuli of the carotid body: more than a O2 sensor. Respir Physiol Neurobiol 157: 12–21, 2007 [DOI] [PubMed] [Google Scholar]
- 199. Kurth CD, Wagerle LC. Cerebrovascular reactivity to adenosine analogues in 0.6–07 gestation and near-term fetal sheep. Am J Physiol Heart Circ Physiol 262: H1338–H1342, 1992 [DOI] [PubMed] [Google Scholar]
- 200. Lahiri S, Mitchell CH, Reigada D, Roy A, Cherniack NS. Purines, the carotid body and respiration. Respir Physiol Neurobiol 157: 123–129, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the carotid body. Prog Biophys Mol Biol 91: 249–286, 2006 [DOI] [PubMed] [Google Scholar]
- 202. Lasely RD, Hegge JO, Noble MA, Mentzer RM. Evidence that cytosolic and ecto 5′-nucleotidases contribute equally to increased interstitial adenosine concentration during porcine myocardial ischemia. Basic Res Cardiol 94: 199–207, 1999 [DOI] [PubMed] [Google Scholar]
- 203. Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch 460: 525–542, 2010 [DOI] [PubMed] [Google Scholar]
- 204. Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal 18: 499–507, 2006 [DOI] [PubMed] [Google Scholar]
- 205. Levitsky KL, López-Barneo J. Developmental change of T-type Ca2+ channel expression and its role in rat chromaffin cell responsiveness to acute hypoxia. J Physiol 587: 1917–1929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol 495: 561–571, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol 110: 261–268, 1997 [DOI] [PubMed] [Google Scholar]
- 208. Long WQ, Anthonisen NR. Aminophylline partially blocks ventilatory depression with hypoxia in the wake cat. Can J Physiol Pharmacol 72: 673–678, 1994 [DOI] [PubMed] [Google Scholar]
- 209. Longo LD. Respiratory gas exchange in the placenta. In: Handbook of Physiology. The Respiratory System. Gas Exchange. Bethesda, MD: Am. Physiol. Soc; 1987, sect. 3, vol. IV, chapt. 18, p. 351–402 [Google Scholar]
- 210. Lopes JM, Davis GM, Mullahoo K, Aranda JV. Role of adenosine in the hypoxic ventilatory response of the newborn piglet. Pediatr Pulmonol 17: 50–55, 1994 [DOI] [PubMed] [Google Scholar]
- 211. López-Barneo J, Ortega-Sáenz P, Pardal R, Pascual A, Piruat JI, Durán R, Gómez-Díaz R. Oxygen sensing in the carotid body. Ann NY Acad Sci 1177: 119–131, 2009 [DOI] [PubMed] [Google Scholar]
- 212. Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structure changes in obstructive sleep apnea. Sleep 31: 967–977, 2008 [PMC free article] [PubMed] [Google Scholar]
- 213. Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic defects in congenital central hypoventilation syndrome. J Appl Physiol 98: 958–969, 2005 [DOI] [PubMed] [Google Scholar]
- 214. MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol 587: 1931–1942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. MacFarlane PM, Wilkerson JER, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164: 263–271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Maeda T, Koos BJ. Adenosine A1 and A2a receptors modulate insulinemia, glycemia, and lactatemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 296: R693–R701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Martin-Body RL, Johnston BM. Central origin of the hypoxic depression of breathing in the newborn. Respir Physiol 71: 25–32, 1988 [DOI] [PubMed] [Google Scholar]
- 218. Maulik D, Arbeille P, Kadado T. Hemodynamic foundation of umbilical arterial Doppler waveform analysis. Biol Neonate 62: 280–289, 1992 [DOI] [PubMed] [Google Scholar]
- 219. Mayer CA, Haxhiu MA, Martin RJ, Wilson CG. Adenosine A2A receptors mediate GABAergic inhibition of respiration in immature rats. J Appl Physiol 100: 91–97, 2006 [DOI] [PubMed] [Google Scholar]
- 220. Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without fetal intrauterine growth restriction. Placenta 24: 219–226, 2003 [DOI] [PubMed] [Google Scholar]
- 221. McQueen DS, Ribeiro JA. On the specificity and type of receptor involved in carotid chemoreceptor activation by adenosine in the cat. Br J Pharmacol 80: 347–354, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. McQueen DS, Ribeiro JA. Pharmacological characterization of the receptor involved in chemoexcitation induced by adenosine. Br J Pharmacol 88: 615–620, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Mentzer RM, Ely SW, Lasley RD, Mainwaring RD, Wright EM, Jr, Berne RM. Hormonal role of adenosine in maintaining patency of the ductus arteriosus in fetal lambs. Ann Surg 202: 223–230, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Mielke G, Benda N. Cardiac output and central distribution of blood flow in the human fetus. Circulation 103: 1662–1668, 2001 [DOI] [PubMed] [Google Scholar]
- 225. Milley JR. Effect of insulin on the redistribution of cardiac output in the fetal lamb. Pediatr Res 22: 168–72, 1987 [DOI] [PubMed] [Google Scholar]
- 226. Momoi N, Tinney JP, Liu LJ, Elshershari H, Hoffmann PJ, Ralphe JC, Keller BB, Tobita K. Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. Am J Physiol Heart Circ Physiol 294: H2248–H2256, 2008 [DOI] [PubMed] [Google Scholar]
- 227. Montandon G, Bairam A, Kinkead R. Neonatal caffeine induces sex-specific developmental plasticity of the hypoxic respiratory chemoreflex in adult rats. Am J Regul Integr Comp Physiol 295: R922–R934, 2008 [DOI] [PubMed] [Google Scholar]
- 228. Montandon G, Horner RL, Kinkead R, Bairam A. Caffeine in the neonatal period induces long-lasting changes in sleep and breathing in adult rats. J Physiol 587: 5493–5507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Monteiro EC, Ribeiro JA. Ventilatory effects of adenosine mediated by carotid body chemoreceptors in the rat. Naunyn Schiedeberg's Arch Pharmacol 335: 143–148, 1987 [DOI] [PubMed] [Google Scholar]
- 230. Monteiro EC, Ribeiro JA. Adenosine deaminase and adenosine uptake inhibitions facilitate ventilation in rats. Naunyn Schiedeberg's Arch Pharmacol 340: 230–238, 1989 [DOI] [PubMed] [Google Scholar]
- 231. Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol 4: 141–156, 2003 [DOI] [PubMed] [Google Scholar]
- 232. Moore PJ, Clarke JA, Hanson MA, Daly MD, Ead HW. Quantitative studies of the vasculature of the carotid body in fetal and newborn sheep. J Dev Physiol 15: 211–214, 1991 [PubMed] [Google Scholar]
- 233. Moore PJ, Hanson MA. The role of the peripheral arterial chemoreceptors in the rapid response of the pulmonary vasculature of the late gestation fetus to changes in PaO2. J Dev Physiol 16: 133–138, 1991 [PubMed] [Google Scholar]
- 234. Moore PJ, Parkes MJ, Hanson MA. Unilateral cooling in the region of the locus coeruleus blocks the fall in respiratory output during hypoxia in anesthetized neonatal sheep. Exp Physiol 81: 983–994, 1996 [DOI] [PubMed] [Google Scholar]
- 235. Morrow RJ, Adamson SL, Bull SB, Ritchie JWK. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 161: 1055–1060, 1989 [DOI] [PubMed] [Google Scholar]
- 236. Morrow RJ, Adamson SL, Bull SB, Ritchie JWK. Acute hypoxemia does not affect the umbilical artery velocity waveform in fetal sheep. Obstet Gynecol 75: 590–593, 1990 [PubMed] [Google Scholar]
- 237. Morrow RJ, Adamson SL, Bull SB, Ritchie JWK. Hypoxic acidemia, hyperviscosity, and maternal hypertension do not affect the umbilical artery velocity waveform in fetal sheep. Am J Obstet Gynecol 163: 1313–1320, 1990 [DOI] [PubMed] [Google Scholar]
- 238. Moss IR, Brown KA, Laferrière A. Recurrent hypoxia in rats during development increases subsequent respiratory sensitivity to fentanyl. Anesthesiology 105: 715–718, 2006 [DOI] [PubMed] [Google Scholar]
- 239. Moss TJ, Davey MG, McCrabb GJ, Harding R. Development of ventilatory responsiveness to progressive hypoxia and hypercapnia in low-birth-weight lambs. J Appl Physiol 81: 1555–1561, 1996 [DOI] [PubMed] [Google Scholar]
- 240. Muijsers GJJM, Hasaart THM, Ruissen CJ, van Huisseling H, Peeters LLH, de Haan J. The response of the umbilical and femoral artery pulsatility indices in fetal sheep to progressively reduced uteroplacental blood flow. J Dev Physiol 13: 215–221, 1990 [PubMed] [Google Scholar]
- 241. Mulligan E, Lahiri S, Storey BT. Carotid body O2 chemoreception and mitochondrial oxidative phosphorylation. J Appl Physiol 51: 433–446, 1981 [DOI] [PubMed] [Google Scholar]
- 242. Mustafa S, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 193: 161–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Myatt L. Control of vascular resistance in the human placenta. Placenta 13: 329–341, 1992 [DOI] [PubMed] [Google Scholar]
- 244. Myatt L. Placental adaptive responses and fetal programming. J Physiol 572: 25–30, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol 96: 367–374, 2004 [DOI] [PubMed] [Google Scholar]
- 246. Nishida N, Blood AB, Hunter CJ, Bragg S, Williams J, Pearce WJ, Power GG. Role of prostanoids in the regulation of cerebral blood flow during normoxia and hypoxia in the fetal sheep. Pediatr Res 60: 524–529, 2006 [DOI] [PubMed] [Google Scholar]
- 247. Nock ML, Difiore JM, Arko MK, Martin RJ. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J Pediatr 144: 291–5, 2004 [DOI] [PubMed] [Google Scholar]
- 248. Nurse CA, Buttigieg J, Brown S, Holloway AC. Regulation of oxygen sensitivity in adrenal chromaffin cells. Ann NY Acad Sci 1177: 132–139, 2009 [DOI] [PubMed] [Google Scholar]
- 249. Nyakas C, Buwalda B, Luitten PG. Hypoxia and brain development. Prog Neurobiol 49: 1–51, 1996 [DOI] [PubMed] [Google Scholar]
- 250. Ogunyemi DA, Koos BJ, Arora CP, Castro LC, Mason BA. Adenosine modulates hypoxia-induced atrial natriuretic peptide release in fetal sheep. Am J Physiol Heart Circ Physiol 269: H282–H289, 1995 [DOI] [PubMed] [Google Scholar]
- 251. Okubo S, Mortola JP. Long-term respiratory effects of neonatal hypoxia in the rat. J Appl Physiol 64: 952–958, 1988 [DOI] [PubMed] [Google Scholar]
- 252. Okubo S, Mortola JP. Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol Regul Integr Comp Physiol 259: R836–R841, 1990 [DOI] [PubMed] [Google Scholar]
- 253. Ortega-Sáenz P, Pascual A, Gomez-Díaz R, López-Barneo J. Acute oxygen sensing in hemeoxygenase-2 null mice. J Gen Physiol 128: 405–411, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Ortega-Sáenz P, Pascual A, Piruat JI, López-Barneo J. Mechanisms of acute oxygen sensing by the carotid body: lessons from genetically modified animals. Respir Physiol Neurobiol 157: 140–147, 2007 [DOI] [PubMed] [Google Scholar]
- 255. Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006 [DOI] [PubMed] [Google Scholar]
- 256. Patel J, Landers K, Mortimer RH, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta 31: 951–957, 2010 [DOI] [PubMed] [Google Scholar]
- 257. Paulick RP, Meyers RL, Rudolph CD, Rudolph AM. Venous responses to hypoxemia in the fetal lamb. J Dev Physiol 14: 81–88, 1990 [PubMed] [Google Scholar]
- 258. Paulick RP, Meyers RL, Rudolph CD, Rudolph AM. Umbilical and hepatic venous responses to circulating vasoconstrictive hormones in fetal lamb. Am J Physiol Heart Circ Physiol 260: H1205–H1213, 1991 [DOI] [PubMed] [Google Scholar]
- 259. Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 296: R735–R742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol 104: 1287–1294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol 100: 731–738, 2006 [DOI] [PubMed] [Google Scholar]
- 262. Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol 174: 292–298, 2010 [DOI] [PubMed] [Google Scholar]
- 263. Peeters LL, Sheldon RE, Jones MD, Jr, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol 135: 637–646, 1979 [DOI] [PubMed] [Google Scholar]
- 264. Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci 107: 10719–10724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29: 4903–4910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenga GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Peyronnet J, Roux JC, Géloën A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol 524: 525–537, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Peyronnet J, Roux JC, Mamet J, Perrin D, Lachuer J, Pequignot JM, Dalmaz Y. Developmental plasticity of the carotid chemoafferent pathway in rats that are hypoxic during the prenatal period. Eur J Neurosci 26: 2865–2872, 2007 [DOI] [PubMed] [Google Scholar]
- 269. Picard N, Guenin S, Larnicol N, Perrin Y. Maternal caffeine ingestion during gestation and lactation influences respiratory adaptation to acute alveolar hypoxia in newborn rats and adenosine A2A and GABAA receptor mRNA. Neuroscience 156: 630–639, 2008 [DOI] [PubMed] [Google Scholar]
- 270. Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmac Ther 65: 215–239, 1995 [DOI] [PubMed] [Google Scholar]
- 271. Powell FL, Kim CB, Johnson RS, Fu Z. Oxygen sensing in the brain. Adv Exp Med Biol 648: 369–376, 2009 [DOI] [PubMed] [Google Scholar]
- 272. Power GG, Dale PS, Nelson PS. Distribution of maternal and fetal blood flow within cotyledons of the sheep placenta. Am J Physiol Heart Circ Physiol 241: H486–H496, 1981 [DOI] [PubMed] [Google Scholar]
- 273. Power GG, Longo LD, Wagner HN, Kuhl DE, Forster RE. Uneven distribution of maternal and fetal placental blood flow as demonstrated using macroaggregates, and its response to hypoxia. J Clin Invest 46: 2053–2063, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol 91: 17–23, 2006 [DOI] [PubMed] [Google Scholar]
- 275. Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via HIF-mediated ROS. Respir Physiol Neurobiol 174: 230–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Prabhakar NR, Peng YJ, Kumar GK, Pawar A. Altered carotid body function by intermittent hypoxia in neonates and adults: Relevance to recurrent apneas. Respir Physiol Neurobiol 157: 148–153, 2007 [DOI] [PubMed] [Google Scholar]
- 277. Pretlove SJ, Fox CE, Khan KS, Kilby MD. Noninvasive methods of detecting fetal anemia: a systematic review and meta-analysis. BJOG 116: 1558–1567, 2009 [DOI] [PubMed] [Google Scholar]
- 278. Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update 16: 415–431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Rajakumar A, Brandon HM, Daftary A. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25: 763–769, 2004 [DOI] [PubMed] [Google Scholar]
- 280. Read MA, Boura ALA, Walters WAW. Vascular actions of purines in the foetal circulation of the human placenta. Br J Pharmacol 110: 454–460, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Reeves SR, Gozal D. Changes in ventilatory adaptations associated with long-term intermittent hypoxia across the age spectrum in the rat. Respir Physiol Neurobiol 150: 135–143, 2006 [DOI] [PubMed] [Google Scholar]
- 282. Reeves SR, Gozal D. Respiratory and metabolic responses to early postnatal chronic intermittent hypoxia and sustained hypoxia in the developing rat. Pediatr Res 60: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 283. Reeves SR, Mitchell GS, Gozal D. Early postnatal chronic intermittent hypoxia modifies hypoxic respiratory responses and long-term facilitation in adult rats. Am J Physiol Regul Integr Comp Physiol 290: R1664–R1671, 2006 [DOI] [PubMed] [Google Scholar]
- 284. Regnault TRH, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta 28: 714–723, 2007 [DOI] [PubMed] [Google Scholar]
- 285. Reid DL, Davidson SR, Phernetton Rankin JH. Adenosine causes biphasic response in the ovine fetal placental vasculature. J Dev Physiol 13: 237–240, 1990 [PubMed] [Google Scholar]
- 286. Reuss ML, Rudolph AM. Distribution and recirculation of umbilical and systemic venous blood flow in fetal lambs during hypoxia. J Dev Physiol 2: 71–84, 1980 [PubMed] [Google Scholar]
- 287. Reviriego J, Alonso MJ, Ibanez C, Marin J. Action of adenosine and characterization of adenosine receptors in human placental vasculature. Gen Pharmacol 21: 227–233, 1996 [DOI] [PubMed] [Google Scholar]
- 288. Reyes EP, Fernandez R, Larrain C, Zapata P. Effects of combined cholinergic-purinergic block upon cat carotid body chemoreceptors in vitro. Respir Physiol Neurobiol 156: 17–22, 2007 [DOI] [PubMed] [Google Scholar]
- 289. Reyes EP, Fernandez R, Larrain C, Zapata P. Carotid body chemosensory activity and ventilatory chemoreflexes in cats persist after combined cholinergic-purinergic block. Respir Physiol Neurobiol 156: 23–32, 2007 [DOI] [PubMed] [Google Scholar]
- 290. Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Genet Metab 74: 160–171, 2001 [DOI] [PubMed] [Google Scholar]
- 291. Rizzo G, Capponi A, Arduini D, Romanini C. The value of fetal arterial, cardiac and venous flows in predicting pH and blood gases measured in umbilical blood at cordocentesis in growth retarded fetuses. Br J Obstet Gynaencol 102: 963–969, 1995 [DOI] [PubMed] [Google Scholar]
- 292. Rizzo G, Capponi A, Vendola M, Arduini D. Low cardiac output to the placenta: an early hemodynamic adaptive mechanism in intrauterine growth restriction. Ultrasound Obstet Gynecol 32: 155–159, 2008 [DOI] [PubMed] [Google Scholar]
- 293. Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol 61: 1254–1260, 2008 [DOI] [PubMed] [Google Scholar]
- 294. Rodrigo J, Fernández AP, Serrano J, Peinado MA. The role of free radicals in cerebral hypoxia and ischemia. Free Radic Biol Med 39: 26–50, 2005 [DOI] [PubMed] [Google Scholar]
- 295. Roy A, Baby SM, Wilson DF, Lahiri S. Rat carotid body chemosensory discharge and glomus cell HIF-1α expression in vitro: regulation by a common oxygen sensor. Am J Physiol Regul Integr Comp Physiol 293: R829–R836, 2007 [DOI] [PubMed] [Google Scholar]
- 296. Roy A, Rozanov C, Mokashi A, Daudu P, Al-Mehdi AB, Shams H, Lahiri S. Mice lacking in gp91 phox subunit of NAD(P)H oxidase showed glomus cell [Ca2+]i and respiratory responses to hypoxia. Brain Res 872: 188–193, 2000 [DOI] [PubMed] [Google Scholar]
- 297. Ruderman NB, Saha AK. Metabolic syndrome: adenosine monophosphate-activated protein kinase and malonyl coenzyme A. Obesity 14, Suppl 1: 25S–33S, 2006 [DOI] [PubMed] [Google Scholar]
- 298. Rudolph AM. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res 57: 811–821, 1985 [DOI] [PubMed] [Google Scholar]
- 299. Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res 81: 713–722, 2009 [DOI] [PubMed] [Google Scholar]
- 300. Runold M, Cherniak NS, Prabhakar NR. Effect of adenosine on isolated and superfused cat carotid body activity. Neurosci Lett 113: 111–114, 1990 [DOI] [PubMed] [Google Scholar]
- 301. Salihagic-Kadic A, Medic M, Jugovic D, Kos M, Latin V, Kusan JM, Arbeille P. Fetal cerebrovascular response to chronic hypoxia-implications for prevention of brain damage. J Matern Fetal Neonatal Med 19: 387–396, 2006 [DOI] [PubMed] [Google Scholar]
- 302. Salvador HS, Koos BJ. Effects of regular and decaffeinated coffee on fetal breathing and heart rate. Am J Obstet Gynecol 160: 1043–7, 1989 [DOI] [PubMed] [Google Scholar]
- 303. Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Sato T, Obata T, Yamanaka Y, Arita M. Stimulation of α1-adrenoceptors and protein C-mediated activation of ecto-5′-nucleotidase in rat hearts in vivo. J Physiol 503: 119–127, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Sauvannakitt D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol 101: 2837–2847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Sawa R, Asakura H, Power GG. Changes in plasma adenosine during simulated birth of fetal sheep. J Appl Physiol 70: 1524–1528, 1991 [DOI] [PubMed] [Google Scholar]
- 307. Schocken DD, Schneider MN. Use of multiple radioligands to characterize adenosine receptors in human placenta. Placenta 7: 339–348, 1986 [DOI] [PubMed] [Google Scholar]
- 308. Sebastião AM, Ribeiro JA. Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol 48: 167–189, 1996 [DOI] [PubMed] [Google Scholar]
- 309. Sebastião AM, Ribeiro JA. Tuning and fine-tuning of synapses with adenosine. Curr Neuropharmacol 7: 180–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24: 97–106, 2008 [DOI] [PubMed] [Google Scholar]
- 311. Shinagawa T, Suzuki S, Sawa R, Yoneyama Y. Maternal plasma adenosine and endothelin-1 levels in twin gestation complicated by preeclampsia. Arch Gynecol Obstet 267: 72–75, 2002 [DOI] [PubMed] [Google Scholar]
- 312. Simon MC, Liu L, Barnhart BC, Young RM. Hypoxia-induced signaling in the cardiovascular system. Annu Rev Physiol 70: 51–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Siristatidis C, Salamalekis E, Kassanos D, Loghis C, Creatsas G. Evaluation of fetal intrapartum hypoxia by middle cerebral and umbilical artery Doppler velocimetry with simultaneous cardiotocography and pulse oximetry. Arch Gynecol Obstet 270: 265–270, 2004 [DOI] [PubMed] [Google Scholar]
- 314. Sladek M, Parker RA, Grgaard JB, Sundell HW. Long-lasting effects of prolonged hypoxemia after birth on the immediate ventilatory response to changes in arterial partial pressure of oxygen in young lambs. Pediatr Res 34: 821–828, 1993 [DOI] [PubMed] [Google Scholar]
- 315. Slotkin TA, Seidler FJ. Adrenomedullary catecholamine release in the fetus and newborn: secretory mechanisms and their role in stress and survival. J Dev Physiol 10: 1–16, 1988 [PubMed] [Google Scholar]
- 316. Sobrevia L. Activation of A2-purinoceptors by adenosine stimulates l-arginine transport (system y+) and nitric oxide synthesis in human fetal endothelial cells. J Physiol 499: 135–140, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochem Biophys Acta 1797: 1171–1177, 2010 [DOI] [PubMed] [Google Scholar]
- 318. Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 90: 4299–4308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Gudermann T, Schultz R, Seeger W, Grimminger F, Weissmann N. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J 32: 1639–1651, 2008 [DOI] [PubMed] [Google Scholar]
- 320. Spinillo A, Montanari L, Roccio M, Zanchi S, Tzialla C, Stronati M. Progostic significance of interaction between abnormal umbilical and middle cerebral artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Acta Obstet Gynecol Scand 88: 159–166, 2009 [DOI] [PubMed] [Google Scholar]
- 321. Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am J Physiol Lung Cell Mol Physiol 277: L645–L652, 1999 [DOI] [PubMed] [Google Scholar]
- 322. Stonestreet BS, Boyle LD, Papparella A, Berard DJ. Circulatory and metabolic effects of α-adrenergic blockade in the hyperinsulinemic ovine fetus. J Soc Gynecol Invest 3: 241–249, 1996 [DOI] [PubMed] [Google Scholar]
- 323. Szentmiklos AJ, Ujfalusi A, Cseppento A, Nosztray K, Kovacs P, Szabo JZ. Adenosine receptors mediate both contractile and relaxant effects of adenosine in main pulmonary artery of guinea pigs. Naunyn Schmiedebergs Arch Pharmacol 351: 417–425, 1995 [DOI] [PubMed] [Google Scholar]
- 324. Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Therapeut 91: 133–147, 2001 [DOI] [PubMed] [Google Scholar]
- 325. Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology 25: 272–279, 2010 [DOI] [PubMed] [Google Scholar]
- 326. Tchirikov M, Eisermann K, Rybakowski C, Schröder HJ. Doppler ultrasound evaluation of ductus venosus blood flow during acute hypoxemia in fetal lambs. Ultrasound Obstet Gynecol 11: 426–431, 1998 [DOI] [PubMed] [Google Scholar]
- 327. Tchirikov M, Schröder HJ, Hecher K. Ductus venosus shunting in fetal venous circulation: regulatory mechanisms, diagnostic methods and medical importance. Ultrasound Obstet Gynecol 27: 452–461, 2006 [DOI] [PubMed] [Google Scholar]
- 328. Tchirikov M, Strohner M, Scholz A. Cardiac output and blood flow volume redistribution during acute maternal hypoxia in fetal sheep. J Perinat Med 38: 387–392, 2010 [DOI] [PubMed] [Google Scholar]
- 329. Teitel DF, Iwamoto HS, Rudolph AM. Changes in the pulmonary circulation during birth-related events. Pediatr Res 27: 372–278, 1990 [DOI] [PubMed] [Google Scholar]
- 330. Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010 [DOI] [PubMed] [Google Scholar]
- 331. Terashvili M, Pratt PF, Gebremedhin D, Narayanan J, Harder DR. Reactive oxygen species cerebral autoregulation in health and disease. Pediatr Clin North Am 53: 1029–137, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Thakor AS, Giussani DA. Calcitonin gene-related peptide contributes to the umbilical haemodynamic defence response to acute hypoxaemia. J Physiol 563: 309–317, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Thakor AS, Giussani DA. Effects of acute acidemia on the fetal cardiovascular defense to acute hypoxemia. Am J Physiol Regul Integr Comp Physiol 296: R90–R99, 2009 [DOI] [PubMed] [Google Scholar]
- 334. Thompson RJ, Farragher SM, Cutz E, Nurse CA. Developmental regulation of O2 sensing in neonatal adrenal chromaffin cells from wild-type and NADH-oxidase-deficient mice. Plugers Arch Eur J Physiol 444: 539–548, 2002 [DOI] [PubMed] [Google Scholar]
- 335. Thornburg KL, O′Tierney PF, Louey S. The placenta is a programming agent for cardiovascular disease. Placenta 24: S54–S59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Thornburg KL, Reller MD. Coronary flow regulation in the fetal sheep. Am J Physiol Regul Integr Comp Physiol 277: R1249–R1260, 1999 [DOI] [PubMed] [Google Scholar]
- 337. Tomimatsu T, Lee SJ, Peña JP, Ross JM, Lang JA, Longo LD. Maternal caffeine administration and cerebral oxygenation in near-term fetal sheep. Reprod Sci 14: 588–594, 2007 [DOI] [PubMed] [Google Scholar]
- 338. Tong W, Chen W, Ostrowski RP, Ma Q, Souvenir R, Zhang L, Zhang JH, Tang J. Maternal hypoxia increases the activity of MMPs and decreases the expression of TIMPS in the brain of neonatal rats. Develop Neurobiol 70: 182–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Tong J, Zhu MJ, Underwood KR, Hess BW, Ford SP, Du M. AMP-activated protein kinase and adipogenesis in sheep fetal skeletal muscle and 3T3–L1 cells. J Anim Sci 86: 1296–1305, 2008 [DOI] [PubMed] [Google Scholar]
- 340. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007 [DOI] [PubMed] [Google Scholar]
- 341. Tune JD. Control of coronary blood flow during hypoxemia. Adv Exp Med Biol 618: 25–39, 2007 [DOI] [PubMed] [Google Scholar]
- 342. Vandier C, Conway AF, Landhauer RC, Kumar P. Presynaptic action of adenosine on a 4-aminopyridine-sensitive current in the rat carotid body. J Physiol 515: 419–429, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. von Versen-Höynck F, Rajakumar A, Bainbridge SA, Gallaher MJ, Roberts JM, Powers RW. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta 30: 434–442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Vyas S, Nicolaides KH, Campbell S. Doppler examination of the middle cerebral artery in anemic fetuses. Am J Obstet Gynecol 162: 1066–1068, 1990 [DOI] [PubMed] [Google Scholar]
- 345. Waites BA, Ackland GL, Noble R, Hanson MA. Red nucleus lesions abolish the biphasic respiratory response to isocapnic hypoxia in decerebrate young rabbits. J Physiol 495: 217–225, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Waleh N, Kajino H, Marrache AM, Ginzinger D, Roman C, Seidner SR, Moss TJ, Fouran JC, Vazquez-Tello A, Chemtob S, Clyman RI. Prostaglandin E2-mediated relaxation of the ductus arteriosus: effects of gestational age and G protein-coupled receptor expression, signaling, and vasomotor control. Circulation 110: 2326–2332, 2004 [DOI] [PubMed] [Google Scholar]
- 347. Wall MJ, Dale N. Auto-inhibition of rat parallel fibre-Purkinje cell synapses by activity-dependent adenosine release. J Physiol 581: 553–565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Wang XY, Bisgard GE. Postnatal growth of the carotid body. Respir Physiol Neurobiol 149: 181–190, 2005 [DOI] [PubMed] [Google Scholar]
- 349. Ward JPT. Oxygen sensors in context. Biochim Biophys Acta 1777: 1–14, 2008 [DOI] [PubMed] [Google Scholar]
- 350. Weaver DR. A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res 20: 313–327, 1993 [DOI] [PubMed] [Google Scholar]
- 351. Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci 66: 3539–3554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Weiner CP. The relationship between umbilical artery systolic/diastolic ratio and umbilical blood gas measurements in specimens obtained by cordocentesis. Am J Obstet Gynecol 162: 1198–1202, 1990 [DOI] [PubMed] [Google Scholar]
- 353. Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol 174: 182–191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Weir EK, Cabrera JA, Hahapatra S, Peterson DA, Hong Z. The role of ion channels in hypoxic pulmonary vasoconstriction. Adv Exp Med Biol 661: 3–14, 2010 [DOI] [PubMed] [Google Scholar]
- 355. Wendler CC, Amatya S, McClaskey C, Ghatpande S, Fredholm BB, Rivkees SA. A1 adenosine receptors play an essential role in protecting the embryo against hypoxia. Proc Natl Acad Sci USA 104: 9697–9702, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Wendler CC, Busovsky-McNeal M, Ghatpande S, Kalinowski A, Russell KS, Rivkees SA. Embryonic caffeine exposure induces adverse effects in adulthood. FASEB J 23: 1273–1278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Williams BA, Hanson MA. The role of the carotid chemoreceptors in the respiratory response of newborn lambs to different pairs of breaths of air and a hypoxic gas. J Dev Physiol 13: 157–164, 1990 [PubMed] [Google Scholar]
- 358. Wilson CG, Martin RJ, Jaber M, Abu-Shaweesh J, Jafri A, Haxhiu MA, Zaidi S. Adenosine A2A receptors interact with GABAergic pathways to modulate respiration in neonatal piglets. Respir Physiol Neurobiol 141: 201–211, 2004 [DOI] [PubMed] [Google Scholar]
- 359. Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296: H539–H549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem 282: 8092–8098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci USA 92: 295–299, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Xu F, Xu J, Tse F, Tse A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid body. Am J Physiol Cell Physiol 290: C1592–C1598, 2006 [DOI] [PubMed] [Google Scholar]
- 363. Xu MH, Gong YS, Su MS, Dai SS, Bao SZ, Li N, Zheng RY, Chen JF, Wang XT. Absence of adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. J Vasc Res 48: 171–183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Yaar R, Jones MR, et al. Chen JF. Animal models for the study of adenosine receptor function. J Cell Physiol 202: 9–20, 2005 [DOI] [PubMed] [Google Scholar]
- 365. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 366. Yamamoto M, Nishimura M, Kobayashi S, Akiyama Y, Miyamoto K, Kawakami Y. Role of endogenous adenosine in hypoxic ventilatory response in humans: a study with dipyridamole. J Appl Physiol 76: 196–203, 1994 [DOI] [PubMed] [Google Scholar]
- 367. Yan X, Koos BJ, Kruger L, Linden J, Murray TF. Characterization of [125]ZM241385 binding to adenosine A2A receptors in pineal gland of sheep brain. Brain Res 1096: 30–39, 2006 [DOI] [PubMed] [Google Scholar]
- 368. Yang JN, Björklund O, Lindström-Törnqvist K, Lindgren E, Eriksson TM, Kahlström J, Chen JF, Schwarzschild MA, Tobler I, Fredholm BB. Mice heterozygous for both A1 and A2A adenosine receptor genes show similarities to mice given long-term caffeine. J Appl Physiol 106: 631–639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Yang JN, Chen JF, Fredholm BB. Physiological roles of A1 and A2A adenosine receptors in regulating heart rate, body temperature, and locomotion as revealed using knockout mice and caffeine. Am J Physiol Heart Circ Physiol 296: H1141–H1149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Yoneyama Y, Sawa R, Suzuki S, Doi D, Yoneyama K, Otsubo Y, Araki T. Relationship between plasma malondialdehyde levels and adenosine deaminase activities in preeclampsia. Clin Chim Acta 322: 169–173, 2002 [DOI] [PubMed] [Google Scholar]
- 371. Yoneyama Y, Sawa R, Suzuki S, Miura A, Kobayashi H, Doi D, Yoneyama K, Araki T. Relationship between adenosine deaminase activities and cytokine-producing T cells in women with preeclampsia. Clin Biochem 35: 303–306, 2002 [DOI] [PubMed] [Google Scholar]
- 372. Yoneyama Y, Suzuki S, Sawa R, Kiyokawa Y, Power GG, Araki T. Plasma adenosine levels and P-selectin expression on platelets in preeclampsia. Obstet Gynecol 97: 366–370, 2001 [DOI] [PubMed] [Google Scholar]
- 373. Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Miura A, Kuwabara Y, Ishino H, Kiyokawa Y, Doi D, Yoneyama K, Kobayashi H, Araki T. Plasma 5′-nucleotidase activities in women with pre-eclampsia. Gynecol Obstet Invest 54: 168–171, 2002 [DOI] [PubMed] [Google Scholar]
- 374. Yoneyama Y, Suzuki S, Sawa R, Shin S, Power GG, Araki T. The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 174: 267–271, 1996 [DOI] [PubMed] [Google Scholar]
- 375. Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Relationship between adenosine and T-helper 1/T-helper 2 imbalance in women with preeclampsia. Obstet Gynecol 99: 641–646, 2002 [DOI] [PubMed] [Google Scholar]
- 376. Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Increased plasma adenosine concentrations and the severity of preeclampsia. Obstet Gynecol 100: 1266–1270, 2002 [DOI] [PubMed] [Google Scholar]
- 377. Yoneyama Y, Watatsuki M, Sawa R, Kamois S, Takahashi H, Shin S, Power GG, Araki T. Plasma adenosine concentrations in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 170: 684–688, 1994 [DOI] [PubMed] [Google Scholar]
- 378. Yuan G, Nanduri Y, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expresión by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Zaidi SIA, Jafri A, Martin RJ, Haxhiu MA. Adenosine A2A receptors are expressed by GABAergic neurons of medulla oblongata in developing rat. Brain Res 1071: 42–53, 2006,. [DOI] [PubMed] [Google Scholar]
- 380. Zapata P. Is ATP a suitable co-transmitter in carotid body arterial chemoreceptors? Respir Physiol Neurobiol 157: 106–115, 2007 [DOI] [PubMed] [Google Scholar]
- 381. Zezula J, Freissmuth M. The A2A-adenosine receptor: a GPCR with unique features? Br J Pharmacol 153, Suppl 1: S184–S190, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]