Abstract
β-Hydroxy-β-methylbutyrate (HMB) is a leucine metabolite shown to reduce protein catabolism in disease states and promote skeletal muscle hypertrophy in response to loading exercise. In this study, we evaluated the efficacy of HMB to reduce muscle wasting and promote muscle recovery following disuse in aged animals. Fisher 344×Brown Norway rats, 34 mo of age, were randomly assigned to receive either Ca-HMB (340 mg/kg body wt) or the water vehicle by gavage (n = 32/group). The animals received either 14 days of hindlimb suspension (HS, n = 8/diet group) or 14 days of unloading followed by 14 days of reloading (R; n = 8/diet group). Nonsuspended control animals were compared with suspended animals after 14 days of HS (n = 8) or after R (n = 8). HMB treatment prevented the decline in maximal in vivo isometric force output after 2 wk of recovery from hindlimb unloading. The HMB-treated animals had significantly greater plantaris and soleus fiber cross-sectional area compared with the vehicle-treated animals. HMB decreased the amount of TUNEL-positive nuclei in reloaded plantaris muscles (5.1% vs. 1.6%, P < 0.05) and soleus muscles (3.9% vs. 1.8%, P < 0.05). Although HMB did not significantly alter Bcl-2 protein abundance compared with vehicle treatment, HMB decreased Bax protein abundance following R, by 40% and 14% (P < 0.05) in plantaris and soleus muscles, respectively. Cleaved caspase-3 was reduced by 12% and 9% (P < 0.05) in HMB-treated reloaded plantaris and soleus muscles, compared with vehicle-treated animals. HMB reduced cleaved caspase-9 by 14% and 30% (P < 0.05) in reloaded plantaris and soleus muscles, respectively, compared with vehicle-treated animals. Although, HMB was unable to prevent unloading-induced atrophy, it attenuated the decrease in fiber area in fast and slow muscles after HS and R. HMB's ability to protect against muscle loss may be due in part to putative inhibition of myonuclear apoptosis via regulation of mitochondrial-associated caspase signaling.
Keywords: aging, cachexia, muscle wasting, apoptosis
sarcopenia is the age-associated loss of muscle mass and function (28, 29), which is central to the care of geriatric individuals (17). It impairs mobility and increases the susceptibility to muscle injury (30), thereby leading to a decrease in the independence and the quality of life in the elderly (15), as well as to increase the risk for serious falls (85, 86). In addition, sarcopenia exacerbates the loss of muscle function and the quality of life in aged individuals with restricted mobility or extended hospitalization. While sarcopenia is multifactorial, apoptosis has been shown to play a role in the events that lead to muscle loss with aging.
Several laboratories have shown that apoptosis is a significant contributor to muscle degeneration and sarcopenia (1–3, 22, 45, 49, 50, 57, 70, 72, 76, 80, 84). However, apoptosis in skeletal muscle is uniquely different from cell death, which has been described classically in other tissues and cells. Most importantly, skeletal muscle is multinucleated so that the removal of one myonucleus by apoptosis will not produce wide-spread or extensive muscle cell death. However, elimination of a myonucleus will lead to loss of gene expression within the cytoplasmic domain that had been controlled by that myonucleus. This loss of gene control cannot be fully assumed by an adjacent myonucleus, and after some threshold of nuclear loss, the muscle fiber will undergo atrophy. Evidence that not all myonuclei in a single myofiber become apoptotic during muscle loss has been observed in experimental denervation and denervation-associated diseases (31, 68).
Three primary apoptotic pathways have been implicated in apoptotic signaling of skeletal muscle fibers These include mitochondria-dependent (intrinsic), death receptor-mediated (extrinsic), and sarcoplasmic/endoplasmic reticulum calcium, stress-induced pathways (8). Several lines of data suggest that the intrinsic mitochondrial-associated pathway has an important role in apoptotic signaling in muscle under conditions of aging (16, 22) and under cardiovascular pathologies (59, 60). Furthermore, apoptotic signaling increases in aged muscles during periods of disuse (9, 23, 46, 49, 73, 76). However, it is not known to what extent apoptotic signaling is altered during muscle reloading in aged animals after disuse.
Aging reduces the ability of muscle to recover after immobilization or disuse in humans (38, 83) and animals (39, 94). Furthermore, muscle growth is suppressed with aging, because the extent of hypertrophy is attenuated in muscles from aged animals compared with young adult animals (5, 20, 21, 69), and this reduced muscle growth with aging is associated with increased apoptotic signaling (7, 8, 71). As reloading after immobilization has been shown to reduce mitochondrial-associated apoptotic signaling in skeletal muscle (88) of young adult animals, and younger animals have better recovery following disuse than old animals (39, 94), we were interested in identifying approaches that would reduce apoptotic signaling and thereby improve muscle structure and function during recovery following disuse in old animals.
One potential candidate for improving muscle mass and growth under various conditions is β-hydroxy-β-methylbutyrate (HMB). HMB, a metabolite of the essential branched-chain amino acid leucine, has been found to reduce muscle wasting associated with trauma and cancer cachexia (11, 14, 55, 87). Furthermore, HMB supplementation has been reported to improve muscle function in the mdx mouse model for Duchenne muscular dystrophy, which has a high degree of muscle degeneration and regeneration (55). Furthermore, HMB has been shown to attenuate fiber atrophy and damage during limb immobilization of adult mice (82).
HMB has been reported to reduce muscle atrophy and increase muscle hypertrophy by inhibiting muscle degradation (77), in part, from greater Akt phosphorylation (42) and improved anabolic signaling via the m-TOR pathway (11). However, although less well studied, HMB has also has been shown to reduce apoptosis in human myoblasts under conditions of serum-starvation or staurosporine-induced apoptosis (42). We have recently found that serum starvation induces myoblast apoptosis that was accompanied by an increased mitochondrial release of cytochrome-c, cleaved caspase-9, and apoptosis-inducing factors as a result of increased mitochondrial-associated apoptotic signaling (Y. Wang, unpublished observations). Taken together, the findings from Kornasio et al. (42) and our lab indicate that HMB might be a potential candidate that will protect against mitochondrial-associated apoptotic signaling.
It is well established that muscle atrophy induced by hindlimb suspension (HS) in rodents is associated with mitochondrial-associated apoptotic signaling (9, 23, 46, 49, 73), and reloading after immobilization has been shown to reduce mitochondrial-associated apoptotic signaling in skeletal muscle (88). Together, these observations lead us to ask whether HMB might also protect against mitochondrial-associated apoptotic signaling after HS and subsequent reloading of skeletal muscle in rats. In this study, we tested the hypothesis that HMB would reduce myonuclear apoptosis in the hindlimb muscles of aged rats in response to disuse and reloading following disuse. Our data show that HMB attenuated the decrease in muscle fiber area in both fast and slow muscles after HS. Furthermore, HMB reduced muscle atrophy and improved muscle force during reloading after disuse and also reduced myonuclear apoptosis and abundance of proapoptotic proteins Bax and caspase-3 during recovery of hindlimb muscles of aged rats after disuse.
MATERIALS AND METHODS
Animal care.
Sixty-four male Fisher 344×Brown Norway rats, 34 mo of age, were obtained from the National Institute on Aging colony that was housed at Harlan (Indianapolis). The animal care standards were followed by adhering to the recommendations for the care of laboratory animals as advocated by the American Association for Accreditation of Laboratory Animal Care and following the policies and procedures detailed in the Guide for the Care and Use of Laboratory Animals published by the US Department of Health and Human Services and proclaimed in the Animal Welfare Act (PL89–544, PL91–979, and PL94–279). All experimental procedures carried approval from the Institutional Animal Care and Use Committee from the West Virginia University.
HS and reloading after HS.
The rats were randomly assigned to ambulatory control (n = 32), hindlimb suspended (HS; n = 16), or reloaded (R; n = 16) groups. HS was conducted for 14 days as described previously (58). Briefly, orthopedic tape was applied along the proximal one-third of the tail, which was then placed through a wire harness that was attached to a fishlike swivel at the top of a specially designed HS cage. This provided the rats with 360° of movement around the cage. Sterile gauze was wrapped around the orthopedic tape and was subsequently covered with a thermoplastic material (Vet-Lite; Veterinary Specialty Products, Boca Raton, FL). The exposed tip of the tail was monitored to ensure that it remained pink, indicating that HS did not interfere with blood flow to the tail. The suspension height was monitored daily and adjusted, to prevent contact between the hindlimb and any supportive surface in the cage. The suspension angle did not exceed 30 degrees. The forelimbs maintained contact with a grid floor, which allowed the animals to move, groom themselves, and obtain food and water freely. In the R group, the rats were released from HS and allowed normal cage ambulation for 14 days. In the control group, the rats maintained normal mobility, and they moved freely around their cages.
Nutritional treatment with HMB.
Previous studies have reported reduced muscle wasting in rodents in response to cancer cachexia with HMB supplemented at 250 mg/kg body wt (78). This is approximately six times the dose of most studies in humans (62). Our pilot experiments indicated that low doses of HMB were not an effective deterrent for suppressing muscle loss or apoptotic signaling in muscles of aged rats during HS, whereas a dose of 340 mg/kg that was ∼8 times that of human dosing was effective. Therefore, eight conscious animals in each experimental group received 340 mg/kg of Ca-HMB (TSI Health Sciences) dissolved in distilled water (170 mg/ml), or 1 ml of the vehicle (distilled water) by gavage feeding. Animals in the HS and R groups were pretreated with Ca-HMB or the vehicle for 1 wk prior to HS. This allowed the animals to readily accommodate to gavage when they were tail suspended. The animals were given free access to a diet of Purina 5008 rat chow (Ralston Purina, St. Louis, MO) and water over the course of the study.
Research design.
Muscle data were obtained from animals treated with HMB or the vehicle. Two groups of ambulatory nonsuspended control animals were used. Sixteen cage control animals received the vehicle (CC-Veh), and 16 additional animals were cage controls that received HMB (CC-HMB). The cage control animals that were examined 14 days after the initiation of the study were controls for the HS groups (HS-Con; n = 8). A second cage control group of 8 animals/diet group was examined 28 days after the initiation of the study (R-Con; n = 8). These animals were used as controls for the animals that received HS for 14 days followed by 14 days of reloading. Eight animals per diet group were examined after 14 days of HS, and another eight animals per diet group were examined after 14 days of HS followed by 14 days of recovery.
Force measurements.
All force measurements were made while the animals were anesthetized with 98% oxygen and 2% isoflurane gas (53, 65). The animals were placed supine on the heated XY positioning table using a custom-built rat dynamometer (19) with the left foot secured to the footplate at an ankle angle of 90 degrees. Vertical forces were translated to a load cell transducer in the load cell fixture on the footplate. Platinum stimulating electrodes (Grass Medical Instruments, Quincy, MA) were inserted subcutaneously to span the tibial nerve in the popliteal fossa. The maximal isometric force of the plantar flexor muscle group was evaluated by stimulating the tibial nerve using supramaximal square-wave pulses that were 4 V, 100 Hz for 3 s, using a SD9 stimulator (Grass Medical Instruments). Maximal force was determined using LabVIEW-based software. The maximal forces for three isometric contractions were averaged for each data point. Maximal isometric force measurements were made before 14 days of HS (day 0), after 14 days of HS, and 14 days after reloading the hindlimbs following the 14-day HS period.
Body weight and tissue preparation.
Each animal was weighed at the beginning of the experiment, following 14 days of HS and after 14 days of reloading. At the end of the experimental period and with the animals deeply anesthetized, the soleus and the plantaris muscles were removed from both limbs and then blotted and weighed. Following this procedure, the animals were killed by removing the heart. A block obtained from the midbelly of the muscle was embedded in optimal cutting temperature compound (Tissue-Tek; Andwin Scientific, Addison, IL), snap frozen in liquid nitrogen-cooled isopentane, and stored at −80°C. The remainder of the muscle was snap frozen in liquid nitrogen and stored at −80°C until needed for subsequent analyses.
Identification of apoptotic nuclei.
The 10-μm-thick frozen cross sections from soleus and plantaris muscles were mounted on charged microscope slides (Fisher Scientific, Pittsburgh, PA). Apoptotic nuclei were identified by labeling the sections with fluorescent labeling of terminal dUTP nick-end labeling (TUNEL) (cat. no. 11684795910; Roche Applied Science, Indianapolis, IN) and lamina, using methods that were modified slightly from that previously reported for our lab (75). Briefly, the tissue sections were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100 in PBS. The tissue was then incubated at 4°C over night with a rat anti-lamina monoclonal antibody (cat. no. MAB 1914; Millipore, Billerica, MA) to visualize the basal lamina of each muscle fiber. The sections were then incubated with donkey anti-rat rhodamine-conjugated second antibody (cat. no. 712-025-150; Jackson ImmunoResearch Laboratories, West Grove, PA) along with the TUNEL reaction mixture in a humidified chamber at 37°C for 1 h in the dark. Omission of the terminal deoxynucleotide transferase enzyme in the TUNEL reaction mixture on one of the tissue sections on each slide was included as a negative control. The sections were mounted with 4′,6-diamidino-2-phenylindole (DAPI) to visualize all nuclei (Vectashield mounting medium; Vector Laboratories, Burlingame, CA) and viewed under a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Microimaging Thornwood, NY). The number of TUNEL- and DAPI-positive nuclei that were immediately adjacent to, or beneath the basal lamina were counted. Data were expressed as an apoptotic index that was calculated by counting the number of TUNEL-positive nuclei divided by the total number of nuclei (i.e., DAPI-positive nuclei). The apoptotic index was determined from ∼1,200 fibers that were obtained from four nonoverlapping regions of each tissue cross section.
Fiber morphology.
The muscle fiber cross-sectional area (CSA) was determined by planimetry from 750 to 1,200 fibers that were obtained from four nonoverlapping regions of each tissue cross section stained for lamina. Fiber CSA was calculated by ImageJ software (National Institutes of Health). Muscle fiber CSA was not subdivided by fiber type. However, the plantaris muscle in Fisher Brown Norway rats are composed primarily of type II fibers (32), whereas the soleus is composed of ∼90% type I fibers (32, 81). Thus the sampling of fiber CSA likely represented primarily type II fibers in the plantaris muscle and type I fibers in the soleus muscle.
Western blot analysis immunoblots.
Approximately 75 μg of muscle were homogenized in ice-cold RIPA buffer (1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 10 mM Tris; pH 7.4), containing protease inhibitors (cat. no. P8340; Sigma-Aldrich, St. Louis, MO), and phosphatase inhibitors (cat. nos. P2850, P5726; Sigma-Aldrich). The muscle homogenates were centrifuged at 1,000 g for 5 min at 4°C, and the protein content of the supernatant was measured (cat. no. 500-0116; Bio-Rad, Hercules, CA). Then 40 μg of protein were loaded into each well of a 4–12% gradient polyacrylamide gel (cat. no. NP0335BOX; Invitrogen, Carlsbad, CA) and separated by routine SDS-PAGE for 1 h at 120 V. The proteins were transferred to a nitrocellulose membrane for 1.5 h at 25 V. Nonspecific protein binding was blocked by incubating the membranes in 5% nonfat milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) at room temperature. The membranes were incubated (1:1,000) overnight at 4°C, with primary antibodies directed against Bcl-2 (cat. no. 2876; Cell Signaling Technology, Boston, MA), Bax (cat. no. 2772; Cell Signaling), cleaved caspase-3 (cat. no. 9664; Cell Signaling), and cleaved caspase-9 (cat. no. 9509; Cell Signaling). The membranes were washed in TBST and incubated in appropriate dilutions of secondary antibodies (diluted in 5% nonfat milk) conjugated to horseradish peroxidase (Sigma-Aldrich). The signals were developed using a chemiluminescent substrate (Lumigen TMA-6; Lumigen, Southfield, MI) and visualized by exposing the membranes to X-ray films (BioMax MS-1; Eastman Kodak). Digital records were captured with a Kodak 290 camera, and protein bands were quantified using 1D analysis software. The bands were quantified as optical density X-band area and expressed in arbitrary units.
Statistical analysis.
The results are reported as means ± SD. Differences in means between groups were determined by a two-way (2 × 2) ANOVA with the factors of diet (HMB vs. vehicle) and treatment (HS, cage control). A repeated-measures ANOVA was used for longitudinal data analyses of the animals in the recovery group, for assessments before HS, after HS, and in recovery. Bonferroni post hoc analyses were performed between significant means. Then χ2 analyses were conducted between experimental groups for the fiber area frequency data. Statistical significance was set at P < 0.05.
RESULTS
Body weight.
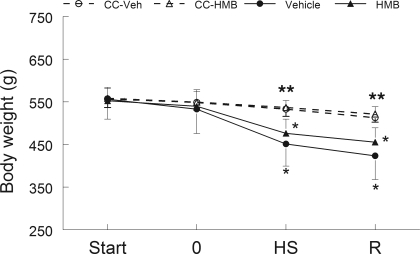
Animal body weight did not differ among the experimental groups at the beginning of the study (Supplementary Table S1; see the online version of this article for supplemental data). The body weight of the CC-Veh and CC-HMB animals did not change over the course of the study. Figure 1 presents longitudinal data for animals in the recovery group and their respective control groups. By contrast, 14 days of HS significantly lowered the animals' body weight by ∼15% (P < 0.05) after treatment in both HMB and water-treated groups (n = 16/group), but there was no difference between treatment groups. Longitudinal assessments of the body weight did not differ between HS and the reloading period of the animals that were in both treatment groups (n = 8 per group) (Fig. 1).
Fig. 1.
Body weight was determined longitudinally in animals at 4 different time points. The first was prior to giving the animals any dietary intervention (Start). The second (time 0), was after the animals were given either β-hydroxy-β-methylbutyrate (HMB) or the vehicle (water) by gavage for 7 days. Final points were after the animals had undergone 14 days of hindlimb suspension (HS) and after 14 days of HS followed by 14 days of reloading (R). Cage control-vehicle-treated (CC-Veh) and cage control-HMB (CC-HMB)-treated animals did not receive hindlimb suspension but were examined at the same time points as the other animals. Body weight was not significantly different between the HMB and vehicle control groups at any time point. An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *Body weight was significantly lower (P < 0.05) than the time points before HS for the same treatment. **Body weight was significantly lower (P < 0.05) in vehicle and HMB-treated groups after both HS and R compared with CC-Veh or CC-HMB groups.
Maximal isometric force.
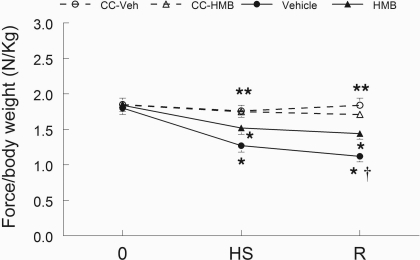
HMB attenuated the loss of force during recovery after unloading, but it did not prevent the decline in force production after HS. Figure 2 shows the longitudinal data for animals in the recovery group compared with the cage control animals for the two diet groups. Maximal isometric force was not different among the groups prior to HS. For animals in the R group, 14 days of HS reduced maximal in vivo plantarflexor isometric force by 34.3% in vehicle-treated animals and by 23.7% in HMB-treated animals (n = 16 per group); however, this did not represent a statistically significant difference between HMB and control groups. In contrast, there was a significantly greater loss in maximal isometric plantarflexor force of the vehicle-treated animals (42.4%) than HMB-treated animals (27.3%) in the R group after reloading (n = 8 per group) (P < 0.01, Fig. 2) compared with the respective cage control groups. The force data for animals that were examined and euthanized after 14 days of HS, did not differ from animals in the R group that were examined after 14 days of HS (Supplementary Table S2).
Fig. 2.
Isometric force. Maximal isometric force production was measured longitudinally in vivo in the plantar flexor muscles before experimental intervention (time 0), after 14 days of HS, and after 14 days of HS followed by 14 days of R. Animals received either HMB, or the vehicle (water) daily, for 7 days before time 0 and throughout the experimental period. An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. †P < 0.05, vehicle vs. HMB; *force/body weight was significantly lower (P < 0.05) compare to points before HS for the same treatment; **force/body weight was significantly lower (P < 0.05) in vehicle and HMB-treated groups after both HS and R compared with CC-Veh or CC-HMB groups.
Muscle wet weight.
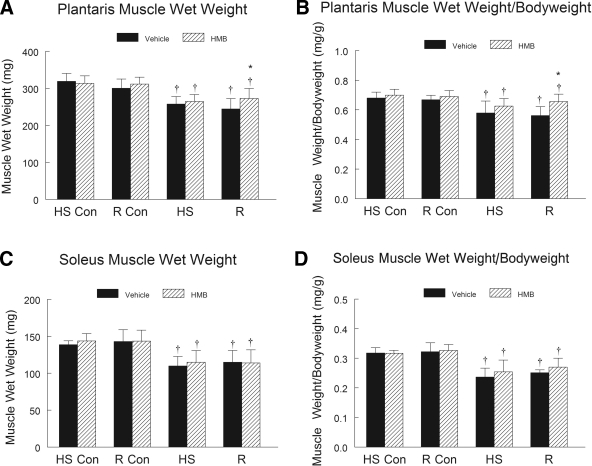
HMB did not reduce the extent of HS-induced atrophy, but it did improve muscle wet weight in the plantaris muscles of the recovery group that were reloaded after HS, relative to the vehicle control animals. HS induced a significant decrease in the wet weight of the plantaris (19%) and soleus (15%) muscles (P < 0.001) of both HMB and vehicle-treated animals (n = 16 per group). Gastrocnemius muscle wet weight was not significantly reduced by HS and did not change during recovery in either diet group (Supplementary Table S3). There was no significant difference in the extent of plantaris or soleus muscle mass loss between the experimental groups after 14 days of HS. Reloading prevented any further decline in soleus and plantaris muscle wet weight, but it did not fully reverse the muscle loss induced by HS. HMB treatment significantly improved plantaris muscle weight after 14 days of reloading relative to vehicle-treated animals (n = 8 per group) (Fig. 3A). However, HMB did not provide any protective effect against HS-induced loss in the soleus muscle wet weight, nor did it improve soleus muscle wet weight recovery after 14 days of reloading, relative to the vehicle-treated animals (Fig. 3B).
Fig. 3.
Muscle wet weight was obtained in plantaris (A) and soleus (C) muscles of control animals for the HS group (HS-Con), the R group (R-Con), and in experimental animals after 14 days of HS or after 14 days of HS followed by 14 days of R. The ratio of muscle wet weight to body weight is presented for the plantaris (B) and the soleus (D) muscles of the aged animals after each condition. Animals received HMB or the vehicle (water) daily by gavage, for a total of 21 days (HS-Con and HS) or for 32 days (R-Con and R). An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *P < 0.05, vehicle vs. HMB within the same condition; †P < 0.05 HS or R vs. control animals for that experimental condition.
Changes of muscle fiber CSA.
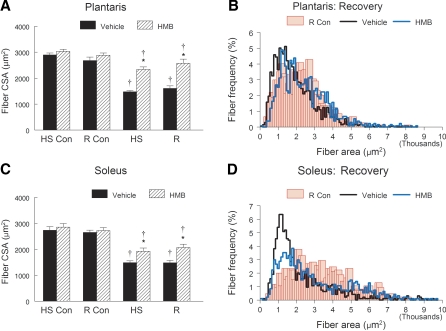
HMB appeared to reduce the extent of fiber atrophy in both the slow soleus and the fast plantaris muscles that occurred after HS or reloading. HS dramatically decreased mean fiber CSA in both plantaris (Fig. 4A) and soleus (Fig. 4B) hindlimb muscles. However, the HS-induced decrease in fiber CSA of the vehicle-treated animals was greater than in the HMB-treated animals for plantaris (48.8% vs. 26.4%, P < 0.05) and soleus muscles (45.6% vs. 32.5%, P < 0.05). HMB did not further improve fiber CSA in either plantaris (Fig. 4A) or soleus (Fig. 4B) muscles after 14 days of reloading compared with HS. The fiber area-to-fiber frequency distribution for the plantaris (Fig. 4C) and soleus (Fig. 4D) are shown for the recovery group. After 14 days of recovery, the muscle fibers in both the plantaris and soleus were still shifted to the left in the fiber area-to-frequency distribution, relative to the distribution of the control muscles. The χ2 analyses showed that the frequency of fibers < 1,500 μm2 was significantly greater in the plantaris and soleus muscles of vehicle-treated animals compared with HMB-treated animals that had recovered for 14 days following HS. The distribution of the fiber area-to-frequency distribution after HS (Fig. 4, C and D) was similar to that shown for recovery (data not shown).
Fig. 4.
Fiber cross-sectional area (CSA) was obtained by planimetry in the plantaris (A) and the soleus (C) muscles of control animals for the HS group (HS-Con), the recovery group (R-Con), and in experimental animals after 14 days of HS, or after 14 days of HS followed by 14 days of R. Animals received HMB or the vehicle (water) daily by gavage, for 7 days of pretreatment, followed by 14 days (HS-Con and HS) or for 28 days (R-Con and R) of the respective interventions. Fiber area-to-fiber frequency distribution is shown in 100-μm2 bin widths for the plantaris (B) and soleus (D) muscles of animals in the R group. An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *P < 0.05, vehicle vs. HMB within the same condition; †P < 0.05. HS or R vs. control animals for that experimental condition.
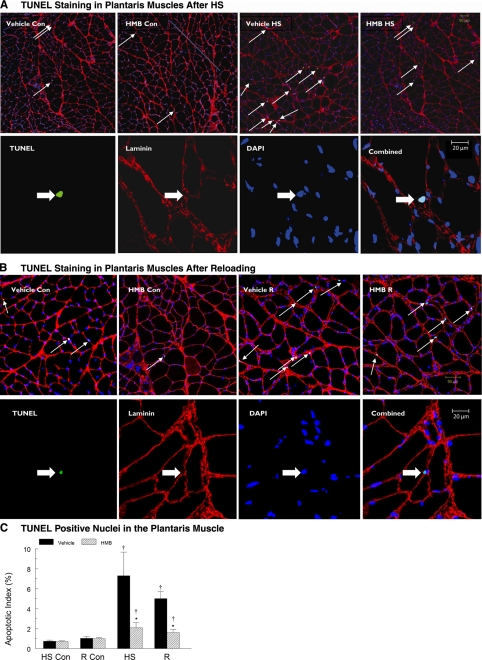
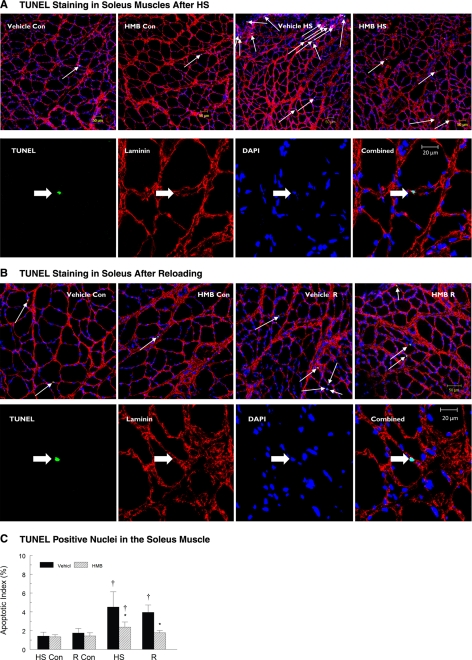
Apoptotic myonuclei as identified by TUNEL labeling.
The apoptotic index as indicated by the number of TUNEL-positive myonuclei to total myonuclei, was significantly increased by HS, and reloading following HS, relative to muscles from cage control animals, but HMB attenuated the apoptotic index in both HS and recovery muscles. HS significantly increased the number of TUNEL-positive nuclei in both plantaris (Fig. 5A) and soleus (Fig. 6A) muscles. Although there were some regional differences within tissue sections, TUNEL-positive nuclei occurred throughout each tissue cross section that was obtained from plantaris and soleus muscles following HS. The apoptotic index was significantly increased in plantaris (9.9-fold, P < 0.05) and soleus (3.2-fold, P < 0.05) muscles of vehicle-treated animals compared with ambulatory control animals. Although HMB treatment suppressed myonuclear apoptosis, it did not eliminate it. HS increased the apoptotic index in both plantaris (3.0-fold, P < 0.05) and soleus (1.8-fold, P < 0.05) muscles of HMB-treated animals compared with ambulatory control animals. The apoptotic index was significantly greater (P < 0.05) in both plantaris (Fig. 5C) and soleus muscles from vehicle compared with HMB-treated animals (Fig. 6C). Apoptosis, as identified by TUNEL labeling, remained high during reloading following HS, especially in vehicle-treated muscles (Figs. 5B and 6B). HMB significantly suppressed TUNEL labeling in the myonuclei of reloaded plantaris (Fig. 5C) and soleus (Fig. 6C) muscles relative to vehicle-treated animals. The ratio of TUNEL-positive myonuclei to total myonuclei in reloaded, HMB-treated soleus was not different from the ratio of TUNEL-positive to total myonuclei in soleus cross sections from in cage control animals (Fig. 6C).
Fig. 5.
A, top row: representative tissue sections from the plantaris muscle with fluorescent staining for TUNEL (green) to identify apoptotic nuclei in control and hindlimb suspended muscles. DAPI identified all nuclei (blue). Basal lamina (red) was identified to confirm that the TUNEL-positive nuclei were myonuclei. Conditions were vehicle control (Vehicle-Con), HMB control (HMB-Con), vehicle after 14 days of HS (Vehicle-HS), and HMB after 14 days of HS (HMB-HS). Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. Bottom row: a higher magnification showing the individual markers for TUNEL, laminin, DAPI, and the combined images. Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. B, top row: representative tissue sections from the plantaris muscle, with fluorescent staining for TUNEL (green) to identify apoptotic nuclei in control and reloaded muscles. DAPI identified all nuclei (blue). Basal lamina (red) was identified to confirm that the TUNEL-positive nuclei were myonuclei. Conditions were the same as in A. Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. Bottom row: a higher magnification showing the individual markers for TUNEL, laminin, DAPI, and the combined images. Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. C: apoptotic index was calculated from tissue cross sections of the plantaris muscle by determining the ratio of TUNEL-positive nuclei to total nuclei in plantaris muscles of control animals for the HS group (HS-Con), the recovery group (R-Con), and in experimental animals after 14 days of HS or after 14 days of HS followed by 14 days of reloading (R). Only nuclei that were directly below or touching the basal lamina were counted. Animals received HMB or the vehicle (water) daily by gavage, for a total of 21 days (HS-Con and HS) or for 32 days (R-Con and R). An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *P < 0.05, Vehicle vs. HMB within the same condition; †P < 0.05, HS or R vs. control animals for that experimental condition.
Fig. 6.
A, top row: representative tissue sections from the soleus muscle with fluorescent staining for TUNEL (green) to identify apoptotic nuclei in control and hindlimb suspended muscles. DAPI identified all nuclei (blue). Basal lamina (red) was identified to confirm that the TUNEL-positive nuclei were myonuclei. Conditions were vehicle control (Vehicle-Con), HMB control (HMB-Con), vehicle after 14 days of HS (Vehicle-HS), and HMB after 14 days of HS (HMB-HS). Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. Bottom row: a higher magnification showing the individual markers for TUNEL, laminin, DAPI, and the combined images. Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. B, top row: representative tissue sections from the soleus muscle, with fluorescent staining for TUNEL (green) to identify apoptotic nuclei in control and reloaded muscles. DAPI identified all nuclei (blue). Basal lamina (red) was identified to confirm that the TUNEL-positive nuclei were myonuclei. Conditions are the same as in A. Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. Bottom row: a higher magnification showing the individual markers for TUNEL, laminin, DAPI, and the combined images. Arrows show TUNEL-positive nuclei lying below or immediately adjacent to the basal lamina of the muscle fibers. C: apoptotic index was calculated from tissue cross sections of the soleus muscle by determining the number ratio of TUNEL-positive nuclei to total nuclei in soleus muscles of control animals for the HS group (HS-Con), the recovery group (R-Con), and in experimental animals after 14 days of HS or after 14 days of HS followed by 14 days of reloading (R). Only nuclei that were directly below or touching the basal lamina were counted. Animals received either HMB or the vehicle (water) daily by gavage, for a total of 21 days (HS-Con and HS) or for 32 days (R-Con and R). An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *P < 0.05, vehicle vs. HMB within the same condition; †P < 0.05, HS or R vs. control animals for that experimental condition.
Apoptotic signaling proteins.
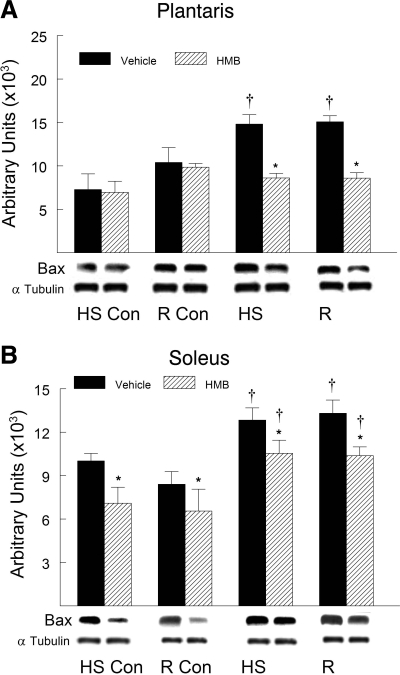
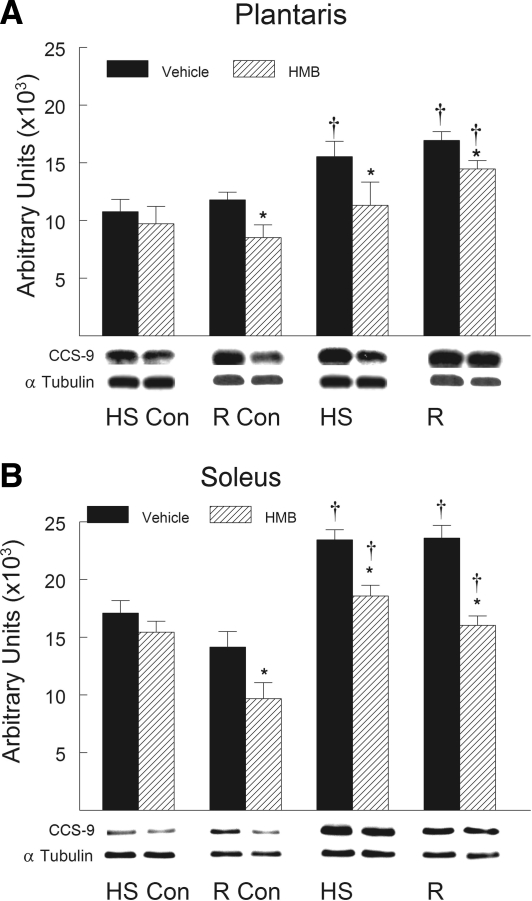
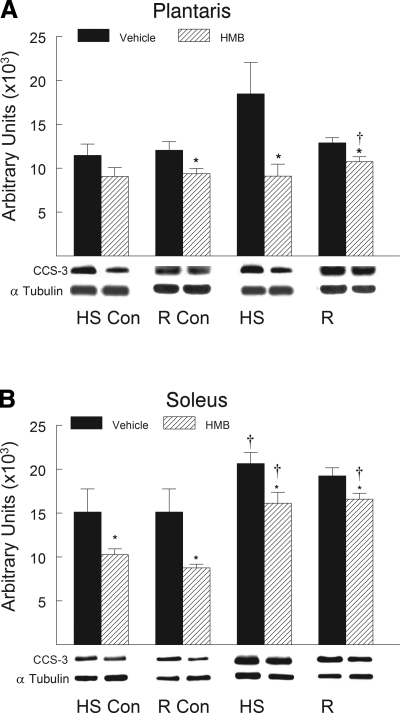
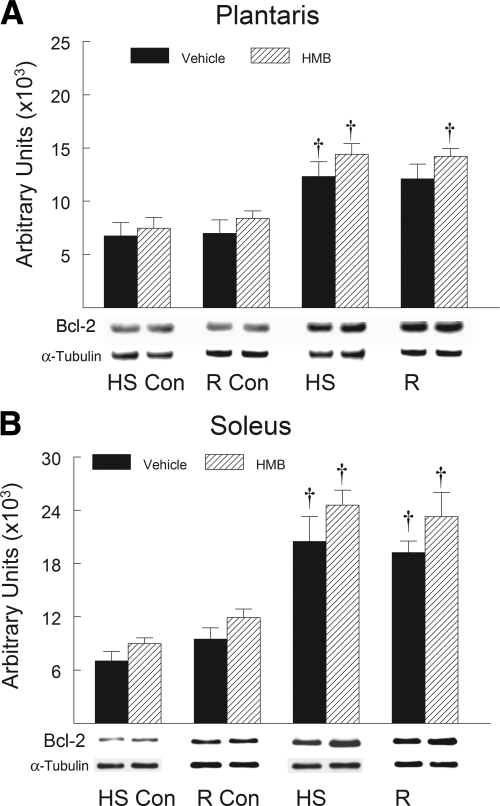
HMB treatment suppressed the HS-induced increase in the proapoptotic proteins after HS and reloading compared with vehicle-treated animals. Proapoptotic proteins associated with mitochondrial apoptotic signaling significantly increased in abundance in hindlimb muscles after HS and remained elevated during reloading. Proapoptotic proteins including Bax (Fig. 7), cleaved caspase-9 (Fig. 8), and cleaved caspase-3 (Fig. 9) all significantly increased (P < 0.05) after both HS and reloading. HMB significantly suppressed the protein abundance for Bax (Fig. 7), cleaved caspase-9 (Fig. 8), and cleaved caspase-3 (Fig. 9) in plantaris and soleus muscles after both HS and reloading conditions (P < 0.05). The abundance of proapoptotic signaling proteins was similar in the plantaris (Figs. 7A, 8A, and 9A) and soleus (Figs. 7B, 8B, and 9B) muscles after HS and reloading, and this was not altered by HMB. It was interesting to note that HMB significantly reduced the protein abundance of Bax (Fig. 7B) and cleaved caspase-3 (Fig. 9B) in control muscles of ambulatory cage control animals (HS-Con and R-Con). Both HS and reloading after HS significantly increased the anti-apoptotic protein Bcl-2 in plantaris (Fig. 10A) and soleus (Fig. 10B) muscles by ∼100% (P < 0.05), but there was no significant difference between HMB and vehicle-treated muscles after either HS or reloading after 14 days of HS.
Fig. 7.
Bax protein abundance was determined by Western blot analysis in the plantaris (A) and soleus (B) muscles of rats under control, HS, or reloading conditions. Groups include: controls for the HS group (HS-Con), controls for the recovery group (R-Con), and in experimental animals after 14 days of HS or after 14 days of HS followed by 14 days of reloading (R). Animals received HMB or the vehicle (water) daily by gavage, for a total of 21 days (HS-Con and HS) or for 32 days (R-Con and R). Eight animals were in each diet and experimental group. α-Tubulin was used as a loading control. Data were normalized to α-tubulin and were expressed as means ± SD. An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *P < 0.05, vehicle vs. HMB within the same condition; †P < 0.05, HS or R vs. control animals for that experimental condition.
Fig. 8.
Cleaved caspase-9 protein abundance was determined by Western blot analysis in the plantaris (A) and soleus (B) muscles of rats under control, HS, or reloading conditions. Groups include controls for the HS group (HS-Con), controls for the recovery group (R-Con), and in experimental animals after 14 days of HS or after 14 days of HS followed by 14 days of reloading (R). Animals received HMB or the vehicle (water) daily by gavage, for a total of 21 days (HS-Con and HS) or for 32 days (R-Con and R). Eight animals were in each diet and experimental group. α-Tubulin was used as a loading control. Data were normalized to α-tubulin and were expressed as means ± SD. An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *P < 0.05, vehicle vs. HMB within the same condition; †P < 0.05, HS or R vs. control animals for that experimental condition.
Fig. 9.
Cleaved caspase-3 protein abundance was determined by Western blot analysis in the plantaris (A) and soleus (B) muscles of rats under control, HS, or reloading conditions. The groups include controls for the HS group (HS-Con), controls for the recovery group (R-Con), and in experimental animals after 14 days of HS or after 14 days of HS followed by 14 days of reloading (R). The animals received HMB or the vehicle (water) daily by gavage, for a total of 21 days (HS-Con and HS) or for 32 days (R-Con and R). Eight animals were in each diet and experimental group. α-Tubulin was used as a loading control. Data were normalized to α-tubulin and were expressed as means ± SD. An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. *P < 0.05, vehicle vs. HMB within the same condition; †P < 0.05, HS or R vs. control animals for that experimental condition.
Fig. 10.
Bcl-2 protein abundance was determined by Western blot analysis in the plantaris (A) and soleus (B) muscles of rats under control, HS, or reloading conditions. The groups include controls for the HS group (HS-Con), controls for the recovery group (R-Con), and in experimental animals after 14 days of HS or after 14 days of HS followed by 14 days of reloading (R). The animals received HMB or the vehicle (water) daily by gavage, for a total of 21 days (HS-Con and HS) or for 32 days (R-Con and R). Eight animals were in each diet and experimental group. α-Tubulin was used as a loading control. Data were normalized to α-tubulin and were expressed as means ± SD. An ANOVA followed by Bonferroni post hoc analyses was used to evaluate the differences between the group means. †P < 0.05, HS or R vs. control animals for that experimental condition.
DISCUSSION
HMB supplementation has been proposed to prevent muscle damage (41), lower protein degradation (77), and directly stimulate skeletal muscle protein synthesis (91). In addition, HMB has been shown to reduce the extent of apoptosis in muscle cells in vitro (42), although the potential for HMB to moderate apoptosis in skeletal muscle in vivo has not been previously studied. In this study, we provide the first evidence for a positive influence of HMB on the reduction of proapoptotic signaling in response to 14 days of hindlimb-induced muscle disuse and also in response to 14 days of muscle reloading following HS in aged rats. Although HMB could not fully prevent HS-induced body weight or muscle loss in response to unloading, it: 1) prevented further force loss during reloading after unloading, 2) improved muscle mass in the plantaris muscles that were reloaded after HS, 3) blunted the extent of fiber atrophy in both fast and slow skeletal muscles in response to unloading and reloading after disuse, 4) significantly attenuated myonuclear apoptosis induced by HS, 5) decreased the apoptotic index after reloading following HS in both plantaris and soleus muscles; and 6) reduced mitochondrial apoptotic signaling as indicated by lower levels of cleaved caspase-3, cleaved caspase-9, and Bax protein abundance in reloaded plantaris and soleus muscles.
HMB protects loss of muscle function.
Studies from our lab and others (6, 58, 74–76, 91) have demonstrated that HS dramatically reduces skeletal muscle mass and strength in both young and aged rats. In contrast to young adult animals, which recover plantaris and soleus muscle weight within 14 days of reloading after 14 days of HS (Fig. S1), the data in the present study (Fig. 3) show that there is no recovery of muscle mass of previously unloaded muscles after 14 days of HS followed by 14 days of reloading (recovery) in the soleus of aged Fisher Brown Norway rats, although there is some improvement in the plantaris weight and muscle weight/body weight of HMB-treated aged rats after 14 days of reloading (Fig. 3). The failure to recover muscle lost during HS after reloading of the hindlimbs in aged rats may be explained, in part, by elevated proapoptotic signaling proteins in these muscles after both HS and reloading following HS. However, in young rats, full recovery of muscle mass during reloading after HS is accompanied by a reduction in proapoptotic signaling (Supplementary Figs. S2, S3, and S4). HMB treatment did not improve this recovery in young animals, but it did increase (but not fully) muscle mass in the plantaris of aged rats during the 14-day recovery period following HS. While the extent of recovery of reloaded muscles following HS was similar in HMB and vehicle-treated animals, we could not determine from this study whether the recovery of muscle mass following HS in young animals occurred more rapidly in the HMB-fed group compared with the vehicle-treated group.
While the gastrocnemius muscle is the greatest contributor to plantar flexion force, HS and reloading after HS did not significantly alter gastrocnemius muscle mass (Supplementary Table S3). Changes in plantar flexion isometric force after HS, and in the R group, must be primarily the result of changes in function of the soleus and plantaris muscles. Our data are consistent with observations from young adult rats (90) and mice (34), which show that reloading after disuse did not improve the production of muscle force. Nevertheless, HMB helped to prevent any further reduction in maximal isometric force in the plantar flexor muscles following reloading after HS compared with the vehicle-treated animals (Fig. 2). The age-associated increase in the potential for muscle injury during reloading (12) and/or the widespread diminished ability of muscles from aged animals to respond positively to loading paradigms (5, 18) likely also contribute to the lack of functional improvement with reloading. We did not complete a full-time response curve of the responses for either HS or recovery following HS. Therefore, it is not possible to know whether the muscle mass was lost in both vehicle and HMB groups during the early parts of reloading and then was restored more fully or rapidly during the subsequent days of recovery in the HMB group vs. the control group or whether HMB provided a protection against the loss of muscle mass and muscle function during reloading. Either scenario would be consistent with the modest protection that HMB provides against loading-induced muscle damage and repair (92).
HMB did not alter the body weight of young adult animals during HS or during the recovery period after HS (Supplementary Table S4). Furthermore, HMB did not improve the recovery of muscle mass in young animals after hindlimb unloading, because muscle restoration was complete by 14 days following HS in young adult rats (Supplementary Table S5; Supplementary Fig. S1). In contrast, HMB reduced the extent of recovery-induced atrophy during reloading after HS in the plantaris muscle of aged animals (Fig. 3A). This was a muscle-specific effect, because soleus muscle wet weight was not different in the HMB and vehicle groups during recovery, whereas plantaris muscle wet weight was greater in the HMB vs. the vehicle-treated group. Nevertheless, fiber CSA was greater in the HMB group in both the plantaris and soleus muscles during recovery.
It was interesting to find a lack of preservation of muscle wet weight after HS (Fig. 3) in the soleus or plantaris muscles, despite preservation of muscle fiber CSA. We had expected that any changes in muscle wet weight would be reflected by similar changes in muscle force production. However, isometric force seemed to track muscle fiber CSA more closely than muscle wet weight. For example, in aged animals, isometric plantarflexor force declined with HS, as did plantaris and soleus fiber CSA, yet force was greater in HMB-treated muscles after reloading, and fiber CSA was also greater in both plantaris and soleus muscles from HMB-treated animals compared with control animals. Although speculative, several possibilities could explain this. First, HS may have reduced total plantaris and soleus fiber number in the muscles of aged animals (perhaps via apoptosis), which in turn, may have contributed to overall lower muscle volume and mass (35), and this was not prevented by HMB. If this occurred, then we would not expect that muscles in old animals could recover without adding back the lost fibers (which might be difficult or impossible), and therefore fiber hypertrophy would be the primary mechanism leading to improved muscle mass and function during reloading. Some compensation of increasing fiber size by HMB during HS might buffer the loss of force, if the cross bridges were all capable of generating maximal force [i.e., moving from the weakly bound to strongly bound positions (47)]. This may have been the case (Fig. 2), although this was not statistically significant. Secondly, if some fibers fused together during remodeling associated with HS or reloading following HS, there could be larger fiber sizes without any change in muscle mass. Likewise, with reloading, new fiber accumulation (33) or fiber splitting could have occurred as, potentially, the result of incomplete fiber repair as shown in human muscles after extreme loading conditions (27) and in disease (61). Furthermore, fiber hypertrophy in split or new fibers could then have contributed to increased muscle mass and force in the plantaris during reloading after disuse. In contrast, the larger fiber CSA in the soleus would not be expected to contribute to any improvement in muscle mass or force if this only reflected an increase in the number of fused fibers. Another possibility is that activated satellite cells fused to existing muscle fibers and began protein synthesis during the atrophic process in an attempt to offset muscle wasting, but this would result in minimal changes in muscle wet weight until sufficient time had occurred to accumulate new protein. In addition, the activated satellite cells during HS would at least initially express immature myosin isoforms (10, 93), which would not have contributed to marked improvement in muscle force, whereas during recovery, there would have been sufficient time for the myosin isoforms in the plantaris to mature and become expressed, fused to the existing fibers, and fully integrated in sarcomeres so that they could both enlarge fiber CSA and contribute to the production of force. It is possible that maturation of the soleus muscle isoforms was incomplete, and this would reduce the contribution to improving overall muscle function. It was however, interesting that the loss of muscle force production tracked the changes in fiber CSA better than muscle wet weight. This observation may have important implications because it suggests that fiber CSA may be a better predictor of muscle function (isometric force) than muscle wet weight. We did not identify CSA for individual fiber types, but rather provided a random sampling across the predominately type II myosin containing plantaris muscle and the type I myosin containing soleus muscle. It is possible that one fiber type might be a better indicator of changes in muscle function, but we cannot determine this from the present data. As the gastrocnemius muscle did not atrophy during HS or hypertrophy during reloading following HS (Supplementary Table S1), the changes in force were presumably the result of remodeling of the plantaris and soleus muscles, but since the plantaris muscle is larger, it would make the greatest contribution to the changes in isometric muscle force in the hindlimb of the aged rats.
Apoptosis with muscle disuse and aging.
The incidence of apoptosis (Supplementary Fig. S2) and proapoptotic signaling (Supplementary Figs. S3 and S4) are very low in muscles from young animals, and although apoptosis increases with HS, it is still lower than that observed for muscles of aged animals. Apoptosis has been suggested to play an important role in the development of aging-associated sarcopenia, and apoptotic signaling is elevated during periods of muscle disuse (4, 23, 46, 54). Results of the present study are consistent with previous data that have shown that muscle atrophy induced by unloading, increases apoptotic signaling in both the plantaris and the soleus muscles of aged Fisher 344×Brown Norway rats (54, 70, 76). The inability to improve muscle mass or strength in response to reloading, may be the result of an aging-associated decline in myonuclei (in part as a result of elevated myonuclear apoptosis), and therefore, a lower transcriptional capability in muscles of aged animals (52). This speculation is supported by our observations in the present study that apoptosis is elevated in hindlimb muscles of aged rats during conditions of both unloading and reloading. In the present study, HS significantly increased the number of TUNEL-positive nuclei to total nuclei in cells from both plantaris and soleus muscles of aged animals, and this was accompanied by an increase in the proapoptotic proteins Bax, caspase-9, and caspase-3 (Figs. 7–9), which together are involved in the mitochondria-dependent apoptotic signaling pathway (9). Interestingly, the anti-apoptotic protein content of Bcl-2 was also increased after HS and reloading (Fig. 10). We cannot rule out the possibility that apoptotic signaling may have occurred by a combination of muscle and nonmuscle cells (e.g., endothelial cells) that reside inside muscles (i.e., within the basal lamina) in vivo. Furthermore, the apoptotic index could overestimate the number of nuclei that would be lost to apoptosis, if DNA damage was eventually repaired in some of the TUNEL-positive nuclei. However, the immunocytochemical data clearly showed that the TUNEL-positive nuclei that were quantified were of myonuclear or satellite cell origins and not nonmuscle nuclei (Figs. 5–6). Furthermore, we anticipate that many of the TUNEL-positive nuclei would result in loss of nuclei in unloaded and reloaded muscles as a result of apoptosis, and this would reduce the ability to maintain transcriptional regulation of muscle proteins, and thereby potentially contribute to fiber atrophy. Apoptosis was much less severe in muscles of young adult animals (Supplementary Fig. S2) compared with muscles in aged animals (Fig. 5, 6), and this was consistent with lower deficits in function in young animals (Supplementary Table S5) compared with aged animals that were subjected to the same conditions (Fig. 2; Supplementary Table S2). Together, these findings suggest that apoptosis contributes to muscle loss of aged hosts during stresses (either unloading or reloading after disuse), but it plays little importance in remodeling of muscle of young hosts that are subjected to similar stresses.
The data in this study suggests that there is not a linear relationship between increased signaling for nuclear apoptosis and muscle mass. This is because the outcome of apoptosis in multinucleated skeletal muscle differs from apoptosis in the classical one cell systems (8, 9). In skeletal muscle, nuclei can be eliminated without a substantial change in muscle mass or loss of muscle fibers. After some critical threshold (and we do not know at what point that exactly occurs) fiber atrophy would need to occur, as the existing nuclei would presumably be unable to maintain the given fiber size (8, 9). After fiber CSA has been decreased as a result of elimination of enough nuclei, there would presumably be some measurable loss of muscle wet weight. However, we would anticipate that the changes in fiber CSA would precede and be more sensitive to apoptotic changes in muscle than would muscle wet weight. Thus, changes in fiber CSA should be better predictors of apoptotic changes in muscle compared with muscle wet weight, and the data in this study supports this possibility. A second factor that offsets the loss of muscle mass that would have occurred as a result of an increase in proapoptotic signaling is the observed increase in anti-apoptotic proteins such as Bcl-2 (Fig. 10; Supplementary Figs. S3 and S4).
In this study, we show that even during muscle reloading following 14 days of unloading, there was apoptosis ongoing in both plantaris and soleus muscles, albeit less than that occurring during HS alone. Thus, the common stimulus triggering apoptosis in skeletal muscles of old animals is unlikely to be solely due to the presence or absence of contractile tension in muscles or tension that is sensed by the extracellular matrix. One potential candidate for triggering apoptosis under both unloading and loaded conditions is the high level of oxidative stress that exists in both unloaded (44, 74) and acutely loaded (65–67) muscles of aged rats. Another possibility is a systemic elevation of cytokines in response to loading or unloading (56, 58).
HMB regulation of apoptosis in unloading and reloading.
HMB supplementation has been previously shown to blunt muscle loss in critically ill patients (43), including patients with inflammatory diseases (37, 48, 51). This is due, at least in part, to reductions in proteasome activity (36). In addition, there is evidence that HMB has the potential to improve strength gains in response to resistance exercise in 70-yr-old males and females (89). Our data build upon these observations by showing that HMB can reduce unloading-reloading muscle fiber area losses in fast skeletal muscle, and this is associated with reduced apoptotic signaling in aged fast (i.e., plantaris) skeletal muscles. For example, HMB significantly attenuated the increase of the proapoptotic proteins (e.g., Bax, cleaved caspase-9, cleaved caspase-3) and the number of apoptotic nuclei in both plantaris and soleus muscles after HS and reloading in aged rats although the positive effects of HMB were less dramatic in the soleus muscle. Thus, the effect of HMB appeared to suppress apoptotic signaling to a greater extent in the predominantly fast myosin-containing plantaris muscle compared with the predominately slow myosin-containing soleus muscle. The data from this study do not provide an explanation for this finding. While speculative, it is possible that the plantaris muscle is more sensitive to HMB. Perhaps the degree of apoptosis was more severe in the plantaris compared with the soleus muscle (apoptotic index, 9.9-fold vs. 3.2-fold).
Although beyond the scope of this study, additional studies are required to determine whether the levels of oxidative stress or cytokines are similar in the fast plantaris and slow soleus muscles of aged animals under conditions of unloading or reloading, and whether HMB affects these potential triggers for apoptosis. This is a possible mechanism worth examining because HMB is known to downregulate lipopolysaccharide-induced oxidative stress (64) as well as the cytokine TNF-α-induced (25, 26) NF-κB (63) activation in cachectic muscle. Oxidative stress would be expected to be greater in the plantaris than the soleus, because slow muscles like the soleus have a greater antioxidant capacity (40) (e.g., greater mitochondria-localized manganese superoxide dismutase, glutathione, etc.) than fast muscles like the plantaris. In this scenario, the soleus would have a lower need for buffering oxidative stress, and therefore HMB would have a lower impact in the soleus than in the plantaris muscle. Another possibility is that the mechanisms that regulate muscle loss are different in the fast plantaris and slow soleus muscles. For example, the frequency of TUNEL-positive myonuclei appeared to be greater in the plantaris vs. the soleus muscle after HS and R conditions. It is therefore possible that multiple apoptotic pathways, including cytokine and mitochondrial pathways, were activated in the atrophied plantaris muscle (58, 73), whereas primarily mitochondrial-associated signaling was more prevalent in the soleus muscle during HS and R (46). Although speculative, it is feasible that HMB may have been more effective at suppressing apoptotic pathways associated with muscle loss in the plantaris compared with pathways regulating muscle loss in the soleus of aged rats.
The apoptotic index was not altered by HMB in control muscles. However, it appears that part of the basal apoptotic signaling pathway was suppressed by HMB treatment. This can be seen by significantly lower abundances of cleaved capase-9 and cleaved caspase-3 in plantaris and soleus muscles of control animals, which had been supplemented for 5 wk (R-Con animals). In some cases, the shorter supplemental period of 3 wk (HS-Con animals) failed to have lower caspase signaling in the plantaris muscle compared with the vehicle ambulatory control animals. Presumably, resetting the basal levels of proapoptotic signaling protein abundance by HMB (as seen in the control animals) should protect the subsequent apoptotic stresses that would be introduced to the skeletal muscles. Thus, some of the benefit of HMB suppressed apoptotic signaling in HS and reloading conditions, may be a resetting of the apoptotic threshold.
Mitochondrial apoptotic signaling proteins are altered by HMB treatment, and therefore it is likely that mitochondrial structure and/or functions improved by HMB during severe stresses, such as reloading after HS. This is possible since HMB stabilizes sarcolemma HMG-CoA reductase (reviewed in Ref. 91). Mevalonic acid, produced from HMG-CoA reductase is a precursor of coenzyme Q and dolichols, which play a major role in mitochondrial electron transport function and myocyte proliferation. Additional studies are needed to determine whether HMB affects apoptosis by stabilizing the function of mitochondria in skeletal muscle under conditions of disuse and reloading following hindlimb unloading. Although apoptosis is an important signaling process that occurs during unloading, clearly apoptosis is not the only contributor to muscle wasting, especially in the soleus muscle where muscle loss is typically more severe than in the plantaris during disuse.
Perspectives and Significance
Although HMB did not prevent skeletal muscle loss induced by HS in aged Fisher 344×Brown Norway rats, HMB did improve muscle recovery following HS and subsequent reloading. As muscle force should be proportional to the physiological fiber (or muscle) CSA, if fiber size is better maintained by HMB during the stress of reloading, then there should be a greater number of cross bridges, which would have the potential to improve force production. The protective mechanism(s) by which HMB exerts its effects during unloading and improves recovery following reloading may be due, in part, to the inhibition of myonuclear apoptosis, specifically, by suppressing the mitochondrial-caspase signaling pathway. For example, a reduction in the level of apoptotic signaling during the period of unloading is a possible mechanism that improves muscle recovery after unloading in HMB-treated animals as a result of having a greater number of nuclei and therefore improved transcriptional control of protein synthesis. Nevertheless, it is unlikely that the positive effects of HMB on muscle mass and function under conditions of hindlimb unloading induced disuse and reloading after hindlimb unloading are solely due to changes in apoptotic signaling. In addition to a reduction in the mitochondrial-associated apoptotic signaling, other possible mechanisms of HMB action include enhancing protein synthesis via the mammalian target of rapamycin pathway (24) and/or depression of protein degradation through inhibition of the ubiquitin-proteosome pathway (13, 79). Another alternative is that HMB might promote increased activation of satellite cells and/or differentiation of activated satellite cells (42) in response to unloading or reloading to replace nuclei that are eliminated by apoptosis. Additional work is needed to determine whether part of the preservation of muscle mass and strength by HMB is on muscle cell proliferation and differentiation, in addition to improving cell survival by reducing apoptosis. In addition, it is possible that if the dose or timing of HMB had differed from that which was used in the present study (e.g., giving HMB only at the onset of reloading) the responses might differ from the results that we report in this study. Nevertheless, our present data suggest that HMB should be further evaluated and considered as part of a potential therapeutic strategy along with more moderate muscle loading to prevent muscle loss in aging and perhaps other conditions of muscle wasting.
GRANTS
The West Virginia University Microscope Imaging Facility is supported by the Mary Babb Randolph Cancer Center and National Institutes of Health Grant 5P20RR016440-09. Funding for this project was provided by Abbott Laboratories.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Hua Zhao, Brian Bennett, and Eric Scheller for technical assistance provided in this study. We also acknowledge assistance from Karen H. Martin, PhD and the West Virginia University Microscope Imaging Facility.
REFERENCES
- 1. Adhihetty PJ, Hood DA. Mechanisms of apoptosis in skeletal muscle. Basic Appl Myol 13: 171–179, 2003 [Google Scholar]
- 2. Adhihetty PJ, O'Leary MF, Hood DA. Mitochondria in skeletal muscle: adaptable rheostats of apoptotic susceptibility. Exerc Sport Sci Rev 36: 116–121, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1α on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol 297: C217–C225, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol 273: C579–C587, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol 283: C66–C76, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Alway SE, Lowe DA, Chen KD. The effects of age and hindlimb suspension on the levels of expression of the myogenic regulatory factors MyoD and myogenin in rat fast and slow skeletal muscles. Exp Physiol 86: 509–517, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Alway SE, Martyn JK, Ouyang J, Chaudhrai A, Murlasits ZS. Id2 expression during apoptosis and satellite cell activation in unloaded and loaded quail skeletal muscles. Am J Physiol Regul Integr Comp Physiol 284: R540–R549, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Alway SE, Morissette MR, Siu PM. Aging and apoptosis in muscle. In: Handbook of the Biology of Aging, edited by Masoro EJ, Austad S. New York: Elsevier, 2011 [Google Scholar]
- 9. Alway SE, Siu PM. Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev 36: 51–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Appell HJ. Morphology of immobilized skeletal muscle and the effects of a pre- and postimmobilization training program. Int J Sports Med 7: 6–12, 1986 [DOI] [PubMed] [Google Scholar]
- 11. Aversa Z, Bonetto A, Costelli P, Minero VG, Penna F, Baccino FM, Lucia S, Rossi FF, Muscaritoli M. β-hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Oncol 38: 713–720, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Baker BA, Cutlip RG. Skeletal muscle injury versus adaptation with aging: novel insights on perplexing paradigms. Exerc Sport Sci Rev 38: 10–16, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Baptista IL, Leal ML, Artioli GG, Aoki MS, Fiamoncini J, Turri AO, Curi R, Miyabara EH, Moriscot AS. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 41: 800–808, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Baracos VE. Management of muscle wasting in cancer-associated cachexia: understanding gained from experimental studies. Cancer 92: 1669–1677, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Baumgartner RN. Body composition in healthy aging. Ann NY Acad Sci 904: 437–448, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7: 2–12, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Cohen HJ. In search of the underlying mechanisms of frailty. J Gerontol A Biol Sci Med Sci 55: M706–M708, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Cutlip RG, Baker BA, Geronilla KB, Mercer RR, Kashon ML, Miller GR, Murlasits Z, Alway SE. Chronic exposure to stretch-shortening contractions results in skeletal muscle adaptation in young rats and maladaptation in old rats. Appl Physiol Nutr Metab 31: 573–587, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Cutlip RG, Stauber WT, Willison RH, McIntosh TA, Means KH. Dynamometer for rat plantar flexor muscles in vivo. Med Biol Eng Comput 35: 540–543, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve 27: 339–347, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Degens H, Alway SE. Control of muscle size during disuse, disease, and aging. Int J Sports Med 27: 94–99, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med 35: 473–483, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol 291: R1730–R1740, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by β-hydroxy-β-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab 293: E923–E931, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab 295: E1409–E1416, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-α and angiotensin II by β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab 295: E1417–E1426, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Eriksson A, Lindstrom M, Carlsson L, Thornell LE. Hypertrophic muscle fibers with fissures in power-lifters; fiber splitting or defect regeneration? Histochem Cell Biol 126: 409–417, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Evans W. Functional and metabolic consequences of sarcopenia. J Nutr 127: 998S–1003S, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci 50 Spec No: 5–8, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci 50 Spec No: 124–129, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Fidzianska A. Suicide muscle cell programme-apoptosis. Ultrastructural study. Folia Neuropathol 40: 27–32, 2002 [PubMed] [Google Scholar]
- 32. Fisher JS, Brown M. Immobilization effects on contractile properties of aging rat skeletal muscle. Aging (Milano) 10: 59–66, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Giddings CJ, Gonyea WJ. Morphological observations supporting muscle fiber hyperplasia following weight-lifting exercise in cats. Anat Rec 233: 178–195, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Hanson AM, Stodieck LS, Cannon CM, Simske SJ, Ferguson VL. Seven days of muscle re-loading and voluntary wheel running following hindlimb suspension in mice restores running performance, muscle morphology and metrics of fatigue but not muscle strength. J Muscle Res Cell Motil 31: 141–153, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Herbst A, Pak JW, McKenzie D, Bua E, Bassiouni M, Aiken JM. Accumulation of mitochondrial DNA deletion mutations in aged muscle fibers: evidence for a causal role in muscle fiber loss. J Gerontol A Biol Sci Med Sci 62: 235–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holecek M, Muthny T, Kovarik M, Sispera L. Effect of β-hydroxy-β-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol 47: 255–259, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Hsieh LC, Chien SL, Huang MS, Tseng HF, Chang CK. Anti-inflammatory and anticatabolic effects of short-term β-hydroxy-β-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr 15: 544–550, 2006 [PubMed] [Google Scholar]
- 38. Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol 109: 1628–1634, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci 64: 618–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol 73: 1854–1859, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Knitter AE, Panton L, Rathmacher JA, Petersen A, Sharp R. Effects of β-hydroxy-β-methylbutyrate on muscle damage after a prolonged run. J Appl Physiol 89: 1340–1344, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. β-Hydroxy-β-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 1793: 755–763, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Kuhls DA, Rathmacher JA, Musngi MD, Frisch DA, Nielson J, Barber A, MacIntyre AD, Coates JE, Fildes JJ. β-Hydroxy-β-methylbutyrate supplementation in critically ill trauma patients. J Trauma 62: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med 35: 9–16, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Lees SJ, Zwetsloot KA, Booth FW. Muscle precursor cells isolated from aged rats exhibit an increased tumor necrosis factor- α response. Aging Cell 8: 26–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288: R1288–R1296, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 331: 1439–1443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with β-hydroxy-β-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr 24: 442–454, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta 1800: 235–244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev 129: 542–549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. May PE, Barber A, D'Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, arginine, and glutamine. Am J Surg 183: 471–479, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Mozdziak PE, Pulvermacher PM, Schultz E. Muscle regeneration during hindlimb unloading results in a reduction in muscle size after reloading. J Appl Physiol 91: 183–190, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Murlasits Z, Cutlip RG, Geronilla KB, Rao KM, Wonderlin WF, Alway SE. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp Gerontol 41: 398–406, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Oishi Y, Ogata T, Yamamoto KI, Terada M, Ohira T, Ohira Y, Taniguchi K, Roy RR. Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol (Oxf) 192: 381–395, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Payne ET, Yasuda N, Bourgeois JM, Devries MC, Rodriguez MC, Yousuf J, Tarnopolsky MA. Nutritional therapy improves function and complements corticosteroid intervention in mdx mice. Muscle Nerve 33: 66–77, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Pistilli EE, Alway SE. Systemic elevation of interleukin-15 in vivo promotes apoptosis in skeletal muscles of young adult and aged rats. Biochem Biophys Res Commun 373: 20–24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pistilli EE, Siu PM, Alway SE. Molecular regulation of apoptosis in fast plantaris muscles of aged rats. J Gerontol A Biol Sci Med Sci 61: 245–255, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pistilli EE, Siu PM, Alway SE. Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol 292: C1298–C1304, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Quadrilatero J, Bloemberg D. Apoptosis repressor with caspase recruitment domain is dramatically reduced in cardiac, skeletal, and vascular smooth muscle during hypertension. Biochem Biophys Res Commun 391: 1437–1442, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Quadrilatero J, Rush JW. Evidence for a pro-apoptotic phenotype in skeletal muscle of hypertensive rats. Biochem Biophys Res Commun 368: 168–174, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Rowinska-Marcinska K, Szmidt-Salkowska E, Fidzianska A, Zalewska E, Dorobek M, Karwanska A, Hausmanowa-Petrusewicz I. Atypical motor unit potentials in Emery-Dreifuss muscular dystrophy (EDMD). Clin Neurophysiol 116: 2520–2527, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Rowlands DS, Thomson JS. Effects of β-hydroxy-β-methylbutyrate supplementation during resistance training on strength, body composition, and muscle damage in trained and untrained young men: a meta-analysis. J Strength Cond Res 23: 836–846, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Russell ST, Eley H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal 19: 1797–1806, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Russell ST, Tisdale MJ. Mechanism of attenuation by β-hydroxy-β-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem 330: 171–179, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Cutlip RG, Alway SE. Aging-dependent regulation of antioxidant enzymes and redox status in chronically loaded rat dorsiflexor muscles. J Gerontol A Biol Sci Med Sci 63: 1015–1026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Haff GG, Cutlip RG, Alway SE. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp Gerontol 45: 882–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ryan MJ, Jackson JR, Hao Y, Williamson CL, Dabkowski ER, Hollander JM, Alway SE. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci 65: 815–831, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Simic G, Seso-Simic D, Lucassen PJ, Islam A, Krsnik Z, Cviko A, Jelasic D, Barisic N, Winblad B, Kostovic I, Kruslin B. Ultrastructural analysis and TUNEL demonstrate motor neuron apoptosis in Werdnig-Hoffmann disease. J Neuropathol Exp Neurol 59: 398–407, 2000 [DOI] [PubMed] [Google Scholar]
- 69. Siu PM, Alway SE. Age-related apoptotic responses to stretch-induced hypertrophy in quail slow-tonic skeletal muscle. Am J Physiol Cell Physiol 289: C1105–C1113, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Siu PM, Alway SE. Id2 and p53 participate in apoptosis during unloading-induced muscle atrophy. Am J Physiol Cell Physiol 288: C1058–C1073, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Siu PM, Alway SE. Aging alters the reduction of pro-apoptotic signaling in response to loading-induced hypertrophy. Exp Gerontol 41: 175–188, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Siu PM, Alway SE. Deficiency of the Bax gene attenuates denervation-induced apoptosis. Apoptosis 11: 967–981, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol 289: R1015–R1026, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J Appl Physiol 105: 1695–1705, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 288: C338–C349, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Siu PM, Pistilli EE, Murlasits Z, Alway SE. Hindlimb unloading increases muscle content of cytosolic but not nuclear Id2 and p53 proteins in young adult and aged rats. J Appl Physiol 100: 907–916, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Slater GJ, Jenkins D. β-Hydroxy-β-methylbutyrate (HMB) supplementation and the promotion of muscle growth and strength. Sports Med 30: 105–116, 2000 [DOI] [PubMed] [Google Scholar]
- 78. Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced proteolysis in skeletal muscle by β-hydroxy-β-methylbutyrate in cancer-induced muscle loss. Cancer Res 65: 277–283, 2005 [PubMed] [Google Scholar]
- 79. Smith HJ, Wyke SM, Tisdale MJ. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by β-hydroxy-β-methylbutyrate. Cancer Res 64: 8731–8735, 2004 [DOI] [PubMed] [Google Scholar]
- 80. Smith MI, Huang YY, Deshmukh M. Skeletal muscle differentiation evokes endogenous XIAP to restrict the apoptotic pathway. PLoS ONE 4: e5097, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Snow LM, McLoon LK, Thompson LV. Adult and developmental myosin heavy chain isoforms in soleus muscle of aging Fischer Brown Norway rat. Anat Rec A Discov Mol Cell Evol Biol 286: 866–873, 2005 [DOI] [PubMed] [Google Scholar]
- 82. Soares JM, Povoas S, Neuparth MJ, Duarte JA. The effects of β-hydroxy-β-methylbutyrate (HMB) on muscle atrophy induced by immobilization (Abstract). Med Sci Sports Exerc 33: 140, 2001 [Google Scholar]
- 83. Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 107: 1172–1180, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Tews DS. Muscle-fiber apoptosis in neuromuscular diseases. Muscle Nerve 32: 443–458, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Tinetti ME. Where is the vision for fall prevention? J Am Geriatr Soc 49: 676–677, 2001 [DOI] [PubMed] [Google Scholar]
- 86. Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci 53: M112–M119, 1998 [DOI] [PubMed] [Google Scholar]
- 87. van Someren KA, Edwards AJ, Howatson G. Supplementation with β-hydroxy-β-methylbutyrate (HMB) and α-ketoisocaproic acid (KIC) reduces signs and symptoms of exercise-induced muscle damage in man. Int J Sport Nutr Exerc Metab 15: 413–424, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Vazeille E, Codran A, Claustre A, Averous J, Listrat A, Bechet D, Taillandier D, Dardevet D, Attaix D, Combaret L. The ubiquitin-proteasome and the mitochondria-associated apoptotic pathways are sequentially downregulated during recovery after immobilization-induced muscle atrophy. Am J Physiol Endocrinol Metab 295: E1181–E1190, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Vukovich MD, Stubbs NB, Bohlken RM. Body composition in 70-year-old adults responds to dietary β-hydroxy-β-methylbutyrate similarly to that of young adults. J Nutr 131: 2049–2052, 2001 [DOI] [PubMed] [Google Scholar]
- 90. Widrick JJ, Maddalozzo GF, Hu H, Herron JC, Iwaniec UT, Turner RT. Detrimental effects of reloading recovery on force, shortening velocity, and power of soleus muscles from hindlimb-unloaded rats. Am J Physiol Regul Integr Comp Physiol 295: R1585–R1592, 2008 [DOI] [PubMed] [Google Scholar]
- 91. Wilson GJ, Wilson JM, Manninen AH. Effects of β-hydroxy-β-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab (Lond) 5: 1–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wilson JM, Kim JS, Lee SR, Rathmacher JA, Dalmau B, Kingsley JD, Koch H, Manninen AH, Saadat R, Panton LB. Acute and timing effects of β-hydroxy-β-methylbutyrate (HMB) on indirect markers of skeletal muscle damage. Nutr Metab (Lond) 6: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yoshimura K, Harii K. A regenerative change during muscle adaptation to denervation in rats. J Surg Res 81: 139–146, 1999 [DOI] [PubMed] [Google Scholar]
- 94. Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp Gerontol 36: 125–140, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.