Abstract
The influence of the sympathetic nervous system (SNS) upon vascular resistance is more profound in muscles comprised predominately of low-oxidative type IIB vs. high-oxidative type I fiber types. However, within muscles containing high-oxidative type IIA and IIX fibers, the role of the SNS on vasomotor tone is not well established. The purpose of this study was to examine the influence of sympathetic neural vasoconstrictor tone in muscles composed of different fiber types. In adult male rats, blood flow to the red and white portions of the gastrocnemius (GastRed and GastWhite, respectively) and the soleus muscle was measured pre- and postdenervation. Resistance arterioles from these muscles were removed, and dose responses to α1-phenylephrine or α2-clonidine adrenoreceptor agonists were determined with and without the vascular endothelium. Denervation resulted in a 2.7-fold increase in blood flow to the soleus and GastRed and an 8.7-fold increase in flow to the GastWhite. In isolated arterioles, α2-mediated vasoconstriction was greatest in GastWhite (∼50%) and less in GastRed (∼31%) and soleus (∼17%); differences among arterioles were abolished with the removal of the endothelium. There was greater sensitivity to α1-mediated vasoconstriction in the GastWhite and GastRed vs. the soleus, which was independent of whether the endothelium was present. These data indicate that 1) control of vascular resistance by the SNS in high-oxidative, fast-twitch muscle is intermediate to that of low-oxidative, fast-twitch and high-oxidative, slow-twitch muscles; and 2) the ability of the SNS to control blood flow to low-oxidative type IIB muscle appears to be mediated through postsynaptic α1- and α2-adrenoreceptors on the vascular smooth muscle.
Keywords: adrenergic receptors, clonidine, phenylephrine, blood flow
the ability to match blood flow to oxygen uptake (V̇o2) in skeletal muscle varies according to the predominant myosin heavy chain isoform and fiber phenotype present (i.e., type I; type IIA, B, and D/X) (6, 7, 23). Differences in V̇o2 are likely due to a combination of tissue oxidative enzymatic activity and the fiber-type-dependent regulation of mitochondrial O2 consumption (8, 34). However, the regulation of vascular resistance, and thus O2 delivery, in muscles composed of different fiber types is less clear.
Within skeletal muscle there are a myriad of factors that influence vascular resistance at rest and during exercise, including the local muscle fiber metabolism and the consequent release of vasoactive metabolites, circulating vasoactive substances, local myogenic tone, and sympathetic postganglionic neural activity (21, 27, 35). The sympathetic nervous system (SNS) appears to have a fiber-type-dependent influence on vasoreactivity in skeletal muscle (23), since there is a greater vasoconstriction in muscle comprised of low-oxidative type IIB fibers during sympathetic neuron stimulation (16, 19). Similarly, Gray (17) demonstrated that resistance vessels from muscle with low-oxidative type IIB fibers were more responsive to topically applied norepinephrine than those from highly oxidative type I muscle. In vivo, pharmacological blockade of α-receptors increases blood flow in muscle composed predominantly of type IIB fibers, whereas flow remains unchanged in muscle comprised primarily of type I fibers (24).
Although these studies demonstrate a differential autonomic control of resistance vessel tone in muscles composed of low-oxidative, fast-twitch and high-oxidative, slow-twitch fibers, vasomotor control of resistance vessels (i.e., arterioles) from highly-oxidative, fast-twitch muscle is relatively unknown. Understanding vascular control mechanisms in this muscle type is important because type IIA and -IIX fibers account for ∼23% of skeletal muscle mass (10) and this fiber type receives the highest blood flow during exercise of the locomotory muscles [e.g., red portion of the gastrocnemius muscle (GastRed)] (3, 5, 33). Given the heterogeneous composition of the majority of skeletal muscle, it is difficult to discriminate vascular adrenergic regulation in muscles of specific fiber type. Therefore, the purpose of this study was to investigate the differential autonomic control of blood flow and resistance vessel tone in skeletal muscle composed primarily of different fiber types, i.e., the soleus (84% type I), the high-oxidative GastRed (48% type IIA and 13% type IIX), and the low-oxidative white portion of the gastrocnemius (GastWhite; 92% type IIB) (10). Blood flow was measured in the soleus, and GastRed and GastWhite during sequential deprivation of metabolic, sympathetic neural, and sympathetic humoral influences. Within the resistance vasculature of skeletal muscle there are α2-adrenergic receptors located on both the smooth muscle and vascular endothelium (for a review see Ref. 38), which can result in vasoconstrictor or vasodilator influences, respectively, with activation. Therefore, to further delineate the effects of the vascular endothelium on fiber-type-associated adrenergic vasomotor control, arterioles from each of these muscles were isolated and α1- and α2-adrenoreceptor function was studied in vitro.
MATERIALS AND METHODS
Six-month-old male Sprague-Dawley rats (482 ± 9 g) were used in this study. All procedures were approved by the Institutional Animal Care and Use Committees at the University of Georgia (where blood flow experiments were performed) and Texas A&M University (where isolated vessel experiments were performed). Rats were housed two per cage at 23°C, and maintained on a 12:12-h light-dark cycle. All rats were fed Purina rat chow and water ad libitum.
Blood flow measurements.
Blood flow to the entire hindlimb, as well as individual hindlimb muscles, was determined using the radionuclide-tagged microsphere technique (25). Initially, rats were anesthetized with isoflurane (2.5%/O2 balance), and Silastic catheters (ID: 0.6 mm, OD: 1.0 mm) were implanted in the right carotid and caudal (tail) arteries. The carotid artery catheter was advanced 2- to 3-mm rostral to the aortic valve and secured. The tail artery catheter was advanced toward the bifurcation of the descending aorta and secured. The tail artery catheter was connected to a 5-ml glass syringe, which was attached to a withdrawal pump (model 907; Harvard, Apparatus, Holliston, MA). The carotid artery catheter was connected to a pressure transducer (model BP100; ADInstruments) to monitor mean arterial pressure. A midline abdominal incision was then made to expose the descending aorta and bifurcation of the iliac arteries. An ultrasonic flow probe (Transonic Systems; Ithaca, NY) was placed around the descending aorta just proximal to the bifurcation of the common iliac arteries for measuring absolute volume flow rates.
Surgical procedures for denervation.
Surgical denervation was performed according to the methods described by Delp and Armstrong (9). Briefly, a 1-cm incision was made through the skin and connective tissue at the junction of the semitendinsus, biceps femoris, and gastrocnemius muscles on the posterior aspect of the right hindlimb. The sciatic nerve was isolated using glass probes, and a silk suture (4-0) was loosely looped and tied around the nerve. The incision was then stapled closed for rats used in protocol 1 (see below). All anesthetized animals were placed on a heating pad, and body temperature (measured via a rectal thermometer) was maintained at 37°C.
Radiolabeled (46Sc, 113Sn, and 85Sr) microspheres (15-μm diameter; DuPont/NEN; Boston, MA) were used for blood flow measurements as previously described (5, 11). Prior to infusion, the microspheres were agitated by sonication, and 30 s prior to the microsphere infusion, blood withdrawal from the caudal artery was initiated at 0.25 ml/min. The right carotid artery catheter was disconnected from the pressure transducer, and ∼2.5 × 105 microspheres of a specified radiolabel were infused into the ascending aorta and flushed with warmed saline to assure clearance of the beads. Blood withdrawal from the caudal artery continued for 45 s after the microsphere infusion.
Following the microsphere infusion, the rats were killed with an overdose of pentobarbital sodium (>80 mg/kg) via the right carotid artery catheter. After verifying correct placement of the carotid catheter, the left and right soleus, gastrocnemius, plantaris, tibialis posterior, flexor digitorum longus, flexor hallicus longus, tibialis anterior, and extensor digitorum longus muscles and kidneys were dissected free. The gastrocnemius and tibialis anterior muscles were then sectioned into red (GastRed, TARed), mixed, and white (GastWhite, TAWhite) portions of the muscle, which correspond to the predominately high-oxidative type IIA and IIX fibers (GastRed, TARed), and the predominately low-oxidative type IIB fibers (GastWhite, TAWhite) (24). The radioactivity level of the tissues was determined by a three-channel gamma scintillation counter (model 5230; Packard Auto Gamma Spectrometer) set to record the peak energy activity of each isotope for 5 min. Total blood flow to each tissue was calculated by the reference sample method (20, 22) and expressed in milliliters per minute per 100 g of tissue. Vascular resistance was calculated by dividing mean arterial pressure by blood flow and expressed in millimeters of mercury per milliliter per minute per 100 g of tissue. Adequate mixing of the microspheres was verified by demonstrating a < 15% difference in blood flows between the right and left kidneys.
Blood flow protocol I.
The first protocol was designed to examine blood flow to muscles composed of different fiber types after a reduction in metabolic activity from conscious standing to an anesthetized and paralyzed condition (n = 8). Animals were instrumented for blood flow measurements, and allowed 4 h to recover as Flaim et al. (15) demonstrated that cardiac or circulatory dynamics, regional blood flow, arterial blood gases, and acid-base status are stable in the awake unrestrained rat 1–6 h after gas anesthesia. Microspheres were injected during conscious standing. After microsphere infusion, animals were anesthetized (isoflurane 2.5%/O2 balance) and a tracheotomy was performed for artificial ventilation. Briefly, the trachea was isolated and an 8-cm polyethylene tube (ID: 1.4 mm, OD: 1.9 mm) was secured 1 cm into the trachea. Under isoflurane anesthesia (2.5%/O2 balance), neuromuscular blockade was induced with an intra-arterial infusion of 10 mg/kg gallamine triethiodide. In a separate group of animals (n = 7) this dose of gallamine triethiodide was found to block 97.9% of the maximal twitch tension of the soleus, plantaris, and gastrocnemius muscles without compromising mean arterial pressure (data not shown). After 50–75% of the dose of gallamine triethiodide was infused, the rats were connected to a rodent respirator (model 683; Harvard, Apparatus) and ventilated (88 breaths/min, 2.25–2.5 ml tidal volume) to achieve an arterial PaO2 ∼90 mmHg. Blood flow was then measured in the anesthetized and paralyzed condition.
Blood flow protocol II.
The second protocol was designed to examine blood flow to muscles composed of different fiber types after removal of sympathetic neural influences in the absence of α-motoneuronal activation. All experiments were performed under isoflurane anesthesia (2.5%/O2 balance) with the animals connected to the rodent respirator as described above. The group of animals (n = 8) was used to follow temporal changes in abdominal aortic blood flow after denervation of one hindlimb to identify the time of the peak blood flow response. Neuromuscular blockade was induced with the infusion of 10 mg/kg gallamine triethiodide. Aortic blood flow, heart rate, and mean arterial pressure were measured for 5 min after neuromuscular blockade. With the use of a silk loop, the sciatic nerve was quickly isolated and severed unilaterally with microscissors. Temporal changes in aortic blood flow, heart rate, and mean arterial pressure were continuously monitored for 60 min.
Once the peak blood flow response was identified (20–30 s postdenervation) in the first group, a second group of animals (n = 9) was used to measure the distribution of flow with radiolabeled microspheres to the left and right leg muscles during the predenervation, 30 s postdenervation (peak blood flow response), and 5 min postdenervation (relative steady state). The anaesthetized rats were infused with gallamine triethiodide (10 mg/kg) and 5 min later blood flow distribution, aortic flow, and mean arterial pressure were measured. Next, the sciatic nerve was severed as described above, and blood flow distribution, aortic flow, and mean arterial pressure were measured 30 s and 5 min postdenervation.
Blood flow protocol III.
This protocol was designed to examine blood flow to muscles composed of different fiber types during stimulation (group 1; n = 8) and/or inhibition (group 2; n = 8) of adrenergic receptors. Instrumentation for measuring blood flow and denervation was performed as described above. In the group from protocol II above (i.e., instrumented for aortic flow determination), after the 5-min postdenervation period various pharmacological interventions were performed, and the temporal responses were measured to align microsphere infusion with a blood pressure and abdominal aortic flow steady state with the interventions. Subsequently, in the group for this protocol, blood flow was determined under the following conditions: 1) 1 min after infusion (via carotid artery catheter) of the α1-adrenoreceptor agonist phenylephrine (0.3 mg/kg) (group 1), 2) 3 min after prazosin α1-antagonist) infusion (3.0 mg/kg) (group 2), and 3) 1 min after phenylephrine infusion (0.3 mg/kg) in the presence of prazosin inhibition (group 2). At least 10 min of baseline aortic flow, heart rate, and mean arterial pressure were required before any compound(s) was administered.
Isolated microvessel preparation.
In a separate set of animals (n = 24) adrenergic vasoconstrictor responses of isolated resistance arterioles from the GastRed, GastWhite, and soleus muscles were investigated.
Animals were anesthetized with pentobarbital sodium (85 mg/kg ip) and killed by exsanguination. The gastrocnemius-plantaris-soleus muscle complex was carefully excised from each leg and placed in cold (4°C) physiological saline solution (PSS) containing (in mM): 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 3.0 MOPS buffer and 1 g/100 ml BSA at pH 7.4. Soleus and gastrocnemius muscle first-order (1A) arterioles were then isolated with the aid of a dissecting microscope (Olympus SVH10) as previously described (29, 32). In soleus muscles, 1A arterioles were defined as the first branch off the feed artery perforating the muscle. In gastrocnemius muscles, 1A arterioles were defined as the first branch off the feed artery that runs over the superficial (GastWhite) or into the deep (GastRed) portions of the muscle. The arterioles (length, 0.5–1.0 mm) were cleared of surrounding muscle fibers, removed from the muscle, and placed in Lucite chambers containing MOPS-buffered PSS equilibrated to room air. The arterioles were cannulated on both ends to glass micropipettes and secured with ophthalmic nylon suture (Alcon 11-0). After cannulation, the chambers were transferred to the stage of an inverted microscope (model IX70; Olympus) equipped with a video camera (model BP310; Panasonic), video caliper (Microcirculation Research Institute, Texas A&M), and data acquisition system (MacLab) for recording of luminal diameter. Intraluminal pressure was set at 75 cm H2O to coincide with pressures used in previous in vitro studies of skeletal muscle arterioles (1, 12). Leaks were detected by pressurizing the vessel and determining whether vessel diameter was maintained. Arterioles that exhibited leaks were discarded. Arterioles free of leaks were warmed to 37°C and allowed to develop spontaneous tone during a 30–60 min equilibration period, upon which arterioles were discarded unless at least 20% baseline tone was achieved prior to the addition of vasoactive agents. Sensitivity of the arterioles to agonists was assessed by calculating the dose eliciting 50% of the maximal vasoconstriction (EC50).
To determine whether there are fiber-type differences in adrenergic vasoconstrictor function of the skeletal muscle arterioles, responses of 1A arterioles were determined to the cumulative addition of either the α2-adrenoreceptor agonist clonidine (10−9 to 10−5 M) or the α1-adrenoreceptor agonist phenylephrine (10−9 to 10−4 M). A second paired series of studies was performed to determine whether differences in α1- or α2-vasoconstriction were mediated through the vascular endothelium. For these studies, the endothelium was removed from arterioles by passing 3–5 ml of air through the lumen of the vessel as described previously (12). To ensure complete denudation of the endothelium, arterioles were exposed to the endothelium-dependent vasodilator acetylcholine (3 × 10−5 M), and any vessel that exhibited vasodilatation > 5% was excluded. Following exposure to acetylcholine, the vessels were washed several times with PSS and allowed to establish spontaneous tone. Dose-response relations to either the cumulative addition of clonidine (10−9 to 10−5 M) or phenylephrine (10−9 to 10−4 M) were performed in the absence of the endothelium.
Blood gas analysis.
Arterial pH and the PaO2 were measured with a pH/blood gas analyzer (model 170; Corning). Body temperature and hemoglobin concentration measurements were used to correct pH and PaO2 values.
Data analysis.
Responses were recorded as actual diameters and expressed as a percentage of possible vasoconstriction according to the following formula: vasoconstriction (%maximal response) = (Db − Ds)/Db × 100, where Ds is the steady-state inner diameter recorded after addition of an agonist, Db is the initial baseline inner diameter before the first addition of a pharmacological agonist, and Dm is the maximal intraluminal diameter obtained in Ca2+-free PSS. Comparison of data as a percentage of the maximal vasoconstriction normalizes for potential differences in maximal diameter or spontaneous tone among vessels.
Spontaneous tone is expressed at a percentage of maximal intraluminal diameter according to the formula: spontaneous tone (%) = (Dm − Db)/Dm × 100.
Statistical analysis.
Repeated-measures ANOVA was used to determine differences among blood flow for predenervation and 30 s and 5 min postdenervation. ANOVA was also used to determine differences for dose-response diameters in isolated vessels, to detect differences within (dose), and between (muscle fiber type) factors. Post hoc analysis was performed by Duncan's multiple range test to determine the significance of differences among means. A one-way ANOVA was used to determine the significance of differences among vessel characteristics and blood flow from conscious standing (protocol I, group 1) to anesthetized condition (protocol I, group 2). All data are presented as means ± SE. Significance was set at P ≤ 0.05.
RESULTS
Hemodynamics and neuromuscular blockade.
With administration of 10 mg/kg gallamine triethiodide there was no change in heart rate, mean arterial pressure, or abdominal aortic blood flow. Arterial pH was 7.46 ± 0.01 and arterial Po2 was 88.5 ± 3.9 mmHg, and both variables remained unaltered throughout the entire pre- and postdenervation period (data not shown).
Protocol I: blood flow from conscious standing to the anesthetized condition.
Blood flow decreased and resistance increased in all muscles studied from conscious standing to an anesthetized and paralyzed condition (Table 1). The most dramatic decreases in blood flow occurred in the most oxidative muscles. Specifically, there was a 94% and 86% reduction in blood flow to the soleus and GastRed, respectively. In GastWhite, which is composed predominately of type IIB fibers (10), blood flow decreased by ∼53%.
Table 1.
Hemodynamic data during conscious standing (CS) and anesthesia plus neuromuscular blockade (AN)
| CS, n = 8 | AN, n = 7 | |
|---|---|---|
| Heart rate, beats/min | 373 ± 8 | 320 ± 11* |
| MAP, mmHg | 114 ± 2 | 104 ± 5 |
| Blood flow, ml·min−1·100 g−1 | ||
| Soleus | 148 ± 12 | 8 ± 2* |
| Red gastrocnemius | 69 ± 15 | 10 ± 2* |
| Mixed gastrocnemius | 24 ± 3 | 9 ± 2* |
| White gastrocnemius | 15 ± 2 | 7 ± 2* |
| Plantaris | 26 ± 7 | 11 ± 2* |
| Tibialis posterior | 36 ± 5 | 9 ± 3* |
| Flexor digitorum longus | 29 ± 3 | 8 ± 2* |
| Flexor hallicus longus | 25 ± 4 | 9 ± 3* |
| Red tibialis anterior | 66 ± 6 | 10 ± 2* |
| White tibialis anterior | 21 ± 4 | 9 ± 2* |
| Extensor digitorum longus | 32 ± 4 | 10 ± 1* |
Values are means ± SE.
P < 0.05 vs. mean during CS.
Protocol II: blood flow and denervation.
After denervation, aortic flow increased from 17 ± 2 ml/min (predenervation) to a peak of 26 ± 3 ml/min (P < 0.05) at 30 s. At 1 min postdenervation, aortic flow remained ∼15% above baseline at 24 ± 3 ml/min (P < 0.05; Fig. 1A) and remained unchanged through 60 min postdenervation. Mean arterial pressure and heart rate did not change at any time point from predenervation to 60-min postdenervation (range 104 ± 7 to 108 ± 4 mmHg).
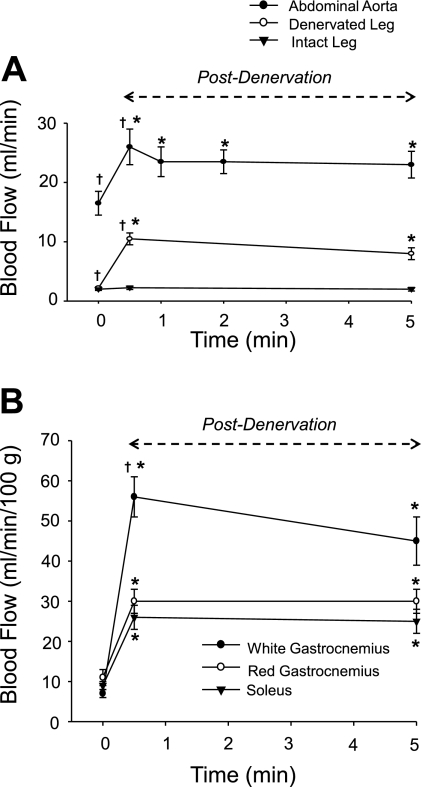
Fig. 1.
A: mean absolute (ml/min) abdominal aortic, denervated, and intact leg muscle blood flow during predenervation and 30 s and 5 min postdenervation. B: mean relative (ml·min−1·100 g−1) blood flow in the soleus and red (GastRed) and white (GastWhite) portions of the gastrocnemius muscle after denervation. Blood flow to the contralateral (i.e., intact) muscles did not change over the 5-min period. *P < 0.05 vs. predenervation blood flow (time 0). †P < 0.05 vs. blood flow at 5 min postdenervation.
The increase in abdominal aortic flow was equivalent to the summed increase in flow measured with microspheres to all denervated muscles (Fig. 1A). Blood flow increased in all individual denervated leg muscles from predenervation to 30 s postdenervation (Table 2). Of the GastWhite, GastRed, and soleus muscles, the greatest increases in blood flow occurred in GastWhite (8.5-fold increase), with an 2.7-fold increase in the GastRed and soleus muscle (Fig. 1B).
Table 2.
Hemodynamic data during anesthesia and neuromuscular blockade (AN) and at 30 s and 5 min postdenervation
| AN | 30 s | 5 min | |
|---|---|---|---|
| Heart rate, beats/min | 341 ± 8 | 351 ± 13 | 349 ± 12 |
| MAP, mmHg | 108 ± 7 | 110 ± 6 | 111 ± 5 |
| Abdominal aorta blood flow, ml/min | 17 ± 2 | 26 ± 3* | 23 ± 3*† |
| Blood flow, ml·min−1·100 g−1 | |||
| Soleus | 9 ± 1 | 26 ± 3* | 25 ± 3* |
| Red gastrocnemius | 11 ± 2 | 30 ± 3* | 30 ± 3* |
| Mixed gastrocnemius | 11 ± 2 | 46 ± 4* | 39 ± 5* |
| White gastrocnemius | 7 ± 1 | 56 ± 5* | 45 ± 6*† |
| Plantaris | 11 ± 3 | 47 ± 8* | 38 ± 7* |
| Tibialis posterior | 8 ± 1 | 92 ± 17* | 74 ± 23* |
| Flexor digitorum longus | 8 ± 2 | 75 ± 14* | 66 ± 15* |
| Flexor hallicus longus | 8 ± 2 | 86 ± 19* | 63 ± 18* |
| Red tibialis anterior | 9 ± 1 | 40 ± 9* | 33 ± 4* |
| White tibialis anterior | 10 ± 2 | 81 ± 15* | 45 ± 9*† |
| Extensor digitorum longus | 8 ± 1 | 98 ± 21* | 61 ± 11*† |
Values are means ± SE.
P < 0.05 vs. anesthesia and neuromuscular blockade (AN);
P < 0.05 vs. 30 s postdenervation value.
At 5 min postdenervation, abdominal aortic blood flow decreased by 3 ml/min vs. the 30 s postdenervation, but remained elevated compared with predenervation (Table 2; Fig. 1A). The total summed flow to the denervated leg muscles had a similar decrease in flow at 5 min postdenervation, whereas in the contralateral muscles (i.e., normal leg), flow remained unchanged (Fig. 1A).
Protocol III: muscle vascular resistance during adrenergic receptor stimulation/inhibition.
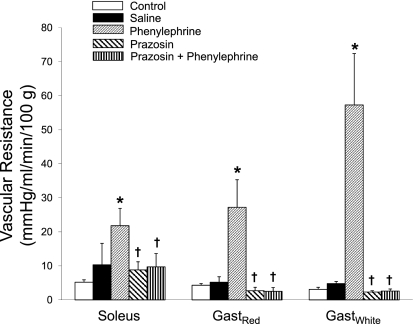
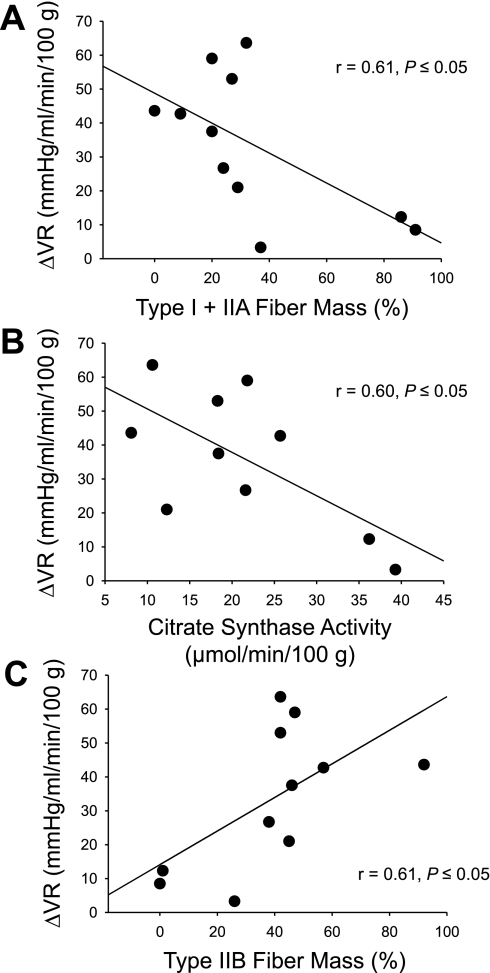
In all denervated muscles, there was an increase in vascular resistance with stimulation of α1-adrenoreceptors via systemic phenylephrine infusion. Relative to baseline control blood flow (measured at 5 min postdenervation in protocol II) the greatest increase in resistance occurred in GastWhite (∼19-fold), with a more moderate increase in GastRed (∼6-fold) and soleus (∼4.5-fold) muscles (Fig. 2). Changes in resistance to normally innervated muscles with phenylephrine paralleled the fold differences found in the denervated muscles (data not shown). When comparing the relationships between vascular resistance changes with phenylephrine infusion and the fiber type composition or oxidative capacity of the individual hindlimb muscles listed in Table 2, there was 1) an indirect relationship with the percentage of type I and IIA fiber mass (Fig. 3A) and oxidative capacity (Fig. 3B), and 2) a direct relationship with the percentage of type IIB fiber mass (Fig. 3C) of the individual muscles.
Fig. 2.
Mean vascular resistance in denervated muscles during control (i.e., 5 min postdenervation) and with administration of saline, phenylephrine, prazosin, or prazosin + phenylephrine. *P < 0.05 vs. control and saline; †P < 0.05 vs. phenylephrine.
Fig. 3.
Relationships between the % sum of type I and IIA fibers (A), citrate synthase activity (B), and % sum of type IIB fibers (C) of the individual muscles listed in Table 1 of the rat hindlimb and the change in vascular resistance (ΔVR) after infusion of phenylephrine. Based on fiber-type composition and citrate synthase activity [reported by Delp and Duan (10)].
There was no significant change in vascular resistance in any muscle with the administration of the α1-adrenoreceptor antagonist prazosin (Fig. 2); prazosin treatment was sufficient to block the vasoconstrictor effects of phenylephrine in all muscle analyzed (Fig. 2). Mean arterial pressure increased from control (103 ± 8 mmHg) to phenylephrine treatment (196 ± 4 mmHg; P < 0.05); prazosin treatment resulted in a decreased pressure (63 ± 5 mmHg; P < 0.05).
In vitro studies.
The maximal intraluminal diameter of arterioles from the soleus muscle (131 ± 9 μm) was smaller than that of the GastRed (157 ± 7 μm) and GastWhite (158 ± 11 μm) (P < 0.05). There was no difference in spontaneous tone between muscles with (soleus, 33 ± 2%; GastRed, 32 ± 3%; GastWhite, 30 ± 3%) or without (soleus, 34 ± 3%; GastRed, 36 ± 3%; GastWhite, 32 ± 3%) the endothelium.
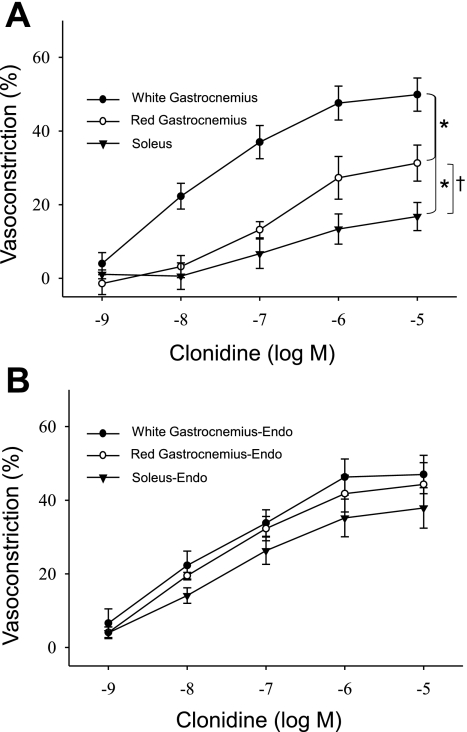
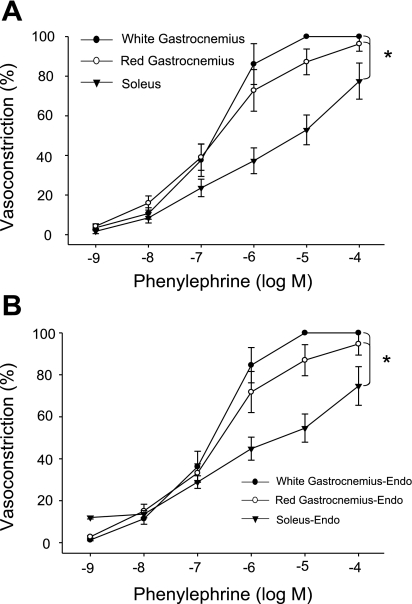
In response to the α2-adrenoreceptor agonist clonidine, there were fiber-type-associated differences in vasoconstriction; the GastWhite demonstrated the greatest maximal vasoconstriction of the three muscle types and a greater sensitivity (EC50; Table 3) to clonidine vs. the soleus muscle arteriole (Fig. 4A). In addition, there was greater maximal vasoconstriction of the GastRed arteriole compared with that of the soleus (Fig. 4A); there were no differences in sensitivity to clonidine between these two muscle types. All differences in the percent vasoconstriction and sensitivity to clonidine among muscle types were abolished with the removal of the endothelium (Fig. 4B). Endothelium removal did not change the maximal response or sensitivity to clonidine in GastWhite arterioles, whereas there was an enhanced vasoconstriction and sensitivity in GastRed and soleus arterioles with denudation.
Table 3.
Arteriolar sensitivity (EC50) to clonidine and phenylephrine before and after removal of the endothelium
| Before Removal | After Removal | |
|---|---|---|
| Clonidine | ||
| Soleus | 5.5E−7 ± 2.3E−7 | 3.1E−8 ± 7.1E−9† |
| Red gastrocnemius | 1.1E−7 ± 3.2E−7 | 3.5E−8 ± 1.9E−8† |
| White gastrocnemius | 4.5E−8 ± 3.1E−8* | 2.8E−8 ± 1.1E−8 |
| Phenylephrine | ||
| Soleus | 4.2E−6 ± 2.3E−6 | 2.7E−6 ± 2.1E−6 |
| Red gastrocnemius | 8.9E−7 ± 4.8E−7* | 7.5E−7 ± 3.9E−7* |
| White gastrocnemius | 4.4E−7 ± 3.2E−7* | 4.1E−7 ± 2.2E−7* |
All values are in molar concentration as means ± SE; E, represents the logarithmic values of the sensitivity measurements.
P < 0.05 vs. soleus;
P < 0.05 vs. endothelium intact for same muscle type.
Fig. 4.
Dose response relations to cumulative additions of the α2-receptor agonist clonidine in arterioles from the soleus, gastrocnemius (Gast)Red, and GastWhite muscle. A: % vasoconstriction to cumulative doses of clonidine with the endothelium intact. B: % vasoconstriction to cumulative doses of clonidine with the endothelium removed. *P < 0.05 vs. GastWhite; †P < 0.05 vs. soleus.
Maximal vasoconstrictor responses and sensitivity (EC50; Table 3) to the to the α1-adrenoreceptor agonist phenylephrine were greater in arterioles from GastWhite and GastRed than that of the soleus muscle (Fig. 5A). Removal of the endothelium did not alter these differences (Fig. 5B).
Fig. 5.
Dose response relations to cumulative additions of the α1-receptor agonist phenylephrine in arterioles from the soleus, GastRed and GastWhite muscle. A: % vasoconstriction to cumulative doses of phenylephrine with the endothelium intact. B: % vasoconstriction to cumulative doses of phenylephrine with the endothelium removed. *P < 0.05 vs. vessel responses from GastRed and GastWhite.
DISCUSSION
The purpose of this study was to investigate the potential for differential vasomotor control of resistance vessels in skeletal muscle composed of different fiber types. The main findings are 1) there exists a large metabolic control of blood flow in muscles composed of type I, IIA, and IIX vs. IIB fibers; 2) removal of sympathetic nerve influence via denervation induces a greater increase in blood flow to muscle of type IIB fiber composition than either type I or IIA and IIX; 3) in vivo, with selective α1-adrenergic receptor stimulation there is a direct relationship between changes in vascular resistance and the percentage of type IIB fiber composition of the individual muscles measured herein, 4) endothelial modulation of vascular tone through α2-adrenergic receptor stimulation is greatest in muscle composed of type I fibers and least in muscle composed of type IIB fibers; and 5) soleus muscle arterioles have a lower α1-adrenergic receptor-mediated vasoconstriction vs. arterioles from the GastRed and GastWhite. Therefore, vasoconstriction of arterioles from IIA and IIX muscle to α1-adrenoreceptor stimulation is most similar to that of arterioles from muscle composed of type IIB fibers. Conversely, arterioles from muscle composed of high-oxidative type IIA and IIX fibers demonstrate vasoconstrictor responses to α2-adrenoreceptor stimulation that are more similar to that of arterioles from muscle composed of high-oxidative type I fibers than muscle containing low-oxidative, glycolytic-type IIB fibers. These data indicate that the vasoconstrictor responsiveness of the resistance vasculature in type IIA and IIX muscle is intermediate to that of the low-oxidative and fast-glycolytic muscles. Similarly, the magnitude of control exerted through the sympathetic nerves and adrenergic stimulation over the range of blood flows occurring in muscle is relatively small in muscles composed of the high-oxidative type I, IIA, and IIX fibers, whereas the magnitude of adrenergic control over the range of flows occurring in the low-oxidative type IIB muscle is quite large (Fig. 6).
Fig. 6.
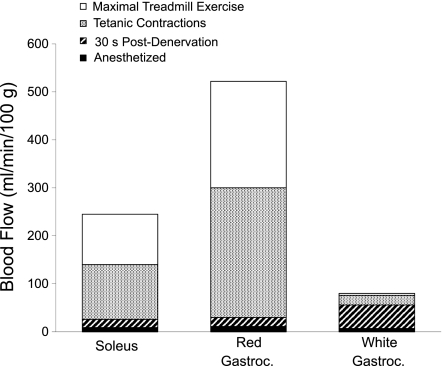
Comparisons of the blood flow response measured in the present study after denervation to that measured during maximal exercise [data from Armstrong and Laughlin (4); Musch et al., (33); or with tetanic contractions of Mackie and Terjung (28)]. With removal of sympathetic neural tone, blood flow in the GastWhite increased to near-maximal reported values.
Changes in blood flow from conscious standing to anesthetized.
There is evidence to suggest that local metabolite release is different among muscle composed of different fiber types (18, 28). For example, muscles composed of a high percentage of type I fibers generate greater muscle hyperemia to the vasodilator substance adenosine than muscles with predominately type IIB fibers (26). This greater hyperemia to adenosine could reflect greater production of adenosine by the type I fibers, greater sensitivity of the resistance vasculature to adenosine in type I muscle, or a combination of the two. In vivo differences in the quantity and types of vasoactive metabolites among muscles could result in variations in the level of vascular tone and the mechanism(s) through which vascular tone is achieved in muscle. In the present study, a reduction in muscular activity from the conscious standing to the anesthetized condition resulted in a 2,100%, 310%, and 209% increase in vascular resistance in the soleus, GastRed, and GastWhite muscle, respectively (Table 1). It is probable that the difference in the change in resistance among the muscle types simply reflects the relative changes in motor unit activity from the conscious to anesthetized state. The soleus muscle, for example, which is near maximally active during postural maintenance (25, 36, 39), would presumably have had the greatest decrease in muscle fiber activity from the standing to the anesthetized paralyzed condition. In contrast, the motor units in the GastWhite are relatively inactive during conscious standing (12, 18, 28), which is supported by the relative smaller change in blood flow from conscious standing to the anesthetized condition.
Removal of sympathetic neural influence.
The differential influence of the SNS in controlling blood flow to type I and IIB muscle has long been noted (16, 25, 35). Peripheral nerve section was used in the present study to estimate neurally mediated sympathetic tone in the absence of evoked muscular activity. Denervation resulted in a rapid increase in blood flow through the abdominal aorta with a peak response at ∼20–30 s postdenervation, followed by a small decrease at 1 min (Fig. 1) that remained stable for 60 min postdenervation. The change in the total sum of blood flow to all denervated leg muscle measured with microspheres from predenervation to 30 s and 5 min postdenervation could quantitatively account for the change in abdominal aortic flow measured with an ultrasonic flow probe (Fig. 1A). The GastWhite, which has the largest proportion of type IIB fibers of the muscles studied, demonstrated the greatest hyperemic response 30 s postdenervation. When comparing denervated to maximal exercising blood flow, the hyperemic response in the denervated GastWhite increased to ∼75% of the highest blood flow reported to this muscle during maximal exercise (4, 33) or with tetanic contractions (28). In the GastRed and soleus muscle, the blood flow with denervation reached only 4 and 7%, respectively, of the reported maximal exercising blood flows for these muscles (Fig. 6). This suggests that 1) sympathetic vasoconstrictor tone is greater in muscles composed of type IIB than in type I, IIA, and IIX fibers; and 2) the SNS has the potential to modulate virtually the entire adaptive range of blood flow measured in conscious exercising animals in muscles comprised of mainly type IIB fibers. Indeed, Thomas et al. (37) have demonstrated that sympathetic vasoconstrictor tone is abolished in maximally contracting muscles composed of highly glycolytic type IIB fibers. These same authors have found that sympathetically mediated vasoconstrictor tone is maintained in the more oxidative type I fibers due, in part, to the production of nitric oxide (6, 33).
α-Adrenergic receptor function in isolated vessels.
The observations that resistance vessels from the GastWhite (type IIB muscle) demonstrate greater maximal vasoconstriction and sensitivity with α2-receptor agonism (vs. soleus and GastRed; Fig. 4) may be explained by a greater postsynaptic α2-adrenergic receptor density (13, 14, 25) or location. In intact muscle, there are no apparent differences in adrenergic innervation density to resistance vessels in type IIB and type I muscle (2). However, within the resistance vasculature of skeletal muscle there are α2-adrenergic receptors located on both the smooth muscle and vascular endothelium (for a review see Ref. 38), which result in vasoconstrictor or vasodilator influences, respectively, with activation. Therefore, the vascular tone of a given resistance vessel in response to a selective α2-adrenergic receptor agonist is determined by the net contribution of these opposing vasoconstrictor and vasodilator influences. Recognizing this, we chose not to infuse a selective α2-adrenoreceptor agonist into the intact animal as we could not discriminate the relative contributions from endothelial vs. smooth muscle α2-adrenergic receptor stimulation on the net blood flow response. However, we were able to investigate the modulatory role of the vascular endothelium with selective the α2-adrenergic receptor stimulation in vitro. In arterioles from muscle composed of type I and type IIA and IIX fiber types, the α2-adrenergic receptors on the endothelium appear to have a greater vasodilator influence to oppose the vasoconstriction (Fig. 4A), since removal of the vascular endothelium resulted in no differences in maximal vasoconstriction among the three fiber type muscles (Fig. 4B). The fact that removing the endothelium did not alter the sensitivity or maximal vasoconstriction to clonidine in GastWhite (Fig. 4) supports the notion that the endothelial α2-receptors play a minimal role in setting vascular tone in this muscle type. It should be noted that Aaker and Laughlin (1) found no difference in vasoconstriction to norepinephrine in arterioles from the GastRed and GastWhite. It is possible, however, that since norepinephrine stimulates both α1- and α2-adrenoreceptors, the contribution of α2-adrenoreceptors located on the endothelium was masked by a dominant vasoconstriction elicited by α1- and α2-adrenoreceptors located on the smooth muscle.
With respect to α1-adrenoreceptor function in the intact muscle, the infusion of phenylephrine increased vascular resistance to the greatest extent in the GastWhite (Fig. 2). However, there were no differences in the sensitivity or maximal vasoconstriction of isolated arterioles between the GastWhite and GastRed, and arterioles from both these muscle types demonstrated greater responsiveness to phenylephrine than soleus muscle arterioles (Fig. 5). In the present study, we investigated vasomotor control in situ from a major resistance artery (i.e., 1A arterioles); however, the change in vascular resistance with phenylephrine infusion in vivo reflects the net effect of all the resistance vasculature. Therefore, given the regional heterogeneity of α-adrenoreceptor subtypes in skeletal muscle arterial networks (31), it is possible that the downstream resistance vessels in the GastWhite may be more sensitive to phenylephrine compared with those of the GastRed.
Conclusion.
The present investigation demonstrates that sympathetic vasoconstrictor tone is greater in muscles composed of type IIB than type I, IIA, and IIX fibers. Furthermore, the GastRed appears to be more similar to the soleus with α2-adrenoreceptor stimulation and to the GastWhite with α1-adrenergic stimulation. With respect to the modulating effect of the SNS on blood flow, the GastRed and soleus are very similar in that denervation resulted in blood flow increasing to < 7% of maximal reported values. The ability of the SNS to maintain tighter control of blood flow in muscle with predominately type IIB fibers appears to be primarily mediated though α1- and α2-adrenergic receptors located on the vascular smooth muscle with little effect of endothelial α2-adrenoreceptors. In addition, modulation of sympathetic nerve activity to the muscle composed of type IIB fibers appears to have the potential to control virtually the entire adaptive range of blood flow to this muscle type.
GRANTS
This work was supported by the National Institutes of Health Grants AG-31317 and ROI-HL-077224, National Aeronautics and Space Administration Grants NNX-09AP06G and NNX-08AQ62G, and Florida Biomedical Research Program 1BN-02.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to α1- adrenergic constriction than gastrocnemius arterioles. J Appl Physiol 92: 1808–1816, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Allum WH, Cotter M, Hudlicka O. Functional characteristics of the vascular bed in long-term stimulated fast muscles. J Physiol 242: 137P–138P, 1974 [PubMed] [Google Scholar]
- 3. Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol 62: 1285–1298, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 189–208, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol 59: 1322–1328, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Behnke BJ, Delp MD, McDonough P, Spier SA, Poole DC, Musch TI. Effects of chronic heart failure on microvascular oxygen exchange dynamics in muscles of contrasting fiber type. Cardiovasc Res 61: 325–332, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol 549: 597–605, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crow MT, Kushmerick MJ. Correlated reduction of velocity of shortening and the rate of energy utilization in mouse fast-twitch muscle during a continuous tetanus. J Gen Physiol 82: 703–720, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delp MD, Armstrong RB. Blood flow in normal and denervated muscle during exercise in conscious rats. Am J Physiol Heart Circ Physiol 255: H1509–H1515, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Delp MD, Manning RO, Bruckner JV, Armstrong RB. Distribution of cardiac output during diurnal changes of activity in rats. Am J Physiol Heart Circ Physiol 261: H1487–H1493, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res 66: 393–401, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Drew GM, Whiting SB. Evidence for two distinct types of postsynaptic α-adrenoreceptors in vascular smooth muscle in vivo. Br J Pharmacol 67: 207–215, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faber JE. In situ analysis of α-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res 62: 37–50, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods 11: 1–39, 1984 [DOI] [PubMed] [Google Scholar]
- 16. Folkow B, Halicka HD. A comparison between “red” and “white” muscle with respect to blood supply, capillary surface area and oxygen uptake during rest and exercise. Microvasc Res 1: 1–14, 1968 [Google Scholar]
- 17. Gray SD. Responsiveness of the terminal vascular bed in fast and slow skeletal muscle to α-adrenergic stimulation. Angiologica 8: 285–296, 1971 [DOI] [PubMed] [Google Scholar]
- 18. Hilton SM, Hudlicka O, Marshall JM. Possible mediators of functional hyperemia in skeletal muscle. J Physiol 282: 131–147, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hilton SM, Jeffries MG, Vrbova G. Functional specializations of the vascular bed of soleus. J Physiol 206: 543–562, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol Heart Circ Physiol 239: H443–H449, 1980 [DOI] [PubMed] [Google Scholar]
- 21. Johnson PC. The myogenic response. In: Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am. Physiol. Soc., 1980, sect. 2, vol. II, chapt. 15, p. 409–442 [Google Scholar]
- 22. Kindig CA, Poole DC. A comparison of the microcirculation in the rat spinotrapezius and diaphragm muscles. Microvasc Res 55: 249–259, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Lambert DG, Thomas GD. α-Adrenoceptor constrictor responses and their modulation in slow-twitch and fast-twitch mouse skeletal muscle. J Physiol 563: 821–829, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laughlin MH, Armstrong RB. Adrenoreceptor effects on rat muscle blood flow during treadmill exercise. J Appl Physiol 62: 1465–1472, 1987 [DOI] [PubMed] [Google Scholar]
- 25. Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol 257: H1507–H1515, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 16, p. 705–769 [Google Scholar]
- 28. Mackie BG, Terjung RL. Blood flow to different skeletal muscle fiber types during contraction. Am J Physiol Heart Circ Physiol 245: H265–H275, 1983 [DOI] [PubMed] [Google Scholar]
- 29. McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol 89: 398–405, 2000 [DOI] [PubMed] [Google Scholar]
- 30. McDonough P, Behnke BJ, Musch TI, Poole DC. Recovery of microvascular Po2 during the exercise off-transient in muscles of different fiber type. J Appl Physiol 96: 1039–1044, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Moore AW, Jackson WF, Segal SS. Regional heterogeneity of α-adrenoreceptor subtypes in arteriolar networks of mouse skeletal muscle. J Physiol 588: 4261–4274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Musch TI, McAllister RM, Symons JD, Stebbins CL, Hirai T, Hageman KS, Poole DC. Effects of nitric oxide synthase inhibition on vascular conductance during high speed treadmill exercise in rats. Exp Physiol 86.6: 749–757, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184: 81–100, 1998 [PubMed] [Google Scholar]
- 35. Shepherd JL, Vanhoutte PM. Local modulation of adrenergic neurotransmission. Circulation 64: 655–666, 1981 [DOI] [PubMed] [Google Scholar]
- 36. Smith JL, Edgerton VR, Betts B, Collatos TC. EMG of slow and fast ankle extensors of cat during posture, locomotion, and jumping. J Neurophysiol 40: 503–513, 1977 [DOI] [PubMed] [Google Scholar]
- 37. Thomas GD, Hansen J, Victor RG. Inhibition of α2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol 266: H920–H929, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Vanhoutte PM. Endothelial adrenoceptors. J Cardiovasc Pharmacol 38: 796–808, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscle during locomotion in freely moving cats. J Neurophysiol 41: 1103–1216, 1978 [DOI] [PubMed] [Google Scholar]