Abstract
The neurokinin 3 receptor (NK3R) is a G protein-coupled receptor that is expressed in brain and is highly expressed by magnocellular vasopressinergic neurons in both the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus. Hyperosmolarity causes a ligand-mediated internalization of NK3Rs to the cytoplasm and to the nuclei of vasopressinergic PVN neurons. This receptor activation-dependent pathway is presumed to be a means to directly transmit synaptic signals from the cell membrane to the nucleus. The present study evaluated in vivo the subnuclear domains that associate with NK3R. Rats were administered 2 M NaCl (intragastric) or no intragastric load, and 40 min later, the PVN was dissected and nuclei were isolated. Using double-immuno-transmission electron microscopy (TEM), we show that, compared with controls, hyperosmolarity causes a significant increase in NK3R Immunogold beads in the nucleus of PVN neurons. Furthermore, NK3R spatially colocalized with histone H4 and with highly acetylated H4 in nuclei isolated from the PVN of rats administered 2 M NaCl, but not in nuclei from control rats. Next, coimmunoprecipitation experiments showed that acetylated H4, as well as acetylated H3, were pulled down with NK3R in the PVN nuclear enriched fraction from rats treated with 2 M NaCl, but not from control rats. In response to hyperosmolarity, NK3R is transported to the nucleus of PVN neurons and associates with transcriptionally active chromatin, where it may influence the transcription of genes.
Keywords: epigenetics, vasopressin release, nuclear trafficking
vasopressin (vp) is synthesized by magnocellular neurons in the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus, and is released from the posterior pituitary. Extracellular hyperosmolarity and hypovolemia are two physiological cues that elicit the release of VP (2). The release of VP in response to these physiological challenges involves multiple receptors that are expressed on VP neurons. The majority of VP magnocellular neurons expresses the tachykinin neurokinin 3 receptor (NK3R) (13, 14, 46). The NK3R is a typical membrane-bound, G protein-coupled receptor (GPCR) and is coupled to the pertussin-toxin insensitive Gq/G11 and Gs (35). Following ligand binding, GPCRs, including NK3R, are desensitized and internalized to the cytoplasm (8, 11, 31). Internalization of NK3R to the cytoplasm of magnocellular neurons in the SON and PVN occurs in response to application of a NK3R agonist, hypotension, and hyperosmolarity (21, 26).
Typically, GPCRs are either recycled to the membrane or degraded following internalization to the cytoplasm (17, 45, 61). This is not the case for NK3R and several other GPCRs. Following internalization, confocal microscopy showed that NK3Rs are trafficked to the nuclei of SON and PVN neurons shortly after the onset of hypotension and hyperosmolarity, respectively (21, 26), a result confirmed by Western blot analysis of NK3R protein in isolated nuclei from PVN neurons (28). Subsequently, the subnuclear localization of NK3R was identified using immuno-transmission electron microscopy (TEM). TEM established that following the hyperosmotic challenge, NK3R was within the nucleus and mainly dispersed in the less electron-dense regions (28). In addition, NK3R Immunogold was also detected in the nucleolus and along the inner nuclear membrane of isolated nuclei (28).
Dominant within the nucleoplasm is dispersed chromatin, a DNA-protein complex that comprises core histone proteins (H2A, H2B, H3, and H4) wrapped by 146 bp of DNA (37, 47). The posttranslational modification of histones by acetylation, phosphorylation, and methylation, affects the chromatin structure and the accessibility of DNA (1, 18, 24). Acetylation of the histone tail relaxes chromatin structure and is generally linked to transcriptional activation (1, 24). Histone H4 can be acetylated at four lysines, and acetylation of histones is catalyzed by histone acetyltransferases (HAT) (60). Acetylation of H4 can occur at a single, double, triple and tetra lysines, and acetylation of multiple lysines proceeds in a specific order (62). Acetylated histones, particularly H4 and H3, are concentrated at active gene loci (24, 60), and mutation of H4 and H3 severely reduces gene activation (16, 63); combining the mutations produces a greater deficit than either mutation alone (63).
Immuno-TEM established that NK3R is translocated to the nucleoplasm of PVN neurons following acute hyperosmotic stimulation (28). Identifying the subnuclear domains that associate with NK3R is essential in determining the functional impact of the activity-dependent translocation of NK3R into the nucleus. In the present study, we show using double immuno-TEM and coimmunoprecipitation that NK3R colocalizes and associates with acetylated histone H4 and H3 in PVN neurons following an acute hyperosmotic challenge. This association was not present in nuclei isolated from control rats. Thus, once activated in response to a physiological stimulus, NK3Rs are translocated into the nucleus and associate with histones to potentially affect chromatin structure and gene expression.
METHODS
Animals and Treatment
All animal experiments were carried out in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the University of Wyoming Institutional Animal Care and Use Committee. Male Charles River rats (Charles River Laboratories, Wilmington, MA) (∼300 g) were housed individually in hanging stainless-steel cages and maintained in a controlled environment (25°C, 12:12-h light-dark) with food and water available ad libitum.
Rats were handled and adapted prior to the experiments to reduce stress. Rats were hand-held and an infant feeding tube was gently inserted into the stomach. Rats were given either an intragastric load of 2 M NaCl (6 ml; n = 2) or a sham load (n = 2). Rats were then returned to their home cages for 40 min and then deeply anesthetized with pentobarbital sodium (70 mg/kg body wt, ip). Previous publications from this laboratory show that plasma osmolarity increases to ∼320 mOsmol, plasma VP levels peak, and NK3R are translocated to the nucleus at 40 min following the 2 M NaCl treatment (21, 28). Once deeply anesthetized, rats were decapitated. The brain was removed and a 2-mm coronal section containing the PVN was collected. The dorsal and ventral boundaries of the PVN were identified using a surgical microscope; the PVN area was dissected from the surrounding tissue and was chopped into small pieces (28). During the whole process, the PVN tissue (∼25 mg) was kept cold on ice.
Immuno-Electron Microscopy
Nuclear isolation.
PVN nuclei were isolated using established protocols (28). PVN tissue was transferred into a Dounce homogenizer containing 1.0 ml homogenization buffer [0.31 M sucrose, 3 mM CaCl2, 2 mM MgCl2, 1.0 mM EDTA, 0.1% Triton X-100, 1.0 mM dithiothreitol (DTT), 10 mM Tris·HCl; pH 8.0] with Halt protease inhibitor (Pierce, Rockford, IL). The PVN tissue was homogenized with 30 strokes of loose and tight pestles sequentially. The homogenate was centrifuged at 1,000 g for 15 min at 4°C for 2 times in 1.0 ml homogenization buffer, and the supernatant was discarded. The pellet was resuspended in 0.75 ml homogenization buffer and then mixed thoroughly with 0.75 ml sucrose buffer (1.8 M sucrose, 10 mM MgCl2, 1.0 mM EDTA, 1.0 mM DTT, 10 mM Tris·HCl, pH 8.0). The 1.5-ml homogenate was overlaid on 1.0 ml sucrose buffer and centrifuged at 30,000 g for 60 min at 4°C in a swinging bucket rotor (Optima MAX Ultracentrifuge) to isolate nuclei. The supernatant was discarded, and the isolated nuclear pellet was then resuspended in 0.75 ml homogenization buffer and centrifuged at 1,000 g for 15 min at 4°C twice. The nuclear pellet was collected and fixed with 4% paraformaldehyde and 0.25% glutaraldehyde in PBS (0.1 M, pH 7.4) for 1 h at room temperature. After fixation, the nuclear pellet was rinsed in dH2O and dehydrated in a concentration series of ethanol (50%, 70%, 85%, 95%, 100%, 5 min each). The nuclear pellet was embedded in LR White resin and allowed to polymerize at 60°C overnight.
Sections (50 nm) were cut using an ultramicrotome. Three or four sections were mounted on each formvar-coated nickel mesh grid (Electron Microscopy Sciences, Hatfield, PA). To decrease the possibility that the same nucleus was collected and mounted on the grids, multiple sections were discarded between each saved section. To inactivate the residual aldehydes, sections were first incubated with 0.1 M glycine in PBS, and then rinsed in PBS containing 0.1% Triton X-100 for 5 min. The sections were blocked again in incubation buffer (PBS with 0.2% Tween-20, 0.2% BSA-C) containing 5% normal goat serum for 40 min. Next, sections were incubated in incubation buffer containing the Histone H4 antibody (1:20) or the Pan-acetylated Histone H4 antibody (1:20) for 2 h. The sections were rinsed in incubation buffer (2 × for 5 min) and then incubated in the secondary antibody conjugated to 6-nm gold beads (goat anti-rabbit, 1:30 for 2 h). After rinsing with PBS, the sections were blocked in incubation buffer containing 5% donkey serum for 40 min and then incubated with the sheep anti-NK3R antibody (5 μg/ml) for 2 h. The sections were rinsed in buffer and then incubated in buffer containing 5% donkey serum for 20 min. Last, sections were incubated in donkey anti-sheep secondary antibody conjugated to 15-nm gold beads (1:30) for 2 h. The grids were rinsed with incubation buffer for 5 min and then PBS (2 × for 5 min). The sections were fixed with 8% glutaraldehyde for 1 h and then rinsed in dH2O. Sections were stained with 2% uranyl acetate for 30 min and lead citrate for 30 s. Grids were viewed on a Hitachi H-7000 Electron Microscope.
The Immunogold labeling process was completed under room temperature. Three different methodological controls were incorporated into the analyses. First, the NK3R primary antibody was omitted from the procedure. Second, gold beads located within areas of nuclei-free resin were considered as a background (nonspecific) labeling control. Third, isolated nuclei from the control rats not administered with 2 M NaCl served as normal controls.
Electron micrographs for isolated nuclei were captured with a Gatan high-resolution 4 K × 4 K digital camera and Gatan Digital Micrograph software (Pleasanton, CA). For each isolated nucleus, one image containing the whole nucleus was taken at low magnification (6,000×). Multiple images of the same nucleus were taken at high magnification (20,000×) and then stitched together with Adobe Photoshop Cs2 (San Jose, CA) to provide a high-resolution image of the entire nucleus for quantification. In addition, images were taken at higher magnifications [40,000× (100-nm scale bar in figures), 100,000× (50-nm scale bars in figures)] to visualize and measure the distance separating adjacent 6 nm- and 15-nm gold beads.
Seven nuclei were randomly selected per grid and three grids per animal were collected and analyzed. The number of 15-nm gold beads was counted by a third person, who was blind as to the experimental manipulation. The area of the nucleus was measured using the National Institutes of Health's ImageJ software, and the density of 15-nm gold beads was expressed as the number of gold beads divided by the area of the nucleus (beads/μm2). Background Immunogold labeling was determined by counting the number of gold beads in areas of nuclei-free resin surrounding the nuclei.
The spatial colocalization of NK3R (15-nm gold bead) and H4 or pan-acetylated H4 (6-nm gold bead) was determined. Energy transfer-based methods show that molecules that are close enough to generate an energy transfer are within 60 nm of each other (20). Also, Immunogold beads that are conjugated to the secondary antibody are ∼30 nm from the epitope (3). Hence, for two epitopes that are in close spatial association the Immunogold beads conjugated to the secondary antibody can be separated by up to 60 nm. On the basis of this information, if a 6-nm gold bead and a 15-nm gold bead were located within 60 nm of each other, they were defined to be colocalized. The numbers of 15-nm gold beads colocalized and not colocalized with 6-nm gold beads were counted. The percentage of NK3R 15-nm beads that colocalized with H4 and pan-acetylated H4 was calculated by dividing the number of colocalized 15-nm gold beads by the total number of 15-nm gold beads for each nucleus.
Coimmunoprecipitation and Western Blot Analysis
For immunoprecipitation, the sheep anti NK3R antibody was conjugated to Pierce MagnaBind Carboxyl-Derivatized Beads using the methods provided by the manufacturer (Thermo Scientific, Rockford, IL). MagnaBind beads (400 μl) were transferred into a precooled microcentrifuge tube and washed with 1.0 ml PBS 3 times. Sheep NK3R antibody (400 μg) was diluted in conjugation buffer {0.1 M [2(N-Morpholino) ethanesulfonic acid], 0.9% NaCl, pH 4.7} to the final concentration of 5 μg/μl and added to the beads. The cross-linker 1-ethyl-3(3-dimethylaminopropyl) carbodiimide hydrochloride (4 mg) was dissolved in conjugation buffer (400 μl) and added to the bead-antibody mixture. The solution was placed on a rotating mixer for 60 min at room temperature. The beads were then rinsed 3 times in 1 ml PBS, and the antibody-bead conjugate was then stored in 400 μl PBS at 4°C for subsequent Co-IP studies.
To address the interactions between nuclear NK3R and histones, a nuclear coimmunoprecipitation was performed. Rats were administered either 2 M NaCl (n = 9) or a sham load (n = 9), and killed 40 min later. Coimmunoprecipitation experiment was run in triplicate with the PVN tissue from three rats in each condition being pooled together to obtain a sufficient amount of extracted nuclear protein for the nuclear Co-IP and Western blot studies. The Active Motif Universal Co-IP kit (Carlsbad, CA) was used to isolate a nuclear enriched pellet for the Co-IP. The manufacturer's instructions were used with slight modifications. Briefly, the PVN was isolated, as described earlier, and placed in a Dounce Homogenizer with 1 ml of complete hypotonic buffer (Active Motif) and homogenized with 25 strokes of both the loose and tight pestles on ice. The samples were incubated for 15 min on ice, transferred to a chilled microcentrifuge tube, and centrifuged 850 g for 10 min at 4°C. The pellet was resuspended in 1 ml of complete hypotonic buffer and incubated for 15 min on ice. Detergent (50 μl) was added to the sample and mixed by pipetting up and down. The samples were then centrifuged 14,000 g for 30 s, and the supernatant was discarded. The nuclear enriched pellet was suspended in 200 μl of complete digestion buffer and 3 μl of the DNA enzymatic shearing cocktail was added to the sample. The sample was incubated for 30 min at 37°C in a water bath and gently vortexed every 2 min. EDTA (4 μl, 0.5 M) was added to the sample to stop the reaction. The sample was left on ice for 5 min and then centrifuged at 14,000 g for 10 min. The supernatant containing the nuclear proteins was transferred to a fresh prechilled 1.7-ml microcentrifuge tube and saved for the Co-IP reaction.
The nuclear protein supernatant was incubated with 75 μl of the anti-NK3R antibody-bead conjugate that was prepared earlier and 500 μl of complete Co-IP/wash buffer for 18 h at 4°C on a rotating mixer. The beads were then washed with 500 μl complete Co-IP wash buffer 4 times, and resuspended in 45 μl Laemmli buffer (containing 100 mM DTT). After being denatured at 99°C for 10 min, the eluted sample was loaded onto a 4–16% PAGE gel for Western blot and separated by electrophoresis (room temperature) at a constant 200 V for 35 min. Proteins were subsequently transferred to a PVDF membrane (0.2-μm pore size, Bio-Rad Laboratories, Hercules, CA) at a constant 100 V for 60 min at ∼4°C. The membrane was then incubated in blocking buffer [5% blotting grade blocker nonfat dry milk (Bio-Rad) in Tris-buffered saline with 0.25% Tween-20 (TBST)] for 60 min at room temperature. Afterward, the membrane was incubated with the primary antibody in blocking buffer at room temperature for 1 h and then washed three times in TBST. The membrane was then incubated in blocking buffer with secondary antibody at room temperature for 1 h. For the NK3R immunoblotting, both the sheep anti-NK3R (1 μg/ml) and the Life Span rabbit anti-NK3R (1:1,000) were used to show that the NK3R was precipitated from the nuclear enriched pellet. For the detection of acetylated H3, anti-acetylated lys9 H3 primary antibody (1 μg/ml), and chicken anti-rabbit horseradish peroxidase (HRP) (1: 5,000) secondary antibody were used; acetylated H4 was detected using the primary antibody (1: 500) and chicken anti-rabbit HRP (1: 5,000). Between each procedure, the membrane was rinsed 3 times in TBST for 10 min each. Freshly prepared Super-Signal West Femto kit (Pierce) was used for visualizing the protein bands. Images were captured using a Gel Doc XRS digital imaging system, and antigen signal (pixel density) was quantified using Quantity One analysis software (Bio-Rad).
Last, as a negative control, the membrane was stripped a final time and probed for Lamin B2. Membranes were incubated in blocking buffer with mouse monoclonal anti-Lamin B2 (1 μg/ ml) for 1 h, rinsed three times in TBST, and then incubated in chicken anti-mouse HRP (1:20,000) for 1 h at room temperature. The membrane was processed using the Super-Signal Femto kit and imaged using the Gel Doc XRS and analysis software.
Antibodies
For immuno-TEM, the antibodies against histone H4 and pan-acetylated H4 (Active Motif) were raised in rabbit. The histone H4 antibody detected H4 regardless of the posttranslational modifications (acetylation, methylation, etc.), while the pan-acetylated H4 required acetylation of greater than three lysines. The acetylated H3 antibody used in coimmunoprecipitation detected acetylation at lys9 and was purchased from Cell Signaling (Danvers, MA). Three antibodies against NK3R were used: two rabbit anti-NK3R antibodies, the K7 (kindly provided by Dr. James Krause), and the Life Span (Seattle, WA) anti-TACR3 antibody both targeted amino acid (aa) 434–452 of the NK3R. The third NK3R antibody was raised in sheep against aa 180–192 on the 2nd extracellular loop of the NK3R. The NK3R antibodies did not cross react with other tachykinin receptors and the specificity of the anti NK3R antibodies was confirmed by Jensen et al. (28) using siRNA and immunoprecipitation. Briefly, the specificity of the K7 antibody was established by injecting siRNA that was targeted against the NK3R unilaterally into the PVN. Twenty four hours after the injection, NK3R was not detected using the K7 antibody in the injected PVN, but it was detected in the contralateral, control PVN. Detection of the NK3R protein by the K7 antibody recovered in the days following the siRNA injection. The specificity of the sheep anti-NK3R antibody for the NK3R was demonstrated by the observation that the K7 antibody (specificity validated by siRNA) and the sheep anti-NK3R antibodies, although targeting different regions of the NK3R, both label the same band on Western blots. Furthermore, using a pull-down assay, the sheep anti-NK3R and the Life Span anti-NK3R antibodies immunoprecipitated the same weight protein that was detected by the K7 antibody. Hence, the sheep NK3R and the Life Span anti-NK3R antibodies recognize the same protein as does the K7 antibody. Collectively, the experiments establish that the three NK3R antibodies are specific to our target protein-NK3R and meet the published criteria for antibody specificity (7, 25, 38, 50, 52–54).
The anti-Lamin B2 (raised in mouse) was purchased from Invitrogen (Camarillo, CA, USA). Six-nanometer gold beads conjugated goat anti-rabbit IgG secondary antibody were purchased from Jackson ImmunoResearch (West Grove, PA); 15-nm gold conjugated donkey anti-sheep IgG secondary antibody was purchased from Electron Microscopy Science (Hatfield, PA). Goat anti-rabbit and rabbit anti-goat IgG HRP, and Restore PLUS Western Blot stripping buffer were purchased from Pierce Thermo Scientific. Universal Magnetic Co-IP Kit was purchased from Active Motif (Carlsbad, CA). Magnabind carboxyl-derivatized beads and Pierce 3-color protein molecular weight marker were purchased from Pierce Thermo (Rockford, IL).
For quantification of the protein bands in the coimmunoprecipitation experiment, the pixel density for each of the protein bands was determined using Quantity One (Bio-Rad) software. The histone bands that were detected in the 2 M NaCl-treated rats were outlined, the pixel density was determined, and the user-defined boxes were superimposed on the corresponding molecular weights in the control lanes. The relative density was expressed as the density of the target band or corresponding control band divided by the density of the local background. The density of the acetylated H3 and H4 protein bands on the Western blots was averaged over the three replications. The relative density was expressed as the density of the target band or corresponding control band divided by the density of the global background.
Statistical Analysis
Nonparametric tests (Mann-Whitney U-test) were used to compare the number of NK3R Immunogold particles and the frequency of association of NK3R and H4 of experimental (2 M NaCl load) to that of control rats. Densitometry of acetylated histone H3 and H4 was compared in control rats and rats treated with 2 M NaCl using Student's t-tests.
RESULTS
Immuno-TEM: NK3R and H4 Colocalize in Nuclei Following Hyperosmotic Challenge
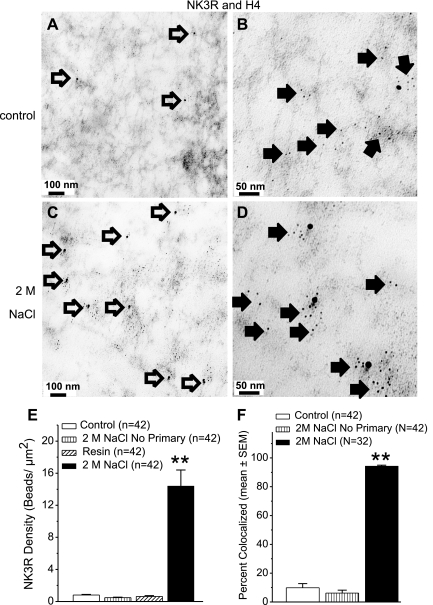
Isolated nuclei were readily identified in the sections, and the sections were free from contamination (e.g., membrane fragments, other cytoplasmic organelles; Fig. 1A). At 40,000, 15-nm beads can be easily identified in the nucleus (Fig. 1, B and C), and 100,000× magnification showed that H4 (6-nm gold beads) was dispersed in the nucleoplasm with NK3R (Fig. 2, B and D). The density of NK3R Immunogold (15 nm) beads within the nuclei (n = 42) from the control rats was low (0.8 ± 0.07 beads/μm2), similar to the resin area surrounding the nucleus (0.6 ± 0.1 beads /μm2; Fig. 2). Similarly, when the NK3R primary antibody was omitted, only a few 15-nm gold beads were detected (0.5 ± 0.06 beads/μm2). Overall, the presence of NK3R in nuclei (n = 42) from rats treated with 2 M NaCl (14.4 ± 2.0 beads/μm2) was significantly greater than that of control rats (P < 0.0001; Fig. 2). NK3R Immunogold was mainly dispersed in the nucleoplasm. In addition, NK3R Immunogold beads could be found along the nuclear membrane and nucleolus. Approximately 70% of magnocellular neurons express NK3R (13) and further examination showed that hypothalamic nuclei from rats administered with 2M NaCl could be subdivided on the basis of whether or not NK3R Immunogold labeling was present. Ten nuclei (<1 beads /μm2) showed NK3R labeling similar to resin and controls, and the remaining 32 nuclei showed that hyperosmotic challenge increased the 15-nm gold bead density to 18.8 ± 1.9 beads/μm2. Further, within these nuclei (n = 32) isolated from rats administered with 2 M NaCl 94.3 ± 0.7% of the NK3R Immunogold beads colocalized with the 6-nm gold beads for H4 (Fig. 2). This was significantly greater than in control nuclei, where only 9% of the limited NK3R colocalized with H4 (P < 0.0001). These results demonstrate that upon acute hyperosmotic challenge, NK3R moves to the nucleus where it colocalizes with chromatin.
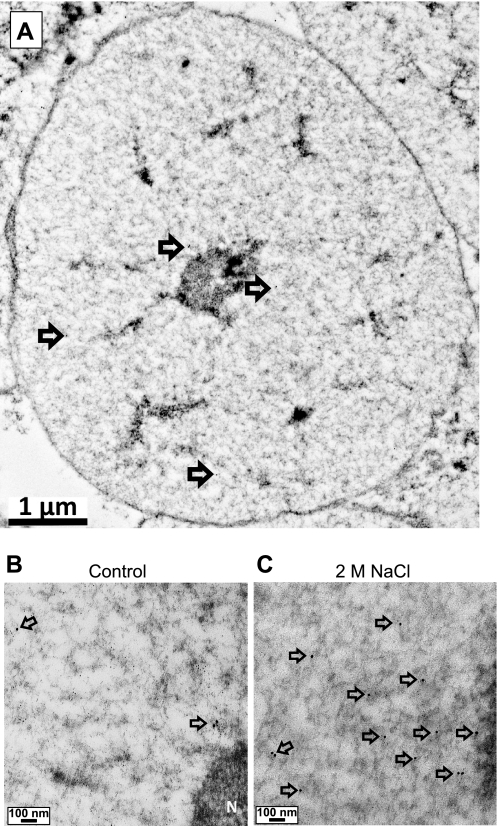
Fig. 1.
Low-power micrographs of isolated nuclei. A: illustration of a cell nucleus taken at low (6,000×) power to illustrate that intact nuclei were captured using the procedure. The nuclear membrane, nucleolus, and several 15-nm gold beads (arrows) may be detected. At 40,000×, a few NK3R 15-nm gold beads (arrows) are seen in the nucleus isolated from a control rat (B) and multiple NK3R 15 nm gold beads (arrows) are seen in the nucleus from a rat treated with 2 M NaCl (C). At these powers, however, 6-nm beads are difficult to discern.
Fig. 2.
Transmission electron micrographs (TEMs) and quantification of NK3R and H4. Immuno-electron micrographs from control (A and B) and 2 M NaCl-loaded rats (C and D). NK3R Immunogold beads (15 nm, open arrows) are observed at 40,000× (A and C). At 100,000× (B and D) H4 Immunogold beads (6 nm, closed arrows) are apparent and colocalize with 15-nm NK3R Immunogold beads (D). E: graph shows the density of 15-nm gold beads in control nuclei, nuclei from rats treated with 2 M NaCl but no primary antibody, nuclei from rats treated with 2 M NaCl, and resin areas. Vertical bars represent the mean ± SE in each group. **P < 0.0001 compared with the control group. F: percentage of 15-nm gold beads colocalized with 6-nm gold beads in the nuclei from control rats, rats treated with 2 M NaCl but no primary antibody, and rats treated with 2 M NaCl. Vertical bars represent the mean ± SE in each group. **P < 0.0001 compared with the control groups.
Immuno-TEM: NK3R and Pan-Acetylated H4
H4 can undergo several posttranslational modifications, and the H4 antibody detected H4, regardless of the modification. As mentioned above, acetylation of H4 at the lysine residue is a marker for gene activation and H4 has multiple lysine acetylation sites. Therefore, the association of NK3R and polyacetylated (pan-acetylated) H4 was determined. Polyacetylated H4 was readily detected in the nuclei from control rats and rats treated with 2 M NaCl (Fig. 3, B and D). Forty two nuclei from control and 42 nuclei from rats treated with 2 M NaCl were analyzed. There were very few NK3R Immunogold beads within the nuclei from the control rats or nuclei from rats treated with 2 M NaCl for which the NK3R antibody was omitted (0.8 ± 0.08/μm2 and 0.5 ± 0.06/μm2, respectively; Fig. 3). The density of NK3R and polyacetylated H4 in the resin surrounding the nuclei were both <1 bead/μm2.
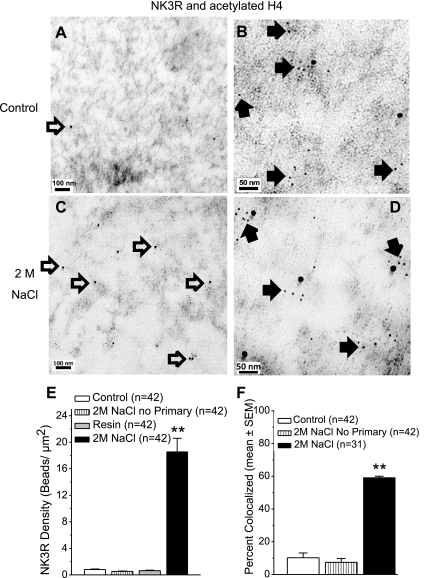
Fig. 3.
TEMs and quantification of NK3R and acetylated H4. Immuno-electron micrographs from control (A and B) and 2 M NaCl-loaded rats (C and D). NK3R Immunogold beads (15 nm, open arrows) are observed at 40,000× (A and C). At higher power (100,000×) acetylated H4 Immunogold beads (6 nm, closed arrows) are apparent and associate with 15-nm NK3R Immunogold beads (D). E: graph shows the density of 15-nm gold beads in the nuclei from: control rats, rats treated with 2 M NaCl but no primary antibody, rats treated with 2 M NaCl, and resin area. Vertical bars represent the mean ± SE in each group. **P < 0.0001 compared with the control group. F: percentage of 15-nm gold beads colocalized with 6-nm gold beads in the nuclei from control rats, rats treated with 2 M NaCl but no primary antibody, and rats treated with 2 M NaCl. Vertical bars represent the mean ± SE in each group. **P < 0.0001 compared with the control groups.
Among the 42 nuclei isolated from PVN tissue of 2 M NaCl-treated rats and processed for NK3R and polyacetylated H4, 11 nuclei had a density of NK3R Immunogold beads (<1 bead/ μm2) that was comparable to control nuclei and resin. The density of NK3R Immunogold beads in the remaining 31 nuclei (18.6 ± 2.0/μm2) was significantly greater than that observed in control nuclei (P < 0.0001; Fig. 3). The majority of NK3R Immunogold beads was colocalized with polyacetylated H4 (59.2 ± 0.8%, n = 31) in nuclei from rats treated with 2 M NaCl, but only 10.2 ± 0.03% were found to colocalize in nuclei from control rats (P < 0.0001). Similar to that seen with H4, the distance separating the 15-nm and 6-nm Immunogold beads was frequently less than 11 nm (Fig. 3, B and D).
Co-IP: NK3R and Acetylated H4 and Acetylated H3
Immuno-TEM showed that NK3R and acetylated H4 colocalize following the hyperosmotic challenge. We next tested whether nuclear NK3R physically associates with acetylated H4. In addition, histone modifications function in a combinatorial manner, and we, therefore, tested whether NK3R associates with another histone that is subject to acetylation, histone H3. The pan-acetylated H4 antibody used in immuno-TEM did not detect protein using Western blot analysis. Therefore, another antibody against acetylated H4 lys16 (Active Motif) was confirmed in Western blot analysis and used in coimmunoprecipitation. The detection of H4 acetyl Lys 16 indicates that this lysine was acetylated but does not rule out that additional lysines were also acetylated.
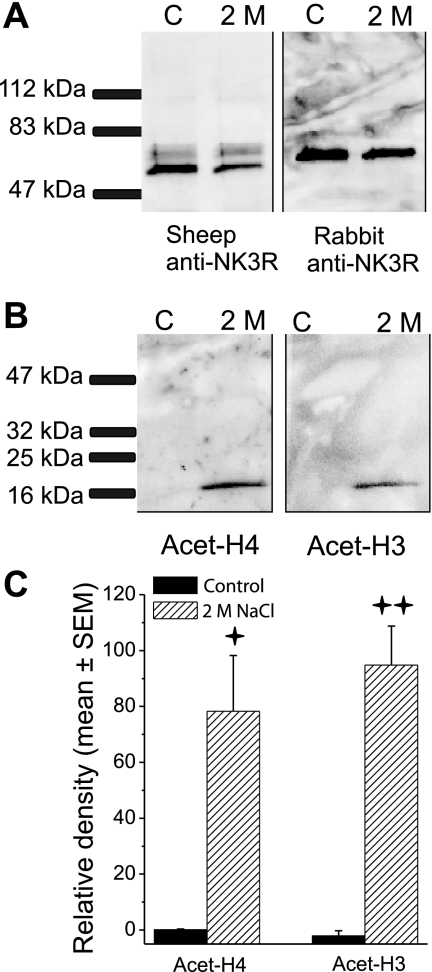
Nuclear proteins that were pulled down with the sheep NK3R antibody conjugated to magnetic beads were then probed for the presence of NK3R protein using the sheep anti-NK3R and the Life Span, rabbit anti-NK3R. Both antibodies detected the protein immunoprecipitated from control rats and rats treated with 2 M hypertonic saline. Although these two antibodies recognize different amino acid sequences, they both detected NK3R at ∼65–67 kDa in the enriched nuclear samples. In addition, the sheep anti NK3R detected a second, lighter-weight band (∼59 kDa) in the enriched nuclear samples from control and 2 M NaCl-treated rats. The Active Motif protocol yields a nuclear enriched sample, but it is not a purified nuclear preparation, and the NK3R that was detected in the nuclear enriched samples (Fig. 4A) may be from cytoplasmic and nuclear fractions. Nuclear proteins were pulled down with the sheep NK3R antibody conjugated to magnetic beads and then probed for acetylated histones H3 and H4 (Fig. 4, B and C). Acetylated H3 and H4 coimmunoprecipitated with NK3R in the nuclear enriched sample from rats challenged with 2 M NaCl, and the density of the histone bands were significantly greater than that in control rats, t's (4) >4.6, P’s < 0.01 (Fig. 4C). In mature cells, H3 and H4 are nuclear proteins (19), and the observation that NK3R immunoprecipitated both histones in 2 M NaCl-treated rats could only occur after NK3R was translocated into the nucleus. To check for nondiscriminant protein-protein interactions, the membranes were checked for the presence of Lamin B2, a nuclear membrane protein. Lamin B2 was undetectable in the nuclear Co-IP membranes (Western blots not shown).
Fig. 4.
Coimmunoprecipitation. A: proteins that were pulled down in the nuclear enriched sample with the sheep NK3R antibody conjugated to magnetic beads were probed for NK3R protein using the sheep anti-NK3R and the Life Span, rabbit anti-NK3R. Both antibodies detected the protein immunoprecipitated from control rats and rats treated with 2 M hypertonic saline. B: membrane was stripped and probed for acetylated histone H4; then it was stripped and probed for acetylated H3. Acetylated H4 and H3 pulled down with NK3R in nuclear enriched sample from 2 M NaCl-treated rats (2 M) but not in control rats (C). C: coimmunoprecipitation experiment was run in triplicate with the PVN tissue from three rats in each condition being pooled together. Histograms reflect the means ± SE of the three samples in each condition. Quantification of the densitometry showed that significantly more acetylated H4 and H3 immunoprecipitated with NK3R in the enriched nuclear samples from rats treated with 2 M NaCl than control rats. Vertical bars represent the mean ± SE in each group. +P < 0.01 ++P < 0.001.
DISCUSSION
NK3Rs are internalized into the cytoplasm after ligand-mediated activation following hypotension, hyperosmolarity, and agonist (senktide) injection (21, 26). The internalization of the NK3R follows the traditional GPCR pathway, whereby following ligand binding, the receptors are internalized where they are either degraded by enzymes in lysosomes or recycled to the membrane (17, 36, 61). However, in addition to these pathways, after ligand-induced activation, confocal microscopy revealed that NK3R was detected within the nuclei of VP neurons in the PVN and SON (21, 26). Subsequently, we utilized immuno-TEM to describe the subnuclear distribution of NK3R following an acute hyperosmotic challenge (28). The present results using an NK3R antibody raised in sheep against the second extracellular loop replicate our earlier findings using a different NK3R antibody (raised in rabbit against the COOH terminus) that NK3R was dispersed within the nucleoplasm 40 min following administration of hypertonic saline (28). Our present results show that nuclei isolated from the PVN of control rats lacked NK3R (less than 1 bead/μm2), and there was a significant increase in the presence of NK3R in the PVN nuclei of rats administered with 2 M NaCl (>14 beads/μm2). Therefore, the nuclear translocation of NK3R to the nucleus of neurons in the PVN tissue is largely driven by synaptic events triggered by the hyperosmotic stimulus.
NK3Rs expressed in other brain areas are also trafficked to the nucleus. For example, under basal conditions, NK3R is found within the nucleus of neurons in the ventral tegmental area of rat (VTA) (40) and globus pallidus of squirrel monkeys (41). Limited NK3R is found in nuclei of SON neurons under basal conditions, but nuclear NK3R increases significantly in response to hypotension (26). It appears that in some regions, such as the SON and PVN, nuclear translocation of NK3R is activity dependent; that is, translocation is in response to identifiable stimuli, such as hypotension and hyperosmolarity (21, 26, 28). The synaptic inputs and stimuli that influence translocation of NK3R to the nuclei of other areas, such as the VTA and globus pallidus, remains to be identified, or alternatively, if there is a steady state, constitutive transport of NK3R into the nuclei in these areas.
Our current results confirm our previous findings that hyperosmolarity causes the translocation of NK3R into the nuclei of PVN neurons. This raises the question of how a large, hydrophobic membrane receptor moves through the cytoplasm and into the nucleus? The transport of large proteins through the nuclear pore complex (NPC) requires that the target protein contains a nuclear localization signal (NLS) (57). The NLS binds to the importin family of nuclear transport proteins. Once bound to the target protein, importins enable movement of the protein through the NPC and into the nucleus (12). The NK3R contains a putative NLS region (amino acids 348–351; Ref. 39), and Jensen et al. (29) showed that importin β coimmunoprecipitated with NK3R in PVN tissue isolated from rats administered an acute hyperosmotic challenge, but did not coimmunoprecipitate in control rats. Furthermore, immunoneutralization of importin-β-1 significantly decreased the nuclear translocation of NK3R in CLU209 cells, a hypothalamic embryonic cell line (29). The results indicate that the importin pathway mediates the nuclear transport of NK3R in response to acute hyperosmotic challenge. But does the full-length receptor or some fraction of the peptide translocate to the nucleus and how does the hydrophobic NK3R protein move in the cytoplasm to associate with the importins? First, the full-length NK3R appears to be translocated to the cell nucleus. Antibodies directed against the NH2-terminal (aa 6–18), the 2nd extracellular loop (aa 180–192), and COOH terminus (AA 434–452) all detected nuclear NK3R in nuclei of hypothalamic neurons (28). Similarly, the molecular weights of epidermal growth factor receptor (EGFR) that are detected in the cytoplasm and nucleus are the same (43), suggesting that it is transported to the nucleus in the full form. Chromatin immunoprecipitation assays show that following application of EGF, membrane EGF receptors (EGFRs) are translocated to the nucleus where EGFR associates with specific promoter regions to affect proliferative activities (43, 48). In the case of EGFR, transport of the full-length receptor involves the ligand-mediated internalization and transport of the receptor to the endoplasmic reticulum (ER). Once in the ER, the receptor is extracted from the membrane by the ER-associated degradation (ERAD) pathway. The retrotranslocation of EGFR to the cytosol requires the association of the protein with the ERAD protein, Sec61 translocon, and knock-down of Sec61 disrupts the nuclear transport of EGFR, in vitro (42). Within the cytoplasm, the hydrophobic receptor could then associate with chaperone proteins and/or importins that mask the hydrophobic region of the protein (42). We previously showed that NK3R associates with importin β-1 for translocation to the nucleus (29). The ER degradation pathway is one hypothesized route via which full-length proteins, such as EGFR and possibly NK3R, may be translocated to the nucleus (42, 49).
In the present experiments, the PVN tissue block contained the magnocellular and parvocellular areas of the PVN, as well as parts of the anterior and medial preoptic nucleus (28). Not all of these areas express NK3R. Although the actual tissue sample was heterogeneous, the expression of NK3R is restricted to neurons, and furthermore, it is highly expressed in the magnocellular divisions of the PVN with some NK3R present in parvocellular portions (13, 14, 21, 23). Ding et al. (13) indicated that slightly over 70% of NK3R expressing neurons were positive for VP. Furthermore, confocal microscopy showed that NK3Rs are translocated to the nuclei of VP neurons in the PVN following administration of 2 M NaCl (21). Collectively, this information leads to the inference that many of the isolated nuclei showing nuclear NK3R following the hyperosmotic challenge are from vasopressinergic magnocellular neurons.
The primary issue addressed in this paper is the possible target and function of NK3R within the nucleus. Double-labeling immuno-TEM shows that following the hyperosmotic challenge, NK3R moves into the nucleus where it colocalizes with H4. Indeed, 94% of the nuclear NK3R was colocalized with H4. Further, we show that following the hyperosmotic challenge NK3R colocalized with acetylated H4, a marker of gene activation (30, 51). Approximately 60% of the NK3R Immunogold beads colocalized with acetylated H4. The magnitude of the colocalization was less than that observed with H4, and this may reflect the properties of the polyacetylated H4 antibody. As mentioned above, H4 can be acetylated at multiple sites, and the antibody can preferentially recognize H4 that is acetylated at 4 or 5 different lysines. As such, any less acetylated H4 or H4 that had undergone other posttranslational modifications would not be detected. Nevertheless, over half of the NK3R was colocalized with highly acetylated H4, indicating that NK3R is present at transcriptionally active portions of chromatin.
To confirm a protein-protein interaction (NK3R and histone), coimmunoprecipitation studies were conducted. PVN tissue was taken from control rats and rats administered 2 M NaCl, and NK3R was immunoprecipitated from enriched nuclear samples. Samples from control and treated rats were probed with a sheep anti-NK3R and a rabbit anti-NK3R. Antibodies from different species that targeted different amino acid sequences detected NK3R at ∼65–67 kDa in the enriched nuclear samples. The second, lighter band (∼59 kDa) detected by the sheep anti-NK3R may reflect, in part, variable glycosylation modifications and differences in affinity of the antibody for the complexed and noncomplexed forms of NK3R.
The enriched nuclear preparation protocol from Active Motif yields a high concentration of nuclear proteins, but it also contains cytoplasmic proteins. NK3R that was immunoprecipitated in the enriched nuclear samples from control rats was likely cytoplasmic NK3R. Support for this conclusion is provided by the current immuno-TEM images showing that NK3R Immunogold beads were minimal and at levels equal to that seen in resin in nuclei isolated from control rats. In contrast, nuclei isolated from rats treated with 2 M NaCl displayed a high density of NK3R Immunogold beads. These immuno-TEM observations in control rats and rats treated with 2 M NaCl replicate our previous report (28). In addition, when a stringent nuclear isolation protocol was used, NK3R was not detected by Western blot in nuclear samples isolated from control nuclei that were free of cytoplasmic proteins; NK3R was readily detected in uncontaminated nuclear samples from rats treated with 2 M NaCl (28). In the present experiment, NK3R detected in the nuclear enriched samples from rats treated with 2 M NaCl reflects both nuclear and cytoplasmic protein.
Immunoprecipitated nuclear enriched samples were probed for acetylated H4 and H3. In the control rats, although NK3R was detected in the enriched sample, it was likely of cytoplasmic origin and as such, NK3R did not associate with either acetylated H3 or acetylated H4; neither immunoprecipitated with NK3R. A high density of NK3R was detected in the nuclei isolated from rats treated with 2 M NaCl and immuno-TEM showed that NK3R colocalized with acetylated H4. Acetylated H4 immunoprecipitated in the nuclear enriched samples from rats treated with 2 M NaCl. Coimmunoprecipitation and immuno-TEM used different NK3R antibodies that were raised in two different species and against two quite different epitopes. Both NK3R antibodies and techniques provided complementary results showing an association of NK3R and acetylated H4. The use of a different antibody (species and epitope) and approach serves to confirm that the immuno-TEM staining is NK3R. In addition, acetylated H3 was shown to physically associate with nuclear NK3R. Previous papers show that hyperosmolarity causes the translocation of NK3R to the nucleus (21, 28), where it could associate with acetylated H3 and H4. Acetylation of H4 lys16, in particular, plays a determining role in the level of chromatin compaction (55).
Histone acetylation is critical in the epigenetic control of gene regulation. Since NK3R associated with both acetylated H4 and acetylated H3, one possibility is that the NK3R possesses intrinsic HAT activity. Sequence examination revealed that there was no correspondence of the NK3R sequence with that of other proteins with known HAT activity. NK3R also lacks the traditional DNA-binding domains, such as zinc fingers. As such, NK3R acting alone would not appear to be capable of influencing histone acetylation or gene activity. However, as a complex with other factors, NK3R could influence chromatin structure. One possibility is that NK3R protein has an activational role. For example, HBO1 (histone acetyltransferase binding to ORC) is a nuclear protein that has no or little intrinsic HAT activity, but as a complex with another protein has considerably higher HAT activity and acetylates histone H3, and H4 lys16 (27). Alternatively, nuclear NK3R could have a targeting role by recruiting HATs to promoter regions. The association of proteins with histone acetyltransferases is proposed as a mechanism to selectively target chromatin acetylation (5). The nuclear receptor coactivator 6 is a nuclear protein that similarly lacks HAT activity and modulates chromatin structure by recruiting histone acetyltransfereases to the promoter (44). As such, the association of HATs with NK3R might be required for the promoter-specific recruitment of HAT function to specific histones in promoter regions of genes activated by hyperosmolarity. Thus, two possible actions of nuclear NK3R await further confirmation.
Nuclear NK3R associated with acetylated H3 and H4. The question becomes what specific genes may be affected by NK3R. Acute hyperosmolarity causes the local intra-PVN release of an NK3R ligand (21, 22). Ligand binding to the receptor causes the internalization and translocation of NK3R to the nucleus (21, 28). Although hyperosmolarity alters the expression levels of multiple mRNAs (6, 34), local injections of NK3R agonists into the SON activate a very limited set of genes (33). In particular, both acute hyperosmolarity and NK3R agonists activate c-Fos (22, 58). The induction of c-fos involves the acetylation of H3 and H4 at the c-fos promoter (10, 59). Evidence suggests a role for NK3R activity in the expression levels of c-fos mRNA and protein. Haley and Flynn (22) showed that intra-PVN injections of a selective NK3R antagonist significantly reduced c-Fos expression in the PVN. Also, injections of NK3R agonists induce extensive c-Fos expression in magnocellular neurons (15, 58). While the effects of NK3R agonists and blockade of NK3R on c-Fos could be due to affecting intracellular signaling cascades, it could potentially reflect the direct effects of nuclear NK3R.
GPCRs provide messages to the nucleus by several direct and indirect downstream signaling mechanisms. First, NK3Rs and several GPCRs provide a direct signal to the nucleus in that the receptor itself is translocated to the nucleus (4). As the present results indicate, once in the nucleus, NK3R may have a role in transcriptional regulation by associating with acetylated H4 and acetylated H3. Second, extracellular signals that are initially transduced by activating receptors at the plasma membrane may be transmitted to the nucleus by cytoplasmic signaling cascades. In this case, ligand binding leads to the endocytosis of the activated receptor, and the initiation of protein-protein interactions and signaling cascades. The most established are those indirect mechanisms leading to the recruitment of transcription factors. In the case of the neurokinin receptors, following activation, NK3R activates phosopholipase C signaling pathway to generate inositol 1,4,5-trisphosphate and diacylglycerol (35). These molecules ultimately activate Ca2+-regulated transcription factors, such as cAMP response element-binding protein (56). The third mechanism by which a GPCR may indirectly influence nuclear function is related to the endocytosis process itself. Activation of GPCRs causes cytoplasmic β arrestin to move to the cell membrane to colocalize with the receptor, where it promotes receptor endocytosis (9). For example, stimulation of the delta-opioid receptor results in an arrestin-mediated internalization and the translocation of β-arrestin to the nucleus. There, β arrestin recruits P300 to acetylate H4, leading to the transcription of specific genes, including c-fos (32). Chromatin is a direct target of both NK3R and β-arrestin, and like β-arrestin, NK3R may target acetylate histones at the c-fos promoter to stimulate c-fos transcription.
Perspectives and Significance
Our results suggest a new role for the membrane-bound GPCR NK3R in altering chromatin structure in the brain. In response to acute hyperosmolarity, membrane-bound NK3Rs are activated, internalized to the cytoplasm, and translocated to the nucleoplasm. The nuclear NK3R then plays a role in potentially acetylating H3 and H4, relaxing chromatin structure, and affecting transcription. This model illustrates the dynamic and complex nature of NK3R function in PVN neurons area after an acute physiological challenge. Although the present focus is on the PVN, the same may apply to multiple other brain regions that express NK3R. Indeed, NK3R is detected within the nuclei of neurons in the globus pallidus and ventral tegmental area (40, 41). Additional techniques were not used to identify whether nuclear NK3R in these brain areas associated with transcriptionally active chromatin, but the presence of the NK3R in nuclei of neurons in a variety of brain regions suggests that NK3R may affect gene activation under multiple conditions. These data support an interesting and novel mechanism, that NK3R, and perhaps other GPCRs, moves to the nucleus and directly interacts with chromatin to potentially affect transcription.
GRANTS
This research was supported by National Institutes of Health Grant R01-NS57823 and Grant P20-RR15640 from the National Center for Research Resources awarded to F. W. Flynn.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The technical assistance provided by Sean Bell, Matthew Fournier, Kellee D. Sundstrom, and Donald Pratt is greatly appreciated.
REFERENCES
- 1. Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA sythesis. Proc Natl Acad Sci USA 51: 786–794, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong WE, Sladek CD. Evidence for excitatory actions of histamine on supraoptic neurons in vitro: mediation by an H1-type receptor. Neuroscience 16: 307–322, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Bergersen LH, Storm-Mathisen J, Gundersen V. Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc 3: 144–152, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res 28: 15–28, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Brownell JE, Allis CD. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev 6: 176–184, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev 81: 1197–1267, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Burry RW. Specificity controls for immunocytochemical methods. J Histochem Cytochem 48: 163–166, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Chawla MK, Gutierrez GM, Young WS, III, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol 384: 429–442, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol 66: 61–79, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Clayton AL, Rose S, Barratt MJ, Mahadevan LC. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J 19: 3714–3726, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colin I, Blondeau C, Baude A. Neurokinin release in the rat nucleus of the solitary tract via NMDA and AMPA receptors. Neuroscience 115: 1023–1033, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Davis LI. The nuclear pore complex. Annu Rev Biochem 64: 865–896, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Ding YQ, Lu BZ, Guan ZL, Wang DS, Xu JQ, Li JH. Neurokinin B receptor (NK3)-containing neurons in the paraventricular and supraoptic nuclei of the rat hypothalamus synthesize vasopressin and express Fos following intravenous injection of hypertonic saline. Neuroscience 91: 1077–1085, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol 364: 290–310, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Ding YQ, Shi J, Su LY, Xu JQ, Su CJ, Guo XE, Ju G. Intracerebroventricular injection of senktide-induced Fos expression in vasopressin-containing hypothalamic neurons in the rat. Brain Res 882: 95–102, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Durrin LK, Mann RK, Kayne PS, Grunstein M. Yeast histone H4 N-terminal sequence is required for promoter activation in vivo. Cell 65: 1023–1031, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Ferguson SS, Zhang J, Barak LS, Caron MG. Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci 62: 1561–1565, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Fukuda H, Sano N, Muto S, Horikoshi M. Simple histone acetylation plays a complex role in the regulation of gene expression. Brief Funct Genomic Proteomic 5: 190–208, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Gabler C, Blank N, Hieronymus T, Schiller M, Berden JH, Kalden JR, Lorenz HM. Extranuclear detection of histones and nucleosomes in activated human lymphoblasts as an early event in apoptosis. Ann Rheum Dis 63: 1135–1144, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gurevich VV, Gurevich EV. GPCR monomers and oligomers: it takes all kinds. Trends Neurosci 31: 74–81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 290: R1242–R1250, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Haley GE, Flynn FW. Blockade of NK3R signaling in the PVN decreases vasopressin and oxytocin release and c-Fos expression in the magnocellular neurons in response to hypotension. Am J Physiol Regul Integr Comp Physiol 295: R1158–R1167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hatae T, Kawano H, Karpitskiy V, Krause JE, Masuko S. Arginine-vasopressin neurons in the rat hypothalamus produce neurokinin B and co-express the tachykinin NK-3 receptor and angiotensin II type 1 receptor. Arch Histol Cytol 64: 37–44, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Hebbes TR, Thorne AW, Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J 7: 1395–1402, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmseth S, Lehre KP, Danbolt NC. Specificity controls for immunocytochemistry. Anat Embryol (Berl) 211: 257–266, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Howe HE, Somponpun SJ, Sladek CD. Role of neurokinin 3 receptors in supraoptic vasopressin and oxytocin neurons. J Neurosci 24: 10103–10110, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iizuka M, Stillman B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J Biol Chem 274: 23027–23034, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Jensen D, Zhang Z, Flynn FW. Trafficking of tachykinin neurokinin 3 receptor to nuclei of neurons in the paraventricular nucleus of the hypothalamus following osmotic challenge. Neuroscience 155: 308–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen DD, Sundstrom K, Flynn FW. Expression of the nuclear transport protein importin ss-1 and its association with the neurokinin 3 receptor in the rat hypothalamus following acute hyperosmotic challenge. Neuroscience 170: 1020–1027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jenuwein T, Allis CD. Translating the histone code. Science 293: 1074–1080, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Johnson HM, Subramaniam PS, Olsnes S, Jans DA. Trafficking and signaling pathways of nuclear localizing protein ligands and their receptors. Bioessays 26: 993–1004, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell 123: 833–847, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Kawasaki M, Ponzio TA, Yue C, Fields RL, Gainer H. Neurotransmitter regulation of c-fos and vasopressin gene expression in the rat supraoptic nucleus. Exp Neurol 219: 212–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawasaki M, Yamaguchi K, Saito J, Ozaki Y, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y. Expression of immediate early genes and vasopressin heteronuclear RNA in the paraventricular and supraoptic nuclei of rats after acute osmotic stimulus. J Neuroendocrinol 17: 227–237, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol 28: 721–738, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci 15: 87–114, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98: 285–294, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Larsson LI. Peptide immunocytochemistry. Prog Histochem Cytochem 13: 1–85, 1981 [PubMed] [Google Scholar]
- 39. Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O'Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem 279: 7901–7908, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Lessard A, Savard M, Gobeil F, Jr, Pierce JP, Pickel VM. The neurokinin-3 (NK3) and the neurokinin-1 (NK1) receptors are differentially targeted to mesocortical and mesolimbic projection neurons and to neuronal nuclei in the rat ventral tegmental area. Synapse 63: 484–501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levesque M, Parent R, Parent A. Cellular and subcellular localization of neurokinin-1 and neurokinin-3 receptors in primate globus pallidus. Eur J Neurosci 23: 2760–2772, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell 18: 1064–1072, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 3: 802–808, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Mahajan MA, Samuels HH. Nuclear receptor coactivator/coregulator NCoA6(NRC) is a pleiotropic coregulator involved in transcription, cell survival, growth and development. Nucl Recept Signal 6: e002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci USA 92: 2622–2626, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB, Lorens SA. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience 89: 1269–1290, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Olins AL, Olins DE. Spheroid chromatin units (v bodies). Science 183: 330–332, 1974 [DOI] [PubMed] [Google Scholar]
- 48. Rakowicz-Szulczynska EM, Otwiaska D, Rodeck U, Koprowski H. Epidermal growth factor (EGF) and monoclonal antibody to cell surface EGF receptor bind to the same chromatin receptor. Arch Biochem Biophys 268: 456–464, 1989 [DOI] [PubMed] [Google Scholar]
- 49. Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J Cell Biol 152: 1307–1312, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci 26: 8017–8020, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem 70: 81–120, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol 493: 477–478, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Saper CB. A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem 57: 1–5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol 465: 161–163, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76: 75–100, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252: 1427–1430, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Smith AE, Kalderon D, Roberts BL, Colledge WH, Edge M, Gillett P, Markham A, Paucha E, Richardson WD. The nuclear location signal. Proc R Soc Lond B Biol Sci 226: 43–58, 1985 [DOI] [PubMed] [Google Scholar]
- 58. Smith ME, Flynn FW. Distribution of Fos-like immunoreactivity within the rat brain following intraventricular injection of the selective NK(3) receptor agonist senktide. J Comp Neurol 426: 413–428, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci 24: 5603–5610, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Verdone L, Agricola E, Caserta M, Di ME. Histone acetylation in gene regulation. Brief Funct Genomic Proteomic 5: 209–221, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels 5: 193–199, 1997 [PubMed] [Google Scholar]
- 62. Zhang K, Williams KE, Huang L, Yau P, Siino JS, Bradbury EM, Jones PR, Minch MJ, Burlingame AL. Histone acetylation and deacetylation: identification of acetylation and methylation sites of HeLa histone H4 by mass spectrometry. Mol Cell Proteomics 1: 500–508, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Zhang W, Bone JR, Edmondson DG, Turner BM, Roth SY. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J 17: 3155–3167, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]