Abstract
We have shown previously that an intravenous injection of oxytocin (OT) in ovariectomized (OVX) rats initiates a circadian rhythm of prolactin (PRL) secretion similar to that observed after cervical stimulation (CS). In this study, we investigated the pathway through which OT triggers the PRL rhythm. We first tested whether an intracerebroventricular injection of OT could trigger the PRL secretory rhythm. As it did not, we injected OT intravenously while an OT receptor antagonist was infused intravenously. This antagonist completely abolished the PRL surges, suggesting that a peripheral target of OT is necessary for triggering the PRL rhythm. We hypothesized that OT may induce PRL release, which would be transported into the brain and trigger the rhythm. In agreement with this, OT injection increased circulating PRL by 5 min. To test whether this acute increase in PRL release would induce the PRL rhythm, we compared the effect of intravenously administered thyrotropin-releasing hormone (TRH) and OT. Although TRH injection also increased PRL to a comparable level after 5 min, only OT-injected animals expressed the PRL secretory rhythm. Motivated by prior findings that bilateral resection of the pelvic nerve blocks CS-induced pseudopregnancy and OT-induced facilitation of lordosis, we then hypothesized that the OT signal may be transmitted through the pelvic nerve. In fact, OT injection failed to induce a PRL secretory rhythm in pelvic-neurectomized animals, suggesting that the integrity of the pelvic nerve is necessary for the systemic OT induction of the PRL secretory rhythm in OVX rats.
Keywords: oxytocin receptor, pseudopregnancy, lactotrophs
mating or cervical stimulation (CS) induces a circadian rhythm of prolactin (PRL) secretion in female rats, consisting of nocturnal (0300) and diurnal (1700) surges. The rhythm persists for 10–12 days, which is about half the duration of pregnancy (19). As the rhythm in secretion continues for several days without additional stimuli, it has been suggested that a hypothalamic “memory” is activated by CS and acts to sustain the daily PRL surges (17). This rhythm can be produced in ovariectomized (OVX) rats, demonstrating that the memory does not require ovarian steroids (43). Despite these findings, the mechanism by which the memory is triggered is not understood.
PRL secretion by lactotrophs is tonically inhibited by hypothalamic dopamine (DA) (3, 11). Whereas the full generation of PRL surges requires a decrease in DA inhibition, the actions of one or more stimulating factors are also needed (16, 18). Several lines of evidence suggest that oxytocin (OT) may act as a PRL-releasing factor to induce PRL surges in several physiological paradigms (23, 39, 40), including mating. Because it has been shown that there is an immediate release of OT (34) after CS in rats, we hypothesized that this initial burst of OT could be responsible for triggering the PRL surges induced by CS. In agreement with this, we observed that a single intravenous injection of OT in OVX rats was indeed able to induce a PRL secretory rhythm and a DA release pattern similar to that initiated by CS (13). Early in vivo work demonstrated that the concentration of PRL in the blood is increased within 15–20 min after mating or artificial CS (7, 44). Complementing these in vivo studies, we and others have demonstrated that OT stimulates PRL secretion in vitro when administered to anterior pituitary cells in culture (28) through a calcium-dependent mechanism (12, 45). This led to the hypothesis that the OT-induced PRL rhythm could be triggered by the PRL released by lactotrophs following OT injection.
To evaluate the role of PRL in mediating the OT- or CS-induced circadian PRL rhythm, we recently tested whether a bolus of PRL was “sufficient” to trigger the rhythm by injecting ovine PRL (oPRL) into OVX rats. We found that either peripheral or central oPRL injections were able to trigger a PRL rhythm that is similar to that induced by OT or CS (21). The concentration of oPRL required to trigger the rhythm was much larger when injected peripherally than when injected centrally, consistent with a mechanism that involves a central action of PRL. Peripheral PRL presumably enters the central nervous system through a carrier-mediated transport system in the choroid plexus (47).
The objective of this study was to clarify the mechanism through which OT triggers the PRL rhythm when injected into OVX rats. We hypothesized the existence of a neurogenic component to mediate the effects of systemic OT on the initiation of the PRL secretory rhythm. The integrity of the pelvic nerves has been previously inferred to be necessary for the initiation of the mating-induced surges of PRL, since pelvic neurectomy prior to mating blocks pregnancy (44) and pseudopregnancy (10). As pelvic nerve integrity is also required for OT-induced lordosis facilitation (33), in this study, we sought to test the effect of pelvic neurectomy on the OT-induced PRL secretory rhythm in OVX rats.
METHODS
Animals
Adult female Sprague-Dawley rats weighing 250–300 g (Charles River, Raleigh, NC) were kept in a Laboratory Animal Resources care facility, housed in groups of three in plastic cages under a 12:12 light-dark cycle (lights on at 0600) and controlled temperature (25° C). Food and water were provided ad libitum. All rats were OVX bilaterally through a single ventral midline incision under isoflurane anesthesia (Aerrane; Baxter, Deerfield, IL) and allowed to recover for at least 1 wk. At the end of this and other surgical procedures, rats were treated with a single intraperitoneal injection of an anti-inflammatory analgesic (Metacam, Boehringer Ingelheim Vetmedica, St. Joseph, MO; 1 mg/kg). Animal procedures were approved by the Florida State University Animal Care and Use Committee.
Experimental Design
Experiment 1: central vs. peripheral OT action to trigger rhythmic PRL secretion.
We first injected saline or OT intracerebroventricularly to determine whether central OT could trigger the PRL secretory rhythm in OVX rats. In a second set of OVX animals, we performed an intravenous OT injection in the presence of an OT antagonist that had been infused peripherally for ∼24 h to prevent OT binding to its receptors in the anterior pituitary. The control group also received an intravenous OT injection, but no OT antagonist was infused. Both central and peripheral OT injections were performed at 1600, the same time used in our previous work, in which a single OT injection in OVX animals induced the PRL secretory rhythm (13). Blood samples were drawn every 2–4 h on days 2 and 3 to determine the dynamics of PRL secretion.
Experiment 2: correlation between immediate PRL release and the PRL secretory rhythm.
Animals were injected with OT or thyrotropin-releasing hormone (TRH) vs. saline intravenously at 1600, and blood samples were taken 5 and 10 min later, to evaluate the acute changes in PRL levels in plasma after the injections. Blood samples were additionally drawn every 2–4 h, on days 2 and 3 after the injections to assess the occurrence of the PRL secretory rhythm.
Experiment 3: effect of pelvic neurectomy on the OT-induced rhythmic secretion of PRL.
OVX animals had their pelvic nerve sectioned bilaterally (see Pelvic Neurectomy) and after 1 wk of recovery were subjected to OT injection iv at 1600. Sham animals were dissected down to the level of the pelvic nerve, but no sectioning was performed. Blood samples were taken 5 and 10 min immediately after the injection and every 2–4 h on days 2 and 3 for the measurement of PRL levels.
Intracerebroventricular Cannulation
Under ketamine (Ketaset; Fort Dodge, IA; 49 mg/ml) and xylazine (Anased; Lloyd Laboratories, Shenandoah, IA; 1.8 mg/ml) anesthesia (100 μl/100 g body wt), animals were positioned in a stereotaxic apparatus with the incisor bar at −3.3 mm. For the central injection of OT, a 22-gauge guide cannula (C313G; Plastics One, Roanoke, VA) was implanted in the right cerebral lateral ventricle (coordinates: 1.0 mm posterior to bregma, 1.6 mm lateral to the midline, and 3.2–3.7 mm below the outer surface of the skull). The correct vertical positioning of the cannula in the lateral ventricle was determined by displacement of the meniscus in a water manometer that detects pressure differences among compartments. The cannula, protected with a plastic-capped mandril (C313DC; Plastics One), was attached to the bone with stainless-steel screws and acrylic cement. After surgery, all rats were allowed to recover for 1 wk in individual cages.
Jugular Vein Catheter Implantation and Blood Samples
One week after intracerebroventricular cannulation, rats were anesthetized with isoflurane, and a catheter (Micro-Renathane; MRE-040, 0.040′′ OD × 0.025′′ ID; Braintree Scientific, Braintree, MA) filled with sterile saline (0.9% NaCl; Teknova, Hollister, CA) was inserted through the external jugular vein into the right atrium, fitted subcutaneously, and exteriorized at the back of the animal, as previously described (20). All stainless-steel surgical instruments were immersed in chlorhexidine disinfectant (Novalsan; Fort Dodge, IA) until surgery. After the surgery, the catheter tube was filled with gentamicine sulfate (Alexis, San Diego, CA) to prevent bacterial growth and to maintain catheter patency (46). On the morning of the first day of the experiment, an extension of the catheter tubing filled with saline was connected to the jugular catheter, and the rats were left undisturbed in their cages. Blood samples of 300 μl were withdrawn into plastic heparinized syringes, and the same volume of sterile 0.9% NaCl was injected through the catheter immediately after removal of each blood sample.
Injections and Infusions
All drugs were dissolved in 0.9% NaCl. OT (H-2510, Bachem Americas, Torrance, CA; 0.3 μg/5 μl) or saline was injected intracerebroventricularly under light isoflurane anesthesia via a stainless-steel needle (0.2-mm diameter) connected by PE-10 polyethylene tubing to a Hamilton syringe (Hamilton, Reno, NV) controlled by an injection pump (KDS100, KD Scientific, Holliston, MA) calibrated to dispense 5-μl solution/min. After each injection, the needle was left inside the cannula for an additional 60 s to avoid solution reflux. This dose of OT has been shown to elicit known central effects of OT, such as induction of maternal behavior in virgin rats (36). Peripheral OT (5 μg/200 μl), TRH (1 μg/200 μl, P1319, Sigma, St. Louis, MO), or saline administration was performed intravenously using the jugular vein catheter implantation described above.
For the peripheral infusions, 200 μl of the OT antagonist solution (225 μg /200 μl, desGly-NH2-d(CH2)5[d-Tyr2,Thr4]OVT; GenScript, Scotch Plains, NJ) (30) was inserted in each osmotic pump (AP-2001D; Alzet, Durect, Cupertino, CA) and infused at a rate of 9 μg/h for 1 day. This peptide OT antagonist, unlike nonpeptide OT antagonists, has limited penetration through the blood-brain barrier (8). Infusion of the OT antagonist alone in OVX rats did not modify PRL levels over the next 1–3 days (data not shown).
Pelvic Neurectomy
Immediately after ovariectomy, the mid-ventral incision was extended from the pubis toward the xyphoid process. The bifurcation of the vena cava into the common iliac veins was first located and then followed 1 cm caudally to the site of origin of the internal iliac veins. These veins run perpendicular to the long axis of the body, toward the rat's dorsum and were visualized by gentle retraction of the surrounding muscle with sterilized cotton swabs. The pelvic nerve was located ∼5 mm from the origin of the internal iliac vein, running rostrocaudally across its axis, perpendicular to the vein, deep in the space between the internal iliac vein and the adjacent muscles (for detailed explanation, see Ref. 10). The nerve was transected bilaterally with fine scissors under a Zeiss surgical microscope. Sham surgery consisted of dissection down to the level of the pelvic nerve, but no sectioning was performed. As bladder evacuation is compromised by pelvic neurectomy, bladders were emptied manually twice daily until the end of the experiment. The same procedure was conducted in sham animals to ensure identical handling procedures, but little urine was expelled in these animals. Bladder distention due to urine retention was used as the indicator of successful neurectomy.
Radioimmunoassay
Blood samples were centrifuged at 1,200 g for 15 min at 4°C; the plasma was separated and frozen at −20°C until assayed. Plasma PRL was determined by RIA using a kit provided by Dr. Albert F. Parlow through the National Hormone and Pituitary Program (Torrance, CA). 125I was purchased from PerkinElmer Life Sciences (Shelton, CT), and PRL-I-6 was radioiodinated by the chloramine-T method. The antiserum for PRL was anti-rat PRL-S9 and the reference preparation was PRL-RP3. The lower limit of detection was 0.10 ng/ml and the intra- and inter-assay coefficients were less than 4 and 12%, respectively.
Statistical Analysis
Data are expressed as means ± SE. Statistical differences were determined by two-way ANOVA followed by the Bonferroni post hoc test. Comparisons among times within the same experimental group were analyzed by one-way ANOVA followed by the Newman-Keuls post hoc test. P < 0.05 was considered statistically significant.
RESULTS
OT Acts Peripherally to Induce the PRL Secretory Rhythm in OVX Rats
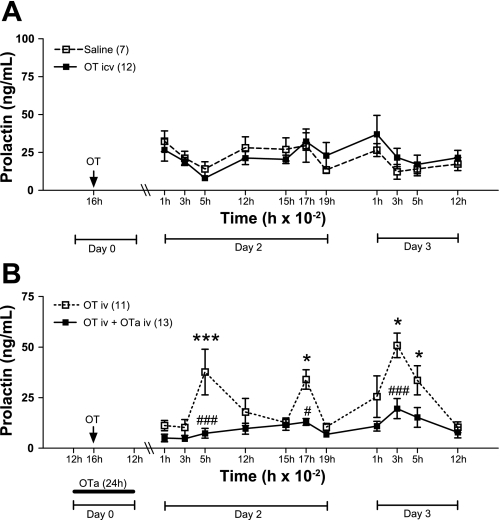
The intracerebroventricular injection of OT (0.3 μg) had no effect on PRL levels in OVX rats (Fig. 1A). However, peripheral injection of OT (5 μg) triggered a two-pulse per day PRL rhythm (Fig. 1B), as demonstrated previously (12). This suggests that OT acts peripherally, not centrally, in initiating this PRL rhythm. In another set of experiments an OT receptor antagonist (OTa) was infused peripherally for 24 h during the time of OT injection. In these animals, the surges were completely blocked (Fig. 1B, P < 0.001 for the nocturnal surges and P < 0.05 for the diurnal surges), even after clearance of the antagonist. This suggests that a peripheral target of OT, possibly anterior pituitary lactotrophs, is necessary for triggering the OT-induced PRL rhythm.
Fig. 1.
Central (intracerebroventricular) vs. peripheral (intravenous) effect of oxytocin (OT) to induce the prolactin (PRL) secretory rhythm. A: ovariectomized (OVX) rats were injected with OT (0.3 μg, n = 12) or vehicle (n = 7) intracerebroventricularly at 1600 of day 0 (arrow). B: OVX rats were injected intravenously with OT (5 μg) at 1600 of day 0 (arrow). In 13 rats, the OT antagonist (OTa, 9 μg/h) was infused intravenously beginning around 1200 of day 0 for 24 h. Blood samples were withdrawn during the next 2 days to observe the PRL diurnal and nocturnal surges. Data are presented as means ± SE. #P < 0.05 and ###P < 0.001 vs. control group at the same time. *P < 0.05, ***P < 0.001 vs. 0100 of day 2 PRL in the same experimental group.
An Acute Release of PRL Is not Sufficient to Trigger the PRL Secretory Rhythm
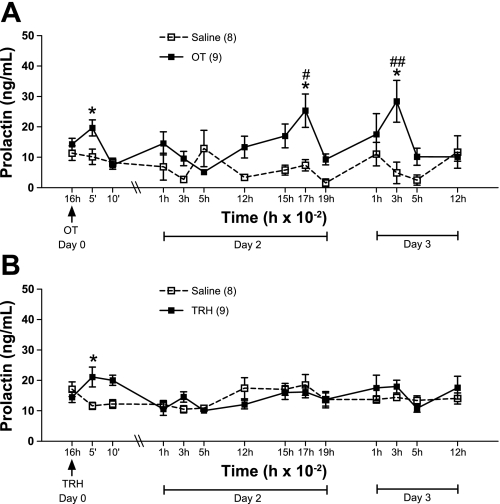
The immediate blood sampling after the peripheral OT injection revealed an acute increase in the circulating concentration of PRL after 5 min (Fig. 2A, P < 0.05). As expected, the PRL secretory rhythm was induced in these animals, with elevated PRL at 1700 and 0300 (P < 0.05). We repeated the same experimental design, but injecting TRH instead of OT, into another group of animals. TRH is known to evoke PRL release from lactotrophs (25, 26). The results are shown in Fig. 2B. Although TRH induced an acute release of PRL comparable to that observed after OT injection (P < 0.05), no increase of PRL concentration was observed in the subsequent blood samples. This suggests that the acute release of PRL does not mediate the OT-induced triggering of the PRL secretory rhythm.
Fig. 2.
Correlation between immediate PRL release and the PRL secretory rhythm. A: OVX rats were injected with saline (n = 8) or OT (n = 9) iv at 1600 of day 0 (arrow). Blood samples were taken immediately before and 5 and 10 min following peripheral injection and, thereafter, during the next 2 days to observe the PRL diurnal and nocturnal surges. B: same experimental design was repeated injecting rats with saline (n = 8) or TRH (n = 9). Data are presented as means ± SE. #P < 0.05 and ##P < 0.01 vs. control group at the same time. *P < 0.05 vs. 1600 PRL in the same experimental group.
Pelvic Neurectomy Blocks the OT-Induced PRL Rhythm
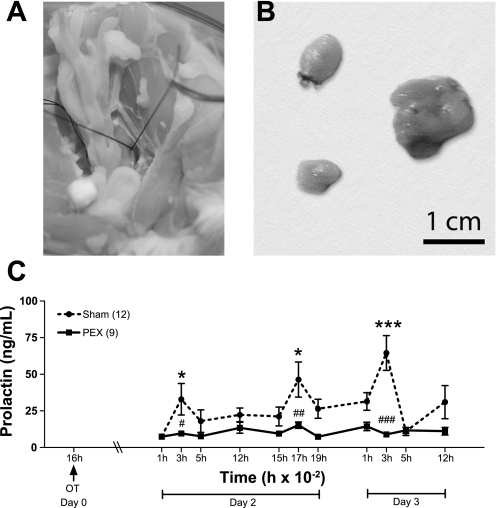
In a final series of experiments, resection of the pelvic nerve (shown in Fig. 3A) was performed. Successful pelvic neurectomy was assessed by comparing bladder size (Fig. 3B). Sham animals exhibited normal size bladders (left), while pelvic neurectomized animals presented distension of the bladder (right), due to interference with the micturition reflex. The results of peripheral OT injection to animals subjected to pelvic neurectomy are shown in Fig. 3C. OT administration at 1600 in sham-operated animals increased the concentration of PRL at the expected times in subsequent days (Fig. 3C): 0300 (P < 0.05) and 1700 (P < 0.01) on day 2, and 0300 on day 3 (P < 0.001). Bilateral transection of the pelvic nerves completely abolished the OT-induced increases of PRL occurring on subsequent days [Fig. 3C, P < 0.05 (day 2), and P < 0.001 (day 3)].
Fig. 3.
The effect of pelvic neurectomy on the OT-induced PRL secretory rhythm. A: photograph showing the location of the pelvic nerve in a perfused rat. B: comparison between bladders from two sham-operated animals (left) and one from a pelvic neurectomized animal (right). C: OVX rats had their pelvic nerve cut bilaterally (PEX; n = 9) or were submitted to sham surgery (Sham; n =12). After recovery, they were injected with OT intravenously at 1600 of day 0 (arrow). Blood samples were withdrawn immediately after the injection and during the next 2 days to observe the PRL diurnal and nocturnal surges. Data are presented as means ± SE. #P < 0.05 ##P < 0.01, and ###P < 0.001 vs. sham group at the same time. *P < 0.05 and ***P < 0.001 vs. 1600 PRL in the same experimental group.
DISCUSSION
We intended to clarify the mechanism by which a bolus injection of OT triggers a PRL secretory rhythm that mimics the rhythm observed after CS in OVX rats. Our first hypothesis was that OT could act centrally to induce this rhythm. This proposition was ruled out, since an intracerebroventricular injection of OT known to induce maternal behavior in virgin rats was ineffective in starting the rhythmic secretion of PRL. Therefore, it is very unlikely that the peripheral injection of OT acts at the central nervous system (CNS) to trigger the PRL rhythm. Our results corroborate the fact that OT has poor blood-brain barrier permeability (24). In contrast, central OT release is known to be involved in several reproductive behaviors, including facilitation of the onset of sexual and maternal behavior (22). CS has been shown to alter OT receptor affinity and density in the medial preoptic area (9) and to increase the expression of c-Fos in OT neurons of the parvicellular paraventricular nucleus. These neurons release OT centrally and into the median eminence (38) and hence to the anterior pituitary through the long portal vessels, which very likely influences circadian PRL secretion. Thus, although our results show that an intracerebroventricular injection of OT does not initiate a PRL rhythm, we must point out that a central role for OT on CS or mating-induced PRL surges is possible. In addition, our mathematical model also suggests that the CS-induced PRL rhythm requires activation of hypothalamic OT neurons (5).
Our next experiments confirmed that OT must be acting on a peripheral target to trigger the PRL rhythm, as peripheral infusion of an OT receptor antagonist completely blocked the occurrence of the OT-induced PRL surges. The surges were abolished even after the clearance of the antagonist, suggesting that the antagonist effectively prevented OT from triggering the memory of this rhythm. These results differ from our results in OVX rats subjected to CS, in which peripheral infusion of an OT antagonist initially abolished the CS-induced PRL surges that nonetheless returned after clearance of the drug (31). Thus, the current evidence indicates that peripheral OT can trigger the rhythmic PRL surges, but it is not necessary to trigger the CS-induced PRL rhythm.
Convincing evidence has been published for a stimulatory role of OT on PRL secretion in rats, and several reports have shown that PRL may gain access to the brain through a receptor-mediated mechanism (27, 29, 47). Even though evidence has been presented to support an immediate release of PRL after CS (7, 42) or mating (44), it is not yet clear that this PRL release is related to the incidence of pseudopregnancy or pregnancy. More recent studies show that the PRL levels are higher 1 h after mating among females that become pseudopregnant than among those in which mating did not induce pseudopregnancy (15). Conversely, the diurnal surges and pseudopregnancy smears are observed in CS rats treated with ergocornine (a blocker of PRL release) (48), consistent with the early assumptions that activation of the corpora lutea of pregnancy or pseudopregnancy does not require a PRL surge within the first 12 h after mating, that occurs in delayed pseudopregnancy (2).
Given the requirement for a peripheral action of OT and its stimulatory effect on PRL, our next proposition was that OT might act directly on the lactotrophs, inducing PRL secretion, which, in turn, would act in the CNS to trigger its own rhythm. This hypothesis was based on our prior observations that central PRL injection initiated the circadian PRL rhythm (6, 21). If this hypothesis is true, 1) OT injection must result in acute PRL secretion, and 2) other factors that stimulate PRL secretion should also induce the PRL secretory rhythm. We tested this possibility in experiment 2. OT increased peripheral plasma PRL 5 min after its injection and induced the PRL secretory rhythm on subsequent days, supporting preceding in vivo findings that peripheral administration of OT results in a rapid release of PRL (28). Injection of TRH in another group of animals also induced acute PRL secretion, similar to that induced by the OT injection. However, TRH did not elicit the PRL secretory rhythm on the following days, suggesting that the PRL increase observed after OT peripheral injection is not sufficient to trigger the PRL secretory rhythm. Alternatively, the acute PRL increase may act in concert with other peripheral effects of OT to trigger the memory and initiate the rhythm.
These results led us to the hypothesis that other peripheral targets of OT injection might be more relevant than the anterior pituitary lactotrophs to induce the PRL secretory rhythm. Systemic or peripheral administration of OT has been demonstrated to facilitate lordosis in steroid-treated OVX rats (1), and this facilitation is blocked after pelvic neurectomy (33). In female rats, the internal genitalia are innervated primarily by the hypogastric nerves (sympathetic) and pelvic nerves (sympathetic and parasympathetic) that terminate peripherally in the pelvic or uterine cervical ganglia (37). Visceral afferent fibers of the pelvic nerve are more sensitive than hypogastric nerve fibers to uterine and cervical mechanostimulation (4). Electrical stimulation of the pelvic nerve elicited contractions in both cervix and uterus (41) and increased intravaginal pressure (35). Accordingly, recordings from the pelvic nerve activity showed that it responds to mechanical stimulation of the vagina and to stretching of the cervix during parturition (37). Pelvic neurectomy blocks induction of pseudopregnancy (10), presumably by blocking the PRL increase after mating (44). The integrity of the pelvic nerve is also necessary for the pacing behavior during mating (14). Since the cervix and uterus are targets of peripheral OT, it is reasonable to hypothesize that intravenous OT administration could result in increased pelvic nerve activity and neurotransmission to the CNS. Interestingly, the OT-induced facilitation of lordosis is blocked after removal of either the cervix or uterus, demonstrating that both are important components in the peripheral mechanism transmitting the signal to the CNS (32). Consistently with this, our results demonstrated that pelvic neurectomy also blocked the OT-induced PRL rhythmic secretion, suggesting that the pelvic nerves may relay the signals triggered by the OT injection to the CNS to elicit lordosis and rhythmic PRL secretion.
Perspectives and Significance
The mating stimulus induces a circadian PRL rhythm that rescues the corpora lutea of the rodent estrous cycle and prolongs their ability to secrete progesterone (19). This is critical for successful pregnancy and implantation. This rhythm of two surges per day can be induced by artificial CS in the absence of gonadal steroids, which indicates that this reflex operates through a memory triggered independently of gonadal steroids. In this study, we show that an OT injection can also initiate a circadian PRL rhythm through peripheral, not central, actions of OT. The peripheral signal is transmitted to the CNS through the pelvic nerve. In this regard, peripheral OT can cause contractions of the cervix and uterus which, in turn, may signal the CNS through the pelvic nerve, thus initiating the circadian PRL rhythm. This possibility would be consistent with our results and needs to be tested. An interesting question is to what extent the pathway for the response initiated by cervical stimulation and that initiated by OT injection overlap. Oxytocin is released into the plasma after CS, a response known as the Ferguson reflex in humans (34), so it is plausible that OT acts as the trigger for the CS-induced rhythm. However, the blockade of OT receptors abrogated the OT-induced surges, but not those induced by CS, indicating that the latter stimulus might activate additional receptor systems and sensory pathways to those targeted by OT. Thus, although the mechanisms for triggering the OT-induced and the CS-induced PRL secretory rhythm may both involve the pelvic nerve, additional sensory afferents are likely utilized in the CS pathway. These additional elements, as well as the brain areas involved in both the CS and OT induction of the PRL rhythm, will be the focus of future experiments.
GRANTS
This work was supported by the National Institutes of Health Grants DK 43200 and DA 19356.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Natalia Dmitrieva for her advice with the pelvic neurectomy technique, Dr. De'Nise McKee for her contribution in the first experiments of this research, Mr. Charles Badland for his photo art assistance, and Patrick Fletcher for valuable discussions and ideas.
REFERENCES
- 1. Arletti R, Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides 6: 247–253, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Beach JE, Tyrey L, Everett JW. Prolactin secretion preceding delayed pseudopregnancy in rats after electrical stimulation of the hypothalamus. Endocrinology 103: 2247–2251, 1978 [DOI] [PubMed] [Google Scholar]
- 3. Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22: 724–763, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol 69: 533–544, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Bertram R, Egli M, Toporikova N, Freeman ME. A mathematical model for the mating-induced prolactin rhythm of female rats. Am J Physiol Endocrinol Metab 290: E573–E582, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertram R, Helena CV, Gonzalez-Iglesias AE, Tabak J, Freeman ME. A tale of two rhythms: the emerging roles of oxytocin in rhythmic prolactin release. J Neuroendocrinol 22: 778–784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bishop W, Orias R, Fawcett CP, Krulich L, McCann SM. Plasma gonadotropins and prolactin in pseudopregnancy in the rat. Proc Soc Exp Biol Med 137: 1411–1414, 1971 [DOI] [PubMed] [Google Scholar]
- 8. Boccia ML, Goursaud AP, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Horm Behav 52: 344–351, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caldwell JD, Jirikowski GF, Greer ER, Stumpf WE, Pedersen CA. Ovarian steroids and sexual interaction alter oxytocinergic content and distribution in the basal forebrain. Brain Res 446: 236–244, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Carlson RR, De Feo V. Role of the pelvic nerve vs. the abdominal sympathetic nerves in the reproductive function of the female rat. Endocrinology 77: 1014–1022, 1965 [DOI] [PubMed] [Google Scholar]
- 11. DeMaria JE, Livingstone JD, Freeman ME. Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology 67: 377–383, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology 145: 3386–3394, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME. Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab 290: E566–E572, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erskine MS. Pelvic and pudendal nerves influence the display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav Neurosci 106: 690–697, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Erskine MS, Kornberg E. Acute luteinizing-hormone and prolactin responses to paced mating stimulation in the estrous female rat. J Neuroendocrinol 4: 173–179, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 80: 1523–1631, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Freeman ME, Smith MS, Nazian SJ, Neill JD. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology 94: 875–882, 1974 [DOI] [PubMed] [Google Scholar]
- 18. Gibbs DM, Neill JD. Dopamine levels in hypophysial stalk blood in the rat are sufficient to inhibit prolactin secretion in vivo. Endocrinology 102: 1895–1900, 1978 [DOI] [PubMed] [Google Scholar]
- 19. Gunnet JW, Freeman ME. The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev 4: 44–61, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 36: 391–392, 1974 [DOI] [PubMed] [Google Scholar]
- 21. Helena CV, McKee DT, Bertram R, Walker AM, Freeman ME. The rhythmic secretion of mating-induced prolactin secretion is controlled by prolactin acting centrally. Endocrinology 150: 3245–3251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Insel TR, Young L, Wang Z. Central oxytocin and reproductive behaviours. Rev Reprod 2: 28–37, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Johnston CA, Negro-Vilar A. Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology 122: 341–350, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Kang YS, Park JH. Brain uptake and the analgesic effect of oxytocin—its usefulness as an analgesic agent. Arch Pharm Res 23: 391–395, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Keith LD, Tam B, Ikeda H, Opsahl Z, Greer MA. Dynamics of thyrotropin-releasing hormone-induced thyrotropin and prolactin secretion by acutely dispersed rat adenohypophyseal cells. Evidence for ‘all-or-none’ secretion by heterogeneous secretory units, each with a specific response threshold. Neuroendocrinology 43: 445–452, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Lamberts SW, MacLeod RM. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev 70: 279–318, 1990 [DOI] [PubMed] [Google Scholar]
- 27. Lerant A, Freeman ME. Ovarian steroids differentially regulate the expression of PRL-R in neuroendocrine dopaminergic neuron populations: a double label confocal microscopic study. Brain Res 802: 141–154, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Lumpkin MD, Samson WK, McCann SM. Hypothalamic and pituitary sites of action of oxytocin to alter prolactin secretion in the rat. Endocrinology 112: 1711–1717, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Mangurian LP, Walsh RJ, Posner BI. Prolactin enhancement of its own uptake at the choroid plexus. Endocrinology 131: 698–702, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY. Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int J Pept Protein Res 46: 244–252, 1995 [DOI] [PubMed] [Google Scholar]
- 31. McKee DT, Poletini MO, Bertram R, Freeman ME. Oxytocin action at the lactotroph is required for prolactin surges in cervically stimulated ovariectomized rats. Endocrinology 148: 4649–4657, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moody KM, Adler NT. The role of the uterus and cervix in systemic oxytocin-PGE2 facilitated lordosis behavior. Horm Behav 29: 571–580, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Moody KM, Steinman JL, Komisaruk BR, Adler NT. Pelvic neurectomy blocks oxytocin-facilitated sexual receptivity in rats. Physiol Behav 56: 1057–1060, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Moos F, Richard P. [Level of oxytocin release induced by vaginal dilatation (Ferguson reflex) and vagal stimulation (vago-pituitary reflex) in lactating rats (author's transl)]. J Physiol (Paris) 70: 307–314, 1975 [PubMed] [Google Scholar]
- 35. Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res 490: 85–94, 1989 [DOI] [PubMed] [Google Scholar]
- 36. Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA 76: 6661–6665, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters LC, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res 408: 199–204, 1987 [DOI] [PubMed] [Google Scholar]
- 38. Polston EK, Centorino KM, Erskine MS. Diurnal fluctuations in mating-induced oxytocinergic activity within the paraventricular and supraoptic nuclei do not influence prolactin secretion. Endocrinology 139: 4849–4859, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Samson WK, Lumpkin MD, McCann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology 119: 554–560, 1986 [DOI] [PubMed] [Google Scholar]
- 40. Sarkar DK. Immunoneutralization of oxytocin attenuates preovulatory prolactin secretion during proestrus in the rat. Neuroendocrinology 48: 214–216, 1988 [DOI] [PubMed] [Google Scholar]
- 41. Sato Y, Hotta H, Nakayama H, Suzuki H. Sympathetic and parasympathetic regulation of the uterine blood flow and contraction in the rat. J Auton Nerv Syst 59: 151–158, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Sirinathsinghji DJ, Audsley AR. Endogenous opioid peptides participate in the modulation of prolactin release in response to cervicovaginal stimulation in the female rat. Endocrinology 117: 549–556, 1985 [DOI] [PubMed] [Google Scholar]
- 43. Smith MS, Neill JD. A “critical period” for cervically-stimulated prolactin release. Endocrinology 98: 324–328, 1976 [DOI] [PubMed] [Google Scholar]
- 44. Spies HG, Niswender GD. Levels of prolactin, LH and FSH in the serum of intact and pelvic-neurectomized rats. Endocrinology 88: 937–943, 1971 [DOI] [PubMed] [Google Scholar]
- 45. Tabak J, Gonzalez-Iglesias AE, Toporikova N, Bertram R, Freeman ME. Variations in the response of pituitary lactotrophs to oxytocin during the rat estrous cycle. Endocrinology 151: 1806–1813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thrivikraman KV, Huot RL, Plotsky PM. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Protoc 10: 84–94, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Walsh RJ, Slaby FJ, Posner BI. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 120: 1846–1850, 1987 [DOI] [PubMed] [Google Scholar]
- 48. Wuttke W, Meites J. Induction of pseudopregnancy in the rat with no rise in serum prolactin. Endocrinology 90: 438–443, 1972 [DOI] [PubMed] [Google Scholar]