Abstract
Dieting is the most common approach to losing weight for the majority of obese and overweight individuals. Restricting intake leads to weight loss in the short term, but, by itself, dieting has a relatively poor success rate for long-term weight reduction. Most obese people eventually regain the weight they have worked so hard to lose. Weight regain has emerged as one of the most significant obstacles for obesity therapeutics, undoubtedly perpetuating the epidemic of excess weight that now affects more than 60% of U.S. adults. In this review, we summarize the evidence of biology's role in the problem of weight regain. Biology's impact is first placed in context with other pressures known to affect body weight. Then, the biological adaptations to an energy-restricted, low-fat diet that are known to occur in the overweight and obese are reviewed, and an integrative picture of energy homeostasis after long-term weight reduction and during weight regain is presented. Finally, a novel model is proposed to explain the persistence of the “energy depletion” signal during the dynamic metabolic state of weight regain, when traditional adiposity signals no longer reflect stored energy in the periphery. The preponderance of evidence would suggest that the biological response to weight loss involves comprehensive, persistent, and redundant adaptations in energy homeostasis and that these adaptations underlie the high recidivism rate in obesity therapeutics. To be successful in the long term, our strategies for preventing weight regain may need to be just as comprehensive, persistent, and redundant, as the biological adaptations they are attempting to counter.
Keywords: energy restriction, diet-induced obesity, obesity prone, weight loss, energy balance
in the united states, over 60% of adults and close to 20% of children are overweight or obese (35, 174). A number of effective weight loss strategies are available, but most are only transiently effective over a period of 3 to 6 mo. Less than 20% of individuals that have attempted to lose weight are able to achieve and maintain a 10% reduction over a year (128). Over one-third of lost weight tends to return within the first year, and the majority is gained back within 3 to 5 years (3, 246). A number of reasons have been proposed for the high incidence of weight regain (69, 246), and several point to the biological response to weight loss.
The objective of this review is to examine the role of this biological response in the process of weight regain. Biological regulation is first discussed in relation to other nonbiological pressures that affect body weight as weight is gained, lost, and regained. The specific biological adaptations to the most common form of dieting, an energy-restricted low-fat diet, are then discussed in more detail. Previous reviews provide extensive descriptions of the normal adaptive response to energy restriction. We do not attempt to recapitulate those efforts here. Rather, the unique perspective of this review summarizes those adaptations that have been confirmed or specifically observed in the overweight or obese. Because there are unique features of the adaptive response with obesity (138, 251, 252), understanding the integrative nature of the response in the obese is pertinent to therapeutic development. Although homeostatic systems are clearly operational in both lean and obese individuals and, in both, underlie the tendency for lost weight to be regained, the central and peripheral adaptations involved in weight recovery can differ with one's starting weight. These differences may provide insight as to what components of the homeostatic system would be most effectively targeted in those who have the most difficulty controlling their weight. To this end, we examined weight loss studies in clinically overweight and obese adults, in diet-induced, polygenic animal models of obesity, and with dietary (nonsurgical) interventions involving an energy-restricted, low-fat diet. We would assert that, in contrast to its subtle, permissive role in the development of obesity, biology has a more prominent, causal role in weight regain after energy-restricted weight loss. Countering this metabolic drive to regain lost weight may be the most significant challenge for obesity therapeutics in the coming decades.
Our Biology in the Context of Other Pressures Affecting Body Weight
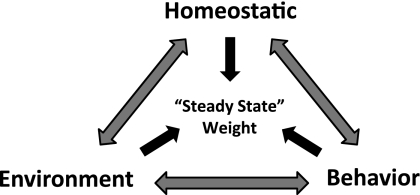
Body weight is affected by biological, environmental, and behavioral pressures, all of which are inherently influenced by genetics (Fig. 1). These pressures interact with one another, and their integrated effect establishes a “steady state” weight in adults. Humans exhibit a broad weight range, because of the variability in each of these pressures and the genetic diversity that underlies them. Changing any one of these pressures can alter the steady-state weight. To appreciate the biological influence, it is helpful to conceptualize how the homeostatic system changes with respect to these other pressures during the development of obesity, during weight loss, and during weight regain. For this purpose, we use a steady-state weight graph linked to an energy balance (Fig. 2A). The three pressures that affect body weight are positioned atop the balance, and the fulcrum of the balance represents the underlying genetic disposition. Moving the pressures to either side of the fulcrum tips the balance toward weight gain (to the left) or weight loss (to the right). The biological component (homeostatic pressures) is responsible for reestablishing a balance when the environmental or behavioral pressures change, preventing the organism from wasting away or from gaining weight indefinitely.
Fig. 1.
Pressures affecting the steady-state weight. The three pressures, all influenced by the underlying genetic disposition, interact with one another, culminate in a steady state weight at which the body resides. Changing any one of these pressures can alter this steady state weight.
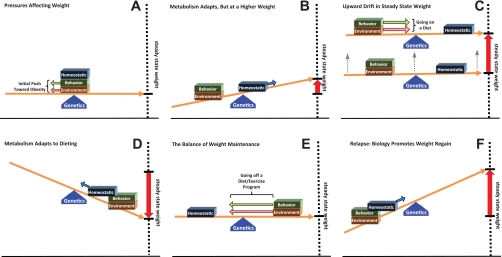
Fig. 2.
Biology's influence during obesity development, treatment, and relapse. Homeostatic systems adapt to prevent perpetual weight loss or gain when environmental and behavioral pressures change. The interplay of this balance between the changes in nonhomeostatic pressures and homeostatic adaptations is shown for the initial development and progression of obesity (A–C), during energy-restricted weight loss (D), during weight maintenance after weight loss (E), and during the relapse to obesity (F). Biology may play a more subtle, permissive role during the initial development of obesity, but it becomes a driving force for weight regain after weight loss.
Over the past 50 years, our environment has become more obesogenic, favoring behavioral choices that increase the intake of energy in excess of energy requirements (99). When an individual is subjected to these pressures, stored energy accumulates, and metabolism gradually adapts to prevent perpetual weight gain (Fig. 2B). At some point, for most people, these biological adaptations reestablish a balance with the obesogenic pressures, and a new, albeit higher, steady-state weight is achieved (Fig. 2C).
In rodents, the variability in this metabolic defense against perpetual weight gain when challenged with similar obesogenic pressures (high-fat, high-carbohydrate feeding; limited physical activity) is what separates the obesity-resistant (OR) from the obesity-prone (OP) (140). Both initially experience a positive energy imbalance, but the OR sense the nutrient overload, increase fat oxidation, elevate expended energy, and reestablish energy balance (106, 107). OP rodents, on the other hand, continue to eat to excess until expenditure increases from their accumulated mass to reestablish energy balance. This polygenic predisposition for biology to respond (OR), or not respond (OP), to obesogenic pressures leads to the diet-induced obese (DIO) and the diet resistance (DR) phenotypes. The DIO/DR model is reflective of the human condition from the perspective that some people become obese and others do not, when obesogenic pressures appear to be similar. In the graphic representation, a polygenic predisposition for obesity would be akin to the fulcrum shifting right of center. If sufficiently extreme, the genetic disposition could make it practically impossible for homeostatic counter-regulatory measures to reestablish a new steady state. Weight would continue to creep up.
This upward drift in weight (Fig. 2C) is observed in some DIO models. The drift occurs with some permanence in the homeostatic system, continually retuning it to target the newly achieved level of adiposity (138, 139, 148, 149). There is little evidence to suggest that circumstances would be any different for humans, when immersed in an environment with readily available foods high in fat and simple sugars and permissive to physically inactivity. Both OP-like and OR-like responses would emerge. Adding highly palatable and rewarding components to the diet recruits hedonic systems in the brain to eat even more, further challenging homeostatic systems to adapt and reestablish a steady state (136, 139). Although some weight loss can occur by returning to a lower-energy diet that is less palatable, satisfactory amounts of weight loss usually do not occur without intentionally restricting energy intake.
The most common diet is the restricted consumption of low-fat foods, which is sometimes, but not always, accompanied by regular exercise. In our graphic model, these dramatic changes in environmental and behavioral pressures initially work in concert with the biological adaptations to induce weight loss (Fig. 2D). However, the homeostatic system immediately begins to adapt to prevent the individual from wasting away. At some point, a balance is achieved at a lower, steady state weight, and the individual hits the well-known “wall” or plateau in their weight loss efforts. To lose more weight, the environmental and behavioral strategies must be stepped up a notch. To maintain the reduced weight, these weight loss strategies must be maintained indefinitely (Fig. 2E), as the homeostatic system does not appear to “reset” at this lower weight, at least in DIO rodents, even with long-term weight reduction (149). Unfortunately, most people view their weight loss program as a transient change in their lifestyle and dietary habits or have difficulty in sustaining the changes that they have made to lose the weight (52, 69). If the strategies are not maintained, the biological adaptations become a driving force for weight regain (Fig. 2F).
Two critical points emerge from this graphical description of the interplay between these pressures affecting body weight. Initially, our biology, or this homeostatic system, is actually working for us in an attempt to minimize the impact of obesogenic environmental and behavioral pressures, although with better results in OR than in OP individuals. Second, because of the upward retuning of the homeostatic system, at least for those prone to obesity, metabolic conditions after weight loss may not be the same as they were prior to gaining the weight in the first place. Instead of working in our favor to prevent weight gain, biology becomes one of the driving pressures that underlie weight regain.
Adaptations in Homeostatic Control
A complex picture of the biological system controlling body weight regulation has emerged over the last several decades. In its simplest version, it is dominated by a feedback loop between the brain and periphery. The brain receives signals from the periphery regarding energy stores (long term) and nutrient availability (short term), and on the basis of these integrated signals, adjusts energy balance to meet both the long-term storage and short-term nutrient status objectives of energy homeostasis. This feedback system adapts when energy intake is cognitively (in humans) or forcefully (in animal models) restricted, and the response, in general, promotes a positive energy imbalance, the replenishment of energy stores, and an increase in nutrient availability. Numerous components of the homeostatic system contribute to this normal physiological response. The various components are inherently linked, and the integrated adaptations work in concert. Obesity imposes additional adjustments in energy homeostasis, which make some aspects of this adaptive response distinctly different (138, 251, 252). For this reason, we limit this summary of the homeostatic adaptations to those that have been observed in the overweight or obese humans, as well as in DIO models of the human condition, in response to an energy-restricted, low-fat diet.
Adipose Signals: Low Reserves and an Empty Fuel Tank
Adiposity signals do not remain proportional to fat mass when it is changing.
Two hallmarks of energy-restricted weight loss in obese and overweight individuals are a decline in fasting leptin and insulin (Table 1). Leptin and insulin have been termed “adiposity signals,” because their levels generally reflect fat mass, and they convey this signal of peripheral energy storage to the brain when energy balance is maintained (16, 19). Reduced leptin levels are more intuitive, as leptin is secreted from adipose tissue. Lower insulin levels can be explained by improved insulin sensitivity (109, 124, 142, 158) and a reduced fasting and postprandial response in glucose-dependent insulinotropic polypeptide (121, 238). The low levels of leptin and insulin levels have been traditionally thought to convey a message of “depleted energy” stores to key regulatory areas in the brain (Fig. 3A). As our understanding of these hormones has increased, the role of these hormones in the homeostatic response to weight reduction has become more complex.
Table 1.
Effects of weight loss on adipose signals, appetite sensations, and signals of nutrient availability in obese and overweight humans
| PreMeal/Fasting |
Meal/day Long/Glucose Load |
||||
|---|---|---|---|---|---|
| ⇑ | ⇓ | ⇑ | ⇓ | No effect observed | |
| Adipose signals | |||||
| Insulin | (18, 34, 50, 51, 53, 54, 95, 97, 100, 109, 117, 133, 142, 153, 175, 196, 201, 238) | (42, 48, 171, 188, 193, 238) | (2, 94, 95) | ||
| Leptin | (18, 34, 50, 51, 94, 95, 97, 117, 142, 152, 153, 196, 201, 221, 228) | ||||
| Appetite sensations | |||||
| Hunger* | (4, 40, 53, 59, 61, 63, 84, 95, 238) | (61, 117) | (42, 233) | ||
| Satiety** | (4, 53) | (40, 42, 59, 61, 63, 84, 95, 117, 187, 233, 238) | |||
| Hunger/satiety signals | |||||
| Ghrelin | (94, 95, 163, 164, 254) | (51, 164) | (18, 50, 82, 133, 142, 153) | ||
| PYY | (18, 71, 142, 180) | (71, 233) | (164, 175, 233) | ||
| CCK | (18) | (95, 121) | (42) | (143, 155, 164) | |
| GLP-1 | (18) | (238) | (1, 2) | (129, 175) | |
| Circulating nutrients | |||||
| Glucose | (1, 48, 54, 117, 133, 171, 175, 201, 219, 221, 238) | (1, 42, 48, 133, 153, 166, 171, 188, 219, 238) | (18, 53, 109, 193) | ||
| TG | (34, 38, 48, 53, 95, 100, 171, 181, 219, 221) | (48, 109, 153, 187, 213) | (18, 51, 142) | ||
| FFA | (34, 142, 221, 238) | (1, 42, 48, 109, 204, 238) | (2, 153, 166, 219) | ||
Listed studies reflect observations in overweight and obese humans during weight maintenance after energy restricted weight loss.
Visual Analog Scale, corresponding to hunger, desire to eat, or prospective food consumption.
Visual Analog Scale, corresponding to satiety, feeling of fullness, or satisfaction with appetite.
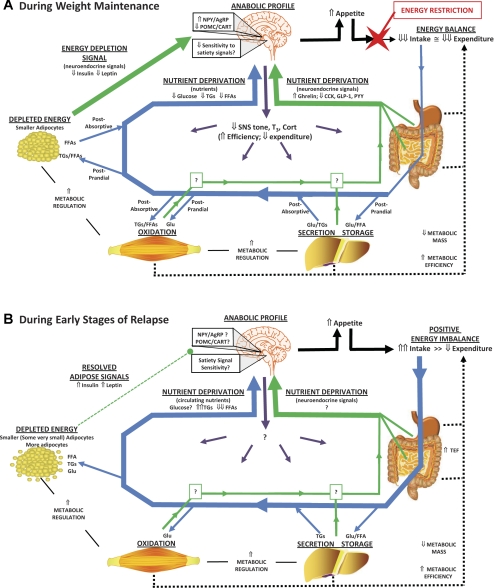
Fig. 3.
Homeostatic adaptations to an energy-restricted, low-fat diet. A: adaptations in the homeostatic system that are observed during weight maintenance, while in energy balance, are summarized. Neuroendocrine signals from the periphery (green arrows) convey a message of energy depletion (low leptin and insulin) and low nutrient availability (favoring signals of hunger over satiety/satiation) to the brain. Trafficking of absorbed nutrients (glucose, Glu; free fatty acids, FFA; triglycerides, TGs) to and from circulation is shown for both postprandial and postabsorptive metabolic states (blue arrows). Enhanced nutrient clearance reduces postprandial excursions in Glu and TGs and potentiates the postprandial suppression of FFAs, which may also convey a signal of nutrient deprivation to the brain. The signals of energy depletion and nutrient deprivation create an “anabolic” neural profile in the hypothalamus and hindbrain, increasing appetite (solid black arrows) and sending efferent signals to enhance metabolic efficiency in peripheral tissues (purple arrows). Both the reduced metabolic mass and enhanced metabolic efficiency reduce expended energy (dotted black lines). A large energy gap is created between appetite and expenditure, and food intake must be cognitively (in humans) or forcefully (in animals) restricted to maintain the reduced weight. B: when overfeeding occurs, a persistent postprandial state is created and the traditional signals of energy depletion resolve. Glu becomes the primary fuel for energy production, and the rate of clearance of all nutrients is maximized. This enhanced rate of nutrient clearance may be potentiated in some cases by the formation of very small adipocytes. Glu and TG levels in circulation become a function of the high rate of absorption from the gut and the high rate of clearance in peripheral tissues, while FFAs levels in circulation become persistently suppressed. Little is known about how the neuroendocrine signals of nutrient status, the neural profile of the hypothalamus and hindbrain, and the efferent signals sent to the periphery change during this dynamic state of weight gain. With more food absorbed and metabolized, the suppressed energy expenditure resolves to some extent because of an increase in thermic effect of food (TEF). However, the energy gap between appetite and expended energy continues until adipose depots achieve a cell size frequency distribution profile that was present prior to weight loss. At present, circulating FFAs represent the only known signal that changes during this dynamic state of weight regain in a manner that would be permissive to continued overfeeding, while having an inverse relationship with the size of adipocytes in peripheral depots.
Recent studies have revealed that leptin, and perhaps insulin, reflects both the amount of adipose tissue and the size of the constituent adipocytes (6). Smaller adipocytes secrete less leptin and result in lower circulating levels for a given fat mass. Smaller adipocytes are also more insulin sensitive (25, 146), which presumably means they require lower circulating levels of insulin to impart the same metabolic control. Energy-restricted weight loss in the obese reduces cell size, not number (91, 148, 151, 160, 184). While total mass declines, the maximal capacity to store energy (the projected mass if all adipocytes were filled to capacity) remains the same. The decline in total fat mass and the reduced percentage of total capacity that is filled would compound the reduction in insulin and leptin. Consistent with this assertion, a number of studies have observed that leptin and insulin are reduced to a greater extent than would be expected for the amount of fat mass that is lost after weight is stabilized (110, 140, 145, 148, 196). The integrated adipose signal conveyed to the brain and peripheral tissues is, therefore, that energy reserves are low and the fuel tank is far from full (Fig. 3A).
To make the role of leptin and insulin more complex, they reflect adipose stores only when subjects are in energy balance. When an imbalance occurs, leptin and insulin reflect the metabolic state (anabolic or catabolic) of adipose tissue, as it deposits or mobilizes energy. When weight is forcefully perturbed by overfeeding, leptin and insulin are predictably elevated as weight is gained (85). However, when again allowed to eat ad libitum, both leptin and insulin resolve much faster than when the excess weight is lost. The same has been observed with energy-restricted weight loss. Leptin is lower during weight loss than it is once weight is stabilized at the reduced weight (196), and a single day of overfeeding leads to the complete resolution of insulin and ∼80% of the reduction in leptin in postobese rats (Fig. 4). Given that it takes several weeks of overfeeding for lost weight to return in this model, leptin and insulin, by themselves, do not appear to sustain the signal of energy depletion as the weight is regained.
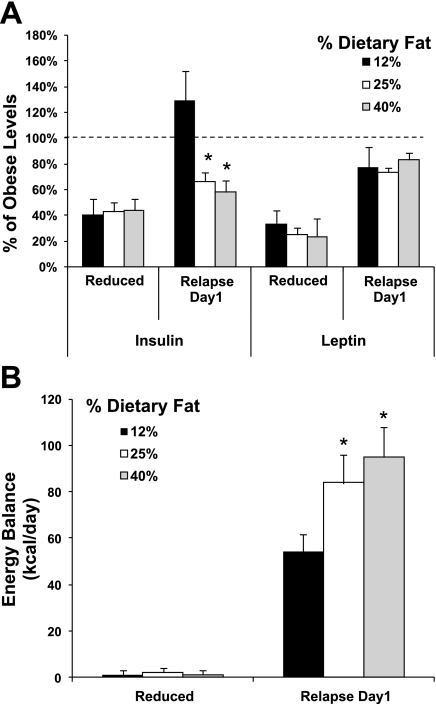
Fig. 4.
Effect of dietary fat on the resolution of insulin and leptin and the energy gap at the maintenance-relapse transition. A: insulin and leptin, expressed as a percentage of obese controls, are shown for energy-restricted, weight-maintained rats before (weight reduced and in energy balance) and after the first day of relapse. Data were drawn from three similar DIO rat weight loss studies of relapse on diets containing 12%, 25%, and 40% kcal fat. B: energy gap at this transition from weight maintenance to relapse is estimated from the positive energy balance on the first day of relapse. [Data were adapted from Am J Physiol Regul Integr Comp Physiol 294: R1117–R1129, 2008 and two similar ongoing studies with higher levels of dietary fat (n = 6–10/group)]. *Significantly different from 12% kcal fat diet, P < 0.001.
These observations, however, should not discount a role for low leptin and insulin in weight regain. Overfeeding with diets higher in fat significantly blunts the resolution of insulin (Fig. 4A), even though these animals experience an energy imbalance 50 to 100% higher (Fig. 4B). Other factors may also be potentiating the conveyance or reception of their energy depletion signal as relapse progresses. Furthermore, postobese humans rarely gorge themselves continually back to obesity, like our animal models. Intermittent periods of weight stability, in between bouts of overfeeding, may allow these signals to normalize and reassert their influence as descriptors of energy stores under energy balance conditions. Therefore, the decline of these adipose signals represent significant adaptations that contribute to weight regain (Fig. 3A), but their impact during sustained periods of overfeeding is somewhat in question (Fig. 3B). Later in this review, we present a novel alternative for how this energy depletion signal is conveyed during the dynamic metabolic state of weight regain.
Drive to Regain in the Brain
The two areas of the brain that are traditionally considered the homeostatic regions controlling weight are the hypothalamus (22, 113) and the hindbrain (249). Here, we do not attempt to review in detail every region of these and other areas of the brain that are involved in weight regulation nor describe every aspect of the normal response to calorie restriction. Rather, we summarize aspects of the adaptive response and their consequences that have been confirmed or observed to be different in overweight and obese subjects in response to an energy-restricted, low-fat diet.
The hypothalamus: converging peripheral signals of energy depletion.
The arcuate nucleus (ARC) of the hypothalamus undergoes wholesale changes in neuronal activity and neuropeptide expression in response to an energy deficit (202), integrating numerous signals from the periphery as it develops the overall picture of energy depletion. Key aspects of this general response persist after long-term weight reduction from an obese state, including increased expression of ARC neuropeptide Y (NPY) (24, 137, 168, 248, 251) and Agouti-related peptide (AgRP) (168, 251), as well as decreased expression of proopiomelanocortin (POMC) (137). Concomitant elevations in the firing of NPY/AgRP neurons and reductions in the firing rate of POMC neurons would be expected with this gene expression profile. These changes are the hypothalamic hallmark of an “anabolic” state, which would lead to a positive energy imbalance and weight gain (Fig. 3A).
The ARC projects this signal of energy depletion to other areas of the hypothalamus. In DIO models, first-order projections to the paraventricular nucleus (PVN) reduce the expression of corticotrophin-releasing hormone (137). Other projections impart modest changes in neural peptide profiles in the lateral hypothalamus (168, 252), which interfaces with the cortico-limbic system. Still other projections extend into the ventromedial hypothalamus (VMH), known for its high density of nutrient (glucose, fatty acids) sensing neurons (218). DIO rodents exhibit fewer glucose-sensing neurons in the VMH (218) and lower VMH brain-derived neurotrophic factor expression (253). Neither VMH-associated abnormality resolves after weight loss in DIO models, and their influence on weight gain may reemerge after weight loss, as other homeostatic circuits adapt to weight loss (135, 253). While the interaction between the ARC and the dorsomedial nucleus (DMN) of the hypothalamus is not clearly understood (24, 123), DMN NPY expression is higher (137) and DMN cocaine- and amphetamine-regulated transcript expression is lower (252), after weight loss from obesity. Other components of homeostatic body weight regulation in the hypothalamus may indeed be changed after energy-restricted weight loss, but they have yet to be reported in DIO models of obesity.
While confirmation of these hypothalamic adaptive responses in obese humans is difficult to come by, a plethora of indirect and genetic evidence would suggest that the hypothalamus is inherently involved in the human response to energy deficit (202). In addition, functional magnetic resonance imaging (fMRI) studies have reported altered neural activity in the hypothalamus in postobese humans, which is normalized after 3 wk of leptin replacement therapy (194). For these reasons, the hypothalamus remains a critical node for integrating and processing the homeostatic signal of peripheral energy depletion (Fig. 3A). However, we have yet to understand whether these same adaptive responses are sustained during the dynamic state of weight regain (Fig. 3B).
The hindbrain: reduced sensitivity to satiety signals.
The energy depletion signal from the hypothalamus likely translates into a reduced sensitivity to satiety signals, increased meal size, and higher food intake (Fig. 3A). The nucleus of the solitary tract and area postrema are among the areas in the hindbrain that receive input via gut-derived CCK and vagal afferent signals. Monogenic obese rats that lack the leptin receptor are less responsive to CCK-induced meal size reduction, and the effects of CCK are partially restored when hypothalamic leptin receptors are generated via adenoviral gene delivery (167). Prior to the development of obesity, the hindbrain of OP rats may be less sensitive to peripheral satiety signals (39, 223). Whether this characteristic reemerges after weight loss to facilitate weight regain needs to be studied. Evidence from human fMRI studies does show weight-reduced subjects have altered neural activity in the brain stem (197), but, unlike other neural responses to weight reduction, this does not normalize with leptin therapy. Taken together, the sensitivity of the brain to gut-derived signals appears to be depressed in the obese (31), but the improved sensitivity after weight loss and during weight regain needs to be confirmed in DIO models of obesity (Fig. 3, A and B).
Integration of homeostatic and nonhomeostatic adaptations.
A host of neural projections, both to and from the hypothalamus, establishes a complex network of cross talk that integrates the homeostatic system with other regions of the brain that are more traditionally considered “nonhomeostatic” (22). The effect of weight loss on these neural networks is beginning to emerge in DIO models (253). In these hedonic-cognitive areas, the reduced-obese human exhibits neural imaging responses to food-related stimuli that would favor increased motivation and drive to eat (46, 197), and many of these responses resolve with leptin therapy (197). However, it is important to recognize that communication between the hypothalamus and these nonhomeostatic areas occurs in both directions. These areas may receive peripheral signals of energy depletion or impose hedonic control to affect how homeostatic centers receive and integrate peripheral signals of energy depletion and nutrient status. Given the importance of cognitive and reward-based eating behaviors in humans (26, 47, 134), their role in the adaptive response to weight loss will continue to be studied.
Neuroendocrine signals: enhancing efficiency of fuel utilization and storage.
The energy depletion signal, via the PVN in some cases, results in a number of neuroendocrine adaptations that ultimately target peripheral tissues (Fig. 3A). Energy-restricted weight loss from obesity is accompanied by a reduced sympathetic (SNS) tone (7, 110, 138, 191, 193, 221), reduced thyroid hormone levels (127, 193, 205, 228), and increased activity of the hypothalamic-pituitary-adrenal (HPA) axis (59, 157, 227, 228). In contrast to the effects on SNS, the effects on thyroid hormones and the HPA axis are observed less consistently and/or are more transiently tied to the early stages of weight loss (103, 228, 245). Collectively, the neuroendocrine changes that occur with weight loss target peripheral tissues that use and store energy, increasing efficiency to conserve energy and readying them for energy repletion (Fig. 3A). In postobese humans, leptin therapy normalizes some of these neuroendocrine responses to weight loss (191). However, more studies are needed to understand the impact of these signals on energy deposition and storage during weight regain (Fig. 3B).
The Energy Gap: Promoting a Positive Energy Imbalance
An elevated appetite.
One of the primary outcomes from the neural adaptations to energy-restricted weight loss in obese individuals is an elevated appetite (Fig. 3, A and B). This is more often detected in premeal or postabsorptive appetite sensations related to hunger, desire to eat, and prospective food consumption, but it has also been observed in postprandial and day-long assessments of these measures (Table 1). Measures of hunger predict future weight regain in weight-reduced individuals (177). Surprisingly, an effect of energy-restricted weight loss on measures of satiety and satiation has rarely been observed (Table 1). This presents, to some extent, a disconnect between basic and clinical scientific communities. Studies in genetically manipulated animals have emphasized the importance of satiety and satiation in energy homeostasis, but hunger and meal initiation appear to be more important in humans and often involve nonhomeostatic cues or motivations. Some of this discrepancy may be due to the difficulty in making a clear distinction between hunger and satiety in laboratory animals. In contrast to the food questionnaires used to gauge hunger in humans prior to a meal, animal studies often simply measure how much food is eaten when food is made available. The overeating that occurs may result from increased hunger and/or a delay of (or a modification of) satiation. However, the more substantial difference between human and animal studies are the nonbiological pressures affecting body weight (Fig. 1), which can work with or against the biologically driven appetite (Fig. 2).
Humans are motivated by psychosocial pressures, with varying degrees of success, to keep weight off. The characteristics of cognitive restraint, disinhibition, and susceptibility to hunger, can affect energy intake (186) and measures of hunger and satiety (89). Those who actively seek out weight loss, and thus more likely to enter a weight loss study, exhibit more restrained eating behavior (27). Moreover, individuals who do lose weight exhibit increased cognitive restraint, lower disinhibition, and lower susceptibility to hunger (2, 40, 42, 76, 114, 179, 247). These cognition-driven eating behaviors undoubtedly lead to variability in the assessment of biology's overall impact and may explain why humans often exhibit different patterns of hyperphagia during postobese weight regain (240). It would be a mistake, however, to discount biology's influence because the presence of these psychosocial pressures makes it difficult to measure.
The picture of biology is much clearer in postobese DIO rodents, which are not subject to the same complex psychosocial influences and environmental variability. Driven mostly by their biological urges, these models consistently show that appetite increases such that they want to eat well in excess of what they were eating before they lost weight (17, 65, 104, 137–139, 148, 151), an effect on appetite that persists until the lost weight is regained. An increase in meal size, rather than number, is involved in the development of the DIO phenotype (73) and in the hyperphagia following energy restriction in normal weight animals (55). We suspect the same will be reported in the DIO during weight regain.
Suppressed energy expenditure.
The significance of the adaptive response in energy expenditure has been less appreciated and somewhat confounded by the lack of consensus on how to normalize and interpret data. The confounding issues include: 1) the methods used to adjust for the loss of tissue that contributes to basal energy requirements; and 2) what body compartments (fat mass, lean mass, both) should be used as the estimated amount of tissue that contributes to basal energy requirements. The recent emergence in preclinical research communities of regression analysis for normalization (5, 33, 112, 149) may help to resolve the first issue. Although this approach has been the general standard in clinical research for some time, its application in animal research has been relatively rare. On the other hand, clinical researchers continue to include fat mass in their regression models to correct for the decline in basal tissue energy requirements when studying metabolic efficiency. The recent work of Kaiyala et al. (112) provides strong evidence that the primary impact of fat mass on energy expenditure is not in its contribution to the basal energy requirements for tissue maintenance, but instead regulatory in nature, as a node in the homeostatic feedback loop controlling body weight. These recent developments should bring both research communities together and move this field toward a unified gold standard for the presentation and interpretation of metabolic data (147).
What is often overlooked because of the aforementioned confusion is the most fundamental adaptation and its implications: total energy expenditure declines (Fig. 3, A and B). This adaptation to weight loss has been shown in numerous studies of overweight and obese humans (8, 60, 62, 131, 132, 159, 189, 192, 196, 242–244) and in polygenic models of obesity (45, 65–67, 148–151, 199), and every component of energy expenditure is affected to some extent (Fig. 5). The mechanisms are linked to both the loss of body mass and enhanced metabolic efficiency.
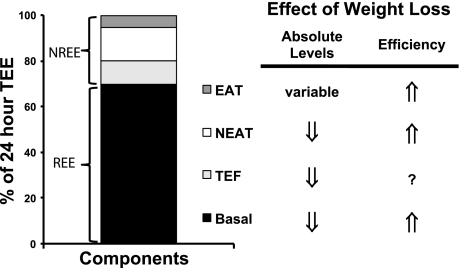
Fig. 5.
Impact of weight loss on the components of total energy expenditure (TEE). Energy-restricted weight loss impacts every component of energy expenditure. The absolute levels of resting energy expenditure (REE) decline from a loss of metabolic mass and enhanced metabolic efficiency. Nonresting energy expenditure (NREE) declines, unless the level of physical activity and its associated exercise activity thermogenesis (EAT) are substantially increased. This increase must overcome the decline in the TEF that results from lower energy consumption and a decline in nonexercise activity thermogenesis (NEAT) related to loss in overall mass. Increased energetic efficiency of EAT, NEAT, and potentially TEF contribute to the overall decline in NREE.
The lower body mass contributes to the decline in expended energy in three ways. First, there is often less lean mass contributing to the basal tissue maintenance costs of the organism. This manifests in a reduced basal metabolic rate (resting energy expenditure, REE), reported for both obese humans (8, 60, 70, 77, 78, 80, 102, 132, 159, 189, 192, 196, 234, 245) and obese animals (45, 66, 149–151) after energy-restricted weight loss. Second, less total mass must be moved during physical activity, so the same activity will be less energetically expensive. If activity levels are the same, this is observed as a decline in the nonresting component of energy expenditure (NREE), which has also been consistently reported after weight loss (66, 132, 191, 192, 196, 242–244). Finally, the loss in fat mass reduces energy expenditure, primarily by altering metabolic efficiency via its role in homeostatic regulation (i.e., reduced leptin, insulin) (112).
This regulatory effect on efficiency affects both basal metabolism and the energy expended during physical activity. The majority of studies show that a portion of the decline in REE remains significant after adjusting for the loss in lean mass (8, 45, 60, 65, 66, 70, 102, 132, 149, 150, 234). The enhanced work efficiency during physical activity impacts both nonexercise and exercise activity thermogenesis (66, 198), at least with activity at lower levels of intensity. These effects ultimately reduce NREE, when activity levels are the same. Leptin therapy reverses these adaptive responses in postobese humans (191, 195).
A more fundamental biological adaptation may occur to reduce NREE by simply reducing the amount of activity. In stark contrast to most rodents (202), DIO rats that have lost weight lower their volitional wheel running (137) and exhibit reduced compliance to daily treadmill exercise during weight regain (151). These observations suggest a reduced biological drive to be physically active, which may be rooted in homeostatic neural pathways that involve the orexin system (126). These neuronal pathways are altered in OP rats, and the lower intrinsic activity levels are thought to contribute to their DIO phenotype when fed a high-fat diet. It is unclear how the orexin neurons and their targets are altered in DIO models after weight loss, but we suspect that at least the preexisting defects are likely to reemerge. Some clinical studies have shown a decline in activity during weight loss (241, 242). However, physical activity and the desire to be physically active, like appetite sensations, are difficult to directly measure in humans. Similar to nonhomeostatic influences of food intake, humans are motivated to increase physical activity in their cognitive pursuit of a lower weight and better health. Success is variable, however, as compliance to exercise prescriptions in combination with dieting is notoriously poor (36). Additional studies are needed to clarify whether reduced activity, or reduced motivation to be physically active, in postobese individuals is indeed part of the biological response to weight loss.
Finally, NREE also declines because less food is consumed. Less food requires less energy for ingestion, absorption, metabolism, and storage, and the consequence is a lower thermic effect of food after energy-restricted weight loss (159, 243). Beyond this predictable change related to the amount of food, however, ingested food can also induce thermogenesis by increasing sympathetic tone (224). Some studies have reported that this facultative thermogenesis, particularly with respect to glucose-induced thermogenesis, declines after energy-restricted weight loss (86, 235), but other studies show the effect is not sustained with long-term weight reduction (132, 235). Regardless, the adaptations, if any, that do persist may be inherently linked to the same mechanisms that underlie enhanced metabolic efficiency at rest and during exercise.
In summary, the reduction in mass and ingested energy, the enhanced metabolic efficiency, and any decline in activity levels, all contribute to lower expended energy (Fig. 3A). Studies in rodents have shown that this hypometabolic state persists during weight regain (Fig. 3B), normalizing only after the lost weight returns or, in some cases, the original weight is surpassed (67, 137, 148). Because much of this effect can be explained by reduced mass, the relevance of this biological response in energy expenditure is often discounted. However, the brain does not reset the appetite to match the lower-energy requirements of the remaining tissue. The lower-energy requirements, whether from the loss of lean tissue or from increased efficiency, become a quantifiable burden that must be overcome in the pursuit to maintain the reduced weight. This may be why the decline in energy expenditure, similar to the elevated hunger, is predictive of weight regain (156).
The energy gap: biology's drive to go off a diet.
Because both sides of the energy balance equation are affected after weight loss, the biological pressure to gain weight is a consequence of both increased appetite and suppressed energy expenditure. We have termed this difference between appetite and expenditure requirements the “energy gap” (108, 148, 149). In humans, it is difficult to quantitatively measure appetite in terms of energy equivalents, so accurately estimating the energy gap becomes challenging. In lieu of this difficulty, the energy gap has also been discussed as the difference between pre-weight loss energy requirements and post-weight loss energy requirements (101). We would suggest this is a conservative estimate of biology's impact, as animal studies clearly show that the energetic equivalent of “appetite” more than surpasses the energy requirements of the obese state. In animal models, both components of this energy gap can be directly measured by assessing energy balance in the weight-reduced state and during a subsequent day of ad libitum feeding (108, 151).
During weight maintenance after weight loss, this energy gap reflects the magnitude of the daily burden that thwarts cognitive efforts to maintain the reduced weight. Our studies indicate that this energy gap at the maintenance-relapse transition is influenced by diet composition (Fig. 4B), by the length of time in weight maintenance after weight loss (149), and by physical activity levels (151). The relative contribution of the adaptations in appetite and expenditure can vary between and even within animal models (92, 93). Regardless of which side of the energy balance equation is most affected, the energy gap imparts a substantial pressure to eat in excess of the energy requirements. Obese humans may exhibit this same variability (240), which could explain the common observation of “responders” and “nonresponders” in clinical studies focused only on one side of the energy balance equation.
Finally, studies in obese humans and rodents suggest that weight regain reflects a first-order growth curve (3, 148, 151). The rate of gain, and, therefore, the energy gap and the energy imbalance it promotes, diminishes during the relapse to obesity as a function of this first-order relationship. In addition to diet and physical activity, both the amount of weight lost and the time in weight maintenance may influence this relationship. The magnitude of the energy gap is greatest at the nadir weight after weight loss (108, 148, 151). Likewise, this energy gap does not dissipate with time in weight maintenance. Rather, studies in DIO models indicate that the magnitude of the energy gap gradually increases the longer they maintain their reduced weight with an energy-restricted diet (149). The implications from these observations are that the biological pressures may strengthen with time and the amount of lost weight, gradually increasing their perceived influence.
The Gut: Sensing the Prandial State
The gut plays a critical role in the homeostatic body weight regulation, as it is the origin of a number of neural, nutrient, and hormonal responses to ingested energy. These gut-derived signals of the nutrient status are reviewed in more detail elsewhere (74, 115, 249). In general, if these signals change in the obese humans in response to energy-restricted weight loss, a collective signal favoring hunger over satiety/satiation is observed (Table 1). However, detection of this adaptive response is much less consistent than that of circulating adipose signals and nutrients, as they may be dependent on the presence of a negative energy balance (2, 30, 42, 71, 133), the amount of weight loss (71), the amount of physical activity (121), the specific derivative of the hormone measured [peptide YY(PYY) vs. PYY3–36; total vs. acetylated ghrelin] (18) or the type of macronutrient restricted in the diet (71, 95). Even under the same conditions of weight loss and maintenance, there is a wide variability in individual responses with respect to these adaptations in hunger and satiety signals (18). As such, these adaptations may be more or less influential, depending upon the genetic disposition and diet composition.
When they do persist into weight maintenance, these altered signals could potentiate the effects of a brain that may already be more sensitive to hunger signals and less sensitive to satiety signals (Fig. 3A). They may also act in the periphery to indirectly alter food intake, insulin secretion, gastric emptying, fuel utilization, and energy expenditure (14, 203, 249, 255). Less is known about vagal afferent signals and a large number of other humoral factors known to regulate appetite and energy balance (88, 178), as they have yet to be studied in the obese after weight loss or during weight regain (Fig. 3, A and B).
Changing gut microbiota-emerging implications for nutrient absorption.
The effect of obesity on gastric emptying is equivocal (98), but some studies show that emptying of solids may be slowed after weight loss (155, 229, 237). Other studies show little effect of weight loss (105). Regardless, the effect in humans appears to be transient, such that it does not persist with long-term weight reduction.
More attention in recent years has been directed to gut microbiota (231). Energy-restricted weight loss results in an increase in the proportion of Bacteroidetes/Firmicutes bacteria in some studies (141, 170) and a diet-dependent reduction of butyrate, producing Firmicutes bacteria in others (68). The implications and relevance of these adaptive responses in gut microbiota are as yet unclear, but there is evidence that microbiome alterations like these can affect energy extraction, metabolism, and adiposity (10, 11, 169). It remains to be seen whether the altered gut microflora after energy-restricted weight loss contributes weight regain in obese humans, or is simply coincidental to changes in diet.
Enhanced Metabolic Flexibility: Improved Nutrient Clearance
Improved metabolic regulation.
Metabolic inflexibility refers to an organismal impairment in regulation, which manifests as the inability to appropriately shift fuel preference in the face of metabolic challenges, like fasting, exercise, a meal, or overfeeding (119, 120, 220). The inflexible state of metabolism results from a discordant attempt of numerous peripheral tissues to respond to changing fuel needs and nutrient availability, yielding little or no discernable response. Metabolic inflexibility is a common characteristic of obesity (119, 220), and energy-restricted weight loss reverses many of its associated impairments in metabolic regulation (48, 81, 158, 188, 219). Because enhanced metabolic flexibility improves the metabolic response to ingested energy, it alters the peripheral signals of nutrient status that are sent to the brain during weight maintenance and during weight regain (Fig. 3, A and B). Because lean subjects do not generally exhibit this global impairment in metabolic regulation, the extent to which an improvement in whole body responses to metabolic challenges, like overfeeding, contributes to the general response to calorie restriction is unclear. For the obese, enhanced metabolic flexibility, like other nodes in homeostatic regulation, may sustain the energy gap by minimizing the negative feedback that these nutrients and their surrogate signals have.
The normal response to a meal or overfeeding, which includes suppressing fat oxidation and increasing carbohydrate oxidation when carbohydrates are sufficiently high in the diet, reemerges after weight loss (12, 23, 108, 149, 150, 187, 211). Dietary fat is preferentially trafficked to adipose and hepatic triglyceride stores rather than being used for energy needs. This enhanced metabolic response to ingested nutrients occurs, in part, from an increase in whole body insulin sensitivity (109, 124, 142, 148, 158). While beneficial for metabolic health, the enhanced insulin sensitivity primes the body for rapid, energetically efficient weight gain during chronic overfeeding (148, 230). As long as the weight-reduced subject restricts intake and stays in energy balance, there is little impact of enhanced metabolic flexibility on 24-h substrate oxidation (32, 150, 151). However, the rapidity and efficiency of nutrient clearance could reasonably hasten the transition to a postabsorptive state, the reemergence of hunger pains, and the persistence of the energy gap.
When overfeeding occurs, the improved response to ingested energy transitions into a sustained suppression of fat oxidation and the trafficking of dietary fat to adipose tissue (108), ensuring the most efficient repletion of adipose stores (75, 210). The energetic cost of depositing excess dietary fat into triglyceride depots is much less than an equivalent carbohydrate or protein (<2% vs. ∼25% of the energy excess) (209). For protein and carbohydrate, additional energy is required to pay for converting these nutrients into fat via de novo lipogenesis. In DIO models, the preferential deposition of dietary fat is observed even in minor bouts of overfeeding, ensuring weight gain occurs at the highest rate and with the greatest energetic efficiency (108). This increased rate and efficiency of weight gain may explain, in part, why weight regain is predicted by improved insulin sensitivity (250), an enhanced response to a glucose load (28), and the preferential use of carbohydrate for energy production (80).
Nutrient availability declines.
Improved insulin sensitivity is often accompanied by lower fasting levels of glucose, free fatty acids (FFAs), and triglycerides (TGs) in circulation (Table 1), but more consistently yields reduced postprandial excursions of glucose and TGs with potentiated postprandial reductions in FFAs (Table 1). These effects are often reflected in lower fasting levels of these nutrients as well (Table 1). This wholesale, consistent change in circulating nutrients undoubtedly imparts some homeostatic influence on the signals of nutrient status (Fig. 3A). Levels of glucose are detected by nutrient-sensing systems in both the periphery (15, 41, 56, 130, 212) and brain (111, 130), with consequences to energy balance and fuel utilization in the periphery. TGs may even be sensed via their putative effects on leptin and insulin transport across the blood-brain barrier (13, 108, 232). However, FFAs present the most intriguing of these nutrients, as their impact on energy balance regulation may be most relevant during the dynamic metabolic state of overfeeding.
FFAs are sensed, just like glucose, in central and peripheral nutrient sensing systems. Dietary fat can impact hypothalamic FFA levels (185), and FFAs reduce subsequent food intake when infused into the gut (79, 144), into the circulation (236), or directly into the brain (44, 173). Unlike glucose and TGs, FFAs in postobese subjects decline postprandially (Table 1) and during weight regain (108). With every meal, every bout of overfeeding, and sustained periods of a positive energy balance, the suppressive effects of FFAs on the energy gap would be minimized. As such, FFAs represent one signal of nutrient status after energy-restricted weight loss that could sustain a message of nutrient deprivation during the dynamic metabolic state of weight regain (Fig. 3B). Later in this review, we connect this aspect of FFA metabolism to peripheral energy repletion and the energy gap.
Liver: Restored Function of a Transient Energy Depot
The nutrients and hormones discussed earlier in this review are known to act directly on the liver (43), and their changes after weight loss have dramatic effects on hepatic metabolism. Relief from the persistent nutrient overload that accompanies the development and progression of obesity could alone improve metabolic regulation in the liver. However, the decline in leptin and insulin is also likely involved, as they support the expression of catabolic and anabolic genes, respectively, in this tissue (29). In addition, an emerging body of evidence suggests that hepatic metabolism is also subject to hypothalamic regulation (20, 83, 165, 182, 183), which needs to be studied in obesity models after energy-restricted weight loss. While our focus is on the overweight and obese, the majority of the adaptations in the liver can be extended to energy restriction in general. As obese individuals are less glucose tolerant with a higher incidence of steatosis than the lean, these adaptive responses may appear to be more dramatic in the overweight and obese.
The nutrient, hormonal, and potentially neural changes that occur with weight loss from an obese state result in a global reduction in hepatic gene expression (100), and this is accompanied by reduced markers of protein synthesis (214). These adaptations are indicative of a gross reduction of this tissue's energy requirements, undoubtedly contributing to the reduced basal energy requirements measured over the whole body (Fig. 3A). The liver's regulation of both carbohydrate and lipid metabolism improves within as little as 48 h of an energy deficit (124), and this improvement persists into weight maintenance (109, 124). Hepatic lipid declines (38, 124, 219), splanchnic glucose uptake increases (206), and glucose production is reduced (124). Secretion of very low density lipoproteins, ApoB-100 particles in circulation, and hepatic-triglyceride lipase, declines (38, 116). In postobese rodents, the suppression of dietary fat oxidation during the dark cycle (when they feed) is reversed later in the light cycle when their energy balance provision of food is gone (108). The return of this diurnal cycle of anabolic and catabolic states reflects the restored function of a transient depot for ingested energy, which can facilitate the rapid clearance of nutrients during bouts of overfeeding (Fig. 3B).
Sustained periods of overfeeding lead to the induction of genes associated with de novo lipogenesis, similar to what is observed in the “catch-up” fat phenomenon in younger animals (49). In our postobese rats, de novo lipogenesis is readily apparent within 12 h of overfeeding, and a substantial retention of this newly formed fat is observed in the liver after 24 h (108). Studies in humans suggest that when hepatic regulation is intact, as it is after weight loss, chronic overfeeding of a low-fat, high-carbohydrate diet leads to the trafficking of glucose to fat depots rather than glycogen pools (161, 162). This is consistent with what is observed in postobese rats during the early stages of weight regain (108). Once converted to fat, this energy cannot be remobilized to support glucohomeostatic objectives. The implications are that the transition to the postabsorptive state would be hastened and the peripheral signal of low nutrient availability promoting further overfeeding would be sent sooner.
Skeletal Muscle: Reduced Energy Requirements and A Preference for Carbohydrate
The same neural, endocrine, and nutrient signals affecting the liver also reduce the energy requirements of skeletal muscle, which has a more substantial impact on daily expended energy because of its overall mass. This increased metabolic efficiency is a general characteristic of the adaptive response to energy restriction and occurs to some extent in all animals, regardless of their adiposity level. What may be different for the obese is the impact on fuel utilization. Like hepatic de novo lipogenesis, adaptations in skeletal muscle facilitate the clearance and dissipation of excess carbohydrate, as glucose becomes the preferred fuel under postprandial conditions (Fig. 3A). Metabolic regulation improves in skeletal muscle, as insulin's stimulation of glucose uptake and its suppression of fat oxidation are enhanced (48, 118, 207, 226). As lean subjects generally have muscles that are more insulin sensitive and already exhibit this preferential use of fuels in response to overfeeding, the shift in fuel utilization and its impact on nutrient clearance may be less apparent. For the obese, the improvement in metabolic regulation could have consequences during maintenance and regain. When energy balance is maintained by cognitive (humans) or forced (animals) restriction, enhanced insulin sensitivity has little effect on 24-h substrate balance or weight gain (32), but the increased clearance rate of circulating nutrients and lower nutrient availability could help create the large energy gap between appetite and expenditure requirements during weight maintenance (Fig. 3A). When overfeeding occurs, more discernable effects on energy balance have been observed (108, 151), as the fuel preference of this tissue ensures that carbohydrate loads are rapidly dissipated through oxidation, and excess nutrients are stored in an energetically efficient manner (Fig. 3B).
While the improvement in insulin sensitivity in muscle is responsible for this postprandial fuel preference, the critical energy sensor in muscle, AMPK (200), also becomes more responsive. Its regulation by leptin, insulin, and sympathetic neural efferents is impaired in DIO models of obesity (154), and some aspects of AMPK regulation appear to resolve after energy-restricted weight loss (81, 108). This fuel preference may be exacerbated by an inherent impairment in the capacity to oxidize fat, which underlies the genetic predisposition for obesity (106, 107). Unlike lean subjects, energy-restricted weight loss in obese subjects fails to increase skeletal muscle oxidative capacity (215, 225, 226) unless the energy-restricted diet is accompanied by regular exercise (96, 207, 208). Mitochondrial respiratory capacity declines (188), glycolytic capacity declines (87, 215), and the expression of enzymes associated with β-oxidation and mitochondrial enzyme activities remain low and, if anything, decline (62, 188, 215, 225). Collectively, these adaptations in muscle underlie the reduced energy requirements, the increased metabolic efficiency, and the enhanced muscle work efficiency observed with energy-restricted weight loss in postobese subjects (192, 198) (Fig. 3A). These adaptations also limit the body's capacity to dissipate excess fat when overfeeding occurs (Fig. 3B).
Adipose Tissue: an Expanding, Empty Fuel Tank, Primed for Filling
Like the liver, adipose tissue experiences a global downregulation of metabolic gene expression in obese subjects in response to energy-restricted weight loss (34). Unlike the liver, much of this adaptation is reversed at the transition to weight maintenance, favoring a gene expression profile that would enhance energy conservation during weight maintenance and the repletion of energy stores during meals or periods of chronic overfeeding (21, 34, 90, 122, 125, 222, 239). Markers of oxidative stress and inflammatory cytokines, which are also known to suppress appetite and increase expenditure, decline (104, 122, 176). The impaired induction of lipogenesis by insulin, glucose, and feeding associated with obesity (57, 58, 64, 162) resolves after energy-restricted weight loss (72, 108, 122, 172, 204). The enhanced metabolic response to ingested energy enhances nutrient clearance during weight maintenance (Fig. 3A) and during sustained periods of overfeeding (Fig. 3B).
The reduction in average adipocyte size rather than a decline in cell number (91, 148, 151, 160, 184) likely contributes to the improved metabolic regulation in this tissue. Total capacity of the tissue to store fat remains the same, but stored energy falls well below capacity (Fig. 6A). Regardless of overall adiposity, smaller adipocytes, compared with larger adipocytes, are more sensitive to the antilipolytic effects of insulin, exhibit a lower basal and catecholamine-induced lipolysis, have a lower rate of turnover of the stored lipid, and express genes favoring energy storage (25, 146, 222). Reducing the size of adipocytes with energy-restricted weight loss primes them to take up and store excess energy when overfeeding occurs. This would presumably contribute to the metabolic drive to regain weight for both lean and obese subjects.
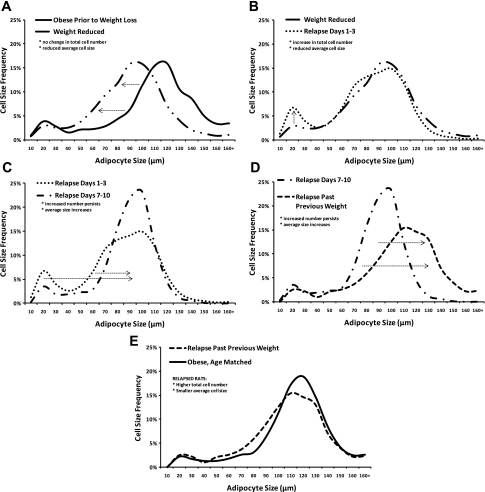
Fig. 6.
The change in adipocyte cell size frequency distribution with weight loss and weight regain. Data from our published weight regain studies in DIO rats (108, 148, 151), as well as ongoing studies, were examined in a combined analysis of retroperitoneal adipocyte cellularity characteristics. In all, the analysis included 21,710 adipocytes from 115 animals at the specified stages of the weight regain process. Cell size frequency distributions, with the effect on average cell size and total number of cells in the depot, are shown for the sequential steps of loss and regain: weight loss and long term (>8 wk) maintenance (A), after the first few days of weight regain (B), after relapse continues into the second week of weight regain (C), and after surpassing their preweight loss weight (D). E: when relapse animals are compared again to obese rats that never lost weight, they have more cells and still have a smaller average size. The profile shift at each stage of weight loss and regain, indicated with dotted arrows, is statistically significant (χ2 analysis, P < 0.05).
The impact on nutrient clearance may be further exacerbated by the formation of new adipocytes (Fig. 6B). Our studies of weight regain in DIO rats that are prone to obesity revealed the emergence of a population of very small (<20 μm) adipocytes during the early stages of weight regain. Their appearance was accompanied by an increase in total number of adipocytes in the depot (108). As weight gain proceeded, preferential hypertrophy of these small cells was observed, while the higher cell number persisted (Fig. 6C). By 10–14 days of relapse, the small cells become indistinguishable from the preexisting adipocytes in the fat pad. As the relapse to obesity continues, hypertrophy occurs more broadly across the entire depot (Fig. 6D), and the increase in cell number continues to persist. Most animals in this DIO model gain more weight than they originally lose before the rate of gain normalizes (148, 151). Yet, after surpassing their previous weight, these relapsed animals still have smaller adipocytes on average and more adipocytes per fat pad than an obese rat that had never lost or regained the weight (Fig. 6E). While substantiating the temporal changes in cell size frequency distribution and total cell number in humans presents a substantial challenge, a hypercellularity phenomenon with similar characteristics has been reported in postobese humans (145). Even so, this relapse-induced hyperplasia of adipose tissue, if it does occur, is likely limited to individuals who have a genetic predisposition for obesity. We have yet to observe an increase in cell number in DR rats or in DIO mice, which tend to relapse to their previous weight. If this does occur in some humans, particularly those who are obese or are prone to obesity, the consequences lie not only in facilitating rapid, energetically efficient regain early in relapse (Fig. 3B), but also in permanently expanding the total storage capacity of the depot.
An Integrated Picture of the Biological Drive to Regain Weight
No single adaptation that we have discussed provides a stand-alone explanation for biological drive to regain weight after weight loss. These adaptations are interdependent and integrated, establishing a system filled with redundancy. Comparing Fig. 3, A and B, it is clear that we have a better understanding of the homeostatic adaptations that are observable during weight maintenance after weight loss. Considerably less is known about the signals that sustain the energy gap during dynamic metabolic states when the well-known adipose signals, leptin and insulin, do not reflect levels of stored energy in the periphery. From the information presented in this review, we have developed one model of how this message is sustained during the relapse process.
The nutrient clearance model for the dynamic metabolic state of weight regain.
The model we propose suggests that the energy-depletion signal from adipose tissue is inherently linked to the cellularity characteristics of adipocytes in the body and is conveyed to the brain by the levels of circulating nutrients (or their neuroendocrine surrogate signals) during postprandial, anabolic conditions (Fig. 7). Energy-depleted adipose tissues have smaller adipocytes with an exceptionally high capacity to clear ingested energy from circulation (Fig. 7, A and B). This capacity would be further enhanced if very small, new fat cells appear at the maintenance/relapse transition (Fig. 7C). The enhanced clearance rate of adipose tissues would attenuate postprandial glucose excursions and potentiate the postprandial suppression of circulating FFAs. During sustained periods of overfeeding, the link between adipose-specific nutrient clearance and circulating levels would become stronger, as storage depots in other tissues (liver, muscle, etc.) fill to capacity. The blunted glucose excursions and suppressed FFAs levels during this sustained, anabolic state may then be sensed by the brain directly, via hypothalamic nutrient sensing neurons, or indirectly, via neuroendocrine signals reflecting nutrient status.
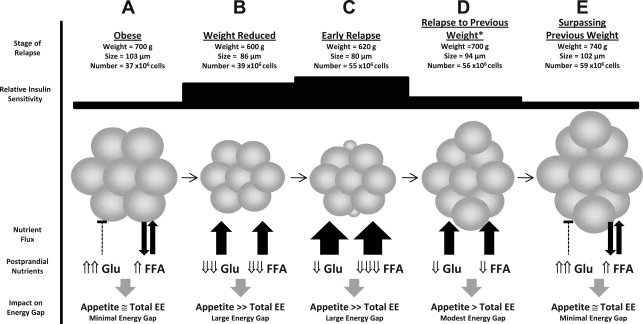
Fig. 7.
Model linking adipocyte cellularity and peripheral nutrient clearance to the energy gap during dynamic weight regain. For reference, weight and cellularity characteristics from the studies presented in Fig. 6 are shown for each stage of weight loss and regain (A–C, E), while hypothetical data for the relapse to the preweight loss weight is extrapolated (D). The metabolic state during weight regain reflects sustained postprandial conditions. The proposed model links relative whole body insulin sensitivity, adipocyte cellularity characteristics, the capacity of adipose tissue to clear excess nutrients, and postprandial nutrient levels in circulation. Circulating levels of these nutrients are sensed by the brain directly or indirectly (via neuroendocrine signals reflecting nutrient status), thereby affecting energy balance. A and B: weight loss from the obese state reduces the size of adipocytes, improving insulin sensitivity, enhancing the clearance of absorbed nutrients, reducing postprandial levels of glucose (Glu) and free fatty acids (FFAs), and minimizing their inhibition of the energy gap. B and C: during the early stages of relapse, the formation of very small, new adipocytes, further enhances insulin sensitivity and the capacity of adipose tissue to clear nutrients from circulation. The rate at which nutrients are ingested and cleared is pitted against the rate at which these nutrients are cleared from circulation by adipose tissue. Circulating glucose levels become a function of this competition between absorption and clearance, while FFA levels are persistently suppressed. FFAs represent the only known signal that would attenuate the energy gap but is persistently suppressed during this dynamic state of weight gain. C and D: after relapsing to the previous weight, the same fat mass is now composed of smaller adipocytes (on average), and insulin sensitivity and nutrient clearance rates are still higher than prior to weight loss. The impact on postprandial nutrients sustains the energy gap as weight gain continues. D and E: clearance rates, the suppressive effects on postprandial nutrients in circulation, and their minimized feedback energy balance regulation gradually resolve as adipocytes expand to the size they were prior to weight loss. With more cells at the same average size, total fat mass is higher than what it was prior to weight loss.
With this model, the adipose tissue's capacity to clear excess energy is pitted against the rate at which nutrients are ingested and absorbed. Excessively large bouts of overfeeding may temporarily overwhelm the clearance rate, elevating glucose to the extent it negatively affects appetite despite FFAs levels remaining suppressed. When adipose tissues eventually catch up, circulating glucose and its associated neuroendocrine markers would decline, appetite would increase, and additional bouts of overfeeding would ensue. At some point, a regular, sustained supply of energy would be achieved to match the high clearance rate of adipose tissues. As the adipocytes expand, their capacity to clear excess energy diminishes and the suppressive effects on circulating nutrients under dynamic (postprandial) states of metabolism would decline. If adipocyte hyperplasia occurs early in relapse, as it does in our DIO rats, relapsing to the previous weight would return the animal to the same fat mass, but the adipocytes on average would be smaller (Fig. 7D). Rates of nutrient clearance would remain somewhat elevated and the impact on postprandial nutrients would persist, gradually declining as the adipocytes become larger, more insulin resistant, and less capable of clearing excess energy. As adipocytes become filled to capacity, postprandial glucose excursions would reflect those prior to weight loss and postprandial suppression of FFAs would be abolished. The high levels of these nutrients, or their neuroendocrine mediators, would feed back to the brain, minimize the energy gap, and normalize the rate of weight gain (Fig. 7E).
This “nutrient clearance” model is generally consistent with preclinical and clinical observations of energy-restricted weight loss from the obese state, providing a reasonable picture of how the energy gap is sustained as weight regain occurs. However, we acknowledge that it is not substantiated, nor is it likely to be substantiated, at every step with definitive proof. As highly integrated as body weight regulation is, biology's drive to regain lost weight will certainly not be this simple and straightforward. Even so, the model serves the purpose of placing the focus on the dynamic metabolic state associated with the sustained positive energy imbalance. We have presented circulating nutrients as the conveyor of this message, but changes in proinflammatory cytokine secretion from adipose tissue provide an equally plausible mediator coincident to cellularity and postprandial nutrient levels in the blood. Alternatively, neural afferents projecting into critical energy balance neural networks in the brain (15, 217) may sense and convey the signal of energy depletion in a more direct manner. While these and a number of other possibilities should be considered, maintaining the focus on the dynamic metabolic state during the process of weight regain will be important to advance our understanding of the process of weight regain.
Perspectives and Significance: Countering the Biological Drive to Regain Weight
While the homeostatic influence on body weight plays a more subtle, permissive role in the development of obesity, biological pressures emerge after weight loss to impart a more prominent influence on the process of weight regain (Fig. 2). It is the dieting and the deviation from the “steady-state” weight that awakens the body's defense system. The biological response is persistent, saturated with redundancies, and well focused on the objective of restoring the body's depleted energy reserves. Any weight loss strategy that fails to acknowledge and plan for this emerging metabolic influence is likely to have little success in facilitating long-term weight reduction.
Even so, the overarching message about our biology's response to weight loss should not be misconstrued into a conciliatory surrender to the inevitability of weight regain. The biological drive to regain lost weight can be countered with environmental, behavioral, and pharmaceutical interventions (Fig. 2E). Composition of the weight maintenance diet (high protein, low carbohydrate; type of dietary fat) has a significant impact on several aspects of this homeostatic response (9, 37, 216), as does the amount of physical activity and regular programmed exercise (137, 151). Promising combination pharmacotherapy, targeting more than one component of the homeostatic system is also on the horizon (190). By acknowledging that these homeostatic pressures emerge, we can proactively develop and implement regain prevention strategies to counter their influence. To ensure success, the regain prevention strategies will likely need to be just as comprehensive, persistent, and redundant, as the biological adaptations they are attempting to counter.
GRANTS
This work was supported by grants from the National Institutes of Health (DK038088 to P. S. MacLean) and P30DK45820.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Adam TC, Jocken J, Westerterp-Plantenga MS. Decreased glucagon-like peptide 1 release after weight loss in overweight/obese subjects. Obes Res 13: 710–716, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Adam TC, Lejeune MP, Westerterp-Plantenga MS. Nutrient-stimulated glucagon-like peptide 1 release after body-weight loss and weight maintenance in human subjects. Br J Nutr 95: 160–167, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of U.S. studies. Am J Clin Nutr 74: 579–584, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Anton SD, Han H, York E, Martin CK, Ravussin E, Williamson DA. Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet 22: 141–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30: 1322–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Arner P, Spalding KL. Fat cell turnover in humans. Biochem Biophys Res Commun 396: 101–104, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol Regul Integr Comp Physiol 269: R222–R225, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Astrup A, Gotzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr 69: 1117–1122, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Axen KV, Axen K. Longitudinal adaptations to very low-carbohydrate weight-reduction diet in obese rats: body composition and glucose tolerance. Obesity (Silver Spring) 18: 1538–1544, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718–15723, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104: 979–984, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ballor DL, Harvey-Berino JR, Ades PA, Cryan J, Calles-Escandon J. Decrease in fat oxidation following a meal in weight-reduced individuals: a possible mechanism for weight recidivism. Metabolism 45: 174–178, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53: 1253–1260, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Barazzoni R, Zanetti M, Cattin MR, Visintin L, Vinci P, Cattin L, Stebel M, Guarnieri G. Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity (Silver Spring) 15: 2614–2623, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318: 34–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res 848: 114–123, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Beck B, Richy S. Dietary modulation of ghrelin and leptin and gorging behavior after weight loss in the obese Zucker rat. J Endocrinol 202: 29–34, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Beck EJ, Tapsell LC, Batterham MJ, Tosh SM, Huang XF. Oat beta-glucan supplementation does not enhance the effectiveness of an energy-restricted diet in overweight women. Br J Nutr 103: 1212–1222, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res 59: 267–285, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, Lefevre AL, Cruciani-Guglielmacci C, Magnan C, Yu F, Niswender K, Irani BG, Holland WL, Clegg DJ. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest 119: 2577–2589, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berman DM, Nicklas BJ, Ryan AS, Rogus EM, Dennis KE, Goldberg AP. Regulation of lipolysis and lipoprotein lipase after weight loss in obese, postmenopausal women. Obes Res 12: 32–39, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol 59: 55–92, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Bessesen DH, Rupp CL, Eckel RH. Dietary fat is shunted away from oxidation, toward storage in obese Zucker rats. Obes Res 3: 179–189, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol 285: R1030–R1036, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Bjorntorp P, Carlgren G, Isaksson B, Krotkiewski M, Larsson B, Sjostrom L. Effect of an energy-reduced dietary regimen in relation to adipose tissue cellularity in obese women. Am J Clin Nutr 28: 445–452, 1975 [DOI] [PubMed] [Google Scholar]
- 26. Born JM, Lemmens SG, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, Westerterp-Plantenga MS. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes (Lond) 34: 172–181, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Boschi V, Iorio D, Margiotta N, D'Orsi P, Falconi C. The three-factor eating questionnaire in the evaluation of eating behaviour in subjects seeking participation in a dietotherapy programme. Ann Nutr Metab 45: 72–77, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Boule NG, Chaput JP, Doucet E, Richard D, Despres JP, Bouchard C, Tremblay A. Glucose homeostasis predicts weight gain: prospective and clinical evidence. Diabet Metab Res Rev 24: 123–129, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Brabant G, Muller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity. FASEB J 19: 1048–1050, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Brennan IM, Seimon RV, Luscombe-Marsh ND, Otto B, Horowitz M, Feinle-Bisset C. Effects of acute dietary restriction on gut motor, hormone and energy intake responses to duodenal fat in obese men. Int J Obes (Lond) 35: 448–456, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 151: 4745–4755, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Burstein R, Prentice AM, Goldberg GR, Murgatroyd PR, Harding M, Coward WA. Metabolic fuel utilisation in obese women before and after weight loss. Int J Obes Relat Metab Disord 20: 253–259, 1996 [PubMed] [Google Scholar]
- 33. Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Capel F, Klimcakova E, Viguerie N, Roussel B, Vitkova M, Kovacikova M, Polak J, Kovacova Z, Galitzky J, Maoret JJ, Hanacek J, Pers TH, Bouloumie A, Stich V, Langin D. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 58: 1558–1567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med 30: 415–444, vii, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Catenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nat Clin Pract Endocrinol Metab 3: 518–529, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caton SJ, Yinglong B, Burget L, Spangler LJ, Tschop MH, Bidlingmaier M. Low-carbohydrate high-fat diets: regulation of energy balance and body weight regain in rats. Obesity (Silver Spring) 17: 283–289, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Chan DC, Watts GF, Gan S, Wong AT, Ooi EM, Barrett PH. Nonalcoholic fatty liver disease as the transducer of hepatic oversecretion of very-low-density lipoprotein-apolipoprotein B-100 in obesity. Arterioscler Thromb Vasc Biol 30: 1043–1050, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Chandler PC, Wauford PK, Oswald KD, Maldonado CR, Hagan MM. Change in CCK-8 response after diet-induced obesity and MC3/4-receptor blockade. Peptides 25: 299–306, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Chaput JP, Drapeau V, Hetherington M, Lemieux S, Provencher V, Tremblay A. Psychobiological effects observed in obese men experiencing body weight loss plateau. Depress Anxiety 24: 518–521, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Chaput JP, Tremblay A. The glucostatic theory of appetite control and the risk of obesity and diabetes. Int J Obes (Lond) 33: 46–53, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Chearskul S, Delbridge E, Shulkes A, Proietto J, Kriketos A. Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am J Clin Nutr 87: 1238–1246, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Cherrington AD, Moore MC, Sindelar DK, Edgerton DS. Insulin action on the liver in vivo. Biochem Soc Trans 35: 1171–1174, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Coccurello R, Caprioli A, Bellantuono S, D'Amato FR, Conti R, Giannessi F, Borsini F, Moles A. Effects of the increase in neuronal fatty acids availability on food intake and satiety in mice. Psychopharmacology 210: 85–95, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Corbett SW, Stern JS, Keesey RE. Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr 44: 173–180, 1986 [DOI] [PubMed] [Google Scholar]
- 46. Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLos One 4: e6310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav 99: 538–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Corpeleijn E, Mensink M, Kooi ME, Roekaerts PM, Saris WH, Blaak EE. Impaired skeletal muscle substrate oxidation in glucose-intolerant men improves after weight loss. Obesity (Silver Spring) 16: 1025–1032, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Crescenzo R, Bianco F, Falcone I, Prisco M, Dulloo AG, Liverini G, Iossa S. Hepatic mitochondrial energetics during catch-up fat after caloric restriction. Metabolism 59: 1221–1230, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martinez JA, Casanueva FF. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab 95: 5037–5044, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 293: 43–53, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85: 1023–1030, 2007 [DOI] [PubMed] [Google Scholar]
- 54. de Luis DA, Sagrado MG, Conde R, Aller R, Izaola O. The effects of two different hypocaloric diets on glucagon-like peptide 1 in obese adults, relation with insulin response after weight loss. J Diabetes Complications 23: 239–243, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Del Prete E, Balkowski G, Scharrer E. Meal pattern of rats during hyperphagia induced by long-term food restriction is affected by diet composition. Appetite 23: 79–86, 1994 [DOI] [PubMed] [Google Scholar]
- 56. Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes Metab 36: 257–262, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Delzenne N, Ferre P, Beylot M, Daubioul C, Declercq B, Diraison F, Dugail I, Foufelle F, Foretz M, Mace K, Reimer R, Palmer G, Rutter G, Tavare J, Van Loo J, Vidal H. Study of the regulation by nutrients of the expression of genes involved in lipogenesis and obesity in humans and animals. Nutr Metab Cardiovasc Dis 11: 118–121, 2001 [PubMed] [Google Scholar]
- 58. Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab 282: E46–E51, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Doucet E, Imbeault P, St.-Pierre S, Almeras N, Mauriege P, Richard D, Tremblay A. Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int J Obes Relat Metab Disord 24: 906–914, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Doucet E, St.-Pierre S, Almeras N, Despres JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. Br J Nutr 85: 715–723, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Doucet E, St-Pierre S, Almeras N, Tremblay A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite 40: 137–143, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Doucet E, Tremblay A, Simoneau JA, Joanisse DR. Skeletal muscle enzymes as predictors of 24-h energy metabolism in reduced-obese persons. Am J Clin Nutr 78: 430–435, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Drapeau V, King N, Hetherington M, Doucet E, Blundell J, Tremblay A. Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite 48: 159–166, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Ducluzeau PH, Perretti N, Laville M, Andreelli F, Vega N, Riou JP, Vidal H. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in type 2 diabetes. Diabetes 50: 1134–1142, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Dulloo AG, Calokatisa R. Adaptation to low calorie intake in obese mice: contribution of a metabolic component to diminished energy expenditures during and after weight loss. Int J Obes 15: 7–16, 1991 [PubMed] [Google Scholar]
- 66. Dulloo AG, Girardier L. 24-hour energy expenditure several months after weight loss in the underfed rat: evidence for a chronic increase in whole-body metabolic efficiency. Int J Obes Relat Metab Disord 17: 115–123, 1993 [PubMed] [Google Scholar]
- 67. Dulloo AG, Girardier L. Adaptive changes in energy expenditure during refeeding following low-calorie intake: evidence for a specific metabolic component favoring fat storage. Am J Clin Nutr 52: 415–420, 1990 [DOI] [PubMed] [Google Scholar]
- 68. Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 32: 1720–1724, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 6: 67–85, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Elliot DL, Goldberg L, Kuehl KS, Bennett WM. Sustained depression of the resting metabolic rate after massive weight loss. Am J Clin Nutr 49: 93–96, 1989 [DOI] [PubMed] [Google Scholar]
- 71. Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of weight loss by a low-fat diet and a low-carbohydrate diet on peptide YY levels. Int J Obes (Lond) 34: 1239–1242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Faraj M, Jones P, Sniderman AD, Cianflone K. Enhanced dietary fat clearance in postobese women. J Lipid Res 42: 571–580, 2001 [PubMed] [Google Scholar]
- 73. Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol 6: 444–453, 2010 [DOI] [PubMed] [Google Scholar]
- 75. Flatt JP, Tremblay A. Energy expenditure and substrate oxidation. In: Handbook of Obesity, edited by Bray GA, Bouchard C, James WPT. New York: Marcel Decker, 1998, p. 513–537 [Google Scholar]
- 76. Foster GD, Wadden TA, Swain RM, Stunkard AJ, Platte P, Vogt RA. The eating inventory in obese women: clinical correlates and relationship to weight loss. Int J Obes Relat Metab Disord 22: 778–785, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Franssila-Kallunki A, Rissanen A, Ekstrand A, Ollus A, Groop L. Effects of weight loss on substrate oxidation, energy expenditure, and insulin sensitivity in obese individuals. Am J Clin Nutr 55: 356–361, 1992 [DOI] [PubMed] [Google Scholar]
- 78. Franssila-Kallunki A, Rissanen A, Ekstrand A, Ollus A, Groop L. Weight loss by very-low-calorie diets: effects on substrate oxidation, energy expenditure, and insulin sensitivity in obese subjects. Am J Clin Nutr 56: 247S–248S, 1992 [DOI] [PubMed] [Google Scholar]
- 79. French SJ, Conlon CA, Mutuma ST, Arnold M, Read NW, Meijer G, Francis J. The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology 119: 943–948, 2000 [DOI] [PubMed] [Google Scholar]
- 80. Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr 57: 35–42, 1993 [DOI] [PubMed] [Google Scholar]
- 81. Galgani JE, Heilbronn LK, Azuma K, Kelley DE, Albu JB, Pi-Sunyer X, Smith SR, Ravussin E. Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes 57: 841–845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Garcia JM, Iyer D, Poston WS, Marcelli M, Reeves R, Foreyt J, Balasubramanyam A. Rise of plasma ghrelin with weight loss is not sustained during weight maintenance. Obesity (Silver Spring) 14: 1716–1723, 2006 [DOI] [PubMed] [Google Scholar]
- 83. German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150: 4502–4511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gilbert JA, Drapeau V, Astrup A, Tremblay A. Relationship between diet-induced changes in body fat and appetite sensations in women. Appetite 52: 809–812, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Gloy VL, Lutz TA, Langhans W, Geary N, Hillebrand JJ. Basal plasma levels of insulin, leptin, ghrelin, and amylin do not signal adiposity in rats recovering from forced overweight. Endocrinology 151: 4280–4288, 2010 [DOI] [PubMed] [Google Scholar]
- 86. Golay A. Blunted glucose-induced thermogenesis: a factor contributing to relapse of obesity. Int J Obes Relat Metab Disord 17 Suppl 1: S23–S27, 1993 [PubMed] [Google Scholar]
- 87. Goldsmith R, Joanisse DR, Gallagher D, Pavlovich K, Shamoon E, Leibel RL, Rosenbaum M. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol 298: R79–R88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gourcerol G, Wang L, Wang YH, Million M, Tache Y. Urocortins and cholecystokinin-8 act synergistically to increase satiation in lean but not obese mice: involvement of corticotropin-releasing factor receptor-2 pathway. Endocrinology 148: 6115–6123, 2007 [DOI] [PubMed] [Google Scholar]
- 89. Green SM, Delargy HJ, Joanes D, Blundell JE. A satiety quotient: a formulation to assess the satiating effect of food. Appetite 29: 291–304, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Gummesson A, Jernas M, Svensson PA, Larsson I, Glad CA, Schele E, Gripeteg L, Sjoholm K, Lystig TC, Sjostrom L, Carlsson B, Fagerberg B, Carlsson LM. Relations of adipose tissue CIDEA gene expression to basal metabolic rate, energy restriction, and obesity: population-based and dietary intervention studies. J Clin Endocrinol Metab 92: 4759–4765, 2007 [DOI] [PubMed] [Google Scholar]
- 91. Gurr MI, Jung RT, Robinson MP, James WP. Adipose tissue cellularity in man: the relationship between fat cell size and number, the mass and distribution of body fat and the history of weight gain and loss. Int J Obes 6: 419–436, 1982 [PubMed] [Google Scholar]
- 92. Gutman R, Choshniak I, Kronfeld-Schor N. Defending body mass during food restriction in Acomys russatus: a desert rodent that does not store food. Am J Physiol Regul Integr Comp Physiol 290: R881–R891, 2006 [DOI] [PubMed] [Google Scholar]
- 93. Hambly C, Simpson CA, McIntosh S, Duncan JS, Dalgleish GD, Speakman JR. Calorie-restricted mice that gorge show less ability to compensate for reduced energy intake. Physiol Behav 92: 985–992, 2007 [DOI] [PubMed] [Google Scholar]
- 94. Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jorgensen JO. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol 56: 203–206, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Hayes MR, Miller CK, Ulbrecht JS, Mauger JL, Parker-Klees L, Gutschall MD, Mitchell DC, Smiciklas-Wright H, Covasa M. A carbohydrate-restricted diet alters gut peptides and adiposity signals in men and women with metabolic syndrome. J Nutr 137: 1944–1950, 2007 [DOI] [PubMed] [Google Scholar]
- 96. He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res 12: 761–769, 2004 [DOI] [PubMed] [Google Scholar]
- 97. Heini AF, Lara-Castro C, Kirk KA, Considine RV, Caro JF, Weinsier RL. Association of leptin and hunger-satiety ratings in obese women. Int J Obes Relat Metab Disord 22: 1084–1087, 1998 [DOI] [PubMed] [Google Scholar]
- 98. Hellmig S, Von Schoning F, Gadow C, Katsoulis S, Hedderich J, Folsch UR, Stuber E. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol 21: 1832–1838, 2006 [DOI] [PubMed] [Google Scholar]
- 99. Hetherington MM, Cecil JE. Gene-environment interactions in obesity. Forum Nutr 63: 195–203, 2010 [DOI] [PubMed] [Google Scholar]
- 100. Hietaniemi M, Jokela M, Rantala M, Ukkola O, Vuoristo JT, Ilves M, Rysa J, Kesaniemi Y. The effect of a short-term hypocaloric diet on liver gene expression and metabolic risk factors in obese women. Nutr Metab Cardiovasc Dis 19: 177–183, 2009 [DOI] [PubMed] [Google Scholar]
- 101. Hill JO, Peters JC, Wyatt HR. Using the energy gap to address obesity: a commentary. J Am Diet Assoc 109: 1848–1853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hill JO, Sparling PB, Shields TW, Heller PA. Effects of exercise and food restriction on body composition and metabolic rate in obese women. Am J Clin Nutr 46: 622–630, 1987 [DOI] [PubMed] [Google Scholar]
- 103. Ho JT, Keogh JB, Bornstein SR, Ehrhart-Bornstein M, Lewis JG, Clifton PM, Torpy DJ. Moderate weight loss reduces renin and aldosterone but does not influence basal or stimulated pituitary-adrenal axis function. Horm Metab Res 39: 694–699, 2007 [DOI] [PubMed] [Google Scholar]
- 104. Huang P, Li S, Shao M, Qi Q, Zhao F, You J, Mao T, Li W, Yan Z, Liu Y. Calorie restriction and endurance exercise share potent anti-inflammatory function in adipose tissues in ameliorating diet-induced obesity and insulin resistance in mice [Online]. Nutr Metab 7: 59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hutson WR, Wald A. Obesity and weight reduction do not influence gastric emptying and antral motility. Am J Gastroenterol 88: 1405–1409, 1993 [PubMed] [Google Scholar]
- 106. Jackman MR, Kramer RE, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab 291: E1083–E1091, 2006 [DOI] [PubMed] [Google Scholar]
- 107. Jackman MR, Maclean PS, Bessesen DH. Energy expenditure in obesity prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol 299: R1097–R1105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, MacLean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol 294: R1117–R1129, 2008 [DOI] [PubMed] [Google Scholar]
- 109. James AP, Watts GF, Barrett PH, Smith D, Pal S, Chan DC, Mamo JC. Effect of weight loss on postprandial lipemia and low-density lipoprotein receptor binding in overweight men. Metabolism 52: 136–141, 2003 [DOI] [PubMed] [Google Scholar]
- 110. Jequier E. Leptin signaling, adiposity, and energy balance. Ann NY Acad Sci 967: 379–388, 2002 [DOI] [PubMed] [Google Scholar]
- 111. Jordan SD, Konner AC, Bruning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci 67: 3255–3273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20: 68–100, 1999 [DOI] [PubMed] [Google Scholar]
- 114. Karlsson J, Hallgren P, Kral J, Lindroos AK, Sjostrom L, Sullivan M. Predictors and effects of long-term dieting on mental well-being and weight loss in obese women. Appetite 23: 15–26, 1994 [DOI] [PubMed] [Google Scholar]
- 115. Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol 587: 19–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kasim S, Jen KL. Effects of weight loss and weight maintenance on the serum lipids, lipoprotein lipase and hepatic triglyceride lipase activities in obese rats. Int J Obes 13: 279–288, 1989 [PubMed] [Google Scholar]
- 117. Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr 68: 794–801, 1998 [DOI] [PubMed] [Google Scholar]
- 118. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999 [DOI] [PubMed] [Google Scholar]
- 119. Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr 22: 325–346, 2002 [DOI] [PubMed] [Google Scholar]
- 120. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000 [DOI] [PubMed] [Google Scholar]
- 121. Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O'Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab 296: E1269–E1274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 95: 2111–2119, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab 296: E282–E290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 136: 1552–1560, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kolehmainen M, Vidal H, Ohisalo JJ, Pirinen E, Alhava E, Uusitupa MI. Hormone-sensitive lipase expression and adipose tissue metabolism show gender difference in obese subjects after weight loss. Int J Obes Relat Metab Disord 26: 6–16, 2002 [DOI] [PubMed] [Google Scholar]
- 126. Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 294: R699–R710, 2008 [DOI] [PubMed] [Google Scholar]
- 127. Kozlowska L, Rosolowska-Huszcz D. Leptin, thyrotropin, and thyroid hormones in obese/overweight women before and after two levels of energy deficit. Endocrine 24: 147–153, 2004 [DOI] [PubMed] [Google Scholar]
- 128. Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, Sciamanna CN. Long-term weight loss maintenance in the United States. Int J Obes (Lond) 34: 1644–1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med 16: 392–395, 2010 [DOI] [PubMed] [Google Scholar]
- 131. Larson DE, Ferraro RT, Robertson DS, Ravussin E. Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr 62: 735–739, 1995 [DOI] [PubMed] [Google Scholar]
- 132. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 133. Lejeune MP, Hukshorn CJ, Saris WH, Westerterp-Plantenga MS. Effects of very low calorie diet induced body weight loss with or without human pegylated recombinant leptin treatment on changes in ghrelin and adiponectin concentrations. Physiol Behav 91: 274–280, 2007 [DOI] [PubMed] [Google Scholar]
- 134. Lemmens SG, Schoffelen PF, Wouters L, Born JM, Martens MJ, Rutters F, Westerterp-Plantenga MS. Eating what you like induces a stronger decrease of ‘wanting’ to eat. Physiol Behav 98: 318–325, 2009 [DOI] [PubMed] [Google Scholar]
- 135. Levin BE. Factors promoting and ameliorating the development of obesity. Physiol Behav 86: 633–639, 2005 [DOI] [PubMed] [Google Scholar]
- 136. Levin BE. Why some of us get fat and what we can do about it. J Physiol 583: 425–430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 286: R771–R778, 2004 [DOI] [PubMed] [Google Scholar]
- 138. Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 278: R231–R237, 2000 [DOI] [PubMed] [Google Scholar]
- 139. Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 282: R46–R54, 2002 [DOI] [PubMed] [Google Scholar]
- 140. Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998 [DOI] [PubMed] [Google Scholar]
- 141. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 142. Lien LF, Haqq AM, Arlotto M, Slentz CA, Muehlbauer MJ, McMahon RL, Rochon J, Gallup D, Bain JR, Ilkayeva O, Wenner BR, Stevens RD, Millington DS, Muoio DM, Butler MD, Newgard CB, Svetkey LP. The STEDMAN project: biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. Omics 13: 21–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lieverse RJ, van Seters AP, Jansen JB, Lamers CB. Relationship between hunger and plasma cholecystokinin during weight reduction with a very low calorie diet. Int J Obes Relat Metab Disord 17: 177–179, 1993 [PubMed] [Google Scholar]
- 144. Litherland NB, Thire S, Beaulieu AD, Reynolds CK, Benson JA, Drackley JK. Dry matter intake is decreased more by abomasal infusion of unsaturated free fatty acids than by unsaturated triglycerides. J Dairy Sci 88: 632–643, 2005 [DOI] [PubMed] [Google Scholar]
- 145. Lofgren P, Andersson I, Adolfsson B, Leijonhufvud BM, Hertel K, Hoffstedt J, Arner P. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab 90: 6207–6213, 2005 [DOI] [PubMed] [Google Scholar]
- 146. Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia 48: 2334–2342, 2005 [DOI] [PubMed] [Google Scholar]
- 147. Maclean PS. Comment on: Kaiyala et al. (2010) Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes;59:1657–1666 Diabetes 60:e3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 290: R1577–R1588, 2006 [DOI] [PubMed] [Google Scholar]
- 149. MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R1306–R1315, 2004 [DOI] [PubMed] [Google Scholar]
- 150. MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R288–R297, 2004 [DOI] [PubMed] [Google Scholar]
- 151. MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, Giles ED, Brown IE, Hill JO. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 297: R793–R802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern P.A., Friedman J. M. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161, 1995 [DOI] [PubMed] [Google Scholar]
- 153. Maraki MI, Aggelopoulou N, Christodoulou N, Anastasiou CA, Toutouza M, Panagiotakos DB, Kavouras SA, Magkos F, Sidossis LS. Lifestyle intervention leading to moderate weight loss normalizes postprandial triacylglycerolemia despite persisting obesity. Obesity 19, 968–976, 2011 [DOI] [PubMed] [Google Scholar]
- 154. Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 281: 18933–18941, 2006 [DOI] [PubMed] [Google Scholar]
- 155. Mathus-Vliegen EM, van Ierland-van Leeuwen ML, Bennink RJ. Influences of fat restriction and lipase inhibition on gastric emptying in obesity. Int J Obes (Lond) 30: 1203–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 156. McGuire MT, Wing RR, Klem ML, Lang W, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol 67: 177–185, 1999 [DOI] [PubMed] [Google Scholar]
- 157. McKibbin PE, Cotton SJ, McMillan S, Holloway B, Mayers R, McCarthy HD, Williams G. Altered neuropeptide Y concentrations in specific hypothalamic regions of obese (fa/fa) Zucker rats. Possible relationship to obesity and neuroendocrine disturbances. Diabetes 40: 1423–1429, 1991 [DOI] [PubMed] [Google Scholar]
- 158. McLaughlin T, Schweitzer P, Carter S, Yen CG, Lamendola C, Abbasi F, Reaven G. Persistence of improvement in insulin sensitivity following a dietary weight loss programme. Diabetes Obes Metab 10: 1186–1194, 2008 [DOI] [PubMed] [Google Scholar]
- 159. Miles CW, Wong NP, Rumpler WV, Conway J. Effect of circadian variation in energy expenditure, within-subject variation and weight reduction on thermic effect of food. Eur J Clin Nutr 47: 274–284, 1993 [PubMed] [Google Scholar]
- 160. Miller WH, Jr, Faust IM, Goldberger AC, Hirsch J. Effects of severe long-term food deprivation and refeeding on adipose tissue cells in the rat. Am J Physiol Endocrinol Metab 245: E74–E80, 1983 [DOI] [PubMed] [Google Scholar]
- 161. Minehira K, Bettschart V, Vidal H, Vega N, Di Vetta V, Rey V, Schneiter P, Tappy L. Effect of carbohydrate overfeeding on whole body and adipose tissue metabolism in humans. Obes Res 11: 1096–1103, 2003 [DOI] [PubMed] [Google Scholar]
- 162. Minehira K, Vega N, Vidal H, Acheson K, Tappy L. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int J Obes Relat Metab Disord 28: 1291–1298, 2004 [DOI] [PubMed] [Google Scholar]
- 163. Mizia-Stec K, Zahorska-Markiewicz B, Olszanecka-Glinianowicz M, Janowska J, Mucha Z, Holecki M, Gasiora Z. Ghrelin as a potential blood pressure reducing factor in obese women during weight loss treatment. Endokrynologia Polska 59: 207–211, 2008 [PubMed] [Google Scholar]
- 164. Moran LJ, Noakes M, Clifton PM, Wittert GA, Le Roux CW, Ghatei MA, Bloom SR, Norman RJ. Postprandial ghrelin, cholecystokinin, peptide YY, and appetite before and after weight loss in overweight women with and without polycystic ovary syndrome. Am J Clin Nutr 86: 1603–1610, 2007 [DOI] [PubMed] [Google Scholar]
- 165. Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem 279: 31139–31148, 2004 [DOI] [PubMed] [Google Scholar]
- 166. Mori Y, Mamori S, Tajima N. Weight loss-associated changes in acute effects of nateglinide on insulin secretion after glucose loading: results of glucose loading on 2 consecutive days. Diabetes Obes Metab 7: 182–188, 2005 [DOI] [PubMed] [Google Scholar]
- 167. Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen-sensitive mechanism. Peptides 25: 667–674, 2004 [DOI] [PubMed] [Google Scholar]
- 169. Muccioli GG, Naslain D, Backhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis [Online]. Mol Syst Biol 6: 392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri M, Moreno LA, Martin-Matillas M, Campoy C, Marti A, Moleres A, Delgado M, Veiga OL, Garcia-Fuentes M, Redondo CG, Sanz Y. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 33: 758–767, 2009 [DOI] [PubMed] [Google Scholar]
- 171. Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol 58: 181–189, 2003 [DOI] [PubMed] [Google Scholar]
- 172. Oberkofler H, Fukushima N, Esterbauer H, Krempler F, Patsch W. Sterol regulatory element binding proteins: relationship of adipose tissue gene expression with obesity in humans. Biochim Biophys Acta 1575: 75–81, 2002 [DOI] [PubMed] [Google Scholar]
- 173. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275, 2002 [DOI] [PubMed] [Google Scholar]
- 174. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 175. Olivan B, Teixeira J, Bose M, Bawa B, Chang T, Summe H, Lee H, Laferrere B. Effect of weight loss by diet or gastric bypass surgery on peptide YY3–36 levels. Ann Surg 249: 948–953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Palming J, Sjoholm K, Jernas M, Lystig TC, Gummesson A, Romeo S, Lonn L, Lonn M, Carlsson B, Carlsson LM. The expression of NAD(P)H:quinone oxidoreductase 1 is high in human adipose tissue, reduced by weight loss, and correlates with adiposity, insulin sensitivity, and markers of liver dysfunction. J Clin Endocrinol Metab 92: 2346–2352, 2007 [DOI] [PubMed] [Google Scholar]
- 177. Pasman WJ, Saris WH, Westerterp-Plantenga MS. Predictors of weight maintenance. Obes Res 7: 43–50, 1999 [DOI] [PubMed] [Google Scholar]
- 178. Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, Raybould HE. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab 296: E898–E903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Pekkarinen T, Takala I, Mustajoki P. Two-year maintenance of weight loss after a VLCD and behavioural therapy for obesity: correlation to the scores of questionnaires measuring eating behaviour. Int J Obes Relat Metab Disord 20: 332–337, 1996 [PubMed] [Google Scholar]
- 180. Pfluger PT, Kampe J, Castaneda TR, Vahl T, D'Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AF, Koebnick C, Weickert MO, Otto B, Spranger J, Tschop MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3–36. J Clin Endocrinol Metab 92: 583–588, 2007 [DOI] [PubMed] [Google Scholar]
- 181. Phillips SA, Choe CC, Ciaraldi TP, Greenberg AS, Kong AP, Baxi SC, Christiansen L, Mudaliar SR, Henry RR. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes Res 13: 1321–1329, 2005 [DOI] [PubMed] [Google Scholar]
- 182. Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116: 1081–1091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54: 3182–3189, 2005 [DOI] [PubMed] [Google Scholar]
- 184. Portillo MP, Cantoral R, Macarulla MT. Effects of dietary fat content on adiposity during energy restriction in genetically obese rats. Reprod Nutr Dev 39: 189–199, 1999 [DOI] [PubMed] [Google Scholar]
- 185. Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296: E1003–E1012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Provencher V, Drapeau V, Tremblay A, Despres JP, Lemieux S. Eating behaviors and indexes of body composition in men and women from the Quebec family study. Obes Res 11: 783–792, 2003 [DOI] [PubMed] [Google Scholar]
- 187. Raben A, Andersen HB, Christensen NJ, Madsen J, Holst JJ, Astrup A. Evidence for an abnormal postprandial response to a high-fat meal in women predisposed to obesity. Am J Physiol Endocrinol Metab 267: E549–E559, 1994 [DOI] [PubMed] [Google Scholar]
- 188. Rabol R, Svendsen PF, Skovbro M, Boushel R, Haugaard SB, Schjerling P, Schrauwen P, Hesselink MK, Nilas L, Madsbad S, Dela F. Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism 58: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 189. Ravussin E, Burnand B, Schutz Y, Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr 41: 753–759, 1985 [DOI] [PubMed] [Google Scholar]
- 190. Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 17: 1736–1743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 88: 906–912, 2008 [DOI] [PubMed] [Google Scholar]
- 193. Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 71: 1421–1432, 2000 [DOI] [PubMed] [Google Scholar]
- 194. Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. Energy intake in weight-reduced humans. Brain Res 1350: 95–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87: 2391–2394, 2002 [DOI] [PubMed] [Google Scholar]
- 196. Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 82: 3647–3654, 1997 [DOI] [PubMed] [Google Scholar]
- 197. Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118: 2583–2591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003 [DOI] [PubMed] [Google Scholar]
- 199. Rozen R, Brigant L, Apfelbaum M. Effects of cycles of food restriction followed by ad libitum refeeding on body composition and energy expenditure in obese rats. Am J Clin Nutr 59: 560–565, 1994 [DOI] [PubMed] [Google Scholar]
- 200. Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298: E751–E760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord 27: 1066–1071, 2003 [DOI] [PubMed] [Google Scholar]
- 202. Sainsbury A, Zhang L. Role of the arcuate nucleus of the hypothalamus in regulation of body weight during energy deficit. Mol Cell Endocrinol 316: 109–119, 2010 [DOI] [PubMed] [Google Scholar]
- 203. Sangiao-Alvarellos S, Vazquez MJ, Varela L, Nogueiras R, Saha AK, Cordido F, Lopez M, Dieguez C. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology 150: 4562–4574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Santosa S, Hensrud DD, Votruba SB, Jensen MD. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr 88: 1134–1141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol 59: 258–262, 2003 [DOI] [PubMed] [Google Scholar]
- 206. Sato F, Tamura Y, Watada H, Kumashiro N, Igarashi Y, Uchino H, Maehara T, Kyogoku S, Sunayama S, Sato H, Hirose T, Tanaka Y, Kawamori R. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab 92: 3326–3329, 2007 [DOI] [PubMed] [Google Scholar]
- 207. Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol 587: 4949–4961, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Schenk S, Horowitz JF. Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am J Physiol Endocrinol Metab 291: E254–E260, 2006 [DOI] [PubMed] [Google Scholar]
- 209. Schutz Y. Concept of fat balance in human obesity revisited with particular reference to de novo lipogenesis. Int J Obes Relat Metab Disord 28 Suppl 4: S3–S11, 2004 [DOI] [PubMed] [Google Scholar]
- 210. Schutz Y. Dietary fat, lipogenesis and energy balance. Physiol Behav 83: 557–564, 2004 [DOI] [PubMed] [Google Scholar]
- 211. Schutz Y, Tremblay A, Weinsier RL, Nelson KM. Role of fat oxidation in the long-term stabilization of body weight in obese women. Am J Clin Nutr 55: 670–674, 1992 [DOI] [PubMed] [Google Scholar]
- 212. Seeley RJ, York DA. Fuel sensing and the central nervous system (CNS): implications for the regulation of energy balance and the treatment for obesity. Obes Rev 6: 259–265, 2005 [DOI] [PubMed] [Google Scholar]
- 213. Sharman MJ, Gomez AL, Kraemer WJ, Volek JS. Very low-carbohydrate and low-fat diets affect fasting lipids and postprandial lipemia differently in overweight men. J Nutr 134: 880–885, 2004 [DOI] [PubMed] [Google Scholar]
- 214. Simon E, del Puy Portillo M, Fernandez-Quintela A, Zulet MA, Martinez JA, Del Barrio AS. Responses to dietary macronutrient distribution of overweight rats under restricted feeding. Ann Nutr Metab 46: 24–31, 2002 [DOI] [PubMed] [Google Scholar]
- 215. Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13: 2051–2060, 1999 [DOI] [PubMed] [Google Scholar]
- 216. Sloth B, Due A, Larsen TM, Holst JJ, Heding A, Astrup A. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Br J Nutr 101: 1846–1858, 2009 [DOI] [PubMed] [Google Scholar]
- 217. Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R501–R511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50: 2673–2681, 2001 [DOI] [PubMed] [Google Scholar]
- 219. Stefan N, Peter A, Cegan A, Staiger H, Machann J, Schick F, Claussen CD, Fritsche A, Haring HU, Schleicher E. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia 51: 648–656, 2008 [DOI] [PubMed] [Google Scholar]
- 220. Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc 63: 363–368, 2004 [DOI] [PubMed] [Google Scholar]
- 221. Straznicky NE, Grima MT, Eikelis N, Nestel PJ, Dawood T, Schlaich MP, Chopra R, Masuo K, Esler MD, Sari CI, Lambert GW, Lambert EA. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J Clin Endocrinol Metab 96: E503–E508, 2011 [DOI] [PubMed] [Google Scholar]
- 222. Svensson PA, Gabrielsson BG, Jernas M, Gummesson A, Sjoholm K. Regulation of human aldoketoreductase 1C3 (AKR1C3) gene expression in the adipose tissue. Cell Mol Biol Lett 13: 599–613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Swartz TD, Duca FA, Covasa M. Differential feeding behavior and neuronal responses to CCK in obesity-prone and -resistant rats. Brain Res 1308: 79–86, 2010 [DOI] [PubMed] [Google Scholar]
- 224. Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev 36: 391–397, 1996 [DOI] [PubMed] [Google Scholar]
- 225. Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab 287: E1076–E1081, 2004 [DOI] [PubMed] [Google Scholar]
- 226. Toledo FG, Menshikova EV, Azuma K, Radikova Z, Kelley CA, Ritov VB, Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57: 987–994, 2008 [DOI] [PubMed] [Google Scholar]
- 227. Tomiyama AJ, Mann T, Vinas D, Hunger JM, Dejager J, Taylor SE. Low calorie dieting increases cortisol. Psychosom Med 72: 357–364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Torgerson JS, Carlsson B, Stenlof K, Carlsson LM, Bringman E, Sjostrom L. A low serum leptin level at baseline and a large early decline in leptin predict a large 1-year weight reduction in energy-restricted obese humans. J Clin Endocrinol Metab 84: 4197–4203, 1999 [DOI] [PubMed] [Google Scholar]
- 229. Tosetti C, Corinaldesi R, Stanghellini V, Pasquali R, Corbelli C, Zoccoli G, Di Febo G, Monetti N, Barbara L. Gastric emptying of solids in morbid obesity. Int J Obes Relat Metab Disord 20: 200–205, 1996 [PubMed] [Google Scholar]
- 230. Tremblay A, Boule N, Doucet E, Woods SC. Is the insulin resistance syndrome the price to be paid to achieve body weight stability? Int J Obes (Lond) 29: 1295–1298, 2005 [DOI] [PubMed] [Google Scholar]
- 231. Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol 587: 4153–4158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology 149: 3592–3597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Valderas JP, Irribarra V, Boza C, de la Cruz R, Liberona Y, Acosta AM, Yolito M, Maiz A. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. J Clin Endocrinol Metab 95: 1069–1075, 2010 [DOI] [PubMed] [Google Scholar]
- 234. Valtuena S, Blanch S, Barenys M, Sola R, Salas-Salvado J. Changes in body composition and resting energy expenditure after rapid weight loss: is there an energy-metabolism adaptation in obese patients? Int J Obes Relat Metab Disord 19: 119–125, 1995 [PubMed] [Google Scholar]
- 235. Van Gaal LF, Vansant GA, De Leeuw IH. Factors determining energy expenditure during very-low-calorie diets. Am J Clin Nutr 56: 224S–229S, 1992 [DOI] [PubMed] [Google Scholar]
- 236. Vandermeerschen-Doize F, Paquay R. Effects of continuous long-term intravenous infusion of long-chain fatty acids on feeding behaviour and blood components of adult sheep. Appetite 5: 137–146, 1984 [DOI] [PubMed] [Google Scholar]
- 237. Verdich C, Madsen JL, Toubro S, Buemann B, Holst JJ, Astrup A. Effect of obesity and major weight reduction on gastric emptying. Int J Obes Relat Metab Disord 24: 899–905, 2000 [DOI] [PubMed] [Google Scholar]
- 238. Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25: 1206–1214, 2001 [DOI] [PubMed] [Google Scholar]
- 239. Viguerie N, Vidal H, Arner P, Holst C, Verdich C, Avizou S, Astrup A, Saris WH, Macdonald IA, Klimcakova E, Clement K, Martinez A, Hoffstedt J, Sorensen TI, Langin D. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia 48: 123–131, 2005 [DOI] [PubMed] [Google Scholar]
- 240. Votruba SB, Blanc S, Schoeller DA. Pattern and cost of weight gain in previously obese women. Am J Physiol Endocrinol Metab 282: E923–E930, 2002 [DOI] [PubMed] [Google Scholar]
- 241. Wang X, Lyles MF, You T, Berry MJ, Rejeski WJ, Nicklas BJ. Weight regain is related to decreases in physical activity during weight loss. Med Sci Sports Exerc 40: 1781–1788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Weigle DS. Contribution of decreased body mass to diminished thermic effect of exercise in reduced-obese men. Int J Obes 12: 567–578, 1988 [PubMed] [Google Scholar]
- 243. Weigle DS, Brunzell JD. Assessment of energy expenditure in ambulatory reduced-obese subjects by the techniques of weight stabilization and exogenous weight replacement. Int J Obes 14 Suppl 1: 69–77; discussion 77–81, 1990 [PubMed] [Google Scholar]
- 244. Weigle DS, Sande KJ, Iverius PH, Monsen ER, Brunzell JD. Weight loss leads to a marked decrease in nonresting energy expenditure in ambulatory human subjects. Metabolism 37: 930–936, 1988 [DOI] [PubMed] [Google Scholar]
- 245. Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr 72: 1088–1094, 2000 [DOI] [PubMed] [Google Scholar]
- 246. Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med 33: 34–40, 2007 [DOI] [PubMed] [Google Scholar]
- 247. Westerterp-Plantenga MS, Kempen KP, Saris WH. Determinants of weight maintenance in women after diet-induced weight reduction. Int J Obes Relat Metab Disord 22: 1–6, 1998 [DOI] [PubMed] [Google Scholar]
- 248. Williams G, Shellard L, Lewis DE, McKibbin PE, McCarthy HD, Koeslag DG, Russell JC. Hypothalamic neuropeptide Y disturbances in the obese (cp/cp) JCR:LA corpulent rat. Peptides 13: 537–540, 1992 [DOI] [PubMed] [Google Scholar]
- 249. Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab 93: S37–S50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Yost TJ, Jensen DR, Eckel RH. Weight regain following sustained weight reduction is predicted by relative insulin sensitivity. Obes Res 3: 583–587, 1995 [DOI] [PubMed] [Google Scholar]
- 251. Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy-restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav Brain Res 205: 50–56, 2009 [DOI] [PubMed] [Google Scholar]
- 252. Yu Y, South T, Wang Q, Huang XF. Differential expression of hypothalamic CART mRNA in response to body weight change following different dietary interventions. Neurochem Int 52: 1422–1430, 2008 [DOI] [PubMed] [Google Scholar]
- 253. Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience 160: 295–306, 2009 [DOI] [PubMed] [Google Scholar]
- 254. Zahorska-Markiewicz B, Mizia-Stec K, Olszanecka-Glinianowicz M, Janowska J. Effect of weight reduction on serum ghrelin and TNFα concentrations in obese women. Eur J Intern Med 15: 172–175, 2004 [DOI] [PubMed] [Google Scholar]
- 255. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 107: 7467–7472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]