Abstract
The midbrain dopaminergic neuronal groups A8, A9, A10 and A10dc occupy, respectively, the retrorubral field (RRF), substantia nigra compacta (SNc), ventral tegmental area (VTA) and ventrolateral periaqueductal gray (PAGvl). Collectively, these structures give rise to a mixed dopaminergic and non-dopaminergic projection system that essentially permits adaptive behavior. Yet, knowledge is incomplete regarding how the afferents of these structures are organized. While the VTA is known to get numerous afferents from cortex, basal forebrain and brainstem and the SNc is widely perceived as receiving inputs mainly from the striatum, the afferents of the RRF and PAGvl have yet to be addressed comprehensively. This study was done to provide an account of those connections and seek a better understanding of how afferents might contribute to the functional interrelatedness of the VTA, SNc, RRF and PAGvl. Ventral midbrain structures received injections of retrograde tracer and resulting retrogradely labeled structures were targeted with injections of anterogradely transported Phaseolus vulgaris-leucoagglutinin. While all injections of retrograde tracer into the VTA, SNc, RRF or PAGvl produced labeling in many of a long list of structures extending from the cortex to caudal brainstem, pronounced labeling of structures comprising the central division of the extended amygdala occurred following injections that involved the RRF and PAGvl. The anterograde tracing supported this finding and, interestingly, the combination of retrograde and anterograde labeling data also confirmed reports from other groups indicating that the SNc receives robust input from many of the same structures that innervate the VTA, RRF and PAGvl.
Keywords: retrorubral field, ventral tegmental area, substantia nigra compacta, periaqueductal gray, amygdala, bed nucleus of stria terminalis
Catecholamine-containing neurons and axon pathways in the rat brain were mapped with the aid of aldehdye-induced fluorescence in the mid-nineteen sixties and since have ranked continuously among the most studied of all brain structures. Dopaminergic neuronal groups A8, A9, A10 (Dahlström and Fuxe, 1964; Anden et al., 1964, 1965; 1966a; 1966b; Björklund et al., 1984) and A10dc (Hökfelt et al., 1984) occupy, respectively, the retrorubral field (RRF), substantia nigra compacta (SNc), ventral tegmental area (VTA) and ventral and lateral parts of the periaqueductal gray (PAGvl). While these structures are invariably referred to as “dopaminergic”, all are supplemented by indeterminate proportions of intermixed dopaminergic neurons co-expressing glutamate (Lapish et al., 2006, Hnasko et al., 2010; Tecuapetla et al., 2010) and non-dopaminergic neurons (Swanson, 1982) expressing gamma-aminobutyric acid (GABA, Steffensen et al., 1998) or glutamate (Yamaguchi et al., 2007; Dobi et al., 2010) that may give rise to long projections and interact with dopaminergic neurons locally. The output from these structures, which we will herein continue to refer to as the midbrain dopaminergic complex, contributes to a broad range of functions, including but not limited to locomotor activation (e.g., Kelly et al., 1975), stimulus and response reinforcement (e.g., Wise, 2004) and fear conditioning (Guarraci et al., 1999, 2000; Fadok et al., 2009) and thus is essential to the neural processes that subserve adaptive behavior.
Whereas the SNc projects mainly to the dorsal striatum, the VTA innervates the ventral striatum, septum, basal forebrain, basal amygdala, ventral hippocampus and medial prefrontal cortex (Fallon et al., 1978; Fallon and Moore, 1978; Beckstead et al., 1979; Swanson, 1982; Lindvall and Björkland, 1983; Phillipson and Griffiths, 1985; Fallon and Loughlin, 1985; 1995; Fallon, 1988; Del Fava et al., 2007) and the RRF projects to dorsal and ventral striatum as well as the piriform and entorhinal cortices (Deutch et al., 1988). The VTA and RRF also project to the extended amygdala (Deutch et al., 1988; Hasue and Shammah-Lagnado, 2002), which includes the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BST) and some related structures (de Olmos and Ingram, 1972; Alheid and Heimer, 1988; de Olmos and Heimer, 1999). However, the PAGvl, which is reported to contain neurons that utilize L-DOPA in place of dopamine (Hökfelt et al., 1984; Misu et al., 1996), appears to have the most catecholaminergic neurons projecting to extended amygdala (Hasue and Shammah-Lagnado, 2002).
As regards afferents, the VTA is most studied among the components of the midbrain dopaminergic complex. VTA inputs arise in multiple structures in the cortex, basal forebrain and brainstem (Nauta and Domesick, 1978; Phillipson, 1979; Wallace et al., 1989; 1992; Carr and Sesack, 1999; 2000; Zahm et al., 2001; Fadel and Deutch, 2002; Philpot et al., 2005; Olmechenko and Sesack, 2005; 2006; 2007; 2009; 2010; Balcita-Pedicino and Sesack, 2007; Ferreira et al., 2008; Olmechenko et al., 2009) reported to comprise a highly interconnected network (Geisler and Zahm, 2005; 2006; Geisler et al., 2007). The SNc, in contrast, is widely regarded as getting input mainly from the dorsal striatum (Beckstead et al., 1979; Gerfen et al., 1985; Jimenez-Castellanos and Graybiel, 1989), despite numerous reports of a lateralward spread through the SNc of a variety of afferents that occupy the medial forebrain bundle (Nauta and Domesick, 1978; Nauta et al., 1978; Krettek and Price, 1978; Nauta and Domesick, 1984; van der Kooy et al., 1984; Zahm and Heimer, 1990; 1993; Gonzales and Chesselet,1990; Heimer et al., 1991b; Rosen et al., 1991; Zahm et al., 1999; Dong et al., 2001; Gastard et al., 2002; Coizet et al., 2010; Geerling et al., 2010). Behind the SNc, the medial forebrain bundle arches through the lateral RRF and then turns caudalward to pass along and emit fibers into the PAGvl. The afferents of neither the RRF nor PAGvl have been comprehensively described in the literature. The experiments described herein and accompanying literature review thus were done primarily to identify the afferents of the RRF and PAGvl and secondarily to develop a better picture of how the midbrain dopaminergic complex, considered as a whole, is innervated.
Materials and Methods
Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing 225–300 g were used in accordance with guidelines mandated in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The rats were housed on a 12 hr light-dark cycle in groups of four until surgeries were performed, after which they were singly housed. Access to food and water was provided ad libitum to all rats throughout the study. Unless stated otherwise, chemicals were purchased from Sigma Chemical Company (St. Louis, MO).
Tracer injections
Several minutes after being given intraperitoneal injections of a cocktail, consisting of 45% ketamine (100 mg/ml), 35% xylazine (20 mg/ml) and 20% physiological saline at a dose of 0.16 ml/100g of body weight, rats were placed in a Kopf stereotaxic instrument. The skulls were exposed and small bore holes were created to allow selected brain structures to be targeted by filament-containing borosilicate glass pipettes (O.D. − 1.0 mm) pulled to tip diameters of 10–25 µm and containing the retrograde tracer Fluorogold (FG; Fluorochrome, Inc., Englewood, CO; 1% in 0.1M cacodylate buffer, pH 7.4) or an anterograde tracer, either Phaseolus vulgaris-leucoagglutinin (PHA-L; Vector, Burlingame, CA, 2.5% in 0.01 M phosphate buffer) or biotinylated dextran amine (BDA, Molecular Probes, Inc., Eugene, OR, 10% in 0.01 M phosphate buffer). A silver wire inserted into the pipettes contacted the solution containing the tracer, which was ejected into the brain substance using positive current pulses (7 s on, 7 s off, for 15 minutes) of 1 µA (for FG) or 4 µA (for PHA-L and BDA). After surgery the rats were kept warm until they awakened.
The analysis utilized 16 injections of FG (e.g., Figs. 1 and 2) evaluated in relation to the midbrain dopaminergic complex, which was revealed with the aid of tyrosine hydroxylase immunoreactivity (Fig. 1). Two of the injections were centered in the PAGvl and one, three and six injections occupied the medial, middle and lateral RRF, respectively. In addition, two injections in the lateral half of the SNc were used, as were two, prepared for an earlier study (Geisler and Zahm, 2005), that occupied about the center of the VTA. A further two control injections into the SNr (05105, 05106) and many into various parts of the midbrain tegmentum near the dopaminergic complex were also studied (06093; 06162, 07120 – red nucleus and adjacent tegmentum; 06014 – tegmentum lateral to red nucleus and above RRF; 06167 – tegmentum below caudal RRF and ventromedial to the pedunculopontine tegmental nucleus; 07086; 07114; 07115, 07130, 08019, 08020, 08023 – rostromedial tegmental nucleus; 07124, 08026 – paramedian raphe; 08022 – midline between rostromedial tegmental nuclei; 07118 – interpeduncular nucleus; 07119 – medial lemniscus; 07126 – oculomotor nucleus). In addition, 31 cases were studied in which PHA-L or BDA injection sites were centered in various forebrain and brainstem structures selected on the basis of the retrograde labeling, such as, e.g., the prefrontal cortex (PHA-L, 04265 and 04266), accumbens (PHA-L, 98003, 98005), ventral pallidum (BDA, 94031, 94032, 94040, 94051, and 94052), sublenticular extended amygdala (PHA-L, 95109), lateral preoptic area (PHA-L, 03153, 06148, 06149, 09108, 09025), lateral hypothalamus (PHA-L, 96049, 96108, 96199), lateral habenula (PHA-L, 07142, 08002, 08004, 08011), zona incerta (PHA-L, 95108), entopeduncular nucleus (PHA-L, 96134) ventral tegmental area (PHA-L, 07046 and 07077) and substantia nigra compacta (PHA-L, 07004 and 07014) and rostromedial tegmental nucleus (PHA-L, 06076, 06096, 07033). Targeting of FG and PHA-L injections was done with the aid of stereotaxic coordinates which were initially acquired from the atlas of Paxinos and Watson (2007) and refined empirically. Most abbreviations used in the text, figures and tables are from Paxinos and Watson (2007).
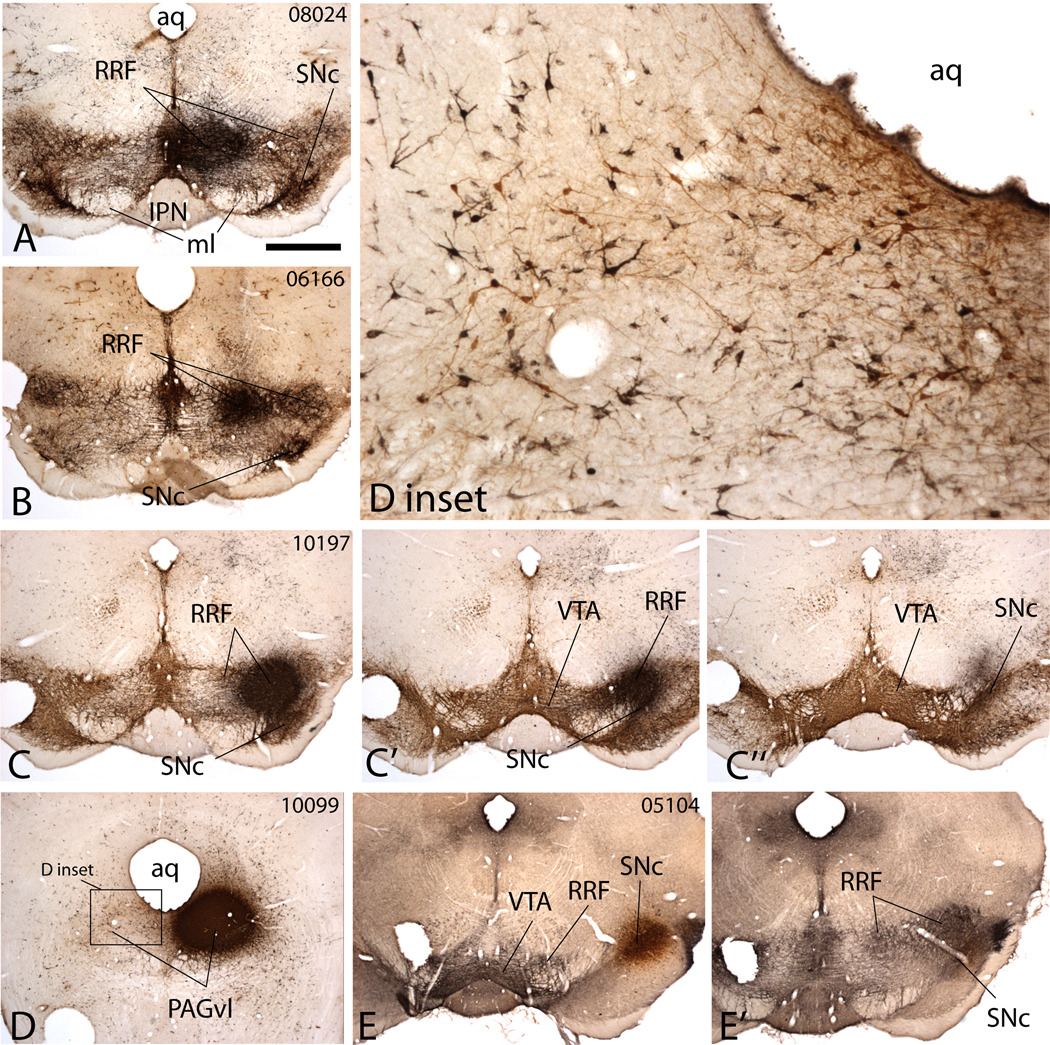
Figure 1.
Micrographs illustrating Fluorogold (FG) injection sites in the medial (A), middle (B) and lateral (C-C”) retrorubral field (RRF), ventrolateral periaqueductal gray (PAGvl, D and D inset) and lateral substantia nigra compact (SNc, E and E’). The sections were processed to exhibit both FG and tyrosine hydroxylase (A–D) or neurotensin (E and E’) immunoreactivities, which illustrate the extent of the ventral mesencephalic dopaminergic complex comprising the ventral tegmental area (VTA), substantia nigra compacta (SNc) and retrorubral field (RRF). In A through D, TH immunoreactivity is brown and the FG injection site black. In E and E’ neurotensin immunoreactivity is black and the FG injection site brown. A and B show injection sites relatively confined to the medial and middle RRF, respectively. C-C” shows an injection site in the lateral RRF that minimally involves the SNc. D inset reveals by symmetry that the PAGvl injection site (D) occupies a part of the periaqueductal gray that contains numerous TH-immunoreactive neurons (brown stained neurons), indicative of the dopaminergic district A10dc. E and E’ illustrate an FG injection site in the SNc (E) that minimally involves the RRF (E’). Case numbers are provided in the upper right corners of the leading panel for each case to facilitate identification of cases shown in subsequent illustrations and discussed in the text. Additional abbreviations: aq – cerebral aqueduct; IPN – interpeduncular nucleus; ml – medial lemniscus. Scale bar: 1 mm in A, B, C-C”, D and E-E’; 200 µm in D inset.
Figure 2.
Micrographs illustrating retrogradely labeled neurons in the bed nucleus of stria terminalis (BST, A and A’), medial division of the central nucleus of the amygdala (CeAm, B and B’) and hypothalamic paraventricular nucleus (Pa, C and C’) of case 10197 resulting from an injection of FG into the lateral retrorubral field shown in Fig. 1C-C”. Panels A’, B‘ and C’ are enlargements of the boxes in corresponding panels A, B and C. Retrogradely labeled neurons (some examples indicated by arrows) exhibit black nickel-enhanced DAB immunoperoxidase product. Note that the labeling in the CeAm (B and B’) is continuous with that in the medial preoptic area (MPO) and lateral preoptic area (LPO). The sections were counterstained with nitric oxide synthase immunoreactivity shown in brown to help differentiate structures within the sections. Additional abbreviations: AH – anterior hypothalamus; BSTl – bed nucleus of stria terminalis, lateral division; BSTm – bed nucleus of stria terminalis, medial division; CeAl – central nucleus of the amygdala, lateral division; CPu – caudate-putamen; EPN – entopeduncular nucleus; GP – globus pallidus; LH – lateral hypothalamic area; to – optic tract; VP – ventral pallidum. Scale bar: 1 mm in A, B and C; 200 µm in A’, B’ and C’.
Fixation of brains and immunocytochemistry
Three days after FG injections, ten days after PHA-L injections and five days after BDA injections, the rats were deeply anesthetized (as above) and perfused transaortically, first with 0.01 M Sorensen's phosphate buffer (SPB; pH 7.4) containing 0.9% sodium chloride and 2.5% sucrose, followed by 0.1 M SPB (pH 7.4) containing 4% paraformaldehyde and 2.5% sucrose. The brains were removed, post-fixed, infiltrated with 25% sucrose and sectioned frozen at 50 µm. Five adjacent series of sections were collected, with each thus reflecting the structure of the entire brain from frontal pole to caudal medulla in sections spaced at intervals of 250 µm. Each series of sections was stored in a separate glass vial at −20° C in a cryoprotectant consisting of SPB containing 30% sucrose (by weight) and 30% ethylene glycol (by volume).
One series of sections from each case was immersed in SPB containing 0.1% Triton X-100 (SPB-t) and polyclonal antibodies raised against the relevant tracer, i.e., either anti-FG made in rabbit and used at a dilution of 1:5000 or anti-PHA-L made in goat used at a dilution of 1:10,000. The following day, after thorough rinsing in SPB-t, the sections were immersed for an hour in SPB-t containing biotinylated antibodies made in donkey against rabbit (for FG) or goat (for PHA-L) at a dilution of 1:200 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Afterward, the sections were rinsed in SPB and then immersed in SPB containing avidin-biotin-peroxidase complex (ABC - Vector Laboratories, Burlingame, CA) at a dilution of 1:200, also for an hour. Cases with BDA injections were pretreated by immersion in 1% aqueous sodium borohydride for 15 minutes followed by thorough rinsing and then placed immediately in SPB-t containing ABC reagents at a dilution of 1:200. After additional thorough rinsing in SPB, the sections were immersed for 20–30 min in 0.05 M SPB (pH 7.4) containing 0.05% DAB, 0.04% ammonium chloride, 0.2% β-D-glucose, and 0.0004% glucose oxidase, which generates an insoluble brown reaction product, or, if the sections were destined to be reacted with a second primary antibody, in 0.025 M Tris buffer (pH 8.0) containing 0.015% 3,3’–diaminobenzidine (DAB), 0.4% nickel ammonium sulfate and 0.003% hydrogen peroxide, which generates an insoluble black reaction product.
Sections intended for additional immunocytochemical processing were then further rinsed in SPB and immersed in SPB-t containing, anti-nitric oxide synthase (NOS) or anti-neurotensin (NT) made in rabbit or anti-tyrosine hydroxylase (TH) made in mouse, all used at a dilution of 1:5000. The following morning the sections were rinsed in SPB-t and immersed for one hour in SPB-t containing a donkey antibody against mouse or rabbit IgGs, as appropriate, each used at a dilution of 1:200 (Jackson). Following further rinsing in SPB the sections were immersed for one hour in SPB containing, respectively, mouse or rabbit peroxidase-anti-peroxidase (PAP) at a dilution of 1:3000 (MP Biomedicals, Solon, OH), after which they were again rinsed thoroughly. Then the sections were immersed for 20–30 min in 0.05 M SPB (pH 7.4) containing 0.05% DAB, 0.04% ammonium chloride, 0.2% β-D-glucose, and 0.0004% glucose oxidase (brown reaction product) or 0.025 M Tris buffer (pH 8.0) containing 0.015% 3,3’–diaminobenzidine (DAB) and 0.003% hydrogen peroxide (also brown) and, after rinsing, mounted onto gelatin coated slides, dehydrated through a series of ascending concentrations of ethanol, transferred into xylene, and coverslipped with Permount (Fisher, Pittsburgh, PA).
Antibody Characterization
The primary antibodies used in the study and their sources are listed in Table 1. Information on the characterization of the antibodies and specificity controls is given below.
Table 1.
Primary Antibodies
| Antiserum | Immunogen | Source (cat. no.) | Working dilution |
|---|---|---|---|
| Rabbit polyclonal anti-Fluorogold (FG) | Fluorogold itself | Bioscience Research Reagents, a division of Millipore, Temecula, CA (AB153) | 1:5000 – 1:10,000 |
| Rabbit polyclonal anti-neurotensin (NT) | Synthetic neurotensin (ELYENLPRRPYIL) conjugated to bovine thryroglobulin with glutaraldehyde | Immunostar, Hudson, WI (20072) | 1:5000 – 1:10,000 |
| Rabbit polyclonal anti-nitric oxide synthase (Nos) | Amino acids 251–270 of nitric oxide synthase (GDNDRVFNDLWGKDNVPVILC) conjugated to keyhole limpet cyanin | Sigma Chemical Company, St. Louis, MO (N7155) | 1:5000 – 1:10,000 |
| Rabbit polyclonal anti-Phaseolus vulgaris leucoagglutinin (PHA-L) | Phaseolus vulgaris-leucoagglutinin (E & L) itself | Vector Laboratories, Burlingame, CA (AS-2224) | 1:5000 – 1:10,000 |
| Mouse monoclonal anti-tyrosine hydroxylase (TH) | Tyrosine hydroxylase from PC12 cells | Bioscience Research Reagents, a division of Millipore, Temecula, CA (MAB318) | 1:5000 – 1:10,000 |
anti-FG
Immunoprocessed sections from brains lacking FG injections were devoid of reaction product and immunoreactivity was abolished by preabsorption with FG (10 µg/ml). In brains that received injections of FG, immunostaining was observed only at the injection sites, in retrogradely labeled neurons and, occasionally, in microglial cells, which, however, are readily distinguished by morphology from labeled neurons. The vendor states that AB153 also reacts with amino-stilbamidine in frozen, 4% PFA fixed tissues.
anti-NT
NT immunoreactivity was abolished in our hands by preabsorption with the cognate peptide (10 µg/ml). Furthermore, the antibody stained rat forebrain and ventral midbrain sections identically to descriptions in literature (e.g., Uhl et al., 1977).
anti-Nos
Nos immunoreactivity was abolished in our hands by preabsorption with the cognate peptide (10 µg/ml). Furthermore, the antibody stained rat forebrain and ventral midbrain sections identically to descriptions in literature (e.g., Rodrigo et al., 1994).
anti-PHA-L
Immunoprocessed sections from brains lacking PHA-L injections were devoid of PHA-L reaction product and immunoreactivity was abolished by preabsorption with PHA-L (10 µg/ml). In brains that received injections of PHA-L, immunostaining was observed only at the injection sites and in anterogradely labeled axons.
anti-TH
In the vendor’s Western blots, 1:1000 dilution of the lot used in the study (LV1377069) detected tyrosine hydroxylase on 10 µg of mouse and rat brain lysates. The antibody stained a single band consistent with a protein of approximately 59–61 kDa. It did not react with the following on Western blots: dopamine-beta-hydroxylase, phenylalanine hydroxylase, tryptophan hydroxylase, dehydropteridine reductase, sepiapterin reductase or phenethanolamine-N-methyl transferase (PNMT). In our hands, the antibody stained rat forebrain and ventral midbrain sections identically to descriptions in literature (e.g., Lindvall et al., 1983; Hökfelt et al., 1984).
Maps and photomicrographs
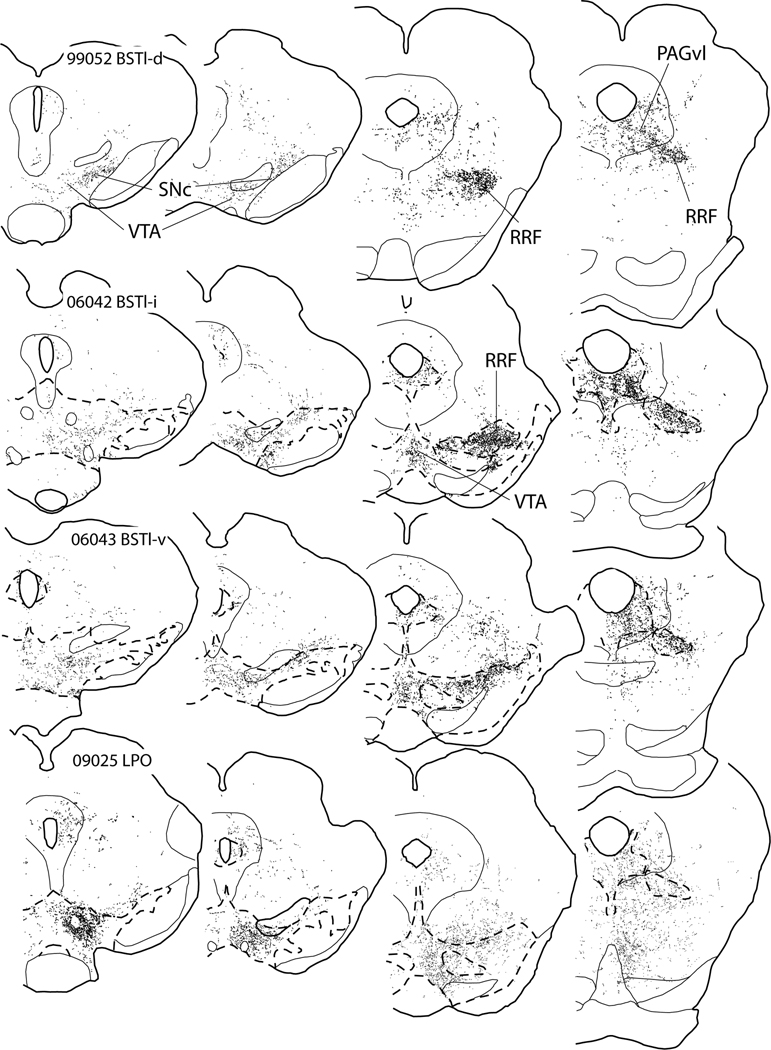
Retrograde and anterograde labeling was plotted in representative frontal sections throughout the CNS (excluding spinal cord) with the 10X or 20X objective under brightfield optics (Nikon Optiphot) with the aid of the AccuStage digitizing system and MDPlot software (AccuStage™, Shoreview, MN) or the Neurolucida dedicated hardware-software platform (MBF Bioscience, Williston, VT). Maps of retrograde labeling included 4 cases representing the medial (case 08024), middle (case 06166) and lateral (case 10197) RRF and PAGvl (case 10099) of which 31, 27, 27, and 26 levels were plotted, respectively. Sixteen levels of case 06166 (16 levels) are shown in the paper (Fig. 3) as are parts (5 levels) of cases 10197 and 10099 (Fig. 4). Anterograde tracing was mapped using the Neurolucida system in a manner such that only axonal varicosities, i.e., puncta (Figure 5A’) were plotted to the complete exclusion of non-varicose, presumably mainly non-synaptic, parts of labeled axons. Varicosities were recognized as distinct, punctate swellings or dilatations of PHA-L filled axons that often were more intensely immunoreactive than adjacent non-varicose parts of labeled axons (see arrowed structures in Fig. 5A’). For anterograde tracing, mapping was limited to the midbrain dopaminergic complex. The following cases and levels were mapped: for BSTl-d - 00022 (6 levels), 99083 (5 levels); for BSTl-i - 99052 (6 levels), 06142 (6 levels), for BSTl-v - 06042 (6 levels), 06043 (4 levels); 06060 (6 levels); for CeAm-m - 00061 (4 levels); for CeAm-l - 11105 (4 levels); for LPO - 03153 (4 levels); 09017 (4 levels), 09018 (4 levels), 09025 (5 levels); for VP - 94052 (6 levels).
Figure 3.
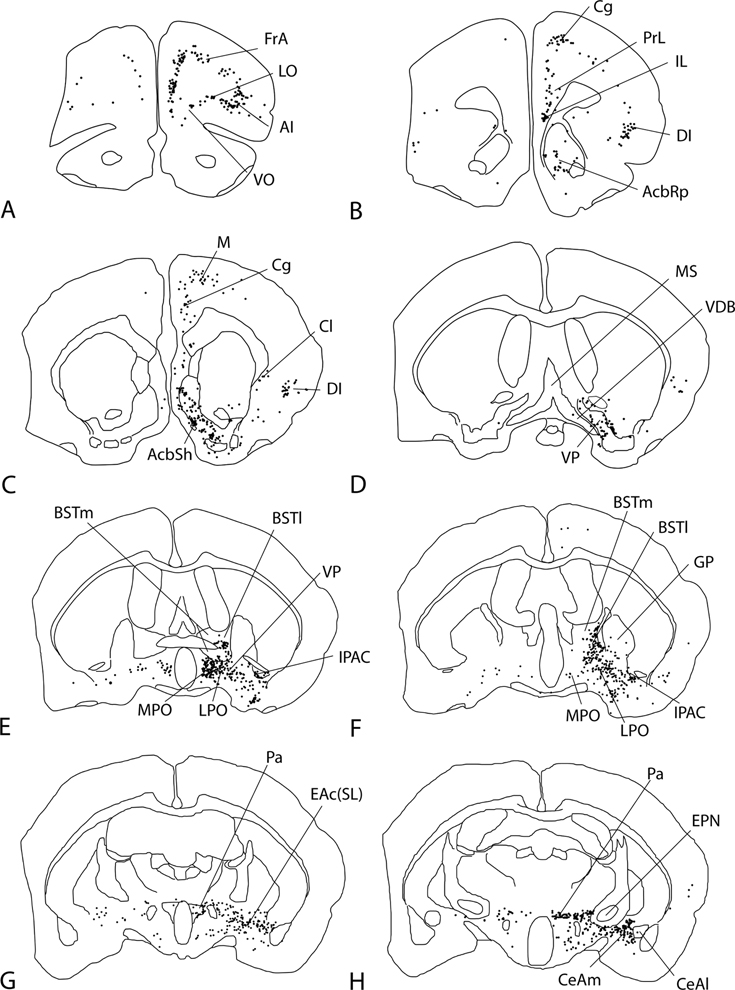
Map of retrograde labeling following an injection of Fluorogold into the middle (case 06166) of the retrorubral field. The injection sites is shown in Figure 1B and panel L. Each dot represents a retrogradely labeled neuron. For abbreviations, see list.
Figure 4.
Maps of retrograde labeling following injections of Fluorogold (FG) into the lateral retrorubral field (RRFl, case 10197, panels A–E’) and ventrolateral periaqueductal gray (PAGvl, case 10099, panels F–J). The injection sites are shown in Figures 1C-C” and 1D, respectively. Each dot represents a retrogradely labeled neuron. Additional abbreviations: AcbC – accumbens core, AcbSh – accumbens shell, BSTl – bed nucleus of stria terminalis, lateral division, BSTm – bed nucleus of stria terminalis, medial division, CeAm – medial division of the central nucleus of the amygdala, CeAl – lateral division of the central nucleus of the amygdala, CPu – caudate-putamen, EAc(SL) – central division of extended amygdala, sublenticular part, GP – globus pallidus, MPO – medial preoptic area, Pa – hypothalamic paraventricular nucleus, Pe – periventricular nucleus, Pf – parafascicular nucleus, PSTh – parasubthalamic nucleus, Sol – nucleus of the tractus solitarius, ZI – zona incerta.
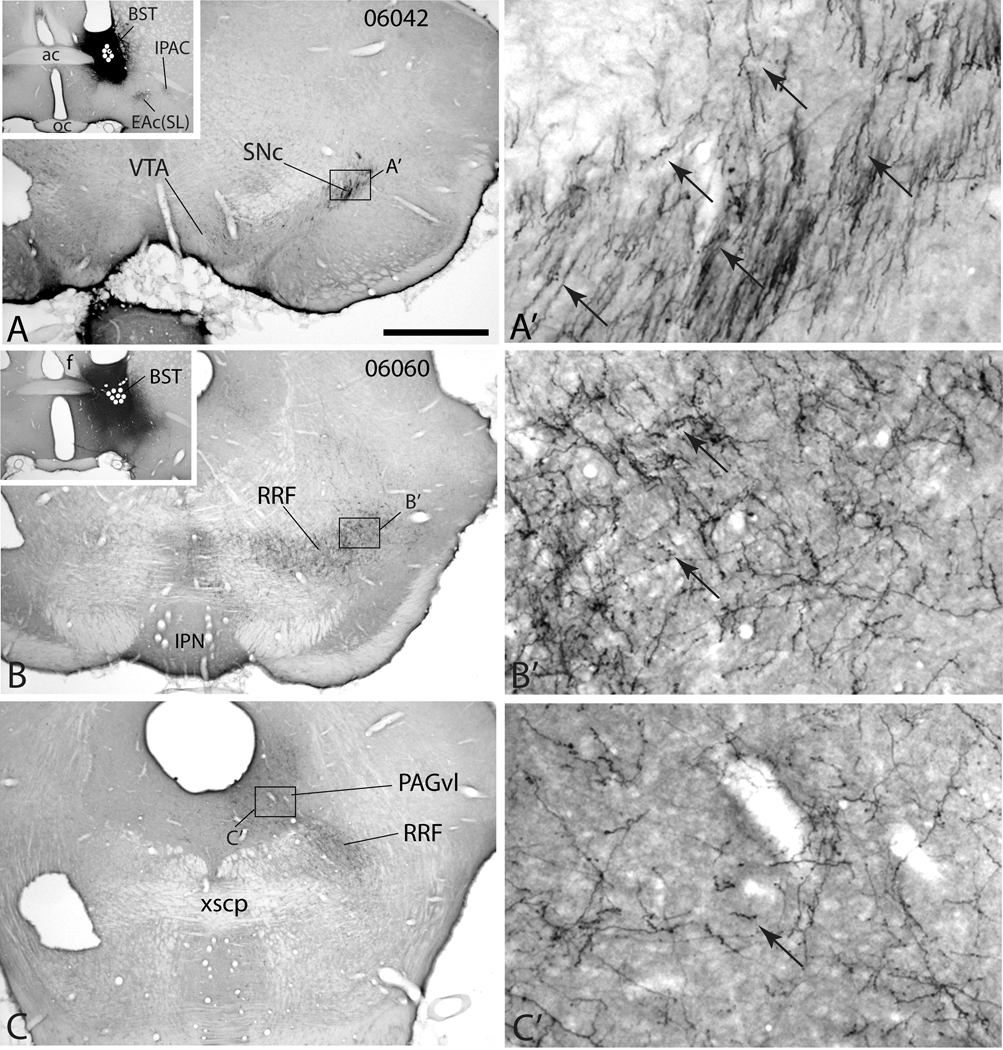
Figure 5.
Micrographs illustrating the distribution of anterogradely labeled axons in the midbrain dopaminergic complex in case 06042 (A-A’) in which an injection of PHA-L (black injection site) was placed in the lateral division of the bed nucleus of stria terminalis (BST, inset in A) and case 06060 (B–C’) with a PHA-L injection in the lateral division of the BST at a slightly more ventral position. White dots indicate the positions of PHA-L impregnated neurons marking the injection sites. A’, B’ and C’ are enlargements of the respective boxes in A, B and C. In case 06042 a rostral section through the midbrain dopaminergic complex (A and A’) exhibits dense anterograde labeling in the substantia nigra compacta (SNc) mostly in the form of non-varicose presumably passing fibers, although some varicosities (puncta) are visible (arrows). Little anterograde labeling is visible in the ventral tegmental area (VTA) in this case at this level. In case 06060, anterograde labeling spreads homogeneously through the retrorubral field (RRF, B and B’) and is more varicose (a few examples are arrowed). The ventrolateral periaqueductal gray substance (PAGvl) also contains a moderately dense plexus of varicose labeled fibers (C and C’). Additional abbreviations: ac – anterior commissure, EAc(SL) – central division of extended amygdala, sublenticular division; f – fornix; IPAC – interstitial nucleus of the posterior limb of the anterior commissure; IPN – interpeduncular nucleus; xscp – crossing of the superior cerebellar peduncle. Scale bar: 1 mm in A, B and C; 100 µm in A’, B’ and C’.
Digital micrographs were captured with a Q Imaging Fast 1394 digital camera and adjusted mainly for brightness and contrast with Adobe Photoshop (CS2) software. The plates were constructed using Adobe Illustrator (CS2) software.
Quantitation of anterogradely labeled axonal varicosities
Having been mapped with the aid of the Neurolucida system, varicosities residing in the VTA, SNc, RRF, and PAGvl, which were identified with the aid of tyrosine hydroxylase immunoreactivity in the same or an adjacent section, were circumscribed and counted with the aid of NeuroExplorer software (MBF Bioscience) and expressed as varicosities/mm2 within the circumscribed area. This provides density of axonal varicosities specifically within the area of distribution of the projection, rather than relative to the area of the entire structure, an appropriate measurement because topographically distributed projections often do not fill an entire structure. Each evaluated projection was so quantitated in at least 3 levels of the midbrain dopaminergic complex and the measurements were averaged. The averages were used to provide means and SEMs for the various cases as listed in the preceding section. Where appropriate, group means were tested a with one-way ANOVA followed by post-hoc evaluation using Fishers LSD test.
Results
Retrograde tracing
Injections of FG into medial, middle and lateral parts of the RRF and the PAGvl (Fig. 1) produced remarkably similar distributions of labeled neurons within the prefrontal cortex, striatopallidum, preoptic area, lateral hypothalamus, zona incerta, mesopontine tegmentum and medial brainstem reticular formation, but, most prominently, in the extended amygdala (EA) and a network of forebrain and brainstem structures with which it is reciprocally interconnected (Figs. 2–4; Tables 2 and 3). Labeling observed following such injections was mainly ipsilateral, but at more caudal levels possessed a more robust contralateral representation, culminating in the mesopontine tegmentum at about half the ipsilateral density. The basic organization is mapped in Figure 3 from a case with an FG injection in the middle part of the RRF (see Fig. 1B for injection site). A few significant deviations from the basic pattern are noted in the following text and some of these are illustrated in Figure 4. Widespread forebrain and brainstem retrograde labeling was observed following many control injections of FG listed in the Materials and Methods section, but in no case, with the exception of the caudolateral SNc (see below and Table 2), did the pattern approximate that consistently observed after injections in the RRF and PAGvl.
Table 2.
Retrograde labeling following injections of Fluoro-Gold
| Retrogradely labeled structures | Injection sites | ||||||
|---|---|---|---|---|---|---|---|
| VTA | RRF | PAGvl | SN | ||||
| med | mid | lat | c | r | |||
| Cortex | |||||||
| frontal association | o | xxx | xx | xxx | xxx | xxx | o |
| medial orbital | o | x | x | xx | xxx | o | o |
| lateral orbital | o | xx | x | xx | xxx | o | o |
| prelimbic | xxx | xxx | xx | xxx | xxx | o | o |
| infralimbic | xxx | xxx | xx | xxx | xxx | o | o |
| dorsal peduncular | xxx | xxx | xx | xx | xxx | o | o |
| anterior cingulate | xx | xxx | xx | xx | xx | o | o |
| agranular insular | x | xxx | xxx | xxx | xxx | xxx | xx |
| claustrum | xx | xx | x | xxx | xx | xxx | x |
| dysgranular insular | o | o | x | xxx | xx | xxx | o |
| granular insular | o | o | x | xxx | xx | xx | o |
| second somatosensory | o | o | o | xx | o | xx | xxx |
| primary somatosensory | o | o | o | xx | o | xx | xxx |
| second motor | o | o | o | xx | o | o | xx |
| primary motor | o | o | o | xx | o | o | xx |
| Basal ganglia | |||||||
| accumbens rostral pole | xxx | xx | o | xxx | xx | xx | x |
| accumbens medial shell | xxx | xxx | x | o | x | xxx | o |
| accumbens lateral shell | x | x | xx | xxx | xxx | xx | x |
| accumbens core | xxx | o | x | xxx | o | xxx | xxx |
| ventral pallidum | xxx | xxx | xx | xx | xxx | x | o |
| caudate-putamen (mainly v and vl) | x | o | o | xxx | xx | xxx | xxx |
| globus pallidus | xx | x | o | xxx | xx | xxx | xxx |
| entopeduncular nucleus | o | xx | x | o | xx | xx | o |
| subthalamic nucleus | o | o | o | xxx | o | xxx | xxx |
| substantia nigra compacta | xxx | xx | xx | xxx | xxx | xxx | xx |
| substantia nigra reticulata | x | xx | xx | xxx | xxx | xxx | xx |
| Septum and extended amygdala | |||||||
| lateral septum | xxx | xx | xx | o | x | o | o |
| medial septum-diagonal band | xxx | xx | xx | o | x | o | o |
| bed nucleus of stria terminalis, med-d | xx | xxx | xxx | xxx | x | xxx | o |
| bed nucleus of stria terminalis, lat-d | x | x | x | xxx | xxx | xxx | o |
| bed nucleus of stria terminalis, med-v | xx | xxx | xxx | xxx | x | xx | o |
| bed nucleus of stria terminalis, lat-v | x | xxx | xxx | xxx | xxx | xx | o |
| marginal nucleus of Shu et al. (1988) | o | o | o | xxx | o | xxx | xxx |
| sublenticular extended amgydala | xxx | xxx | xxx | xxx | xxx | xxx | xx |
| anterior amygdaloid area | xxx | xxx | xxx | xxx | xxx | xxx | xx |
| central amygdaloid nucleus, lat | xx | xxx | xxx | xxx | o | xxx | x |
| central amygdaloid nucleus, med | xx | xxx | xxx | xxx | xxx | xxx | xx |
| Hypothalamus | |||||||
| median preoptic nucleus | xx | xx | x | o | xxx | o | o |
| lateral preoptic area | xxx | xxx | xx | xxx | xxx | xxx | o |
| medial preoptic area | xxx | xxx | xxx | xxx | xxx | xxx | o |
| lateral hypothalamic area | xxx | xxx | xxx | xxx | xxx | xxx | o |
| paraventricular nucleus | xx | xx | xx | xxx | xxx | xx | o |
| posterior hypothalamic area | xxx | xxx | xx | o | xxx | x | o |
| central gray | xxx | xxx | xxx | xxx | xxx | xxx | xxx |
| supramammillary nucleus | xxx | xx | o | o | xxx | o | o |
| mammillary body | x | xx | o | o | o | o | o |
| Thalamus and epithalamus | |||||||
| lateral habenula | xxx | xxx | xx | xx | xxx | xxx | o |
| parafascicular nucleus | xxx | xxx | xxx | xxx | xxx | xxx | o |
| ventromedial nucleus | x | xxx | xx | xx | o | xx | xx |
| posterior intralaminar nucleus | xx | xxx | x | xx | xxx | xx | xx |
| Other structures (ordered rostrocaudally) | |||||||
| zona incerta | xxx | xxx | xxx | xxx | xxx | xxx | xx |
| parasubthalamic nucleus | x | x | o | xxx | xxx | xxx | o |
| ventral tegmental area | xx | xx | x | xx | xxx | xx | xx |
| interpeduncular nucleus | nd | x | o | o | o | o | o |
| rostromedial tegmental nucleus | xxx | x | xx | xxx | xx | xxx | xx |
| superior colliculus, deep layers | xx | xxx | xxx | xxx | xx | xxx | xx |
| periaqueductal gray | xxx | xxx | xxx | xxx | xxx | xxx | xx |
| deep mesencephalic nucleus | xx | xxx | xxx | xxx | xxx | xxx | xx |
| dorsal raphe | xxx | xxx | xx | xx | xxx | xx | xx |
| pedunculopontine tegmental nucleus | xxx | xxx | xxx | xxx | xxx | xxx | xx |
| laterodorsal tegmental nucleus | xxx | xxx | xxx | xx | xxx | xx | x |
| median raphe | xxx | xx | o | x | xx | o | o |
| parabrachial nucleus | xx | xxx | xxx | xxx | xxx | xxx | xx |
| cuneiform nucleus | xxx | xx | xxx | xxx | xxx | x | xx |
| pontine reticular nucleus, oralis | xx | xxx | xx | xxx | xxx | xx | x |
| pontine reticular nucleus, caudalis | x | xxx | xx | xxx | xxx | xx | x |
| principal nucleus V, ventrolateral (contralat) | o | xx | x | xx | xx | xx | o |
| Evaluated cases | 99039 | 08024 | 06108 | 05103 | 10098 | 05104 | 05096 |
| 99060 | 06157 | 05105 | 10099 | 05106 | 05095 | ||
| 06166 | 10090 | ||||||
| 10091 | |||||||
| 10096 | |||||||
| 10097 | |||||||
Representative injection sites are illulstrated in Figure 1. o = absent or trace retrograde labeling reflecting 0–5 labeled cells/structure. + = 6 to 15 labeled neurons/section. ++ = 16–50 labeled neurons/section. +++ = more than 50 labeled neurons/section/structure. This list references labeling of ipsilateral structures following the indicated injections, listed in approximate rostocaudal sequence. Representative contralateral labeling is illustrated in Figure 3. Abbreviations: c – compacta; d- dorsal; lat – lateral; lat-d – lateral division, dorsal part; lat-v – lateral division, ventral part; med – medial; med-d – medial division, dorsal part; med-v – medial division ventral part; mid – middle; PAG – periaqueductal gray; RRF – retrorubral field; SN – substantia nigra; v – ventral; vl – ventrolateral; VTA – ventral tegmental area.
Table 3.
| Injection site | Number of retrogradely labeled structures | |||||
|---|---|---|---|---|---|---|
| xxx | xx | x | o | Total | x index | |
| RRF lat | 43 | 15 | 1 | 8 | 60 | 160 |
| PAGvl | 39 | 14 | 4 | 11 | 57 | 149 |
| RRF med | 35 | 15 | 7 | 11 | 57 | 142 |
| SNc | 32 | 18 | 3 | 15 | 53 | 135 |
| VTA | 28 | 15 | 10 | 14 | 53 | 124 |
| RRF mid | 22 | 16 | 13 | 17 | 51 | 111 |
| SNr | 9 | 20 | 6 | 32 | 35 | 73 |
Data are transposed from Table 2 such that columns 2–4 from the left list the numbers of retrogradely labeled structures with xxx, xx, x and o ratings following Fluoro-Gold injections in the listed Injection Sites. The column entitled “Total” gives the numbers of structures with labeling, i.e., the sum of the values in columns xxx, xx and x. The “x index” is equivalent to the value in column xxx multiplied by 3 plus that in column xx multiplied by 2 plus that in column x and provides a relative estimate of the overall magnitude of retrograde labeling for each injection site. The injection sites are listed in order of descending magnitude of the retrograde labeling that they produced. Abbreviations: dorsal, caudal; lat – lateral; med – medial; mid – middle; RRF - retrorubral field; rvlPAG – rostral ventrolateral periaqueductal gray; SNc and SNr - substantia nigra compacta and reticulata; VTA - ventral tegmental area.
An abundance of retrogradely labeled neurons was present in the ipsilateral and, to a lesser extent, contralateral, frontal cortex, including the frontal association, prelimbic, infralimbic, dorsal peduncular and dysgranular insular areas and the claustrum (Fig. 3A–D). Less dense labeling was present in the ipsilateral anterior cingulate cortex (Fig. 3B and C) and fewer labeled neurons, scattered within the dorsolateral convexity of the frontal cortex were observed only after injections in the lateral RRF (Fig. 4B and C).
Sparse to moderate retrograde labeling was observed in the accumbens, mainly in the rostral pole and ventromedial part of the shell (Figs. 3B and C), except following lateral RRF injections, which produced very dense labeling not only in the accumbens core, rostral pole and ventromedial and lateral shell (Fig. 4A), but also a ventralmost tier of the caudate-putamen extending from the lateral ventricle to the external capsule (Fig. 4A–C). Labeled neurons in the caudate-putamen and globus pallidus (Fig. 4C), however, may have resulted from the nearly unavoidable involvement of the lateral part of the substantia nigra in lateral RRF FG injection sites (see Fig. 1C-C”). To test this idea, we made a number of control PHA-L injections into the ventral caudate-putamen and failed to produce significant anterograde labeling in the RRF or PAGvl (not shown).
Sparse labeling was observed in the subcommissural ventral pallidum after PAGvl and RRF injections (Fig. 3D and 4B). Significantly, the density of labeling at the edges of and adjacent to the ventral pallidum exceeded that in the ventral pallidum itself. This was true both above the VP, in relation to the interstitial nucleus of the posterior limb of the anterior commissure (IPAC), a component of the extended amygdala (Shammah-Lagnado et al., 2001), and below it, in a stratum of immunocytochemically extended amygdala (EA)-like (also Shammah-Lagnado et al., 2001) tissue that intervenes between the ventral pallidum and diagonal band complex (Fig. 3D). Dense labeling of IPAC extended over the caudal dorsolateral convexity of the subcommissural pallidum into the so-called ventral pocket of the striatum (Figs. 3D and E). The BST, notably excepting its medial division, contained dense labeling (Figs. 2A and A” and 3E and F) and an additional band of dense labeling stretching across the sublenticular region (Figs. 3E–G) merged with dense labeling in the medial division of the CeA (Fig. 3H). In contrast, the medial amygdaloid nucleus (Fig. 3I), like the medial division of the BST (Fig. 3E and F), contained no retrogradely labeled neurons. See Discussion for notes on BST subnuclei and other subdivisions.
Labeling in the ventral pallidum, EA surrounding it, and BSTl-v merged caudally with remarkably dense labeling in the lateral (Figs. 3E–G) and medial (Figs. 3E and 4C and F) preoptic areas. The parvicellular division of the paraventricular nucleus of the hypothalamus, which is abundantly interconnected with EA, contained many retrogradely labeled neurons mostly in its caudal part (Figs. 3G and H and 4G and H). The lateral hypothalamic area, including the parasubthalamic nucleus, which has strong reciprocal interconnections with various EA structures (Goto and Swanson, 2004), contained dense numbers of FG labeled cells following injections into the RRF (Figs. 3I and 4D) and PAGvl (Fig. 4I). Dense accumulations of labeled neurons were present in the mediobasal and periventricular hypothalamus following FG injections into the PAGvl (Fig. 4G and H) but not RRF (Fig. 3F–I and 4B and C).
In the diencephalon, the lateral habenular nucleus (LHb) contained many retrogradely labeled neurons, while the medial habenula was empty (Fig. 3I). In the caudal diencephalon, dense accumulations of labeled neurons were present in the thalamic parafascicular nucleus, zona incerta and central gray following all of the RRF and PAGvl injections (Figs. 3I and J and 4D and I).
Labeled neurons were scattered fairly densely through much of the mesencephalic and rostral pontine tegmentum after injections of FG into the RRF, but the most prominent pontomesencephalic labeling occupied the PAGvl (Fig. 3K). Conversely, injections of FG into the PAGvl produced labeling in RRF neurons. Injections into the medial RRF produced dense bilateral labeling in the deep layers of the superior colliculus (not shown), which was not observed after FG injections into the PAGvl or other parts of the RRF and thus may reflect projections to nearby structures, such as the rostromedial tegmental nucleus (Jhou et al., 2009). Moderate to dense labeling was present in the substantia nigra reticulata (Fig. 3K), compacta (Fig. 3L) and, to a lesser extent, VTA (Fig. 3K) after all of the PAGvl and RRF injections. Some retrogradely labeled neurons located in dopaminergic structures following injections of FG into the RRF and PAG exhibited co-localized tyrosine hydroxylase immunoreactivity, but most did not. Dense retrograde labeling was present in the parabrachial nucleus (Fig. 3N), which is abundantly interconnected with the extended amygdala, and additional moderate labeling in the pons and medulla was most dense in the medial, gigantocellular part of the reticular formation (Figs. 3O–P). Labeled neurons were also present in the nucleus of the solitary tract, with which forebrain extended amygdaloid structures are interconnected (Fig. 4E and J). All RRF and PAGvl injections produced labeled neurons in the deep cerebellar nuclei (Fig. 3O).
The retrograde labeling results are further summarized in Tables 2 and 3, which also show data for comparative purposes from some VTA cases archived from a previous study (Geisler and Zahm, 2005) and some injections into the substantia nigra compacta (see, e.g., Fig. 1E and E’) and reticulata. Table 2 gives estimates of the numbers of retrogradely labeled neurons produced in various structures by different injections and documents the abundance of retrograde labeling in structures comprising and interconnected with the EA following FG injections in the RRF, PAG and SNc. Table 3 provides the numbers of structures labeled by each of the injection sites and shows that all parts of the complex produce robust retrograde labeling in many cortical and subcortical sites, with the RRF and PAGvl ranking as the most diversely innervated of the midbrain dopaminergic structures.
Anterograde tracing
Extended amygdala and preoptic area
Following injections of PHA-L into structures comprising the EA, including the BST, CeA and sublenticular region, robust, topographically organized anterograde labeling was observed in the ventral midbrain dopaminergic complex, mainly in the RRF and PAGvl, but also in the VTA and SNc. The general pattern was for labeled axons to descend through the lateral hypothalamus in the medial forebrain bundle and, upon reaching the VTA and SNc, turn lateralward as varyingly compact bundles (Fig. 5A and A’ and 6A and B) enroute to the RRF (Fig. 5B and B’ and 6C and D) and PAGvl (Fig. 5C and C’ and 6D). Depending upon where in the BST the injection site was, labeled projections varied with regard to topography and how terminally branched and ‘varicose’, i.e., beaded in appearance, were the axons (Fig. 5A’, B’ and C’). Terminal branching and axonal varicosities, a.k.a. “puncta”, are a reflection of the functional potency of labeled axons (see Methodological considerations in the Discussion). In view of this revealing feature of PHA-L and BDA labeling, all of the included maps of anterogradely transported PHA-L were created by using the Neurolucida hardware-software platform to plot exclusively axonal varicosities, i.e., puncta (arrowed structures in Figure 5A’), such that non-varicose, presumably mainly non-synaptic, parts of labeled axons are excluded from the maps.
Figure 6.
Micrographs illustrating case 06042 (also shown in Fig. 5) in sections processed for tyrosine hydroxylase (brown reaction product) and PHA-L (black reaction product) immunoreactivity, which reveals that anterograde labeling following the large PHA-L injection shown in the inset in Fig. 5A is present mainly within the midbrain dopaminergic complex. Additional abbreviations: PAGvl – ventrolateral periaqueductal gray; RRFc – caudal part of the retrorubral field. Scale bar: 1 mm.
The projection from the BST to the midbrain dopaminergic complex is topographically organized. PHA-L injections into BSTl-d (Fig. 7A) produced bundles of labeled axons that turn lateralward quite rostrally in the VTA, thus achieving a position in the lateral part of the SNc at a rostral level. Mapping of puncta proximal to the RRF after BSTl-d injections revealed mainly passing fibers with few axonal varicosities (Fig. 8A and B). A plexus of highly varicose labeled axons was present in the RRF, mainly in its lateral part (Fig. 8C). The PAGvl also contained numerous highly varicose labeled axons following injections in BSTl-d (Fig. 8D). Injections of PHA-L slightly more ventral in BSTl (Fig. 7B) produced labeling in axons that turn lateralward further caudal in the VTA, spread more broadly (i.e., medialward) within the RRF (Fig. 8G), and, particularly in the SNc and to some extent the VTA, were more varicose (Fig. 8E and F). Far ventral BST injections of PHA-L (Fig. 7C) completed a trend toward more widespread varicose labeling in the SNc, RRF, PAGvl and VTA (Fig. 8I–L), although the density of labeled puncta in the VTA consistently remained significantly less than in the SNc, RRF and PAGvl. Interestingly, injections of PHA-L into the lateral preoptic area (LPO) beneath the BST (Fig. 7D) produced a pattern of anterograde labeling in the ventral mesencephalon similar to that seen after far ventral BST injections, albeit more diffuse and substantially more concentrated in the VTA (Fig. 8M–P). Indeed, the pattern seen in the RRF and VTA after LPO injections could be imagined as a culmination of the transformations in the labeling pattern associated with moving injection sites more ventrally in BSTl.
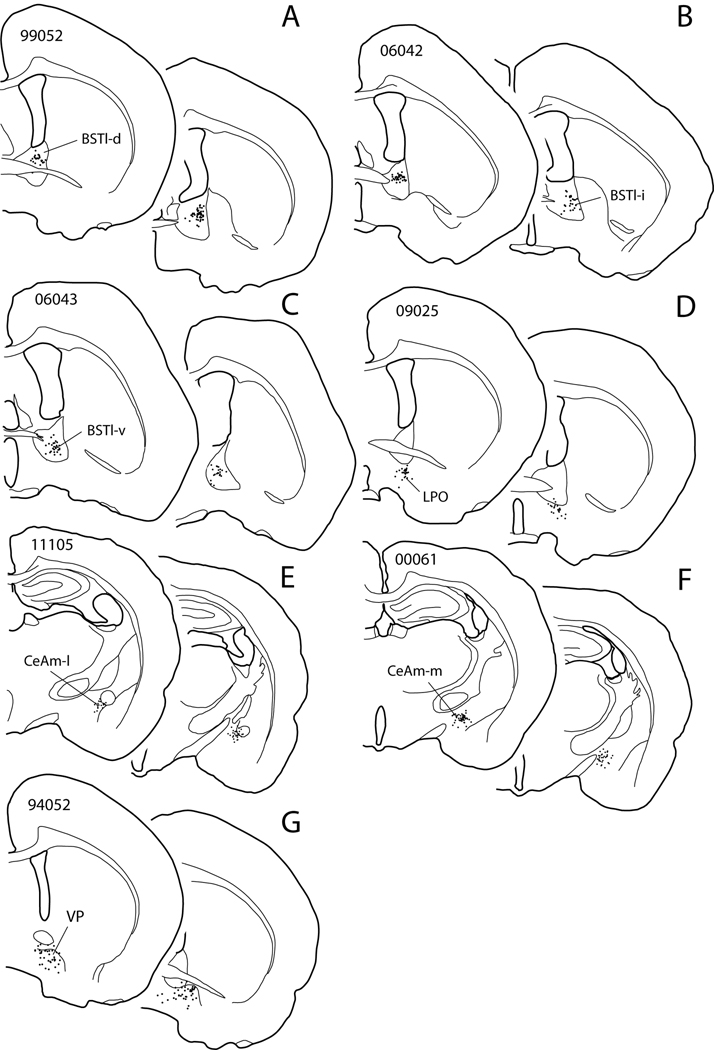
Figure 7.
Diagram showing PHA-L injection sites (A–F) and a BDA injection (G) in cases discussed in the text and illustrated in figures 8 (A–D), 9 (E, F) and 12. Two levels are shown for each injection. Case numbers are given on the more rostral sections of the pairs. Dots indicate PHA-L impregnated neurons, which are thought to give rise to the labeled projection. Abbreviations: BSTl-d, BSTl-i and BSTl-v – bed nucleus of stria terminalis, lateral division, dorsal, intermediate and ventral parts, respectively; CeAm-l and CeAm-m – central nucleus of the amygdala, lateral and medial parts, respectively; LPO – lateral preoptic area; VP – ventral pallidum.
Figure 8.
Diagrams illustrating anterograde labeling associated with PHA-L injections at dorsal (99052 BSTl-d), intermediate (06042 BSTl-i) and ventral (06043 BSTl-v) parts of the lateral division of the bed nucleus of the stria terminalis (BSTl-d, BSTl-i and BSTl-v, respectively) and in the lateral preoptic area (09025 LPO). The injection sites are shown in Fig. 7A–F. Broken lines circumscribe the tyrosine hydroxylase-rich midbrain dopaminergic complex, these having been circumscribed from adjacent serial sections processed to illustrate TH immunoreactivity. Sections processed for TH immunoreactivity were unavailable for case 09052 (A–D). Note the increasing medialward spread of anterograde labeling in the RRF and particularly into the VTA with more ventral injections and that the anterograde labeling after ventral BST injections most resembles that observed following injections into the LPO, particularly with respect to the VTA. For abbreviations, see list.
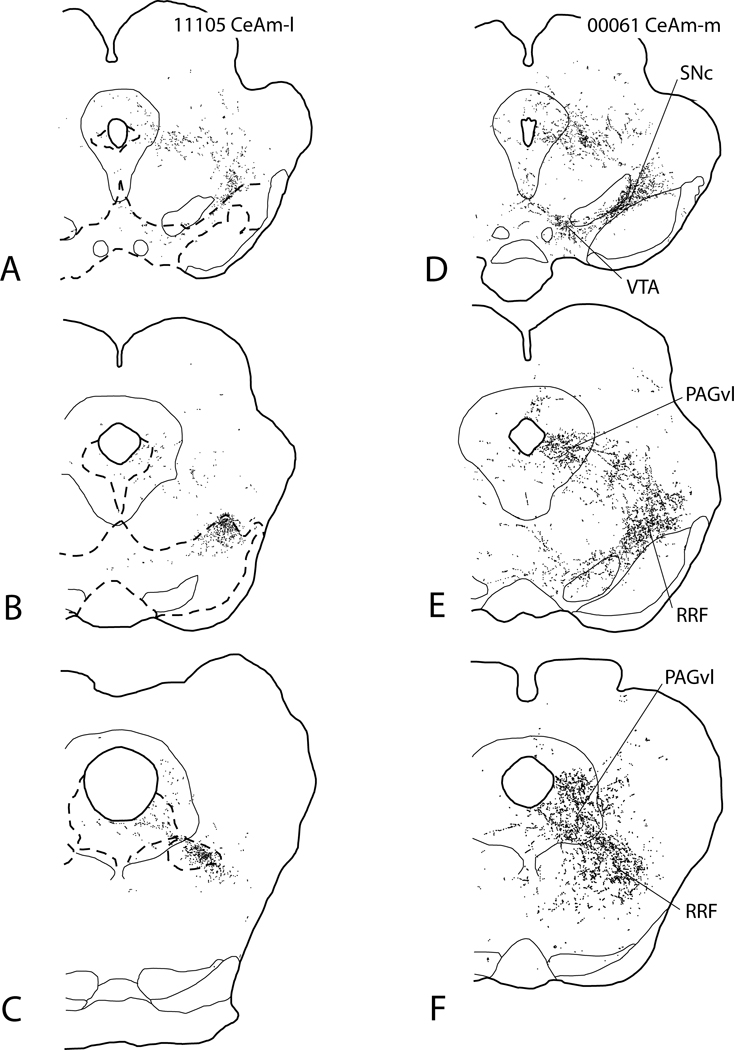
Injections of PHA-L into CeAm (Fig. 9, injection sites shown in Fig. 7E and F) produced a pattern of anterograde labeling in the VTA, SNc, RRF and PAGvl that is topographically organized and nearly identical to that observed after BSTl injections. Injections into CeAm-l produced labeled fibers that, like BSTl-d injections, turn lateralward early, have few puncta in the SNc and almost none in the VTA, but ramify extensively in the lateral RRF and, albeit less so, in the PAGvl (Fig. 9A–C). In contrast, injections into CeAm-m produced a pattern of labeled fibers (Fig. 9D–F) resembling that observed after BSTl-v injections, i.e., widespread varicose labeling in the SNc, RRF and PAGvl (Fig. 8I–L) with moderate labeling of puncta in the VTA. Like the pattern of terminations in the midbrain after ventral BST injections resembled that of the LPO, the pattern observed after CeAm-m injections resembled that seen following injections into the LH, which lies just medial to CeAm-m and gives rise to projections to the midbrain dopaminergic complex much like those from LPO (see below under Prelimbic cortex and lateral hypothalamus).
Figure 9.
Diagrams illustrating anterograde labeling associated with PHA-L injections in the lateral (A–C) and medial (D–F) parts of the medial division of the central nucleus of the amygdala (CeAm-l and CeAm-m, respectively). The injection sites are shown in Fig. 7E and F. Broken lines circumscribe the tyrosine hydroxylase-rich midbrain dopaminergic complex, having been circumscribed from adjacent serial sections processed to for TH immunoreactivity. Sections processed for TH immunoreactivity were unavailable for case 00061 (A–C). Note the increasing medialward spread of anterograde labeling in the RRF and particularly the VTA with more medial injections and that the anterograde labeling after CeAm-m injections more resembles that observed following injections into the LPO (see Fig. 8M–P). For abbreviations, see list.
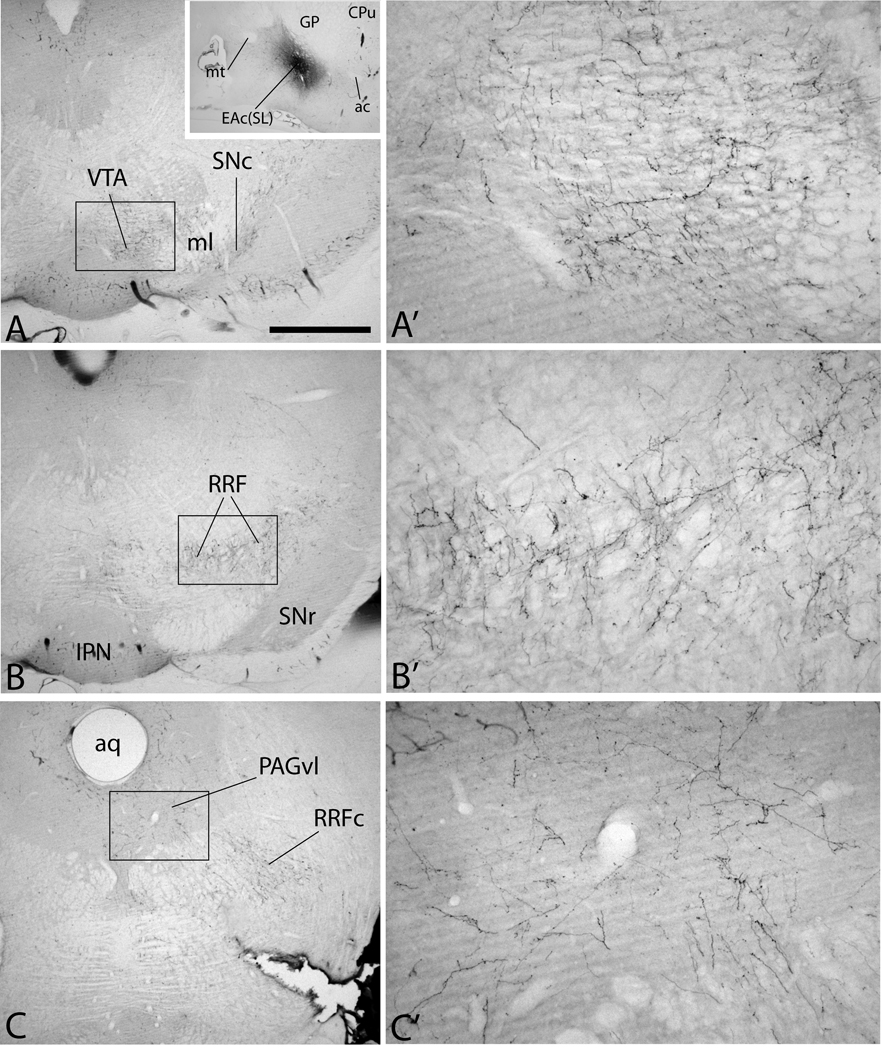
One available injection of PHA-L into the sublenticular part of the EA (case 95109, Fig. 10A, inset) produced broadly dispersed, robust, varicose anterograde labeling in the SNc, medial and lateral RRF (Fig. 10B and B’), PAGvl (Fig. 10C and C’) and modest varicose labeling in the VTA (Fig. 10A and A’).
Figure 10.
Micrographs illustrating case 95108 in which an injection of PHA-L into the central division of the extended amygdala in the sublenticular region [EAc(SL)] resulted in abundant anterogradely labeled axons in the midbrain dopaminergic complex. Numerous labeled fibers were present in the ventral tegmental area (VTA, A and A’), including in a substantial number of varicose labeled axons. Labeled fibers were also observed in the substantia nigra compacta (SNc in A), retrorubral field (RRF in B and B’) and lateral and ventrolateral parts of the periaqueductal gray substance (PAGvl in C and C’). For abbreviations, see list. Scale bar: 1 mm in A, B and C; 100 µm in A’, B’, and C’; 2.5 mm in the inset.
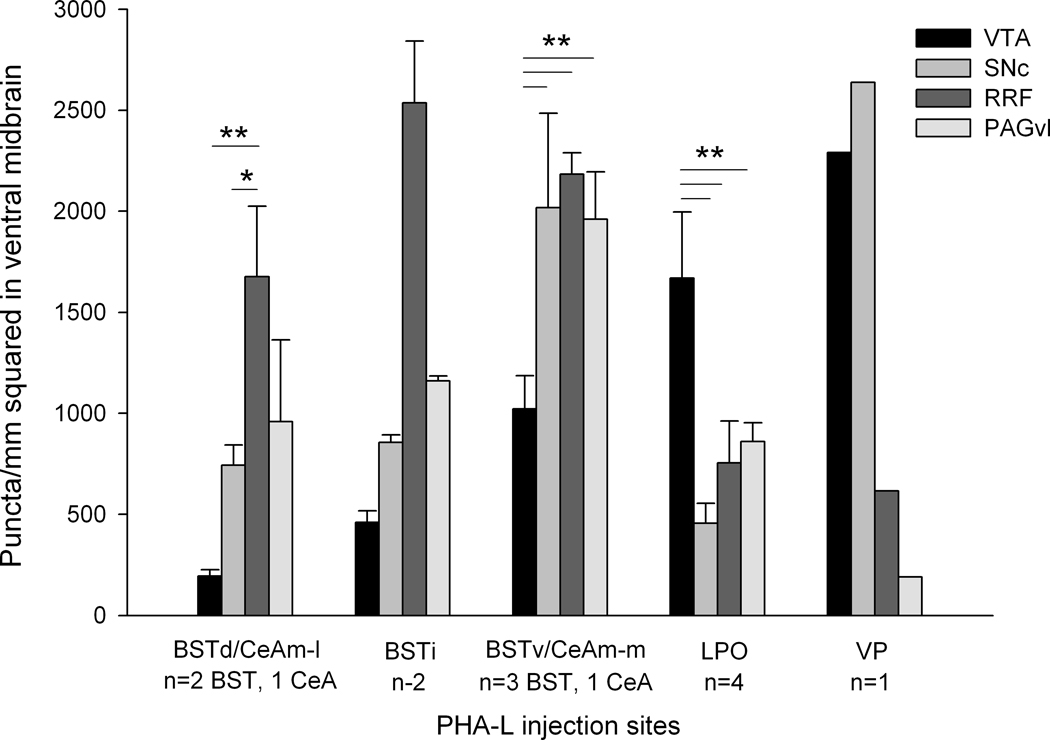
With the aid of the Neurolucida hardware-software platform, we were able to count the numbers of puncta plotted and derive measures of areal density in the various parts of the midbrain dopaminergic complex following injections of anterograde tracer in the BST, CeA, LPO and VP (see Quantitation of anterogradely labeled axonal varicosities in Materials and Methods). The data, shown in Figure 11, reveal that central extended amygdala structures (only BST and CeA projections were quantitated) richly innervate the RRF and PAGvl, in contrast to the LPO, which more strongly innervates the VTA, and ventral striatopallidum, which strongly innervates the VTA and medial SNC.
Figure 11.
Graph showing results measuring the densities of labeled axonal varicosities, or “puncta” in the ventral tegmental area (VTA), substantia nigra compacta (SNc), retrorubral field (RRF) and ventral and lateral sectors of the periaqueductal gray substance (PAGvl) following injections of PHA-L at dorsal (BSTd), intermediate (BSTi) and ventral (BSTv) levels of the bed nucleus of stria terminalis, lateral (CeAm-l) and medial (CeAm-m) part of the medial division of the central nucleus of the amygdala, lateral preoptic area (LPO) and ventral pallidum (VP). The VP was injected with biotinylated dextran amine (BDA) rather than PHA-L. Data were evaluated by a one way ANOVA followed by Fisher’s LSD post-hoc test only in groups of 3 or more. * indicates p< 0.05; ** indicates p<0.01.
In contrast to the central division of the EA, PHA-L injections into the medial division of the EA produced no anterograde labeling in the ventral mesencephalon. For example, PHA-L injected into the medial division of the BST produced massive labeling in the mediobasal hypothalamus, but none in the midbrain dopaminergic complex (cases 99120 and 06029, data not shown). Likewise, an injection of PHA-L into the medial amygdaloid nucleus produced no anterogradely labeled fibers in the midbrain (case 00060, not shown), consistent with another published report (Canteras et al., 1995).
Ventral pallidum
Injections of PHA-L into the medial part of the subcommissural ventral pallidum produced robust anterograde labeling of fibers mainly in the VTA and, to a much lesser extent in medial parts of the RRF. Few fibers traversing the SNc were observed following such injections. In contrast, PHA-L injections into more lateral parts of the subcommissural ventral pallidum produced dense labeling of the lateral VTA, dorsomedial SNr and overlying parts of the SNc. Many varicose and non-varicose axons fibers traversed the SNc. The former contributed to scattered tufts of terminal axonal branching within the SNc. Slight to moderate labeling of axons in the RRF was observed following VP injections and a few labeled fibers were seen in the PAGvl.
A number of cases were available in which the anterogradely and retrogradely transported tracer, biotinylated dextran amine (BDA), was injected into the ventral pallidum. In such cases, BDA presumably is transported anterogradely not only in axons of ventral pallidal neurons that picked up the tracer at the injection site, but also in the axon collaterals of back-filled striatal neurons (Chen and Aston-Jones, 1998; Zahm, 2006), of which many project to the ventral mesencephalon (Lu et al., 1998). In these cases, dense, highly varicose anterograde labeling, presumably reflecting overlapping pallidomesencephalic and striatomesencephalic projection fibers, was prominent in the VTA, SNc and SNr (Fig. 12A–B), by comparison to the modest numbers of labeled axons in the RRF (Fig. 12C) and PAGvl (fig. 12D). One injection site, for the case illustrated in Figure 12, is shown in Fig. 7G and only that case was quantitated and included in Fig. 11.
Figure 12.
Diagrams illustrating case 94052 in which an injection of BDA into the ventral pallidum (see Figure 7G for injection site) resulted in robust anterograde labeling in the ventral tegmental area (VTA in A and B) and substantia nigra compacta (SNc in A and B). A lesser amount of anterograde labeling was present in the retrorubral field (RRF in C and D) and PAGvl (D). Broken lines circumscribe the tyrosine hydroxylase-rich midbrain dopaminergic complex, having been circumscribed from adjacent serial sections processed for TH immunoreactivity.
Lateral habenula
Injections of PHA-L (cases 070142, 08002, 08004 and 08011, not shown) produced moderate, varicose labeling in the VTA and a strong patch of varicose labeling in the RMTg, which abuts the VTA caudally. In addition, sparse varicose anterograde labeling was observed in the SNc, RRF and PAGvl.
Zona incerta
A PHA-L injection into the zona incerta (case 95108, Fig. 13A) produced a very dense plexus of labeled axons in the mesencephalic tegmentum mainly dorsal to the VTA and SNc (Fig. 13B). Although the interpretation of case 95108 would have benefited from demonstration of tyrosine hydroxylase immunoreactivity to precisely indicate boundaries of the RRF (estimated as outlined in Fig. 13C–E), the territories known to contain dense tyrosine hydroxylase immunoreactivity, such as the VTA, SNc and RRF, were mainly, but not entirely, avoided by labeled axons in case 95108. An extensive plexus of labeled fibers was present in the lateral PAG, but the ventrolateral PAG (outlined in Fig. 13F), where numerous tyrosine hydroxylase immunoreactive neurons are present (Fig. 1D and 1D inset), was relatively devoid of such labeled fibers.
Figure 13.
Micrographs illustrating case 95108 in which an injection of PHA-L into the zona incerta (ZI) resulted in dense retrograde labeling in the midbrain tegmentum that largely avoided the midbrain dopaminergic complex shown at the level of the ventral tegmental area (VTA in B), retrorubral field (C, D and E) and periaqueductal gray (PAGvl in F). The white dots in A indicate the position of neurons impregnated with PHA-L immunoreactivity. Broken lines designate the dorsal/dorsolateral margin of the VTA in B and approximate boundaries of the retrorubral field (C, D and E) and ventrolateral periaqueductal gray (PAGvl in F). Note Anterograde labeling within the PAGvl diminishes ventrally where many of the tyrosine hydroxylase immunoreactive neurons that project to the central division of the extended amygdala are located. For abbreviations, see list.: Scale bar: 1 mm.
Prelimbic cortex and lateral hypothalamus
Following a PHA-L injection into either the deep layers of the prelimbic cortex (cases 04062, and 04066, not shown) or lateral hypothalamus (cases 96049, 96108, and 96199, not shown), a moderately dense plexus of labeled varicose axons pervaded the VTA. A significant number of these veered laterally to pass into the SNc, mostly as fibers of passage lacking numerous axonal varicosities in the case of PrL, but moderately varicose after LH injections. PrL injections produced a moderately dense plexus of labeled axons located just rostral to the lateral RRF, mainly in the posterior intralaminar thalamic nucleus. For both injection sites, sparse projections containing immunoreactive PHA-L were present throughout the RRF, however, and these appeared to be part of a more widespread sparse distribution of labeled fibers occupying much of the mesencephalic reticular nucleus. A modest plexus of labeled axons was present in the PAGvl following injections of PHA-L into the PrL and LH.
Ventral mesencephalon
A number of cases with PHA-L injections into sites in the ventral midbrain within and around the dopaminergic complex were made (see Materials and Methods for cases). Most injections produced some varicose anterograde labeling in the midbrain dopaminergic complex and this tended to be greater for sites located within the complex. Very dense anterograde labeling was present in the VTA and SNc and, to a lesser extent, the RRF and PAG following injections in the RMTg (see also Jhou et al., 2009).
Discussion
The combination of results from retrograde and anterograde axonal tracing described herein reveals that projections from the EAc stand out as predominant among manifold inputs to the RRF and PAGvl (Fig. 3, Table 2). Robust RRF- PAGvl inputs also arise in the hypothalamic paraventricular nucleus, lateral hypothalamus, parasubthalamic nucleus, parabrachial nucleus and nucleus of the tractus solitarius (Sol), which happen to be structures with which the core structures of the EA, the BST, i.e., the sublenticular EA and CeA, are most extensively interconnected. Indeed, Aston-Jones and colleagues (Mejias-Aponte et al., 2009) reported that the peak density of noradrenergic projections from Sol to the ventral mesencephalon is in the RRF. This interconnectivity of EAc structures with the RRF-PAGvl supports the conclusion that the RRF and PAGvl together represent a sector of the midbrain dopaminergic complex serving prominently as a node for outputs from EAc. These results complement earlier published findings by Hasue and Shammah-Lagnado (2004) indicating that projections originating in the PAGvl, including the dorsal raphe nucleus embedded within it, and the RRF, contain the majority of tyrosine hydroxylase-immunoreactive neurons providing innervation to the EA (see also Table in Zahm and Trimble, 2008).
Methodological considerations
The retrograde labeling results reported here are subject to potential confounds, such as uptake of tracer by damaged and undamaged fibers-of-passage and spread of injected tracer to unintended structures, that have been discussed at length in our recent papers (Geisler and Zahm, 2005; Jhou et al., 2009). However, in our present and recent evaluations of inputs to ventral mesencephalic structures (e.g., Geisler and Zahm, 2005; Jhou et al., 2009), we almost always observed that structures with retrograde labeling, when targeted by anterogradely transported PHA-L, do exhibit some varicosity-decorated labeled axons in the structures of interest. Sometimes sparse inputs to the target structure comprise but part of a more widely distributed projection, as do, e.g., prelimbic cortical, lateral preoptic area (Fig. 8M–P) and lateral hypothalamic projections to the RRF/PAGvl shown in the present study. Alternatively, input from a strongly innervated adjacent structure may encroach into the target structure, e.g., as the robust ZI projection to the dorsolateral PAG invades the PAGvl (Fig. 13F). It should also be acknowledged that the uptake of FG by axons is vigorous (Brog et al., 1993; Geisler and Zahm, 2005), such that FG uptake even by a sparse projection may give rise to abundant retrogradely labeled neurons. Conversely, a relatively small number of retrogradely labeled neurons, such as are observed, e.g., in the mesopontine rostromedial tegmental nucleus following FG injections into the VTA and SNc, may give rise to a very dense projection field (Jhou et al., 2009). Thus, because purely spurious retrograde labeling is rare in our experience, we conclude that the capacity of retrograde tracing to reveal all of the potential afferents of a structure has value that cannot be overestimated. The possibility that retrogradely labeled sites might later be shown to lack, or, alternatively, have particularly robust inputs to the midbrain dopaminergic complex enhances rather than negates the usefulness of a comprehensive map of the retrograde labeling (as in Fig. 3).
Complementary anterograde tracing is an essential control, however, and, in the present investigation, we used new and archived cases with injections of anterograde tracer into numerous structures, illustrating a selection of those that we regarded as more likely to have projections critical to the validity of the conclusions suggested by the retrograde tracing. One example of an apparently spurious retrograde labeling result disclosed by anterograde labeling was the avoidance of the VTA, SNc and RRF by the projection labeled after a PHA-L injection in the ZI (Fig. 13), despite prominent retrograde labeling observed in the ZI following retrograde tracer injections in the RRF (Figs. 3J and 4D and I). Consistent with considerations mentioned earlier, the observed retrograde labeling in ZI likely reflects the sparse but detectable input of ZI to the RRF/PAGvl and uptake of tracer by ZI axons enveloping the VTA, SNc and RRF (Fig. 13).
In describing the anterograde tracing results, we distinguished what we refer to as ‘varicose’ labeled axons, i.e., ones exhibiting a beaded appearance, from those lacking such apparent differentiation. Insofar as axonal swellings typically, although not always, exhibit synapses when viewed with electron microscopy (e.g., Loopuijt and Zahm, 2006; Omelchenko and Sesack, 2009; 2010), varicose projections may be conditionally regarded as likely to reflect functional, synaptic relationships.
Finally, we noted that the retrograde labeling observed within the VTA, SNc, RRF and PAGvl mainly, although not entirely, involved neurons lacking tyrosine hydroxylase immunoreactivity, suggesting a mainly non-dopaminergic local circuitry. It should be pointed out in this regard, that combining DAB and nickel DAB to generate brown and black immunoperoxidase products, respectively, as was done in the present study, is not an entirely satisfactory way to show such double labeling, due to a tendency of these chromogens to obscure each other. Further investigation is necessary to better address the issue of the phenotypes of the interconnections among the different structures comprising the midbrain dopaminergic complex. The importance of this interconnectivity is highlighted by much early evidence identifying the rostral mesencephalon as a critical interface linking cognitively generated directives originated in the forebrain with a hindbrain-spinal cord circuitry autonomously capable of supporting of differentiated, coordinated postural and motoric function (e.g., Ferrier, 1876/1966; Hinsey et al., 1930; Bard and Macht, 1958; Harris, 1958, for reviews).
Extended amygdala
Two divisions of the EA, central (EAc) and medial (EAm), have been described (de Olmos and Ingram, 1972; Alheid and Heimer, 1988; Heimer et al., 1991a; Heimer and Alheid, 1991; de Olmos and Heimer, 1999). EAc consists of the CeA, BSTl and hodologically similar sectors in the sublenticular region and stria terminalis that extend between them. EAc is characterized by robust outputs to the hypothalamic paraventricular nucleus, lateral hypothalamus, parabrachial nucleus and nucleus of the solitary tract, consistent with an involvement in the central regulation of autonomic function. EAm comprises the medial amygdala, medial division of the BST and hodologically similar parts of the sublenticular region and stria terminalis that extend between them. EAm projects robustly to the mediobasal hypothalamus, consistent with a role in the regulation of neuroendocrine function.
Earlier studies from numerous laboratories showed that EAc projects strongly to the dopaminergic midbrain tegmentum (e.g., Krettek and Price, 1978; Price and Amaral, 1981; van der Kooy et al., 1984; Holstege et al., 1985; Gonzalez and Chesselet, 1990; Rosen et al., 1991; Zahm et al., 1999; Fudge and Haber, 2000; Shammah-Lagnado et al., 2001; Gastard et al., 2002; Zahm, 2006). The present investigation has revealed that these projections possess a previously unrecognized degree of specificity for RRF-PAGvl. Indeed, we have shown here that EAc projections involve the VTA and rostromedial SNC to a much lesser extent than the RRF-PAGvl, mainly passing through the VTA and rostromedial SNc with few varicosities and negligible branching before diverging laterally to densely innervate the caudolateral SNc and the RRF and PAGvl. The present results also indicate that EAm does not project to the midbrain dopaminergic complex, consistent with other reports (e.g., Canteras et al., 1995; Dong and Swanson, 2004b).
It should be noted that some authors maintain that EAc-VTA projections are robust (Fudge and Haber, 2000, in the monkey). Furthermore, presumably monosynaptic activations of VTA neurons following stimulation of the BST have been reported in the rat (Georges and Aston-Jones, 2001; 2002; Dumont and Williams, 2004; Massi et al., 2008; Jalabert et al., 2009). Tracer injections positioned in the ventral part of the BST in the present study produced a distinctly broad and uniform spread of tracer within the RRF and moderate anterograde labeling in the VTA, much as Swanson and colleagues also observed a ‘diffuse’ projection pattern after injection of PHA-L into the fusiform nucleus (a ventral BSTl structure) as compared to the oval nucleus, which has a more dorsal position in the BSTl (Ju and Swanson, 1989; Dong et al., 2001). Ventral parts of the BST merge without detectable boundaries into the dorsal part of the preoptic-rostral hypothalamic area, which has very strong projections to the VTA (Phillipson, 1979; Swanson et al., 1984; Simerly and Swanson, 1988; Zahm et al., 2001; Geisler and Zahm, 2005; 2006; Reynolds et al., 2006; present results) that are in significant part glutamatergic (Geisler et al., 2007; Geisler and Wise, 2008). Monosynaptic excitations recorded in the ventral mesencephalon following stimulation of the ventral BST may reflect the existence there of a transitional region possessing characteristics of both BST and the preoptic-rostral hypothalamic region, which would help to reconcile the functional findings not only with the relative scarcity of EAc-VTA projections that we observe, but also with literature indicating that few BST neurons are glutamatergic (McDonald et al., 1989; Takayama and Miura, 1991; Sun and Cassell, 1993; Geisler and Zahm, 2007).
A comment is required regarding BST nomenclature. Based on developmental and cytoarchitecture considerations, Swanson and colleagues subdivided the BST into anterior and posterior divisions (Ju and Swanson, 1989; Ju et al., 1989) and further designated up to 20 named BST areas and better differentiated nuclei, some of which they considered together as groups referencing putative functional interrelationships (e.g., Dong et al., 2000; 2001; Dong and Swanson, 2003). The distributions of immunocytochemical and histochemical markers and input-output patterns in the BST, however, are not well constrained by Ju and Swanson’s (1989) nuclear boundaries, which were predicated mainly on neuron clustering in Nissl preparations. It is true that Dong and colleagues have described a number of connectional differentiations of BST subnuclei in their extensive series of studies with the anterograde tracer PHA-L (Dong et al., 2000; 2001; Dong and Swanson, 2003; 2004a; b; 2006a; b; c), but, viewed overall, those results tend as much to support the conspicuous mediolateral differentiations repeatedly identified by others (e.g., Krettek and Price, 1978; Ricardo and Koh, 1978; Bleier et al., 1979, Schwaber et al., 1982; Weller and Smith, 1982; Sofroniew, 1983; van der Kooy et al., 1984; de Olmos et al., 1985; Holstege et al., 1985; Alden et al., 1994; Alheid et al., 1995) that form the conceptual basis for distinguishing EAc and EAm (refs cited above). Lateral BST injections in the Dong et al. series of papers produced robust anterograde labeling in EAc structures and the midbrain dopaminergic complex (e.g., Dong et al., 2000; 2001; Dong and Swanson, 2003; 2004a; 2006c). For instance, they (Dong et al., 2000) reported a dense BST-nigral projection from Ju and Swanson’s (1989) juxtacapsular nucleus that much resembles the robust innervation of the SNc that we report arises in BSTl and CeAm (see also Gonzalez and Chesselet; Vankova et al., 1992). Conversely, their medial injections (e.g., Dong and Swanson, 2004b), like ours (data not shown) into their anterodorsal area (a structure in BSTm) showed dense interconnections only with EAm structures and lacked projections to the midbrain tegmentum, just as they reported for the medial amygdaloid nucleus (Canteras et al., 1995), another EAm component. Dong and colleagues also studied several nuclei far ventral in the BST complex with projections reminiscent of neither (or both) EAc or EAm (Dong and Swanson, 2006a; b). These structures are not readily categorized, but their projections may reflect the transitional character of this ventral region interposed between the main part of the BST and the preoptic-rostral hypothalamic continuum. In view of these considerations, we have described our findings in the BST with reference to its mediolateral and dorsoventral dimensions.
Striatopallidum
Voluminous basal ganglia inputs to the substantia nigra compacta arise in the caudate nucleus, putamen and globus pallidus (Nauta and Mehler, 1966; Grofova and Rinvik, 1970; Beckstead et al., 1979; Gerfen, 1985; Jimenez-Castellanos and Graybiel, 1989). Those to the VTA originate principally in the nucleus accumbens (Swanson and Cowan, 1975; Conrad and Pfaff, 1976; Powell and Leman, 1976; Zahm et al., 2001; Geisler and Zahm, 2005) and ventral pallidum (Groenewegen et al., 1993). Nauta et al. (1978), however, observed that fibers projecting from the nucleus accumbens to the VTA veer lateralward to also innervate much of the SNc, prompting Nauta and Domesick (1984) to maintain that the projection “is distributed in greatest volume to the medial half of the pars compacta”.….and…. “impulses from the nucleus accumbens could be thought to affect the nigral innervation, and hence the functional state, of the entire medial half of the striatum.” A projection from the lateral part of the accumbens continues strongly into the lateral RRF and, more sparsely, the PAGvl (Nauta et al., 1978; Zahm and Heimer, 1993; Usuda et al., 1998; Shammah-Lagnado et al., 2001). Indeed, we can confirm with some unpublished PHA-L cases very strong projections from the lateral shell of the Acb to the lateral part of the RRF and quite modest projections from medial Acb to the medial and middle RRF. Injections of PHA-L into the ventral pallidum in the present and an earlier published study (Groenewegen et al., 1993) also produced prominent anterograde labeling in the VTA that turns lateralward to traverse and innervate less robustly the SNc and RRF and, to a yet lesser extent, the PAGvl, further reinforcing the idea that the ventral striatopallidum innervates primarily the VTA, but, less densely, the entire midbrain dopaminergic complex.
Septum and lateral preoptic area (LPO)
The lateral septum, a main terminus of outputs from the hippocampus conveyed mainly via the subiculum (Jakab and Leranth, 1995), has meager projections to the ventral midbrain and these are essentially limited to the VTA (Geisler and Zahm, 2005). In contrast, the LPO, the main recipient of outputs from the lateral septum (Staiger and Nurnberger, 1991; Jakab and Leranth, 1995; Risold and Swanson, 1997; Zahm, 2006), gives rise to projections to the VTA that are among the densest it receives (Zahm et al., 2001; Geisler and Zahm, 2005; 2006; Geisler et al., 2007). The functional potency of the pathway from the LPO to the VTA is reflected in the observation that locomotion is robustly stimulated when the LPO is unilaterally infused with bicuculline, a GABA A receptor antagonist that activates by disinhibition (Reynolds et al., 2006, see also Sinnamon, 1993). The present data indicate that the LPO also provides a moderately dense innervation of the SNc, RRF and PAGvl, such that the influence of the LPO could be conveyed throughout the entire midbrain dopaminergic complex. To our knowledge specific functional implications of LPO projections to SNc, RRF and PAGvl have not been addressed experimentally.
Functional considerations
That the dominant input to the RRF-PAGvl comes from the EAc and numerous autonomic effector sites in the hypothalamus and brainstem that are EAc projection targets (e.g., Zahm et al., 1999; Dong et al., 2000; 2001; Dong and Swanson, 2004a; b; 2006c; Goto and Swanson, 2004; Gastard et al., 2005) suggests that the RRF-PAGvl subserves the synthesis of a broad variety of emotional/motivational, orienting, bodily maintenance and homeostatic responses to which the EAc is well known to contribute (e.g., Applegate et al., 1983; Pascoe and Kapp, 1985; LeDoux et al., 1988; Davis et al., 1997; Davis and Shi, 1999; Nader et al., 2001; Sullivan et al., 2004; Wilensky et al., 2006; Price et al., 1987; Rizvi et al., 1991; Goto and Swanson, 2004). Indeed, a convincing case for linkage of the CeA and PAGvl precisely in this manner has been established (Rizvi et al., 1991), based in part on the intimate interconnectivity of the CeA and PAGvl and in part on the demonstration by Bandler and colleagues that stimulation of PAGvl projections to the ventral medulla elicits both behavioral and autonomic components of defensive/aversive responses (Carrive et al., 1987; 1988; 1989; Bandler and Carrive, 1988). Along similar lines, reportedly strong non-dopaminergic downstream projections from the RRF provide an anatomical link consistent with RRF modulation of brainstem somatomotor and visceromotor structures (von Krosigk and Smith, 1991; von Krosigk et al., 1992). Electrical and chemical stimulation of the RRF elicits forepaw and orofacial movements in cats and rats (Arts et al., 1998; Arts and Cools, 1998; 1999, 2000) and a projection from CeA to the SNc/RRF in rats has been implicated in Pavlovian responding (Gallagher et al., 1990; Hall et al., 2001; Lee et al., 2005; 2006; 2008; El-Amamy and Holland, 2007). In addition, evidence has been reported for an involvement of CeA interconnections with mainly the caudolateral SNc in the modulation of associative learning (Holland et al., 2000; Gallagher and Holland, 1994; Han et al., 1997; Lee et al., 2005; Holland and Gallagher, 2006).
How the activity of ascending ‘mesotelencephalic’ neurons is modulated by afferents is at present poorly understood, but certainly must in some way reflect how the afferents are organized within the midbrain dopaminergic complex. Clearly one would want to know the extent to which various afferent systems synaptically contact dopaminergic neurons, but where this has been addressed with electron microscopy (e.g., Groenewegen et al., 1994; Omelchenko and Sesack, 2005; 2006; 2009; 2010; Omelchencko et al., 2009), inputs invariably were shown to terminate on dopaminergic and non-dopaminergic neurons, which creates difficult issues requiring more sophisticated quantitation than has so far been brought to bear on the question. While it seems likely that some extended amygdala projections directly contact dopaminergic neurons, this remains to be shown. Conversely, we observed many PHA-L labeled puncta within the confines of the midbrain dopaminergic complex but relatively far from dopaminergic neurons, consistent with an innervation that is distributed among multiple cell types.
Concluding remarks
The present study reveals that each of Alheid and Heimer’s (1988) cortico-subcortical functional-anatomical macrosystems is mainly interconnected with a particular sector of the midbrain dopaminergic complex. That is to say, these data encourage the conceptualization of a midbrain dopaminergic complex in which dominant sources of inputs to its classically designated divisions - VTA, SNc and RRF (here regarded as complexed with the PAGvl) – comprise mainly projections from distinct groups of deep telencephalic nuclei. The VTA is innervated by ventral striatopallidum and the septal-preoptic system, SNc by dorsal striatopallidum and the RRF-PAGvl by the extended amygdala. The present data also reinforce a complementary concept - that so-called “limbic” afferents, such as those arising in the septal-preoptic system, ventral striatopallidum and extended amygdala, reach and thus presumably contribute to information processing in all parts of the midbrain dopaminergic complex, including the substantia nigra pars compacta, as has been advocated by others previously (e.g., Nauta et al., 1978; Gonzales and Chesselet, 1990; additional authors cited in the Introduction). We suspect that the interaction of these two aspects of organization underlies the tremendous versatility and subtlety with which the activity of neurons comprising the midbrain dopaminergic complex appears to be modulated.
Acknowledgements
The authors are grateful to Beth A. DeGarmo for superb technical assistance. The work was supported by USPHS grant NIH NS-23805.
List of Abbreviations
- ac
anterior commissure
- AcbC
accumbens core
- AcbRp
accumbens rostral pole
- AcbSh
accumbens shell
- AH
anterior hypothalamic area
- AI
agranular insular cortex
- Aq
cerebral aqueduct
- BDA
biotinylated dextran amine
- BSTl
bed nucleus of stria terminalis, lateral division
- BSTl-d
BSTl, dorsal part
- BSTl-i
BSTl, intermediate (mid-dorsoventral) part
- BSTl-v
BSTl, ventral part
- BSTm
bed nucleus of stria terminalis, medial division
- BSTv
bed nucleus of stria terminalis, ventral division
- CeA
central nucleus of the amygdala
- CeAm
medial division of the central nucleus of the amygdala
- CeAm-l
CeAm, lateral part
- CeAm-m
CeAm, medial part
- CeAl
lateral division of the CeA
- Cg
cingulate cortex
- Cl
claustrum
- CN
deep cerebellar nuclei
- CPu
caudate-putamen
- CPuvl
ventrolateral caudate putamen
- DI
dysgranular insular cortex
- DP
dorsal peduncular cortex
- EAc(SL)
central division of extended amygdala, sublenticular part
- EPN
entopeduncular nucleus
- FG
Fluorogold
- f
fornix
- FrA
frontal association cortex
- FS
fundus striati
- Gi
gigantocellular reticular nucleus
- GI
granular insular cortex
- GP
globus pallidus
- ic
internal capsule
- IL
infralimbic cortex
- InG
intermediate gray layer of the superior colliculus
- IPAC
interstitial nucleus of the posterior limb of the anterior commissure
- IPN
interpeduncular nucleus
- LH
lateral hypothalamic area
- LHb
lateral habenula
- MHb
medial habenula
- LS
lateral septum
- LO
lateral orbital cortex
- LPO
lateral preoptic area
- M
motor cortex
- MHb
medial habenula
- ml
medial lemniscus
- MPO
medial preoptic area
- MRN
mesencephalic reticular nucleus
- MS
medial septum
- mt
mammillothalamic tract
- NOS
nitric oxide synthase
- oc
optic chiasm
- ot
optic tract
- Pa
paraventricular nucleus of the hypothalamus
- PAG
periaqueductal gray substance
- PAGvl
ventral and lateral parts of the periaqueductal gray substance
- PBl
parabrachial nucleus, lateral division
- Pe
periventricular hypothalamus nucleus
- Pf
parafascicular nucleus
- PH
posterior hypothalamic area
- PHA-L
Phaseolus vulgaris-leucoagglutinin
- PPTg
pedunculopontine tegmental nucleus
- PrL
prelimbic cortex
- PSTh
parasubthalamic nucleus
- PvP
posterior part of the thalamic paraventricular nucleus
- RN
red nucleus
- RRF
retrorubral field
- RRFc
caudal part of RRF
- RRFl
lateral part of RRF
- RRFm
medial part of RRF
- sm
stria medullaris
- SNc
substantia nigra compacta
- SNcd
dorsal tier of SNc
- SNr
substantia nigra reticulata
- Sol
nucleus of the tractus solitarius
- VDB
vertical limb of the diagonal band
- VO
ventral orbital cortex
- VP
ventral pallidum
- VTA
ventral tegmental area
- xscp
decussation of the cerebellar peduncle
- ZI
zona incerta
- 3
third ventricle
Contributor Information
Daniel S. Zahm, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, 1402 S. Grand Blvd., Saint Louis, MO, 63104, Berlin, Germany
Anita Y. Cheng, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, 1402 S. Grand Blvd., Saint Louis, MO, 63104, Berlin, Germany
Tristan J. Lee, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, 1402 S. Grand Blvd., Saint Louis, MO, 63104, Berlin, Germany
Comeron W. Ghobadi, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, 1402 S. Grand Blvd., Saint Louis, MO, 63104, Berlin, Germany
Zachary M. Schwartz, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, 1402 S. Grand Blvd., Saint Louis, MO, 63104, Berlin, Germany
Stefanie Geisler, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, 1402 S. Grand Blvd., Saint Louis, MO, 63104, Berlin, Germany.
Kenneth P. Parsely, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, 1402 S. Grand Blvd., Saint Louis, MO, 63104, Berlin, Germany
Clemens Gruber, Institut für Integrative Neuroanatomie, Centrum 2, Charité - Universitätsmedizin, Philippstrasse 12, D-10115, Berlin, Germany.
Ruediger W. Veh, Institut für Integrative Neuroanatomie, Centrum 2, Charité - Universitätsmedizin, Philippstrasse 12, D-10115, Berlin, Germany
Literature Cited
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. Second Edition. San Diego: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Andén NE, Carlsson A, Dahlström A, Fuxe K, Hillarp NÅ, Larsson K. Demonstration and mapping out of nigro-neostriatal dopaminergic neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Andén NE, Dahlström A, Fuxe K, Larsson K. Mapping out of catecholaminergic and 5-hydroxytryptamine neurons innervating the telencephalon and diencephalon. Life Sci. 1965;4:1275–1279. doi: 10.1016/0024-3205(65)90076-7. [DOI] [PubMed] [Google Scholar]
- Andén NE, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U. Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand. 1966a;67:313–326. [Google Scholar]
- Andén NE, Fuxe K, Hamberger B, Hökfelt T. A quantitative study on the nigro-striatal dopamine neuron system in the rat. Acta Physiol Scand. 1966b;67:306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983;31:353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Arts MP, Bemelmans FF, Cools AR. Role of the retrorubral nucleus in striatally elicited orofacial dyskinesia in cats: effects of muscimol and bicuculline. Psychopharmacology (Berl) 1998;140:150–156. doi: 10.1007/s002130050752. [DOI] [PubMed] [Google Scholar]
- Arts MP, Cools AR. Bilateral 6-hydroxydopamine lesion in the dopaminergic A8 cell group produces long-lasting deficits in motor programming of cats. Behav Neurosci. 1998;112:102–115. doi: 10.1037//0735-7044.112.1.102. [DOI] [PubMed] [Google Scholar]
- Arts MP, Cools AR. 6-hydroxydopamine lesion in the A8 cell group of cats produces a short-lasting decreased accuracy in goal-directed forepaw-movements. Behav Brain Res. 1999;103:13–21. doi: 10.1016/s0166-4328(99)00023-6. [DOI] [PubMed] [Google Scholar]
- Arts MP, Cools AR. D1 and D2 dopamine receptor agonists improve deficits in motor programming of cats with a 6-hydroxydopamine lesion in the A8 cell group. Behav Brain Res. 2000;108:73–84. doi: 10.1016/s0166-4328(99)00133-3. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Bandler R, Carrive P. Integrated defense reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Research. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bard P, Macht MB. The behaviour of chronically decerebrate cats. In: Wolstenholme GEW, O'Connor CM, editors. Ciba Foundation Symposium on the Neurological Basis of Behavior; J & A Churchill, LTD; London. 1958. pp. 55–75. [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Research. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Handbook of Chemical Neuroanatomy. Part I. Volume 2. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- Bleier R, Cohn P, Siggelkow . A cytoarchitectonic atlas of the hypothalamus and hypothalamic third ventricle of the rat. In: Morgane PJ, Panksepp J, editors. Anatomy of the Hypothalamus. Handbook of the Hypothalamus. Vol. 1. New York: Marcel-Dekker; 1979. pp. 137–220. [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the "accumbens" part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Terminals from the rat prefrontal cortex synapse on mesoaccumbens VTA neurons. Ann N Y Acad Sci. 1999;877:676–678. doi: 10.1111/j.1749-6632.1999.tb09299.x. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RA. Anatomical evidence that hypertension associated with the defense reaction in the cat is mediated by a direct projection from a restricted portion of the midbrain periaqueductal grey to the subretrofacial nucleus of the medulla. Brain Research. 1988;460:339–345. doi: 10.1016/0006-8993(88)90378-2. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RA. Somatic and autonomic integration in the midbrain of the unanesthetized decerebrate cat: a distinctive pattern evoked by excitation of neurones in the subtentorial portion of the midbrain periaqueductal grey. Brain Research. 1989;483:251–258. doi: 10.1016/0006-8993(89)90169-8. [DOI] [PubMed] [Google Scholar]
- Carrive P, Dampney RA, Bandler R. Excitation of neurones in a restricted portion of the midbrain periaqueductal grey elicits both behavioural and cardiovascular components of the defense reaction in the unanaesthetised decerebrate cat. Neurosci Lett. 1987;81:273–278. doi: 10.1016/0304-3940(87)90395-8. [DOI] [PubMed] [Google Scholar]
- Chen S, Aston-Jones G. Axonal collateral-collateral transport of tract tracers in brain neurons: false anterograde labeling and useful tool. Neuroscience. 1998;82:1151–1163. doi: 10.1016/s0306-4522(97)00336-9. [DOI] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience. 2010;168:263–272. doi: 10.1016/j.neuroscience.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Auto radiographic tracing of nucleus accumbens efferents in the rat. Brain Research. 1976;113:589–596. doi: 10.1016/0006-8993(76)90060-3. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurones. Acta Physiol Scan. 1964;62 Suppl. 232:1–55. [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: Are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear and anxiety? Ann NY Acad Sci. 1999;877:292–308. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Ann NY Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Del-Fava F, Hasue RH, Ferreira JG, Shammah-Lagnado SJ. Efferent connections of the rostral linear nucleus of the ventral tegmental area in the rat. Neuroscience. 2007;145:1059–1076. doi: 10.1016/j.neuroscience.2006.12.039. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Alheid GF, Beltramino CA. Amygdala. In: Paxinos G, editor. The Rat Nervous System. Forebrain and Midbrain. New York: Academic Press; 1985. pp. 223–334. [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. An experimental study. J Comp Neurol. 1972;146:303–334. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, Baldino F, Jr, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann N Y Acad Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Research. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004a;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004b;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006a;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006b;494:108–141. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006c;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur J Neurosci. 2007;25:1557–1567. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH. Topographic association of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE. Monoamine innervation of cerebral cortex and a theory of the role of monoamines in cerebral cortex and basal ganglia. Cerebral Cortex. 1985;6:41–127. [Google Scholar]
- Fallon JH, Loughlin SE. Substantia nigra. In: Paxinos G, editor. The Rat Nervous System. Forebrain and Midbrain 2nd Edition. Volume 1. Sydney: Academic Press; 1995. pp. 215–237. [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Ferreira JG, Del-Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Ferrier D. The Functions of the Brain. London: Smith Elder; 1876/1966. 1976; reprinted in 1966 by Dawsons of Pall Mall, London. [Google Scholar]
- Fudge JL, Haber SN. Bed nucleus of the stria terminalis and extended amygdala inputs to dopamine subpopulations in primates. Neuroscience. 2001;104:807–827. doi: 10.1016/s0306-4522(01)00112-9. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci USA. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastard MC, Jensen SL, Martin JR, III, Williams EA, Zahm DS. The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Research. 2002;957:207–222. doi: 10.1016/s0006-8993(02)03513-8. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat - anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Neurotensinergic afferents of the ventral tegmental area in the rat: [1] re-examination of the origins and [2] responses to acute psychostimulant drug administration. Eur J Neurosci. 2006;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;236:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales C, Chesselet M-F. Amygdalonigral pathway: an anterograde study in the rat with Phaseolus vulgaris Leucoagglutinin (PHA-L) J Comp Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW. Axonal projections from the parasubthalamic nucleus. J Comp Neurol. 2004;469:581–607. doi: 10.1002/cne.11036. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wouterloud FG. Organization of the projections from the ventral striato-pallidal system to ventral mesencephalic dopaminergic neurons in the rat. In: Percheron, McKenzie JS, Féger J, editors. The Basal Ganglia IV. New ideas and Data on Structure and Function. New York: Plenum Press; 1994. pp. 81–93. [Google Scholar]
- Grofova I, Rinvik E. An experimental electron microscopic study on the striatonigral projection in the cat. Exp Brain Res. 1970;11:249–262. doi: 10.1007/BF01474385. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist onPavlovian fear conditioning. Behav Neurosci. 2000;114:647–651. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Han J-S, McMahan RW, Holland P, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Chairman's opening remarks. In: Wolstenholme GEW, O'Connor CM, editors. Ciba Foundation Symposium on the Neurological Basis of Behavior; J & A Churchill, LTD; London. 1958. pp. 1–3. [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol. 1991;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- Heimer L, de Olmos J, Alheid GF, Zaborszky L. "Perestroika" in the basal forebrain: opening the border between neurology and psychiatry. Prog Brain Res. 1991a;87:109–165. doi: 10.1016/s0079-6123(08)63050-2. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991b;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hinsey JC, Ranson SW, McNattin RF. The role of the hypothalamus and mesencephalon in locomotion. Arch Neurol Psychiat. 1930;23:1–42. [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Mårtensson R, Björklund A, Kleinau S, Goldstein M. Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Handbook of Chemical Neuroanatomy. Part I. Volume 2. Amsterdam: Elsevier; 1984. pp. 277–379. [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Han JS, Gallagher M. Lesions of the amygdala central nucleus alter performance on a selective attention task. J Neurosci. 2000;20:6701–6706. doi: 10.1523/JNEUROSCI.20-17-06701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Leranth C. Septum. In: Paxinos G, editor. The Rat Nervous System. Forebrain and Midbrain 2nd Edition. Volume 1. Sydney: Academic Press; 1995. pp. 405–442. [Google Scholar]
- Jalabert M, Aston-Jones G, Herzog E, Manzoni O, Georges F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM. Compartmental origins of striatal efferent projections in the cat. Neuroscience. 1989;32:297–321. doi: 10.1016/0306-4522(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amgydalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, Gallagher M, Holland PC. Temporally limited role of substantia nigra-central amygdala connections in surprise-induced enhancement of learning. Eur J Neurosci. 2008;27:3043–3049. doi: 10.1111/j.1460-9568.2008.06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. J Neurosci. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Björkland A. Dopamine- and norepinephrine-containing neuron systems: Their anatomy in the rat brain. In: Emson PC, editor. Chemical Neuroanatomy. New York: Raven Press; 1983. pp. 229–256. [Google Scholar]
- Loopuijt LD, Zahm DS. Synaptologic and fine structural features distinguishing a subset of basal forebrain cholinergic neurons embedded in the dense intrinsic fiber network of the caudal extended amygdala. J Comp Neurol. 2006;498:93–111. doi: 10.1002/cne.21044. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–10508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Beitz AJ, Larson AA, Kuriyama R, Sellito C, Madl JE. Co-localization of glutamate and tubulin in putative excitatroy neurons of the hippocampus and amygdala: an immunohistochemical study using monoclonal antibodies. Neuroscience. 1989;30:405–421. doi: 10.1016/0306-4522(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misu Y, Goshima Y, Ueda H, Okamura H. Neurobiology of L-DOPA systems. Prog Neurobiol. 1996;49:415–454. doi: 10.1016/0301-0082(96)00025-1. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8:156–163. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJH, Domesick VB. Afferent and efferent relationships of the basal ganglia. In: Evered D, O'Connor M, editors. Functions of the Basal Ganglia. New York: Raven Press; 1984. pp. 3–23. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Domesick VB. Cross roads of limbic and striatal circuitary: hypothalamo-nigral connections. In: Livingston KE, Hornykiewicz O, editors. Limbic Mechanisms. New York: Plenum Press; 1978. pp. 75–93. [Google Scholar]
- Nauta WJ, Mehler WR. Projections of the lentiform nucleus in the monkey. Brain Research. 1966;1:3–42. doi: 10.1016/0006-8993(66)90103-x. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J Neurosci Res. 2010;88:981–991. doi: 10.1002/jnr.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth edition. Amsterdam: Elsevier; 2007. [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Philpot KB, Dallvechia-Adams S, Smith Y, Kuhar MJ. A cocaine-and-amphetamine-regulated-transcript peptide projection from the lateral hypothalamus to the ventral tegmental area. Neuroscience. 2005;135:915–925. doi: 10.1016/j.neuroscience.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Powell EW, Leman RB. Connections of the nucleus accumbens. Brain Research. 1976;105:389–403. doi: 10.1016/0006-8993(76)90589-8. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;11:1242–1289. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Vol 5. Integrated Systems in the CNS, Part I. Hypothalamus, Hippocampus, Amygdala and Retina. Amsterdam: Elsevier; 1987. pp. 279–388. [Google Scholar]
- Reynolds SM, Geisler S, Berod A, Zahm DS. Neurotensin antagonist acutely and robustly attenuates locomotion that accompanies stimulation of a neurotensin-containing pathway from rostrobasal forebrain to the ventral tegmental area. Eur J Neurosci. 2006;24:188–196. doi: 10.1111/j.1460-9568.2006.04791.x. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Research. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Springall DR, Uttenthal O, Bentura ML, Abadia-Molina F, Riveros-Moreno V, Martinez-Murillo R, Polak JM, Moncada S. Localization of nitric oxide synthase in the adult rat brain. Philos Trans R Soc Lond B Biol Sci. 1994;345:175–221. doi: 10.1098/rstb.1994.0096. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJ, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behav Neurosci. 1991;105:817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol. 2001;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM. Preoptic and hypothalamic neurons and the initiation of locomotion in the anesthetized rat. Prog Neurobiol. 1993;41:323–344. doi: 10.1016/0301-0082(93)90003-b. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Direct reciprocal connections between the bed nucleus of the stria terminalis and dorsomedial medulla oblongata: evidence from immunohistochemical detection of tracer proteins. J Comp Neurol. 1983;213:399–405. doi: 10.1002/cne.902130404. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Nurnberger F. The efferent connections of the lateral septal nucleus in the guinea pig: intrinsic connectivity of the septum and projections to other telencephalic areas. Cell Tissue Res. 1991;264:415–426. doi: 10.1007/BF00319032. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. A note on the connections and development of the nucleus accumbens. Brain Research. 1975;92:324–330. doi: 10.1016/0006-8993(75)90278-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ, Gerfen CR, Robinson P. Evidence for a projection from the lateral preoptic area and substantia innominata to the 'mesencephalic locomotor region' in the rat. Brain Resarch. 1984;295:161–178. doi: 10.1016/0006-8993(84)90827-8. [DOI] [PubMed] [Google Scholar]
- Takayama K, Miura M. Glutamate immunoreactive neurons of the central amygdaloid nucleus projecting to the subretrofactial nucleus of SHR and WKY rats: A double-labeling study. Neurosci Lett. 1991;134:62–22. doi: 10.1016/0304-3940(91)90509-r. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Kuhar MJ, Snyder SH. Neurotensin: immunohistochemical localization in rat central nervous system. Proc Natl Acad Sci U S A. 1977;74:4059–4063. doi: 10.1073/pnas.74.9.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Research. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Vankova M, Arluison M, Leviel V, Tramu G. Afferent connections of the rat substantia nigra pars lateralis with special reference to peptide-containing neurons of the amygdalo-nigral pathway. J Chem Neuroanat. 1992;5:39–50. doi: 10.1016/0891-0618(92)90032-l. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Smith AD. Descending Projections from the Substantia Nigra and Retrorubral Field to the Medullary and Pontomedullary Reticular Formation. Eur J Neurosci. 1991;3:260–273. doi: 10.1111/j.1460-9568.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Smith Y, Bolam JP, Smith AD. Synaptic organization of GABAergic inputs from the striatum and the globus pallidus onto neurons in the substantia nigra and retrorubral field which project to the medullary reticular formation. Neuroscience. 1992;50:531–549. doi: 10.1016/0306-4522(92)90445-8. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci Lett. 1989;97:252–258. doi: 10.1016/0304-3940(89)90606-x. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic and adrenergic cell groups in the rat. Brain Res Bull. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Research. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Rev Neurosci. 2004;5:1–12. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. The evolving theory of basal forebrain functional-anatomical "macrosystems.". Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Berod A. Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience. 2001;104:841–851. doi: 10.1016/s0306-4522(01)00118-x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. The efferent projections of the rostral pole of the nucleus accumbens in the rat: Comparison with the core and shell projection patterns. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR., III Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci. 1999;11:1119–11126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Trimble M. The dopaminergic projection system, basal forebrain macrosystems, and conditioned stimuli. CNS Spectr. 2008;13:32–40. doi: 10.1017/s1092852900016138. [DOI] [PMC free article] [PubMed] [Google Scholar]