Abstract
The mammalian pharynx is a hollow muscular tube that participates in ingestion and respiration, and its size, shape, and stiffness can be altered by contraction of skeletal muscles that lie inside or outside of its walls. MRI was used to determine the interaction between pharyngeal pressure and selective stimulation of extrinsic tongue muscles on the shape of the rat nasopharynx. Pressure (−9, −6, −3, 3, 6, and 9 cmH2O) was applied randomly to the isolated pharyngeal airway of anesthetized rats that were positioned in a 4.7-T MRI scanner. The anterior-posterior (AP) and lateral diameters of the nasopharynx were measured in eight axial slices at each level of pressure, with and without bilateral hypoglossal nerve stimulation (0.1-ms pulse, 1/3 maximal force, 80 Hz). The rat nasopharynx is nearly circular, and positive pharyngeal pressure caused similar expansion of AP and lateral diameters; as a result, airway shape (ratio of lateral to AP diameter) remained constant. Negative pressure did not change AP or lateral diameter significantly, suggesting that a negative pressure reflex activated the tongue or other pharyngeal muscles. Stimulation of tongue protrudor muscles alone or coactivation of protrudor and retractor muscles caused greater AP than lateral expansion, making the nasopharynx slightly more elliptical, with the long axis in the AP direction. These effects tended to be more pronounced at negative pharyngeal pressures and greater in the caudal than rostral nasopharynx. These data show that stimulation of rodent tongue muscles can adjust pharyngeal shape, extending previous work showing that tongue muscle contraction alters pharyngeal compliance and volume, and provide physiological insight that can be applied to the treatment of obstructive sleep apnea.
Keywords: hypoglossal, imaging, pharynx
in mammals, the tongue muscles are crucial for swallowing, mastication, breathing and, in humans, speech. These behaviors also require changes in the size, shape, and stiffness of the nasopharynx, which are accomplished by the actions of tongue and pharyngeal wall muscles. In particular, the tongue makes up part of the anterior pharyngeal wall, and activation of the tongue muscles has a major influence on the geometry of the nasopharynx. There are two main classes of tongue muscles: extrinsic and intrinsic. Extrinsic tongue muscles originate on bone and insert into the tongue body; they include the hyoglossus, which depresses and retracts the tongue, the genioglossus, which protrudes and depresses the tongue, and the styloglossus, which retracts and elevates the tongue (37). There are also four intrinsic muscles, all of which have fibers that are contained exclusively within the tongue body, and their actions are complex and poorly understood. Nevertheless, most tongue movements depend on the coordinated action of all extrinsic and all intrinsic tongue muscles, in accordance with the muscular hydrostat theory of tongue movement (43).
In anesthetized rats, coactivation of the extrinsic muscles stiffens the pharynx (3, 20), while activation of the extrinsic or intrinsic muscles increases pharyngeal airway volume (4, 17, 20, 45). Thus extrinsic and intrinsic tongue muscles play an important role in modulating the size and stiffness of the pharyngeal airway. However, far less is known about the influence of extrinsic tongue muscle activation on the shape of the nasopharynx. A recent study (28) greatly advanced our understanding of the influence of extrinsic tongue muscle activation on the shape of the feline airway. However, airway diameter was measured with fiber-optic laryngoscopy, which is excellent for regional evaluation but is prone to artifacts, because it is a two-dimensional technology (22). Artifacts include inherent barrel distortion, which prevents accurate perception and measurement of the distance between structures of interest, as well as movement artifact, which in the present application could present a particularly vexing problem, inasmuch as the pharynx and tongue move considerably during tongue muscle stimulation. In two recent studies, one in cats (9) and the other in rats (11), changes in shape of the pharynx with tongue muscle stimulation were examined using MRI, but the effect of stimulating retractor muscles or coactivating protrudor and retractor muscles was not examined in either study (9). Thus the means by which contraction of the extrinsic tongue muscles influences upper airway shape in mammals is not completely understood. In the present study, a rodent model used in previous studies (4, 12, 45) is employed to explore the role of extrinsic tongue muscle activation on the shape of the nasopharynx, as revealed with MRI. On the basis of previous work showing that coactivation of tongue protrudor and retractor muscles stiffens the airway, coupled with theoretical results showing that contraction of the genioglossus muscle increases upper airway flow while making the pharynx more circular (31), our hypothesis is that coactivation of protrudor and retractor muscles will make the airway more circular, with independent activation of the medial or lateral branches having less affect. As with all collapsible tubes, the shape of the nasopharynx also depends on transmural pressure, with negative pressure tending to collapse it and positive pressure tending to expand it. Accordingly, an additional goal is to explore in detail the interactions between tongue muscle activation and transmural pressure on the shape of the nasopharynx.
METHODS
Animal preparation.
Eighteen male Sprague-Dawley rats [324.6 ± 10.8 (SE) g body wt] were used for the study. All methods were approved by the Institutional Animal Care and Use Committee at the University of Arizona. The experimental preparation is described in detail elsewhere (4, 17, 45), and the data reported here were obtained from these earlier experiments. The data published previously focused on regional pharyngeal compliance changes with tongue muscle stimulation (45) and on localization of the most collapsible segment of the oral and nasal part of the velopharynx (17); in the present study, the focus is exclusively on changes in airway shape with stimulation of the whole hypoglossal nerve or of its medial or lateral branches. The experimental model used for this study and the earlier studies is shown schematically elsewhere (see Fig. 1 in Ref. 45).
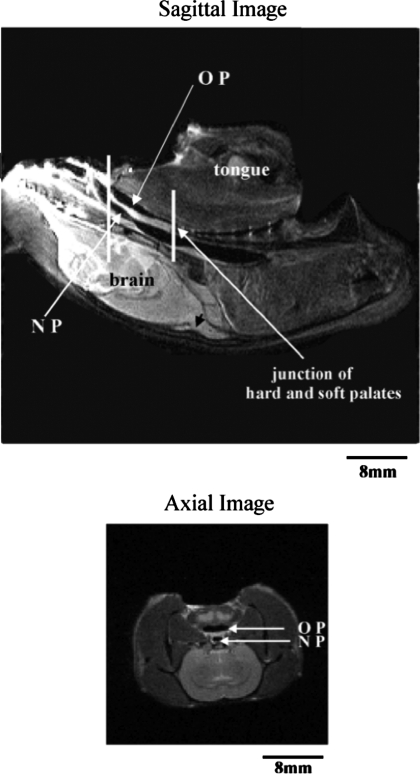
Fig. 1.
Top: sagittal MRI of the rat head, with tongue and brain labeled for orientation. Oropharynx (OP), nasopharynx (NP), and junction of the hard and soft palate are also indicated. Vertical lines show 8-mm region where axial slices were taken. Bottom: representative axial slice through the caudal part of the 8-mm region of interest.
Animals were anesthetized with urethane (1.2 g/kg iv) and breathed through a tracheotomy tube. A second tracheotomy was made ∼5 mm caudal to the vocal folds, through which a nonmagnetic pressure transducer (Millar Instruments) was advanced rostrally to a point ∼3–5 mm caudal to the left naris to measure nasopharyngeal pressure. The left naris remained open to allow airflow; the right naris was sealed with Super Glue. A catheter was advanced through the same tracheotomy to a point just rostral to the vocal folds (i.e., in the hypopharynx) and connected in series to a pressure or vacuum source and a second differential pressure transducer (model DP45-28, Validyne), which provided an estimate of the pressure applied at the hypopharynx. Since the purpose of this study was to examine the influence of pharyngeal transmural pressure and tongue muscle activation on airway shape, comparisons at fixed levels of transmural pressure were necessary. Accordingly, in each animal, the nasopharyngeal pressure was set to six dynamic pressure levels (−9, −6, −3, 3, 6, and 9 cmH2O) by application of suction (via a vacuum source) or positive pressure (compressed air) to the hypopharyngeal catheter. The range of negative pressure values chosen is similar to those encountered physiologically, based on previous measurement of transpulmonary pressure in the rat in eupnea and hypercapnia (7). The positive pressure values were equal and opposite to these values, inasmuch as, in pilot studies, an increase to >9 cmH2O caused no additional pharyngeal dilation. Baseline measurements at atmospheric pressure and under static conditions (i.e., no flow through the isolated nasopharynx) were also made. Although the nasopharynx remained open at all pressure levels tested, different levels of hypopharyngeal pressure were needed to reach the target pressures within and between experiments. The duration of pressure application corresponded to the duration of imaging time, ∼2 min. The application of each pressure level was fully randomized, and all pressure levels were studied in each animal, once with and once without stimulation of the hypoglossal nerves or its main branches.
Platinum wire electrodes, which minimize ferromagnetic distortion (35), were placed around the main trunk of the hypoglossal nerves bilaterally (activation of all tongue muscles). The electrodes were embedded in polyethylene tubing, which was hemisected, forming a “boat,” which allowed a snug fit around the nerve while also insulating the surrounding tissues from current spread (12). To study the effects of protrudor muscle activation, the lateral branches were cut, and to study retractor muscle activation, the medial branches were cut (45). The effectiveness of stimulation was documented before the animal was placed in the magnet, and necessary adjustments were made, as described previously (45). Briefly, the tip of the tongue was attached by string to a force transducer (model F10, Grass), and the current applied to the nerves was slowly increased until protrusion (medial branch stimulation) or retraction (whole nerve or lateral branch stimulation) force was maximal (19). The threshold was defined as the lowest current that caused visible tongue movement (12, 45), and the current above which tongue protrusion or retraction appeared to be maximal defined the maximal current level. A stimulus level of one-third to one-half maximal was used for subsequent stimulation trials (range 50 μA–5 mA).
MRI protocol.
After satisfactory placement of the stimulating electrodes, rats were placed supine on a Plexiglas platen, which secured the head via modified ear bars and a tooth clamp that fixed the superior incisors, as described previously (10, 17, 45). The tip of the tongue was sutured to a small rubber band, positioned at the level of the superior incisors, and attached to the platen. This helped return the tongue to its initial position following stimulation. The platen allowed head position to be maintained within an experiment and standardized across experiments, so that the influence of pressure and muscle stimulation was not the result of movement artifacts or differences in airway position relative to the isocenter of the imaging coil. After satisfactory positioning and head stabilization, rats were placed in a MRI scanner (Bruker Instruments, Billerica, MA) (Fig. 1) and positioned so that the pharyngeal airway was at the isocenter of the gradient coil (4, 12, 45). The electrode lead wires were twisted, shielded, and passed outside the magnet, where they were connected to a stimulator (model S88, Grass) via separate constant-current stimulus isolation units (model PSIU6, Grass). Nerves were stimulated with 0.1-ms pulses at 90 Hz. The MRI protocol involved two sequences: gradient echo fast imaging (GEFI) and rapid acquisition with relaxation enhancement (RARE), as described in detail previously (4, 12, 17, 45). GEFI images were used to visualize tongue movements, to optimize stimulus current levels, and to check the viability of the nerve preparation before and after each set of RARE images was obtained. The settings used for GEFI imaging were as follows: TE = 6.8 ms, TR = 20 ms, flip angle = 30°, number of excitations = 1, field of view = 8 × 8 cm, matrix size = 128 × 128 lines. The GEFI protocol yielded a temporal sequence of 12 images, with each image requiring ∼4 s. Nerve stimulation was delivered during a discrete, 16-s interval that was bracketed by two 16-s intervals without stimulation. This provided four serial images without stimulation, four with stimulation, and four after stimulation. (For an example of a GEFI sequence with whole hypoglossal nerve stimulation, see http://www3.physiology.arizona.edu/labs/rnlab/GEFI.mov, and hit the “play” button to see how the tongue moves with stimulation.)
The RARE technique was used in concert with a stimulus-gated acquisition protocol to obtain axial images of the velopharynx during pressure application, with and without stimulation, achieved by triggering the acquisition software with the stimulator output pulse (4, 12, 17, 45). A 1.7-s stimulus train was delivered every 5 s, resulting in a duty cycle (stimulus on/stimulus off) of 34%. MRI data were collected during the stimulus period by triggering the acquisition software with the stimulator output pulse. Acquisition began 50 ms after the onset of stimulation to allow the muscle to shorten before image acquisition (3). The same protocol was used to obtain baseline images, except in this case the electrode wires were disconnected from the stimulus isolation unit and, therefore, no current was delivered to the muscle nerves. Settings for the RARE protocol were as follows: TE = 6.8 ms, TR = 5 s, field of view = 3.2 × 3.2 cm, matrix size = 128 × 128 lines. This resulted in the collection of 8 of the 128 lines of data during each TR period (i.e., excitation train length = 8). Two excitation periods were used at each pressure, yielding a total imaging time of 160 s [128 lines × 2 excitations × 5 s TR/8 (excitation train length)].
Data analysis.
As described previously (5, 10, 17, 45), a threshold method was used to differentiate the airway from the surrounding tissues using the software program ImageJ (formerly called NIH Image). All the analyses were performed by a laboratory technician who was blinded to the different experimental conditions. With use of a representative sample of axial images, the minimum, maximum, and standard deviation (SD) of the mean pixel values were measured from a region of the black space at the outer boundary of the image. To account for noise in the image, the SD for the black space was subtracted from the minimum pixel value of the black space, and this difference was used as the threshold that differentiated true black space (airways) from tissue surrounding the airway. If the airway could not be isolated using the threshold values, a boundary was inscribed manually to connect the established edges along the curvature of the airway. Then a subprogram within ImageJ was used to compute the lateral and anterior-posterior (AP) diameters within these estimated airway boundaries.
In several experiments, the oropharynx was closed caudally (OP in the sagittal image in Fig. 1), so the quantitative analyses are focused on the nasopharyngeal airway (NP in Fig. 1). Eight consecutive 1-mm-thick axial slices were analyzed starting at the caudal edge of the soft palate (e.g., axial image in Fig. 1) and moving rostrally to the hard-soft palate transition (see vertical lines in the sagittal image of Fig. 1). The four most-caudal and four most-rostral segments were used to divide this segment of the nasopharynx into caudal and rostral segments, respectively. Since measurements on the four rostral and four caudal slices did not vary systematically, data were averaged across the four rostral and four caudal slices, and these average values were used to represent the rostral and caudal nasopharynx, respectively. Averaging also allowed us to avoid potential errors that could result from image distortion in some slices due to slight differences in the angle of each slice relative to the airway long axis (see discussion). The influence of whole nerve (n = 6), medial branch (n = 6), and lateral branch (n = 6) stimulation on AP diameter, lateral diameter, and the lateral-to-AP diameter ratio for each axial image was measured. A lateral-to-AP diameter ratio of 1.0 indicates that the airway is circular, a ratio >1.0 is consistent with a larger AP than lateral diameter, and a ratio <1.0 indicates that the lateral axis exceeds the AP axis. All pressure and stimulation/no-stimulation trials were randomized. Results were analyzed with two-way repeated-measures ANOVA (GraphPad Prism Software, San Diego, CA), with pressure and stimulation/no-stimulation as the main factors. P ≤ 0.05 was considered significant for all tests.
RESULTS
Whole hypoglossal nerve stimulation.
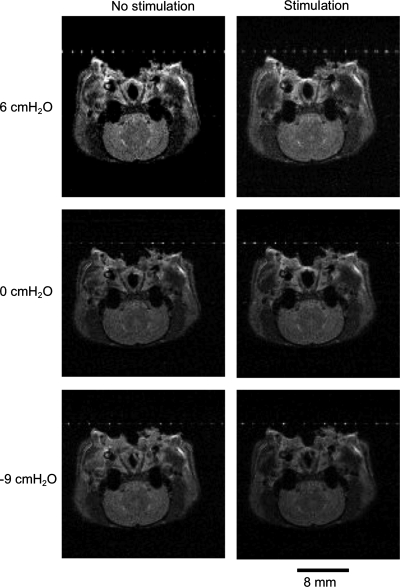
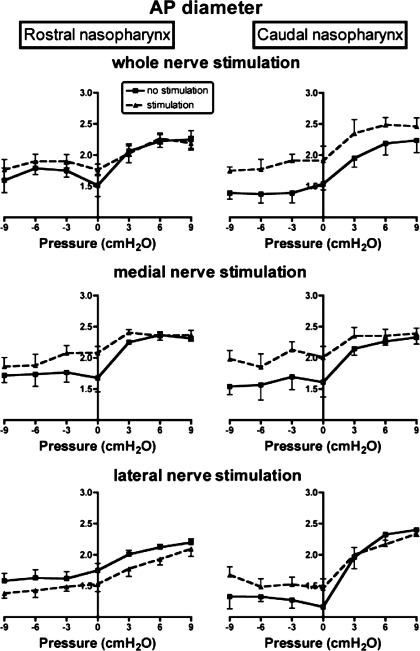
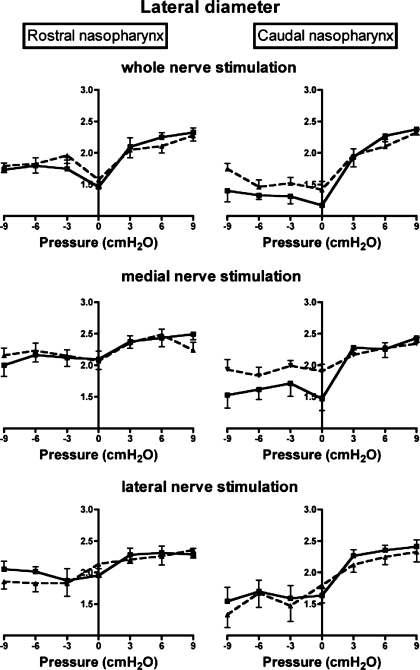
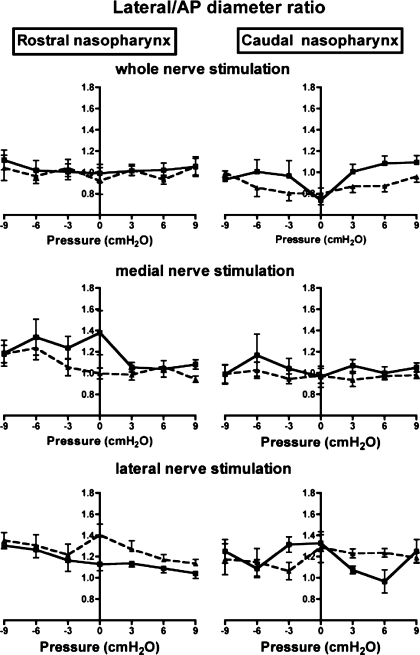
Representative MRIs showing the effects of pharyngeal pressure and nerve stimulation on the nasopharyngeal airway are shown in Fig. 2. Average data on AP diameter, lateral diameter, and lateral-to-AP diameter ratios of the rostral and caudal nasopharynx as a function of pharyngeal pressure and bilateral stimulation of the whole hypoglossal nerves are shown in Figs. 3–5, top. Results of the two-factor ANOVA for each variable are provided in Table 1. There were significant effects of hypoglossal nerve stimulation and pharyngeal pressure on AP diameter, lateral diameter, and the lateral-to-AP diameter ratio, but the interaction between pressure and stimulation was not significant for any of the three outcome variables (Table 1), indicating that the influence of stimulation was independent of pharyngeal pressure. Visual examination of the data shows that stimulation increased AP diameter in the caudal nasopharynx at all pressures, with a small effect at negative pharyngeal pressures in the rostral nasopharynx (Fig. 3, top). Hypoglossal nerve stimulation increased lateral diameter in the caudal nasopharynx at negative airway pressures (Fig. 4, top), but by a modest amount compared with the effects on AP diameter. As a result, stimulation decreased the lateral-to-AP diameter ratio of the caudal nasopharynx at positive and negative pharyngeal pressures (Fig. 5, top), indicating that enlargement of the caudal nasopharynx by whole hypoglossal nerve stimulation was due primarily to expansion in the AP dimension.
Fig. 2.
Representative axial images from the midportion of the caudal nasopharynx showing the influence of pressure and stimulation of the whole hypoglossal nerves on airway shape. Stimulation at zero pressure caused similar expansion in lateral and anterior-posterior (AP) dimensions. Negative pressure narrowed the nasopharynx laterally, with smaller AP narrowing. Stimulation combined with negative pressure caused minimal expansion at this pressure level. Positive pressure caused slight expansion of the airway, and the expansion was symmetrical. Stimulation combined with positive pressure caused no detectable change in airway dimensions in this animal.
Fig. 3.
Influence of pharyngeal pressure and bilateral stimulation of whole (top), medial (middle), and lateral (bottom) hypoglossal nerve branches on AP diameter of the rostral (left) and caudal (right) nasopharynx. Values for rostral and caudal regions of the nasopharynx represent average of four, 1-mm-thick axial slices through the region indicated by vertical lines in Fig. 1.
Fig. 4.
Influence of pharyngeal pressure and bilateral stimulation of whole (top), medial (middle), and lateral (bottom) hypoglossal nerve branches on lateral diameter of the rostral (left) and caudal (right) nasopharynx. Squares, no stimulation; triangles, stimulation.
Fig. 5.
Influence of pharyngeal pressure and bilateral stimulation of whole (top), medial (middle), and lateral (bottom) hypoglossal nerve branches on the lateral-to-AP diameter ratio in the rostral (left) and caudal (right) nasopharynx. Squares, no stimulation; triangles, stimulation.
Table 1.
Results of two-way ANOVA for whole hypoglossal nerve preparations
| df | F | P | |
|---|---|---|---|
| AP diameter (mm) | |||
| Stimulation/no-stimulation | 3 | 8.85 | <0.0001 |
| Pressure | 6 | 18.3 | <0.0001 |
| Interaction | 18 | 0.64 | 0.858 |
| Lateral diameter (mm) | |||
| Stimulation/no-stimulation | 3 | 5.87 | 0.0009 |
| Pressure | 6 | 29.8 | <0.0001 |
| Interaction | 18 | 1.46 | 0.1178 |
| Lateral-to-AP diameter ratio | |||
| Stimulation/no-stimulation | 3 | 4.6 | 0.0042 |
| Pressure | 6 | 2.2 | 0.0455 |
| Interaction | 18 | 0.49 | 0.957 |
For each variable, ANOVA includes data for rostral and caudal nasopharynx. AP, anterior-posterior; df, degrees of freedom.
Stimulation of the medial hypoglossal nerve branches.
Effects of medial hypoglossal nerve branch stimulation and pharyngeal pressure on AP diameter, lateral diameter, and lateral-to-AP diameter ratios in the rostral and caudal nasopharynx are summarized in Figs. 3–5 (middle). The results of the two-factor ANOVA for each variable are provided in Table 2. Pharyngeal pressure and medial nerve branch stimulation had significant effects on AP and lateral nasopharyngeal diameters (Table 2, Figs. 3 and 4), but there was no interaction between stimulation and pressure for either variable (Table 2). Stimulation increased AP diameter in the rostral nasopharynx at atmospheric pressure and at moderately negative pressures, but not at extreme negative or positive pharyngeal pressures (Fig. 3). In contrast, the AP diameter of the caudal nasopharynx increased with stimulation at all negative pressures examined and also at atmospheric and slightly positive pressures. Lateral diameter of the rostral nasopharynx was uninfluenced by stimulation and negative pharyngeal pressure (Fig. 4), although positive pressure slightly increased lateral diameter. Positive pressure caused marked increases in the lateral diameter of the caudal nasopharynx, but negative pressure had little effect (Fig. 4). Medial hypoglossal nerve branch stimulation increased lateral diameter at atmospheric and negative pharyngeal pressures but had no effect at positive pressures (Fig. 4). There was a significant influence of stimulation on the lateral-to-AP diameter ratio (Fig. 5), but the influence of pressure was not significant (Table 2). Examination of data for the rostral nasopharynx shows that stimulation reduced the lateral-to-AP diameter ratio at atmospheric and at moderately negative pharyngeal pressures, but not at positive pressures (Fig. 5). The lateral-to-AP diameter ratio of the caudal nasopharynx fell with stimulation at positive and negative pressures, consistent with larger change in AP than lateral diameter with stimulation (Fig. 5).
Table 2.
Results of two-way ANOVA for medial hypoglossal nerve branch preparations
| df | F | P | |
|---|---|---|---|
| AP diameter (mm) | |||
| Stimulation/no-stimulation | 3 | 7.6 | <0.0001 |
| Pressure | 6 | 19.1 | <0.0001 |
| Interaction | 18 | 0.49 | 0.955 |
| Lateral diameter (mm) | |||
| Stimulation/no-stimulation | 3 | 11.5 | <0.0001 |
| Pressure | 6 | 11.0 | <0.0001 |
| Interaction | 18 | 1.1 | 0.357 |
| Lateral-to-AP diameter ratio | |||
| Stimulation/no-stimulation | 3 | 6.4 | 0.0004 |
| Pressure | 6 | 1.9 | 0.089 |
| Interaction | 18 | 0.74 | 0.764 |
For each variable, ANOVA includes data for rostral and caudal nasopharynx.
Stimulation of the lateral hypoglossal nerve branches.
The influence of bilateral stimulation of the lateral hypoglossal nerve branches and changes in pharyngeal pressure on AP and lateral nasopharyngeal diameters was significant (Table 3, Figs. 3–5, bottom), but the interaction between stimulation and pressure was not significant for either variable. As shown in Fig. 3, AP diameter in the rostral nasopharynx increased with positive pressure, with small but consistent reductions evoked by stimulation of the lateral branches at all pressures. Positive pressure expanded the caudal nasopharynx, but negative pressure was without effect. Stimulation had no influence on caudal nasopharyngeal AP diameter at positive pressures but increased it at negative pressures (Fig. 3).
Table 3.
Results of two-way ANOVA for lateral hypoglossal nerve branch preparations
| df | F | P | |
|---|---|---|---|
| AP diameter (mm) | |||
| Stimulation/no-stimulation | 3 | 3.9 | 0.0069 |
| Pressure | 6 | 12.9 | <0.0001 |
| Interaction | 18 | 0.78 | 0.1338 |
| Lateral diameter (mm) | |||
| Stimulation/no-stimulation | 3 | 4.2 | 0.0098 |
| Pressure | 6 | 34.8 | <0.0001 |
| Interaction | 18 | 1.4 | 0.716 |
| Lateral-to-AP diameter ratio | |||
| Stimulation/no-stimulation | 3 | 1.1 | 0.3487 |
| Pressure | 6 | 1.5 | 0.1743 |
| Interaction | 18 | 0.4 | 0.9845 |
For each variable, ANOVA includes data for rostral and caudal nasopharynx.
Positive pressure had only a small effect on the lateral diameter of the rostral nasopharynx (Fig. 4), and negative pressure was without effect, while stimulation caused slight constriction of this region at negative pharyngeal pressures. Positive pressure increased lateral diameter in the caudal nasopharynx, but stimulation and negative pressure were without apparent effect. Consistent with the small effects of lateral branch stimulation on AP and lateral nasopharyngeal diameters, the lateral-to-AP diameter ratio was not altered significantly by pressure or stimulation (Table 3, Fig. 5).
DISCUSSION
Positive pharyngeal pressure caused similar expansion of AP and lateral diameters of the nasopharynx in all experiments, and as a result airway shape remained constant. Interestingly, negative pharyngeal pressure did not change AP or lateral diameter (and, thus, airway shape), suggesting that pharyngeal muscles, including the tongue muscles, were activated by a negative pressure reflex that prevented nasopharyngeal constriction (but see below). Also, stimulation of tongue protrudor muscles alone or coactivation of protrudor and retractor muscles caused greater AP than lateral expansion, thus making the shape of the nasopharynx slightly more elliptical, with the long axis in the AP direction. These effects tended to be more pronounced at negative pharyngeal pressures and greater in the caudal than the rostral nasopharynx. Thus the tongue muscles play a major role in adjusting nasopharyngeal dimensions in the rat, consistent with previous work in cats (9, 28) and humans (38, 39).
Critique of methods.
Although anatomic differences between the rat and human pharynx are discussed in detail elsewhere (4, 12, 17, 45), there are a few matters that have not been discussed previously. 1) It is not known whether high-intensity stimulation of the muscle nerves damaged the nerve or muscle. Although these tissues were not histologically examined, GEFI analysis ensures that the extent of tongue movement at the beginning and end of the study was approximately the same. However, to match the movement over the course of these long experiments, it was often necessary to adjust stimulation intensity, suggesting that muscle fatigue, or even tissue damage, may have occurred. However, the damage was never severe enough to eliminate the ability to evoke consistent tongue movements. Moreover, the trials were randomized across pressure levels and within stimulation and no-stimulation conditions, so that these effects, if present, did not influence the data systematically. 2) Image distortion occurs as a result of the complex ferromagnetics that accompany metallic structures in the MRI scanner. As described in methods, image distortion was minimized by using platinum wires and by twisting the lead wires. In addition, the use of brief trains of stimulation, with each train triggering an image (effectively, a brief “snapshot”), followed by the averaging of many such snapshots, also minimized image distortion. 3) The angle of the axial MRI slices relative to the airway long axis cannot be guaranteed to be always 90° because of positional changes and tissue movement with neuromuscular stimulation. As a result, images could be distorted, contributing error to the measurements. However, I believe that these effects were minimized, because the airway in the supine rodent is fairly linear; head position was standardized with a stereotaxic frame, so that movements upon nerve stimulation were minimized, and the four rostral and four caudal slices were averaged to mitigate any influence of image distortion that may have been more or less pronounced in a single airway region. 4) Because the rats were anesthetized, there is no assurance that the findings would translate to awake or sleeping animals. It is likely that the airway in the anesthetized rat is relatively compliant and that muscle stimulation had a larger influence than one might expect in the awake or sleeping animal. In addition, the nerve branches were stimulated at 90 Hz, which is above the tetanic fusion frequency for rodent tongue muscles (21). Earlier work showed that urethane-anesthetized rats have brisk protrudor and retractor tongue muscle activity (2, 6, 7, 19, 24, 25), with genioglossus motor unit action potentials firing at up to 80 Hz (26). Nonetheless, tetanic stimulation at 90 Hz evokes more intense contractions than those encountered physiologically. Thus the observations provide insight into how the tongue muscles alter nasopharyngeal airway shape under conditions of intense activation.
Influence of pharyngeal pressure on nasopharyngeal dimensions.
Positive pressure consistently and significantly enlarged lateral and AP diameters in the caudal and rostral regions of the nasopharynx. As a result, the shape of the airway as estimated by the lateral-to-AP diameter ratio remained constant. This finding differs from observations in the anesthetized cat, which show that positive pressure tended to decrease this ratio, particularly in the midportion of the nasopharynx (9). However, the cat has a much more elliptical nasopharynx, with lateral-to-AP diameter ratios ranging from 2–8 compared with 0.8–1.5 in the rat (compare Fig. 5 in the present study with Fig. 4 in Ref. 9). In contrast, negative pressure failed to narrow or change the shape of the nasopharynx, suggesting that pharyngeal muscles were activated by the negative pressure reflex (1, 8, 13, 33, 34, 40) to maintain airway size. In the present study, the stimulating electrodes were placed on the main trunk of the hypoglossal nerve, and selective stimulation of medial or lateral branches was accomplished by section of the opposite branch. Thus, in all conditions, at least one of the tongue muscle groups was intact. Moreover, palatal and pharyngeal constrictor muscles were intact, and both muscle groups have been shown to dilate the pharynx (23, 27, 29, 30). Yet another possibility is that the rodent airway is sufficiently rigid that pressures as low as −9 cmH2O were insufficient to narrow it. In a previous study, the critical closing pressure of the rat nasopharynx averaged −19 cmH2O, suggesting that −9 cmH2O may indeed have been insufficiently negative to significantly narrow the nasopharynx. However, in those studies, the hypoglossal nerves also remained intact, so it is possible that activation of tongue and/or other pharyngeal muscles leads to an overestimation of the actual collapsing pressure. In the anesthetized cat, negative pressure caused a marked reduction in the AP diameter of the nasopharynx, leading to a substantial increase in lateral-to-AP diameter ratio (9). However, the cats in that study were partially paralyzed, and it is very likely that negative pressure failed to elicit a reflex increase in pharyngeal muscle activity.
Compared with a circular airway, an elliptical airway brings the lateral or AP walls closer together, which in theory should make the airway more collapsible under the stress of negative transmural pressure. The lateral-to-AP diameter ratio in the rat nasopharynx was slightly less than 1.0 at atmospheric pressure, with the long axis in the AP dimension, indicating that the rodent nasopharynx is approximately circular, even under unstressed conditions. Since pressure had comparable effects on AP and lateral diameters, airway shape did not change significantly, although the trend was toward a slightly more circular airway with positive and negative pressure. Although similar studies have been done in the cat, negative pressure caused greater expansion in the lateral than the AP dimension, thus increasing the lateral-to-AP diameter ratio (9), while another study showed an increase in this ratio with negative pressure (28). Similarly, the ratio increased with positive pressure in one study (9) but decreased in the other (28). The reasons for the opposing results are not clear, although there were important technical differences, including fiber-optic endoscopy in decerebrate animals in one study (28) and MRI in anesthetized animals in the other (9). The human nasopharynx is more elliptical than the rat nasopharynx, with most studies showing the long axis in the lateral dimension (14, 29, 42, 46), although factors such as adiposity (36), obstructive sleep apnea (32), and differences in imaging methodology (41) can produce conflicting results. Nevertheless, the more circular pharyngeal airway in the rat is consistent with relative resistance to collapse when stressed with negative transmural pressure, compared with the human nasopharynx, which is more elliptical and collapsible in healthy subjects and more so in those with obstructive sleep apnea (15, 16, 32, 36, 38, 39, 47). From a treatment perspective, these data suggest that focusing on interventions that make the airway more circular may be beneficial.
Stimulation of the whole hypoglossal nerve, or its medial and lateral branches, and nasopharyngeal dimensions.
In the caudal nasopharynx, bilateral stimulation of the intact hypoglossal nerves or of the medial branches increased AP diameter at all pressure levels but increased lateral diameter only at negative pressures and to a lesser extent than changes in AP diameter. As a result, the lateral-to-AP diameter ratio decreased at positive and negative pressures, changing shape from more circular to slightly more elliptical, with the long axis oriented in the AP dimension. Given the discussion above, this result implies that while stimulation of the protrudor muscles alone or coactivation of protrudor and retractor muscles expands the nasopharynx, it also makes the airway more elliptical and, thus, slightly less stable. Nonetheless, the ratios were still between 0.8 and 1.1 under all conditions, so in the rat the trade-off between increased airway volume and a slightly less favorable shape appears to be physiologically advantageous, as previous work in the rat shows that stimulation of the whole hypoglossal nerve decreases the critical collapsing pressure of the pharynx from about −19 to −27 cmH2O (20). Interestingly, the influence of stimulating the whole hypoglossal nerves or its medial branches on rostral nasopharyngeal dimensions was limited to a small increase in AP diameter at negative pharyngeal pressures. This is consistent with earlier work in the rat, showing that the caudal nasopharynx is more compliant than the rostral nasopharynx (45). However, over the pressure range used in these experiments, the compliance of both pharyngeal segments is about the same (see the positive pressure-diameter curves in Figs. 3–5), suggesting that the mechanical advantage of the tongue muscles is greater in the caudal than rostral region of the nasopharynx.
Previous work in rats also showed that stimulating the medial branch of the hypoglossal nerves caused similar AP and lateral pharyngeal expansion, with no significant change in the ratio (11). Similar experiments in cats showed that stimulation of the whole hypoglossal nerve increased lateral and AP diameters in the oropharynx and velopharynx at negative airway pressures, with more variable effects at positive airway pressures (28). Stimulation of the medial hypoglossal nerve branches (28) or the genioglossus muscle (9) also increased lateral and AP diameters at negative pressures in the rostral oropharynx (28) and increased AP diameter more than lateral diameter in the midportions of the nasopharynx (9), tending to make the airway more circular. However, the cat airway is more elliptical than the rat airway, with the long axis in the lateral dimension (9, 28). Stimulation of the genioglossus muscle in human subjects with obstructive sleep apnea increased AP diameter in the velopharynx, although data for lateral diameter were not provided (39). Although it is tempting to suggest that coactivation of protrudor and retractor muscles would also dilate and change the shape of the human nasopharynx, the actual outcome is difficult to predict owing to differences in airway shape, tongue muscle fiber orientation, fiber type, and myosin heavy chain expression between humans and lower mammals (44). Taken together, the data suggest that stimulation of the tongue protrudor muscles or coactivation of protrudor and retractor muscles tends to change lateral and AP dimensions, but the effects are dependent on pharyngeal pressure, airway region, and species.
Stimulation of the lateral nerve branches was used to selectively activate the tongue retractor muscles. This maneuver increased AP diameter in the caudal nasopharynx at negative airway pressures, with no effect on lateral diameter or the lateral-to-AP diameter ratio. In the rostral nasopharynx, lateral nerve branch stimulation reduced AP diameter at all pressure levels, with only a slight decrease in lateral diameter at −6 and −9 cmH2O and no changes in the lateral-to-AP diameter ratio. These observations demonstrate that muscles normally associated with airway constriction can also play a role in expanding the caudal regions of the nasopharyngeal airway. As discussed previously (18), lateral nerve branch stimulation not only causes tongue retraction but also evokes powerful depression of the tongue base, an action that is likely responsible for increasing the diameter of the caudal nasopharynx. These observations are supported by data in the cat showing that the rostral and caudal oropharynx expands laterally with stimulation of the tongue retractor muscles (28).
Summary and conclusions.
The rat nasopharynx is nearly circular, and positive pharyngeal pressure evokes similar expansion of AP and lateral diameters, maintaining airway shape. In contrast, negative pharyngeal pressure did not change AP or lateral diameter (and, thus, airway shape), suggesting that a negative pressure reflex activated the tongue muscles. Electrical stimulation of tongue protrudor muscles alone or coactivation of protrudor and retractor muscles caused greater AP than lateral expansion, making the shape of the nasopharynx slightly more elliptical, with the long axis in the AP direction. These effects were more pronounced at negative pharyngeal pressures and greater in the caudal than rostral nasopharynx, consistent with greater compliance in the former (45). These data show that stimulation of rodent tongue muscles can adjust pharyngeal shape, extending previous work showing that tongue muscle contraction alters pharyngeal compliance and volume, and provide physiological insight that can be applied to the treatment of obstructive sleep apnea.
GRANTS
This work was funded by National Institute of Dental and Craniofacial Research Grant DC-07692.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
ACKNOWLEDGMENTS
The author thanks Jennifer Huang and Drs. E. Fiona Bailey and Patrick Janssen for technical assistance.
REFERENCES
- 1. Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol 531: 677–691, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440–449, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bailey EF, Fregosi RF. Pressure-volume behaviour of the rat upper airway: effects of tongue muscle activation. J Physiol 548: 563–568, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey EF, Huang Y, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol 101: 1377–1385, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bailey EF, Huang YH, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol 101: 1377–1385, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bailey EF, Janssen PL, Fregosi RF. Po2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am J Respir Crit Care Med 171: 1403–1407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO2 on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525–534, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry RB, White DP, Roper J, Pillar G, Fogel RB, Stanchina M, Malhotra A. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol 94: 1875–1882, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Brennick MJ, Gefter WB, Margulies SS. Mechanical effects of genioglossus muscle stimulation on the pharyngeal airway by MRI in cats. Respir Physiol Neurobiol 156: 154–164, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Brennick MJ, Ogilvie MD, Margulies SS, Hiller L, Gefter WB, Pack AI. MRI study of regional variations of pharyngeal wall compliance in cats. J Appl Physiol 85: 1884–1897, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Brennick MJ, Pickup S, Dougherty L, Cater JR, Kuna ST. Pharyngeal airway wall mechanics using tagged magnetic resonance imaging during medial hypoglossal nerve stimulation in rats. J Physiol 561: 597–610, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brennick MJ, Trouard TP, Gmitro AF, Fregosi RF. MRI study of pharyngeal airway changes during stimulation of the hypoglossal nerve branches in rats. J Appl Physiol 90: 1373–1384, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579: 515–526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciscar MA, Juan G, Martinez V, Ramon M, Lloret T, Minguez J, Armengot M, Marin J, Basterra J. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J 17: 79–86, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Ferguson KA, Love LL, Ryan CF. Effect of mandibular and tongue protrusion on upper airway size during wakefulness. Am J Respir Crit Care Med 155: 1748–1754, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Fogel RB, Malhotra A, Dalagiorgou G, Robinson MK, Jakab M, Kikinis R, Pittman SD, White DP. Anatomic and physiologic predictors of apnea severity in morbidly obese subjects. Sleep 26: 150–155, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Fregosi RF. Influence of tongue muscle contraction and dynamic airway pressure on velopharyngeal volume in the rat. J Appl Physiol 104: 682–693, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 110: 295–306, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507: 265–276, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519: 601–613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol 74: 547–555, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Helferty JP, Zhang C, McLennan G, Higgins WE. Videoendoscopic distortion correction and its application to virtual guidance of endoscopy. IEEE Trans Med Imaging 20: 605–617, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Isono S, Morrison DL, Launois SH, Feroah TR, Whitelaw WA, Remmers JE. Static mechanics of the velopharynx of patients with obstructive sleep apnea. J Appl Physiol 75: 148–154, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol 89: 1345–1351, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Janssen PL, Williams JS, Fregosi RF. Consequences of periodic augmented breaths on tongue muscle activities in hypoxic rats. J Appl Physiol 88: 1915–1923, 2000 [DOI] [PubMed] [Google Scholar]
- 26. John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med 172: 1331–1337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuna ST. Effects of pharyngeal muscle activation on airway size and configuration. Am J Respir Crit Care Med 164: 1236–1241, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Kuna ST. Regional effects of selective pharyngeal muscle activation on airway shape. Am J Respir Crit Care Med 169: 1063–1069, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kuna ST, Bedi DG, Ryckman C. Effect of nasal airway positive pressure on upper airway size and configuration. Am Rev Respir Dis 138: 969–975, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Kuna ST, Vanoye CR. Mechanical effects of pharyngeal constrictor activation on pharyngeal airway function. J Appl Physiol 86: 411–417, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Leiter JC. Analysis of pharyngeal resistance and genioglossal EMG activity using a model of orifice flow. J Appl Physiol 73: 576–583, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Leiter JC. Upper airway shape: Is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med 153: 894–898, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med 161: 1746–1749, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med 165: 71–77, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Marshall MW, Teitelbaum GP, Kim HS, Deveikis J. Ferromagnetism and magnetic resonance artifacts of platinum embolization microcoils. Cardiovasc Intervent Radiol 14: 163–166, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Mayer P, Pepin JL, Bettega G, Veale D, Ferretti G, Deschaux C, Levy P. Relationship between body mass index, age and upper airway measurements in snorers and sleep apnoea patients. Eur Respir J 9: 1801–1809, 1996 [DOI] [PubMed] [Google Scholar]
- 37. McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec 260: 378–386, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Oliven A, O'Hearn DJ, Boudewyns A, Odeh M, De Backer W, van de Heyning P, Smith PL, Eisele DW, Allan L, Schneider H, Testerman R, Schwartz AR. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol 95: 2023–2029, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Oliven A, Tov N, Geitini L, Steinfeld U, Oliven R, Schwartz AR, Odeh M. Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J 30: 718–758, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Pillar G, Fogel RB, Malhotra A, Beauregard J, Edwards JK, Shea SA, White DP. Genioglossal inspiratory activation: central respiratory vs. mechanoreceptive influences. Respir Physiol 127: 23–38, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodenstein DO, Dooms G, Thomas Y, Liistro G, Stanescu DC, Culee C, Aubert-Tulkens G. Pharyngeal shape and dimensions in healthy subjects, snorers, and patients with obstructive sleep apnoea. Thorax 45: 722–727, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 152: 1673–1689, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Smith KK, Kier WM. Tongue tentacles and trunks: the biomechanics of movement in muscular hydrostats. Zool J Linnean Soc 83: 307–324, 1985 [Google Scholar]
- 44. Sokoloff AJ, Yang B, Li H, Burkholder TJ. Immunohistochemical characterization of slow and fast myosin heavy chain composition of muscle fibres in the styloglossus muscle of the human and macaque (Macaca rhesus). Arch Oral Biol 52: 533–543, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Zutphen C, Janssen P, Hassan M, Cabrera R, Bailey EF, Fregosi RF. Regional velopharyngeal compliance in the rat: influence of tongue muscle contraction. NMR Biomed 20: 682–691, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Walsh JH, Leigh MS, Paduch A, Maddison KJ, Armstrong JJ, Sampson DD, Hillman DR, Eastwood PR. Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnea. Sleep 31: 1543–1549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walsh JH, Leigh MS, Paduch A, Maddison KJ, Philippe DL, Armstrong JJ, Sampson DD, Hillman DR, Eastwood PR. Evaluation of pharyngeal shape and size using anatomical optical coherence tomography in individuals with and without obstructive sleep apnoea. J Sleep Res 17: 230–238, 2008 [DOI] [PubMed] [Google Scholar]