Abstract
We tested the hypothesis, motivated in part by a coordinated computational cough network model, that alterations of mean systemic arterial blood pressure (BP) influence the excitability and motor pattern of cough. Model simulations predicted suppression of coughing by stimulation of arterial baroreceptors. In vivo experiments were conducted on anesthetized spontaneously breathing cats. Cough was elicited by mechanical stimulation of the intrathoracic airways. Electromyograms (EMG) of inspiratory parasternal, expiratory abdominal, laryngeal posterior cricoarytenoid (PCA), and thyroarytenoid muscles along with esophageal pressure (EP) and BP were recorded. Transiently elevated BP significantly reduced cough number, cough-related inspiratory, and expiratory amplitudes of EP, peak parasternal and abdominal EMG, and maximum of PCA EMG during the expulsive phase of cough, and prolonged the cough inspiratory and expiratory phases as well as cough cycle duration compared with control coughs. Latencies from the beginning of stimulation to the onset of cough-related diaphragm and abdominal activities were increased. Increases in BP also elicited bradycardia and isocapnic bradypnea. Reductions in BP increased cough number; elevated inspiratory EP amplitude and parasternal, abdominal, and inspiratory PCA EMG amplitudes; decreased total cough cycle duration; shortened the durations of the cough expiratory phase and cough-related abdominal discharge; and shortened cough latency compared with control coughs. Reduced BP also produced tachycardia, tachypnea, and hypocapnic hyperventilation. These effects of BP on coughing likely originate from interactions between barosensitive and respiratory brainstem neuronal networks, particularly by modulation of respiratory neurons within multiple respiration/cough-related brainstem areas by baroreceptor input.
Keywords: baroreceptive input, baroreceptor drive, respiratory motor control, nitroprusside
cough is initiated by stimulation of mechano- and chemosensitive sensory endings of cough receptors and also may be influenced by a cough-related subgroup of rapidly adapting “irritant” receptors and C fibers within tracheobronchial and laryngeal mucosa (23, 24, 148). However, the pattern of coughing (the number of coughs and their strength and timing; Refs. 67, 149) is profoundly affected by other peripheral and central afferent inputs (17, 49), particularly during pathological processes such as infection, inflammation, and allergic reactions (28, 118). Stimuli within the larynx (141) and nose (109, 110) enhance cough induced from the tracheal-bronchial region. Stimulation of cardiac receptors (140), chemoreceptors (138), and pulmonary as well as bronchial C fibers in anesthetized animals (139) reduces coughing. Afferent signaling from muscles, joints, skin, and possibly the viscera may also alter the expression of cough (66, 67, 118).
Coughing induces vigorous intrathoracic and intra-abdominal pressure oscillations and changes of sympathetic and parasympathetic nervous activities (27, 67, 145) that significantly affect the cardiovascular system including dynamic changes of blood pressure (BP) and regional blood flow (60, 67). Coughing is associated with peaks in systemic arterial blood pressure during systole and cough expulsions followed by post-tussive hypotension (unpublished observations; Refs. 67, 127). This relationship involves central reflex mechanisms (27, 127, 145); it is observed also in neuromuscular-blocked decerebrate animals (unpublished observations). However, very little is known about the effects of systemic BP and baroreceptor afferent input on the excitability and patterning of cough. Available data were mostly obtained with stimulation of multiple sensory afferents resulting in expression of the chemoreflex (96, 140), and the results suggested either no changes (139, 140) or only transient alterations of the cough reflex (96) during reduced BP.
The respiratory neuronal network is a crucial component in the generation of cough and the transmission of its central motor pattern to the respiratory muscles (54, 119, 123–125). There is a close relationship between the control of the respiratory and cardiovascular systems. It is well established that an increase in blood pressure resulting in the baroreflex (83, 98, 135) can prolong expiration, significantly reduce breathing frequency, and reduce inspiratory drive by an action on selected populations of brainstem respiratory neurons (2, 35, 72, 76, 107) sensitive to afferent impulses originating from baroreceptors. Thus baroreceptor reflex feedback mechanisms that modulate breathing may also limit cough intensity and/or number. Hence, changes in cough excitability and/or the pattern of coughing due to the stimulation of baroreceptors (and alternatively by their unloading) are consistent with the multifunctional role of the respiratory pattern generator in controlling cough and breathing.
Motivated by this consideration, we undertook a computational modeling study of the respiratory/cough neuronal network and in vivo experiments to test the role of blood pressure changes in modulation of cough motor pattern. The study also allowed us to address a more general hypothesis that this model could be used to predict, not just motor patterns and neuronal responses of the brainstem respiratory network, but regulation of this system as well. Models simulating concurrent cough and baroreceptor perturbations of breathing predicted that an increase of mean systemic arterial BP would alter the motor pattern and excitability of the cough reflex.
METHODS
In vivo experiments.
Experiments were performed on 20 cats (5.1 ± 0.3 kg; 5 females and 15 males). Animals were anesthetized with pentobarbital sodium (Nembutal; 35 mg/kg iv), and supplementary doses were administered (1–3 mg/kg iv) as needed. Atropine (0.1 mg/kg iv) was given at the beginning of the experiment to reduce secretions. The trachea, femoral artery, and vein were cannulated. The femoral artery and vein catheters carried a small expandable balloon located near the tip. The catheters were introduced and positioned within the descending aorta and/or within inferior vena cava. A balloon catheter was also inserted into the esophagus for the measurement of esophageal pressure. Animals were allowed to breathe spontaneously a gas mixture of 40% oxygen, balance nitrogen. Arterial BP within the descending aorta (proximal from the balloon), esophageal pressure, end-tidal CO2 concentration (ETCO2), and body temperature were continuously monitored (body temperature was maintained at 37.5 ± 0.5°C by a heating pad). Periodically, samples of arterial blood were removed for blood gas and pH analysis. Electromyograms of respiratory muscles were recorded with bipolar insulated fine wire electrodes by the technique of Basmajian and Stecko (7). The electromyograms of expiratory transversus abdominis (ABD) muscles, inspiratory parasternal muscles, laryngeal abductor posterior cricoarytenoid, and laryngeal adductor thyroarytenoid muscles were recorded. The details of electrode positioning were as previously described (113). Two animals that were included in this study were also used in another series of experiments in which microinjection of neurochemicals into the medulla was performed. These two animals were placed prone in a stereotaxic apparatus and the dorsal surface of the medulla was exposed surgically. The surface of the brainstem was covered by warm paraffin oil (113). All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the University of Florida Institutional Animal Care and Use Committee.
Tracheobronchial cough was elicited by mechanical stimulation of the intrathoracic airways with a thin polyethylene catheter or a stimulation device formed from a polyethylene catheter connected to 1–3 nylon fiber loops. This stimulator was inserted into the trachea (moved and rotated at ∼2 Hz) for periods of 10 s (in 3 cats for 20 s) to elicit repetitive coughing. Cough was defined by a large burst of inspiratory-related parasternal muscles electromyographic activity immediately followed by a burst of expiratory ABD electromyographic activity and by a related inspiratory-expiratory waveform of esophageal pressure. These criteria separated cough from other airway defensive behaviors such as augmented breath and the expiration reflex that are also inducible from the trachea (114).
All electromyograms were amplified, filtered (300–5,000 Hz), rectified, and integrated (time constant of 200 ms). The number of coughs in response to mechanical stimulation of the trachea (average number of coughs per 10-s stimulation), amplitudes of parasternal, ABD, and laryngeal muscles electromyographic moving averages, amplitudes of esophageal pressure during appropriate phases of cough, duration of inspiratory and expiratory phases of cough (CTI and CTE), total cough cycle duration (CTtot), and duration of ABD electromyographic activity during cough were analyzed. The cough inspiratory phase was defined as the period from the onset of parasternal muscles electromyographic activity until its maximum during cough. The cough expiratory phase was defined as the interval from the maximum of parasternal muscles activity to the onset of the next parasternal electromyographic burst (15). This phase consists of active E1 (CTE1; from the maximum of parasternal activity to the offset of cough-related ABD activity) and quiescent E2 (CTE2; from the offset of cough ABD activity to the next parasternal muscles activation) periods (147). The activities of posterior cricoarytenoid and thyroarytenoid muscles were analyzed separately in each of four laryngeal cough phases (111). In addition, we analyzed the delays from the stimulation onset to the beginning of the cough-related parasternal and ABD activity in animals where BP was altered by inflation of the embolectomy balloons. The inflation of the embolectomy catheter balloon within the descending aorta resulted in an increase of BP after a delay of ∼2 s. Blood pressure was reduced ∼5 s after inflation of the embolectomy balloon within vena cava inferior. Cough stimulation was applied one breath after BP either rose or fell due to inflation of the embolectomy balloons. When pressure in the balloons was released to terminate each BP challenge trial, BP returned to control values within a few seconds. The duration of BP alterations caused by balloon inflations never exceeded 30 s. Basic cardiorespiratory parameters of BP, heart rate (HR), respiratory rate (RR), inspiratory esophageal pressure amplitudes, and ETCO2 were measured within three consecutive respiratory cycles just before and after the inflation of catheter balloons. We also recorded RR, esophageal pressure amplitudes, ETCO2 from the first breath following the BP change (but before the onset of coughing), BP, and HR within all periods of altered BP. In five additional cats, we reduced BP by injecting nitroprusside (72 ± 8 μg/kg; range 60 to 100 μg/kg iv). In nitroprusside-injected cats, we analyzed the cardiorespiratory data before the intravenous injection, ∼30–60 s later (just before the second postinjection cough trial), and 5 to 20 min after the nitroprusside injection during the recovery period.
The characteristics of coughs during alterations in BP (BP challenge trial − the cough trial with inflated embolectomy catheter balloon) were compared with parameters of coughs before and after BP challenge. Control cough trials were separated from the BP challenge cough trial by ∼1 min. This pattern of stimulation and BP alteration was conducted 2–4 times in 15 animals. A slightly different protocol was employed in 11 animals. When a stable cough baseline was attained after ∼20 consecutive cough stimulation trials, we conducted three to four control cough trials (each separated by ∼1 min) followed by three to four cough trials with altered BP (the balloon was inflated only during the stimulation, not during the 1-min periods between the trials) and followed by another three to four postchallenge cough trials. No differences were observed between the characteristics of coughing during control pre- and post-BP altered trials; therefore, the pre- and postchallenge control cough trials were pooled. The data obtained from this protocol were not different from that obtained from animals where control and BP challenge trials were alternated (the 15 cats described previously); data from the two protocols were therefore pooled.
Results are expressed as a mean values ± SE. For statistical analysis, repeated-measures ANOVA with Student-Newman-Keuls post test, Friedman test with Dunn posttest, paired and unpaired t-test, Wilcoxon matched pairs test, or Mann-Whitney test were employed as appropriate. The differences of variables were considered significant if P < 0.05.
Computational model and simulation.
Network simulations were performed using a previously described simulation program and environment (6, 119) based on the SYSTM11 program by MacGregor (84). Briefly, interacting populations were simulated with an “integrate-and-fire” (IF) approach. The I-Driver (pre-I) population of pre-Bötzinger complex neurons was implemented with conditional INaP-dependent bursting properties using the approach of Breen et al. (20), which reproduces bursting behavior similar to implementations in the Hodgkin-Huxley style (22). The number of neurons in a population ranged from 99 to 300. Excitability of each neuron was determined by an injected current; noise was added to provide variability in the activity of each neuron and the synaptic inputs (external drives) from “fiber populations.” At each integration step (0.5 ms), the simulator updated state variables for membrane potential (Eij), spike-generation threshold (THij), postaction potential potassium conductance (GKij), and synaptic conductances (Gijk) for each neuron (j) of each population (i) and each synaptic type (k). When the membrane potential exceeded the cell's threshold, an action potential was generated, the input conductances were activated, and all target cells received (after conduction times) input synaptic currents defined by weights of synaptic connections and type of synapses. The model and initial parameters for simulations were as descibed in Rybak et al. (119); specific modifications and enhancements are given in the results and Supplemental Tables S1–S4 (Supplemental Material for this article is available online at the J Appl Physiol web site). The program was implemented with the C language and run on 64-bit Intel multiprocessor-based computers under the Linux operating system.
RESULTS
Computational model and simulations.
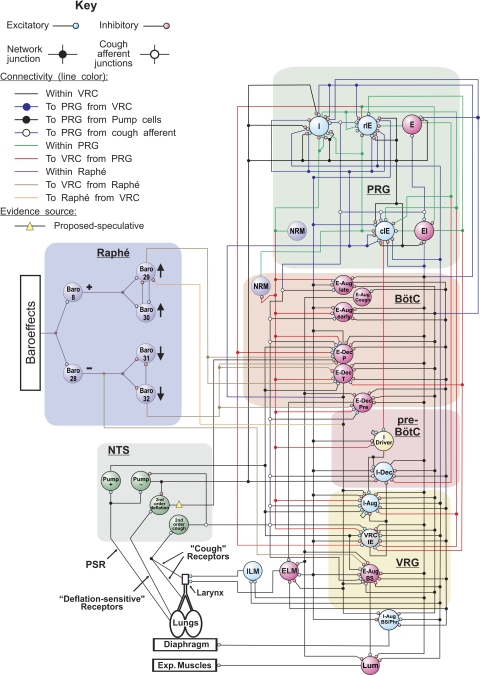
We extended a computational model of the pontomedullary respiratory network (119) capable of reproducing the cough motor pattern. That previous model incorporated connectivity based on prior and coordinated in vivo experiments. It also instantiated speculative connectivity to support the earlier hypotheses that activation of airway cough receptors changes firing activity of second order solitary tract nucleus (NTS) neurons that directly and/or indirectly affect several populations of respiratory neurons in the ventral respiratory column (VRC) and pontine respiratory group (PRG). These evoked changes reconfigure the respiratory network to produce cough motor pattern, acting (at least in part) through the same VRC neurons involved in providing drive to respiratory muscles during normal breathing (123–125).
The present model (Fig. 1) incorporated a circuit “module” for baroreceptor modulation of breathing based on in vivo multiarray recordings and correlational linkages of baroresponsive neurons (76). The new circuit includes both excitatory and disinhibitory raphé neuron influences acting on VRC decrementing expiratory (E-Dec) neurons, including a “tonic” E-Dec population with inhibitory actions on inspiratory premotor bulbospinal neurons. The model also incorporates actions of the E-Dec-tonic population based on recently identified functional interactions (78, 102).
Fig. 1.
Schematic of the pontomedullary respiratory/cough network model showing simulated populations and network connections. Large colored circles represent simulated populations. To facilitate the tracing of pathways, regional connections are color coded and dots mark branch points of divergent projections. This model includes specific enhancements of a prior model (119) as described in results, including an evidence-based raphé circuit with baroreceptor-related neuorns interconnected with the pontomedullary respiratory network and more speculative functional connections (see key). Added neuronal populations and model parameters for cell properties and connections are detailed in Supplemental Tables S1-S4. Aug, units with augmenting activity; BötC, Bötzinger complex; BS, bulbo-spinal; c, caudal; Dec, units with decrementing activity; E, expiratory neurons; E-Dec-pre, E-Dec premotor units excitatory to ELM; EI, neurons with a peak firing rate during the expiratory-inspiratory phase transition and with a Dec activity in inspiratory phase; ELM, expiratory laryngeal motoneurons; I, inspiratory neurons; I driver, excitatory inspiratory neurons with activity onset in the late expiratory phase with conditional bursting pacemaker properties; IE, neurons with a peak firing rate during the inspiratory-expiratory phase transition and with a Dec activity in expiratory phase; ILM, inspiratory laryngeal motoneurons; Lum, lumbar; NRM, nonrespiratory modulated neurons; NTS, solitary tract nucleus; P, phasic; Phr, phrenic; pre-BötC, pre-Bötzinger complex; PRG, pontine respiratory group; PSR, pulmonary stretch receptors; r, rostral; T, tonic; VRC or VRG, ventral respiratory column or group.
Other changes include 1) addition of a separate excitatory premotor population (“E-Dec-pre”) to drive the expiratory laryngeal motoneurons (3), and 2) implementation of a speculative vagal afferent pathway for inhibition of the E-Dec-Phasic population. This “deflation-sensitive” afferent pathway represents a class of possible network mechanisms that could contribute to a biasing inhibition of E-Dec neurons postulated to be lost with vagotomy (37, 108). Specific changes incorporated in the present model (Fig. 1) include modifications and additions in neuron population parameters (Supplemental Tables S1–S3) and in the connectivity between the populations (Supplemental Table S4); these tables also include information for populations and connections that relay the perturbations to the network model.
The model produced changes in inspiratory and expiratory motor patterns observed experimentally. When disrupted by simulated “vagotomy,” the deflation-sensitive afferent circuit operating in parallel with slowly adapting pulmonary stretch receptors contributed to a prolongation of both inspiratory and expiratory phases (not shown). Simulated baroreceptor stimulation prolonged the expiratory phase and reduced inspiratory motor drive (not shown), reproducing in vivo observations (e.g., Ref. 76) and prior modeling results (77).
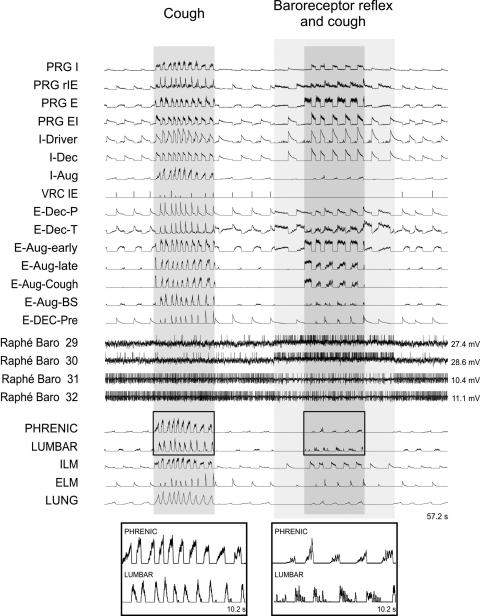
Repetitive cough-like motor patterns generated by the enhanced model were altered when evoked during concurrent activation of simulated baroreceptor reflex influences (Fig. 2). Specific changes with coactivation of the two system inputs included reductions in the firing rates and integrated traces of model motor outputs, prolongation of individual phases of cough motor pattern, and fewer coughs (Fig. 2 and Table 1). These results from the model led to specific predictions consistent with data obtained from the in vivo experiments described in the next section.
Fig. 2.
Integrated firing rates of selected simulated VRC and PRG neuronal populations with laryngeal and main “pump” respiratory muscles motor output during simulated repetitive cough in the absence (Cough) and presence of the baroreceptor influences (Baroreceptor reflex and cough); integrated phrenic and lumbar nerve outputs are shown enlarged at bottom. Membrane potentials of 4 raphé cells are also shown. Note that as in other types of “integrate-and-fire” neuron models, these populations do not actually generate action potential-like spikes but only identified moments of threshold crossings represented graphically by assigning vertical spike-like lines. For abbreviations, see Fig. 1 legend; LUNG represents lung volume (inflations up).
Table 1.
Proportional parameters of cough-like motor patterns during simulated trials generated by the model during concurrent activation of simulated baroreceptor reflex influences
| Coactivation of Simulated Cough and Baroreceptor Reflex Influences | |
|---|---|
| Cough number, % | 52.2 ± 1.8‡ |
| Integrated phrenic amplitude, % | 37.9 ± 4.8‡ |
| Integrated lumbar amplitude, % | 37.9 ± 1.0‡ |
| Cough TI, % | 118.9 ± 4.8* |
| Cough TE, % | 230.5 ± 8.3‡ |
| Cough TE1, % | 224.2 ± 5.3‡ |
| Cough TE2, % | 257.0 ± 45.6† |
| Cough cycle duration, % | 178.7 ± 4.6‡ |
Data are means ± SE. Proportional parameters are expressed as percentages of control. Results of 5 simulations using the enhanced model are shown. Different seeds for random number generation were used for each simulation, resulting in altered firing patterns of fibers within a population, different connection patterns and conduction times between 2 populations, and changes in the noise added to each neuron. Cough TI, Cough TE, Cough TE1, Cough TE2, inspiratory, all expiratory, active expiratory, and quiescent expiratory cough phase duration.
P < 0.05,
P < 0.01, and
P <0.001 vs. control values.
In vivo experiments.
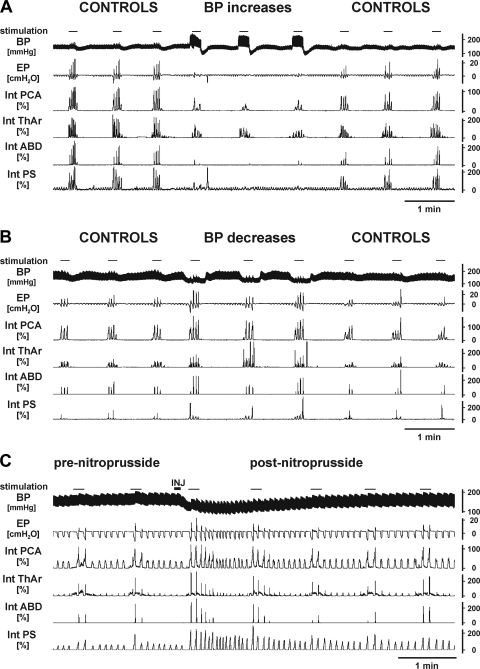
An inflation of the balloon within the descending aorta resulted in an increase of BP associated with significant attenuation of coughing (Fig. 3 and Table 2). Blood pressure was elevated by 37.4 ± 5.8 mmHg, from 147.0 ± 4.8 to 184.4 ± 6.2 mmHg (range of 13 to 73 mmHg; P < 0.001; 12 cats), and this alteration in BP reduced cough number (from 3.86 ± 0.27 to 2.25 ± 0.24; P < 0.001), cough-related inspiratory (from 6.3 ± 1.1 to 4.7 ± 1.2 cmH2O; P < 0.05) and expiratory amplitudes of esophageal pressure (from 9.7 ± 2.6 to 6.8 ± 2.0 cmH2O; P < 0.001), the magnitudes of parasternal muscles and ABD electromyographic moving averages, as well as the maximum of posterior cricoarytenoid muscle electromyographic moving average during the expulsive phase of cough compared with control coughs (Fig. 3 and Table 2). Coughs during increased BP had prolonged CTI (from 1.21 ± 0.15 to 1.44 ± 0.15 s; P < 0.05), CTE (from 2.34 ± 0.27 to 3.16 ± 0.46 s; P < 0.01), CTE2 (from 1.30 ± 0.28 to 2.02 ± 0.52 s; P < 0.05), and CTtot (from 3.63 ± 0.32 to 4.60 ± 0.50 s; P < 0.01) compared with control (Table 2). The latency from the beginning of stimulation to the onset of cough-related parasternal muscles and ABD activities increased during elevated BP from 1.24 ± 0.15 to 2.78 ± 0.71 s (P < 0.05) and from 2.66 ± 0.21 to 4.63 ± 0.78 s (P < 0.02), respectively.
Fig. 3.
Original records of control coughing (CONTROL) and coughing during elevated (BP increases; A) and lowered blood pressure (BP decreases; B) due to the inflation of balloons within the aorta and vena cava, respectively. Coughing after iv injection (INJ) of nitroprusside is shown in C. Aortic balloons were inflated for ∼5 s and vena cava balloons for ∼8 s before mechanical stimulation of the tracheobronchial mucosa was applied. BP, arterial blood pressure; EP, esophageal pressure; Int PCA, Int ThAr, Int ABD, Int PS, electromyographic moving averages of laryngeal abductor posterior cricoarytenoid muscle, laryngeal adductor thyroarytenoid muscle, abdominal muscles, and parasternal muscle, respectively. Moving average waveforms are expressed as percentages of the average amplitudes during control prechallenge coughs.
Table 2.
Proportional parameters of coughing during trials in which BP was altered
| Increased BP | Decreased BP | Nitroprusside | |
|---|---|---|---|
| Number of cats | 12 | 10 | 5 |
| BP, % | 126.1 ± 4.3‡ | 78.7 ± 3.8‡ | 65.0 ± 6.8† |
| Cough number, % | 58.9 ± 6.9‡ | 145.9 ± 18.7‡ | 159.8 ± 28.0* |
| Cough inspiratory EP, % | 70.1 ± 8.3* | 124.0 ± 9.9* | 103.4 ± 6.9 |
| Cough expiratory EP, % | 65.3 ± 7.7‡ | 104.9 ± 10.7 | 116.0 ± 18.9 |
| Cough inspiratory PS, % | 60.9 ± 11.5† | 169.9 ± 30.7* | 153.8 ± 16.1* |
| Cough expiratory ABD, % | 65.4 ± 8.6† | 123.7 ± 10.0* | 111.4 ± 14.8 |
| Cough inspiratory PCA. % | 83.1 ± 8.1 | 108.3 ± 2.8* | 105.4 ± 7.0 |
| Cough expiratory PCA, % | 74.3 ± 7.9† | 116.5 ± 9.7 | 103.8 ± 8.0 |
| Cough compressive ThAr, % | 81.9 ± 8.8 | 105.7 ± 6.8 | 95.0 ± 19.1 |
| Cough late ThAr, % | 110.4 ± 16.4 | 87.3 ± 6.2 | 83.4 ± 26.1 |
| Cough TI, % | 111.1 ± 6.4* | 92.4 ± 4.8 | 92.8 ± 4.7 |
| Cough TE, % | 137.2 ± 10.5† | 72.5 ± 6.6† | 80.2 ± 7.4 |
| Cough TE1, % | 110.7 ± 10.0 | 92.5 ± 3.3 | 93.4 ± 5.1 |
| Cough TE2, % | 184.9 ± 36.5* | 60.9 ± 7.5‡ | 65.2 ± 9.8* |
| Cough cycle duration, % | 127.8 ± 7.7† | 76.0 ± 6.1† | 84.6 ± 5.9 |
| Duration of cough ABD, % | 100.9 ± 16.3 | 86.7 ± 4.0† | 108.2 ± 5.8 |
Values are means ± SE. Proportional parameters are expressed as percentages of control. BP, blood pressure measured during coughing; EP, amplitudes of esophageal pressure; PS and ABD, amplitudes of parasternal and abdominal muscles electromyographic moving averages; PCA and ThAr, amplitudes of posterior cricoarytenoid and thyroarytenoid muscles electromyographic moving averages in particular phase of cough.
P < 0.05,
P <0.01, and
P <0.001 vs. control values.
Experimental reduction of BP by the inflation of the balloon within the inferior vena cava was accompanied by enhancement of coughing (Fig. 3 and Table 2). The BP was reduced by 34.6 ± 6.3 mmHg (from 161.6 ± 6.5 to 127.0 ± 7.9 mmHg, the range of 13 to 75 mmHg; P < 0.001; 10 cats), and this resulted in increased cough number (from 2.93 ± 0.39 to 3.83 ± 0.34; P < 0.001); cough-related inspiratory esophageal pressure amplitudes (from 7.0 ± 1.7 to 8.3 ± 1.8 cmH2O; P < 0.05); parasternal, inspiratory posterior cricoarytenoid muscles, and ABD electromyographic moving average amplitudes along with shorter CTE (from 4.64 ± 1.30 to 2.96 ± 0.61 s; P < 0.01), CTE2 (from 3.43 ± 1.27 to 1.80 ± 0.56 s; P < 0.001), and CTtot (from 6.28 ± 1.33 to 4.40 ± 0.63 s; P < 0.01); and the duration of cough-related ABD discharge (from 1.51 ± 0.23 to 1.42 ± 0.25 s; P < 0.05) compared with control coughs (Table 2). The latencies from the beginning of stimulation to the onset of parasternal muscles and ABD cough activities during reduced BP were decreased compared with controls from 1.68 ± 0.43 to 1.04 ± 0.29 s (P < 0.02) and from 3.31 ± 0.56 to 2.57 ± 0.36 s (P < 0.02), respectively.
Intravenous injections of nitroprusside reduced BP for ∼2 min (maximum changes of 54.8 ± 10.1 mmHg, from 159.0 ± 6.4 to 104.3 ± 13.2; P < 0.01). These BP reductions were accompanied by increased cough number (from 3.91 ± 0.46 to 5.78 ± 0.27; P < 0.05), enhanced cough-related parasternal muscles electromyographic moving average amplitudes, and a shorter CTE2 (from 1.16 ± 0.70 to 0.63 ± 0.31 s; P < 0.05) compared with control (Table 2). In addition, there was a tendency for shorter CTE and CTtot (Table 2; P < 0.06); however, the differences were not statistically significant (Table 2). The shorter CTtot and longer cough-related ABD discharge during reduced BP by nitroprusside led to an increased relative duration of ABD activity (expressed as percentage of CTtot, from 34.6 ± 8.6 to 41.8 ± 7.4%; P < 0.05).
Neither increases nor decreases of BP produced by the inflation of balloons within blood vessels (and coughing induced during these BP modifications) resulted in significant alterations of cardiorespiratory parameters including BP, HR, RR, respiratory esophageal pressure amplitudes, and ETCO2 (Fig. 3 and Table 3) immediately after the periods of altered BP. However, during increased BP HR and RR were reduced (Table 3); when BP was decreased, a higher HR, RR, respiratory esophageal pressure amplitudes, and lower ETCO2 were observed (Table 3). Similarly, within the period 30 to 60 s after the intravenous injection of nitroprusside, we observed tachycardia with tachypnea (and hyperpnea) and significant decreases of ETCO2 (Fig. 3 and Table 3).
Table 3.
Basic cardiorespiratory parameters analyzed before, during, and after the alterations of systemic arterial blood pressure by balloon inflations as well as before, after, and late (5–20 min) recovery following the intravenous injection of nitroprusside
| Mean BP, mmHg | HR, beats/min | RR, breath/min | EP ampl, cmH2O | ETCO2, mmHg | |
|---|---|---|---|---|---|
| Pre-BP up | 142 ± 5 | 200 ± 5 | 17.4 ± 1.3 | 2.3 ± 0.3 | 37.0 ± 2.3 |
| BP up | 187 ± 5c | 180 ± 8a | 14.5 ± 1.2b | 2.1 ± 0.3 | 37.8 ± 2.0 |
| Post-BP up | 142 ± 5f | 198 ± 5d | 17.4 ± 1.1e | 2.6 ± 0.5 | 38.1 ± 2.3 |
| Pre-BP down | 154 ± 6 | 199 ± 3 | 16.2 ± 1.4 | 2.6 ± 0.7 | 38.9 ± 2.2 |
| BP down | 117 ± 8c | 216 ± 5c | 18.2 ± 1.1c | 3.2 ± 0.8b | 32.7 ± 1.1c |
| Post-BP down | 164 ± 8f | 199 ± 3e | 15.5 ± 1.3f | 2.8 ± 0.7d | 38.9 ± 2.4f |
| Prenitroprusside | 161 ± 7 | 190 ± 11 | 18.5 ± 1.8 | 4.2 ± 0.9 | 34.5 ± 1.7 |
| Postnitroprusside | 88 ± 10c | 210 ± 4b | 22.6 ± 1.3a | 4.7 ± 1.2 | 30.6 ± 1.3b |
| Recovery | 158 ± 8f | 183 ± 7e | 16.3 ± 2.1e | 4.3 ± 1.1 | 35.1 ± 1.3e |
Values are means ± SE. BP, arterial blood pressure; HR, heart rate; RR, respiratory rate; EP ampl, respiratory esophageal pressure amplitude; ETCO2, end expiratory content of carbon dioxide.
P < 0.05,
P < 0.01, and
P < 0.001, respectively, compared with pre-BP alteration or preinjection control.
P < 0.05,
P < 0.01, and
P < 0.001, respectively, compared with BP alteration or early postnitroprusside injection.
DISCUSSION
This study demonstrates that changes in blood pressure modify the excitability and the pattern of tracheobronchial cough in anesthetized cats. The results are consistent with predictions derived from simulations of a computational model of the brainstem respiratory-cough neuronal network. The amplitudes of pump and upper airway motor unit bursts were diminished, durations of cough phases were prolonged, and cough frequency was reduced. The in vivo experiments also found that coughing was enhanced when BP was reduced.
To our knowledge, this is the first report investigating the effects of experimental BP changes and therefore altered baroreceptor afferent inputs on cough. Pressure-sensitive nerve endings are located primarily within the aortic arch, carotid sinuses, heart atria, and ventricles (29, 134). Afferent signaling from these receptors is related to the actual BP within the corresponding segment of the cardiovascular system, and this afferent feedback, when significantly altered, results in appropriate adjustments in the control of the cardiovascular and respiratory systems (14, 33, 34, 44, 107). Inflation of balloons within the descending aorta or inferior vena cava reduced the rate of blood flow distal to the obstruction resulting in increases or decreases of BP in the arterial circulation proximal to the balloons (39, 137). This and other similar techniques have been widely used for the stimulation vs. unloading of baroreceptors and are considered to be transient, specific, and sufficiently selective (47, 76, 103). Short duration occlusions of the aorta and vena cava activate/deactivate baroreceptors without profound stimulation of other receptors and cause no ischemic damage to downstream tissues. Far longer reductions in blood flow than those used in our study (15–25 s) are required to observe functional changes within the tissues distal to the occlusion (1, 62, 65, 81, 150), although some signs of ischemia can be detected within the first 10 s (68). The activities of pressure-sensitive and other receptors within the tissues (50, 53) could be affected during our BP changes by the temporarily modified perfusion (85, 106). However, observed alterations of coughing conformed well to changes in “central” BP and consequently to the baroreceptor inputs from main baroreceptive areas. Cardiovascular changes in our animals were short lasting and their magnitude was limited (the mean BP continually >75 mmHg). Vigorous coughing itself markedly modifies blood circulation including pulmonary and brain BP and perfusion of the organs (60, 67), but no distinguishable changes in coughing were reported during cough bouts (16, 67, 113).
Different methods of baroreceptor stimulation produce opposite hemodynamic changes in the systemic circulation and consequently stimulate other afferents in differing ways (19, 76, 120). Phenylephrine and carotid clamping produce an elevation in systemic arterial BP, and increased pressure in isolated carotid sinus elicits a reduction in BP (21). An occlusion of the descending aorta by the method employed in the present study has an opposite effect on arterial BP proximal and distal to the balloon. Nevertheless, these different methods result in very similar responses, e.g., changes of respiratory motor output and firing characteristics of recorded neurons including respiratory units following aorta occlusion, increased carotid sinus pressure, and injection of phenylephrine (76), or corresponding respiratory drive changes to phrenic and hypoglossal nerves (120) during occlusion carotid clamping and occlusions of aorta vs. vena cava, strongly suggesting minor role of other receptors than baroreceptors. The baroreceptor stimulation-related responses are largely eliminated by baroreceptor denervation (117, 143) and are relatively unaffected by vagotomy (120). Pulmonary afferents were certainly much more vigorously stimulated by mechanical airway probing and by large inspiratory-expiratory efforts during cough than by alterations in BP. It is unlikely that the circulatory changes in our animals, including pulmonary (1) and/or cerebral circulation (14, 82), resulted in effects (besides the baroreceptive ones) that would noticeably affect cough. It is also unlikely that chemoreceptors were markedly stimulated during our BP changes (Table 3). Large magnitudes of hypoxia and/or hypercapnia are necessary to significantly inhibit cough (99, 138). Stimulation of chemoreceptors has been reported during reduced BP (13, 70), but this change in BP enhanced coughing in our animals. Nitroprusside (iv) administration was secondarily employed to reduce BP in our study. The effects of nitroprusside trials and vena cava inflation balloon trials on coughing corresponded well. However, sodium nitroprusside may well have inhibited cough by an additional mechanism involving activation of BK(Ca) channels (71).
Cardiovascular and respiratory responses induced by baroreflexes are well documented, including their variations with different experimental conditions and species differences (14, 19, 21, 64, 72, 76, 98). We observed bradycardia and isocapnic bradypnea associated with BP increases and tachycardia, tachypnea, and hypocapnic hyperventilation with reduced BP (Table 3). We also found reduced coughing due to the increased BP and enhancement of the cough reflex when BP was decreased. Our computational model with baroresponsive neuronal populations generated respiratory and cough patterns predictive of key features of the in vivo experimental observations. Reduction of coughing with increased baroreceptor input may reflect a negative feedback system that limits increases in BP associated with (even fictive) cough expulsions.
Reflex cough is produced by a complex multilevel brainstem neuronal network (17, 105, 125) that involves respiratory as well as nonrespiratory modulated neurons (18, 55, 124). Several lines of evidence, including multiple single neuron recordings during fictive cough evoked by mechanical stimulation of the trachea, spike train cross-correlation, Fos expression, and lesioning and simulation experiments (17, 55, 59, 100, 115, 123–125), all indicate that the central pattern of cough is produced by circuit elements of the respiratory central pattern generator (CPG) (common respiratory/cough CPG). A primary component of the respiration/cough producing neuronal network is the respiratory rhythm-generating neuronal circuit within the rostral Bötzinger complex/pre-Bötzinger complex region of the VRC (123, 131). Respiratory-related neurons with various classes of discharge patterns are found in this area (10, 11, 31, 43, 74, 75, 121). Most of these neurons (from 80 to 85%) have altered firing rates or patterns during coughing; most are synchronous with particular phases of cough (123, 124). However, both eupneic breathing and reflex coughing also require the NTS (8, 95, 125, 133), VRC (10, 97, 113, 116, 124, 125, 133), medullary raphé (4, 57, 75), medullary lateral tegmental field (58, 63), and PRG (112, 119, 122, 126), as well as functioning premotor and motor pathways. Respiratory as well as nonrespiratory neurons in cough-related areas of VRC, NTS, raphé (72, 73, 76, 91), lateral tegmental field (48), and PRG (5) responded to baroreceptor stimulation. The computational model included specific “compartments” with neuronal populations known to be responsive to baroreceptor stimulation in vivo; these populations are located within the raphé, VRC, and PRG (Fig. 1 and Supplemental Table S1).
The control of the cardiovascular and respiratory neuronal systems is closely related (34, 107). Reciprocal cardiorespiratory modulation is observed within the neuronal network; for example, respiratory motor (and premotor) activity is BP pulse modulated (35, 36, 144). Considering the percentage of cough-related respiratory neurons within the VRC and the fact that >35% of respiratory neurons in this region are barosensitive (72, 76), there is significant overlap in cough- and baroreceptor-related neuronal populations (i.e., >15% of cough-related respiratory neurons are affected by baroreceptor stimuli). Stimulation of baroreceptors reduced the firing rate of a majority of VRC inspiratory neurons including some bulbo-spinal ones (72, 76), a finding consistent with reduced inspiratory motor drive (during eupnea as well as during coughing; Figs. 1 and 3 and Table 2). Expiratory neurons respond to increased baroreceptor drive in a more variable manner (reflected also in the simulations; Fig. 2); a number of them responded only by prolongation of the active period, others by increased or decreased activity (46, 72, 76). Among expiratory neurons, most of E-Dec units increased firing rate due to baroreceptor stimulation (76). Some of these neurons presumably participate in the determination of expiratory phase duration during eupnea and cough (6, 17, 123). Synaptic connections from barosensitive raphé neuronal populations in the present computational model (Fig. 1) resulting in excitation and/or disinhibition of E-Dec neurons within VRC (Fig. 2) were derived from these studies. Baroreceptor stimulation decreased the firing rates of some E-Dec neurons and most augmenting expiratory (E-Aug) cells within the VRC (76). In the present model, this reduced activity is a consequence of inhibitory actions of E-Dec populations on E-Aug neurons (Fig. 1). It has been proposed that a subpopulation of E-Aug and perhaps some E-Dec neurons provide excitatory drive during the expiratory phase of cough (18, 123, 124). Possible relationships among baroreceptor drive, cough expiratory effort, and the duration of the cough expiratory phase (Fig. 3 and Table 2) are consistent with these concepts. In addition, from 25 (76) to 53% (72) of nonrespiratory cells within the VRC respond to baroreceptor stimuli. Some of these neurons may participate in the control of cough.
Within the medullary raphé, ∼40–50% of neurons are influenced by inputs from baroreceptors (72, 76, 94, 151). Medullary raphé neurons are involved in the transformation and transmission of sensory information (including that from baroreceptors) to neurons in the VRC that modulate breathing (76). The baroreceptor-sensitive components in the present computational model are based on modulation of activity of raphé neurons (see results and Fig. 1). Functional convergence of different afferent inputs on raphé neurons (72, 73, 151) may also include transmission and convergence of vagal pulmonary stretch receptors (125) and/or primary cough-related afferent inputs with baroreceptor afferent feedback. However, the model does not employ any interaction of baroreceptor, cough-related, and/or pulmonary stretch receptor effects on neurons within raphé. Respiratory-related neuronal assemblies in the medullary midline and VRC interact (2, 72, 75, 76, 93). For example, cross-correlations of raphé and VRC E-Dec neurons (supposedly contributing to the development of expiratory phase) suggested excitatory functional interactions originating from the raphé neuron (76). There is also a direct modulatory pathway from raphé to phrenic and ABD motoneurons (12, 40).
Neurons within the PRG participate in normal respiratory rhythmogenesis (10, 119, 122, 136) and the production of reflex coughing (112, 126). PRG neurons receive direct inputs from pulmonary stretch receptors and rapidly adapting receptors through NTS relay neurons (37, 41, 128). The baroreflex is depressed from the dorsolateral pons by the modulation of neural transmission within the NTS (42, 52). Ponto-medullary transection significantly affects breathing and coughing (56, 132) but also alters the baroreflex, particularly its respiratory component (5). It is not known if PRG baroresponsive neurons are involved in the modulation of cough or whether such neurons affect cough-related neurons in other areas. However, synaptic connectivity of the model network predicts baroresponsive PRG neurons and their involvement in cough generation or modulation (Fig. 1).
The present model does not incorporate the medullary lateral tegmental field, which contains sympathoinhibitory and sympathoexcitatory neurons that may represent a source of basal sympathetic activity (30, 48). Neurons in the lateral tegmental field respond to increased carotid sinus pressure (48) and are involved in the baroreflex (30). Respiratory and nonrespiratory cells of lateral tegmental field (45, 63) also participate in the expression of airway reflex behaviors (38, 58, 59).
Primary tracheobronchial cough afferents terminate on the second order interneurons mostly within the commissural and medial subdivisions of NTS (95, 125), similar to other airways rapidly adapting and “irritant” receptors (61, 69, 80, 95). However, recent findings (25, 26) on guinea pigs suggest that the NTS terminal sites for cough and other airway receptors of rapidly adapting subgroup may differ. The second order cough NTS interneurons are the only brainstem neurons thought to receive direct input from cough sensory afferents. These neurons represent the first stage of cough central processing and transmit afferent information to all other cough-related brainstem areas (124, 125; Fig. 1). The afferent signals from baroreceptors are transmitted mainly to the neurons of the medial, dorsomedial, commissural, and lateral NTS (32, 72, 88–90, 106, 129) but also either directly or indirectly to other regions of the brainstem (72, 76, 79, 91). In the present model, modulation of baroreceptors induces activity changes of raphé neurons, but the displayed network does not define a particular NTS population as transmitting this afferent signal to the raphé population and/or processing of this information in the NTS. However, the effects of baroreceptor feedback to these raphé neurons (Fig. 1) are presumed to come from NTS neurons. More than 45% of respiratory neurons and almost 40% of nonrespiratory units within the NTS altered their discharge during baroreceptor stimulation (72). Various patterns of spatial and temporal synaptic interactions on NTS neurons were observed between the inputs from baroreceptors, laryngeal mechanoreceptors, and chemoreceptors (72, 88–90) or other sensory afferents (106). Widespread interconnections with NTS neurons from other cardiorespiratory related brainstem areas can significantly affect the processing of peripheral afferent signals, including those from baroreceptors and cough-related afferents at the second order NTS units (34, 42, 51, 101, 142, 146). In addition, in the cat some carotid sinus nerve terminals within the dorsolateral NTS expressed immunoreactivity for substance P (86). Substance P-sensitive NTS neurons are significantly involved in production of tracheal-bronchial cough in anesthetized rabbits (95) and guinea pigs (87). However, given that substance P can also act extrasynaptically and that it promotes cough (95), it is unlikely to have contributed to the suppression of cough observed during intervals of elevated BP.
The activity of baroreceptive afferent fibers may reduce the drive into and/or from the second order cough interneurons resulting in attenuated inputs to the respiratory/cough CPG from these neurons. This potential suppression of the second order cough interneurons would result in a slower transition from breathing to coughing, lower cough number, reduced intensity of coughing, and likely also in prolongation of the cough cycle (17). We observed these changes in our experiments (Table 2 and Fig. 3) and simulations (Fig. 2). However, important interactions between cough and baroreceptor related neuronal networks may occur between components of these networks outside of the NTS as discussed, a view consistent with the performance of the model, which does not contain any NTS population other than those transmitting stretch receptor and cough-related signals. The present model of respiratory/cough neuronal network does not employ any direct interaction of baroreceptor and cough inputs at the level of the NTS. It has been also shown that there is limited convergence (129) and/or the integration of convergent afferent inputs (e.g., cardiac, carotid chemoreceptor, and C fibers) with baroreceptor signals on NTS neurons. However, this study did not investigate potential convergence of pulmonary afferents other than C-fiber and baroreceptor inputs on NTS neurons. Thus separate pathways for different stimuli via NTS may dominate (34). Stimuli that significantly modify breathing (e.g., from pulmonary stretch, rapidly adapting, laryngeal, chemo-, and baroreceptors) transmitted and processed by NTS cells alter the discharge of neurons that participate in circuits of the respiratory CPG (10, 72, 73, 92, 104). Changes in laryngeal motor activation during BP-altered coughs (Table 2) suggest significantly altered discharge patterns of respiratory/cough CPG neurons as well. Although the NTS neurons functionally interact with laryngeal motoneurons within the nucleus ambiguous (9, 130), a general excitation or inhibition of this pathway (e.g., by baroreceptive stimulation) is unlikely to result in differential effects on laryngeal motor outputs during various phases of cough (Table 2). Thus respiratory modifications of the baroreceptor reflex (88) and likely complex respiratory responses during the baroreflex may be largely due to elements of the cardiovascular and respiratory networks that are outside of the NTS.
The computational model of the respiratory/cough network includes connections between populations of neurons inferred from many studies of neurons and their interactions recorded during (fictive) breathing. Model simulations have previously generated several alterations of the respiratory pattern similar to those observed with physiological perturbations in vivo, including cough (119). The model and simulations reported here predicted reductions in the frequency and amplitude of coughs evoked during elevated blood pressure that were confirmed in vivo. The results also suggest new predictions on the discharge profiles of network populations during coughing and altered baroreceptor drive.
In conclusion, our results show that the alterations of blood pressure can modify a majority of the characteristics of coughing. There was a negative relationship between baroreceptor drive and coughing (similar to that with breathing) that likely originates from an interaction between baroresponsive and respiratory brainstem neuronal networks. We suggest that the primary mechanism consists of modulation of discharge characteristics of respiratory neurons within multiple brainstem areas involved in the control and generation of coughing and breathing, particularly those participating in respiratory/cough central pattern generation. Our computational model simulations support this conclusion. In addition, our experimental data support the plausibility of the motivating model that produced motor patterns and network behavior consistent with in vivo observations.
GRANTS
Support for this project was provided by National Heart, Lung, and Blood Institute Grants RO1-HL-70125, R33-HL-89104, R01-HL-103415, and R33-HL-89071.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Amorim FF, Pinheiro BVP, Beppu OS, Romaldini H. Effect of saline infusion for the maintenance of blood volume on pulmonary gas exchange during temporary abdominal aortic occlusion. Brazil J Med Biol Res 40: 333–341, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Arata A, Hernandez YM, Lindsey BG, Morris KF, Shannon R. Transient configurations of baroresponsive respiratory-related brainstem neuronal assemblies in the cat. J Physiol 525: 509–530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones controlling laryngeal motoneurone discharge during eupnoea and fictive cough in the cat. J Physiol 534: 565–581, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Medullary raphe neuron activity is altered during fictive cough in the decerebrate cat. J Appl Physiol 94: 93–100, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Baekey DM, Dick TE, Paton JFR. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93: 803–816, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulations of a ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biol Cybern 70: 311–327, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Basmajian JV, Stecko G. The role of muscles in arch support of the foot. J Bone Joint Surg Am 45: 1184–1190, 1963 [PubMed] [Google Scholar]
- 8. Berger A, Cooney AA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J Appl Physiol 52: 131–140, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Barillot JC, Bianchi AL, Gogan P. Laryngeal respiratory motoneurons: morphology and electrophysiological evidence of separate sites for excitatory and inhibitory synaptic inputs. Neurosci Lett 47: 107–112, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Bianchi AL, Gestreau C. The brainstem respiratory network: an overview of a half century of research. Respir Physiol Neurobiol 168: 4–12, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Billig I, Card JP, Yates BJ. Neurochemical phenotypes of MRF neurons influencing diaphragm and rectus abdominis activity. J Appl Physiol 94: 391–398, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Biscoe TJ, Bradley GW, Purves MJ. The relation between carotid body chemoreceptor discharge, carotid sinus pressure and carotid body venous flow. J Physiol 208: 99–120, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bishop B. Carotid baroreceptor modulation of diaphragm and abdominal muscle activity in the cat. J Appl Physiol 36: 12–19, 1974 [DOI] [PubMed] [Google Scholar]
- 15. Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol 86: 1017–1024, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Bolser DC, Reier PJ, Davenport PW. Responses of the anterolateral abdominal muscles during cough and expiratory threshold loading in the cat. J Appl Physiol 88: 1207–1214, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing. A holarchical system? Respir Physiol Neurobiol 152: 255–265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bongianni F, Mutolo D, Fontana GA, Pantaleo T. Discharge patterns of Bötzinger complex neurons during cough in the cat. Am J Physiol Regul Integr Comp Physiol 274: R1015–R1024, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Borison HL, Borison R, Sadig T. Open-loop comparison of carotid sinus reflex respiratory and circulatory effects in cats. J Physiol 336: 113–129, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breen BJ, Gerken WC, Butera RJ. Hybrid integrate-and-fire model of a bursting neuron. Neur Comput 15: 2843–2862, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Brunner MJ, Sussman MS, Greene AS, Kallman CH, Shoukas AA. Carotid sinus baroreceptor reflex control of respiration. Circ Res 51: 624–636, 1982 [DOI] [PubMed] [Google Scholar]
- 22. Butera RJ, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex: I. Bursting pacemaker neurons. J Neurophysiol 82: 382–397, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetised guinea-pigs. J Physiol 557: 543–558, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Canning BJ. Anatomy and neurophysiology of the cough reflex. Chest 129: 33S–47S, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Canning BJ, Chou Cough Sensors YL. I. Physiological and pharmacological properties of the afferent nerves regulating cough. In: Pharmacology and Therapeutics of Cough 23, Handbook of Experimental Pharmacology 187, edited by Chung KF, Widdicombe JG. Heidelberg: Springer-Verlag, 2009, p. 23–47 [DOI] [PubMed] [Google Scholar]
- 26. Canning BJ, Mori N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J 24: 3916–3926, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cardone C, Bellavere F, Ferri M, Fedele D. Autonomic mechanisms in the heart rate response to coughing. Clin Sci (Lond) 72: 55–60, 1987 [DOI] [PubMed] [Google Scholar]
- 28. Chung KF, Widdicombe JG, Boudhey HA. Cough: Causes, Mechanisms and Therapy. Oxford: Blackwell, 2003 [Google Scholar]
- 29. Ciriello J, Hrycyshyn AW, Calaresu FR. Horseradish peroxidase study of brain stem projections of carotid sinus and aortic depressor nerves in the cat. J Auton Nerv Syst 4: 43–61, 1981 [DOI] [PubMed] [Google Scholar]
- 30. Clement ME, McCall RB. Impairment of baroreceptor reflexes following kainic acid lesions of the lateral tegmental field. Brain Res 618: 328–332, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Connelly CA, Dobbins AG, Feldman JL. Pre-Bötzinger complex in cats: respiratory neuronal discharge patterns. Brain Res 590: 337–340, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Czachurski J, Dembowsky K, Seller H, Nobiling R, Taugner R. Morphology of electrophysiologically identified baroreceptor afferents and second order neurones in the brainstem of the cat. Arch Ital Biol 126: 129–144, 1988 [PubMed] [Google Scholar]
- 33. Daly M, De B. Interactions between respiration and circulation. In: Handbook of Physiology. The Respiratory System. Circulation and Nonrespiratory Functions. Bethesda, MD: Am Physiol Soc, 1985, sect 3, vol 1, 529–594 [Google Scholar]
- 34. Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Dick TE, Morris KF. Quantitative analysis of cardiovascular modulation in respiratory neural activity. J Physiol 556: 959–970, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Arterial pulse modulated activity is expressed in respiratory neural output. J Appl Physiol 99: 691–698, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol 586: 4265–4282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dyachenko YE. Spontaneous and evoked activity of bulbar respiratory and onrespiratory neurons during the expiration reflex. Neirofiziologija 22: 670–680, 1990 [PubMed] [Google Scholar]
- 39. Ehrlich W, Rodriguez-Lopez E. Cardiovascular changes in awake dogs caused by suddenly induced aortic hypertension. Cardiology 63: 253–269, 1978 [DOI] [PubMed] [Google Scholar]
- 40. Ellenberger HH, Feldman JL. Origins of excitatory drive within the respiratory network: anatomical localization. Neuroreport 5: 1933–1936, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Ezure K, Otake K, Lipski J, She RB. Efferent projections of pulmonary rapidly adapting receptor relay neurons in the cat. Brain Res 564: 268–278, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Felder RB, Mifflin SW. Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circ Res 63: 35–49, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Feldman JL, Mitchell GS, Nattie E. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fregosi RF. Changes in the neural drive to abdominal expiratory muscles in hemorrhagic hypotension. Am J Physiol Heart Circ Physiol 266: H2423–H2429, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Fung ML, Tomori Z, St John WM. Medullary neuronal activities in gasping induced by pharyngeal stimulation and hypoxia. Respir Physiol 100: 195–202, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Gabriel M, Seller H. Excitation of expiratory neurones adjacent to the nucleus ambiguous by carotid sinus baroreceptor and trigeminal afferents. Pflügers Arch 313: 1–10, 1969 [DOI] [PubMed] [Google Scholar]
- 47. Gao K, Mason P. The discharge of a subset of serotonergic raphe magnus cells is influenced by baroreceptor input. Brain Res 900: 306–313, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Gebber GL, Barman SM. Lateral tegmental field neurons of cat medulla: a potential source of basal sympathetic nerve discharge. J Neurophysiol 54: 1498–1512, 1985 [DOI] [PubMed] [Google Scholar]
- 49. Hanacek J, Tatar M, Widdicombe J. Regulation of cough by secondary sensory inputs. Respir Physiol Neurobiol 152: 282–297, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Haouzi P, Huszczuk A, Gille JP, Chalon B, Marchal F, Crance JP, Whipp BJ. Vascular distension in muscles contributes to respiratory control in sheep. Respir Physiol 99: 41–50, 1995 [DOI] [PubMed] [Google Scholar]
- 51. Hay M, Bishop VS. Interactions of area postrema and solitary tract in the nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 260: H1466–H1473, 1991 [DOI] [PubMed] [Google Scholar]
- 52. Hayward LF. Midbrain modulation of the cardiac baroreflex involves excitation of lateral parabrachial neurons in the rat. Brain Res 1145: 117–127, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huszczuk A, Yeah E, Innes JA, Solarte I, Wasserman K, Whipp BJ. Role of muscular perfusion and baroreception in the hyperpnea following muscle contraction in dog. Respir Physiol 91: 207–226, 1993 [DOI] [PubMed] [Google Scholar]
- 54. Iscoe S. Control of abdominal muscles. Prog Neurobiol 56: 433–506, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Jakus J, Tomori Z, Stransky A. Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol Bohemoslov 34: 127–136, 1985 [PubMed] [Google Scholar]
- 56. Jakus J, Tomori Z, Boselová L, Nagyová B, Kubinec V. Respiration and airway reflexes after transversal brain stem lesions in cats. Physiol Bohemoslov 36: 329–340, 1987 [PubMed] [Google Scholar]
- 57. Jakuš J, Stránsky A, Poliacek I, Baráni H, Bosel'ová L. Effects of medullary midline lesions on cough and other airway reflexes in anaesthetized cats. Physiol Res 47: 203–213, 1998 [PubMed] [Google Scholar]
- 58. Jakus J, Stransky A, Poliacek I, Barani H, Boselova L. Kainic acid lesion to the lateral tegmental field of medulla. Effects on cough, expiration and aspiration reflexes in anesthetized cats. Physiol Res 49: 387–398, 2000 [PubMed] [Google Scholar]
- 59. Jakus J, Poliacek I, Halasova E, Murin P, Knocikova J, Tomori Z, Bolser DC. Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir Physiol Neurobiol 160: 289–300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Javorka K, Javorka M, Tonhajzerova I. Coughing and its cardiovascular effects. Acta Med Martin 5: 16–21, 2005 [Google Scholar]
- 61. Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in cat. II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol 193: 467–508, 1980 [DOI] [PubMed] [Google Scholar]
- 62. Kim SJ, Ghaleh B, Kudej RK, Huang CH, Hintze TH, Vatner SF. Delayed enhanced nitric oxide-mediated coronary vasodilation following brief ischemia and prolonged reperfusion in conscious dogs. Circ Res 81: 53–59, 1997 [DOI] [PubMed] [Google Scholar]
- 63. King GW, Knox CK. Types and locations of respiratory-related neurons in lateral tegmental field of cat medulla oblongata. Brain Res 295: 301–315, 1984 [DOI] [PubMed] [Google Scholar]
- 64. Koepchen HP. The respiratory-cardiovascular brain stem oscillator in the context of afferent and central excitatory and inhibitory systems. In: Central Interaction Between Respiratory and Cardiovascular Control Systems, edited by Koepchen HP, Hilton SM, Trzebski A. Berlin: Springer-Verlag, 1980, p. 197–205 [Google Scholar]
- 65. Kolenda H, Steffens H, Gefeller O, Hagenah J, Schomburg ED. Critical levels of spinal cord blood flow and duration of ischemia for the acute recovery of segmental spinal cord responses in cats. J Spinal Disord 10: 288–295, 1997 [PubMed] [Google Scholar]
- 66. Kondo T, Kobayashi I, Hayama N, Ohta Y. An increase in the threshold of citric acid-induced cough during chest wall vibration in healthy humans. Jpn J Physiol 48: 341–345, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Korpas J, Tomori Z. Cough and Other Respiratory Reflexes. New York: Karger, 1979 [Google Scholar]
- 68. Kraitchman D, Sampath S, Derbyshire J, Heldman AW, Zerhouni E, Bluemke DA, Prince JL, Osman NF. Detecting the onset of ischemia using real-time HARP. Proc Intl Soc Mag Reson Med 9: 117, 2001 [Google Scholar]
- 69. Kubin L, Davies RO. Sites of termination and relay of pulmonary rapidly adapting receptors as studied by spike-triggered averaging. Brain Res 443: 215–221, 1988 [DOI] [PubMed] [Google Scholar]
- 70. Lahiri S, Nishino T, Mokashi A, Mulligan E. Relative responses of aortic body and carotid body chemoreceptors to hypotension. J Appl Physiol 48: 781–788, 1980 [DOI] [PubMed] [Google Scholar]
- 71. L'Heureux MC, Muinuddin A, Gaisano HY, Diamant NE. Nitric oxide activation of a potassium channel [BK(Ca)] in feline lower esophageal sphincter. World J Gastroenterol 16: 5852–5860, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Responses of simultaneously recorded respiratory-related medullary neurons to stimulation of multiple sensory modalities. J Neurophysiol 82: 176–187, 1999 [DOI] [PubMed] [Google Scholar]
- 73. Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Multimodal medullary neurons and correlational linkages of the respiratory network. J Neurophysiol 82: 188–201, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol 57: 1101–1117, 1987 [DOI] [PubMed] [Google Scholar]
- 75. Lindsey BG, Segers LS, Morris KF, Hernandez YM, Saporta S, Shannon R. Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brainstem midline: evidence from spike train analysis. J Neurophysiol 72: 1830–1851, 1994 [DOI] [PubMed] [Google Scholar]
- 76. Lindsey BG, Arata A, Morris KF, Hernandez YM, Shannon R. Medullary raphe neurones and baroreceptor modulation of the respiratory motor pattern in the cat. J Physiol 512: 863–882, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lindsey BG, Ross A, O'Connor R, Morris KF, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Rybak IA. Modulation and reconfiguration of the pontomedullary respiratory network: A computational modeling study. FASEB J Abstract 610.11, 2007 [Google Scholar]
- 78. Lindsey BG, Ott MM, Nuding SC, Segers LS, O'Connor R, Morris KF. Central chemoreceptors modulate breathing via multipath tuning in ventrolateral respiratory column (VRC) circuits. FASEB J 25: 847.27, 2011 [Google Scholar]
- 79. Lipski J, Mcallen RM, Spyer KM. The sinus nerve and baroreceptor input to the medulla of the cat. J Physiol 251: 61–78, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol 443: 55–77, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Longhurst JC, Benham RA, Rendig SV. Increased concentration of leukotriene B4 but not thromboxane B2 in intestinal lymph of cats during brief ischemia. Am J Physiol Heart Circ Physiol 262: H1482–H1485, 1992 [DOI] [PubMed] [Google Scholar]
- 82. Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension 55: 698–705, 2010 [DOI] [PubMed] [Google Scholar]
- 83. Maass-Moreno R, Katona PG. Species dependence of baroreceptor effects on ventilation in the cat and the dog. J Appl Physiol 67: 2116–2124, 1989 [DOI] [PubMed] [Google Scholar]
- 84. MacGregor RJ. Neural and Brain Modeling. New York: Academic, 1987 [Google Scholar]
- 85. Malpas SC, Ninomiya I. Effect of chemoreceptor stimulation on the periodicity of renal sympathetic nerve activity in anesthetized cats. J Auton Nerv Syst 37: 19–28, 1992 [DOI] [PubMed] [Google Scholar]
- 86. Massari VJ, Shirahata M, Johnson TA, Lauenstein JM, Gatti PJ. Substance P immunoreactive nerve terminals in the dorsolateral nucleus of the tractus solitarius: roles in the baroreceptor reflex. Brain Res 785: 329–340, 1998 [DOI] [PubMed] [Google Scholar]
- 87. Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J Physiol 399: 349–367, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mifflin SW. Absence of respiration modulation of carotid sinus nerve inputs to nucleus tractus solitarii neurones receiving arterial chemoreceptor inputs. J Auton Nerv Syst 42: 191–200, 1993 [DOI] [PubMed] [Google Scholar]
- 90. Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol 271: R870–R880, 1996 [DOI] [PubMed] [Google Scholar]
- 91. Miura M, Kitamura T. Postsynaptic potentials recorded from medullary neurones following stimulation of carotid sinus nerve. Brain Res 162: 261–272, 1979 [DOI] [PubMed] [Google Scholar]
- 92. Morris KF, Arata A, Shannon Lindsey BG R. Inspiratory drive and phase duration during carotid chemoreceptor stimulation in the cat: medullary neurone correlations. J Physiol 491: 241–259, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol 532: 483–497, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Morrison SF, Gebber GL. Raphe neurons with sympathetic-related activity: baroreceptor responses and spinal connections. Am J Physiol Regul Integr Comp Physiol 246: R338–R348, 1984 [DOI] [PubMed] [Google Scholar]
- 95. Mutolo D, Bongianni F, Fontana GA, Pantaleo T. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull 74: 284–293, 2007 [DOI] [PubMed] [Google Scholar]
- 96. Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Cough reflex responses during pulmonary C-fibre receptor activation in anesthetized rabbits. Neurosci Lett 448: 200–203, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Mutolo D, Bongianni F, Cinelli E, Pantaleo T. Role of excitatory amino acids in the mediation of tracheobronchial cough induced by citric acid inhalation in the rabbit. Brain Res Bul 80: 22–29, 2009 [DOI] [PubMed] [Google Scholar]
- 98. Nishino T, Honda Y. Changes in pattern of breathing following baroreceptor stimulation in cats. Jpn J Physiol 32: 183–195, 1982 [DOI] [PubMed] [Google Scholar]
- 99. Nishino T, Hiraga K, Honda Y. Inhibitory effects of CO2 on airway defensive reflexes in enflurane-anesthetized humans. J Appl Physiol 66: 2642–2646, 1989 [DOI] [PubMed] [Google Scholar]
- 100. Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decerebrate cat. J Physiol 480: 309–324, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Onai T, Takayama K, Miura M. Projections to areas of the nucleus tractus solitarii related to circulatory and respiratory responses in cats. J Auton Nerv Syst 18: 163–176, 1987 [DOI] [PubMed] [Google Scholar]
- 102. Ott MM, Nuding SC, Morris KF, Lindsey BG. Correlational linkage maps of rostral medullary neurons and the ventral respiratory column suggest multiple network sites for motor pattern modulation. Program No. 476.4, 2008 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience, 2008 [Google Scholar]
- 103. Pan HL, Zeisse ZB, Pitsillides KF, Longhurst JC. Spatiotemporal aspects of sympathetic C-fiber afferent activity in pressor reflex during abdominal ischemia. Am J Physiol Heart Circ Physiol 272: H1928–H1936, 1997 [DOI] [PubMed] [Google Scholar]
- 104. Pantaleo T, Corda M. Expiration-related neurons in the region of the retrofacial nucleus: vagal and laryngeal inhibitory influences. Brain Res 359: 343–346, 1985 [DOI] [PubMed] [Google Scholar]
- 105. Pantaleo T, Bongianni F, Mutolo D. Central nervous mechanisms of cough. Pulm Pharmacol Ther 15: 227–233, 2002 [DOI] [PubMed] [Google Scholar]
- 106. Person RJ. Somatic and vagal afferent convergence on solitary tract neurons in cat: electrophysiological characteristics. Neuroscience 30: 283–295, 1989 [DOI] [PubMed] [Google Scholar]
- 107. Pilowsky P, Arnolda L, Chalmers J, Llewellyn-Smith I, Minson J, Miyawaki T, Sun QJ. Respiratory inputs to central cardiovascular neurons. Ann NY Acad Sci 783: 64–70, 1996 [DOI] [PubMed] [Google Scholar]
- 108. Pisarri TE, Yu J, Coleridge HM, Coleridge JCG. Background activity in pulmonary vagal C-fibers and its effects on breathing. Respir Physiol 64: 29–43, 1986 [DOI] [PubMed] [Google Scholar]
- 109. Plevkova J, Kollarik M, Brozmanova M, Revallo M, Varechova S, Tatar M. Modulation of experimentally-induced cough by stimulation of nasal mucosa in cats and guinea pigs. Respir Physiol Neurobiol 142: 225–235, 2004 [DOI] [PubMed] [Google Scholar]
- 110. Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal capsaicin challenge on cough reflex in healthy human volunteers. J Physiol Pharmacol 55: 101–106, 2004 [PubMed] [Google Scholar]
- 111. Poliacek I, Stransky A, Jakus J, Barani H, Tomori Z, Halasova E. Activity of the laryngeal abductor and adductor muscles during cough, expiration and aspiration reflexes in cats. Physiol Res 52: 749–762, 2003 [PubMed] [Google Scholar]
- 112. Poliacek I, Jakus J, Stránsky A, Baráni H, Halasová E, Tomori Z. Cough, expiration and aspiration reflexes following kainic acid lesions to the pontine respiratory group in anesthetized cats. Physiol Res 53: 155–163, 2004 [PubMed] [Google Scholar]
- 113. Poliacek I, Corrie LW, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. J Appl Physiol 102: 1014–1021, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Poliacek I, Rose MJ, Corrie LW, Wang C, Jakus J, Barani H, Stransky A, Polacek H, Halasova E, Bolser DC. Short reflex expirations (expiration reflexes) induced by mechanical stimulation of the trachea in anesthetized cats. Cough 28;4: 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Poliaček I, Jakuš J, Halašová E, Baráni H, Muríň P, Bolser DC. Defensive airways reflexes induce widely spreading Fos labeling in the cat brainstem. Acta Med Mart 8: 3–15, 2008 [Google Scholar]
- 116. Poliacek I, Corrie LW, Rose MJ, Wang C, Bolser DC. Influence of microinjections of d,l-homocysteic acid into the Botzinger complex area on the cough reflex in te cat. J Physiol Pharmacol 59, Suppl 6: 585–596, 2008 [PMC free article] [PubMed] [Google Scholar]
- 117. Potts JT, Fuchs IE. Naturalistic activation of barosensitive afferents release substance P in the nucleus tractus solitarius of the cat. Brain Res 893: 155–164, 2001 [DOI] [PubMed] [Google Scholar]
- 118. Redington AE, Morice AH. Acute and Chronic Cough. New York: Taylor & Francis, 2005 [Google Scholar]
- 119. Rybak IA, O'Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Salamone JA, Strohl KP, Weiner DM, Mitra J, Cherniack NS. Cranial and phrenic nerve responses to changes in systemic blood pressure. J Appl Physiol 55: 61–68, 1983 [DOI] [PubMed] [Google Scholar]
- 121. Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995 [DOI] [PubMed] [Google Scholar]
- 122. Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1749–1769, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol 84: 2020–2035, 1998 [DOI] [PubMed] [Google Scholar]
- 124. Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol 525: 207–224, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther 17: 369–376, 2004 [DOI] [PubMed] [Google Scholar]
- 126. Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Pontine respiratory group neuron discharge is altered during fictive cough in the decerebrate cat. Respir Physiol Neurobiol 142: 43–54, 2004 [DOI] [PubMed] [Google Scholar]
- 127. Sharpey-Schafer EP. Effects of coughing on intrathoracic pressure, arterial pressure and peripheral blood flow. J Physiol 122: 351–357, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shaw CF, Cohen MI, Barnhardt R. Inspiratory-modulated neurons of the rostrolateral pons: effects of pulmonary afferent input. Brain Res 485: 179–184, 1989 [DOI] [PubMed] [Google Scholar]
- 129. Silva-Carvalho L, Paton JF, Rocha I, Goldsmith GE, Spyer KM. Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J Neurophysiol 79: 2374–2382, 1998 [DOI] [PubMed] [Google Scholar]
- 130. Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989 [DOI] [PubMed] [Google Scholar]
- 131. Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brainstem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Speck DF, Beck ER. Respiratory rhythmicity after extensive lesions of the dorsal and ventral respiratory groups in the decerebrate cat. Brain Res 482: 387–392, 1989 [DOI] [PubMed] [Google Scholar]
- 134. Spyer KM. The central nervous organization of reflex circulatory control. In: Central Regulution of' Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford Univ Press, 1990, p. 168–188 [Google Scholar]
- 135. Stella MH, Knuth SL, Bartlett D. Respiratory response to baroreceptor stimulation and spontaneous contractions of the urinary bladder. Respir Physiol 124: 169–178, 2001 [DOI] [PubMed] [Google Scholar]
- 136. StJohn WM. Neurogenesis of patterns of automatic ventilatory activity. Prog Neurobiol 56: 97–117, 1998 [DOI] [PubMed] [Google Scholar]
- 137. Stokland O, Miller MM, Ilebekk A, Kiil F. Mechanism of hemodynamic responses to occlusion of the descending thoracic aorta. Am J Physiol Heart Circ Physiol 238: H423–H429, 1980 [DOI] [PubMed] [Google Scholar]
- 138. Tatár M, Korpáš J, Poláček H, Záhradný V. Changes induced by severe hypoxia in respiratory defence reflexes in anaesthetised cats. Respiration 49: 114–121, 1986 [DOI] [PubMed] [Google Scholar]
- 139. Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 402: 411–420, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tatar M, Nagyova B, Widdicombe JG. Veratrine-induced reflexes and cough. Respir Med 85, Suppl A: 51–55, 1991 [DOI] [PubMed] [Google Scholar]
- 141. Tatár M, Karcolová D, Péčová R, Kollárik M, Plevková J, Brozmanová M. Experimental modulation of the cough reflex. Eur Respir Rev 85: 264–269, 2002 [Google Scholar]
- 142. Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol 265: 275–293, 1987 [DOI] [PubMed] [Google Scholar]
- 143. Thurston CL, Randich A. Acute increases in arterial blood pressure produced by occlusion of the abdominal aorta induces antinociception: peripheral and central substrates. Brain Res 519: 12–22, 1990 [DOI] [PubMed] [Google Scholar]
- 144. Tzeng YC, Larsen PD, Galletly DC. Mechanism of cardioventilatory coupling: insights from cardiac pacing, vagotomy, and sinoaortic denervation in the anesthetized rat. Am J Physiol Heart Circ Physiol 292: H1967–H1977, 2007 [DOI] [PubMed] [Google Scholar]
- 145. van Lieshout EJ, van Lieshout JJ, ten Harkel AD, Wieling W. Cardiovascular response to coughing: its value in the assessment of autonomic nervous control. Clin Sci (Lond) 77: 305–310, 1989 [DOI] [PubMed] [Google Scholar]
- 146. Wang Q, Li P. Stimulation of the ventrolateral medulla inhibits the baroreceptor input to the nucleus tractus solitarius. Brain Res 473: 227–235, 1988 [DOI] [PubMed] [Google Scholar]
- 147. Wang C, Saha S, Rose MJ, Davenport PW, Bolser DC. Spatiotemporal regulation of the cough motor pattern. Cough 5: 12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Widdicombe JG. Afferent receptors in the airways and cough. Respir Physiol 114: 5–15, 1998 [DOI] [PubMed] [Google Scholar]
- 149. Widdicombe J, Fontana G. Cough: what's in a name? Eur Respir J 28: 10–15, 2006 [DOI] [PubMed] [Google Scholar]
- 150. Yamada T, Morimoto T, Nakase H, Hirabayashi H, Hiramatsu K, Sakaki T. Spinal cord blood flow and pathophysiological changes after transient spinal cord ischemia in cats. Neurosurgery 42: 626–634, 1998 [DOI] [PubMed] [Google Scholar]
- 151. Yen CT, Blum PS. Response properties and functional organization of neurons in midline region of medullary reticular formation of cats. J Neurophysiol 52: 961–979, 1984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.