Abstract
The vasodilatory effects of insulin account for up to 40% of insulin-mediated glucose disposal; however, insulin-stimulated vasodilation is impaired in individuals with type 2 diabetes, limiting perfusion and delivery of glucose and insulin to target tissues. To determine whether exercise training improves conduit artery blood flow following glucose ingestion, a stimulus for increasing circulating insulin, we assessed femoral blood flow (FBF; Doppler ultrasound) during an oral glucose tolerance test (OGTT; 75 g glucose) in 11 overweight or obese (body mass index, 34 ± 1 kg/m2), sedentary (peak oxygen consumption, 23 ± 1 ml·kg−1·min−1) individuals (53 ± 2 yr) with non-insulin-dependent type 2 diabetes (HbA1c, 6.63 ± 0.18%) before and after 7 days of supervised treadmill and cycling exercise (60 min/day, 60–75% heart rate reserve). Fasting glucose, insulin, and FBF were not significantly different after 7 days of exercise, nor were glucose or insulin responses to the OGTT. However, estimates of whole body insulin sensitivity (Matsuda insulin sensitivity index) increased (P < 0.05). Before exercise training, FBF did not change significantly during the OGTT (1 ± 7, −7 ± 5, 0 ± 6, and 0 ± 5% of fasting FBF at 75, 90, 105, and 120 min, respectively). In contrast, after exercise training, FBF increased by 33 ± 9, 39 ± 14, 34 ± 7, and 48 ± 18% above fasting levels at 75, 90, 105, and 120 min, respectively (P < 0.05 vs. corresponding preexercise time points). Additionally, postprandial glucose responses to a standardized breakfast meal consumed under “free-living” conditions decreased during the final 3 days of exercise (P < 0.05). In conclusion, 7 days of aerobic exercise training improves conduit artery blood flow during an OGTT in individuals with type 2 diabetes.
Keywords: exercise, diabetes, blood flow
the prevalence of obesity and obesity-related diseases, including type 2 diabetes, is rapidly increasing in the United States and around the world. In 2007, nearly 23.6 million Americans had type 2 diabetes, and an additional 57 million had prediabetes (11). Current projections estimate that by 2050, as many as one in every three Americans will have type 2 diabetes (6).
Type 2 diabetes is characterized by insulin resistance, or the inability of insulin to stimulate glucose uptake. Skeletal muscle is the primary sink for glucose disposal, and insulin-mediated skeletal muscle glucose uptake is critically dependent on insulin action in both skeletal muscle and in the vasculature (48). In the endothelium, insulin stimulates the production of the vasodilator nitric oxide and the vasoconstrictor endothelin-1 (16, 33, 35). In the absence of disease, the net result of insulin stimulation is vasodilation, which increases perfusion and delivery of glucose and insulin to target tissues. The hemodynamic effects of insulin typically account for up to 40% of insulin-mediated glucose uptake (3). However, an imbalance in the production of nitric oxide and endothelin-1 appears to blunt insulin-mediated increases in blood flow in obese and insulin-resistant individuals (3, 7, 26).
Aerobic exercise enhances skeletal muscle glucose uptake in individuals with type 2 diabetes (5, 13, 24). Although improvements in insulin-mediated glucose uptake in response to exercise are commonly attributed to changes in glucose transport mechanisms within skeletal muscle cells (14), evidence of gains in endothelium-dependent vasodilation following exercise in individuals with type 2 diabetes (12, 37) suggest insulin-mediated blood flow may be improved in response to exercise and may contribute to increases in skeletal muscle glucose uptake. However, the specific effects of aerobic exercise on insulin-mediated blood flow in individuals with type 2 diabetes remain poorly understood.
Chronic endurance exercise training is associated with greater limb blood flow and glucose disposal in response to insulin infusion in lean, healthy men relative to their sedentary counterparts (15, 19). Further, a single bout of aerobic exercise enhances insulin-mediated limb blood flow in the elderly, an effect which appears to be mediated by enhanced insulin-stimulated nitric oxide production and corresponds with improved whole body insulin-stimulated glucose disposal (18). Importantly, these studies were conducted in relatively healthy populations, and it is unclear whether similar adaptations occur in individuals with type 2 diabetes.
The purpose of this investigation was to test the hypothesis that short-term aerobic exercise training improves conduit artery blood flow following glucose ingestion in individuals with type 2 diabetes. An oral glucose tolerance test (OGTT) was used to stimulate increases in plasma insulin (42) and to more closely replicate postprandial conditions than the hyperinsulinemic-euglycemic clamp technique (28, 34). Femoral blood flow (FBF) was assessed during an OGTT before and after a 7-day aerobic exercise training program in patients with type 2 diabetes. The 7-day training period was chosen because it has been shown to improve skeletal muscle insulin sensitivity in individuals with type 2 diabetes (36, 39) but does not result in substantial cardiovascular and skeletal muscle adaptations commonly associated with chronic exercise training (10, 21). Furthermore, because 7 days of exercise does not typically alter glucose or insulin responses to an OGTT in patients with type 2 diabetes (22), we were able to examine the effects of exercise on FBF, independent of changes in the glucose and insulin responses to the OGTT. Last, to determine whether the exercise program influenced day-to-day glycemic control under “free-living” conditions, the patients were also equipped with continuous glucose monitors to measure postprandial glucose responses to a standardized breakfast meal during the final 3 days of exercise.
METHODS
Participants.
Sedentary (<60 min/wk structured physical activity), overweight or obese (body mass index 25–43 kg/m2) individuals, 30–65 yr of age, with non-insulin-dependent type 2 diabetes were recruited for participation in this study. The study protocol and procedures were approved by the University of Missouri Health Sciences Institutional Review Board, and written informed consent was obtained from all participants before enrollment in the study (ClinicalTrials.gov no. NCT00972452).
Volunteers completed detailed medical history questionnaires and underwent a medical examination and exercise stress test to determine eligibility. Exclusion criteria included smoking, consumption of >14 alcoholic drinks/wk, history or evidence of advanced cardiovascular, renal or hepatic diseases, proteinuria, diabetic retinopathy, nephropathy, or neuropathy, insulin use, orthopedic or other limitations that may interfere with their ability to exercise safely, hyper- or hypothyroidism, and HbA1c >10%.
Graded exercise testing.
All subjects underwent a continuous incremental exercise test on a stationary cycle ergometer (Monarch, Monarch AB, Varberg, Sweden) for screening purposes and to ascertain maximal heart rate to determine target heart rates for the exercise training regimen. Participants maintained a cadence of 60 rotations/min as the resistance was incrementally increased by 20 W every 2 min. Oxygen consumption was determined using indirect calorimetry (TrueOne 2400, Parvo Medics, Sandy, UT), and heart rate was monitored and recorded by electrocardiography. Peak oxygen consumption (V̇o2peak) was obtained when participants met at least three of the following criteria: 1) could no longer maintain proper cadence, 2) respiratory exchange ratio (RER) ≥ 1.10, 3) heart rate within 10 beats of age-predicted maximum, or 4) plateau in oxygen consumption despite increase in workload.
Experimental design.
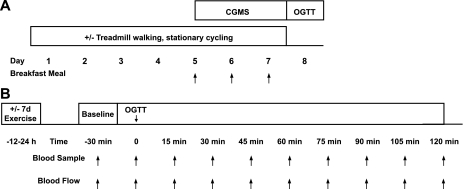
Metabolic and cardiovascular responses to glucose ingestion (OGTT; 75 g glucose) were assessed prior to and following a 7-day aerobic exercise training program in individuals with non-insulin-dependent type 2 diabetes (Fig. 1).
Fig. 1.
Study design. A: continuous glucose monitoring (CGMS) was used to quantify postprandial glucose responses to a standardized breakfast meal in patients with type 2 diabetes (n = 11) before and during the final 3 days of a 7-day exercise training program (60 min/day, 60–75% HRR). B: glucose, insulin, and femoral blood flow (FBF) were assessed before and at 15-min intervals following an oral glucose tolerance test (OGTT) at baseline and after the exercise training program.
Exercise training.
Participants completed 60 min of supervised aerobic exercise training at 60–75% of heart rate reserve (HRR) for seven consecutive days. Each exercise session consisted of 20 min treadmill walking, 20 min stationary cycling, and 20 min treadmill walking. Heart rate was continuously monitored using Polar heart rate monitors, and exercise intensity was adjusted at 5-min intervals to maintain heart rate within the desired range. Whereas the effects of a single bout of exercise on insulin sensitivity and glucose tolerance in patients with type 2 diabetes are disputable (36, 39), prior studies have established that 7 days of aerobic exercise training at this intensity is sufficient to enhance insulin sensitivity in individuals with type 2 diabetes, but does not produce measurable adaptations associated with chronic exercise training, such as increases in skeletal muscle mitochondrial content or capillary density, increases in stroke volume, or significant alterations in body weight or composition or other traditional biomarkers of health (cholesterol, fasting blood glucose) (10, 21). Importantly, this allowed us to determine the effects of exercise on FBF during an OGTT independent of the secondary effects of classic chronic exercise training adaptations.
Experimental protocol.
On experimental days, participants were instructed to refrain from medication use. Diet was standardized for 3 days before the OGTT to ensure adequate glycogen repletion. Additional snacks were provided during each day of exercise training to compensate for the energy cost of exercise and to avoid confounding effects of negative energy availability. All postexercise OGTTs were performed within 12–20 h of the last exercise bout, as previously described (17).
Subjects were positioned supine in a quiet, climate-controlled room (22–23°C) between 6:00 and 8:00 am after an overnight (10–12 h) fast and were instrumented for measures of heart rate, arterial blood pressure, respiration, and FBF. An intravenous catheter was placed in an antecubital vein. Following ≥30 min of quiet rest, baseline variables and blood samples were collected. The participants then ingested a standard 75-g glucose drink within 2 min, and all variables were measured for 5 min at 15-min intervals for the next 120 min. Venous blood samples were collected every 15 min, and the resulting plasma was stored at −80°C for subsequent analysis of plasma glucose, insulin, and C-peptide concentrations. The OGTT served to increase plasma insulin concentrations, allowing us to examine changes in limb blood flow under physiologically relevant conditions, as previously described (1, 3, 28, 42).
Experimental measurements.
Heart rate was recorded continuously by a lead II electrocardiogram (Quinton Q710, Bothell, WA). Arterial blood pressure was obtained by auscultation of the brachial artery using an automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY). Mean arterial blood pressure (MAP) was calculated as MAP = [(2 × diastolic blood pressure) + systolic blood pressure]/3. Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position around the abdomen to ensure relatively normal breathing during data collection periods (Pneumotrace, UFI, Morro Bay, CA).
FBF was measured in the right leg using a duplex Doppler ultrasound system (Logiq 7, GE Medical Systems, Milwaukee, WI) equipped with a 7-MHz linear array transducer, as previously described (51, 52). Briefly, the common femoral artery was imaged 2 cm proximal to the bifurcation of the superficial and deep branches. Simultaneous measurements of femoral artery blood velocity and vessel diameter were performed using the same probe in pulsed-wave mode, operating at a linear frequency of 5 Hz. All measurements were obtained with the probe fixed in position using a custom-designed clamp to maintain an insonation angle ≤ 60°. Photographs were taken at baseline to record the precise position of the transducer on each subject's leg and were used to reproduce the arrangement during the return visit. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Intensity weighted mean velocity (Vmean) values were then calculated using commercially available software (Logiq 7). Arterial diameter was measured at a perpendicular angle to the axis of the vessel. Using arterial diameter and Vmean, blood flow in the femoral artery was calculated as: blood flow = Vmeanπ(diameter/2)2 × 60, where blood flow is in milliliters per minute. Ultrasound parameters were unchanged during the course of the study, and all measurements were made by the same ultrasonographer who established day-to-day reproducibility of resting FBF measures with an intraclass correlation coefficient of 0.905 (P < 0.05).
Blood analysis.
Baseline blood samples were sent to a commercial laboratory for analysis of lipids and HbA1c (Boyce and Bynum Pathology Labs, Columbia, MO). Serum samples collected during the OGTT were analyzed for glucose using the glucose oxidase method (Sigma, St. Louis, MO) and insulin and C-peptide by enzyme-linked immunosorbent assays (Immulite 1000 Analyzer, Siemens, Deerfield, IL). The glucose, insulin, and C-peptide area under the curve (AUC) were calculated by the trapezoidal method. C-peptide was measured as a marker of insulin secretion. The homeostasis model assessment (HOMA-IR) was calculated as previously described (31). The Matsuda insulin sensitivity index (ISI) was calculated as previously described to provide an estimate of whole body insulin sensitivity that correlates with indexes of insulin sensitivity obtained from the hyperinsulinemic-euglycemic clamp (30).
Continuous glucose monitoring.
Glucose responses to a standardized breakfast meal under free-living conditions were assessed over 3 days at baseline and during the final 3 days of the exercise program using continuous glucose monitoring systems (CGMS: iPro CGM, Medtronic Diabetes, Minneapolis, MN). This method was modified from prior reports (29). The evening prior to initiating each monitoring period, a glucose sensor was inserted subcutaneously in the abdominal region and connected to the CGMS monitor. The monitor continuously recorded interstitial glucose concentrations at 5-min intervals over the next 3 days. Participants were instructed to record the exact start and end time at which the breakfast meal was ingested each morning and to perform and record the results of ≥4 finger stick glucose readings (Accu-check Compact Plus, Roche Diagnostics) each day for calibration of the CGMS. The breakfast meal comprised a commercially available meal replacement drink and breakfast bar and contained 462 kcal (60% coming from carbohydrate, 24% from fat, and 16% from protein). At the end of each monitoring period, record books were collected, and data from the CGMS were processed using Solutions Software for CGMS iPro (Medtronic Diabetes). Glucose responses to the breakfast meal were averaged across the 3-day pre- and postexercise monitoring periods.
Anthropometric measures.
Height, weight, and body composition (dual energy X-ray absorptiometry, Hologic QDR 4500A) were measured at baseline.
Statistical analysis.
Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Initial analyses revealed no significant sex-by-treatment interactions; therefore, the data were pooled for analysis. Differences in metabolic and cardiovascular responses to the OGTT (glucose, insulin, C-peptide, FBF, heart rate, and MAP) and breakfast meal (glucose) were detected using two-way repeated-measures ANOVA (SAS proc mixed), with the main effects being treatment (before and after 7 days of exercise) and time (15 min intervals between 0 and 120 min following the OGTT, 30-min intervals between 0 and 120 min following ingestion of the breakfast meal). Tukey post hoc testing was applied where significant main effects were detected. The effects of the 7-day aerobic exercise training program on all other variables (fasting lipids, HOMA, Matsuda ISI, glucose AUC) were determined using paired t- tests. Statistical significance was set at P < 0.05, and data are expressed as means ± SE.
RESULTS
Eleven participants (6 men, 5 women) completed the study protocol. Baseline participant characteristics are described in Table 1. Adherence to the exercise program was 100%. Participants accumulated 6,046 ± 893 steps per day at baseline, and, excluding steps taken during exercise, 6,091 ± 942 steps per day during the exercise intervention. Energy expended during exercise was estimated to be ∼446 ± 33 kcal/day, and, when combined with supplemental snacks provided during the exercise training period, resulted in an energy availability of approximately −136 ± 66 kcal/day (Table 2). Plasma lipids did not change in response to 7 days of exercise training, nor did fasting glucose, insulin, or C-peptide (Table 3). Likewise, changes in HOMA-IR, an index of insulin resistance calculated from fasting glucose and insulin concentrations, were not detected in response to the short-term exercise regimen.
Table 1.
Baseline participant characteristics
| Baseline Characteristic | Value |
|---|---|
| Age, yr | 53 ± 2 |
| Sex, M/F | 6/5 |
| Weight, kg | 95.5 ± 5.9 |
| BMI, kg/m2 | 33.7 ± 1.4 |
| Fat, % | 34.5 ± 2.1 |
| V̇o2peak, ml · kg−1 · min−1 | 22.5 ± 1.4 |
| HbA1c, % | 6.63 ± 0.18 |
| Time since diagnosis, yr | 5 ± 1 |
| Hypoglycemic medications | |
| Any | n = 10 |
| Biguanides | n = 8 |
| Sulfonylureas | n = 5 |
| Combination | n = 3 |
| Statins | n = 8 |
| ACE inhibitors | n = 7 |
| Angiotensin receptor blockers | n = 1 |
| Diuretics | n = 3 |
| Others | |
| Antidepressants | n = 3 |
| Aspirin | n = 5 |
Data are expressed as means ± SE; n = no. of subjects using each type of medication. BMI, body mass index; V̇o2peak, peak oxygen consumption; HbA1c, hemoglobin A1c; ACE, angiotensin-converting enzyme.
Table 2.
Estimated energy availability
| Baseline | 7-Day Exercise | |
|---|---|---|
| Energy intake, kcal/day | 1,857 ± 151 | 2,167 ± 143 |
| Macronutrients | ||
| Carbohydrate, % of kcal | 60 ± 2 | 63 ± 3 |
| Fat, % of kcal | 26 ± 2 | 26 ± 3 |
| Protein, % of kcal | 14 ± 1 | 11 ± 1 |
| Energy cost of exercise, kcal/day | 446 ± 33 | |
| Energy availability, kcal/day | −136 ± 66 |
Data are expressed as means ± SE. Estimated energy intake and energy expenditure. 7-day exercise, 7-day aerobic exercise training program.
Table 3.
Blood chemistry
| Baseline | 7-Day Exercise | |
|---|---|---|
| Fasting glucose, mmol/l | 6.87 ± 0.45 | 6.58 ± 0.41 |
| Fasting insulin, pmol/l | 75.0 ± 11.81 | 71.54 ± 9.02 |
| Fasting C-peptide, nmol/l | 1.05 ± 0.09 | 0.99 ± 0.10 |
| HOMA | 3.36 ± 0.53 | 3.11 ± 0.47 |
| Total cholesterol, mmol/l | 4.11 ± 0.22 | 3.68 ± 0.2 |
| LDL cholesterol, mmol/l | 2.38 ± 0.2 | 2.17 ± 0.17 |
| HDL cholesterol, mmol/l | 0.94 ± 0.07 | 0.93 ± 0.05 |
| TG, mmol/l | 1.68 ± 0.22 | 1.35 ± 0.20 |
Data are expressed as means ± SE. Clinical chemistry at baseline and following the 7-day aerobic exercise program. HOMA, homeostatis model assessment, HOMA; LDL, low-density lipoprotein cholesterol, HDL, high-density lipoprotein; TG, triglycerides.
Glucose and insulin responses to OGTT.
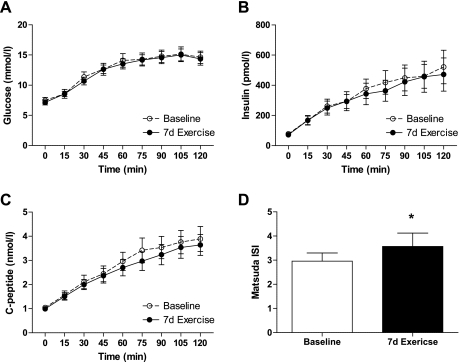
Seven days of exercise training did not produce significant changes in the glucose, insulin, or C-peptide responses to the OGTT (Fig. 2). However, the Matsuda insulin sensitivity index, an estimate of whole body insulin sensitivity derived from glucose and insulin responses to the OGTT, increased significantly (P < 0.05; Fig. 2) due to modest declines in plasma insulin in response to the OGTT. These data indicate less insulin was needed to dispose of the 75-g glucose load following the 7-day exercise program.
Fig. 2.
Glucose (A), insulin (B), and C-peptide (C) responses to 75-g oral glucose tolerance test in obese, sedentary volunteers with type 2 diabetes before (baseline) and after 7 days of aerobic exercise training (7d Exercise). D: Matsuda insulin sensitivity index (ISI), an estimate of insulin sensitivity calculated from glucose and insulin responses to the OGTT. *Significantly different from baseline (P < 0.05).
FBF.
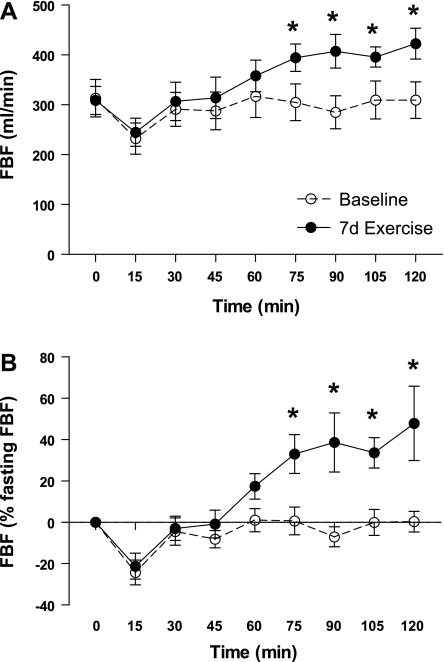
Fasting FBF did not change in response to 7 days of exercise training (313 ± 38 vs. 309 ± 28 ml/min; see Fig. 4). Similarly, as expected, exercise training did not produce significant changes in baseline heart rate or mean arterial blood pressure (MAP) (Table 4). Before exercise training, FBF did not change significantly following glucose ingestion (−22.3 ± 0.7, +3.5 ± 0.8, −28.3 ± 0.7, −3.9 ± 0.6 ml/min, change from fasting FBF at 30, 60, 90, and 120 min, respectively). However, after the week-long aerobic exercise training program, absolute FBF during the OGTT increased by −1.9 ± 0.7, +49.4 ± 0.6, +98.4 ± 0.9*, +114.0 ± 1.1* ml/min above fasting FBF at 30, 60, 90, and 120 min, respectively (*P < 0.05 compared with corresponding preintervention time point; Fig. 3). Figure 3A depicts absolute FBF during the OGTT, and Fig. 3B depicts relative changes in FBF during the OGTT. These increases in FBF to the OGTT were attributed to increases in blood velocity as no change in femoral artery diameter was observed. Both before and after 7 days of exercise, initial, statistically insignificant declines in FBF (−81.8 ± 0.8 and −64.0 ± 0.7 ml/min from fasting FBF, respectively) were observed at minute 15. FBF then returned to baseline between 15 and 30 min during both phases, but continued to increase between minutes 45 and 120 only after 7 days of exercise. The improvement in FBF during the OGTT following exercise training was not accompanied by changes in heart rate or MAP following glucose ingestion (Table 4).
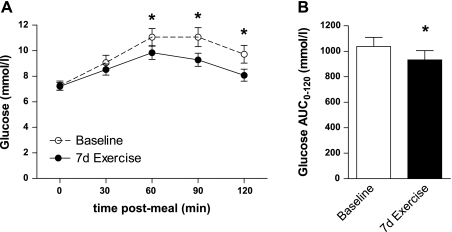
Fig. 4.
A: mean postprandial glucose responses to a standardized breakfast meal in obese, sedentary volunteers with type 2 diabetes over 3 days before (baseline) and during the final 3 days of a 7-day aerobic exercise training program (7d Exercise). B: glucose AUC0–120; glucose area under the curve. *Significantly different from baseline (P < 0.05).
Table 4.
Cardiovascular measures
| Time, min |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | |
| HR, beats/min | |||||||||
| Baseline | 72 ± 3 | 74 ± 3 | 77 ± 3 | 76 ± 3 | 75 ± 4 | 75 ± 3 | 75 ± 3 | 76 ± 3 | 78 ± 3 |
| 7-day Exercise | 68 ± 3 | 72 ± 3 | 71 ± 3 | 72 ± 3 | 72 ± 3 | 72 ± 3 | 72 ± 3 | 72 ± 3 | 73 ± 3 |
| MAP, mmHg | |||||||||
| Baseline | 90 ± 1 | 88 ± 1 | 87 ± 2 | 87 ± 2 | 87 ± 2 | 87 ± 2 | 86 ± 2 | 86 ± 2 | 87 ± 2 |
| 7-day Exercise | 87 ± 2 | 88 ± 3 | 86 ± 3 | 86 ± 2 | 85 ± 2 | 86 ± 3 | 86 ± 3 | 86 ± 3 | 85 ± 3 |
Data are expressed as means ± SE. Cardiovascular responses to the oral glucose tolerance test at baseline and following the 7-day aerobic exercise program.HR, heart rate; MAP, mean arterial pressure.
Fig. 3.
Absolute (A) and relative (%change from fasting; B) FBF following a 75-g oral glucose tolerance test in obese, sedentary volunteers with type 2 diabetes before (baseline) and after 7 days of aerobic exercise training (7d Exercise). *Significantly different from corresponding time point at baseline (P < 0.05).
Glucose responses to a standardized meal.
In agreement with the improvement in the Matsuda ISI, 7 days of exercise training attenuated glucose responses to a standard breakfast meal measured by CGMS at 60, 90, and 120 min postmeal during the final 3 days of exercise training (Fig. 4, P < 0.05). The glucose AUC0–120 measured by CGMS was similarly reduced (Fig. 4, P < 0.05).
DISCUSSION
The primary novel finding of this study is that 7 days of aerobic exercise training increases FBF following glucose ingestion in patients with well-controlled type 2 diabetes. We also observed gains in the Matsuda ISI, an index of insulin sensitivity derived from glucose and insulin responses to the OGTT. These data suggest that 7 days of aerobic exercise training improves FBF during an OGTT in individuals with type 2 diabetes, an effect we suspect may be mediated by improvements in insulin-mediated blood flow (1, 3, 42).
To our knowledge, this study is the first to determine the impact of short-term exercise training on conduit artery blood flow following glucose ingestion in patients with well-controlled type 2 diabetes. Prior work has demonstrated that the hemodynamic effects of insulin are blunted in patients with type 2 diabetes (3, 26), and our data concur. Our group and others have described increases in limb blood flow in response to insulin infusion or mixed meal ingestion in healthy volunteers (41, 51), yet, in the current study, despite exhibiting higher plasma insulin concentrations than those previously reported in healthy controls (51), we observed no change in FBF during an OGTT in patients with type 2 diabetes prior to the exercise intervention. The data presented here provide evidence that increases in conduit artery blood flow following glucose ingestion are attenuated in sedentary patients with type 2 diabetes, but suggest that improvements occur rapidly in response to daily exercise.
We have previously demonstrated that chronic exercise preserves microvascular reactivity to insulin in a rodent model of obesity and type 2 diabetes (32), and others have shown that 2 wk of voluntary wheel running improves insulin-mediated capillary recruitment and glucose uptake in the hindlimb of male hooded Wistar rats (38). Clinical evidence for the role of exercise in maintaining or improving insulin-mediated blood flow comes primarily from studies establishing that increases in limb blood flow during insulin infusion are improved in the trained leg of healthy volunteers following 3 wk of single-leg knee extensor training (17) and following 10 wk of single leg cycling in healthy and insulin-resistant volunteers (13). However, it should be noted that improvements in insulin-mediated blood flow after exercise training are not universally observed (42), and one bout of single-leg cycling does not produce significant changes in insulin-mediated limb blood flow in healthy volunteers or patients with type 2 diabetes (13). Inconsistencies in the literature may be attributable to differences in the populations studied, interventions employed, methods used to measure blood flow, performing measures of blood flow in trained vs. untrained limbs, and/or the duration of the intervention (8).
Whereas chronic exercise training produces a number of physiological adaptations, including changes in fitness, body composition, and skeletal muscle capillary density and oxidative capacity, which influence blood flow responses to feeding or insulin infusion (20, 25, 44, 45) these factors are not altered significantly in response to acute or short-term exercise training (10, 21). Thus, in studies employing chronic exercise training, it is difficult to distinguish the specific effects of exercise from those of changes in these and other factors. The data presented here provide clear evidence that short-term exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes, independent of changes in fitness, adiposity, skeletal muscle capillary density and oxidative capacity, or energy balance.
Insulin is widely recognized as a vasodilator (7, 13, 16, 40). Although the effects of other responses to glucose ingestion, such as the production of counterregulatory hormones, sympathetic activation, and/or increases in circulating glucose on blood flow, cannot be explicitly excluded in the present study, the role of insulin in mediating increases in blood flow has been well established (23). Local insulin infusion increases forearm blood flow (9), insulin stimulates vasodilation in isolated vessels (16, 32), and vasodilation in response to insulin infusion occurs more rapidly in the denervated limbs of patients who have undergone regional sympathectomy (40). Thus, in accord with a number of prior studies, we consider insulin to be the key physiological mediator of changes in blood flow observed during the OGTT (1, 3, 42). More importantly, our rationale for utilizing the OGTT was to more closely simulate postprandial conditions (34). Although some studies have attempted to mimic postprandial conditions by establishing “physiological” insulin concentrations using the hyperinsulinemic-euglycemic clamp, insulin infusion alters sympathetic activation as well as circulating levels of catecholamines, fatty acids, and amino acids (27, 43, 50) while failing to adequately mimic the neural, hormonal, and temporal responses to meal ingestion (34).
Perspectives
A number of studies have demonstrated a strong correlation between limb blood flow and glucose uptake across a broad range of insulin infusion rates in healthy and insulin-resistant individuals (2–4, 13, 15, 26). Insulin-stimulated blood flow and glucose uptake have also been shown to colocalize (46) and appear to be functionally linked (9), suggesting improvements in insulin-mediated blood flow may contribute to enhanced insulin-stimulated glucose uptake in response to exercise.
In the present study, blood flow increased by nearly 50% during the OGTT following 7 days of exercise training. Consistent with previous studies utilizing the OGTT to assess changes in glucose tolerance with short-term exercise training (22), we did not observe significant changes in the glucose or insulin responses to the OGTT. However, glucose tolerance and insulin sensitivity are not equivalent, and studies employing the hyperinsulinemic-euglycemic clamp technique consistently report improvements in insulin sensitivity in patients with type 2 diabetes following 7 days of exercise training (22, 24, 49). Likewise, the Matsuda ISI, an estimate of whole body insulin sensitivity (30), increased after the 7-day exercise training program in the present study, suggesting that although glucose tolerance was not significantly affected by the exercise intervention, insulin sensitivity may have improved. Interestingly, we also observed a significant decrease in the glucose response to ingestion of a standard breakfast meal in free-living nonlaboratory conditions, as measured by CGMS. Although not the primary focus of this experiment, these data suggest peripheral glucose handling may have been improved in response to the 7 days of exercise training although this effect was not captured by the OGTT. Overall, further investigation is warranted to determine whether improvements in leg blood flow during an OGTT contribute to changes in insulin sensitivity and glycemic control.
Although the mechanistic underpinnings of the improvement in FBF during the OGTT following 7 days of exercise observed here are not yet clear, it is plausible that improved nitric oxide bioavailability may play a role. In the endothelium, insulin stimulates the production of the vasodilator nitric oxide and the vasoconstrictor endothelin-1 (16, 33, 35). In the absence of disease, the net result of insulin stimulation is vasodilation, which increases perfusion and delivery of glucose and insulin to target tissues. However, an imbalance in the production of nitric oxide and endothelin-1 appears to blunt insulin-mediated increases in blood flow in obese and insulin-resistant individuals (3, 7, 26). Additionally, insulin-mediated blood flow is nearly ablated by the coadministration of a nitric oxide synthase inhibitor (47). In this regard, increases in phospho-eNOS were associated with improvements in microvascular reactivity to insulin in response to voluntary wheel running in a rodent model of obesity and type 2 diabetes (32). Correspondingly, other measures of nitric-oxide dependent vasodilation are improved in patients with type 2 diabetes in response to aerobic exercise (12, 37) specifically in vessels which experience increases in shear stress during exercise (13). Although we did not obtain traditional measures of endothelial function (i.e., blood flow responses to hyperemia or acetylcholine) in this study, these earlier reports suggest that enhanced nitric oxide bioavailability may have contributed to the improved blood flow responses to the OGTT. Further investigation is warranted to determine the precise mechanisms.
Of note, the population studied here included patients with well-controlled type 2 diabetes who were free from advanced micro- and macro vascular diseases. Additional studies are needed to determine whether similar responses are observed in patients with uncontrolled type 2 diabetes or those with cardiovascular comorbidities as well as to identify the precise mode, intensity, and frequency of exercise that optimizes metabolic and hemodynamic responses to insulin.
In summary, a 7-day aerobic exercise program dramatically improved femoral artery blood flow following glucose ingestion in individuals with type 2 diabetes. The 7-day exercise training program also produced small but significant gains in the Matsuda ISI, an index of insulin sensitivity. In addition, we observed decreases in the glucose response to a standard breakfast meal measured by continuous glucose monitors. Collectively, these data suggest that 7 days of exercise training produces favorable hemodynamic responses to glucose ingestion in patients with type 2 diabetes.
GRANTS
This project was funded by the University of Missouri Institute of Clinical and Translational Sciences (C. R. Mikus), National Institutes of Health Grants T32-AR-048523 (C. R. Mikus) and DK-076636 (P. J. Fadel), Diabetes Action Research and Education Foundation and the Veterans Hospital Administration Career Development Award (J. P. Thyfault). This work was supported with resources and the use of facilities at the Harry S Truman Memorial VHA in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AKNOWLEDGMENTS
In addition to thanking the study participants for their time and commitment, we would like to thank Angelina Taylor for her contributions to data entry and management. We also thank Charla Jay and Peggy Nigh for their technical assistance.
REFERENCES
- 1. Arciero PJ, Smith DL, Calles-Escandon J. Effects of short-term inactivity on glucose tolerance, energy expenditure, and blood flow in trained subjects. J Appl Physiol 84: 1365–1373, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol Endocrinol Metab 271: E1067–E1072, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab 70: 1525–1533, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96: 786–792, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bordenave S, Brandou F, Manetta J, Fedou C, Mercier J, Brun JF. Effects of acute exercise on insulin sensitivity, glucose effectiveness and disposition index in type 2 diabetic patients. Diabetes Metab 34: 250–257, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8: 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295: E732–E750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 284: E241–E258, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cleland SJ, Petrie JR, Ueda S, Elliott HL, Connell JM. Insulin-mediated vasodilation and glucose uptake are functionally linked in humans. Hypertension 33: 554–558, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Cox JH, Cortright RN, Dohm GL, Houmard JA. Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol 86: 2019–2025, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Cumming DC, Wall SR, Galbraith MA, Belcastro AN. Reproductive hormone responses to resistance exercise. Med Sci Sports Exerc 19: 234–238, 1987 [PubMed] [Google Scholar]
- 12. De Filippis E, Cusi K, Ocampo G, Berria R, Buck S, Consoli A, Mandarino LJ. Exercise-induced improvement in vasodilatory function accompanies increased insulin sensitivity in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab 91: 4903–4910, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes 44: 1010–1020, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, Galbo H. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes 43: 862–865, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Ebeling P, Bourey R, Koranyi L, Tuominen JA, Groop LC, Henriksson J, Mueckler M, Sovijarvi A, Koivisto VA. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest 92: 1623–1631, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56: 464–471, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Frosig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JF. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes 56: 2093–2102, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56: 1615–1622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hardin DS, Azzarelli B, Edwards J, Wigglesworth J, Maianu L, Brechtel G, Johnson A, Baron A, Garvey WT. Mechanisms of enhanced insulin sensitivity in endurance-trained athletes: effects on blood flow and differential expression of GLUT 4 in skeletal muscles. J Clin Endocrinol Metab 80: 2437–2446, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Hedman A, Andersson PE, Reneland R, Lithell HO. Insulin-mediated changes in leg blood flow are coupled to capillary density in skeletal muscle in healthy 70-year-old men. Metabolism 50: 1078–1082, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Houmard JA, Cox JH, MacLean PS, Barakat HA. Effect of short-term exercise training on leptin and insulin action. Metabolism 49: 858–861, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Kang J, Robertson RJ, Hagberg JM, Kelley DE, Goss FL, DaSilva SG, Suminski RR, Utter AC. Effect of exercise intensity on glucose and insulin metabolism in obese individuals and obese NIDDM patients. Diabetes Care 19: 341–349, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Kearney MT, Cowley AJ, Stubbs TA, Macdonald IA. Effect of a physiological insulin infusion on the cardiovascular responses to a high fat meal: evidence supporting a role for insulin in modulating postprandial cardiovascular homoeostasis in man. Clin Sci (Lond) 91: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 297: E151–E156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 85: 1844–1852, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Liang C, Doherty JU, Faillace R, Maekawa K, Arnold S, Gavras H, Hood WB., Jr Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest 69: 1321–1336, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94: 3543–3549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manders RJ, Van Dijk JW, van Loon LJ. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 42: 219–225, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol 109: 1203–1210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montagnani M, Quon MJ. Insulin action in vascular endothelium: potential mechanisms linking insulin resistance with hypertension. Diabetes Obes Metab 2: 285–292, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294: E15–E26, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10: 523–530, 2007 [DOI] [PubMed] [Google Scholar]
- 36. O'Gorman DJ, Karlsson HK, McQuaid S, Yousif O, Rahman Y, Gasparro D, Glund S, Chibalin AV, Zierath JR, Nolan JJ. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia 49: 2983–2992, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, Goto Y, Yoshimasa Y, Miyamoto Y. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb 17: 828–833, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes 50: 2659–2665, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Rogers MA, Yamamoto C, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care 11: 613–618, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Sartori C, Trueb L, Nicod P, Scherrer U. Effects of sympathectomy and nitric oxide synthase inhibition on vascular actions of insulin in humans. Hypertension 34: 586–589, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Scheede-Bergdahl C, Olsen DB, Reving D, Boushel R, Dela F. Insulin and non-insulin mediated vasodilation and glucose uptake in patients with type 2 diabetes. Diabetes Res Clin Pract 85: 243–251, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Straznicky NE, Lambert GW, McGrane MT, Masuo K, Dawood T, Nestel PJ, Eikelis N, Schlaich MP, Esler MD, Socratous F, Chopra R, Lambert EA. Weight loss may reverse blunted sympathetic neural responsiveness to glucose ingestion in obese subjects with metabolic syndrome. Diabetes 58: 1126–1132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tack CJ, Lenders JW, Willemsen JJ, van Druten JA, Thien T, Lutterman JA, Smits P. Insulin stimulates epinephrine release under euglycemic conditions in humans. Metabolism 47: 243–249, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Utriainen T, Holmang A, Bjorntorp P, Makimattila S, Sovijarvi A, Lindholm H, Yki-Jarvinen H. Physical fitness, muscle morphology, and insulin-stimulated limb blood flow in normal subjects. Am J Physiol Endocrinol Metab 270: E905–E911, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Utriainen T, Malmstrom R, Makimattila S, Yki-Jarvinen H. Methodological aspects, dose-response characteristics and causes of interindividual variation in insulin stimulation of limb blood flow in normal subjects. Diabetologia 38: 555–564, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Utriainen T, Nuutila P, Takala T, Vicini P, Ruotsalainen U, Ronnemaa T, Tolvanen T, Raitakari M, Haaparanta M, Kirvela O, Cobelli C, Yki-Jarvinen H. Intact insulin stimulation of skeletal muscle blood flow, its heterogeneity and redistribution, but not of glucose uptake in non-insulin-dependent diabetes mellitus. J Clin Invest 100: 777–785, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab 296: E11–E21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Winnick JJ, Sherman WM, Habash DL, Stout MB, Failla ML, Belury MA, Schuster DP. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab 93: 771–778, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Young CN, Deo SH, Kim A, Horiuchi M, Mikus CR, Uptergrove GM, Thyfault JP, Fadel PJ. Influence of endurance training on central sympathetic outflow to skeletal muscle in response to a mixed meal. J Appl Physiol 108: 882–890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis 211: 390–392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]