Abstract
Deep inspirations modulate airway caliber and airway closure and their effects are impaired in asthma. The association between asthma and obesity raises the question whether the deep inspiration (DI) effect is also impaired in the latter condition. We assessed the DI effects in obese and nonobese nonasthmatics. Thirty-six subjects (17 obese, 19 nonobese) underwent routine methacholine (Mch) challenge and 30 of them also had a modified bronchoprovocation in the absence of DIs. Lung function was monitored with spirometry and forced oscillation (FO) [resistance (R) at 5 Hz (R5), at 20 Hz (R20), R5-R20 and the integrated area of low-frequency reactance (AX)]. The response to Mch, assessed with area under the dose-response curves (AUC), was consistently greater in the routine challenge in the obese (mean ± SE, obese vs. nonobese AUC: R5: 15.7 ± 2.3 vs. 2.4 ± 2.0, P < 0.0005; R20: 5.6 ± 1.4 vs. 1.4 ± 1.2, P = 0.027; R5-R20: 10.2 ± 1.6 vs. 0.9 ± 0.1.4, P < 0.0005; AX: 115.6 ± 22.0 vs. 1.5 ± 18.9, P < 0.0005), but differences between groups in the modified challenge were smaller, indicating reduced DI effects in obesity. Given that DI has bronchodilatory and bronchoprotective effects, we further assessed these components separately. In the obese subjects, DI prior to Mch enhanced Mch-induced bronchoconstriction, but DI after Mch resulted in bronchodilation that was of similar magnitude as in the nonobese. We conclude that obesity is characterized by increased Mch responsiveness, predominantly of the small airways, due to a DI effect that renders the airways more sensitive to the stimulus.
Keywords: airway responsiveness, lung inflation, bronchodilation, bronchoprotection
in the past two decades, the prevalence of both asthma and obesity has risen significantly (37). Epidemiologic studies of cross-sectional and longitudinal design have demonstrated an increased frequency of asthma in obese as opposed to nonobese individuals (8, 11, 23, 37, 41, 59). Weight gain appears to be associated with an increased relative risk for asthma while weight loss has the opposite effect (1, 7). Despite these observations, the nature of the association between asthma and obesity has yet to be elucidated. Several groups have used measurements of airway hyperresponsiveness (AHR) to better define the relationship between the two entities, but the results have not always been in accord (35, 50, 53, 67). An important component of AHR is an impaired response to lung inflation (61). The beneficial effects of lung inflation or deep inspiration (DI) have been studied before (i.e., bronchoprotection) and after (i.e., bronchodilation) administration of a spasmogen (30, 31, 39, 55, 56, 61). While the mechanisms of these effects are largely unknown, both mechanical and neurohumoral etiologies are possible (61). Healthy subjects manifest both phenomena, but in most asthmatics bronchoprotection is absent (31, 56) and bronchodilation, though present, is not as robust (15, 19). The mechanisms of DI-induced bronchoprotection and bronchodilation are thought to differ since they are not equally impaired in asthma and since the airway smooth muscle (ASM) is presumably in a different state at the time of the DI in relation to administration of the spasmogen (i.e., smooth muscle is contracted in the case of post-Mch DI, whereas it is in a baseline state in the case of pre-Mch DI). To understand the relationship between obesity and asthma, it is essential to first understand lung physiology in obesity, independent of an asthma diagnosis. In this context, we examined whether the effects of DI are altered in obese, compared with nonobese individuals. Although other investigators have examined AHR in obese nonasthmatics, only Boulet and colleagues (6) sought to directly evaluate the role that DI may play in this phenomenon. Boulet's protocol included two single-dose Mch challenges, one of which was conducted with 20 min of DI avoidance prior to administration of the spasmogen. The results indicated that DI avoidance enhanced the Mch-induced reduction in lung function only in nonobese subjects, suggesting to Boulet that “obesity alters airway function.” Holguin and colleagues (27) showed that DI caused bronchoconstriction in obese individuals. However, their study was conducted in asthmatic subjects and was performed in the absence of Mch.
The purpose of our investigation was threefold. We first studied airway responsiveness to Mch in the presence vs. absence of DI in obese and nonobese nonasthmatics to assess for an abnormal response to lung inflation in the former group. Second, since we confirmed that there was an impaired effect of lung inflation in obesity, we next attempted to localize the abnormality to a defect in bronchoprotection vs. bronchodilation. Third, using forced oscillation testing, we examined whether the lung inflation impairment was attributable to predominantly small or large airways dysfunction.
METHODS
Subjects
Thirty-six healthy individuals were recruited from the Mount Sinai Medical Center and the New York City area by advertisement. Subjects had to meet the following inclusion criteria: age 18–60 yr, current nonsmokers (within the last year) with less than 5 pack-years lifetime tobacco use, no physician diagnosis or symptoms of allergic rhinitis or asthma, no history of other chronic illness (e.g., hypertension or diabetes), no more than 2 positive skin prick tests to a panel of 10 common aeroallergens, no airway obstruction by spirometry (45), and normal gas exchange [single breath diffusion capacity (DLCO)]. Body mass index (BMI) requirements for inclusion in the study were <25 kg/m2 (nonobese group) and ≥30 kg/m2 (obese group). Subjects were not studied within 4 wk of having an upper respiratory infection and were asked to avoid caffeine on all visit days. The study was approved by the Institutional Review Board of the Mount Sinai School of Medicine, and informed, written consent was obtained from each subject.
Lung Function Measurements
Screening pulmonary function was performed using a Sensormedics 6200 body plethysmograph (Sensormedics, Yorba Linda, CA) while all subsequent testing was done via a Jaeger Impulse Oscillation System (IOS) spirometer (Jaeger USA, Yorba Linda, CA) according to standard protocol (40, 70). Routine lung function data included forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC, total lung capacity (TLC), residual volume (RV), functional residual capacity (FRC), expiratory reserve volume (ERV), inspiratory capacity (IC), slow vital capacity (SVC), and DLCO. FRC, ERV, SVC, and IC were directly measured, while TLC and RV were calculated (i.e., TLC from FRC + IC and RV from FRC − ERV).
Forced oscillation (FO) was performed as previously described (62). Subjects supported their cheeks with their hands and breathed normally through a mouthpiece that stabilized tongue position to minimize oral resistance. The mouthpiece was connected to a pneumotachometer, and small pressure oscillations were “forced” by a loudspeaker attached to the other end of the tube. Respiratory resistance (R) and reactance (X) were calculated from oscillatory components of flow and pressure (24) at 5–35 cycles/s (Hz). Large and small airways mechanics were inferred from responses at high (20 Hz) and low (5–15 Hz) frequencies, respectively. Low-frequency oscillations at the mouth are transmitted to the lung periphery while those at 20 Hz and higher frequencies are limited to larger airways (20, 47). Four parameters were evaluated: 1) R at 5 Hz (R5), a global index influenced by both small and large airways; 2) R at 20 Hz (R20), an index of large airways R; 3) R5-R20, an index of frequency dependence of R. Specifically, since this parameter represents the difference between global resistance and large airways resistance, it is reflective of small airways resistance and; 4) integrated area of low frequency reactance (AX)(24, 25). This parameter includes all negative values of X (a measure of elastic load against which subjects breathe) between 5 Hz and the frequency at which X = 0 (resonant frequency). AX is an index of small airways obstruction complementary to frequency dependence of R (14, 24, 25, 33, 34, 42, 43). Three trials of FO (consisting of 30 s of tidal breathing at FRC) were performed. FO tracings were inspected to check that correlations (coherence) among pressure and flow phase and amplitude appeared acceptable (coherence > 0.8). Mean values from the three trials for each oscillometric index were calculated and used for analysis.
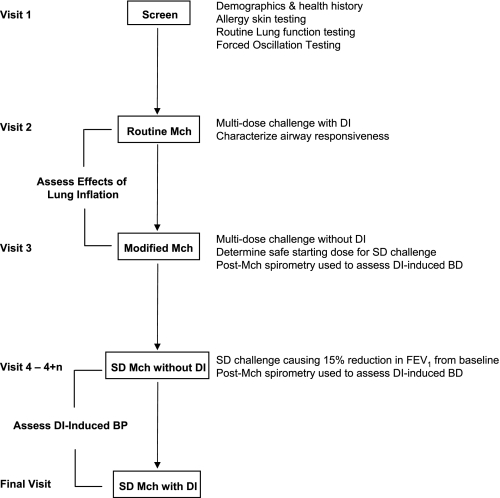
Study Design (Fig. 1)
Fig. 1.
This flow chart outlines the design of the study. Subjects underwent screening by medical history, allergy skin testing, routine lung function, and forced oscillation. They subsequently underwent 4 different types of methacholine (Mch) challenges to assess the effects of lung inflation and to characterize deep inspiration (DI)-induced bronchodilation (BD) and DI-induced bronchoprotection (BP). In the single-dose (SD) Mch challenges, there were multiple visits as indicated (n = 0–3).
Visit 1:
All subjects underwent a screening evaluation including a medical questionnaire (assessing nasal and lower respiratory symptoms, major health issues, and smoking history), measurement of height and weight for BMI calculation, allergy skin testing, and routine lung function testing. Skin testing to a battery of 10 common aeroallergens was done using the epicutaneous method per standard protocol (28). Subjects who met inclusion criteria underwent forced oscillation testing. In addition, only subjects with FEV1 > 60% predicted proceeded to Visit 2. The same baseline spirometry requirement was observed on each of the subsequent visits.
Visit 2:
Routine Mch bronchoprovocation was performed to assess airway responsiveness during a multi-dose challenge protocol including DI as follows: FO and spirometry were measured at baseline and 3 min after inhalation of saline and subsequent concentrations of Mch (inhaled by 5 deep breaths using a dosimeter and nebulizer) starting from 0.025 mg/ml and increasing in half log increments up to 25 mg/ml. At each dose level, a single trial of FO followed by three repeatable forced expirations from TLC was performed (the expiration with the highest FEV1 was used for analysis). The challenge ended after a 20% reduction in FEV1 from the postsaline value was attained (i.e., determining the provocative concentration of Mch causing a 20% reduction in FEV1 from post-saline baseline or PC20 Mch) (2) or after the highest concentration of Mch was inhaled.
Visit 3:
A modified Mch challenge was performed with three purposes: 1) to compare Mch reactivity in the routine vs. modified protocols to assess the effects of lung inflation (DI) in the two subject groups, 2) to determine a safe starting concentration for subsequent single-dose challenges (see additional Mch challenge protocols below), and 3) as one of two ways to assess DI-induced bronchodilation (see below). The modified challenge (60) was conducted using the same starting concentrations and increments of Mch as in the routine challenge. At each dose level (administered as 5 tidal breaths), a single trial of FO and three “partial” forced expirations from end-tidal inspiration to RV were performed. The response to Mch was monitored using the partial FEV1/FVC. Mch was administered until the partial FEV1/FVC fell to 0.5–0.55, the participant developed uncomfortable chest symptoms, or the highest concentration was reached. Prior to and after the end of the challenge, routine spirometry involving DIs was performed. DI-induced bronchodilation was determined by the routine spirometric maneuvers at the end of the challenge and was defined as the difference between the third and the first post-Mch spirograms (using both FEV1 and FVC).
Visit 4 (multiple visits for some subjects):
This visit assessed for DI-induced bronchoprotection, as well as for DI-induced bronchodilation using single-dose Mch challenge. The protocol was modified from that of Kapsali et al. (31). On Visit 4, subjects performed one trial of FO followed by routine spirometry (3 trials). They then avoided DI for 20 min by breathing quietly and not sighing or talking. After that, a single dose of Mch (estimated based on individual dose-response in the modified challenge) was inhaled (by 5 tidal breaths) followed in 3 min by repeat FO and spirometry (3 trials). If >15% reduction in FEV1 from baseline was not achieved, subjects returned on nonconsecutive days for repeat challenge with a higher single Mch dose (the concentration was generally doubled at each visit for most subjects up to a maximum of 40 mg/ml) until the >15% reduction in FEV1 threshold was reached. Once the threshold dose was determined, subjects returned for a final visit. At that visit, the challenge protocol and Mch dose were identical except that five DIs were taken immediately prior to administration of Mch. To assess for DI-induced bronchoprotection, the absolute difference in lung function (i.e., FEV1, FVC, and FO indices) post-Mch relative to baseline was calculated for the two threshold dose challenges (1 with and 1 without DIs prior to Mch) and the two challenges were compared. DI-induced bronchodilation from the single-dose Mch challenge was defined as on Visit 3 from the post-Mch routine spirometry (i.e., the difference between the third and the first post-Mch values for both FEV1 and FVC).
Data Analysis
In the routine vs. modified multi-dose Mch challenges, we assessed the change in lung function relative to baseline by calculating the area under the curve (AUC) for each parameter. For both the routine and modified challenges, we contrasted the AUC between the obese and nonobese groups. Within each subject group, we compared the AUC in the routine to that in the modified Mch challenge. In the modified challenge, 18 subjects did not receive the highest Mch concentration (25 mg/ml), because they had already reached the provocation endpoint. For this reason, the dose-response curves were truncated at the concentration of 7.5 mg/ml because data were available at this dose step for all 36 subjects who underwent routine challenge and for 27 of 30 subjects who performed the modified challenge (2 nonobese and 1 obese subject reached the endpoint in the modified challenge at a concentration of 2.5 mg/ml Mch). AUC comparisons reported here are age-adjusted using linear regression. Similar results, not shown, were obtained for crude contrasts using Mann-Whitney U-statistics.
The analysis of bronchoprotection and bronchodilation requires contrasting groups of subjects (obese vs. nonobese) in terms of within-person changes in pulmonary function measurements. To conduct these analyses, we constructed hierarchical linear models of pulmonary function measurements with random effects at the subject level, and fixed effects indicating test condition, obesity, and age. In all cases, statistical significance was defined by a P value <0.05.
The magnitude of bronchodilation by DI may depend in part on the degree of the preceding bronchoconstriction and that, in turn, depends on baseline lung function. To assess whether we needed to correct for baseline measurements, we analyzed repeatability of the baseline measurements across and within visits by examining mean differences, Pearson correlation and intraclass (within subject) correlation coefficients (ICC), and by inspecting Bland-Altman plots. We found acceptable values for both spirometric (between visit ICC for FEV1 and FVC were 0.98 and 0.97, respectively) and FO indices (within visit ICC ranged from 0.85 to 0.96 and the between visit ICC from 0.78 to 0.99).
RESULTS
Of 47 subjects screened, 44 qualified for inclusion in the study and 36 completed routine Mch challenge. Of the three subjects who were excluded, one was overweight but not obese, one had equivocal skin test results, and one disclosed a history of pneumonia. Eight subjects withdrew after screening due to the required time commitment. Demographic and lung function characteristics of the 36 subjects are shown in Table 1. Obese subjects were older. Both groups were predominantly Caucasian, most were female, and almost all were never-smokers. Significant differences in routine lung function between groups included a lower FEV1% predicted, FEV1/FVC, and ERV in obese subjects. All oscillometric indices were significantly higher in the obese group. Five obese and two nonobese individuals had a positive PC20 on routine Mch challenge, but PC20 was <8.0 mg/ml in only three subjects (all obese). To avoid bias, all Mch responsive subjects were included in the analyses presented herein, but analyses were also conducted with exclusion of these subjects and showed equivalent results.
Table 1.
Demographic and lung function characteristics of the 36 subjects who completed routine Mch challenge testing
| Parameter | Nonobese (n = 19) | Obese (n = 17) |
|---|---|---|
| Age, yr | 31 ± 8 | 42 ± 12‡ |
| # (%) Female | 12 (63) | 11 (65) |
| # (%) Caucasian | 14 (74%) | 9 (53%) |
| # (%) Former smokers | 1 (5) | 0 (0) |
| # (%) Skin test negative | 16 (84) | 12 (71) |
| BMI | 22 ± 2 | 35 ± 5*† |
| FEV1, %pred | 101 ± 8 | 93 ± 10* |
| FVC, %pred | 100 ± 8 | 95 ± 10 |
| FEV1/FVC | 0.84 ± 0.05 | 0.80 ± 0.04*† |
| TLC, %pred | 101 ± 13 | 94 ± 15 |
| RV, %pred | 93 ± 33 | 83 ± 29 |
| RV/TLC | 0.26 ± 0.06 | 0.28 ± 0.08 |
| FRC, %pred | 96 ± 16 | 94 ± 25 |
| ERV, %pred | 99 ± 17 | 70 ± 40† |
| SVC, liter | 4.43 ± 1.05 | 3.73 ± 1.07 |
| IC, liter | 2.71 ± 0.76 | 2.65 ± 0.76 |
| R5 | 2.89 ± 0.57 | 4.31 ± 0.80‡ |
| R20 | 2.88 ± 0.58 | 3.45 ± 0.54‡ |
| R5-R20 | 0.01 ± 0.27 | 0.86 ± 0.52‡ |
| AX | 1.91 ± 0.92 | 7.22 ± 4.21‡ |
| # with PC20 | 2 | 5 |
Data shown as mean±SD unless indicated. Demographic and lung function characteristics of the 30 subjects who underwent both routine and modified methacholine (Mch) challenges were not statistically different from those of the 36 subject cohort. BMI, body mass index; FEV1, forced expiratory value in 1 s; FVC, forced vital capacity; RV, residual volume; FRC, functional residual capacity; SVC, slow vital capacity; IC, inspiratory capacity; R5, R20, resistance at 5 and 20 Hz, respectively.
P < 0.05;
P < 0.01;
P < 0.005.
Thirty of thirty-six subjects also completed the modified Mch challenge. The demographic and baseline lung function characteristics of this subset did not differ significantly from that of the larger group. The six subjects who stopped at this juncture included two obese subjects unable to perform high quality maneuvers during the modified protocol and four subjects (i.e., 2 nonobese and 2 obese) who chose not to commit further.
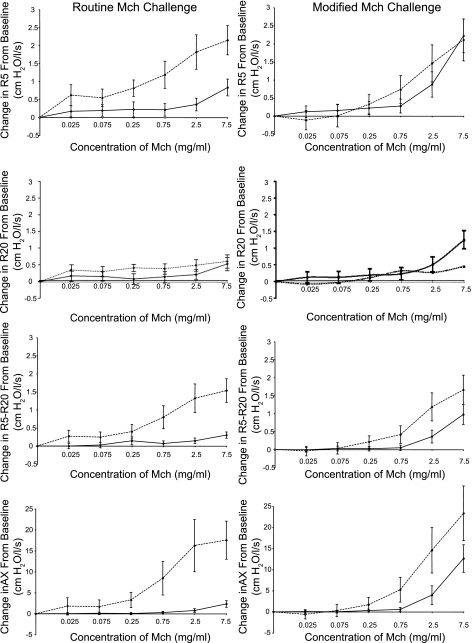
Figure 2 shows the dose-response curves in the two subject groups in routine compared with modified Mch challenge up to a concentration of 7.5 mg/ml [n = 36 subjects for the routine challenge and 27 subjects for the modified challenge (see Data Analysis)]. Data are shown for all FO indices and are expressed as the absolute changes at each concentration from the baseline values. The greatest differences in responsiveness between the obese and nonobese groups are observed in the routine challenge (where DI maneuvers are performed) with FO values being consistently and significantly higher in the obese [mean ± SE, obese vs. nonobese AUC (age adjusted): R5: 15.7 ± 2.3 vs. 2.4 ± 2.0, P < 0.0005; R20: 5.6 ± 1.4 vs. 1.4 ± 1.2, P = 0.027; R5-R20: 10.2 ± 1.6 vs. 0.9 ± 0.1.4, P < 0.0005; AX: 115.6 ± 22.0 vs. 1.5 ± 18.9, P < 0.0005]. The most dramatic differences between the two groups are seen in the overall resistance (R5) and in the small airways indices (R5-R20 and AX). We also performed AUC analysis in the routine challenge for FEV1 (data not shown). Differences in the dose-response curves were not significant using this parameter (P = 0.36). When we repeated our analyses restricted to the 27 individuals who underwent both challenge types (i.e., routine and modified Mch challenges), the results were not substantially different. To assess whether differences in baseline values may have influenced the observed results, we regressed the AUC for each of the reported lung function indices against a dummy variable marking obesity and adjusted for the baseline value of the corresponding parameter. The statistical results were not different from that of our primary analytic methods (P < 0.05 for each FO index and NS for FEV1).
Fig. 2.
The dose-response curves in routine (left) and modified (right) Mch challenge are shown in obese (dashed line) and nonobese (solid line) subjects for all forced oscillation indices. Data (means ± SE) are expressed as the absolute change at each Mch concentration relative to the baseline value and is displayed up to a concentration of 7.5 mg/ml. Airway responsiveness, assessed by area under the curve analysis was consistently increased in the obese group in the routine challenge for all forced oscillation indices shown (P < 0.0005), whereas the dose-response curves of the 2 subject groups were more similar in the modified challenge with significant differences only for the small airways indices (P ≤ 0.003).
In the modified Mch challenge (performed in the absence of DI), obese and nonobese groups do not show large differences even at the highest concentration of Mch. AUC analysis in the modified challenge shows significant difference between groups only for the small airways indices [mean ± SE, obese vs. nonobese: R5-R20: 9.9 ± 1.6 vs. 2.8 ± 1.4, P = 0.002; AX: 123.3 ± 22.6 vs. 29.4 ± 19.6, P = 0.003].
Table 2 shows results of AUC analysis (age-adjusted data) within each group comparing the routine (with DI) to the modified (without DI) Mch challenge. In none of the FO indices is there a significant difference between the two challenges in the obese group. In contrast, the nonobese group is more responsive when DIs are absent with significant differences in AX and a trend toward significance in the other indices.
Table 2.
AUC analysis of differences between routine and modified Mch challenge in the two subject groups
| FO Index | Group | Δ AUC (Modified-Routine) | SE Δ | P Value |
|---|---|---|---|---|
| R5 | Nonobese | 4.37 | 2.36 | 0.065 |
| Obese | −2.79 | 2.66 | 0.296 | |
| R20 | Nonobese | 2.60 | 1.60 | 0.104 |
| Obese | −2.23 | 1.80 | 0.217 | |
| R5-R20 | Nonobese | 1.84 | 1.19 | 0.122 |
| Obese | −0.27 | 1.34 | 0.839 | |
| AX | Nonobese | 27.82 | 13.76 | 0.043 |
| Obese | 7.70 | 15.43 | 0.618 |
Data are age adjusted. AUC, area under the curve; AX, integrated area of low frequency reactance.
Twenty-one individuals participated in the single-dose Mch protocols (12 nonobese and 9 obese). The single-dose Mch concentration needed to induce >15% reduction in FEV1 varied from 10–30 mg/ml and was not statistically different between the two subject groups (P = 0.49). Of the 9 subjects who did not complete these studies, 6 (4 nonobese and 2 obese) did not manifest the required minimal reduction in FEV1 with the highest concentration of Mch approved for use. The remaining three subjects dropped out due to scheduling conflicts.
Table 3 compares DI-induced bronchoprotection in obese vs. nonobese subjects. Age-adjusted data for FEV1, FVC, and all four oscillometric parameters are displayed as the absolute difference post-Mch relative to baseline from the single-dose challenges with and without DI (we also looked at the percent reduction in FEV1 and in FVC from baseline on the day without DI and found no differences between groups). Using our primary statistical methodology, we failed to observe a bronchoprotective effect of DI in the nonobese. However, using an unpaired t-test (not age adjusted), we found significant bronchoprotection by FEV1 only in the nonobese group (P = 0.01). In the obese, DIs actually caused statistically significant bronchoconstriction as assessed by small airways indices (i.e., R5-R20 and AX) in that the effects of Mch were larger in the challenge with DIs, compared with that in which DIs were prohibited.
Table 3.
Assessment of DI-induced bronchoprotection in obese compared with nonobese subjects from the single-dose Mch protocols
| Absolute Difference PostMch Relative to Baseline |
||||
|---|---|---|---|---|
| NonObese |
Obese |
|||
| Without DI | With DI | Without DI | With DI | |
| FEV1, liter | −0.74 ± 0.22 | −0.42 ± 0.22 | −1.09 ± 0.19 | −0.84 ± 0.19 |
| FVC, liter | −0.54 ± 0.23 | −0.28 ± 0.23 | −1.03 ± 0.20 | −0.71 ± 0.20 |
| R5, cmH2O · l−1 · s−1 | 2.21 ± 0.60 | 2.11 ± 0.60 | 2.03 ± 0.48 | 2.60 ± 0.48 |
| R20, cmH2O · l−1 · s−1 | 1.22 ± 0.25 | 1.30 ± 0.25 | 0.63 ± 0.21 | 0.57 ± 0.21 |
| R5-R20, cmH2O · l−1 · s−1*† | 0.99 ± 0.49 | 0.81 ± 0.49 | 1.39 ± 0.39 | 2.03 ± 0.39 |
| AX, cmH2O · l−1 · s−1*† | 14.57 ± 7.48 | 11.47 ± 7.48 | 20.43 ± 5.84 | 27.05 ± 5.84 |
Values are means ±SE. Age-adjusted data shown.
P ≤ 0.005 for deep inspiration (DI) effect in the obese group.
P ≤ 0.04 for DI effect between obese and nonobese.
Results from the two methods that we employed to assess DI-induced bronchodilation (using the modified multi-dose and the single-dose Mch challenges) are shown in Table 4. In both types of Mch challenges, DI-induced bronchodilation was defined by the absolute difference between the third and first post-Mch spirometric maneuvers for both FEV1 and FVC. DI-induced bronchodilation occurred in both obese and nonobese subjects in each challenge type (the difference between the 3rd and 1st maneuver was statistically different from zero by 1-sample t-test). This was particularly impressive in the modified challenge in which the absolute difference between the third and first post-Mch FEV1 was almost one-half a liter in both groups. Prior to age adjustment, there was significantly less DI-induced bronchodilation in the obese group in the single-dose challenge. After age adjustment, however, regardless of the methodology, there was no significant difference in the magnitude of DI-induced bronchodilation between groups for either parameter. Additional analysis, in which we adjusted each individual's degree of bronchodilation for the degree of preceding bronchoconstriction, confirmed the lack of difference in the bronchodilatory effect of DIs between the two groups.
Table 4.
Assessment of DI-induced bronchodilation in obese compared with nonobese subjects from the modified Mch and single-dose Mch protocols
| Absolute Difference PostMch Third Relative to First Spirogram |
||||
|---|---|---|---|---|
| Modified Protocol |
Single-Dose Protocol |
|||
| Nonobese | Obese | Nonobese | Obese | |
| FEV1, liter | 0.49 ± 0.10 | 0.47 ± 0.12 | 0.43 ± 0.13 | 0.27 ± 0.13 |
| FVC, liter | 0.41 ± 0.10 | 0.36 ± 0.13 | 0.35 ± 0.13 | 0.19 ± 0.13 |
Values are means ± SE. Age-adjusted data shown. All values were significantly different from zero (one-sample t-test) except for FVC in the obese in the single-dose protocol.
DISCUSSION
Our results demonstrate that the effects of lung inflation are impaired in obesity in the absence of asthma, because the greatest differences in airway responsiveness between obese and nonobese subjects were observed in routine Mch challenge in which DI was permitted. Airway responsiveness, assessed by AUC analysis, was consistently increased in the obese group under these conditions for all forced oscillation indices studied, whereas the dose-response curves of the two subject groups were more similar in the modified Mch challenge (devoid of DI). Since there are two components to the DI effect (i.e., pre-Mch DI and post-Mch DI effects), we tested each separately to localize the defect in obesity. Our data demonstrate that the post-Mch DI effect is not abnormal in obese individuals since they manifest DI-induced bronchodilation of similar magnitude to nonobese subjects. Using a standard protocol (31), we were unable to consistently find statistically significant DI-induced bronchoprotection in the nonobese. However, in the obese, we found exaggerated bronchoconstriction with pre-Mch DI. Thus our data suggest that the specific impairment in DI response in obesity lies in the pre-Mch effect. Furthermore, it is the small airways that appear to be more dysfunctional in obese individuals. The most dramatic differences in responsiveness between the obese and nonobese in the routine Mch challenge were in the small airways. There was no significant difference based on FEV1. Only the small airways parameters reflected increased bronchoconstriction in the obese with pre-Mch DI. The fact that small airways dysfunction in obesity persisted even in the absence of DI (i.e., based on our modified Mch results) suggests that it is a pervasive impairment related to this condition.
Multiple investigators have studied AHR using Mch and other spasmogens in obesity (13, 35, 50, 67). Some have demonstrated a positive correlation between BMI and AHR (35), although in certain cases, this has been sex dependent (13). In other reports, a relationship between obesity and AHR was not demonstrable (53). One possible explanation for such discrepancies may relate to the use of an arbitrary cut-off value or to other single-value indices to assess AHR. Particularly, when assessing nonasthmatics who are not expected to have AHR, it may be most informative to evaluate the full dose-response curves.
Several studies have investigated airway responsiveness in obesity in relationship to the DI effect. Salome and colleagues (50) showed no difference in maximal response to Mch and in Mch sensitivity (i.e., position of the dose-response curve) in obese and nonobese nonasthmatic subjects. The protocol of this study included multiple deep breaths in addition to those incorporated into the main lung function outcome (i.e., FEV1). In accordance with our findings, Salome and colleagues found similar bronchodilatory responses to DI in obese and nonobese nonasthmatics. Like Salome et al., we found no difference between these two groups in the routine Mch challenge using FEV1 as the outcome. Torchio et al. (67) studied subjects with an age range similar to that of our cohort and with BMIs ranging from 20–56 mg/kg2. They demonstrated a significant positive correlation between BMI and airway responsiveness (assessed as PD50 Mch using expiratory flows measured at 60% of TLC) and a negative correlation between BMI and the magnitude of DI-induced bronchodilation. Although we also initially observed a negative association between BMI and magnitude of DI-induced bronchodilation, this did not persist after age adjustment of data. Torchio's data were not age adjusted and there is no indication whether obese and nonobese subjects differed in age. A second explanation for the absence of the correlation in our data may be a more limited BMI range. Boulet's group (6) compared Mch responsiveness in obese and nonobese subjects in single-dose protocols with and without DI avoidance prior to administration of Mch. Their findings are consistent with our observation that Mch-induced changes in lung function are influenced more by the presence or absence of DI in nonobese compared with obese individuals.
Differences in methodology and subject characteristics (i.e., sex disparities, smoking habits, and presence of respiratory symptoms and atopy) may at least, in part, explain differences in study results. Studies that determine airway responsiveness using only the FEV1 may not distinguish as well between obese and nonobese subjects. This variable does not specifically reflect small airways function, and it is the small airways that seem most dysfunctional in the obese based on our data. Second, since the FEV1 itself incorporates a deep breath and obese subjects have intact DI-induced bronchodilation, repeated FEV1 maneuvers during routine Mch challenge may allow obese subjects to behave more like their nonobese counterparts.
The mechanism(s) of increased airway responsiveness in obesity and of the associated abnormal response to DI remain(s) unknown. In our study, the post-Mch and pre-Mch DI effects appeared to be disassociated. In other words, DI-induced bronchodilation (post-Mch effect) remained intact while there was a paradoxical increase in bronchoconstriction with DI taken prior to Mch (pre-Mch effect). This suggests that the mechanism for the two effects of DI may differ as indicated in studies conducted in asthma (56). One of the most discussed mechanisms for increased airway responsiveness and altered response to DI in obesity relates to the mechanical changes imposed by the condition. Obese individuals have lower resting lung volumes than do non-obese subjects (3, 5, 12, 16, 22, 36, 46, 49, 52) and low amplitude of tidal breathing in obesity may increase respiratory resistance (18, 72) and lead to stiffer airways more vulnerable to increased responsiveness (21, 64, 69). Several studies in nonobese subjects involving alterations in breathing pattern or excursion (17, 66), including our own prior work (60), support the fact that low “dynamic” lung volumes may be one determinant of airway responsiveness.
The low lung volume hypothesis, and especially low amplitude of tidal breathing, cannot fully account for increased airway responsiveness and abnormal DI response in obesity. In Salome's obese cohort (50), lung volumes (i.e., FRC and TLC) were significantly lower yet there was no difference in Mch response (assessed by FEV1) in the obese and nonobese groups. In our study, despite the demonstrated abnormal response to pre-Mch DI in obesity, FRC and tidal volume (the latter measured on the screening day prior to Mch challenge from a subset of 10 nonobese and 9 obese subjects, data not shown) were not lower in the obese group [i.e., we had fewer subjects with severe obesity (BMI > 40 kg/m2) than did Salome]. We did find a significantly lower ERV, and this has been cited as the most common lung function aberration in obese subjects (5). Our low ERV data should be interpreted cautiously since it is hard to explain in the setting of the other lung volume results (i.e., particularly the FRC). It is possible that the use of different reference equations for ERV compared with FRC, RV, and TLC was a confounding factor. It is also conceivable that our low ERV data was attributable to a lower SVC (since IC was similar). The SVC, in turn, was lower presumably due to a slightly lower TLC.
In accord with Salome's data we found a greater change in low frequency reactance induced by Mch in obese individuals, suggesting an increase in lung elastance (10, 38, 50) or “stiffness” particularly of the small airways. Our observation that low frequency reactance was higher in the obese even in the modified Mch challenge (without DI) suggests that lung stiffness may not be explained simply by low lung volume breathing and that there may be other important factors (32, 71) or an intrinsic small airway abnormality. Scichilone and colleagues (58) showed that, in the absence of DI, response to Mch is affected by level of fitness, with athletes being more hyporesponsive. Decreased exercise and fitness in the obese could possibly enhance ASM response to Mch. Alternative explanations for airway dysfunction and stiffness in obesity include thickening of the airway wall due to vascular congestion (48), fat deposition (51), or inflammation [i.e., obesity is a pro-inflammatory state (64, 68) and adipose tissue can produce allergic cytokines (4)]. Inflammation is known to inhibit surfactant production (26), although to our knowledge, human studies on surfactant production or release in obesity have not been conducted. There is evidence (29) in obese rats of alterations of lipid deposition in the lungs that may affect surfactant function. Lung stiffness due to any of these etiologies may predispose obese individuals to airway narrowing or early closure particularly of the small airways (44), increased airway responsiveness, and abnormal response to DI (i.e., pre-Mch DI-induced bronchoconstriction in the small airways).
Airway closure is viewed as an important determinate of AHR (9). Airway closure could at least partially explain our observation that the most dramatic differences in responsiveness between obese and nonobese subjects occurred in the routine compared with modified Mch challenge. In the latter protocol without DI, low lung volume breathing in the nonobese would predispose these subjects to increased airway closure, thus minimizing differences between groups. However, even in the modified challenge, there was increased responsiveness in the obese as assessed from small airways indices. Although we did not directly measure this during Mch challenge, it is possible that there were differences in tidal amplitude of breathing between groups. Fredberg et al. (21) showed that even tidal amplitude can influence responsiveness. Decreased amplitude of tidal breathing in the obese during Mch challenge might have also enhanced peripheral airway closure by “priming” small airways to close in the setting of minimal smooth muscle activation (10).
Thus we found the greatest differences in responsiveness between obese and nonobese in the small airways in both challenge types. Chapman and colleagues (10) suggested that the bronchoprotective effect of DI relates to prevention of closure of peripheral airways and avoidance of increased lung elastance. This latter observation is supported by Lutchen et al. (38) and Schweitzer et al. (54). Loss of bronchoprotection in obesity may relate to a blunting of these effects (i.e., our own data demonstrate an increase in lung elastance with pre-Mch DI). It is possible that the low ERV in our obese subjects meant that they were close to RV when they inhaled Mch in the single-dose protocol. Based on Chapman's findings (10), this may have contributed to loss of bronchoprotection. If, on the other hand, DI could open small airways to some extent as suggested by Holguin et al. (27), there might be increased peripheral Mch deposition with pre-Mch DI. These ideas also seem compatible with our finding that DI prior to Mch actually led to a paradoxical constrictive effect seen only in the small airways.
Airway “stretch” may be less important in DI-induced bronchoprotection (10) than in DI-induced bronchodilation. In the latter situation, under conditions of increased ASM tone, airway stretch is likely instrumental in breaking actin-myosin cross bridges and allowing the smooth muscle to relax. The fact that we observed a similar magnitude of DI-induced bronchodilation in the obese and nonobese, implies that the stretch of DI in the setting of induced constriction is similarly effective in both groups. Airway imaging studies may be needed to address this matter more definitively.
Our study does have limitations. First, our sample size was fairly small and, in some cases, the ability to detect the effects we were examining may have been limited by inadequate power. This was particularly true for results derived from the single-dose challenges with a sample size of only 21 subjects. Second, our obese group was significantly older than the nonobese group. There is a known effect of aging on DI response with a decrease in DI-induced bronchodilation (57). For this reason, we analyzed our data with age adjustments. Third, five of our obese subjects (but also 2 nonobese subjects) showed various degrees of responsiveness to Mch inhalation under routine challenge conditions [i.e., only 3 subjects meeting the conventional cut-off (8 mg/ml) for hyperresponsiveness] and an increase in the number of positive skin tests. We considered that we may have included some asthmatics in the cohort. To account for this, we reanalyzed our data eliminating all subjects who had a PC20 Mch and did not find differences in results. A final limitation in relation to previously published work (31, 55) is that we did not detect DI-induced bronchoprotection by FEV1 or by any of the FO parameters in our nonobese subjects using our primary statistical methodology. This discrepancy with previous work was moderated by the fact that significant bronchoprotection for FEV1 was detectable using an unpaired t-test. The fact that the results differed depending on the statistical methodology suggests that sample size may have been an issue. DI-induced bronchoprotection by FEV1 in healthy individuals can be of variable magnitude (55). It is possible that our small cohort coincidentally included those with less of an effect. The absence of DI-induced bronchoprotection using FO indices in our study is compatible with the findings of others (63). No explanation for this phenomenon can be offered.
In conclusion, our results indicate that the effects of lung inflation are impaired in obesity. We further localized the defect in the DI response to predisposing the airways to an exaggerated effect of Mch and not to a loss of DI-induced bronchodilation. While the mechanisms underlying these observations remain speculative, our data support the idea that peripheral airways dysfunction may be a characteristic of obesity. Our results suggest that, in some respects, the airways of obese individuals behave like those of asthmatics, although they may not have all the bona fide features of the asthma phenotype. From this perspective, we believe that special consideration should be given when asthma is diagnosed in some obese individuals in that they may simply belong to a different clinical phenotype (65). In addition, if asthma is indeed the underlying disorder in an obese patient, it is important to recognize that airway dysfunction may reflect additive or synergistic effects of two conditions. More work is required to elucidate the implications of these situations in clinical management.
GRANTS
This work was supported by the Mount Sinai Pulmonary Divisional Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors express gratitude to Daniel Ceusters for assistance in editing the manuscript.
Present address of A. Desai: Assistant Professor, Department of Medicine at Stony Brook University Medical Center, East Loop Road, Stony Brook, NY 11794 (e-mail: alpa.desai@stonybrook.edu).
REFERENCES
- 1. Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 125: 2046–2052, 2004 [DOI] [PubMed] [Google Scholar]
- 2. American Thoracic Society Statement Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med 161: 309–329, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Barrera F, Reidenberg MM, Winters WL. Pulmonary function in the obese patient. Am J Med Sci 36: 785–795, 1967 [DOI] [PubMed] [Google Scholar]
- 4. Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue interleukin-6 content and serum C463 reactive protein concentrations in obese subjects. Circulation 99: 2221–2222, 1999 [PubMed] [Google Scholar]
- 5. Bedell GN, Wilson WR, Seebohm PM. Pulmonary function in obese persons. J Clin Invest 37: 1049–1060, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boulet L, Turcotte H, Boulet G, Simard B, Robichaud P. Deep inspiration avoidance and airway response to methacholine: Influence of body mass index. Can Respir J 12: 371–6, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 159: 2582–2588, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med 163: 1344–1349, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J 32: 1563–9, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Chapman DG, Berend N, King GG, McParland BE, Salome CM. Deep inspirations protect against airway closure in nonasthmatic subjects. J Appl Physiol 107: 564–569, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol 155: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Cherniak RM. Respiratory effects of obesity. Can Med Assoc J 80: 613–616, 1959 [PMC free article] [PubMed] [Google Scholar]
- 13. Chinn S, Jarvis D, Burney P. Relation of bronchial responsiveness to body mass index in the ECRHS. European Community Respiratory Health Survey. Thorax 57: 1028–1033, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clement J, Lansder F, Van de Woestinjne K. Total resistance and reactance in patients with respiratory complaints with and without airways obstruction. Chest 2: 215–220, 1983 [DOI] [PubMed] [Google Scholar]
- 15. Crimi E, Pellegrino R, Milanese M, Brusasco V. Deep breaths, methacholine, and airway narrowing in healthy and mild asthmatic subjects. J Appl Physiol 93: 1384–1390, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Cullen JH, Formel PE. The respiratory defects in extreme obesity. Am J Med 32: 525–531, 1962 [Google Scholar]
- 17. Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol 62: 1324–1330, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Douglas FG, Chong PY. Influence of obesity on peripheral airways patency. J Appl Physiol 33: 559–563, 1972 [DOI] [PubMed] [Google Scholar]
- 19. Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. J Appl Physiol Respir Environ Exercise Physiol 50: 1079–1086, 1981 [DOI] [PubMed] [Google Scholar]
- 20. Frantz I, Close R. Alveolar pressure swings during high frequency ventilation in rabbits. Pediatr Res 19: 162–166, 1985 [DOI] [PubMed] [Google Scholar]
- 21. Fredberg JJ, Inouye DS, Mijailovich SM, Butler JP. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med 159: 959–967, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Gibson GJ. Obesity, respiratory function and breathlessness. Thorax 55: S41–44, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, Avol E, Peters JM. Obesity and risk of newly diagnosed asthma in school age children. Am J Epidemiol 158: 405–415, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Goldman M. Clinical application of forced oscillation. Pulm Pharmacol Ther 14: 341–350, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Goldman M, Carter R, Klein R, Fritz G, Carter B, Pachucki P. Within- and between-day variability of respiratory impedance using impulse oscillometry in adolescent asthmatics. Pediatr Pulmonol 34: 312–319, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Holfeld JM, Schmiedl A, Erpenbeck VJ, Venge P, Krug N. Eosinophilic cationic protein alters pulmonary surfactant structure and function in asthma. J Allergy Clin Immunol 113: 496–502, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Holguin F, Cribbs S, Fitzpatrick AM, Ingram RH, Jackson AC. A deep breath bronchoconstricts obese asthmatics. J Asthma 47: 55–60, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Horwitz RJ, Lemanske RF., Jr Allergic disease: pathophysiology and immunopathology. In: Allergic Diseases: Diagnosis and Treatment, edited by Lieberman P, Anderson JA. Totowa, NJ: Humana, 1997 [Google Scholar]
- 29. Inselman LS, Wapnir RA, Spencer H. Obesity-induced hyperplastic lung growth. Am Rev Respir Dis 135: 613–616, 1987 [DOI] [PubMed] [Google Scholar]
- 30. Jensen A, Atileh H, Suki B, Ingenito EP, Lutchen KR. Selected contribution: airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspirations. J Appl Physiol 91: 506–515, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Kapsali T, Permutt S, Laube B, Schchilone N, Togias A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol 89: 711–720, 2000 [DOI] [PubMed] [Google Scholar]
- 32. King GG, Brown NJ, Diba C, Thorpe CW, Munoz P, Marks GB, Toelle B, Ng K, Berend N, Salome CM. The effects of body weight on airway caliber. Eur Respir J 25: 896–901, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Klug B, Bisgaard H. Measurement of lung function in awake 2–4 year old asthmatic children during methacholine challenge and acute asthma. Pediatr Pulmonol 21: 290–300, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Landser F, Clement J, Van de Woestijne K. Normal values of total respiratory resistance and reactance determined by forced oscillations: Influence of smoking. Chest 81: 586–91, 1982 [DOI] [PubMed] [Google Scholar]
- 35. Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax 57: 581–585, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luce JM. Respiratory complications of obesity. Chest 74: 626–629, 1980 [DOI] [PubMed] [Google Scholar]
- 37. Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol 108: 729–734, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lutchen KR, Jensen A, Atileh H, Kaczka DW, Israel E, Suki B, Ingenito EP. Airway constriction pattern is a central component of asthma severity: the role of deep inspirations. Am J Respir Crit Care Med 164: 207–215, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Malmberg P, Larsson K, Sundblad BM, Zhiping W. Importance of the time interval between FEV1 measurements in a methacholine provocation test. Eur Respir J 6: 680–686, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pederson OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry from series ATS/ERS task force: standardisation of lung function testing. Eur Respir J 26: 319–338, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Nicolacakis K, Skowronski ME, Coreno AJ, West E, Nader NZ, Smith RL, McFadden ER., Jr Observations on the physiological interactions between obesity and asthma. J Appl Physiol 105: 1533–1541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nielsen K, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function and cold air and methacholine responsiveness in 2 to 5 year old asthmatic children. Am J Respir Crit Care Med 162: 1500–1506, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Nielsen K, Bisgaard H. Lung function response to cold air challenge in asthmatic and healthy children of 2–5 years of age. Am J Respir Crit Care Med 161: 1805–1809, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J 13: 203–210, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pederson OF, Wanger J. Interpretative strategies for lung function tests from series ATS/ERS task force: standardisation of lung function testing. Eur Respir J 26: 948–968, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anaesthesia. Anesth Analg 87: 654–660, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Peslin R, Fredberg J. Oscillation mechanics of the respiratory system. In: Handbook of Physiology,: The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am Physiol Soc, sect. 3, vol. III, 1986 [Google Scholar]
- 48. Rubinstein I, Zamel N, Dubarry L, Hoffstein V. Airflow limitation in morbidly obese, nonsmoking men. Ann Int Med 112: 828–832, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Sahebjami H, Gartside PS. Pulmonary function in obese subjects with a normal FEV1/FVC ratio. Chest 110: 1425–1429, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG. Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obesity 32: 502–509, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Salome CM, King GG, Berend N. Physiology of obesity and effects of lung function. J Appl Physiol 108: 206–211, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol 55: 1269–1276, 1983 [DOI] [PubMed] [Google Scholar]
- 53. Schachter L, Salome C, Peat J, Woolcock A. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 56: 4–8, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schweitzer C, Demoulin B, Bello G, Bertin N, Leblanc AL, Marchal F. Deep inhalation prevents the respiratory elastance response to methacholine in rats. Pediatr Res 59: 646–649, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Scichilone N, Kapsali T, Permutt S, Togias A. Deep inspiration-induced bronchoprotection is stronger than bronchodilation. Am J Respir Crit Care Med 162: 910–916, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med 163: 413–419, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Scichilone N, Marchese R, Catalano F, Togias A, Vignola AM, Bellia V. The bronchodilatory effect of deep inspiration diminishes with aging. Respir Med 98: 838–843, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Scichilone N, Morici G, Zangla D, Chimenti L, Davì E, Reitano S, Paternò A, Santagata R, Togias A, Bellia V, Bonsignore MR. Effects of exercise training on airway responsiveness and airway cells in healthy subjects. J Appl Physiol 109: 288–294, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Shaheen SO, Sterne JAC, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax 54: 396–402, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 96: 2393–2403, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Skloot G, Togias A. Bronchodilation and bronchoprotection by deep inspiration and their relationship to bronchial hyperresponsiveness. Clin Rev Allergy Immunol 24: 55, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Skloot G, Goldman M, Fischler D, Goldman C, Schechter C, Levin S, Teirstein A. Respiratory symptoms and physiologic assessment of ironworkers at the World Trade Center Disaster site. Chest 125: 1248–1255, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Slats AM, Janssen K, van Schadewijk A, van der Plas DT, Schot R, van den Aardweg JG, de Jongste JC, Hiemstra PS, Mauad T, Rabe KF, Sterk PJ. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 176: 121–128, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Tantisira KG, Weiss ST. Complex interactions in complex traits: obesity and asthma. BMJ 320: 827–832, 2000 [PMC free article] [PubMed] [Google Scholar]
- 65. The National Heart, Lung, and Blood Institute's Severe Asthma Research Program Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med 181: 315–323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Torchio R, Gulotta C, Ciacco C, Perboni A, Guglielmo M, Crosa F, Zerbini M, Brusasco V, Hyatt RE, Pellegrino R. Effects of chest wall strapping on mechanical response to methacholine in humans. J Appl Physiol 101: 430–438, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Torchio R, Gobbi A, Gulotta C, Dellaca R, Tinivella M, Hyatt RE, Brusasco V, Pellegrino R. Mechanical effects of obesity on airway responsiveness in otherwise healthy humans. J Appl Physiol 107: 408–416, 2009 [DOI] [PubMed] [Google Scholar]
- 68. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C709 reactive protein levels in overweight and obese adults. JAMA 282: 2131–2135, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Wang L, Cerny FJ, Kufel TJ, Grant BJB. Simulated obesity-related changes in lung volume increases airway responsiveness in lean, nonasthmatic subjects. Chest 130: 834–340, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson D, MacIntyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes from series ATS/ERS task force: standardisation of lung function testing. Eur Respir J 26: 511–522, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol 98: 512–517, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest 103: 1470–1476, 1993 [DOI] [PubMed] [Google Scholar]