Abstract
For decades it was believed that direct and indirect heating (the latter of which elevates blood and core temperatures without directly heating the area being evaluated) increases skin but not skeletal muscle blood flow. Recent results, however, suggest that passive heating of the leg may increase muscle blood flow. Using the technique of positron-emission tomography, the present study tested the hypothesis that both direct and indirect heating increases muscle blood flow. Calf muscle and skin blood flows were evaluated from eight subjects during normothermic baseline, during local heating of the right calf [only the right calf was exposed to the heating source (water-perfused suit)], and during indirect whole body heat stress in which the left calf was not exposed to the heating source. Local heating increased intramuscular temperature of the right calf from 33.4 ± 1.0°C to 37.4 ± 0.8°C, without changing intestinal temperature. This stimulus increased muscle blood flow from 1.4 ± 0.5 to 2.3 ± 1.2 ml·100 g−1·min−1 (P < 0.05), whereas skin blood flow under the heating source increased from 0.7 ± 0.3 to 5.5 ± 1.5 ml·100 g−1·min−1 (P < 0.01). While whole body heat stress increased intestinal temperature by ∼1°C, muscle blood flow in the calf that was not directly exposed to the water-perfused suit (i.e., indirect heating) did not increase during the whole body heat stress (normothermia: 1.6 ± 0.5 ml·100 g−1·min−1; heat stress: 1.7 ± 0.3 ml·100 g−1·min−1; P = 0.87). Whole body heating, however, reflexively increased calf skin blood flow (to 4.0 ± 1.5 ml·100 g−1·min−1) in the area not exposed to the water-perfused suit. These data show that local, but not indirect, heating increases calf skeletal muscle blood flow in humans. These results have important implications toward the reconsideration of previously accepted blood flow distribution during whole body heat stress.
Keywords: positron-emission tomography, skin blood flow, bone blood flow, heat stress
when humans are exposed to acute heat stress several cardiovascular adjustments occur primarily directed toward increasing skin blood flow, which are necessary to adequately dissipate an internal heat load. These responses are accomplished through a combination of local and neurally mediated cutaneous vasodilation, coupled with elevated cardiac output and redistribution of blood flow and volume away from central vascular beds, such as the splanchnic and renal circulations, to the cutaneous circulation (4, 9, 21, 29–31). In addition, skin and muscle sympathetic nerve activities (SNA) increase during heat stress (3, 5, 6, 17, 23, 34), with increases in skin SNA being responsible for sweating and cutaneous vasodilation, while increases in muscle SNA have a less clear end result.
Earlier studies provided evidence that, during both local and indirect whole body heating, increases in forearm blood flow were exclusive to the skin; that is, muscle blood flow did not change to these perturbations (7, 8, 15, 26). In part due to these and related studies, it was reported that the skin has a capacity to increase blood flow upwards to 7–8 l/min during a profound heat stress (7, 27). However, those calculations were based on the assumption that the absence of an increase in forearm muscle blood flow during heat stress is consistent with other areas of the body, such as the legs. This may not be correct given heterogeneity of vascular responses between upper and lower limbs (24), coupled with recent findings by Keller et al. (18) showing increases in calf muscle blood flow, via Xe133 clearance, during local heating. However, use of the Xe133 clearance technique to evaluate muscle blood flow is not without limitations as it is reported to underestimate skeletal muscle blood flow in animals and humans (16). Furthermore, the study by Keller et al. (18) did not investigate the effects of indirect heat stress on limb muscle blood flow.
Positron-emission tomography (PET) is a noninvasive imaging technology, which can be used to measure blood flow and its distribution in muscle and other tissues. Blood flow can be quantified with PET by the use of [15O]H2O (radiowater), which is an intravenously infused inert and freely diffusible tracer. The PET-radiowater technique measures only blood flow in tissues where there is an exchange of water molecules, i.e., where exchange of nutrients and oxygen occurs. This technique has the capacity to quantify perfusion through different tissues (such as skin and skeletal muscle), while providing three-dimensional insight into capillary level blood flow in tissues at rest, as well as in contracting skeletal muscle (13, 32). The objective of the present study was thus to test the hypothesis that both local and indirect whole body heating increase skeletal muscle blood flow as evaluated by PET-radiowater measures.
METHODS
Subjects.
Eight healthy men (25 ± 6 yr old; 182 ± 7 cm; 77 ± 13 kg) volunteered to participate in the study. The purpose and potential risks of the study were explained to the subjects before they gave their written informed consent to participate. The subjects abstained from caffeine-containing beverages for at least 24 h before the experiments as well as avoided strenuous exercise within 48 h before the study. The subjects were not taking any medications. The study and consent were performed according to the Declaration of Helsinki and were approved by the Ethical Committee of the Hospital District of South-Western Finland and National Agency for Medicines.
Instrumentation.
On arrival to the laboratory each subject swallowed a telemetric thermometer pill that transmitted intestinal temperature to a receiver next to the subject (HQ). Mean skin temperature was recorded from the weighted average of six thermocouples attached to the skin (Sable Systems). A sterile thermocouple-based temperature probe was inserted into the gastrocnemius muscle of the right leg, 2–2.5 cm below the surface of the skin, using a small gauge needle. A separate thermocouple was taped to the skin adjacent to the intramuscular insertion site. The antecubital vein of the right hand was cannulated for tracer administration, while the radial artery of the left hand was cannulated for blood sampling. Subjects were then moved to the PET scanner with the calf region in the imaging area. Systolic and diastolic blood pressures were measured via auscultation of the brachial artery (Omron, M5–1, Omron Healthcare, Europe B.V. Hoofddorf, The Netherlands), from which systemic mean arterial pressure (MAP) was calculated.
Study procedures.
Skeletal muscle, skin, and bone blood flows in the calf region (Fig. 1) were measured using PET with [15O]H2O (900 MBq), as described below. The pant leg of a water-perfused suit was placed around the right calf region, which contained the intramuscular temperature probe. Thermoneutral water (∼34°C) was perfused through this region while blood flows from both legs were measured under resting normothermic conditions. This was followed by hot water (47–50°C) being perfused through the pant leg sufficient to increase intramuscular temperature ∼4°C, at which time blood flow from both legs were again measured. Next, the entire water-perfused suit was placed on the subject such that the entire body was exposed to the water-perfused suit except the head, both hands, the calf of the left leg, and both feet. Hot water (47–50°C) was then perfused through the entire suit with a goal to increase intestinal temperature ∼1°C. It is important to emphasize that during the whole body heating component, the calf with the intramuscular temperature probe continued to be directly heated given that the water-perfused suit covered this leg, whereas the water-perfused suit was not covering the contralateral calf (left leg) and thus it received “indirect” heating via elevated blood temperature.
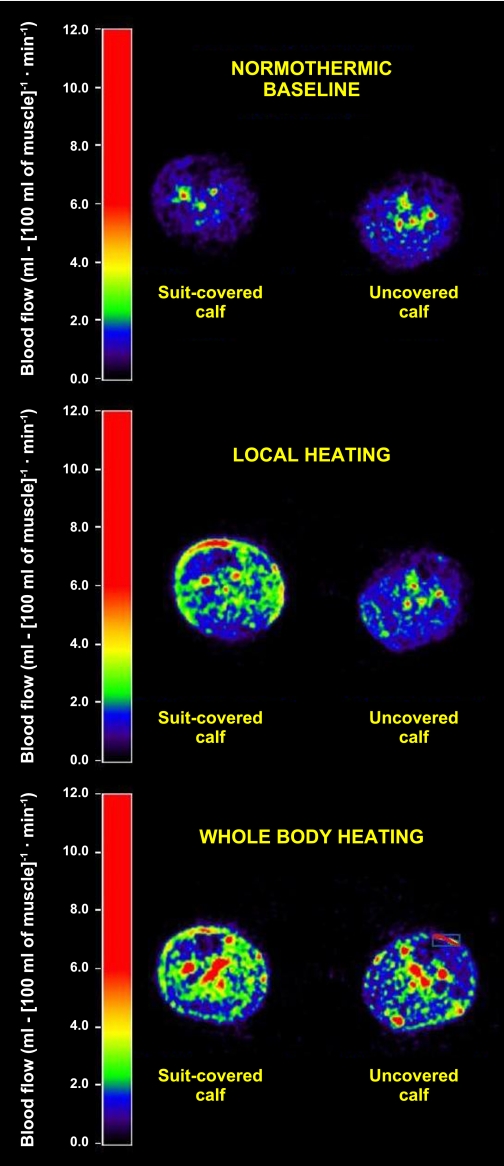
Fig. 1.
Representative cross-sectional positron-emission tomography (PET) blood flow images from the middle calf region at normothermic baseline, during local heating, and during whole body heating.
Blood flow measurements and analysis.
Radiowater positron-emitting tracer [15O]H2O was produced as previously described (33) and an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN) was used in three-dimensional (3D) mode for image acquisition to measure muscle blood flow. Photon attenuation was corrected by 5-min transmission scans performed both at the beginning of the normothermic and heat stress PET evaluations. All data were corrected for dead time, decay, and measured photon attenuation, and the images were reconstructed into a 256 × 256 matrix, producing 2.57 × 2.57 mm in-plane dimensions of voxels with 2.43 mm plane thickness. Scanning began simultaneously with the bolus infusion of the tracer and scanning consisted of the following frames; 6 × 5 s, 12 × 10 s and 7 × 30 s. Arterial blood radioactivity was also sampled continuously with a detector during imaging for blood flow quantification. The data analysis was performed using standard models (14) and methods (11–13, 32). Blood flow was analyzed from calf musculature, with specific regions of interest including the soleus and gastrocnemius muscles, but avoided all apparent blood vessels and bone structures (Fig. 1). Regional vascular conductance and resistance were calculated from the ratio of blood flow to MAP (conductance) or the ratio of MAP to blood flow (resistance). Bone blood flow was evaluated from tibia.
MRI.
Structural MRI was performed approximately 1 wk before the PET study to make PET-MRI fusion images, and thus to accurately exclude large vessels and define the regions of interests for blood flow determinations only from muscle and skin (11–13).
Statistical analysis.
Statistical analyses were performed with SAS 8.2 and SAS Enterprise 4.2 programs (SAS Institute, Cary, NC). Comparisons between conditions (i.e., normothermia, local heating, and whole body heating) for thermal and hemodynamic variables were analyzed using a one-way repeated-measures ANOVA. Likewise, skin and muscle blood flow responses, for each calf, were analyzed using one-way repeated-measures ANOVA between the three thermal conditions. If a significant main effect(s) was found, pairwise differences were identified using the Tukey-Kramer post hoc procedure. Results are expressed as means ± SD, and P ≤ 0.05 was considered statistically significant.
RESULTS
Muscle and intestinal (core) temperatures.
The effects of local and whole body heating on intestinal, muscle, local skin, and mean skin temperatures are shown in Table 1. Calf-only heating increased local skin temperature ∼8°C resulting in an increase in intramuscular temperature from 33.4 ± 1.0 to 37.4 ± 0.8°C, while intestinal temperature did not change. During whole body heating, mean skin temperature was elevated ∼4°C, muscle temperature under the water-perfused suit remained elevated (37.3 ± 0.5°C), and intestinal temperature increased from 37.2 ± 0.2 to 38.2 ± 0.4°C.
Table 1.
Effects of local and whole body heating on intestinal, muscle, skin, and local temperatures
| Normothermic Baseline | Local Heating | Whole Body Heating | |
|---|---|---|---|
| Intestinal temperature, °C | 37.2 ± 0.2 | 37.2 ± 0.3 | 38.2 ± 0.4*† |
| Muscle temperature, °C | 33.4 ± 1.0 | 37.4 ± 0.8* | 37.3 ± 0.5* |
| Mean skin temperature, °C | 34.2 ± 0.5 | 35.6 ± 0.6* | 38.5 ± 0.4*† |
| Local skin temperature, °C | 31.9 ± 0.9 | 39.6 ± 1.0* | 36.9 ± 2.1*† |
Values are means ± SD. Local temperature is the temperature from the probe on the skin adjacent to the intramuscular probe.
P < 0.05 compared with normothermic baseline;
P < 0.05 compared with local heating.
Heart rates, blood pressures, and muscular vascular resistance.
The effects of local and whole body heating on heart rate, blood pressure, and muscular vascular conductance and resistance are shown in Table 2. While systolic blood pressure remained unchanged in all three conditions, diastolic and mean arterial blood pressure were reduced during whole body heating. Muscle vascular resistance of the right calf (covered with the water-perfused suit) decreased during both local and whole body heating, compared with normothermic baseline. However, muscle vascular resistance of the left calf (not covered with the water-perfused suit) was unchanged regardless of the thermal condition. Generally, similar findings but in the opposite direction were observed when these value are reported as muscle vascular conductance.
Table 2.
Effects of local and whole body heating on heart rate, blood pressure, and muscle vascular conductance and resistance
| Normothermic Baseline | Local Heating | Whole Body Heating | |
|---|---|---|---|
| Heart rate, beats/min | 57 ± 7 | 63 ± 9 | 99 ± 9*† |
| Systolic arterial blood pressure, mmHg | 120 ± 13 | 122 ± 12 | 123 ± 9 |
| Diastolic arterial blood pressure, mmHg | 69 ± 8 | 67 ± 6 | 54 ± 7*† |
| Mean arterial blood pressure, mmHg | 86 ± 8 | 85 ± 7 | 77 ± 7*† |
| Muscle vascular conductance (covered), ml · 100 g−1 · min−1 · mmHg−1 | 0.016 ± 0.005 | 0.026 ± 0.013 | 0.025 ± 0.007* |
| (P = 0.06) | |||
| Muscle vascular resistance (covered), mmHg · ml−1 · 100 g · min | 70.1 ± 27.8 | 43.8 ± 14.8* | 44.3 ± 16.7* |
| Muscle vascular conductance (uncovered), ml · 100 g−1 · min−1 · mmHg−1 | 0.018 ± 0.006 | 0.018 ± 0.006 | 0.022 ± 0.006 |
| Muscle vascular resistance (uncovered), mmHg · ml−1 · 100 g · min | 60.5 ± 21.0 | 62.1 ± 20.2 | 48.9 ± 13.7 |
Values are means ± SD. Covered: the right calf that was covered by the water-perfused suit and thus was directly heated during both local and whole body heating; uncovered: the left calf that was not covered by the water-perfused suit and thus was not directly heated at any time.
P < 0.05 compared with normothermic baseline;
P < 0.05 compared with local heating.
Muscle blood flow.
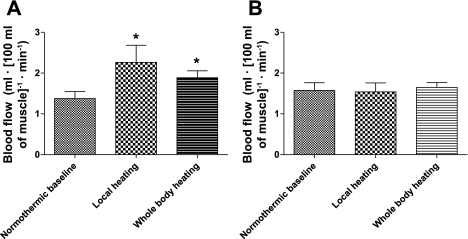
Local heating increased muscle blood flow in the covered calf (P < 0.05) (see Fig. 1 for illustration and Fig. 2A for numerical values) and remained elevated, relative to normothermia, during whole body heating (P = 0.037). However, there was no difference in skeletal muscle blood flow in this calf between local and whole body heating (P = 0.61). The thermal perturbations did not change muscle blood flow from the uncovered calf (P = 0.87 for the main effect of the ANOVA) (Figs. 1 and 2B).
Fig. 2.
Effects of local and whole heating on muscle blood flow. Local heating increased muscle blood flow of the covered leg (A) (*P < 0.05 vs. normothermic baseline). Muscle blood flow was also higher in the covered leg during whole body heating compared with normothermic baseline (P = 0.037) (A). However, in the leg not covered by the water-perfused suit, muscle blood flow did not change from normothermic baseline values regardless of the perturbation (P = 0.87 from the ANOVA) (B).
Skin blood flow.
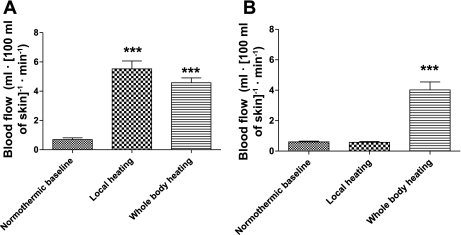
Both local and whole body heating increased skin blood flow from the covered calf (P < 0.001), but there was no difference in skin blood flow between these heating protocols (P = 0.46) (Fig. 3A). Skin blood flow from the uncovered calf was not changed during local heating of the covered calf (Fig. 3B). However, skin blood flow from the uncovered calf during whole body heating was elevated from 0.6 ± 0.2 to 4.0 ± 1.4 ml·100 g−1·min−1 compared with normothermia.
Fig. 3.
Effects of the local and whole body heating on skin blood flow. Both local and whole body heating increased skin blood flow (***P < 0.001) in suit-covered calf (A), while there was no difference in skin blood flow between these heating protocols (P = 0.46). Only whole body heating increased skin blood flow in the calf that was not covered by the water-perfused suit (***P < 0.001 relative to normothermia and local heating of the contralateral leg) (B).
Bone blood flow.
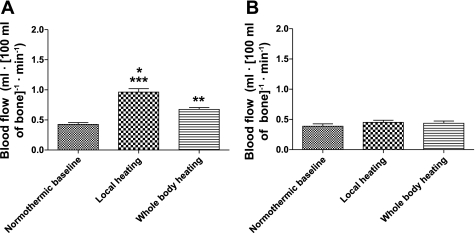
Both local and whole body heating increased bone blood flow of the covered leg (Fig. 4A) (**P < 0.01 and ***P < 0.001 from normothermic baseline). Bone blood flow was higher in the covered leg during local heating compared with whole body heating (*P < 0.05) (Fig. 4A). However, in the leg not covered by the water-perfused suit, bone blood flow did not change from normothermic baseline values regardless of the perturbation (P = 0.2 from the ANOVA) (Fig. 4B).
Fig. 4.
Effects of local and whole body heating on bone blood flow. Both local and whole body heating increased bone blood flow of the covered leg (A) (**P < 0.01, ***P < 0.001 from normothermic baseline). Bone blood flow was higher in the covered leg during local heating compared with whole body heating (*P < 0.05) (A). However, in the leg not covered by the water-perfused suit, bone blood flow did not change from normothermic baseline values regardless of the perturbation (P = 0.2 from ANOVA) (B).
DISCUSSION
These results demonstrate that local heating, which increased muscle temperature by ∼4°C, but not indirect whole body heating, which increased core temperature by ∼1°C, increases skeletal muscle blood flow in humans. Moreover, muscle vascular resistance of the heated calf decreased during both local and whole body heating compared with normothermic baseline, but remained similar to control values in nonheated contralateral calf musculature. We also report for the first time that bone blood flow increases during local heating. These results have important implications for reconsideration of whole body blood flow distribution and skin-specific flow capacities during passive heat stress and perhaps dynamic exercise in the heat.
Local heating responses.
An increase in calf muscle and tibia blood flow during local heating occurred in response to increased intramuscular temperature (from 33.4 ± 1.0 to 37.4 ± 0.8°C), while intestinal temperature remained unchanged. These findings confirm and extend the recent findings by Keller et al. (18) who, by using the xenon-clearance technique, similarly observed increased calf muscle blood flow during local heating. However, both findings are in contrast to classic investigations in which forearm muscle blood flow was unchanged during local heating (15). The reason for the discrepancy may simply be regional differences in vasodilator responsiveness to local heating between the arm and leg. Consistent with this hypothesis, Pawelcyzk and Levine (24) found greater vasoconstrictor responses to intra-arterial phenylephrine administration (an α1-adrenergic agonist) in the leg relative to the arm. Alternatively, differences in experimental techniques, and their associated sensitivities, coupled with relatively small increases in calf muscle blood flow with local heating (∼1 ml·100 g−1·min−1), may contribute to differences between the aforementioned findings.
There are at least three potential mechanisms for the increased human muscle blood flow during prolonged externally delivered local heating. First, increased muscle temperature per se may directly vasodilate resistance vessels in the human microcirculation. This stimulus may also decrease the resistance of upstream feed arteries (1). Although in some ex vivo experiments heating relaxes isolated blood vessels, especially cutaneous veins (2), it is evident that in the majority of animal studies heating per se does not change or slightly increases the tone of (arterial) blood vessels, such as in mesenteric and carotid arteries (19, 22). These latter observations are in line with the understanding that warm blood is shunted from the body's core to skin so that heat can more effectively be released, although clearly sympathetically mediated vasoconstriction is the primary driver of this response. Second, in a recent study which also proposed that heating increases (thigh) muscle blood flow, Pearson et al. (25) suggested that the potent vasodilator and sympatholytic agent ATP might be responsible for this response. Moreover, it is likely that heating directly increases nitric oxide synthase and thus nitric oxide release within the muscle vasculature (10), which would augment muscle blood flow. Thus released substances associated with direct heating may cause the observed dilation of muscle, and bone, vasculature. Third, increased temperature per se is known to increase oxygen consumption of the tissue (i.e., a Q10 effect), which may act as an initiator of metabolically induced vasodilation.
Whole body heating responses.
In the covered leg, muscle and bone blood flow remained elevated during the subsequent whole body heating protocol, although the increase in blood flow from normothermic baseline was slightly, but not significantly, less compared with local heating. Directionally consistent with this tendency for a reduction in muscle blood flow between local and whole body heating conditions, local skin temperature (by ∼2.5°C) and muscle temperature (not statistically significant) were also lower in the covered calf. Although unlikely, it may be that this tendency for a reduction in muscle blood flow from local to whole body heating is due to subtle decreases in muscle temperature, which would support the recent findings of Pearson et al. (25), who suggested that muscle blood flow increases in proportion to increases in muscle temperature. However, in the present results there was no correlation between the increase in muscle temperature and the elevation in muscle blood flow (R2 = 0.08, P = nonsignificant) in the pooled dataset (i.e., combining both local and whole body heating data). Furthermore, the tendency for a reduction in muscle blood temperature between local and whole body heating conditions was very small and with questionable physiological significance.
Whole body heat stress increases muscle sympathetic nerve activity (MSNA) by ∼100% (3, 6, 17, 23). Such an increase should cause a vasoconstrictor bias in the muscle, particularly given recent findings that heat stress does not impair adrenergic vasoconstrictor responsiveness in muscle (18). Perhaps the attenuation in the elevation in muscle blood flow during whole body heat stress in the covered calf, compared with local heating, is due to a slight vasoconstrictor bias in the balance between local heating-induced muscle vasodilation and sympathetically induced vasoconstriction. However, muscle blood flow responses from the nonheated leg during the whole body heat stress are not entirely consistent with this hypothesis. That is, a doubling of MSNA should have reduced muscle vascular conductance and blood flow in the uncovered calf, which was not observed. A possible explanation for this observation may be due to a combination of heat induced increases in muscle temperature (associated with ∼1°C increase in core body temperature and thus blood temperature) causing slight increases in muscle blood flow, coupled with sympathetically mediated vasoconstriction, the net effect being no measurable change in muscle blood flow in the uncovered leg. Alternatively, the absence of a change in muscle blood flow in the uncovered leg may simply be a consequence of the level of muscle heating during whole body heat stress being insufficient to alter muscle blood flow, although this hypothesis does not explain the absence of a reduction in muscle blood flow of this limb despite an approximate doubling of MSNA during whole body heat stress. Finally, the overall stress reaction of the body in response to heat stress may elevate circulating catecholamines, epinephrine most importantly, which could have contributed to elevate muscle blood flow through adrenergic beta receptors.
Muscle and skin blood flows during heat stress: how high do they go?
During passive whole body heat stress, the entire body, with the typical exception of the feet, hands, and head, are exposed to elevated temperatures. Mean skin temperature under the water-perfused suit can exceed 40°C (31), although the magnitude of heat stress in the present study was less than that imposed by Rowell and colleagues. Nevertheless, based on the present findings muscle temperature would increase under the water-perfused suit and, at least in the calf, this would cause an increase in muscle blood flow. During such a heat stress cardiac output has the potential to more than double, approaching 13 l/min in resting humans. This exposure decreases both renal and splanchnic blood flow (21, 29, 30). Based on the previously held hypothesis that muscle blood flow does not change during heat stress, it has long been thought that 100% of the increase in cardiac output, plus the magnitude of the reductions in renal and splanchnic blood flow, equals the increase in skin blood flow that occurs during this exposure (28). Using this calculation, Rowell (27) proposed that the increase in skin blood flow can approach 7.8 l/min during a severe passive heat stress. However, given the present findings that muscle blood flow increases under the water-perfused suit, the aforementioned calculations need to be reconsidered. In the present study, local heating sufficient to increase intramuscular temperature ∼4°C increased muscle blood flow by ∼45%. However, this equated to an absolute increase in muscle blood flow of only ∼1 ml·100 g−1·min−1. If one assumes that an average individual has a muscle mass of 35 kg, and if blood flow to 100% of this mass is increased by 1 ml·100 g−1·min−1, then the overall increase in muscle blood flow to the heat stress would only be ∼350 ml/min. However, this calculation assumes that the observed vascular responses in calf muscle are consistent with other muscles groups, which has yet to be determined. Even if a more severe heat stress caused a doubling of muscle blood flow relative to the present findings, the “error” associated with not including muscle blood flow in the calculation of total skin blood flow would be ∼10% (i.e., 7.1 l/min rather than 7.8 l/min previously proposed by Rowell) (27). Therefore, although clearly muscle blood flow has the potential to increase during resting whole body heat stress, that increase is relatively small and has only a minimal effect on the calculation of total skin blood flow. Conversely, during dynamic exercise, heat associated with metabolism may contribute to exercise muscle hyperemia. The importance of this possibility becomes greater during prolonged and high-intensity exercise when disproportionate increases in muscle blood flow (in relation to metabolic demand) may compromise skin blood flow and thus heat exchange. The observed increase in bone blood flow in response to local heat stress is also likely to affect the calculations of the distribution of cardiac output during passive heating, but due to its low absolute values, the contribution is also only marginal at best. However, although some aspects of bone blood flow have already been characterized (20), this area remains largely unexplored in humans and more research is therefore warranted.
Figure 3 illustrates that during whole body heating, calf skin blood flow from the covered leg increased to ∼5 ml·100 g−1·min−1. If a 70-kg individual has 8–10 kg of skin, and if skin blood flow responses from the calf are representative of whole body skin blood flow, a skin blood flow of 5 ml·100 g−1·min−1 would equate to a whole body skin blood flow of only 400–500 ml/min. This value is substantially less than prior calculations yielding maximum skin blood flows approaching 8 l/min (28). Moreover, Edholm et al. (8) proposed skin blood flows as high as ∼160 ml·100 g−1·min−1 in severely heat-stressed humans. In this respect there are, however, a number of important points that should be made to explain these discrepancies. First, in the prior studies subjects were heated to the limits of their thermal tolerance, with skin temperatures over 40°C and blood temperatures over 39°C. This is in contrast to the much more moderate heat stress in the present protocol. Second, although PET measures blood flow per 100 g of tissue, there are difficulties inherent to any technique in defining the boundary between skin and subcutaneous tissue needed to precisely calculate skin mass of the evaluated or especially whole body area, and thus also total skin blood flow. Thus the partial volume effect may contribute to underestimation of skin blood flow per 100 g tissue due to its thin structure. Third, it is apparent that there are marked heterogeneities in sweating and skin blood flow between regions of the body. It is, therefore, possible that skin blood flow from other regions of the body were greater than that observed in the calf. Finally, it also remains a possibility that PET-derived estimates of skin blood flow underestimate true skin blood flow during both local and whole body heat stress if some of the increases in skin blood flow occur through nonnutritive paths that are undetectable by PET techniques. Nevertheless, it should be highlighted that the relative increase (∼650% or ∼7-fold) in skin blood flow from normothermia to heat stress measured by PET is similar to that observed with laser-Doppler flowmetry and shows the potential for PET to adequately quantify relative changes in regional skin blood flow.
In conclusion, this study shows that local heating increases skeletal muscle blood flow in humans, which is consistent with that previously shown using the Xe133 clearance technique (18). In addition, the present findings do not support the hypothesis that muscle blood flow increases in a limb not directly exposed to a heat stimulus during an ∼1°C increase in core body temperature.
GRANTS
The study was conducted within and funded by the Centre of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research, supported by the Academy of Finland, University of Turku, Turku University Hospital and Abo Academy. The present study was also financially supported by National Heart, Lung, and Blood Institute Grants HL-61388 and HL-84072 and the Finnish Cultural Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
This study could not have been performed without the contribution of the personnel of the Turku PET Centre and authors want to thank them for their excellent assistance during the study.
All authors contributed to the conception and design of the experimental protocol, analysis and interpretation of data, and revision of the manuscript. I. Heinonen wrote the initial draft of the manuscript. C. G. Crandall, K. K. Kalliokoski,and J. Knuuti secured the funding of the project. All authors have read, revised, and approved the final version of the manuscript. All the experiments were performed at Turku PET Centre, Turku, Finland.
REFERENCES
- 1. Bagher P, Segal SS. Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol (Oxf) 202: 271–284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooke JP, Shepherd JT, Vanhoutte PM. The effect of warming on adrenergic neurotransmission in canine cutaneous vein. Circ Res 54: 547–553, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 277: H2348–H2352, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Crandall CG, Gonzalez-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf) 199: 407–423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL, Crandall CG. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 290: H1601–H1609, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol 96: 2103–2108, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Detry JM, Brengelmann GL, Rowell LB, Wyss C. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol 32: 506–511, 1972 [DOI] [PubMed] [Google Scholar]
- 8. Edholm OG, Fox RH, MacPherson RK. The effect of body heating on the circulation in skin and muscle. J Physiol 134: 612–619, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalez-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol 586: 45–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol 285: H333–H340, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, Oikonen V, Nuutila P, Knuuti J, Hellsten Y, Boushel R, Kalliokoski KK. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol 108: 378–386, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Heinonen I, Nesterov SV, Kemppainen J, Nuutila P, Knuuti J, Laitio R, Kjaer M, Boushel R, Kalliokoski KK. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J Appl Physiol 103: 2042–2048, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol 299: R72–R79, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Iida H, Kanno I, Miura S, Murakami M, Takahashi K, Uemura K. Error analysis of a quantitative cerebral blood flow measurement using H2(15)O autoradiography and positron emission tomography, with respect to the dispersion of the input function. J Cereb Blood Flow Metab 6: 536–545, 1986 [DOI] [PubMed] [Google Scholar]
- 15. Johnson JM, Brengelmann GL, Rowell LB. Interactions between local and reflex influences on human forearm skin blood flow. J Appl Physiol 41: 826–831, 1976 [DOI] [PubMed] [Google Scholar]
- 16. Katz SD, Zheng H. Peripheral limitations of maximal aerobic capacity in patients with chronic heart failure. J Nucl Cardiol 9: 215–225, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller DM, Sander M, Stallknecht B, Crandall CG. alpha-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol 588: 3799–3808, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massett MP, Lewis SJ, Bates JN, Kregel KC. Modulation of temperature-induced tone by vasoconstrictor agents. J Appl Physiol 86: 963–969, 1999 [DOI] [PubMed] [Google Scholar]
- 20. McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am 88, Suppl 3: 4–9, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Mustafa S, Thulesius O, Ismael HN. Hyperthermia-induced vasoconstriction of the carotid artery, a possible causative factor of heatstroke. J Appl Physiol 96: 1875–1878, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst 63: 61–67, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol 92: 2105–2113, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Pearson J, Low DA, Stohr E, Kalsi K, Ali L, Barker H, Gonzalez-Alonso J. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol 300: R663–R673, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roddie IC, Shepherd JT, Whelan RF. Evidence from venous oxygen saturation measurements that the increase in forearm blood flow during body heating is confined to the skin. J Physiol 134: 444–450, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974 [DOI] [PubMed] [Google Scholar]
- 28. Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress, edited by Rowell LB. Oxford Univ. Press, 1986, p. 193–212 [Google Scholar]
- 29. Rowell LB, Brengelmann GL, Blackmon JR, Murray JA. Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol 28: 415–420, 1970 [DOI] [PubMed] [Google Scholar]
- 30. Rowell LB, Brengelmann GL, Blackmon JR, Twiss RD, Kusumi F. Splanchnic blood flow and metabolism in heat-stressed man. J Appl Physiol 24: 475–484, 1968 [DOI] [PubMed] [Google Scholar]
- 31. Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969 [DOI] [PubMed] [Google Scholar]
- 32. Ruotsalainen U, Raitakari M, Nuutila P, Oikonen V, Sipila H, Teras M, Knuuti MJ, Bloomfield PM, Iida H. Quantitative blood flow measurement of skeletal muscle using oxygen-15-water and PET. J Nucl Med 38: 314–319, 1997 [PubMed] [Google Scholar]
- 33. Sipilä HT, Clark JC, Peltola O, Teräs M. An automatic [15O]H2O production system for heart and brain studies. J Labelled Compounds Radiopharm 44: S1066–S1068, 2001 [Google Scholar]
- 34. Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole body heating in humans. J Physiol 536: 615–623, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]