Abstract
Cardiac output measurement from arterial pressure waveforms presumes a defined relationship between the arterial pulse pressure (PP), vascular compliance (C), and resistance (R). Cardiac output estimates degrade if these assumptions are incorrect. We hypothesized that sepsis would differentially alter central and peripheral vasomotor tone, decoupling the usual pressure wave propagation from central to peripheral sites. We assessed arterial input impedance (Z), C, and R from central and peripheral arterial pressures, and aortic blood flow in an anesthetized porcine model (n = 19) of fluid resuscitated endotoxic shock induced by endotoxin infusion (7 μg·kg−1·h−1 increased to 14 and 20 μg·kg−1·h−1 every 10 min and stopped when mean arterial pressure <40 mmHg or SvO2 < 45%). Aortic, femoral, and radial artery pressures and aortic and radial artery flows were measured. Z was calculated by FFT of flow and pressure data. R and C were derived using a two-element Windkessel model. Arterial PP increased from aortic to femoral and radial sites. During stable endotoxemia with fluid resuscitation, aortic and radial blood flows returned to or exceeded baseline while mean arterial pressure remained similarly decreased at all three sites. However, aortic PP exceeded both femoral and radial arterial PP. Although Z, R, and C derived from aortic and radial pressure and aortic flow were similar during baseline, Z increases and C decreases when derived from aortic pressure whereas Z decreases and C increases when derived from radial pressure, while R decreased similarly with both pressure signals. This central-to-peripheral vascular tone decoupling, as quantified by the difference in calculated Z and C from aortic and radial artery pressure, may explain the decreasing precision of peripheral arterial pressure profile algorithms in assessing cardiac output in septic shock patients and suggests that different algorithms taking this vascular decoupling into account may be necessary to improve their precision in this patient population.
Keywords: hemodynamics, monitoring, pulse contour, animal model, sepsis
beat-to-beat estimates of left ventricular (LV) stroke volume and cardiac output from the arterial pressure waveform have been studied since the original work of Hamilton and Remington (12) over 60 years ago. Such measurements of cardiac output from the arterial pressure waveform presume a defined relationship between the arterial PP profile, C, and R, or, that if they change, they do so in a uniform fashion. Such cardiac output estimates degrade if these assumptions are incorrect. Recently, commercial devices have evolved to report LV stroke volume and cardiac output from the direct measure of peripheral arterial pressure via an indwelling arterial catheter (3, 5). These devices are presently being used to aid in clinical decision making. However, concern exists as to the accuracy of these devices under conditions wherein peripheral vasomotor tone changes. The arterial pulse is created by LV stroke volume into the central arterial compartment as quantified by a transfer function (12). If arterial elastance and compliance remain constant, then central arterial PP will vary directly with LV stroke volume. Unfortunately, most indwelling arterial catheters sample a more peripheral arterial pressure signal, whose waveform may be altered in unexpected ways as arterial tone, pulse wave velocity, and LV contractility vary. For this reason, it has been concluded that after a 1-h calibration, free period recalibration of these devices may be needed (13). Such repetitive recalibration may not be feasible in the clinical setting.
Continuous cardiac output measurement methodologies typically rely on a real-time analysis of the arterial waveform, with the assumption that changes in the waveform morphology can be related to corresponding changes in blood flow and central vasomotor tone. However, remarkable differences between aortic and peripheral arterial pressure have been reported in patients during the periods shortly after return from cardiopulmonary bypass (18, 22). This resulted in significant out layers in pulse contour cardiac output estimates (14). Also, in the setting of septic shock, profound changes in the vasculature tone occur that may not be uniform across the entire arterial bed. The impact of these vascular tone changes on the accuracy of various arterial pressure contour devices to assess LV stroke volume when vascular reflected waves and central compliance change in unexpected ways is unknown. The relation between blood pressure and flow seen in the central arterial compartment may become dissociated in the periphery under conditions of severe vasomotor shock because of the profound alterations in peripheral vessel compliance and elastance induced by sepsis.
Circulatory changes in vasomotor tone altering vascular resistance and compliance appear to uniformly occur among different cardiovascular stress states, like hemorrhage, hypertension, and heart failure and with the use of vasodilators and ACE inhibitors, because they all similarly modulate the adrenergic receptor response. Thus mathematical models like those used by Pulsion for PiCCOplus, LiDCO for LiDCOplus, and Edwards for FloTrac to calculate stroke volume from arterial pressure waveforms should remain stable in predicting flow from pressure across heart failure, hemorrhage, and their therapies whether pressure is measured at a central or peripheral location. What is not known but highly relevant for the assessment of cardiovascular status in the patients with pathological vasoregulatory disease, like sepsis and end-stage liver failure. Prior work has documented that patients with sepsis may have both pathological vasodilation, owing to the excess synthesis of endogenous vasodilators like nitric oxide, and decreased adrenergic responsiveness, presumably due to receptor downregulation, metabolic block, or competing vasoactive substance counter signaling (1, 2, 6, 9, 29). Accordingly, the accuracy of arterial pressure-derived flow devices can become inaccurate in these common clinical settings. To our knowledge, no analysis of the differential effect of sepsis or sepsis-like states on regional differences in vascular flow characteristics have been reported.
We hypothesized that when acute endotoxic shock in a porcine model resulted in cardiovascular collapse, this would be manifested by a change in vascular impedance characteristics that would decouple the normal pressure pulse propagation from central to peripheral vasculature, thus degrading the ability of peripheral arterial sensing pulse contour devices to estimate cardiac output. To address this issue, we assessed central and peripheral arterial pressure and aortic flow in an acute, anesthetized porcine model in which endotoxin shock was induced by the continuous infusion of low dose endotoxin followed by fluid resuscitation to restore cardiac output to pre-endotoxemic levels.
METHODS
The experimental protocol for this study was approved by the Ethics Committee for experimental animal research at Edwards Lifesciences.
Anesthesia and surgical preparation.
Nineteen female pigs (Yorkshire X) weighing between 75 and 107 kg (mean 89 ± 11 kg) were fasted overnight with free access to water. The animals were anesthetized with intramuscular injection of Telazol (4.4 mg/kg), Ketamine (2.2 mg/kg), and Xylazine (1.1 mg/kg). Neuromuscular blocking drugs were not used. The pigs were then orally intubated, and their lungs were mechanically ventilated (FiO2 0.4; positive end expiratory pressure 3 cmH20, Narkomed 2B, North American Dräger) with a tidal volume of 10 ml/kg at a respiratory rate 12–15 breaths/min, adjusted to maintain arterial Pco2 between 4.77 to 5.33 kPa. Arterial blood gases were monitored on a periodic basis, and acid-base balance was corrected by infusing bolus sodium bicarbonate or by changing ventilator frequency as needed. The other ventilator settings were kept unchanged throughout the experiment. The body temperature was maintained at 37 ± 1°C using blankets and a heating pad. A catheter was placed in the urinary bladder through a cystotomy for measurement of urinary output. Following induction of anesthesia and endotracheal intubation, general anesthesia was maintained with isoflurane (2%) for 15 min followed by a continuous intravenous infusion of proprofol (1%) at 10 ml·kg−1·h−1 in 12 pigs and by continuous intravenous infusion of penthotal (1%) at a rate of 15 ml·kg−1·h−1 in the remaining 7 pigs.
The left external jugular vein was cannulated and a pulmonary artery catheter (Swan-Ganz CCOmbo 777HF8, Edwards Lifesciences, Irvine, CA) was introduced and by pressure guidance positioned in the pulmonary artery for monitoring continuous cardiac output (CCO) and mixed venous saturation (SvO2). The positioning of the catheter was verified with fluoroscopy. A central venous catheter for drug and fluid infusion and for blood sampling was inserted into the superior vena cava. Ringers lactate solution (10 ml·kg−1·h−1) was used as maintenance fluid balance. The left and right femoral veins were used for additional drug and fluid infusion during the resuscitation phase of the experiment as described below.
The left and right radial arteries as well as one femoral artery were surgically exposed. In one radial artery and in the femoral artery, fluid-filled catheters were placed for continuous blood pressure recording. A 1- to 1.5-mm diameter perivascular transit-time ultrasonic flow probe (Transonic System, Ithaca, NY) was placed around the other radial artery. Radial flow was continuously recorded (T206 flow meter, Transonic System). A 5-French catheter was inserted using fluoroscopy through the carotid or the femoral artery into the aortic arch for continuous measurement of central aortic pressure. A midline sternotomy was performed, the pericardium separated, and a 20- to 24-mm diameter perivascular transit-time ultrasonic flow probe (Transonic System) was placed around the aortic root.
Measurements.
The arterial radial, femoral, and aortic catheters were connected to fluid-filled direct pressure transducers (PXMK 1063, Edwards Lifesciences, Irvine, CA) and radial, femoral, and aortic arterial pressures together with an electrocardiogram for heart rate (HR), central venous pressure (CVP), pulmonary artery pressure (PAP), and respiration were measured continuously using a bedside monitor (IntelliVue MP70, Phillips, Boeblingen, Germany). In one experiment, to determine if arterial pressure measures were accurately collected using a fluid-filled catheter, the exact study was repeated but using high fidelity pressure tip-mounted catheters (Mikro-tip, Millar Instruments, Houston, TX) to measure central aortic and radial arterial pressures. All other instrumentation was similar to that described above. A personal computer-based data acquisition system (DAQCardAI-16XE-50, National Instruments, Austin, TX) was connected to the analog outputs of both the bedside monitor and the T206 flow meter. All the signals (radial, femoral, and aortic pressures and radial and aortic flow signals) were digitized continuously at 500 Hz, and the data were saved to disc for offline analysis.
CCO and SvO2 were measured continuously using the Vigilance monitor (Edwards LifeSciences, Irvine CA). Bolus injection cardiac output (ICO) was measured intermittently every 2–3 h or as needed using the same monitor. The CCO, ICO, and SvO2 data were data logged to a laptop from the serial ports of the Vigilance monitor using data-logger software, and the data were saved on a hard drive.
Experimental protocol.
After surgery, the animals were allowed to recover for a period of 30 min during which time no animal displayed arrhythmias, hypotension, or on-going metabolic acidosis. This stabilization period served as baseline for subsequent comparisons. After the 30-min stabilization period, an intravenous infusion of endotoxin (LPS E-Coli 055:B5, Sigma, St. Louis, MO) was started at a rate of 7 μg·kg−1·h−1 and increased stepwise (7, 14, and 20 μg·kg−1·h−1) every 10 min to a final rate of 20 μg·kg−1·h−1. The endotoxin infusion was discontinued when MAP decreased below 40 mmHg or when SvO2 decreased below 45%. This usually occurred 2–3 h after the start of endotoxin infusion. Fluid resuscitation postdecompensation was performed with intravenous infusion of 0.9% sodium chloride or lactated Ringers solution and with 6%Dextran-70 at a rate of 20 mg·kg−1·h−1. Fluids were given until CO ceased to increase. This usually occurred when CO was between 8 and 18 l/min, with most of the animals reaching their CO plateau at 10 l/min. The total amount of fluids that the animals received ranged from 10 to 20 liters.
Data processing.
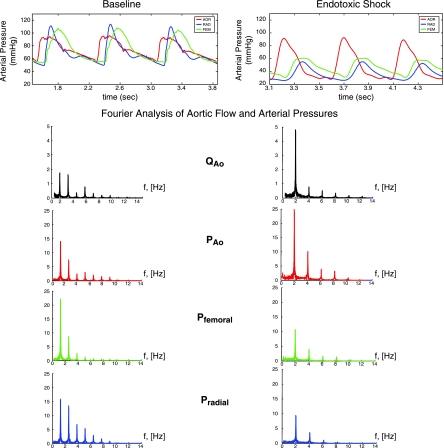
The aortic flow and aortic, femoral, and radial artery pressure data were analyzed in the frequency domain as Fourier series using FFT. A representative example of the pressure waveforms and Fourier analysis of the measured pressure and flow signals during baseline and endotoxemic conditions is shown in Fig. 1. The power spectrum was then computed and the impedance modulus at each harmonic was calculated as the ratio of the respective pressures and flow moduli at that harmonic. Input impedance (Z) was calculated using the mean impedance moduli for the 2nd to 8th harmonics (20):
| (1) |
where P(ω) is the power spectrum of the arterial pressure signal; Q(ω) is the power spectrum of the flow signal; Z(ω) is the arterial input impedance; f is the frequency; ω=2πf is the angular frequency.
Fig. 1.
An example of the Fourier analysis for one animal of central aortic flow (QAo) and the three measured arterial pressures: aortic pressure (PAo), femoral pressure (Pfemoral), and radial pressure (Pradial) for one animal under baseline (left) and endotoxic shock (right) conditions.
The peripheral resistance (R) was defined as the ratio of pressure to flow at 0 Hz term of the spectrum.
The arterial system was modeled with a two-element Windkessel (11). The two elements of this model are the constant arterial compliance (C), representing elastic property of the arterial tree, and a peripheral resistance (R), which drains the capacitor. The aortic flow curve (Q) together with one of the three pressure curves (P) was used as input of this model to characterize the parameters of the model.
The accompanying differential equation is:
| (2) |
In the frequency domain the corresponding input impedance is given by:
| (3) |
The reactive component Xc(ω) of the impedance of the Windkessel model was estimated based on the input impedance Z(ω) and the peripheral resistance estimated in the previous steps above:
| (4) |
Total arterial compliance, C, was then estimated by:
| (5) |
Theoretically, in the two-element Windkessel model, C should be the same throughout all harmonics. However, since the cardiovascular system cannot be simply modeled by a linear model and has also a transmission line of behavior an effect of reflected waves exists. So C is not consistent for all harmonics and could either increase or decrease slightly with the increase of the harmonic number. Thus the total C was computed as the average compliance from the 2nd to 8th harmonics. These harmonics were chosen so that this analysis would be consistent with previous analyses of impedance (8, 20).
Statistical analysis.
Group mean data ± SD were derived for all the P, Q, R, C, and Z parameters. Differences in parameters among animals and between control and endotoxic state within animals were compared by a three-way analysis of variance and a post hoc Student-Neuman-Keuls test. Differences corresponding to a P value <0.05 were considered significant.
RESULTS
All animals tolerated the surgical procedure well without any postoperative instability, arrhythmias, or ongoing intrathoracic bleeding. Data for the one animal with pressure reading using the Millar catheters were indistinguishable from those of the other 19 animals. Radial arterial flow signals were highly variable with ripples, reflections, and high frequency artifacts at baseline and in eight animals displayed no flow during endotoxemia making analysis of radial arterial flow from these data impossible. We attribute these changes to the surgical trauma associated with isolation of the arteries during the initial instrumentation. Thus radial arterial flow data are only reported for 11 of 19 animals and not used for any hemodynamic calculations. The hemodynamic variables during the control and 30 min post-resuscitation in the endotoxic state are summarized in Table 1 and the derived vascular Z, C, and R parameters for those times are summarized in Table 2. Figure 1 displays the regional pressure waveforms forms and Z for one animal for baseline and endotoxemic states. Figure 2 displays the time activity data for all measured variables for one animal and Fig. 3 displays its paired instantaneous calculated Z, R, and C values.
Table 1.
Hemodynamic data
| Baseline |
Endotoxemia |
ANOVA |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | Baseline to endotoxemia | |
| Pulse pressure | |||
| Aortic | 30.7 ± 6.2 | 44.2 ± 13.5 | P = 0.0001; F =23.7 |
| Femoral | 40.9 ± 7.0 | 27.3 ± 13.0 | P = 0.001; F =15.7 |
| Radial | 43.2 ± 9.0 | 33.8 ± 20.4 | P = 0.08; F =3.2 |
| Mean arterial pressure | |||
| Aortic | 75.4 ± 17.7 | 50.5 ± 16.0 | P < 0.0001; F =32.6 |
| Femoral | 76.4 ± 18.0 | 47.8 ± 17.4 | P < 0.0001; F =37.0 |
| Radial | 76.2 ± 16.9 | 47.0 ± 18.4 | P < 0.0001; F =40.4 |
| Systolic pressure | |||
| Aortic | 99.0 ± 21.2 | 80.3 ± 22.9 | P = 0.003; F =11.5 |
| Femoral | 106.6 ± 23.3 | 65.5 ± 25.0 | P < 0.0001; F =37.0 |
| Radial | 108.2 ± 20.4 | 69.1 ± 30.7 | P < 0.0001; F =33.1 |
| Diastolic pressure | |||
| Aortic | 68.2 ± 20.4 | 34.6 ± 12.3 | P < 0.0001; F =67.2 |
| Femoral | 66.3 ± 20.2 | 36.8 ± 10.9 | P < 0.0001; F =58.6 |
| Radial | 65.1 ± 20.5 | 35.4 ± 12.4 | P < 0.0001; F =40.8 |
| Heart rate | 85.4 ± 15.2 | 126.5 ± 23.2 | P < 0.0001; F =53.7 |
| Cardiac output | 6.7 ± 1.5 | 8.3 ± 3.6 | P = 0.08; F =3.4 |
n = 19. All pressures in mmHg, heart rate in beats/min, and cardiac output in l/min.
Table 2.
Derived arterial vascular parameters
| Baseline |
Endotoxemia |
ANOVA |
||
|---|---|---|---|---|
| Input Pressure | Mean ± SD | Mean ± SD | Baseline to endotoxemia | |
| Z | Aortic | 1.5 ± 0.6 | 2.7 ± 1.2 | P = 0.0003; F =23.5 |
| Radial | 1.3 ± 0.4 | 1.0 ± 0.6 | P = 0.03; F =5.8 | |
| R | Aortic | 10.7 ± 3.3 | 6.9 ± 3.5 | P = 0.004; F =11.9 |
| Radial | 10.4 ± 3.2 | 6.4 ± 3.3 | P = 0.004; F =11.8 | |
| C | Aortic | 34 ± 11 | 20 ± 18 | P = 0.02; F =5.8 |
| Radial | 30 ± 18 | 79 ± 64 | P = 0.01; F =8.9 |
n = 19. Z, input impedance (mmHg.min.l−1); R, resistance (mmHg·min·l−1); and C, compliance (ml/mmHg).
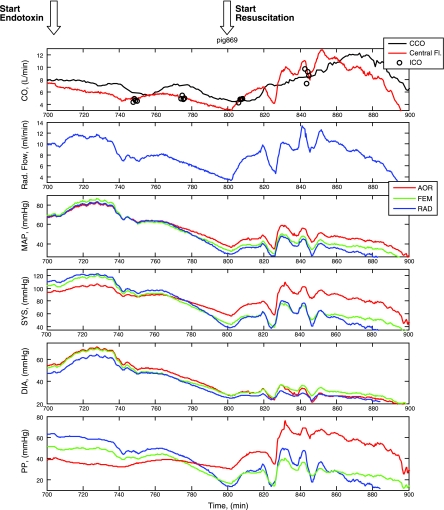
Fig. 2.
A time series display of all the measured hemodynamic variables for one animal from the initial postoperative baseline through endotoxin infusion (red vertical line and horizontal arrow) and resuscitation (green vertical line and horizontal arrow). AOR, aorta; FEM, femoral; RAD, radial; MAP, mean arterial pressure; SYS, systolic arterial pressure; DIA, diastolic arterial pressure; PP, pulse pressure. See results for discussion.
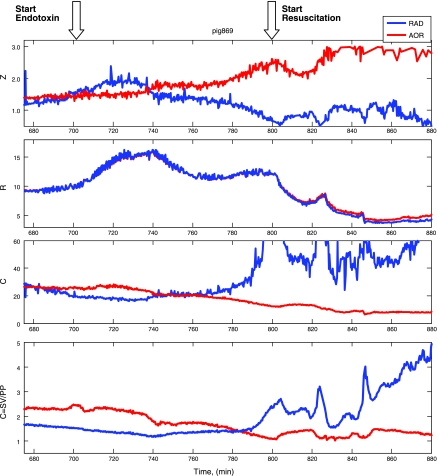
Fig. 3.
A time series display of all the calculated aortic and radial arterial calculated hemodynamic variables for the same animal as in Fig. 2 from the initial postoperative baseline through endotoxin infusion (red vertical line and arrow) and resuscitation (green vertical line and arrow). RAD, radial; Z, impedance (mmHg·min·l−1); R, resistance (mmHg·min·l−1); C, compliance (ml/mmHg). See results for discussion.
Three primary findings are described in Fig. 2 and Table 1. First, at baseline PP progressively increases from aortic to femoral to radial arterial sampling sites. Although mean arterial pressure (MAP) was not different, <1 mmHg, the increases in PP were large (10–15 mmHg). The observed increase in PP is due primarily to an increase in systolic arterial pressure. Second, following the start of the endotoxin infusion, arterial hypotension starts to develop after ∼20 min and is paralleled by a decrease in both aortic and radial arterial flow. Importantly, as MAP decreases aortic PP tends to remain constant, whereas both femoral and radial arterial PP progressively decrease and at some point either at the end of decompensation or early during fluid resuscitation aortic PP exceeds both femoral and radial arterial PP and remains so during resuscitation. The decrease in peripheral PP is due primarily to a greater decrease in systolic pressure in the peripheral sites as diastolic pressure tends to decrease to a similar amount at all sites. Third, during fully developed endotoxic shock following fluid resuscitation aortic and radial arterial blood flows return and usually exceed baseline values while all measures of MAP remain decreased, thus characterizing the hypotensive hyperdynamic septic shock state. Furthermore, during this fully developed hypotensive hyperdynamic state aortic PP markedly exceeds both femoral and radial arterial PP to levels similar to that seen in the opposite direction during control conditions. These dynamic trends shown for one animal in this example persisted across all animals (Table 1).
Analysis of Z, R, and C from aortic pressure and aortic flow and from radial artery pressure and aortic flow during the evolution of hypotensive hyperdynamic shock reveals striking time and loci-specific changes (Fig. 3, Table 2). During baseline Z and R are similar with both aortic and radial pressures as input of the model, whereas calculated C tends to be less with peripheral pressures as input, although not significantly so. However, with the induction of endotoxic shock, the calculated Z separates increasing with aorta pressure as input and decreasing with the radial arterial pressure as input. Since flow and mean arterial pressure are similar for both measurement sites R, although decreasing, remains common in both central and peripheral sites. Accordingly, endotoxemia induces a dissociation in calculated C from central and peripheral pressure, such that the central compartment behaves as if it is getting stiffer (less compliant), whereas the peripheral compartment becomes more compliant. These differences in Z and C with central and peripheral pressures become even more apparent following fluid resuscitation (Table 3).
Table 3.
Effect of endotoxemia on the difference between aortic and radial measured and derived variables
| Baseline Aortic-Radial |
Endotoxemia Aortic-Radial |
ANOVA |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | Baseline to endotoxemia | |
| Pulse pressure | −12.4 ± 5.0 | 10.4 ± 15.6 | P = 0.0001; F =26.1 |
| Mean arterial pressure | −0.8 ± 1.7 | 3.5 ± 4.1 | P = 0.0003; F =19.3 |
| Z | 0.1 ± 0.7 | 1.7 ± 1.4 | P = 0.0002; F =27.6 |
| R | 0.3 ± 0.7 | 0.4 ± 0.5 | P = 1.7; F =0.2 |
| C | 4.6 ± 14 | −60 ± 50 | P = 0.009; F =9.4 |
n = 19. Pressures in mmHg, Z, input impedance (mmHg·min.l−1); R, resistance (mmHg·min.l−1); C, compliance (ml·mmHg−1).
DISCUSSION
This study of acute porcine endotoxemia has two main findings. First, like other studies of acute endotoxic shock associated with fluid resuscitation previously described by us, we report the development of a hypotensive hyperdynamic state (23–25). Furthermore, although not shown, arterial blood gas analysis revealed the progressive onset of metabolic acidosis. Thus we believe our model of acute porcine endotoxic shock is representative of the previously described pathophysiology. It is within the differences between baseline and developed hyperdynamic hypotension that our analyses focus. Second, we describe the novel finding of a switch in the usual amplification of the arterial PP from central to peripheral vascular loci with the development of acute endotoxic shock. The etiology of this PP reversal appears to be due to a differential effect of acute endotoxemia on the central and peripheral derived vascular compliance. Presumably the endotoxin-induced expression of vascular endothelial inducible nitric oxide synthatase (iNOS) results in pathological peripheral vasodilation (31). If this were the case, why then do we only see peripheral loss of vascular stiffness despite a generalized decrease in ohmic resistance? Our study does not allow us to answer that question. However, since central aortic vessels have little smooth muscle relative to their vascular walls whereas peripheral vascular have much reactive smooth muscle architecture it is reasonable that they may be differentially affected by vasodilating substances during endotoxemic shock. Since stroke volume is constant across the arterial tree, if one could simultaneously measure both central and peripheral arterial PP, noting dynamic changes in their ratios would suggest changes in regional vascular compliance. Unfortunately, dual measures of central and peripheral PP are rarely available in clinical practice.
Technical limitations.
Extrapolation of these data to clinical studies should be done with caution because an acute animal preparation under general anesthesia and challenged with an endotoxin infusion for only 2–3 h is only a rough approximation of an awake critically ill patient in circulatory shock with varying levels of sympathetic tone and pre-existing cardiovascular abnormalities and associated exogenous vasopressors infusions. Still, this model has been widely used to assess the circulatory state of humans in septic shock, since pigs have a cardiovascular response similar to that of humans. Thus we predict that if similar measures of central and peripheral pressures and flow are made in patients in septic shock similar directional changes in peripheral vascular compliance and loss of PP amplification will be seen. Accordingly, to the extent that any acute animal model is useful in understanding human pathophysiology, these data should be relevant. In this study we used fluid-filled catheters to measure P and calculated Z. To the extent that overdamping or underdamping of these catheters occurs or their frequency response is inadequate to assess the pressure harmonics, our reported changes in Z may be suspect. But as noted in methods, in one animal we used high-fidelity pressure-tipped catheters to measure central aortic and peripheral P and found identical MAP, PP, and Z values as with the other animals. Thus these data can collectively be shown to reflect their purported changes in regional vascular tone.
We used fluid-filled catheters to measure arterial pressure instead of pressure-tipped catheters. The Edwards Direct Pressure Transducer has a flat frequency response up to 100 Hz. The pressure catheters used to measure redial, femoral, and aortic pressure were short (60 in.) and stiff. The natural frequency of the catheter-tubing-transducer units ranged from 42 to 45 Hz with the damping coefficient of 0.16, which was better than the frequency response previously reported (8, 20). This response is considered adequate, as most of the energy of the signal is contained in the first seven harmonics (20).
Another potential limitation was the use of colloid for fluid resuscitation with a mixture of crystalloid and colloid solutions that could alter blood viscosity. We specifically used a 3:1 volume ratio of crystalloid to colloid so as to minimize any change in blood viscosity due to fluid resuscitation. Although our method minimized changes in blood viscosity relative to pure crystalloid resuscitation, we did not measure blood viscosity in these studies. Still, our resuscitation parallels clinical practice, making these findings as consistent as possible to the clinical setting. Finally, decreases in arterial pressure during acute endotoxemia could independently alter central arterial compliance independent of endotoxin-induced changes in vasomotor tone owing to the curvilinearity of the central vascular compliance relation. But, if anything, systemic hypotension would underfill the aorta increasing its compliance, and we observed a marked decrease in central aortic compliance during endotoxemia. Thus the changes we report cannot be explained by differences in MAP.
In our data analysis we used a linear two-element Windkessel model, although arterial compliance has been reported non-linearly related to arterial pressure (16, 17, 26) and heart rate (15). However, the use of a linear compliance instead of a nonlinear compliance suffices in most Windkessel models (10). Therefore, we chose the more simple approach with a linear Windkessel model. Different methods exist to estimate arterial compliance. A recent review showed nine methods based in the Windkessel approach (34). The most important methods are the decay time method (11), the stroke volume over PP method (7), the area methods (17, 27), the parameter estimation method (32), and the impedance method in the frequency domain (8, 19, 21, 35). We used the generally accepted, robust, and well described impedance method. Furthermore, we used a two-element Windkessel model for the whole heart beat. This model is known for its good properties to describe the flow-pressure relation for the diastolic part of the heart beat. However, it has been shown that this model is inadequate to describe the flow-pressure relation during the systolic part of a heart beat (4). A more accurate model is a three-element Windkessel model, as has been used in model flow pulse contour (33). Still, using the three-element model although reporting cardiac output more accurately would not fundamentally change the conclusion that resistance remains similar but impedance and compliance differentially change with pressure measured at different loci during sepsis.
Clinical implications.
These data have important clinical relevance in the use of arterial waveform analyses to derive estimates of CO in critically ill patients. Presently, all commercially available devices that estimate cardiac output from the arterial PP do so based on a series of assumptions regarding aortic compliance and PP or pulse power transmission to the periphery. Although our study did not attempt to study the accuracy of any of these devices in this model, it is clear that our data reflect the potential degree of vascular compliance and impedance change that can rapidly occur during sepsis. Thus algorithm calibration based on healthy vasculatures without recalibration following significant nonhomogeneous vascular tone changes may display a reporting bias, either under or over estimating true CO. The degree of bias by each of the commercially available devices is unknown. Furthermore, devices that use only internal calibration approaches or one initial calibration and then follow CO trends over hours may be prone to systematic measurement errors due to regional changes in vascular reactivity.
There are two potential solutions to this calibration mismatch. First, as recommended by at least one manufacturer, if clinical conditions rapidly change, a recalibration is required. Recall that in our study the profound changes in impedance and compliance occurred only after the development of profound hypotension, not during the initial stages of changing pressures and flow. Thus the clinical signs of a significant vascular change should be similarly obvious and alert the bedside clinician to recalibrate the device. Second, once vascular decoupling occurred in our animal preparations, it remained remarkably constant up until the terminal event. Thus, if one could characterize the specific dynamic qualities of the decoupled vasculature, one could create a second algorithm to match the decoupled state and use it for the calculation of CO, thus minimizing the reporting bias. One would need to know, however, when the decoupled state occurred, its specific characteristics, and when it resolved for such a two-algorithm approach to be used effectively. It is unknown, however, if different patients existing in this decoupled vascular state would display similar enough flow characteristics to allow a single second algorithm to apply for all. But this two-algorithm approach would be a simpler solution to the vascular decoupling effect of estimating CO from arterial pressure recordings.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-67181.
DISCLOSURES
F. Hatib is an employee of Edwards LifeSciences that makes one of the arterial pulse countour devices that may be impacted by the results of this study. M. Pinsky is a consultant to Edwards LifeSciences, LiDCO Ltd., and Cheetah, all of which make minimally invasive or noninvasive cardiac output estimating devices that may be affected by the results of this study. J. Jansen has nothing to declare.
REFERENCES
- 1. Bernard GR, Reines HD, Halushka PV, Higgins SB, Metz CA, Swindell BB, Wright PE, Watts FL, Vrbanac JJ. Prostacyclin and thromboxane-A2 formation is increased in human sepsis syndrome—Effects of cyclooxygenase inhibition. Am Rev Respir Dis 144: 1095–1101, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Calandra T, Gerain J, Heumann D, Baumgartner JD, Glauser MP. High circulating levels of interleukin-6 in patients with septic shock: Evolution during sepsis, prognostic value, and interplay with other cytokines. Am J Med 91: 23–29, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Chaney JC, Derdak S. Minimally invasive hemodynamic monitoring for the intensivist: current and emerging technology. Crit Care Med 30: 2338–2345, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Cholley BP, Shroff SG, Sandelski J, Korcarz C, Balasia BA, Jain S, Berger DS, Murphy MB, Marcus RH, Lang RM. Differential effects of chronic oral antihypertensive therapies on systemic arterial circulation and ventricular energetics in African-American patients. Circulation 91: 1052–1062, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Della Rocca G, Cecconi M, Costa MG. Mini invasive hemodynamic monitoring: from arterial pressure to cardiac output. SIGNA VITAE 3: S7–S9, 2008 [Google Scholar]
- 6. Ertel W, Morrison MH, Wang P, Ba ZF, Ayala A, Chaudry IH. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann Surg 214: 141–148, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferguson JJ, Julius S, Randall OS. Stroke volume-pulse pressure relationships in borderline hypertension: a possible indicator of decreased arterial compliance. J Hypertens 2: S397–S399, 1984 [PubMed] [Google Scholar]
- 8. Finkelstein SM, Collins VR, Cohn JN. Arterial vascular compliance response to vasodilators by Fourier and pulse contour analysis. Hypertension 12: 380–387, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Foex BA, Quinn JV, Little RA, Shelly MP, Slotman GJ. Differences in eicosanoid and cytokine production between injury/hemorrhage and bacteremic shock in the pig. Shock 8: 276–283, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Fogliardi R, Di Donfrancesco M, Burattini R. Comparison of linear and nonlinear formulations of the three-element windkessel model. Am J Physiol Heart Circ Physiol 271: H2661–H2668, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Frank O. Die grunform des arterielen pulses erste abhandlung: mathematische analyse. Zeitschrift fuer Biologie 37: 483–526, 1899 [Google Scholar]
- 12. Hamilton WF, Remington W. The measurement of the stroke volume from the pressure pulse. Am J Physiol 148: 14–24, 1947 [DOI] [PubMed] [Google Scholar]
- 13. Hamzaoui O, Monnet X, Richard C, Osman D, Chemla D, Teboul JL. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med 36: 434–440, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212–222, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Kornet L, Jansen JR, Nijenhuis FC, Langewouters GJ, Versprille A. The compliance of the porcine pulmonary artery depends on pressure and heart rate. J Physiol 512: 917–926, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langewouters GJ, Wesseling KH, Goedhard WJ. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech 17: 425–435, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol Heart Circ Physiol 251: H588–H600, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Manecke GR, Jr, Parimucha M, Stratmann G, Wilson WC, Roth DM, Auger WR, Kerr KM, Jamieson SW, Kapelanski DP, Mitchell MM. Deep hypothermic circulatory arrest and the femoral-to-radial arterial pressure gradient. J Cardiothorac Vasc Anesth 18: 175–179, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Milnor WR. Hemodynamics. Baltimore, MD: Williams & Wilkins, 1989 [Google Scholar]
- 20. Nichols WW, Conti CR, Walker NE, Milnor WR. Input impedance of the systemic circulation in man. Circ Res 40: 451–458, 1977 [DOI] [PubMed] [Google Scholar]
- 21. Nichols WW, O'Rourke MF. MacDonald's Blood Flow in Arteries. New York: Hodder Arnold, 2005 [Google Scholar]
- 22. Pauca AL, Wallenhaupt SL, Kon ND. Reliability of the radial arterial pressure during anesthesia. Is wrist compression a possible diagnostic test? Chest 105: 69–75, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Pinsky MR. The cardiovascular response in canine endotoxic shock: Effect of ibuprofen pretreatment. Circ Shock 37: 323–332, 1992 [PubMed] [Google Scholar]
- 24. Pinsky MR, Matuschak GM. Cardiovascular determinants of the hemodynamic response to acute endotoxemia in the dog. J Crit Care 1: 18–31, 1986 [Google Scholar]
- 25. Pinsky MR, Roman A, Buurman W, Content J, Vincent JL. Effect of ibuprofen and diethylcarbamazine on the hemodynamic and inflammatory response to endotoxin in the dog. Eur Surg Res 32: 74–86, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Remington JW, Noback CR, Hamilton WF, Gold JJ. Volume elasticity characteristics of the human aorta and prediction of the stroke volume from the pressure pulse. Am J Physiol 153: 298–308, 1948 [DOI] [PubMed] [Google Scholar]
- 27. Sharp MK, Pantalos GM, Minich L, Tani LY, McGough EC, Hawkins JA. Aortic input impedance in infants and children. J Appl Physiol 88: 2227–2239, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Stergiopulos N, Meister JJ, Westerhof N. Simple and accurate way for estimating total and segmental arterial compliance: the pulse pressure method. Ann Biomed Eng 22: 392–297, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Suffredini AF. Endotoxin administration to humans: A model of inflammatory responses relevant to sepsis. In: Mediators of Sepsis edited by Lamy M, Thijs LG. Update Intens Care Emerg Med 16: 13–30, 1992 [Google Scholar]
- 30. Tanigawa K, Kellum KA, Bellomo R, Kim YM, Zar H, Lancaster JR, Pinsky MR, Ondulick B. Nitric oxide metabolism in canine sepsis: Relation to regional blood flow. J Crit Care 14: 186–190, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Toorop GP, Westerhof N, Elzinga G. Beat-to-beat estimation of peripheral resistance and arterial compliance during pressure transients. Am J Physiol Heart Circ Physiol 252: H1275–H1283, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput 47: 131–141, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Westerhof N, Stergiopulos N, Nobel MIM. Snapshots Hemodynamics An Aid For Clinical Research And Graduate Education. New York: Springer, 2005 [Google Scholar]