Abstract
Genetically modified mice with deficiency of the G protein α-subunit (Gsα) in skeletal muscle showed metabolic abnormality with reduced glucose tolerance, low muscle mass, and low contractile force, along with a fast-to-slow-fiber-type switch (Chen M, Feng HZ, Gupta D, Kelleher J, Dickerson KE, Wang J, Hunt D, Jou W, Gavrilova O, Jin JP, Weinstein LS. Am J Physiol Cell Physiol 296: C930–C940, 2009). Here we investigated a hypothesis that the switching to more slow fibers is an adaptive response with specific benefit. The results showed that, corresponding to the switch of myosin isoforms, the thin-filament regulatory proteins troponin T and troponin I both switched to their slow isoforms in the atrophic soleus muscle of 3-mo-old Gsα-deficient mice. This fiber-type switch involving coordinated changes of both thick- and thin-myofilament proteins progressed in the Gsα-deficient soleus muscles of 18- to 24-mo-old mice, as reflected by the expression of solely slow isoforms of myosin and troponin. Compared with age-matched controls, Gsα-deficient soleus muscles with higher proportion of slow fibers exhibited slower contractile and relaxation kinetics and lower developed force, but significantly increased resistance to fatigue, followed by a better recovery. Gsα-deficient soleus muscles of neonatal and 3-wk-old mice did not show the increase in slow fibers. Therefore, the fast-to-slow-fiber-type switch in Gsα deficiency at older ages was likely an adaptive response. The benefit of higher fatigue resistance in adaption to metabolic deficiency and aging provides a mechanism to sustain skeletal muscle function in diabetic patients and elderly individuals.
Keywords: Gsα deficiency, aging, skeletal muscle adaptation, myosin and troponin isoforms
skeletal muscles are composed of slow and fast types of muscle fibers with different contractile and metabolic properties. The slow-twitch type I muscle fibers are rich in mitochondria and possess high oxidative capacity and are resistant to fatigue, whereas the fast-twitch type II muscle fibers have high glycolytic metabolism and fatigue more easily. Skeletal muscle is also an important tissue in glucose and energy metabolism due to its large requirement for nutrients and function as a major site of acute disposal of glucose load. Disruption of glucose uptake in skeletal muscle due to deletion of glucose transporter-4 leads to glucose intolerance and insulin resistance (33). Gsα is a ubiquitously expressed G protein α-subunit that couples receptors to adenylyl cyclase and is required for receptor-mediated intracellular cAMP generation. Genetically modified mice with Gsα deficiency in skeletal muscle (MGsKO) showed reduced glucose tolerance, low muscle mass, and decreased contractile force, along with a fast-to-slow-fiber-type switch (9).
Fiber-type switches indicate an adaptation of skeletal muscle to functional and metabolic demands. In Type 2 diabetic patients, the number of slow oxidative fibers is reduced, whereas the number of fast glycolytic fibers increased (35, 36). This replacement of oxidative fibers by glycolytic fibers is also found in obese patients (41). The muscle fiber-type switching toward low oxidative capacity may contribute to the pathogenesis of metabolic disorders, since it was found to precede the development of obesity and diabetes in animals (38). Exercise results in a shift in changes in the type II fibers from type IIb to IIx/a (10). Endurance training, on the other hand, has been shown to be beneficial in diabetic patients by improving muscle quality and increased cross-sectional area of both type I and type II fibers (6).
Progressive losses of locomotor's function and muscle mass occur in aging. Sarcopenia, the age-related loss of muscle mass, leads to declined muscle strength with reduction of fiber size and number (27). Histological studies of human muscle biopsies reported an increased ratio of type I to type II fibers in aging (26). Considering the established function of type I slow-muscle fibers in the resistance to fatigue (31, 44, 46), maintenance of slow type of fibers would have a benefit to the quality of life of the elderly, with reduced muscle mass in age-associated sarcopenia, as well as diabetic and obese patients.
In the present study, we investigated the hypothesis that the switching to more slow fibers is an adaptive response with functional benefits. Studies of MGsKO mouse soleus muscle showed that, corresponding to the switch of myosin isoforms, the thin-filament regulatory proteins troponin T (TnT) and troponin I (TnI) also had significant changes to their slow isoforms. This fiber-type switching involving both thick- and thin-myofilament protein progressed in the soleus muscles of aging MGsKO mice to express solely slow isoforms of myosin and troponin. Functional characterization found slower contractile and relaxation kinetics and lower force production, but increased fatigue resistance, followed by better recovery in MGsKO soleus muscle, correlating to the higher slow-fiber contents. Since the fiber-type switching did not start in the muscles of neonatal and 3-wk-old MGsKO mice, the fast-to-slow-fiber-type switch appears to be an adaptation in metabolic abnormality and in aging, providing a beneficial mechanism to sustain skeletal muscle function by improving fatigue resistance.
MATERIALS AND METHODS
MGsKO mice.
As described previously (9, 11), mice with floxed Gsα exon 1 allele (E1fl) (7) were bred with transgenic mice bearing a muscle creatine kinase (MCK)-cre allele (Taconic, Hudson, NY) to induce striated muscle-specific disruption of the Gsα gene (MCK-cre, E1fl/fl; MGsKO). Genotyping of wild-type (E1+) and E1fl alleles was performed by polymerase chain reaction using primers flanking the downstream loxP site of the E1fl allele (8). The presence or absence of the MCK-cre transgene was determined by polymerase chain reaction using cre-specific primers (8). The E1fl allele has no effect on Gsα expression or phenotype (8), and, therefore, MCK-cre− or E1+/+ littermates were used as controls. Animals were maintained on a 12:12-h light-dark cycle (6:00 AM/6:00 PM) and standard pellet diet. The experimental protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the Guiding Principles in the Care and Use of Animals, as approved by the Council of the American Physiological Society.
Contractility measurement in isolated mouse soleus muscle.
Contractile function of soleus muscle was analyzed using a protocol modified from our laboratory's previous studies (12) in female control and MGsKO mice at 3 mo (n = 3 each) and 18–24 mo (n = 6 and 7, respectively) of age. The mice were anesthetized with pentobarbital sodium (0.1 mg/g body wt intraperitoneally). Intact soleus muscles were isolated from tendon to tendon, with care to avoid stretch or tissue damage. The muscle was mounted vertically to a dual-mode lever arm force transducer (model 300B, Aurora Scientific) by tying the tendons with no. 3–0 sutures in an organ bath containing 100 ml modified Kreb's solution (118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4, 2.25 mM MgSO4, 2.25 mM CaCl2, and 11 mM d-glucose, continuously gassed with 95% O2-5% CO2, pH 7.4). Contractions were elicited with bipolar pulse electrical stimulation via platinum plate electrodes (1 × 5 cm), positioned 0.75 cm apart flanking the muscle strip using a stimulator (model 701B, Aurora Scientific).
Twitch contractions were elicited with supramaximal pulses (0.1 ms, 28 V/cm), unless specified otherwise. Tetanic contractions were elicited with a train of the same pulses at 120 Hz for 0.7 s. Isometric force data were collected via a digital controller A/D interface (model 604C, Aurora Scientific) and recorded using Chart software (ASI, Aurora Scientific). The assays were carried out at 25°C. Developed twitch and tetanic forces were determined at the optimal muscle length that gave the highest twitch force and calculated as the difference between the maximum contractile force and the baseline tension that was constant throughout all experiments. After 20-min equilibration with one tetanic contraction per minute, a 300-s fatigue protocol was applied with intermittent tetani of 700 ms every second.
SDS-polyacrylamide gel electrophoresis and Western blot analysis.
Total protein extracts were made by homogenizing MGsKO and control mouse soleus, extensor digitorum longus (EDL) and diaphragm muscle tissues in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer containing 2% SDS using a high-speed mechanical homogenizer. After heating at 80°C for 5 min, the samples were clarified by centrifugation and resolved on 14% Laemmli gels with an acrylamide-to-bis-acrylamide ratio of 180:1 and visualized using Coomassie blue staining or transferred to nitrocellulose membranes using a Bio-Rad semidry electrotransfer apparatus for Western blot analysis with anti-TnI (TnI-1) and anti-TnT (CT3 and T12) monoclonal antibodies (MAbs) (19, 30). As described previously (12), the MAbs were diluted in Tris-buffered saline (TBS) containing 0.1% bovine serum albumin (BSA) and incubated with the nitrocellulose membranes at 4°C overnight. After high-stringency washes with TBS containing 0.5% Triton X-100 and 0.05% SDS, the membranes were incubated with alkaline phosphatase-labeled goat anti-mouse IgG second antibody (Sigma) in TBS-BSA and washed as above. The blots were then developed in 5-bromo-4-chloro-3-indolylphosphate nitro blue tetrazolium substrate solution to reveal the protein bands recognized by the anti-TnI or anti-TnT MAb. All muscle tissues used in the contractility studies were recovered for examination by Western blotting to verify the expression of fiber-type-specific TnT and TnI isoforms.
Glycerol-SDS-PAGE analysis of myosin heavy chain isoforms.
Muscle tissues were examined for myosin heavy chain (MHC) isoforms by glycerol-SDS-PAGE (12). Briefly, the SDS-PAGE samples were resolved on 8% polyacrylamide gel with acrylamide-to-bis-acrylamide ratio of 50:1, prepared in 200 mM Tris base, 100 mM glycine (pH 8.8), containing 0.4% SDS and 30% glycerol. The stacking gel contained 4% polyacrylamide with acrylamide-to-bis-acrylamide ratio of 50:1, 70 mM Tris·HCl (pH 6.7), 4 mM EDTA, 0.4% SDS, and 30% glycerol. The upper cathode running buffer was composed of 100 mM Tris base, 150 mM glycine, 0.1% SDS, and 0.01 mM 2-mercaptoethanol. The lower anode running buffer was 50% dilution of the upper running buffer without 2-mercaptoethanol. The 0.75-mm-thick Bio-Rad minigels were run at 100 V in an icebox for 24 h. The resolved protein bands were visualized with Coomassie blue staining.
Immunohistochemistry.
Immunohistochemical examination of mouse soleus muscles was performed as described (12). The isolated muscle tissues were mounted between two pins to keep the original slack length in a small drop of optimum cutting temperature compound (Sakura, Tissue-Tek) and rapidly frozen in isopentane at −159°C. The frozen muscle tissue was then embedded in optimum cutting temperature compound before cryo-sectioning. Thin cross sections of the soleus muscles were fixed in 75% acetone/25% ethanol for 5 min. After being blocked in phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) and 1% BSA for 30 min, the sections were incubated with 1% H2O2 in PBS for 10 min to inactivate endogenous peroxidases. The sections were then washed with PBS-T three times and incubated with anti-MHC I MAb FA2 or SP2/0 myeloma culture supernatant at 4°C overnight. After being washed with PBS-T to remove unbound MAb, the sections were incubated with horseradish peroxidase-labeled anti-mouse second antibody at room temperature for 1 h, washed again, and developed in 3,3′-diaminobenzidine-H2O2 substrate solution in a dark box for 30–60 s. The substrate reaction was stopped by washes in 20 mM Tris·HCl, pH 7.6, for six changes. After counterstaining with hematoxylin for 5 min and washing with water, the sections were immersed in a drop of 50% glycerol in PBS and mounted with Cytoseal (Thermo Scientific). The slides were examined using a Zeiss Observer microscope and photographed.
Data analysis.
Densitometry analysis of SDS gels and Western blots was performed on images scanned at 600 dots/in. resolution using National Institutes of Health Image 1.61 software. Quantitative data were documented as means ± SE. Statistical significance of differences between the mean values was analyzed using two-tail unpaired Student's t-test, unless specified in the figure legends.
RESULTS
Fast-to-slow switch of TnI and TnT isoforms in soleus muscle of MGsKO mice corresponding to the switch from type II to type I fibers.
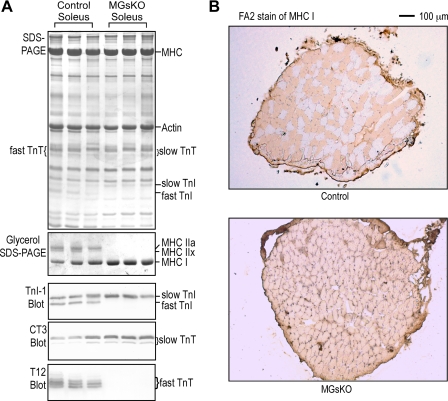
Mouse soleus muscle is a slow-type muscle containing a large proportion of type I slow fibers mixed with significant amount of type IIa fibers. Corresponding to the previously reported switch to a higher level of type I isoform of MHC in MGsKO mouse soleus muscle with increased slow-fiber contents (9), the expression of TnI and TnT also switched to more slow isoforms in 3-mo-old MGsKO mouse soleus muscle compared with that in age-matched controls (Fig. 1). The concurring isoform switch of both thick- and thin-filament proteins (i.e., myosin and troponin) indicated a coordinated fast-to-slow-fiber-type switch in MGsKO mouse soleus muscle.
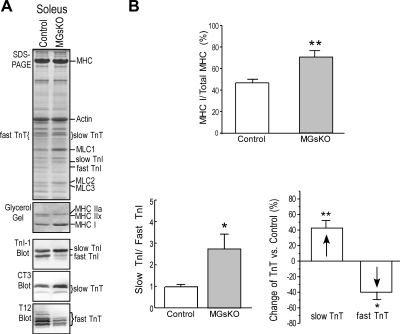
Fig. 1.
Fast-to-slow isoform switches of both thick- and thin-filament proteins in soleus muscle of 3-mo-old genetically modified mice with Gsα deficiency in skeletal muscle (MGsKO). A: glycerol-SDS gel was employed to identify myosin heavy chain (MHC) isoforms, and regular SDS-PAGE/Western blots using anti-troponin I (TnI) monoclonal antibody (MAb) TnI-1, anti-slow and cardiac troponin T (TnT) MAb CT3, and anti-fast TnT MAb T12 were used to identify troponin isoforms. The results showed increases in MHC I, slow TnI, and slow TnT, accompanied by decreases in MHC IIx, fast TnI, and fast TnT in MGsKO soleus muscle compared with age-matched controls. MLC, myosin light chain. B: the fast-to-slow isoform changes in thick- and thin-filament proteins were quantified using densitometry analysis. Values are means ± SE; n = 5 mice each in control and MGsKO groups. *P < 0.05, **P < 0.01 vs. control in Student's t-test.
Minimum phenotype changes in EDL muscles of 3-mo-old MGsKO mice.
The fast-twitch muscle EDL of 3-mo-old MGsKO mice had normal mass, and the myofilament protein contents indicated an unaffected pure fast-fiber type (Fig. 2). No significant change of overall protein profile was found in SDS-PAGE (Fig. 2A). No type I MHC was detected by glycerol-SDS gel. While the major MHC isoform remained to be IIb, there was a detectable increase in MHC IIx in 3-mo-old MGsKO EDL muscle compared with the controls. TnI and TnT also maintained predominantly in fast isoforms, although slow TnI and slow TnT became barely detectable in the 3-mo-old MGsKO EDL muscle (Fig. 2A).
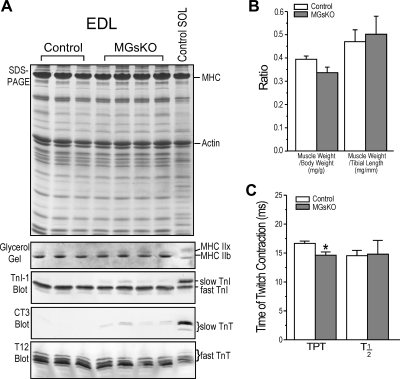
Fig. 2.
Minimum phenotypic change in extensor digitorum longus (EDL) muscle of 3-mo-old MGsKO mice. A: SDS-PAGE, glycerol SDS gel, and Western blots showed that EDL, a normally fast-fiber muscle, of 3-mo-old MGsKO mice had slightly increased MHC IIx and barely detectable slow TnI and slow TnT, in contrast to solely fast myofilament protein isoforms in age-matched controls. SOL, soleus muscle. B: the ratio of EDL muscle weight to body weight or to tibial length showed no statistically significant change in MGsKO vs. control groups (P ≥ 0.097). C: twitch contraction showed shorter time to develop peak tension (TPT) in 3-mo-old MGsKO mouse EDL muscle compared with the control, but no difference in the time from maximum twitch force to half-relaxation, T1/2. Values are means ± SE; n = 4 mice in control and n = 5 mice in MGsKO groups. *P < 0.05 vs. control in Student's t-test.
There was a trend of decreased EDL muscle weight-to-body weight ratio in 3-mo-old MGsKO mice, but no statistical significance was established (Fig. 2B). Tibial length was measured to evaluate the body size and overall growth of the mice. The results showed no difference between MGsKO and control mice (18.9 ± 0.1 vs. 18.7 ± 0.2 mm). When the muscle weight was normalized by tibial length to obtain a muscle cross-sectional area index, no atrophic trend was found in the MGsKO EDL muscle (Fig. 2B). The slight fast-to-slow-fiber-type switch did not have direct functional effect on the MGsKO EDL muscle, as the contractile and relaxation time was not elongated (Fig. 2C).
Higher fatigue resistance of 3-mo-old MGsKO mouse soleus muscle.
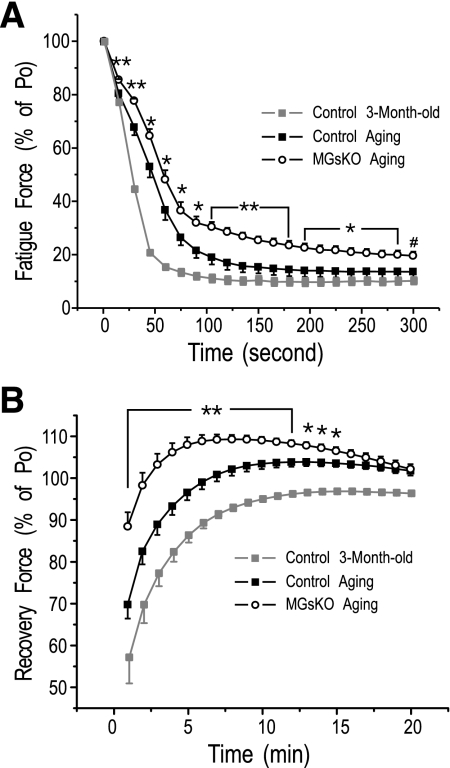
The 300-s intermittent fatigue protocol with 70% duty cycle (700-ms tetanic contraction in each 1,000-ms cycle) showed that MGsKO soleus muscle had less decrease in contractile force from the prefatigue maximum tension than that in the control group (Fig. 3A). During the recovery period, tetanic contractile force returned faster and to a higher level in MGsKO soleus muscle than the controls (Fig. 3B). These results indicated higher fatigue resistance in MGsKO soleus muscle, corresponding to increased slow fibers, which suggests a beneficial adaption.
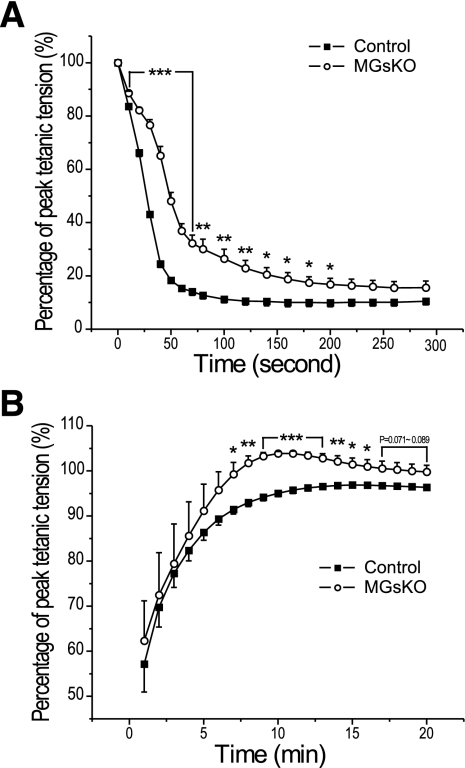
Fig. 3.
Better fatigue resistance and recovery of MGsKO mouse soleus muscle. A: normalized to prefatigue developed tension, the fatigue treatment produced less decrease of force in MGsKO soleus muscle than that in the control group. The absolute forces normalized to muscle mass were 25.42 ± 1.69 mN/mg for the MGsKO and 29.70 ± 0.69 mN/mg for the control groups (9). B: MGsKO soleus muscle recovered faster and to a higher level than the controls in the first 15 min postfatigue treatment. Values are means ± SE; n = 5 mice in control and n = 6 mice in MGsKO groups. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control in Student's t-test.
Normal expression of myofilament protein isoforms in soleus muscle of infantile MGsKO mice.
To evaluate whether the fiber-type switch was a direct consequence of the defect in Gsα signaling in muscle cells or a secondary response to the primary changes, such as muscle atrophy and weakness, MGsKO and control mouse soleus muscles were examined on postnatal days 1, 7, and 21 for the expression of fast- and slow-fiber-specific myofilament protein isoforms. The results in Fig. 4 demonstrated similar patterns of MHC, TnI, and TnT isoform expression during the early postnatal development of MGsKO and control soleus muscles. Normal developmental changes were observed in both groups, including downregulation of embryonic MHC and MHC IIx, appearance of MHC IIa, and upregulation of MHC I. Slow TnI and slow TnT were upregulated, whereas fast TnI and fast TnT downregulated in soleus muscle during postnatal development. Fast TnT also switched to have more of the alternatively spliced low-molecular-weight variants (43) (Fig. 4). Together with our laboratory's previous finding of no difference between the fiber types in soleus and gastrocnemius muscles of 3.5-wk-old MGsKO and control mice (9), the results suggest that Gsα deficiency did not change muscle fiber-type de novo. Our laboratory previously showed that soleus muscle of 3-mo-old MGsKO mice had decreased mass, as measured by the ratio of muscle weight to body weight and muscle cross-sectional area index derived from the muscle weight vs. tibial length (9). Therefore, the fast-to-slow-fiber-type switch in MGsKO soleus muscle could be an adaptive response to low muscle mass, corresponding to less total contractile force.
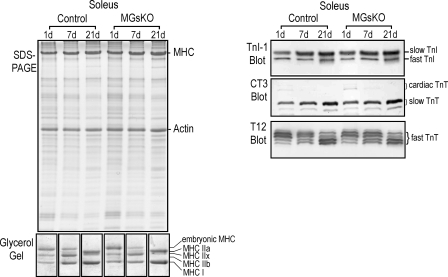
Fig. 4.
Normal expression of myofilament protein isoforms in soleus muscle of infantile MGsKO mice. SDS-PAGE, glycerol-SDS gel, and Western blots showed that days 1, 7, and 21 postnatal MGsKO and control mouse soleus muscles had similar expression and developmental patterns of MHC, TnI, and TnT isoforms. n = 3 mice per time point in each group.
Soleus muscle of aging MGsKO mice switched to contain solely slow fibers.
Since fast-to-slow-fiber-type switch is also a natural process in aging muscles, we further studied fiber-type-specific myofilament protein isoforms and contractile function of muscles from control and MGsKO mice at 1.5–2 yr of age.
Soleus muscles of aging control mice exhibited a switch to express more slow-type isoforms of myofilament proteins, MHC, TnI, and TnT than that in the 3-mo-old control muscles (Fig. 5A and Fig. 1A). This change was much more advanced in aging MGsKO soleus muscles that became expressing solely slow-type MHC I, slow TnI, and slow TnT (Fig. 5A). Consistently, immunohistochemistry using anti-MHC I MAb showed 100% type I slow fibers in the aging MGsKO soleus muscle (Fig. 5B), confirming the switch to purely slow fibers.
Fig. 5.
Aging MGsKO mice contained pure slow fibers in soleus muscle. A: SDS-PAGE, glycerol-SDS gel, and Western blots showed that soleus muscles of 18- to 24-mo-old MGsKO mice expressed solely MHC I, slow TnI, and slow TnT. Aging also produced switches to more slow isoforms of myofilament proteins in control soleus muscle compared with that in the 3-mo-old control group (Fig. 1A). B: immunohistochemistry using MAb FA2 against MHC I showed 100% type I fibers in the soleus muscle of aging MGsKO mice.
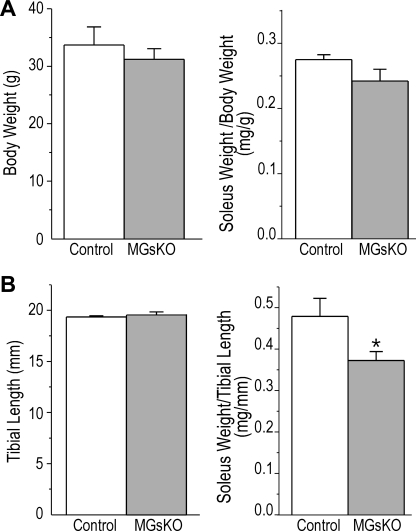
The soleus muscle weight normalized to body weight did not show statistical difference between aging MGsKO and age-matched controls, likely due to the trend of lower body weight in the MGsKO group (Fig. 6A). However, the muscle cross-sectional area index suggested atrophy of MGsKO soleus muscle in aging mice compared with age-matched controls (Fig. 6B).
Fig. 6.
Decrease in soleus muscle mass of aging MGsKO mice. A: aging (18- to 24-mo-old) MGsKO mice had a trend of lower body weight than age-matched controls, and the weight of MGsKO and control soleus muscles normalized to body weight showed no statistical difference. B: no difference was found in tibial length (an indicator of the in vivo muscle length) between MGsKO and control mice at 18–24 mo of age. Muscle cross-sectional area index calculated as the ratio of soleus muscle weight vs. tibial length detected a lower soleus muscle mass in aging MGsKO mice vs. the control with statistical significance (*P < 0.05 vs. control in Student's t-test). Values are means ± SE; n = 6 mice for control and n = 7 mice for MGsKO groups.
Aging MGsKO mouse soleus muscle had decreased contractile force and kinetics, but had higher resistance to fatigue.
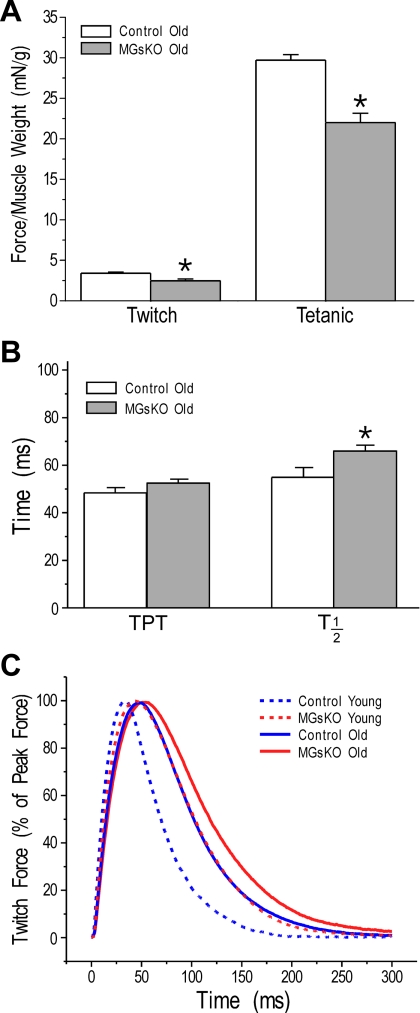
Twitch and tetanic forces normalized to muscle weight were lower in soleus muscle of aging MGsKO mice than that of age-matched controls (Fig. 7A). The time parameters of twitch contractions were longer in aging MGsKO soleus muscle than that in age-matched controls (Fig. 7B). Compared with the 3-mo-old groups, the twitch kinetics of aging MGsKO and control soleus muscles are correlated to the degrees of fast-to-slow switch in muscle fiber types (Fig. 7C).
Fig. 7.
Lower force and contractile kinetics of aging MGsKO mouse soleus muscle. A: twitch and tetanic forces normalized to muscle weight were lower in aging MGsKO soleus muscle compared with age-matched controls. B and C: compared with age-matched controls, kinetics of twitch contraction were also lower in aging MGsKO mouse soleus muscle, as shown by the elongated time parameters (TPT and T1/2). Values are means ± SE; n = 6 mice for control and n = 7 mice for MGsKO groups. *P < 0.05 vs. controls in Student's t-test.
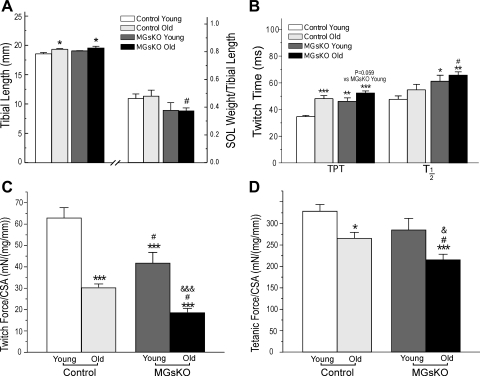
The effects of aging on the loss of muscle mass and on the fast-to-slow-muscle-type switch were further compared between control and MGsKO mouse soleus muscles. As shown previously, MGsKO mice had severely decreased soleus muscle mass at 3 mo of age compared with age-matched controls (9). The tibial length did not change between 3 and 18–24 mo of age (Fig. 8A). Normalized to tibial length, aging produced a significant loss of mass in control soleus muscles (Fig. 8A). The normalized mass of MGsKO soleus muscle at 18–24 mo of age did not further decrease from the level of 3-mo-old MGsKO soleus muscle and was similar to that of age-matched controls (Fig. 8A).
Fig. 8.
Soleus muscle of aging MGsKO mice had compensated mass but slower contraction and lower developed force. A: 3-mo-old and aging control and MGsKO mice showed similar tibial length that was used to normalize the weight of soleus muscle to show an aging-related decrease in soleus muscle mass. While 3-mo-old MGsKO soleus muscle showed significantly lower mass compared with young controls, no further decrease was found in MGsKO soleus muscle during aging. B: twitch contractile time parameters (TPT, T1/2) were elongated in aging control and MGsKO soleus muscles. Twitch (C) and tetanic (D) forces normalized to muscle weight were lower in aging control and MGsKO soleus muscles. Although the aging MGsKO soleus muscles did not show aging-related decrease in mass, their normalized force was lower than that of age-matched control. CSA, cross-sectional area. Values are means ± SE; n = 5 mice each for young control and young MGsKO groups, n = 6 mice for aging control groups, and n = 7 mice for aging MGsKO group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. young controls. #P < 0.05 vs. aging controls in Student's t-test. &P < 0.05, &&&P < 0.001 vs. young MGsKO mice in one-way ANOVA Fisher test.
In the mean time, the switch to more type I slow fibers in aging soleus muscle (Fig. 5) elongated the duration of twitch contraction and relaxation in both control and MGsKO groups compared with the 3-mo-old groups (Fig. 8B). This is consistent with the previous observation that MHC II produced higher contractile velocity than that of MHC I (18). Our results showed that soleus muscles of aging MGsKO and control mice both produced lower twitch (Fig. 8C) and tetanic (Fig. 8D) forces than that of the 3-mo-old groups, which may have resulted from other factors other than the change of myosin isoenzymes. Since the tibial lengths that determine the in situ muscle lengths are similar in the control and MGsKO groups, force normalization to muscle mass should yield the same results as that of normalization to muscle cross-sectional area calculated from muscle weight vs. muscle length.
Supporting the hypothesis that the enhanced fast-to-slow-fiber-type switch in soleus muscle of aging MGsKO mice may have a benefit by functionally compensating for the decreased force production, aging MGsKO soleus muscles exhibited higher resistance to fatigue with better recovery than that of age-matched controls (Fig. 9). Corresponding to the naturally occurring fast-to-slow-fiber-type switch in aging soleus muscles, the controls also showed higher fatigue resistance and recovery in the aging group than that in the 3-mo-old group (Fig. 9).
Fig. 9.
Increased fatigue resistance of aging mouse soleus muscle enhanced in MGsKO. Control aging mouse soleus muscles showed increased fatigue resistance (A) and improved recovery (B) compared with that of 3-mo-old controls. Compared with age-matched controls, aging MGsKO soleus muscles exhibited further higher fatigue resistance (A) and better recovery (B). Values are means ± SE; n = 6 mice for control and n = 7 mice for MGsKO groups. *P < 0.05, **P < 0.01 vs. aging controls in two-tail Student's t-test. #P < 0.05 vs. aging controls in one-tail Student's t-test.
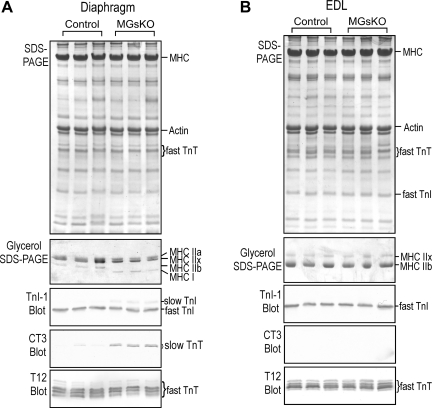
Fast-to-slow-fiber-type switch in diaphragm but not EDL muscle of aging MGsKO mice.
Slow fibers play critical functions in slow- and mixed-fiber-type skeletal muscles. Therefore, we also examined diaphragm as a representative of mixed-fiber muscle. In contrast to the prominent fiber-type switch in aging soleus muscles (Fig. 5), the aging diaphragm muscle of control mice expressed only minimal amounts of slow isoforms of myofilament proteins (Fig. 10A). However, slow isoforms of MHC, TnI, and TnT were clearly detectable in aging MGsKO diaphragm muscle (Fig. 10A). For the continuous contractile activity of the diaphragm muscle in sustaining respiration, the increase in slow-fiber contents in MGsKO diaphragm may as well be a beneficial adaptation to increase the resistance to fatigue against the reduction of muscle mass and power. In contrast, no slow-type myofilament protein isoforms were detectable in aging control or MGsKO EDL muscles (Fig. 10B).
Fig. 10.
Fast-to-slow-fiber-type switch in diaphragm containing mixed types of fibers, but not in EDL muscle containing only fast fibers of aging MGsKO mice. A: SDS-PAGE, glycerol-SDS gel, and Western blots showed that aging MGsKO, but not control, diaphragm muscle had increased MHC I, slow TnI, and slow TnT, with diminished MHC IIb, and decreases in fast TnI and fast TnT. B: no slow-type myofilament protein isoform was detected in EDL muscles of aging control or MGsKO mice.
DISCUSSION
The MGsKO mouse model provided an experimental system to investigate pathophysiological adaptation of skeletal muscle and functional significance. Our studies of the fast-to-slow-fiber-type switch in MGsKO muscle and the beneficial effect on improving fatigue resistance had the following findings for the role of slow fibers in skeletal muscle function.
Fast-to-slow-fiber-type switch in soleus muscle improves fatigue resistance.
Fatigue resistance is a crucial feature of weight-bearing skeletal muscles, such as the soleus. This function is especially important in the elderly and other individuals with skeletal muscle weaknesses (37). Our laboratory's previous studies showed slow-to-fast-fiber-type switches in rat soleus muscle treated with hindlimb unloading (14) and in genetically modified mouse diaphragm muscle in which the expression of slow skeletal muscle TnT was knocked down (12). Both models resulted in decreased fatigue resistance. Further supporting the critical role of slow fibers in weight bearing and respiratory functions, the loss of slow fibers was found in a human nemaline myopathy with lethal respiratory failure (20, 21).
Our present study demonstrated a unique example in which the deficiency of Gsα, the predominant Gα isoform coupled to β-adrenoceptors in skeletal muscles, caused low muscle mass and a fast-to-slow-fiber-type switch in soleus and diaphragm muscles of MGsKO mice. Consistent with the role of β2-adrenergic signaling in determining skeletal muscle fiber types, chronic administration of β2-agonist was shown to increase muscle mass and induced slow-to-fast-muscle fiber switches in rat and mouse (1, 16, 45). As a consequence of the slow-to-fast-fiber-type switch, β2-agonist-induced muscle hypertrophy was accompanied with decreased fatigue resistance (4).
The previous studies were all based on conditions involving slow-to-fast-fiber-type switching. To provide a strong positive evidence for the role of slow fibers in the resistance of skeletal muscle to fatigue, our present study employed a model of fast-to-slow-fiber-type switch and demonstrated that increases in slow fibers resulted in higher resistance to fatigue and better recovery (Figs. 3 and 9).
The fast-to-slow-fiber-type switch is an adaptive response to reduced muscle mass.
Skeletal muscle exhibits a high plasticity in response to chronic changes in physiological environment and activity. Reduction of skeletal muscle mass occurs in aging, starvation, physical inactivity, and various metabolic and neuromuscular disorders (5, 13, 15, 24, 42). Following the observation that skeletal muscles in diabetes undergo loss of mass (40), our laboratory previously demonstrated that MGsKO mice at 3.5 mo of age had impaired glucose tolerance with low muscle mass and low mitochondria content in soleus and gastrocnemius muscles (9).
The low-mass soleus muscle, but not the normal-mass EDL muscle, of 3-mo-old MGsKO mice had significant fast-to-slow-fiber-type switch (Figs. 1 and 2). Compared with that of control mice, MGsKO mice did not have fiber-type change in infantile soleus muscles (Fig. 4) or in 3.5-wk-old soleus muscles when the lower muscle mass began to be detectable (9). These observations suggest that the fast-to-slow-fiber-type switch was not a primary cell signaling effect of Gsα deficiency, but rather a secondary adaptive change in response to low muscle mass and the subsequently less force production. The fast-to-slow-fiber-type switch that accompanies decreased muscle mass during normal aging also supports the hypothesis that fast-to-slow-muscle-type switch is an adaptive response to low muscle mass and muscle weakness. This observation is consistent with the report of Kugelberg (25) that, during the growth of rat soleus muscle, a fast-to-slow-fiber-type switch was in response to the functional overload due to an increasing body-to-muscle weight ratio.
The fast-to-slow-fiber-type switch in soleus muscle of MGsKO mice improved resistance to fatigue, providing a plausible functional compensation for reduced force production. Whereas the muscles becoming slower would possibly impair the quick response to loss of balance, the higher resistance to fatigue remains to be beneficial in weight-bearing muscles. Although our laboratory's previous study (9) demonstrated increased glucose intolerance in MGsKO mice, this diabetes-related systemic defect is unlikely a consequence of increased slow fibers, since mice have very few slow-fiber muscles. Altogether, this compensatory mechanism is especially attractive in diabetic subjects, who tend to have a switch toward more fast-muscle fibers (9, 10, 14, 46).
The fast-to-slow-fiber-type switch in aging muscles was significantly enhanced in MGsKO mice.
An intriguing finding in the present study was that the fast-to-slow-fiber-type switch in normal mouse soleus muscle in aging was significantly enhanced in MGsKO mice, resulting in solely slow fibers in aging soleus muscle (Fig. 5). Whereas one study reported a replacement of slow oxidative type I fibers with fast glycolytic type II fibers during aging in the setting of caloric restriction (3), other studies showed increases in the proportion of type I fibers in aging human muscle (2, 22), corresponding to slower (34) and more economical contractility (39) than that of type II fibers. Consistent with the increased fatigue resistance in aging muscle of healthy human subjects (23), our data showed that aging control mouse soleus muscle had greater fatigue resistance than that of young control soleus. This effect was more profound in aging MGsKO soleus muscle, corresponding to the switch to purely type I fibers (Fig. 9).
As discussed above, reduced muscle mass occurs in aging (37). Our data showed that soleus muscle of aging control mouse had reduced mass compared with the 3-mo-old group. Although low mass was found in soleus muscles of young MGsKO mice compared with age-matched control, it did not advance in aging, and the muscle mass became similar to that of aging controls (Fig. 8). Therefore, the enhanced fast-to-slow-fiber-type switch in MGsKO soleus muscle may not be a simple adaptation to low muscle mass.
Our laboratory has shown that the MGsKO mice have decreased cardiac function with occult heart failure (11). Severe heart failure was found to decrease skeletal muscle function without significant change in muscle fiber-type composition (28). Those changes were only found in glycolytic fast fibers, but not in oxidative slow fibers, and the Gsα-deficient mice studied here did not have clinical signs of severe heart failure. Therefore, the characteristic switch to more slow fibers in the soleus muscle of Gsα-deficient mice is unlikely an indirect consequence of heart failure.
It is worth noting that, although the mass of MGsKO soleus muscle of aging mice was similar to that of the age-matched controls, their force production was significantly reduced (Fig. 8). Although the switching to slow fibers may be related to the lower muscle force observed, many other mechanisms can be the primary cause to decrease force production in old age, such as replacement of contractile material by fat and connective tissue at the whole muscle level, decreased amount of contractile proteins per muscle fiber volume, and altered properties of contractile proteins related to posttranslational modifications.
MGsKO enhances fast-to-slow-fiber-type switch selectively in the slow- and mixed-fiber muscles.
It was interesting to find that Gsα deficiency specifically affected slow and mixed-fiber-type muscles, but not fast-fiber-type muscle. The fast-to-slow-fiber-type switch was highly prominent in soleus muscle. Diaphragm muscle showed a detectable increase in slow myofilament proteins in aging MGsKO mice compared with that in age-matched control. However, EDL, a typical fast-fiber muscle, did not show much fiber-type switch, even in aging MGsKO mice (Fig. 10). Supporting a muscle-type-specific regulation of fiber-type differentiation, previous studies showed no fiber-type switch in EDL muscles in hindlimb-unloading rats, in which significant slow-to-fast-fiber-type switch was found in soleus muscles (44). On the other hand, a fast-to-slow-fiber-type switch was observed in the fast-twitch EDL and tibialis anterior muscles in aging rats, involving type IIb to IIx myosin isoform transition (29). The absence of such fiber-type switch in mouse EDL may reflect the nature of mouse muscles that contain predominantly fast fibers.
These observations suggest a hypothesis that the fiber-type switch may depend on the type of muscle activity. Soleus muscle plays an important role in keeping body posture against gravity, and diaphragm muscle continuously works to maintain respiration. Considering that low muscle mass in MGsKO mice was more profound in slow-fiber-rich muscles, the activity demands would be more predominant in soleus and diaphragm muscles to contribute to the adaptive fast-to-slow-muscle-type switch. The increased slow-fiber contents in these slow- or mixed-fiber muscles in MGsKO mice would, in turn, provide a compensation for the lower muscle force by improving the resistance to fatigue.
The muscle-type-specific fast-to-slow-fiber-type switch in the Gsα-deficient mouse model may also be due to varying levels of β-adrenergic receptors in different types of skeletal muscle. The effect of activating β2-adrenoceptors by β2-agonist has been well documented (32). Slow-twitch muscles, such as soleus, have a higher density of β2-adrenoceptors than that in fast-twitch muscles (33). Therefore, β2-adrenoceptor-Gsα signaling may have a greater effect on muscle mass and fiber-type composition in slow-twitch muscles that were specifically affected in MGsKO mice.
The enhancing effect of Gsα deficiency on aging-related fast-to-slow-fiber-type switching remains to be understood. To investigate the adaptive fast-to-slow-muscle fiber-type switch will lead to an approach to improve the function of atrophic muscle in aging, metabolic abnormality, and other pathological conditions. The data obtained in the present study laid groundwork for further studies on the molecular mechanisms underlying muscle-fiber differentiation and function.
GRANTS
This study was supported by National Institutes of Health (NIH) Grants AR048816 and HL098945 to J.-P. Jin and the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH, US Department of Health and Human Services.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Hui Wang for technical assistance and Dr. Jim Lin, University of Iowa for the T12 MAb.
REFERENCES
- 1. Agbenyega ET, Wareham AC. Effect of clenbuterol on normal and denervated muscle growth and contractility. Muscle Nerve 13: 199–203, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13: 40–47, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J 11: 573–581, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Baker DJ, Constantin-Teodosiu D, Jones SW, Timmons JA, Greenhaff PL. Chronic treatment with the beta(2)-adrenoceptor agonist prodrug BRL-47672 impairs rat skeletal muscle function by inducing a comprehensive shift to a faster muscle phenotype. J Pharmacol Exp Ther 319: 439–446, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Baracos VE. Management of muscle wasting in cancer-associated cachexia: understanding gained from experimental studies. Cancer 92: 1669–1677, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci 4: 19–27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen M, Gavrilova O, Liu J, Xie T, Deng C, Nguyen AT, Nackers LM, Lorenzo J, Shen L, Weinstein LS. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci U S A 102: 7386–7391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest 115: 3217–3227, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen M, Feng HZ, Gupta D, Kelleher J, Dickerson KE, Wang J, Hunt D, Jou W, Gavrilova O, Jin JP, Weinstein LS. G(s)alpha deficiency in skeletal muscle leads to reduced muscle mass, fiber-type switching, and glucose intolerance without insulin resistance or deficiency. Am J Physiol Cell Physiol 296: C930–C940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daugaard JR, Richter EA. Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol Scand 171: 267–276, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Feng HZ, Chen M, Weinstein LS, Jin JP. Removal of the N-terminal extension of cardiac troponin I as a functional compensation for impaired myocardial beta-adrenergic signaling. J Biol Chem 283: 33384–33393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng HZ, Wei B, Jin JP. Deletion of a genomic segment containing the cardiac troponin I gene knocks down expression of the slow troponin T gene and impairs fatigue tolerance of diaphragm muscle. J Biol Chem 284: 31798–31806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89: 823–839, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Hamilton MT, Booth FW. Skeletal muscle adaptation to exercise: a century of progress. J Appl Physiol 88: 327–331, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Hasselgren PO, Fischer JE. Regulation by insulin of muscle protein metabolism during sepsis and other catabolic conditions. Nutrition 8: 434–439, 1992 [PubMed] [Google Scholar]
- 16. Hayes A, Williams DA. Long-term clenbuterol administration alters the isometric contractile properties of skeletal muscle from normal and dystrophin-deficient mdx mice. Clin Exp Pharmacol Physiol 21: 757–765, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Hickey MS, Carey JO, Azevedo JL, Houmard JA, Pories WJ, Israel RG, Dohm GL. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol Endocrinol Metab 268: E453–E457, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Hilber K, Galler S. Mechanical properties and myosin heavy chain isoform composition of skinned skeletal muscle fibres from a human biopsy sample. Pflügers Arch 434: 551–558, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Jin JP, Chen A, Huang QQ. Three alternatively spliced mouse slow skeletal muscle troponin T isoforms: conserved primary structure and regulated expression during postnatal development. Gene 214: 121–129, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Jin JP, Brotto MA, Hossain MM, Huang QQ, Brotto LS, Nosek TM, Morton DH, Crawford TO. Truncation by Glu180 nonsense mutation results in complete loss of slow skeletal muscle troponin T in a lethal nemaline myopathy. J Biol Chem 278: 26159–26165, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Johnston JJ, Kelley RI, Crawford TO, Morton DH, Agarwala R, Koch T, Schaffer AA, Francomano CA, Biesecker LG. A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet 67: 814–821, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93: 1813–1823, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev 37: 3–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krasnoff J, Painter P. The physiological consequences of bed rest and inactivity. Adv Ren Replace Ther 6: 124–132, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci 27: 269–289, 1976 [DOI] [PubMed] [Google Scholar]
- 26. Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand 117: 469–471, 1983 [DOI] [PubMed] [Google Scholar]
- 27. Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6: 588–595, 1983 [DOI] [PubMed] [Google Scholar]
- 28. Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol 170: 599–608, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Larsson L. Maximum shortening velocity and myosin isoforms in single muscle fibers from young and old rats. Am J Physiol Cell Physiol 270: C352–C360, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Lin JJ. Monoclonal antibodies against myofibrillar components of rat skeletal muscle decorate the intermediate filaments of cultured cells. Proc Natl Acad Sci U S A 78: 2335–2339, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linssen WH, Stegeman DF, Joosten EM, Binkhorst RA, Merks MJ, ter Laak HJ, Notermans SL. Fatigue in type I fiber predominance: a muscle force and surface EMG study on the relative role of type I and type II muscle fibers. Muscle Nerve 14: 829–837, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88: 729–767, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Martin WH, III, Murphree SS, Saffitz JE. Beta-adrenergic receptor distribution among muscle fiber types and resistance arterioles of white, red, and intermediate skeletal muscle. Circ Res 64: 1096–1105, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol 71: 1277–1281, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29: 895–900, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100: 8466–8471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 5: 129–142, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1 alpha expression is controlled in skeletal muscles by PPAR beta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab 4: 407–414, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J Physiol 493: 299–307, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun Z, Liu L, Liu N, Liu Y. Muscular response and adaptation to diabetes mellitus. Front Biosci 13: 4765–4794, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68: 1–12, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Jin JP. Primary structure and developmental acidic to basic transition of 13 alternatively spliced mouse fast skeletal muscle troponin T isoforms. Gene 193: 105–114, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol 292: C1192–C1203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeman RJ, Ludemann R, Easton TG, Etlinger JD. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a beta 2-receptor agonist. Am J Physiol Endocrinol Metab 254: E726–E732, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Zierath JR, Hawley JA. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol 2: e348, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6: 924–928, 2000 [DOI] [PubMed] [Google Scholar]