Abstract
MicroRNA (miRNA) levels in brain are altered by sleep deprivation; however, the direct effects of any miRNA on sleep have not heretofore been described. We report herein that intracerebroventricular application of a miRNA-132 mimetic (preMIR-132) decreased duration of non-rapid-eye-movement sleep (NREMS) while simultaneously increasing duration of rapid eye movement sleep (REMS) during the light phase. Further, preMIR-132 decreased electroencephalographic (EEG) slow-wave activity (SWA) during NREMS, an index of sleep intensity. In separate experiments unilateral supracortical application of preMIR-132 ipsilaterally decreased EEG SWA during NREMS but did not alter global sleep duration. In addition, after ventricular or supracortical injections of preMIR-132, the mimetic-induced effects were state specific, occurring only during NREMS. After local supracortical injections of the mimetic, cortical miRNA-132 levels were higher at the time sleep-related EEG effects were manifest. We also report that spontaneous cortical levels of miRNA-132 were lower at the end of the sleep-dominant light period compared with at the end of the dark period in rats. Results suggest that miRNAs play a regulatory role in sleep and provide a new tool for investigating sleep regulation.
Keywords: delta power, electroencephologram, slow-wave activity, RNA interference, non-rapid-eye-movement sleep
micrornas (miRNAs) are small highly conserved non-protein-coding sequences that regulate mRNA stability and translation. miRNA synthesis and catabolism are tightly regulated and coupled to physiological functions (18). They bind to target mRNAs through a microribonuclear protein complex and thereby alter mRNA transcript stability, e.g., miRNA/mRNA complexes are susceptible to degradation by the Argonaute 2 endonuclease (2). Destabilization of mRNA appears to be a major process affecting protein levels (17). However, miRNAs can also promote mRNA stability and/or enhance its translation under certain conditions (48, 49). The regulatory potential for miRNAs is thus large although the roles that miRNAs play in brain functions such as sleep remain understudied.
Multiple mRNAs are altered by sleep (10, 32, 43, 45), and many of the proteins associated with these sleep-linked mRNAs are involved in sleep regulation, e.g., tumor necrosis factor (TNF) (13, 14, 25, 28, 41). Previously we reported that sleep deprivation changes brain miRNA levels; the magnitude and direction of those effects and the specific miRNAs affected depend on the brain region analyzed (11). It is plausible that the sleep-associated miRNAs target the sleep-linked mRNAs and thereby regulate sleep. However, heretofore, direct effects of miRNAs on sleep have not been determined.
Herein, we describe how microinjections of a synthetic miRNA-132 mimetic (hereafter called preMIR-132) affect sleep phenotypes. miRNA-132 was selected because it varies with sleep loss (11) and with the time of day (7). Further, miRNA-132 is linked to circadian rhythm entrainment (7) and to dendritic modifications (51), processes associated with sleep regulation (15, 19, 23, 35, 47). First, we investigated the effects of intracerebroventricular (ICV) microinjections of preMIR-132; during the light phase, it decreased duration of non-rapid-eye-movement sleep (NREMS) and increased duration of rapid eye movement sleep (REMS). It also decreased NREMS electroencephalographic (EEG) slow-wave activity (SWA; 0.5–4.0 Hz), a sleep phenotype indicative of NREMS intensity (4). Next we show that direct unilateral application of the miRNA-132 mimetic onto the surface of the cortex ipsilaterally decreased EEG SWA during NREMS.
If miRNAs are indeed responsible for some aspect of sleep regulation, it is logical to predict that brain miRNA levels fluctuate with sleep and with experimentally induced changes in sleep. To this end, we show that miRNA-132 abundance changes between the onset of the dark period compared with the onset of the light period. We also show that miRNA-132 levels increased in the cortex after cortical application of the miRNA-132 mimetic at the time changes in the NREMS EEG occurred. Collectively, data demonstrate a role for miRNA-132 in the biochemical regulation of sleep.
MATERIALS AND METHODS
Compounds.
A double-stranded Pre-MIR miRNA mimetic for miRNA-132 was obtained from Ambion (Austin, TX; cat. no. AM17100), hereafter referred to as preMIR-132. preMIR-132 is chemically modified for guide strand selection and stability. To determine the extent to which the preMIR-132 modification affected the timing of the potential sleep responses, a 22-bp-long, unmodified or “naked” double-stranded oligonucleotide (naked-132) matching the mature miRNA-132 sequence (5′-UAACAGUCUACAGCCAUGGUCG-3′) was procured from Integrated DNA Technologies (IDT; San Diego, CA). Unless otherwise specified, Ambion's Negative Control-1 for pre-MIRs (cat. no. AM17110) was used as a nonsensical oligonucleotide control. Doses were determined by in vitro work in our laboratory using immortalized H19 cells from which we performed a dose-response curve based on manufacturer suggestions.
Animals.
Animal use was in compliance with National Institutes of Health standards and approved by Washington State University's Institutional Animal Care and Use Committee. All protocols were designed to minimize animal discomfort. Male Sprague-Dawley rats weighing 275–325 g were procured from Taconic Farms (Hudson, NY) and maintained in AAALAC-approved housing at 23 ± 1°C. Rats were given food ad libitum and maintained on a 12:12-h light (110 ± 5 lx)/dark cycle.
Surgery.
Rats used for polysomnographic recording and ICV instrumentation were anesthetized with ketamine-xylazine (87 and 13 mg/kg im, respectively). Rats were provided with a guide cannula at −1.8 mm posterior, −1.5 mm lateral to bregma and −3.2 to −3.7 mm ventral to the skull surface. Actual depth was determined in the following manner. Pyrogen-free saline (PFS) was loaded into a sterilized apparatus consisting of 15-cm Silastic tubing (0.31-mm internal diameter/0.64-mm external diameter; Helix Medical; Carpinteria, CA), attached to a cannula that was secured within a guide cannula and held by the stereotax calipers. The tubing was then taped to the vertical stereotax arm and the cannula lowered to the point where the PFS column began to flow downward, indicating that the lateral ventricle had been breached. Four stainless steel jewelry screws with wire leads were positioned at 2 mm rostral, or 5.0 mm caudal to the coronal suture and ± 3.0 mm on either side of the sagittal suture for EEG recording. An electromyogram (EMG) reference screw was placed 3.0 mm posterior to lambda, and nuchal muscles were penetrated with an EMG wire to detect muscle tonus. All wire leads were inserted into a six-pin plug interface (Plastics One; Roanoke, VA), and dental composite (Patterson Dental; St. Paul, MN) was used to affix the electrode wires, guide cannula, and plug interface in position.
To verify cannula placement rats received acute ICV microinjections of 12.5 ng/μl of ANG II (Sigma; St. Louis, MO) in 4 μl to induce a drinking response. Only rats that consumed >5 ml of water in 25 min were included in the study.
Rats selected for unilateral supracortical injections were anesthetized as above and then provided bilateral guide cannula at 2.0 mm posterior, ± 4.0 mm lateral to bregma, and 1.5 mm ventral to the skull surface. Before cannula placement the dura was ruptured with a 22-gauge hypodermic needle. Guide cannulas were angled at ± 25° with reference to the midsagittal plane to avoid the recording cable. EEG screws were secured 2.0 mm caudal and rostral to each guide cannula tip. The EMG was recorded from an electrode in the nuchal muscle as previously described (24).
After a minimum of 7 days of recovery, animals were anesthetized as before, and 2 μl of 20% lidocaine (Sigma; St. Louis, MO) was microinjected over 5 min. EEG was then recorded for 10–15 min to verify cannula integrity as indicated by a decrease in the low-frequency EEG spectrum in both hemispheres (54). Rats that did not respond to lidocaine were excluded from the study.
Recordings and injections.
Rats were handled for 5 min for each of 5 days during which their obturators were manipulated to habituate the animal to the experimental protocol. Rats were acclimated in the recording chamber with the recording cable attached for at least 24 h before recording began. The tethered cable connecting the differential EEG electrode pairs and EMG electrodes was attached to a six-channel commutator (Plastics One; Roanoke, VA), and cables from the commutator were routed to a Grass model 15 15A54 32-channel amplifier (Grass-Telefactor; West Warwick, RI). All amplified signals were digitized at 128 Hz and EEG was filtered below 0.1 Hz and above 100 Hz while EMG was filtered below 30 Hz and above 3 kHz.
ICV injections.
For ICV injections baseline recordings began at light onset of the first day and continued for 24 h. Thirty minutes before light onset, rats (n = 12) were removed from the recording setup, and a 4-pmol dose of preMIR-negative control sequence was injected in 4 μl over 2.5 min after which the animals were reattached and recording resumed for another 24 h. Then animals received preMIR-132 at the same volume and dose (4 pmol) as the control injection. Recordings were restarted and continued for 72 h based on our earlier work demonstrating sleep effects of siRNAs were limited to 3 days postinjection (42).
Cortical injections.
For the unilateral supracortical injection experiments, baseline recording of EMG and bilateral EEG started at light onset on the first day and continued for 48 h. In the first of these experiments one hemisphere was given preMIR-132 (n = 7) and preMIR-negative control to the contralateral hemisphere. In the second experiment, rats (n = 5) were tested with saline (previously we determined that negative control and saline did not affect SWA or spectrum of any stage) on one side of the cortex and naked-132 on the other. Thirty minutes before light onset on the third recording day, rats were detached from the recording cable, and 100 pmol of preMIR-132 or naked-132 in 2 μl was delivered through the guide cannula to one hemisphere over a 5-min period. At the same time, 100 pmol of negative control sequence or saline in 2 μl was delivered to the opposite hemisphere. Animals were then reattached to the cable, placed inside the chambers, and recording continued for 96 h.
In a follow-up study, eight rats received surgery and treatment with preMIR-132 and negative control as described, but were euthanized at 15 h postinjection (the time point when the preMIR-132-induced effects on NREMS EEG SWA were most pronounced). Cortical tissue was harvested from each cerebral hemisphere from a 2.5-mm radius from the site of injection and placed in RNAlater for polymerase chain reaction (PCR) analyses. In a separate study, rats (n = 6) were given naked-132 or saline in one hemisphere and tissues surrounding the injection sites were sampled 15 h postinjection as above.
EEG analyses.
The vigilance states of wake, NREMS, and REMS were manually scored off-line in 10-s epochs using SleepSign for Animal software (Kissei Comtec, Nagano, Japan) as previously reported (24). Briefly, NREMS was characterized by high-amplitude EEG slow waves in the 0.5- to 4-Hz range, and REMS consisted of low-amplitude waves with a pronounced theta component (6 to 9 Hz) and low EMG activity, whereas waking was characterized by variable low-amplitude EEG waves in multiple frequencies and elevated EMG activity. In the supracortical experiments the manual scoring was done with both hemispheres on-screen, and the hemisphere with the best signal (e.g., fewest artifacts, good amplitude, and characteristic delta and theta spectral patterns) was used. We scored both hemispheric EEGs from five animals; there were no hemispheric differences with time in stage. Moreover, on comparison of epochs with different stage assignments from a single animal most of the epochs with differences appeared artificial. Thus the analysis for time-in-stage on the asymmetry was not performed further.
The EEG signals were subjected to fast Fourier transformation, and power densities were extracted using a Hanning window filter calculated by SleepSign. EEG SWA (0.5–4 Hz) during NREMS, REMS, and wake was averaged into 2-h blocks and normalized to the mean of two 24-h averages of the 48-h baseline period. Treatment days were expressed as a percentage of that baseline average for each animal and used as a reference value to normalize data across the 24-h data collection period for both the baseline day and experimental days. Data for 2-h time bins during control or experimental recordings were then expressed as a percentage of the reference value.
For spectral analyses of ICV-treated animals, the negative control day EEG power spectra were calculated in 1.0-Hz bins in the 1- to 20-Hz frequency range for 0–6 h. EEG power on the negative control and the 0- to 6-h time period on experimental days were expressed as a percent of total negative control power in 1-Hz bins. Alternatively, in the supracortical injection experiments to establish state specificity of the asymmetries induced by preMIR-132, spectral analyses of 0.5- to 20-Hz frequencies were determined for each hemisphere in a 2-h period demonstrating the most prominent and significant change in NREMS EEG SWA. Raw spectra from 20 NREMS- or REMS-scored epochs from 14–16 h postinjection were extracted while sampling epochs distributed throughout the 2-h time period. Similar extractions were also performed 24 h later at 38–40 h to demonstrate the cessation of the observed effect. In the preMIR-132 supracortical injection experiment, 10 and 13 REMS epochs were used in two rats that did not have a total of 20 epochs of REMS within the select 2-h time block.
RNA isolation and cDNA synthesis for primer extension PCR.
RNA isolation from cortical tissues and reverse transcription were performed as previously described (11) except that tissues were stored in RNAlater at the time of collection. Samples were diluted 10-fold with 180 μl 1× TE (10 mM Tris, pH = 7.7) and stored frozen until needed for primer extension quantitative PCR (PE-qPCR). To measure miRNA-132 by qPCR, the primer extension technique was followed as previously described (11). The locked nucleic acid (LNA) primer sequence for miRNA-132 was T+AA+CAGTCTACAGCC [Integrated DNA Technologies, Coralville, IA; + = nucleotide with LNA bridge; (LNA primers are now only available from Exiqon, Woburn, MA)]. Samples were assayed in triplicate and normalized to the housekeeping gene cyclophilin A with the forward primer sequence of aaatgctggaccaaacacaaa and the reverse primer sequence of ctcatgccttctttcaccttc. For PE-qPCR data, the 2−ΔΔCt values that represent PCR threshold cycle differences between the preMIR- or naked-132-treated sides and their respective control-treated sides.
Bioarrays.
Two groups of rats (n = 8 each) were acclimated to the 12:12-h light/dark cycle for 10 days for the bioarray miRNA assay. Animals were euthanized in two cohorts of four rats beginning 30 min before light (dawn) or dark (dusk) onset. Dawn is the beginning of the sleep period in nocturnal rats when sleep propensity is high. At dusk sleep propensity is low as evidenced by rats showing little sleep for the next few hours if left undisturbed. We used dawn and dusk because SWA is maximally different at these two time points, as is their cumulative sleep history.
Following decapitation, the hippocampus, prefrontal cortex, somatosensory cortex, and hypothalamus were dissected using landmarks previously reported (9). Each sample was immersed in RNAlater and placed at 4°C overnight and then stored at −20°C until RNA was isolated for hybridization on microarrays. RNA isolation, fractionation, labeling, and array hybridization were performed with Ambion's isolation kit, labeling kit, and MirVana BioArrays (cat. no. #1560, 1562, and 1564V2, respectively) as previously described (11). The starting quantities of enriched small RNAs varied between structures (29.0–52.2 μg for the prefrontal cortex; 20.0–48.0 μg for the somatosensory cortex; 33.8–48.5 μg for the hypothalamus and 23.5–51.8 μg for the hippocampus). Equal amounts of RNA were applied to each array slide. Thus, for each of the four structures four independent dawn or dusk samples were hybridized according to the manufacturer's protocol. Following hybridization, array slides were scanned and GenepixPro 6.0 was used to digitize and quantify the array data. The dusk or dawn four replicate spot-mean pixel intensities were normalized to the mean pixel intensity of positive control spots specific to each array and expressed as relative optical densities of miRNA-132.
Statistics.
For ICV injections, NREMS and REMS data were analyzed in 12-h time blocks using repeated-measures one-way ANOVAs for light and dark conditions. Slow-wave amplitude was analyzed in 2-h time blocks using four mixed 12 × 2 ANOVA (1 for each recording day) to examine between factors of treatment and within factors of time with SPSS v.17. Power spectrum of the first 6 h on each treatment day was analyzed using 2 × 20 ANOVA with factors of treatment and single hertz frequency bins from 1 to 20. When the omnibus was significant (P < 0.05), a priori unconfounded comparisons of treatment at each time block or particular single hertz frequency were conducted with a reduced alpha of (P < 0.01) to control for family-wise errors.
Student's t-tests were used to analyze 12-h compilations of NREMS EEG SWA and the 2-h power spectra in the 0.5- to 20-Hz range. The 2−ΔΔCt values for PE-qPCR data were also analyzed with Student's t-tests to compare hypothalamic levels of miRNA-132 in preMIR-132- and negative control-treated animals. Alternatively, cortical miRNA-132 levels were analyzed with a one-way ANOVA after which a Newman-Keuls post hoc test was performed to detect differences between saline-, naked-132-, negative control-, or preMIR-132-treated hemispheres. For each miRNA-132 mimetic four mixed 12 × 2 ANOVA (1 for each postinjection day) were employed to examine between factors of treatment and within factors of 2 h-time blocks of NREMS EEG SWA. As before when the omnibus was significant (P < 0.05), a priori unconfounded comparisons of treatment at each time block were conducted.
To control for family-wise error rate in the bioarray data, elimination criteria were constructed to limit the number of comparisons made between dawn and dusk miRNA levels. First, a spot's average signal-to-noise ratio was greater than two. Second, the minimum difference between the dawn and dusk average pixel intensity of each miRNA was at least 10% of the average of the top two miRNA difference scores. Third, the adjusted ratios were >1.5 or <0.5 for inclusion. Student's t-tests on the remaining comparisons were calculated with SPSS v.17 software.
RESULTS
ICV preMIR-132 altered sleep.
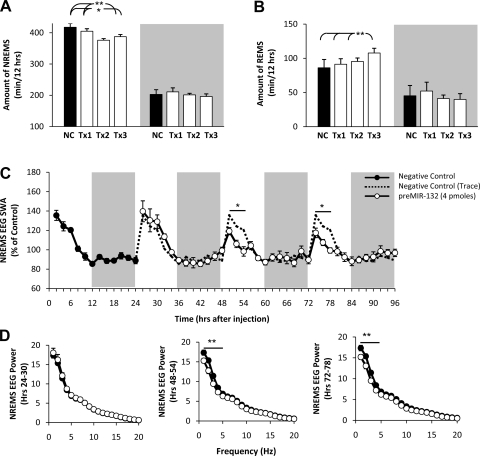
ICV injection of preMIR-132 decreased time spent in NREMS (F3,21 = 9.78, P < 0.01; Fig. 1A) by 20–50 min during postinjection days 1–3 with the largest effects occurring on day 2. The effect was largely isolated to the light periods on treatment days 2 and 3. REMS duration gradually increased during the light phase from postinjection days 1–3 and was significantly increased on day 3 (F3,21 = 12.31, P < 0.01; Fig. 1B) by ∼30 min. In addition, preMIR-132 suppressed NREMS EEG SWA during the first 6 h after light onset on day 2 (F1,14 = 5.95, P < 0.05), and day 3 (F1,14 = 4.67, P < 0.05; Fig. 1C) compared with negative control. For EEG SWA during NREMS, the factors of time and time × treatment interaction were also significant for days 2 and 3. Duration of NREMS and REMS during the dark phase was not altered on any of the postinjection days.
Fig. 1.
Intracerebroventricular (ICV) injection of size-matched scrambled negative control sequence (black bar) and 4 pmol of preMIR-132 (open bars) decreases non-rapid-eye-movement sleep (NREMS) duration (A), enhances rapid eye movement sleep (REMS) duration (B), and decreases NREMS EEG slow-wave activity (SWA) (C). In A and B, NC is size-matched scrambled negative control sequence-treated day; Tx1, Tx2, and Tx3 are preMIR-132-treated days 1, 2, and 3, respectively. In C, dashed lines are replotted negative control values superimposed on treatment days 1–3. D represents NREMS EEG spectral power from the first 6 h after light onset of treatment days 1–3 (*P < 0.05, **P < 0.01; n = 8).
EEG spectral analyses of day 1, 0- to 6-h data, indicated that EEG power during NREMS was not suppressed at any frequency (Fig. 1D). However, statistically significant attenuated spectral values were observed during the initial 6 h following light onset of days 2 and 3 in the 1- to 4-Hz ranges (F1,280 = 27.05, P < 0.01, F1,280 = 36.65, P < 0.01, respectively; Fig. 1D). EEG SWA and spectral power at the same times for wake and REMS-scored epochs were also examined, and no significant changes were detected.
Supracortical injection of preMIR-132 decreased NREMS EEG SWA unilaterally.
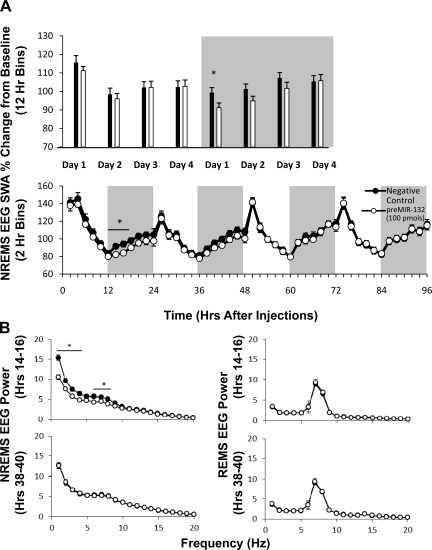
In the first supracortical injection experiment, EEG SWA during NREMS was reduced during the 12-h dark period on postinjection day 1 (t = 2.19, P < 0.05) but not during other 12-h time blocks during the remaining of the 4-day recording period (Fig. 2A, top panel). EEG SWA during REMS or wake episodes was not affected during any 12-h time block after treatment with the preMIR-132 (data not shown). Statistically significant changes occurred during several 2-h blocks of the dark period on day 1 (F1,22 = 6.08, P < 0.05), but not on subsequent days (Fig. 2A, bottom panel). Specifically, application of preMIR-132 attenuated NREMS EEG SWA at 12–18 h postinjection compared with negative control sequence. Moreover, preMIR-132 significantly decreased the power spectrum during NREMS at the 14–16 h postinjection in the 1- to 4-Hz and 6- to 8-Hz ranges. The effects subsided 24 h later during the 38- to 40-h time block. The preMIR-132-induced 2-h time block effects only occurred during NREMS and were not present during REMS or wake over the 4-day recording period (Fig. 2B).
Fig. 2.
Baseline normalized SWA in NREMS-scored epochs following unilateral cortical microinjection of preMIR-132 (100 pmol; open circles or bars) or size-matched scrambled negative control sequence (black circles or bars) to the contralateral side displayed in 12-h (A, top panels) and 2-h blocks (A, bottom panels; n = 12). B: NREMS (left panels) or REMS (right panels) EEG power spectrum at 14–16 h (top panels) and 38–40 h (bottom panels) following preMIR-132 or negative control treatment. The selection of 14- to 16-h time block is based on the NREMS EEG SWA response. The 38- to 40-h time block was 1 day later to show cessation of the effect. (comprised of 20 epochs/2 h; *P < 0.05; n = 10).
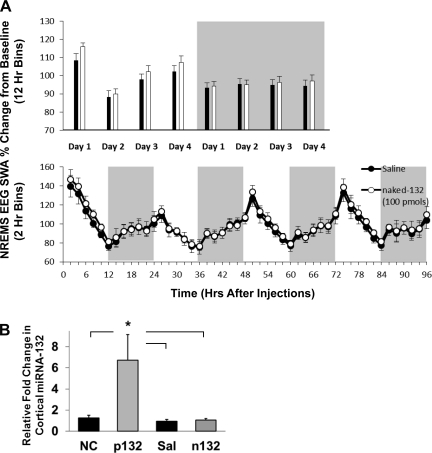
Supracortical injection of naked-132 failed to affect NREMS EEG SWA.
In the second supracortical injection experiment, unilateral application of naked-132 did not alter ipsilateral EEG SWA compared with the contralateral side receiving control injections during NREMS-, REMS-, or wake-scored epochs using 12-h or 2-h blocks (Fig. 3A). To compare the efficacies of the preMIR-132 and naked-132 levels in cortical tissues, rats were microinjected with either mimetic as described and euthanized at 15 h postinjection and miRNA-132 levels were measured. Extracts from preMIR-132-treated hemispheres had an almost sixfold increase in miRNA-132 concentrations compared with the saline-, negative control-, or naked-132-treated hemispheres (F3,26 = 4.84, P < 0.01; Fig. 3B). This difference in residual miRNA-132 levels indicates the efficacy of the preMIR-132 mimetic and also validates the injection procedure.
Fig. 3.
Baseline normalized NREMS EEG SWA following local delivery to the somatosensory cortex of naked-132 (100 pmol; open circles or bars) or vehicle control saline (black circles or bars) in 12-h blocks (A, top panel) or 2-h blocks (A, bottom panel; n = 5). Primer-extension qPCR-quantified levels of miRNA-132 from cortical tissues surrounding the injection site (B). Tissues were harvested at 15 h postinjection of 100 pmol negative control (NC; n = 8), 100 pmol of preMIR-132 (p132; n = 8), saline (Sal; n = 7), or 100 pmol of naked-132 (n132; n = 7; *P < 0.05).
miRNA-132 levels changed with time.
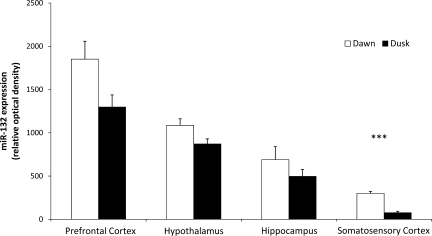
miRNA-132 levels in the hippocampus, prefrontal cortex, somatosensory cortex, and hypothalamus were higher at dawn (high sleep propensity) compared with levels occurring at dusk although only the somatosensory cortex reached significance (P < 0.001; Fig. 4).
Fig. 4.
Data from 4 independent bioarrays comparing miRNA expression at dawn (open bars) vs. dusk (black bars) in prefrontal cortex, hypothalamus, hippocampus, and somatosensory cortex (total of 32 arrays). Results expressed as relative optical density (+SE) of miRNA-132 (***P < 0.001).
DISCUSSION
The major findings reported herein demonstrate a potential role for miRNA-132 in sleep regulation. ICV injection of the preMIR-132 inhibits duration of NREMS and NREMS EEG SWA while it increases duration of REMS. Local injection of the preMIR-132 also inhibits NREMS EEG SWA; these effects were local, occurring only on the side of the injection and not affecting whole animal sleep-state duration. The effects of the preMIR-132 on the EEG are also state specific, occurring only during NREMS, and are time-of-day specific. Spontaneous brain levels of miRNA-132 are also site- and time-of-day specific. Further, after local cortical application of the preMIR-132, miRNA-132 levels are higher at the time of reduced NREMS EEG SWA. Collectively these data provide evidence of miRNA-132 involvement in sleep regulation.
That ICV preMIR-132 increases REMS duration while simultaneously decreasing NREMS durations indicates independent actions of miRNA-132 on these states. Multiple different proteins are involved in the regulation of these two states. For instance, TNF is involved in REMS and NREMS regulation (22). Prolactin, in contrast, is specifically involved in REMS regulation (30, 36). Thus the potential for independent actions on the respective mRNAs exists. However, the complexity of the miRNA actions is great in that it involves multiple target mRNAs, some being degraded and others being stabilized; time-of-day dependent transcription of many of the target mRNAs; and differential rates of translation of each target mRNA. Such complexity renders miRNA biology difficult to relate directly to physiological function. Nevertheless, present results suggest an integrated action of miRNA-132; thus if the duration of one state is increased, necessarily the duration of another state must decrease. Thus the present observation that preMIR-132 has opposite effects on NREMS and REMS, although speculative, may result from its having multiple targets and differential actions of the subsequent proteins on state, suggesting an integrative regulatory mechanism that begins with miRNAs.
The effects of preMIR-132 on NREMS EEG are also state specific. NREMS EEG SWA is often interpreted as a measure of sleep intensity and is the quantitative parameter used to represent process S in the two-process model of sleep (4). High-amplitude waves dominate the EEG during NREMS but not during REMS or waking. In rats, NREMS EEG SWA is highest at dawn, the onset of the sleep period (3). It is also enhanced during the sleep immediately occurring after sleep loss in multiple species, including humans (33). Many endogenous sleep-regulatory substances also enhance NREMS EEG SWA (22). That the effects of miRNA-132 manifest NREMS state-specificity is consistent with the conclusion that its actions are indeed sleep related. Nevertheless, the regulation of EEG SWA is independent of the regulation of duration of NREMS (20). Further, EEG SWA can be induced and/or enhanced pharmacologically, e.g., systemic atropine (5, 38) or by other experimental manipulation such as hyperventilation in awake adolescent humans (52). As a consequence, alternative explanations are conceivable. Regardless, the present data clearly indicate that miRNA-132 affects brain function to the extent that it state-specifically enhances NREMS EEG SWA.
In both supracortical injection studies after both control and treatment injections a decrease in NREMS SWA at 24–26 h was observed. We speculate that this is a possible artifact of injection as it does not occur in the 2-day baseline before the simultaneous hemispheric injections nor does it occur with ICV injection. Regardless, the actions of preMIR-132 after topical application to the cortex are localized, occurring on only the side of the cortex to which it is applied. Consistent with these findings, application of several sleep regulatory substances, including TNF (54), interleukin-1 (53), growth hormone releasing hormone (27), and brain-derived neurotrophic factor (13), to the surface of the cortex also induces unilateral state-specific enhancements of NREMS EEG SWA. The 0.5- to 1.5-Hz component of the EEG survives thalamectomy (40) and is absent in the thalamus of decorticate animals (46), suggesting a local origin of EEG SWA. Moreover in single cortical columns, slow oscillatory field potentials occur in cortical slices (37). A subset of cortical GABAergic neurons that also express neuronal nitric oxide synthase are posited to be involved in the sleep-related EEG delta wave mechanism (16). The time courses of the local EEG effects induced by preMIR-132 are distinct from those occurring after ICV application of preMIR-132 although both are specific to NREMS. Whether these differential time courses reflect site-specific mRNA interactions with miRNA-132 or are a manifestation of target proximity remains unknown. However, after ICV injections, the preMIR-132 is likely available to the hypothalamus and more posterior structures surrounding the ventricles due to the direction of cerebrospinal fluid flow. These structures are involved in global regulation of sleep states. In contrast, diffusion of the preMIR-132 after the localized unilateral injections is likely much more restricted to the immediate area surrounding the injection site. Regardless, collectively, these findings are consistent with the hypothesis that sleep is a property of local neuronal networks (reviewed in 23) and that miRNA-132 serves this process.
At the end of the dark cycle (dawn) miRNA-132 cortical levels are higher as is the propensity to sleep. These data are consistent with a prior report of miRNA-132 expression in the suprachiasmatic nucleus during the daylight hours (7). However, following sleep deprivation sleep propensity is high and cortical miRNA-132 levels are low (11). Such differences are difficult to interpret. If directly applied to the cortex it takes 12 h for the miRNA-132 effects to manifest, and after ICV injections it takes 2 days for miRNA-132 to manifest maximal effects. Other miRNAs are also cyclically expressed (34) and some of them affect miR-132 expression.
The mechanisms responsible for the local miRNA-132 actions on NREMS EEG SWA remain unknown. Neuronal activity induces miRNA-132 (29, 51). Sleep is dependent on prior neuronal activity (23) and sleep loss is associated with enhanced cortical neuronal activity (50). Enhanced neuronal activity is associated with enhanced sleep regulatory substance expression; e.g., TNF expression in cortical columns increases after excessive input afferent activity and application of TNF to such columns increases the probability of the column being in a sleeplike state (8). Mechanistically neuronal/glial activity-induced miRNA-132 could affect multiple mRNAs to alter local network state.
Our studies are severely limited because we have yet to describe the site-specific changes in mRNAs induced by miRNA-132. This is a consequence of the fact that over 1,000 potential targets are predicted for miRNA-132 in silico (http://www.microrna.org/microrna/getTargets.do?organism=10116&matureNamerno-miR-132). To test all such targets is an open-ended endeavor because we lack knowledge of the structure-specific time courses of miRNA-132 actions on mRNAs relevant to sleep and the direction of any such effects. Further, miRNA-induced changes in mRNAs can be direct or indirect, acting either via other miRNAs induced by miRNA-132 or via secondary, tertiary, etc., effects resulting from an action on a specific mRNA. These studies are beyond the scope of the present study because they require large investments in time and money.
There are two reports of miRNA-132 targets that could be indirectly involved in sleep regulation. First, miRNA-132 targets acetylcholine esterase (AChE) mRNA, producing a negative feedback on inflammatory signaling (39). Two proinflammatory cytokines, IL-1 and TNF, are well-established sleep regulatory substances (22, 31). miRNA-132 induces an increase in ACh that in turn suppresses macrophage secretion of TNF. Thus depleted TNF levels could manifest in suppressed NREMS EEG (42) and sleep time (12, 44) as we observed after enhancing brain miRNA-132 levels with preMIR-132. Second, transcriptional coactivator p300 is another reported target of miRNA-132 (26). p300 exhibits high homologies to CREB-binding protein, and their histone transferase activity affects the expression of hundreds of genes (21), several of which are linked to sleep. The involvement of these miRNA-132 targets in our present findings is speculative. Nevertheless, the higher-level regulatory events represented by the orchestrated involvement of miRNAs affecting multiple mRNAs in various brain sites to coordinate sleep responses may become an active area of investigation due to many potential clinical and pharmaceutical applications of such knowledge.
Although this study was not designed to determine relationships between the pharmacokinetics of various forms of miRNAs and their actions on sleep, there is limited evidence of a relationship between the two. Thus the attenuated NREMS EEG SWA observed during the first half of the dark period after cortical preMIR-132 treatment is concurrent with increased cortical levels of miRNA-132. This abundance of miRNA-132 15 h after preMIR-132 administration attests to the stability of one or both of these substances in vivo. However, naked oligos appeared to be more susceptible to RNase degradation because naked-132 failed to increase miRNA-132 levels or alter NREMS EEG power.
The time course of the asymmetrical changes in NREMS EEG SWA following treatment with cortical preMIR-132 is more transient than the EEG effects of other studies that employ RNA interference to target specific genes (6, 42). Perhaps dissimilarities between the temporal dynamics of preMIR-132 and the latter two studies are again due to the properties of the oligos themselves. In those studies, synthetic small interfering RNAs (siRNAs) were used rather than increasing endogenous miRNA strands as was done here. siRNAs are designed to inhibit a single target whereas miRNAs have numerous targets. Alternatively, the effects of ICV preMIR-132 are confined to the light period and are recurrent over a relatively longer time course. This difference could reflect the structure-dependent actions of miRNA-132. For example, ICV injection of the preMIR-132 mimetic could differentially affect hypothalamic levels of miRNA-132 and disrupt targets that regulate the biological clock as has been demonstrated with ICV directed miRNA manipulation in the suprachiasmatic nucleus (1). Following sleep deprivation sleep need is high and cortical miRNA-132 levels are low. At the end of the dark cycle (dawn) sleep need is high in nocturnal rats; however, miRNA-132 cortical levels are also high. Without detailed time courses of miRNA-132 expression and actions it is speculative to relate the dawn/dusk levels to those changes induced by sleep deprivation (11).
In conclusion, it seems likely that miRNAs, such as miRNA-132, are molecular components of sleep regulatory mechanisms. This is a promising direction due to the potential integrative actions resulting from the multiple targets of any specific miRNA. The present results showing clear effects of miRNA-132 on sleep provide a beginning.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-031453 and NS-025378 to J. M. Krueger.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alvarez-Saavedra M, Antoun G, Yanagiya A, Oliva-Hernandez R, Cornejo-Palma D, Perez-Iratxeta C, Sonenberg N, Cheng HY. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum Mol Genet 20: 731–751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 122: 553–563, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Borbely AA. Sleep deprivation: effects on sleep and EEG in the rat. J Comp Physiol 133: 71–87, 1979 [Google Scholar]
- 4. Borbely AA. A two process model of sleep regulation. Hum Neurobiol 1: 195–204, 1982 [PubMed] [Google Scholar]
- 5. Bringmann A. Topographic mapping of the cortical EEG power in the unrestrained rat: peripheral effects of neuroactive drugs. Arch Ital Biol 133: 1–16, 1995 [PubMed] [Google Scholar]
- 6. Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci 24: 2039–2048, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K. microRNA modulation of circadian-clock period and entrainment. Neuron 54: 813–829, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, Krueger JM. Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience 156: 71–80, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Churchill L, Taishi P, Wang M, Brandt J, Cearley C, Rehman A, Krueger JM. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Res 1120: 64–73, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron 41: 35–43, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Davis CJ, Bohnet SG, Meyerson JM, Krueger JM. Sleep loss changes microRNA levels in the brain: a possible mechanism for state-dependent translational regulation. Neurosci Lett 422: 68–73, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci 17: 5949–5955, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci 28: 4088–4095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Floyd RA, Krueger JM. Diurnal variation of TNF alpha in the rat brain. Neuroreport 8: 915–918, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci 29: 1820–1829, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Gerashchenko D, Wisor JP, Burns D, Reh RK, Shiromani PJ, Sakurai T, de la Iglesia HO, Kilduff TS. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci USA 105: 10227–10232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5: 522–531, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Huguenard JR. Anatomical and physiological considerations in thalamic rhythm generation. J Sleep Res 7, Suppl 1: 24–29, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Kapas L, Bohnet SG, Traynor TR, Majde JA, Szentirmai E, Magrath P, Taishi P, Krueger JM. Spontaneous and influenza virus-induced sleep are altered in TNF-alpha double-receptor deficient mice. J Appl Physiol 105: 1187–1198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasper LH, Fukuyama T, Biesen MA, Boussouar F, Tong C, de Pauw A, Murray PJ, van Deursen JM, Brindle PK. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol Cell Biol 26: 789–809, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des 14: 3408–3416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci 9: 910–919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krueger JM, Taishi P, De A, Davis CJ, Winters BD, Clinton J, Szentirmai E, Zielinski MR. ATP and the purine type 2 X7 receptor affect sleep. J Appl Physiol 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kushikata T, Fang J, Krueger JM. Brain-derived neurotrophic factor enhances spontaneous sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol 276: R1334–R1338, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nature Cell Bio 12: 513–519, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Liao F, Taishi P, Churchill L, Urza M, Krueger JM. Localized suppression of cortical growth hormone releasing hormone receptors state specifically attenuates EEG delta waves. J Neurosci 30: 4151–4159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci 18: 1041–1049, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Nudelman AS, Dirocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20: 492–498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Obal F, Jr, Garcia-Garcia F, Kacsoh B, Taishi P, Bohnet S, Horseman ND, Krueger JM. Rapid eye movement sleep is reduced in prolactin-deficient mice. J Neurosci 25: 10282–10289, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci 8: d520–d550, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von Smith R, Kay T, Lian J, Svenson K, Peters LL. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics 28: 232–238, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol 38: 1299–1311, 1975 [DOI] [PubMed] [Google Scholar]
- 34. Pegoraro M, Tauber E. The role of microRNAs (miRNA) in circadian rhythmicity. J Genet 87: 505–511, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Ramesh V, Lakshmana MK, SB, Rao S, Raju TR, Kumar VM. Alterations in monoamine neurotransmitters and dendritic spine densities at the medial preoptic area after sleep deprivation. Sleep Res Online 2: 49–55, 1999 [PubMed] [Google Scholar]
- 36. Roky R, Obal F, Jr, Valatx JL, Bredow S, Fang J, Pagano LP, Krueger JM. Prolactin and rapid eye movement sleep regulation. Sleep 18: 536–542, 1995 [PubMed] [Google Scholar]
- 37. Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Schaul N, Gloor P, Ball G, Gotman J. The electromicrophysiology of delta waves induced by systemic atropine. Brain Res 143: 475–486, 1978 [DOI] [PubMed] [Google Scholar]
- 39. Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31: 965–973, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci 13: 3266–3283, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taishi P, Churchill L, De A, Obal F, Jr, Krueger JM. Cytokine mRNA induction by interleukin-1beta or tumor necrosis factor alpha in vitro and in vivo. Brain Res 1226: 89–98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taishi P, Churchill L, Wang M, Kay D, Davis CJ, Guan X, De A, Yasuda T, Liao F, Krueger JM. TNFalpha siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res 1156: 125–132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taishi P, Sanchez C, Wang Y, Fang J, Harding JW, Krueger JM. Conditions that affect sleep alter the expression of molecules associated with synaptic plasticity. Am J Physiol Regul Integr Comp Physiol 281: R839–R845, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Takahashi S, Kapas L, Seyer JM, Wang Y, Krueger JM. Inhibition of tumor necrosis factor attenuates physiological sleep in rabbits. Neuroreport 7: 642–646, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Terao A, Wisor JP, Peyron C, Apte-Deshpande A, Wurts SW, Edgar DM, Kilduff TS. Gene expression in the rat brain during sleep deprivation and recovery sleep: an Affymetrix GeneChip study. Neuroscience 137: 593–605, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol 76: 4152–4168, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med 5: S16–S19, 2009 [PMC free article] [PubMed] [Google Scholar]
- 48. Vasudevan S, Tong Y, Steitz JA. Cell-cycle control of microRNA-mediated translation regulation. Cell Cycle 7: 1545–1549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 318: 1931–1934, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron 63: 865–878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA 105: 9093–9098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamatani M, Konishi T, Murakami M, Okuda T. Hyperventilation activation on EEG recording in childhood. Epilepsia 35: 1199–1203, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep 28: 177–184, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNFalpha. Brain Res 1009: 129–136, 2004 [DOI] [PubMed] [Google Scholar]