Abstract
Obstructive sleep apnea (OSA) causes intermittent hypoxia (IH) during sleep. Both obesity and OSA are associated with insulin resistance and systemic inflammation, which may be attributable to tissue hypoxia. We hypothesized that a pattern of hypoxic exposure determines both oxygen profiles in peripheral tissues and systemic metabolic outcomes, and that obesity has a modifying effect. Lean and obese C57BL6 mice were exposed to 12 h of intermittent hypoxia 60 times/h (IH60) [inspired O2 fraction (FiO2) 21–5%, 60/h], IH 12 times/h (FiO2 5% for 15 s, 12/h), sustained hypoxia (SH; FiO2 10%), or normoxia while fasting. Tissue oxygen partial pressure (PtiO2) in liver, skeletal muscle and epididymal fat, plasma leptin, adiponectin, insulin, blood glucose, and adipose tumor necrosis factor-α (TNF-α) were measured. In lean mice, IH60 caused oxygen swings in the liver, whereas fluctuations of PtiO2 were attenuated in muscle and abolished in fat. In obese mice, baseline liver PtiO2 was lower than in lean mice, whereas muscle and fat PtiO2 did not differ. During IH, PtiO2 was similar in obese and lean mice. All hypoxic regimens caused insulin resistance. In lean mice, hypoxia significantly increased leptin, especially during SH (44-fold); IH60, but not SH, induced a 2.5- to 3-fold increase in TNF-α secretion by fat. Obesity was associated with striking increases in leptin and TNF-α, which overwhelmed effects of hypoxia. In conclusion, IH60 led to oxygen fluctuations in liver and muscle and steady hypoxia in fat. IH and SH induced insulin resistance, but inflammation was increased only by IH60 in lean mice. Obesity caused severe inflammation, which was not augmented by acute hypoxic regimens.
Keywords: intermittent hypoxia, insulin resistance, obesity, mouse
intermittent hypoxia, a hallmark feature of obstructive sleep apnea (OSA), is believed to be a major factor in the development and progression of metabolic dysfunction (11, 23, 26, 28, 37, 46). However, despite a copious body of literature on metabolic and cardiovascular outcomes, almost nothing is known about changes in tissue oxygen partial pressure (PtiO2) during intermittent hypoxia (3).
The majority of patients with OSA are obese (38, 55, 56). Obesity per se causes insulin resistance, Type 2 diabetes, systemic inflammation, oxidative stress, and adverse cardiovascular outcomes (10). Adipose tissue hypoxia is implicated in the development of insulin resistance and systemic inflammation of obesity (12, 53). Obese individuals show significantly lower oxygen levels in adipose tissue than normal-weight subjects (9, 19, 32). Oxygen levels in other insulin-sensitive tissue, such as liver and skeletal muscle, in obese and lean subjects have not been compared.
The authors of the present study have developed a mouse model of intermittent hypoxia, recapitulating the nocturnal oxygen profile in patients with OSA, and have shown that chronic intermittent hypoxia induces insulin resistance, systemic inflammation, and oxidative stress in mice (14, 17, 35, 42). Nevertheless, multiple aspects of OSA and intermittent hypoxia remain poorly understood. Metabolic outcomes of OSA have been correlated with the severity of the disease, but this relationship has not been sufficiently addressed in the animal model. A more severe nadir of SpO2 (oxygen saturation measured by pulse oximetry) led to more severe hepatic oxidative stress and dyslipidemia at the same frequency of intermittent hypoxia (25), but the effects of frequency of intermittent hypoxia on tissue O2 levels and metabolic outcomes have not been systematically investigated in animal models.
In the present study, lean and obese mice were exposed to two different paradigms of intermittent hypoxia: 12 times/h and 60 times/h, as well as sustained hypoxia and measured 1) oxygen levels in blood, liver, muscle, and epididymal fat in real time; 2) indexes of insulin resistance, including fasting blood glucose and plasma insulin levels, as well as levels of adipokines affecting insulin resistance, leptin and adiponectin; and 3) indexes of oxidative stress and inflammation. Commonly used by investigators regimens of intermittent and sustained hypoxia were chosen (34, 35, 47).
We hypothesized that a regimen of hypoxic exposure determines both oxygen profiles in peripheral tissues and systemic metabolic outcomes, and that obesity has a modifying effect.
METHODS
Ethical approval.
All protocols complied with and were approved by the Johns Hopkins University Animal Care and Use Committee and were in accordance with the current National Institutes of Health guidelines (Guide for the Care and Use of Laboratory Animals).
Experimental animals.
In total, 143 6- to 8-wk-old male lean C57BL/6J mice, purchased from Jackson Laboratory (Bar Harbor, ME), were used in the study. Seventy-two animals were used for all reported metabolic changes, oxidative stress, and inflammation. Forty-eight animals were used for immunoblotting and immunohistostaining for hypoxia probe. Twenty-three animals were used solely for assessing oxygen profiles in arterial blood and metabolically active tissues. Lean mice were fed a regular chow diet, whereas obese mice were fed a high-fat diet (TD 03584, Teklad WI, 5.4 kcal/g, 35.2% fat, 58.4% of kcal from fat). Mice were housed in a light-dark regulated environment with the 12-h light phase administered from 9 AM to 9 PM. All measurements were obtained at an age of 25–30 wk.
Experimental design.
The authors of the present study have developed a mouse model of intermittent hypoxia, as previously described (39). Briefly, a gas control delivery system was designed to regulate the flow of room air, nitrogen, and oxygen into cages. During intermittent hypoxia 60 times/h, the fractional inhaled O2 was reduced from 20.9 to ∼5% over 30 s and then reoxygenated to room air levels in the subsequent 30 s, resulting in 60 hypoxic events per hour. During intermittent hypoxia 12 times/h, the fractional inhaled O2 was reduced from 20.9 to ∼5% within 20 s, kept at ∼5% for the subsequent 15 s, and then rapidly reoxygenated to room air levels. This cycling was repeated every 5 min, resulting in 12 hypoxic events per hour (34). During sustained isobaric hypoxia, the fractional inhaled O2 was reduced to a sustained level of 10%. Normoxic mice had continuous exposure to room air. For all measurements, except for assessing oxygen profiles, mice were exposed for one night (12 h) to normoxia, intermittent hypoxia 12 times/h, intermittent hypoxia 60 times/h, or sustained hypoxia while fasting. Thirty minutes before the end of the exposure, blood glucose was measured in unanesthetized mice by tail bleeding. Immediately after the exposure, mice were anesthetized with 1–2% isoflurane, and blood, liver, muscle, and epididymal fat were obtained for further analysis. In a second set of 11 lean and 12 obese mice without previous conditioning to hypoxia, PtiO2 in liver, muscle and epididymal fat was measured during the different hypoxic regimens using a fast-response oxygen microelectrode (OX-50, Unisense A/S, Denmark; 50-μm diameter, 90% response time <5 s) simultaneously with noninvasive pulse oximetry by applying a Mouse CollarClip (Starr Life Sciences, Oakmont, PA). The oxygen microelectrode was calibrated before and after measurement in each animal, according to the manufacturer's protocol. The mice were anesthetized with 1.5 g/kg urethane intraperitoneally for this procedure. The snout was positioned through a hole into the same type of cage used for the first set of mice. Then the skin over the different tissues was cut open to allow measurement of tissue oxygenation with the probe. The oxygen microelectrode was mounted on a micromanipulator and inserted vertically 2 mm from the surface and then slightly retracted to a point where a stable PtiO2 signal was obtained. All three hypoxic regimens, as well as normoxia, were delivered as described above and recorded in the same position before moving the oxygen microelectrode to a different position in the same tissue or to a different tissue. The duration of measurement was at least 2 min for both normoxia and sustained hypoxia and 5 min for the intermittent hypoxic regimens. Overall, each experimental animal was used for multiple measurements (four experimental regimens in three tissues).
Hypoxia probe.
Immunohistostaining for hypoxia probe was done using Hypoxyprobe-1 kit (HP2–100, Chemicon International), according to the manufacturer's protocol. Briefly, Hypoxyprobe-1 was injected intraperitoneally at 60 mg/kg body wt at 30 min before tissue collection, while exposure to hypoxia or normoxia continued. Fresh tissue was fixed in 10% buffered formalin, dehydrated in ethanol, and embedded in paraffin. Immunohistostaining was performed with the primary Hypoxyprobe-1 antibody conjugated with FITC and a secondary antibody of anti-FITC antibody conjugated to horseradish peroxidase. For Western blotting, fresh liver, muscle, and epididymal fat were frozen immediately in liquid nitrogen. SDS-PAGE and Western blot were performed using Bio-Rad precast gel system. Protein (20 μg) was applied per lane. We used Hypoxyprobe-1 antibody as the primary antibody.
Biochemistry.
Fasting plasma total cholesterol and triglycerides were measured with kits from Wako Diagnostics (Richmond, VA). Blood glucose was tested with Accu-Chek Comfort Curve kit from Roche Diagnostics (Indianapolis, IN). Plasma insulin and leptin were measured with ELISA kits from Millipore (Billerica, MA). Adiponectin was assessed with an ELISA kit from R&D Systems (Minneapolis, MN). Malondialdehyde was measured with an assay from Percipio Biosciences. To measure tumor necrosis factor-α (TNF-α) secretion ex vivo, epididymal fat tissue fragments were incubated for 3 h in M199 medium (Gibco, Langley, OK) containing 1% BSA. TNF-α levels in the medium were assessed with an ELISA kit from R&D Systems (Minneapolis, MN). For real-time polymerase chain reaction (PCR), total RNA was extracted from liver using TRIzol (Invitrogen, Carlsbad, CA), and complementary DNA was synthesized using Advantage RT for PCR kit from Clontech (Palo Alto, CA). Real-time reverse transcriptase PCR was performed with primers and probe specific for TNF-α, as previously described (42). The mRNA expression levels were normalized to 18S ribosomal RNA concentrations using the standard ΔΔCt approach (http://www.ambion.com/techlib/basics/rtpcr/index.html), as our laboratory has previously done (25), and then expressed as a ratio of mRNA in a sample of interest to the average mRNA level in normoxic mice.
Data analysis.
Analyses were performed using Stata version 9.0. Data are presented as means ± SE. Comparison of oxygen levels between lean and obese mice was performed using a two-sample Wilcoxon rank-sum (Mann-Whitney) test. Comparison of mean variance in oxygen levels was performed using a Wilcoxon signed-rank test. Comparison of metabolic and inflammatory indexes between hypoxic and normoxic conditions within lean or obese phenotype were performed using unpaired t-tests. Comparisons of metabolic and inflammatory indexes between lean and obese mice were performed using a general linear model ANOVA. A P value of <0.05 was considered significant.
RESULTS
Lean mice.
In lean mice, preexposure body weights in the normoxic group were lower than in all hypoxic groups (Table 1). The initial weight difference was intentional in anticipation of a greater weight loss in hypoxic groups (16). The body weight at the time of death was similar in all groups, except for mice exposed to sustained hypoxia, which were heavier than other groups. Weight loss during all hypoxic exposures was significantly greater compared with normoxia, with both intermittent hypoxic regimens showing the greatest weight loss.
Table 1.
Basic characteristics
| Lean Mice |
Obese Mice |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Air | IH12 | IH60 | SH | Air | IH12 | IH60 | SH | P Value | |
| N | 18 | 18 | 18 | 18 | 12 | 12 | 12 | 12 | |
| Age, wk | 25–30 | 25–30 | 25–30 | 25–30 | 25–30 | 25–30 | 25–30 | 25–30 | |
| Body weight before exposure, g | 28.7 ± 0.4 | 30.5 ± 0.6* | 31.0 ± 0.6* | 30.2 ± 0.5* | 42.4 ± 1.9 | 43.3 ± 2.1 | 43.0 ± 1.9 | 42.2 ± 1.7 | <0.001 |
| Body weight after exposure, g | 26.9 ± 0.3 | 26.5 ± 0.4 | 26.8 ± 0.4 | 28.2 ± 0.5* | 40.2 ± 1.8 | 39.7 ± 2.0 | 39.4 ± 1.9 | 39.1 ± 1.7 | <0.001 |
| Body weight loss, % | 7.6 ± 0.6 | 12.7 ± 0.6* | 12.8 ± 0.6* | 9.3 ± 0.3 | 5.2 ± 0.4 | 8.4 ± 0.3† | 8.8 ± 0.5† | 7.5 ± 0.5† | <0.001 |
| Plasma triglycerides, mg/dl | 28.4 ± 1.9 | 17.4 ± 1.1* | 21.7 ± 0.9* | 32.0 ± 1.9 | 54.7 ± 2.3 | 47.0 ± 3.7 | 51.4 ± 2.3 | 33.1 ± 2.6† | <0.001 |
| Plasma total cholesterol, mg/dl | 97 ± 2 | 107 ± 3* | 105 ± 3 | 106 ± 5 | 176 ± 10 | 158 ± 8 | 177 ± 11 | 181 ± 11 | <0.001 |
Values are means ± SE; n, no. of mice. Values are shown for normoxia (air), intermittent hypoxia 12 times/h (IH12), intermittent hypoxia 60 times/h (IH60), and sustained hypoxia (SH) in both lean and obese mice.
P < 0.05 compared with lean air group.
P < 0.05 compared with obese air group. The P values in the right column compare lean mice vs. obese mice using a general linear model ANOVA.
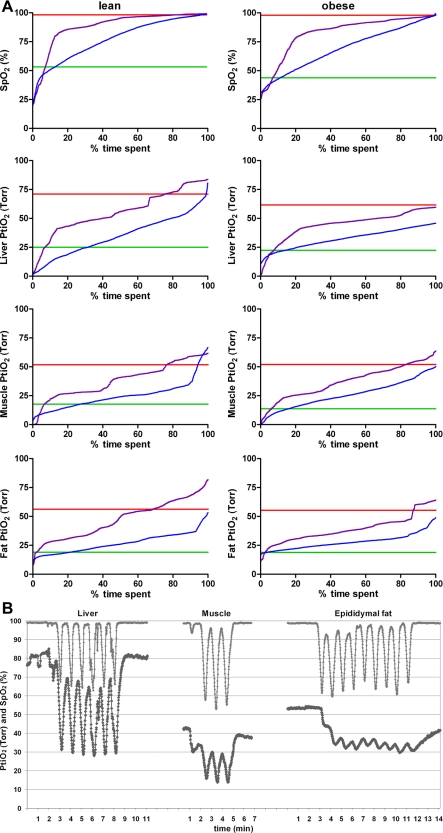
The mean oxygen levels in blood and all organs were highest in normoxia and decreased progressively from normoxia to infrequent intermittent hypoxia (12 times/h), frequent intermittent hypoxia (60 times/h), and sustained hypoxia being the lowest (P < 0.001, Fig. 1A, Table 2). Comparison of PtiO2 in different tissues showed that the mean PtiO2 was significantly higher in the liver than in muscle and fat (P < 0.001). During frequent intermittent hypoxia, high-amplitude swings of PtiO2 were observed in blood and liver and, to a lesser degree, in muscle. The variability of PtiO2 in muscle and epididymal fat during frequent intermittent hypoxia was significantly less compared with SpO2. The lowest variability of PtiO2 during frequent intermittent hypoxia was observed in epididymal fat (Fig. 1, Table 3).
Fig. 1.
A: average distribution of oxygen saturation measured by pulse oximetry (SpO2) and tissue oxygen partial pressure (PtiO2) during normoxic and hypoxic conditions in lean and obese mice. The red curves show normoxic group, the purple curves show infrequent intermittent hypoxia (12 times/h; IH12), the blue curves show frequent intermittent hypoxia (60 times/h; IH60), and the green curves show sustained hypoxia (SH). SpO2 and PtiO2 values were normalized to 100% (%time spent) for each regimen. B: example tracings during frequent intermittent hypoxia in a lean mouse. The top curves show the SpO2 in arterial blood, the bottom curves show the PtiO2 in the different tissues at the same time.
Table 2.
Mean oxygen levels in arterial blood and tissues
| Regimen | Lean Mice | Obese Mice | P Value | |
|---|---|---|---|---|
| SpO2, % | Air | 98.3 ± 0.1 | 98.0 ± 0.1 | 0.090 |
| IH12 | 92.2 ± 2.1 | 84.7 ± 1.7 | 0.022 | |
| IH60 | 79.2 ± 1.2 | 73.4 ± 1.5 | 0.045 | |
| SH | 53.2 ± 1.6 | 43.9 ± 3.3 | 0.055 | |
| Liver PtiO2, Torr | Air | 71.1 ± 3.6 | 61.6 ± 1.4 | 0.028 |
| IH12 | 60.0 ± 7.2 | 52.2 ± 4.0 | NS | |
| IH60 | 39.9 ± 3.1 | 37.8 ± 2.5 | NS | |
| SH | 25.0 ± 2.2 | 22.4 ± 2.4 | NS | |
| Muscle PtiO2, Torr | Air | 51.8 ± 5.7 | 52.0 ± 2.0 | NS |
| IH12 | 37.9 ± 7.8 | 36.7 ± 4.3 | NS | |
| IH60 | 26.0 ± 4.4 | 24.2 ± 2.9 | NS | |
| SH | 17.7 ± 4.4 | 13.7 ± 6.0 | NS | |
| Epididymal fat PtiO2, Torr | Air | 56.3 ± 3.8 | 55.4 ± 3.4 | NS |
| IH12 | 47.1 ± 5.2 | 38.9 ± 3.6 | NS | |
| IH60 | 26.7 ± 2.8 | 28.8 ± 2.0 | NS | |
| SH | 19.0 ± 2.8 | 18.9 ± 3.5 | NS |
Values are means ± SE; n = 11 lean mice, n = 12 obese mice. Oxygen saturation measured by pulse oximetry (SpO2) and tissue oxygen partial pressures (PtiO2) are shown for normoxia (air), IH12, IH60, and SH. NS, not significant. Bolded P values are significant.
Table 3.
Variability in oxygen levels during intermittent hypoxia in arterial blood and tissues
| Nadir |
Peak |
Standard Deviation |
|||||
|---|---|---|---|---|---|---|---|
| Regimen | Tissue | Lean | Obese | Lean | Obese | Lean | Obese |
| IH12 | SpO2, % | 47.0 ± 7.5 | 32.5 ± 3.2 | 96.7 ± 0.9 | 94.1 ± 1.2 | 13.9 ± 2.3 | 16.8 ± 1.2 |
| Liver PtiO2, Torr | 22.0 ± 6.3 | 15.8 ± 5.5 | 65.1 ± 7.0 | 59.4 ± 3.5 | 11.4 ± 1.6 | 11.3 ± 1.2* | |
| Muscle PtiO2, Torr | 9.2 ± 5.1 | 8.0 ± 3.0 | 43.8 ± 9.2 | 43.0 ± 4.6 | 10.0 ± 1.9 | 9.7 ± 0.9* | |
| Epididymal fat PtiO2, Torr | 21.7 ± 3.3 | 21.4 ± 2.7 | 55.8 ± 6.1 | 45.3 ± 3.5 | 10.0 ± 2.3 | 7.0 ± 1.5* | |
| IH60 | SpO2, % | 49.2 ± 3.2 | 44.6 ± 3.1 | 96.6 ± 0.3 | 95.2 ± 1.0 | 16.7 ± 1.0 | 17.6 ± 1.0 |
| Liver PtiO2, Torr | 16.8 ± 3.3 | 20.1 ± 2.7 | 59.9 ± 5.0 | 52.4 ± 4.0 | 13.1 ± 1.8 | 9.4 ± 1.2* | |
| Muscle PtiO2, Torr | 11.2 ± 2.1 | 8.7 ± 3.2 | 37.7 ± 7.3 | 36.8 ± 3.9 | 8.9 ± 2.0* | 9.0 ± 1.3* | |
| Epididymal fat PtiO2, Torr | 21.1 ± 2.5 | 23.9 ± 2.0 | 31.7 ± 4.0 | 33.3 ± 2.7 | 3.7 ± 1.1*† | 3.2 ± 0.8*†‡ | |
Values are means ± SE; n = 11 lean mice, n = 12 obese mice. SpO2and PtiO2 are shown for IH12 and IH60.
P < 0.05 compared with SpO2.
P < 0.05 compared with liver PtiO2.
P < 0.05 compared with muscle PtiO2.
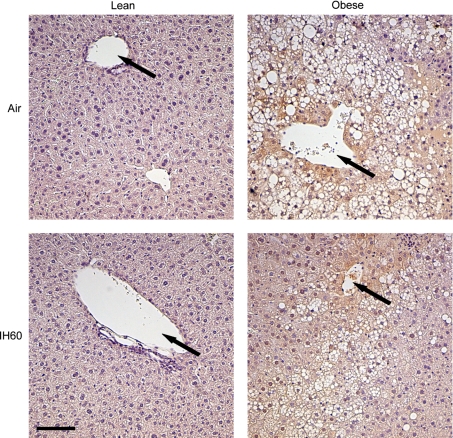
Hypoxyprobe-1 (pimonidazole hydrochloride) was used to assess hypoxia in the different tissues. Following injection, Hypoxyprobe-1 is distributed to all tissues and forms adducts with thiol containing proteins only in those cells that have a PtiO2 < 10 Torr (29). Lean mice demonstrated no evidence of tissue hypoxia in the immunohistostaining (Fig. 2) or immunoblotting (not shown), regardless of hypoxic regimen or control condition.
Fig. 2.
Immunohistostaining of the liver with Hypoxyprobe (pimonidazole) counterstained with hematoxylin. Representative immunohistostaining is shown for both lean and obese mice in the normoxic group (air) and the IH60 group. Original magnification: ×200. Hypoxic areas are stained in brown (pimonidazole). Arrows mark hepatic vein. Scale bar is 50 μm.
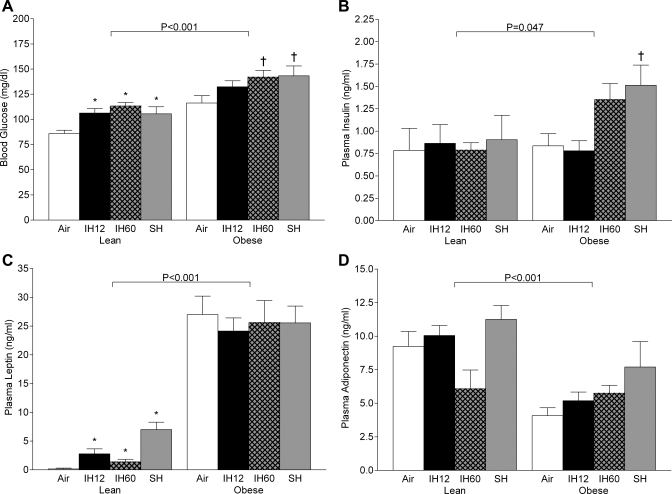
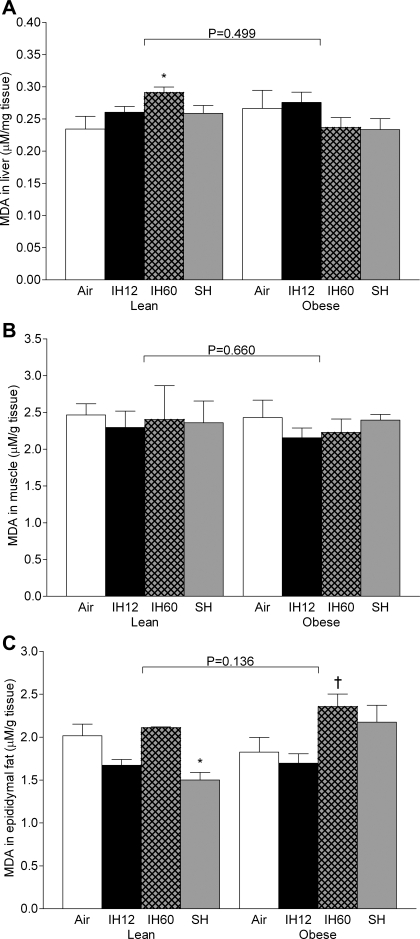
Fasting blood glucose significantly increased by ∼20 mg/dl in all three hypoxic groups compared with normoxia, without changes in plasma insulin (Fig. 3, A and B). Hypoxia significantly increased plasma leptin levels, especially in the sustained hypoxia group (44-fold, Fig. 3C). There was no significant change in adiponectin at any hypoxic condition compared with normoxia (Fig. 3D). Plasma total cholesterol increased during all hypoxic regimens, but only intermittent hypoxia 12 times/h differed significantly from normoxia (Table 1). Plasma triglycerides were decreased in both intermittent hypoxia regimens. Lipid peroxidation was significantly increased by intermittent hypoxia 60 times/h in the liver, but not in muscle or fat (Fig. 4). There was a greater than twofold increase in TNF-α secretion by adipose tissue in the frequent intermittent hypoxia group compared with normoxia (Fig. 5). Hypoxia did not affect TNF-α expression in the liver of lean mice (Fig. 6).
Fig. 3.
Indexes of insulin resistance after different hypoxic regimens and 12-h fast. A: glucose. B: insulin. C: leptin. D: adiponectin. Values are means ± SE. Values are shown for normoxia (air), IH12, IH60, and SH. *P < 0.05 compared with lean air group. †P < 0.05 compared with obese air group.
Fig. 4.
Lipid peroxidation measured by malondialdehyde (MDA) levels after different hypoxic regimens. A: liver. B: muscle. C: epididymal fat. Values are means ± SE. Values are shown for normoxia (air), IH12, IH60, and SH. *P < 0.05 compared with lean air group. †P < 0.05 compared with obese air group.
Fig. 5.
Secretion of tumor necrosis factor-α (TNF-α) by adipose tissue ex vivo after different hypoxic regimens. Values are means ± SE. Values are shown for normoxia (air), IH12, IH60, and SH. *P < 0.05 compared with lean air group.
Fig. 6.
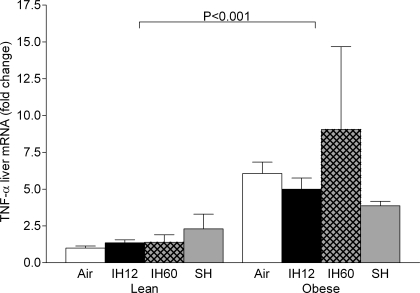
Results of the real-time PCR for TNF-α in liver tissue. Values are means ± SE. TNF-α levels are shown as mRNA expression levels normalized to 18S ribosomal RNA concentrations and then expressed as a ratio to the lean air group.
Obese mice.
Obese mice were intentionally grouped to yield a higher preexposure body weight in both intermittent hypoxia groups due to anticipated weight loss during hypoxic exposure (Table 1). After exposure, body weights were similar in all exposure groups. Hypoxic mice lost more weight than control animals, regardless of the hypoxic regimen. Absolute weight loss was similar to that of lean mice.
Obese mice showed a trend towards lower SpO2 than lean mice at normoxic conditions, although the difference was very small (Table 2). Compared with lean mice, obese animals had lower levels of mean SpO2 during all hypoxic exposures. Of interest, liver PtiO2 at baseline conditions was lower in obese mice than in lean animals, but not during hypoxic exposures. PtiO2 in muscle and fat was similar in both groups, regardless of experimental conditions. As expected, the mean oxygen levels in blood and all organs were highest in normoxia and declined progressively from normoxia to infrequent intermittent hypoxia, frequent intermittent hypoxia, and sustained hypoxia (P < 0.001). As in lean mice, comparison of PtiO2 in different tissues showed that the mean PtiO2 was significantly higher in the liver than muscle and fat (P < 0.001). Similar to lean mice, frequent intermittent hypoxia induced high-amplitude swings of oxygen levels in blood, liver, and muscle, but not in epididymal fat tissue (Table 3). Mean variability during frequent intermittent hypoxia was significantly less in all tissues compared with SpO2. In contrast to lean mice, these differences were also prominent during infrequent intermittent hypoxia. As in lean mice, the lowest variability was observed in epididymal fat during frequent intermittent hypoxia. In the livers of obese mice, Hypoxyprobe-1 staining revealed evidence of hypoxia, predominantly in the areas surrounding hepatic veins, which were present at all conditions, including normoxia (Fig. 2). Hypoxyprobe-1 staining in muscle and fat was negative in lean and obese mice, regardless of the condition (not shown).
As expected, fasting blood glucose was higher in obese than in lean mice at all experimental conditions. As in lean mice, fasting blood glucose was increased by acute hypoxia (Fig. 3A). Fasting insulin levels were increased by frequent intermittent hypoxia and sustained hypoxia, which did not occur in lean mice (Fig. 3B). Obese mice showed a greater than 100-fold increase in leptin levels compared with baseline in lean mice, which were not modified by acute hypoxia (Fig. 3C). Plasma adiponectin levels were decreased by obesity without significant effects of acute hypoxia (Fig. 3D). Plasma total cholesterol and triglyceride levels were higher in obese than lean mice across all experimental conditions. Hypoxic regimens did not affect cholesterol levels, and there was a reduction in triglyceride in the sustained hypoxia group (Table 1). Lipid peroxidation was similar to that in lean mice in all organs. There was a mild increase in malondialdehyde levels in fat tissue during frequent intermittent hypoxia compared with normoxia and infrequent intermittent hypoxia (Fig. 4). There was a 10-fold increase in TNF-α secretion rate by adipose tissue in obese mice compared with lean mice at baseline conditions (Fig. 5), but acute hypoxia had no further impact. There was a four- to sixfold increase in TNF-α liver mRNA levels in obese mice compared with lean mice at baseline conditions (Fig. 6), and acute hypoxia had no further impact.
DISCUSSION
To the best of our knowledge, this is the first study to determine the impact of different regimens of acute hypoxia on tissue oxygen levels in lean and obese mice. Several novel findings resulted from the study. First, frequent intermittent hypoxia caused large-amplitude oxygen swings in the liver, mirroring swings of SpO2, whereas fluctuations of oxygen levels were attenuated in muscle and nearly abolished in adipose tissue. Second, at baseline normoxic conditions, obesity led to lower oxygen levels in the liver, predominantly in the areas surrounding hepatic veins, whereas oxygen levels in muscle and fat did not differ between the lean and obese states. Third, during hypoxic exposures, oxygen levels in liver, muscle, and fat were similar in obese and lean mice. Several additional findings resulted from the study. In lean mice, all types of hypoxia induced insulin resistance and increased circulating leptin levels, but only frequent intermittent hypoxia (60 times/h) increased lipid peroxidation and TNF-α production by adipose tissue. In obese mice, frequent intermittent hypoxia and sustained hypoxia augmented insulin resistance, but obesity-induced increases in plasma leptin levels and TNF-α secretion overwhelmed any effect of acute hypoxia. In the discussion below, we will further elaborate on significance of these findings.
Patterns of hypoxia in different tissues during intermittent hypoxia.
Oxygen levels in various tissues at baseline normoxic conditions have been previously measured in humans and several animal species, including cats, guinea pigs, dogs, rabbits, and rats (49). Mean Po2 values ranged from 1–3 Torr in rat pons to 20–30 Torr in rat liver, and 30–35 mmHg in dog liver to 60–70 mmHg in dog kidneys. We report relatively high baseline liver PtiO2 in lean mice of 71 Torr (Table 2). Using the same type of oxygen electrode and the same technique, Almendros et al. (2) recently reported baseline oxygen levels of 40–45 Torr in rat skeletal muscle and ∼50 Torr in rat visceral fat, which was ∼5 Torr lower than in our study (Table 2). Thus discrepancies between presently reported and previously described baseline values could be attributed to a different type of electrode used in the past (49) or interspecies differences, since none of the previous measurements were done in mice.
An important implication of the present work is that it describes patterns of oxygen in different tissues during intermittent hypoxia. A growing body of clinical and experimental literature previously assumed a priori that fluctuations in blood oxygen observed in intermittent hypoxia and sleep apnea were translated into oxygen swings in different organs and tissues. There was a general assumption that the pathogenesis of intermittent hypoxia and sleep apnea is similar to ischemia-reperfusion injury (22, 23, 31). The effects of intermittent asphyxia and intermittent hypoxia on oxygen levels in cerebral cortex, skeletal muscle, and visceral fat of a rat have recently been examined (2, 3). The report by Almendros et al. (2) suggests that fluctuations of blood oxygen levels during intermittent asphyxia and intermittent hypoxia result in significant oxygen swings in the brain and skeletal muscle, whereas the amplitude of the swings is attenuated in visceral fat. The present study established that intermittent hypoxia indeed generates hypoxia-reoxygenation in liver and skeletal muscle, whereas white adipose tissue exhibits a sustained hypoxia pattern, especially during frequent intermittent hypoxia. The sustained pattern could be attributed to relatively poor perfusion of adipose tissue (32).
Over the last decade, a number of papers have been published attempting to address molecular mechanisms of intermittent hypoxia utilizing cell culture (27, 30, 36, 40, 57). However, exposure regimens were arbitrary in these systems, because PtiO2 in vivo during intermittent hypoxia had been unknown. Based on the present data, a realistic modeling of in vivo conditions in intermittent hypoxia cell culture can be employed.
Effects of different regimens of hypoxic exposure.
Compared with infrequent intermittent hypoxia, frequent intermittent hypoxia and sustained hypoxia induced more severe hypoxia in insulin-sensitive tissues, liver, skeletal muscle, and white adipose tissue (Fig. 1A). Infrequent intermittent hypoxia induced similar SpO2 and PtiO2 nadirs and peaks to frequent intermittent hypoxia, but the mean SpO2 and PtiO2 levels were much higher during the former regimen compared with the latter (Tables 2 and 3). An overshoot in PtiO2 values during intermittent hypoxia compared with normoxia (Fig. 1A) is likely explained by compensatory hyperventilation immediately after the hypoxic episode (33) and a compensatory increase in cardiac output (1, 43). In lean mice, all types of hypoxia caused identical increases in fasting blood glucose without changes in serum insulin levels, suggesting that peripheral tissues are resistant to insulin action and pancreatic β-cells are incapable of an increase in insulin secretion. The authors of the present study have previously performed a euglycemic hyperinsulinemic clamp in mice and have shown that acute intermittent hypoxia (60 times/h over 9 h) induces insulin resistance (14). Hypoxic injury of pancreatic β-cells during intermittent hypoxia 60 times/h has also been reported in mice (52, 54). Louis and Punjabi (26) have utilized an intravenous glucose tolerance test in healthy human volunteers and reported similar finding of decreased insulin sensitivity and the lack of adequate increase in pancreatic insulin secretion after intermittent hypoxia. The present study shows that even infrequent episodic hypoxia (12 times/h) is sufficient to disturb insulin and glucose regulations in lean mice.
Suppression of insulin-sensitizing adipokine adiponectin (20) has been invoked as a possible cause of insulin resistance in OSA and intermittent hypoxia (27). The present study found no evidence that adiponectin is involved in intermittent hypoxiainduced insulin resistance, but the levels of biologically active high-molecular-weight adiponectin were not measured. Mechanisms of pancreatic endocrine insufficiency in intermittent hypoxia are not clear, but one possibility is leptin, which suppresses insulin secretion (21, 44). In lean mice, all types of hypoxia caused striking increases in serum leptin, 17-fold in infrequent intermittent hypoxia, 9-fold in frequent intermittent hypoxia, and 44-fold in sustained hypoxia. The authors of the present study have previously shown that intermittent hypoxia increases leptin levels (24, 35). Notably, human OSA is associated with high levels of circulating leptin (15), which can be decreased by treatment with continuous positive airway pressure (7, 13). Overall, the present study shows that infrequent episodic hypoxia was sufficient to induce hyperleptinemia in lean mice, and that sustained hypoxia induced both more severe tissue hypoxia and higher leptin levels than intermittent hypoxia.
Frequent chronic intermittent hypoxia induces oxidative stress in different organs and systems. In rats, chronic intermittent hypoxia causes myocardial oxidative stress that is associated with cardiac dysfunction (6, 51). In contrast to rats, mice are relatively protected from myocardial oxidative stress during intermittent hypoxia (17), but develop oxidative stress in the liver. In the present study, frequent intermittent hypoxia was the only type of exposure that induced a small (20%) increase in lipid peroxidation in liver tissue of lean mice, whereas muscle and fat were not affected. The unique effect of frequent intermittent hypoxia on the mouse liver could be attributed to more vigorous production of reactive oxygen species during large and frequent swings of PtiO2 levels, which were attenuated in muscle and fat. Liver PtiO2 swings did not occur during sustained hypoxia and happen with a lower frequency during infrequent intermittent hypoxia. Of interest, an increase in liver lipid peroxidation did not occur in obese mice, which might be related to the lower amplitude of oxygen fluctuations. Thus the present findings suggest that an increase in hepatic lipid peroxidation in lean mice may be related to frequent high-amplitude swings of PtiO2.
Another unique feature of frequent intermittent hypoxia was inflammatory response manifested by an increase in TNF-α secretion by adipose tissue. This increase did not occur during sustained hypoxia, despite mean fat PtiO2 being lower than nadirs of PtiO2 in both intermittent hypoxic groups (Tables 2 and 3). The inflammatory response cannot be attributed to hypoxia-reoxygenation, because there was virtually no fluctuation in PtiO2 levels in fat. The present findings suggest that excessive TNF-α secretion during frequent intermittent hypoxia is likely attributable to systemic inflammatory response rather than adipose tissue hypoxia per se.
Effects of obesity.
PtiO2 levels in the liver in lean and obese mice at baseline have not been compared previously. The present study showed that, at normoxic conditions, PtiO2 levels in obese livers were, on average, 10 Torr lower than in lean livers (Table 2). Pimonidazole staining revealed that hypoxic areas were in the centers of hepatic lobules around hepatic veins (zone 3). Previous studies have shown that liver tissue is not homogeneous, and hepatocytes surrounding hepatic vein are markedly more hypoxic than periportal hepatocytes (4, 48). The novelty of the present findings is that zone 3 hypoxia was much more prominent in obese mice. Obese mice had higher baseline fasting glucose levels than lean mice, suggesting higher hepatic glucose output. Hypoxia upregulates a key enzyme of hepatic gluconeogenesis, phosphoenolpyruvate carboxykinase (8), but the role of tissue hypoxia in obesity-induced gluconeogenesis has not been explored. Thus obesity is associated with hypoxia in liver tissue.
Obesity did not induce hypoxia in epididymal fat and skeletal muscle at baseline conditions. While effects of obesity on PtiO2 in skeletal muscle have not been previously studied, the present data in fat differ from previous reports in mice (53). However, Ye et al. (53) measured PtiO2 in ob/ob mice, which are more obese than mice with diet-induced obesity. Lacking leptin, ob/ob mice have poor adipose tissue perfusion (50), because the lack of leptin induced angiogenesis (5, 45). Of note, human studies also consistently show that obesity induces adipose tissue hypoxia (9, 19, 32). It is conceivable that, if PtiO2 would have been measured in the present study in other adipose tissue depots (omental, retroperitoneal, or subcutaneous), hypoxia might have been detected.
The effect of obesity on PtiO2 during hypoxic exposure has been previously unknown. Obese mice showed more severe oxyhemoglobin desaturation than lean mice during intermittent hypoxia (Table 2). In context of this finding, it is surprising that obese and lean mice exhibited the same liver, muscle, and fat oxygen levels during all types of hypoxic exposure. It is conceivable that tissue oxygenation is preserved in obesity by a mechanism yet to be determined.
Another important finding of this study was that, despite a similar impact on tissue oxygen levels in obese and lean mice, metabolic effects of hypoxia in obese animals were modest. We propose that effects of acute hypoxia were likely overwhelmed by obesity. Indeed, obesity induced striking changes in adipokine production and increased systemic inflammation. Compared with lean mice, obese mice showed a 170-fold increase in plasma leptin, 11.5-fold increase in adipose TNF-α secretion, a 4- to 6-fold increase in TNF-α expression in the liver, and a 2-fold decrease in plasma adiponectin. Notably, the magnitude of the impact of obesity greatly exceeded effects of hypoxia. The only metabolic effect of acute hypoxia, which was present in obese mice, was insulin resistance. Unlike lean mice, obese mice nearly doubled serum insulin levels in response to frequent intermittent hypoxia and sustained hypoxia, suggesting that pancreatic β-cell secretion was preserved. Obese mice did not increase leptin levels during intermittent and sustained hypoxia, and the lack of inhibitory action of leptin could be accountable for preserved insulin secretion (21, 44). Overall, obesity did not augment levels of hypoxia in metabolically active tissues during intermittent and sustained hypoxia, and the effects of obesity overwhelmed and masked the majority of proinflammatory and metabolic effects of acute hypoxia, except insulin resistance.
Limitations.
The present work has a number of limitations. First, we measured PtiO2 during acute hypoxic exposure. It is conceivable that chronic intermittent hypoxia will result in a different PtiO2 tissue profile. Metabolic and proinflammatory responses to chronic intermittent hypoxia, a hallmark of OSA, may also differ from acute hypoxia. Second, the sustained hypoxia regimen was more severe than both intermittent hypoxia regimens. However, despite the lowest mean inspired O2 fraction, SpO2, and tissue PtiO2, sustained hypoxia induced similar increases in insulin resistance, whereas inflammatory and oxidative stress responses were less prominent, compared with intermittent hypoxia. Third, PtiO2 measurements were performed under general anesthesia. Nevertheless, during both intermittent hypoxic regimens, SpO2 was rebounding to normoxic values between the hypoxic events, suggesting that the observations may be pertinent for unanesthetized animals. Fourth, the probe measured oxygen tension within a small random area, but PtiO2 varies within each organ and tissue. For instance, in the liver, oxygen tension is lowest in the area surrounding the hepatic vein (18). Immunohistochemistry with Hypoxyprobe was used as a complementary approach. Fifth, immunostaining with Hypoxyprobe has a low sensitivity, detecting only severe hypoxia with PtiO2 < 10 Torr. Finally, OSA leads to nocturnal hypercapnia, whereas, in the present model, mice were either hypocapnic or normocapnic (41).
Conclusions and implications.
Frequent acute intermittent hypoxia caused reciprocal oxygen swings in liver and skeletal muscle, whereas fluctuations of PtiO2 were absent in adipose tissue. Intermittent and sustained hypoxia resulted in similar levels of tissue hypoxia and insulin resistance in lean and obese mice. In lean mice, all types of hypoxia raised serum leptin levels, whereas only frequent intermittent hypoxia increased oxidative stress and adipose inflammation. Obesity induced liver hypoxia at baseline and caused a profound impact on adipokine and TNF-α production, which overwhelmed effects of intermittent hypoxia. The present data suggest that infrequent and frequent intermittent hypoxia have similar effects on insulin resistance and adipokine secretion. Given that high-frequency fluctuations of O2 in cell culture are not possible due to a limited rate of gas perfusion in the media, the present work demonstrates that low-frequency swings of O2 achievable in cell culture have biological relevance for OSA.
GRANTS
This work was supported by Research Fellowship Grant RE 2842/1-1 of the German Research Foundation to C. Reinke; Conselho Nacional de Desenvolvimento Científico e Technológico Grant 200032/2009-7 and Fundação Zerbini, Brazil, to L. F. Drager; and National Institutes of Health (NIH) Grant HL-80105 and American Heart Association Grant 10GRNT3360001 to V. Y. Polotsky. This work was also partly supported by NIH Specialized Center of Clinically Oriented Research Grant 5P50-HL-084945 and by the Mid-Atlantic Nutrition Obesity Research Center (formerly Clinical Nutrition Research Unit of Maryland) NIH Grant P30 DK072488.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Nikki Glynn and Urmila Sreenivasan for assessing cytokine secretion from the acute secretion media. The authors further thank Naresh M. Punjabi for support with statistical analysis and helpful suggestions.
REFERENCES
- 1. Adachi H, Strauss W, Ochi H, Wagner HN., Jr The effect of hypoxia on the regional distribution of cardiac output in the dog. Circ Res 39: 314–319, 1976 [DOI] [PubMed] [Google Scholar]
- 2. Almendros I, Farre R, Planas AM, Torres M, Bonsignore MR, Navajas D, Montserrat JM. Tissue oxygenation in brain, muscle and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia (http://www.journalsleep.org/AcceptedPapers/SP-421-10.pdf) Sleep. In press [DOI] [PMC free article] [PubMed]
- 3. Almendros I, Montserrat JM, Torres M, Gonzalez C, Navajas D, Farre R. Changes in oxygen partial pressure of brain tissue in an animal model of obstructive apnea. Respir Res 11: 3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 72: 889–895, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res 83: 1059–1066, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, Mishima M, Nakamura T, Nakao K, Ohi M. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 100: 706–712, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Choi JH, Park MJ, Kim KW, Choi YH, Park SH, An WG, Yang US, Cheong J. Molecular mechanism of hypoxia-mediated hepatic gluconeogenesis by transcriptional regulation. FEBS Lett 579: 2795–2801, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Fleischmann E, Kurz A, Niedermayr M, Schebesta K, Kimberger O, Sessler DI, Kabon B, Prager G. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes Surg 15: 813–819, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gade W, Schmit J, Collins M, Gade J. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci 23: 51–61, 2010 [PubMed] [Google Scholar]
- 11. Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 299: H925–H931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1 alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour SS, Wiest GH, Hahn EG, Lohmann T, Ficker JH. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J 22: 251–257, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 118: 580–586, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 209: 381–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol 295: R1274–R1281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology 31: 255–260, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology 100: 274–280, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JY, Scherer PE. Adiponectin, an adipocyte-derived hepatic insulin sensitizer regulation during development. Pediatr Endocrinol Rev 1, Suppl 3: 428–431, 2004 [PubMed] [Google Scholar]
- 21. Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 100: 2729–2736, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavie L. Obstructive sleep apnoea syndrome–an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Lavie L, Polotsky V. Cardiovascular aspects in obstructive sleep apnea syndrome–molecular issues, hypoxia and cytokine profiles. Respiration 78: 361–370, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1 alpha. Physiol Genomics 25: 450–457, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol 102: 557–563, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol 106: 1538–1544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magalang UJ, Cruff JP, Rajappan R, Hunter MG, Patel T, Marsh CB, Raman SV, Parinandi NL. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes 117: 129–134, 2009 [DOI] [PubMed] [Google Scholar]
- 28. McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med 175: 190–195, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Mueller-Klieser W, Schlenger KH, Walenta S, Gross M, Karbach U, Hoeckel M, Vaupel P. Pathophysiological approaches to identifying tumor hypoxia in patients. Radiother Oncol 20, Suppl 1: 21–28, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2 alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A 106: 1199–1204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park AM, Nagase H, Kumar SV, Suzuki YJ. Effects of intermittent hypoxia on the heart. Antioxid Redox Signal 9: 723–729, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58: 718–725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng YJ, Prabhakar NR. Reactive oxyen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol 94: 2342–2349, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1 alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Polotsky VY, Savransky V, Bevans-Fonti S, Reinke C, Li J, Grigoryev DN, Shimoda LA. Intermittent and sustained hypoxia induce a similar gene expression profile in the human aortic endothelial cells. Physiol Genomics 41: 306–314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 179: 235–240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165: 677–682, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Reinke C, Bevans-Fonti S, Grigoryev DN, Drager LF, Myers AC, Wise RA, Schwartz AR, Mitzner W, Polotsky VY. Chronic intermittent hypoxia induces lung growth in adult mice. Am J Physiol Lung Cell Mol Physiol 300: L266–L273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112: 2660–2667, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293: G871–G877, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schneider H, Schaub CD, Chen CA, Andreoni KA, Schwartz AR, Smith PL, Robotham JL, O'Donnell CP. Neural and local effects of hypoxia on cardiovascular responses to obstructive apnea. J Appl Physiol 88: 1093–1102, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci U S A 96: 674–679, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science 281: 1683–1686, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Stamatakis K, Sanders MH, Caffo B, Resnick HE, Gottlieb DJ, Mehra R, Punjabi NM. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep 31: 1018–1024, 2008 [PMC free article] [PubMed] [Google Scholar]
- 47. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Terada N, Ohno N, Saitoh S, Ohno S. Immunohistochemical detection of hypoxia in mouse liver tissues treated with pimonidazole using “in vivo cryotechnique”. Histochem Cell Biol 128: 253–261, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Vanderkooi JM, Erecinska M, Silver IA. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol Cell Physiol 260: C1131–C1150, 1991 [DOI] [PubMed] [Google Scholar]
- 50. Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Williams AL, Chen L, Scharf SM. Effects of allopurinol on cardiac function and oxidant stress in chronic intermittent hypoxia. Sleep Breath 14: 51–57, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med 46: 783–790, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol 586: 899–911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 56. Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 162: 893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 2011. (February 1, 2011). doi: 10.1002/jcp.22640 [DOI] [PMC free article] [PubMed] [Google Scholar]