Abstract
Mice deficient in the transcription factor Pet-1−/− have a ∼70% deficiency of brainstem serotonin [5-hydroxytryptamine (5-HT)] neurons and exhibit spontaneous bradycardias in room air at postnatal day (P)5 and P12 and delayed gasping in response to a single episode of anoxia at P4.5 and P9.5 (Cummings KJ, Li A, Deneris ES, Nattie EE. Am J Physiol Regul Integr Comp Physiol 298: R1333–R1342, 2010; and Erickson JT, Sposato BC. J Appl Physiol 106: 1785–1792, 2009). We hypothesized that at a critical age Pet-1−/− mice will fail to autoresuscitate during episodic anoxia, ultimately dying from a failure of gasping to restore heart rate (HR). We exposed P5, P8, and P12 Pet-1−/− mice and wild-type littermates (WT) to four 30-s episodes of anoxia (97% N2-3% CO2), separated by 5 min of room air. We observed excess mortality in Pet-1−/− only at P8: 43% of Pet-1−/− animals survived past the third episode of anoxia while ∼95% of WT survived all four episodes (P = 0.004). No deaths occurred at P5 and at P12, and one of six Pet-1−/− mice died after the fourth episode, while all WT animals survived. At P8, dying Pet-1−/− animals had delayed gasping, recovery of HR, and eupnea after the first two episodes of anoxia (P < 0.001 for each); death ultimately occurred when gasping failed to restore HR. Both high- and low-frequency components of HR variability were abnormally elevated in dying Pet-1−/− animals following the first episode of anoxia. Dying P8 Pet-1−/− animals had significantly fewer 5-HT neurons in the raphe magnus than surviving animals (P < 0.001). Our data indicate a critical developmental window at which a brainstem 5-HT deficiency increases the risk of death during episodes of anoxia. They may apply to the sudden infant death syndrome, which occurs at a critical age and is associated with 5-HT deficiency.
Keywords: 5-hydroxytryptamine, sudden infant death syndrome, hypoxia, gasping, neonate
the sudden infant death syndrome (SIDS) is the sudden death of an infant under 12 mo of age that is unexplained by death scene investigation and autopsy (23). Despite international public campaigns advocating the supine sleep position, SIDS remains the leading cause of infant mortality in the Western world, with an overall incidence in the United States of 0.57/1,000 live births (25). Perhaps the most intriguing aspect of SIDS is the age at which it occurs: 90% of the deaths occur before 6 mo of age, with 75% of these deaths between 2 and 4 mo of age (25). The triple risk hypothesis postulates that SIDS occurs in an infant: 1) with an inherent vulnerability, 2) during a critical period, and 3) when exposed to an environmental stressor that would normally be ameliorated by a physiological response (7). Hypoxia is a stressor that likely plays a key role in SIDS: in a recent report, ∼85% of sudden and unexpected deaths were associated with circumstances implicating hypoxia/asphyxia (29), and pathologic studies of SIDS infants reveal multiple tissue markers of chronic or intermittent hypoxia (18). Episodic apnea and bradycardia, both of which can produce hypoxia, have also been reported in SIDS cases days or even weeks before the final lethal event (33, 34, 41). Severe hypoxia or anoxia leads to bradycardia, a cessation of breathing (apnea), and hypotension (11, 12). This initiates autoresuscitation, which restores heart rate (HR), blood pressure, and breathing. Critical to the success of autoresuscitation is the prompt appearance of gasping along with an associated increase in sympathetic activity during each gasp (5, 26, 40). In some SIDS cases, death occurs when gasping fails to increase HR (33, 34, 41), possibly because of autonomic dysfunction (8, 13, 21).
Evidence from several laboratories (4, 20, 24, 28, 30) suggests that SIDS is associated with multiple serotonergic abnormalities within regions of the medulla oblongata critical for cardiorespiratory responses to hypoxia, including those occurring during autoresuscitation. These abnormalities include a partial deficiency of 5-HT and its key biosynthetic enzyme, tryptophan hydroxylase (2). This could have negative consequences for autoresuscitation, since there is evidence that 5-HT participates in the generation and/or maintenance of gasping (42, 46) as well as autonomic adjustments to physiological stress (35).
We utilize mice deficient in the transcription factor Pet-1 that retain only ∼30–40% of their brainstem 5-HT neurons (14). These mice experience apnea and spontaneous bradycardias in first two postnatal weeks (1, 3) and have delayed gasping at postnatal day (P)4.5 and P9.5 (but no significant mortality) in response to a single episode of anoxia (4). This study tests the hypothesis that at a critical age Pet-1−/− mice will fail to autoresuscitate during episodic anoxia, ultimately from a failure of gasping to restore HR.
METHODS
Animals.
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Dartmouth College. We tested pups from Pet-1+/− breeders maintained on a mixed C57Bl/6 and 129Sv background (14). All animals were provided food and water ad libitum and were housed with a 12-h light-dark cycle at an ambient temperature (TA) of 21–23°C. The cardiorespiratory responses of 57 pups total were studied at 3 postnatal ages; P4–5: 6 litters, Pet-1−/−: n = 10; littermates: n = 12; referred to as “P5”; P7–8: 8 litters, Pet-1−/−: n = 14 (6 of which were tested at P5); littermates: n = 16 (5 of which were tested at P5); referred to as “P8”; and P11–12: 3 litters, Pet-1−/−: n = 6 (3 of which were tested at P8); littermates: n = 8 (4 of which were tested at P8); referred to as “P12”. We chose this age range because we were interested in the role of brainstem 5-HT on the recovery of HR after repeated episodes of anoxia during a period in mouse development that encompasses human infancy and because we had observed bradycardias in Pet-1−/− animals up until P12 (1).
Genotyping.
Pups were ear notched at each respective age. Genotyping on isolated DNA was performed according to a previous study (14) using primers 5'-CGC ACT TGG GGG GTC ATT ATC AC-3′, 5′-CGG TGG ATG TGG AAT GTG TGC-3′, and 5′-GCC TGA TGT TCA AGG AAG ACC TCG G-3′. PCR was performed using an initial 5-min denaturing step at 95°C, followed by 35 cycles of 94°C for 1 min, 62°C for 30 s, and 72°C for 50 s. PCR products generated were a wild-type (WT) allele and Pet-1−/− allele of 209 and 361 base pairs, respectively.
Experimental setup.
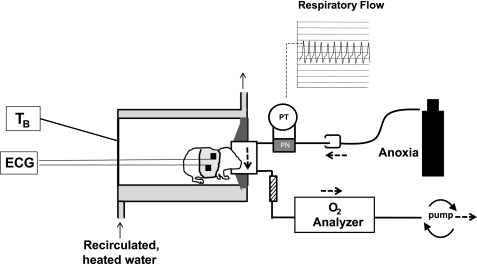
Experiments were performed using a mask-pneumotach setup (Fig. 1). The animal chamber (volume ∼ 40 ml) was constructed from a water-jacketed glass cylinder. Body temperature (TB), held at 36°C ± 0.5°C in all animals throughout the experiment, was controlled by changing the temperature of the water perfusing the glass chamber, thereby changing the TA within the chamber. TA was ∼32–34°C during room air, baseline conditions (higher TA in younger animals). To counter the negative effects of anoxia on thermogenesis, we adjusted TA to ∼34–36°C to maintain TB at ∼36°C during the course of the experiment. Breathing was measured with a head-out system. The head chamber was made by fitting a section of vinyl over the end of syringe tube (volume ∼3 ml), held in place with another rubber gasket that fit into the anterior end of the chamber. The snout of the animal (fur removed) was placed into a small hole in the vinyl and sealed with polyether material (Impregum F Polyether Impression material; 3M, St. Paul, MN).
Fig. 1.
Schematic of experimental setup used to measure cardiorespiratory responses to anoxia in Pet-1−/− mice and littermates. Animals are placed into a glass chamber perfused with warmed water. Respiratory activity is measured by way of a pneumotach (PN) attached to a pressure transducer (PT), with the animals face sealed into a small mask. A high flow rate through the mask permits a rapid delivery of anoxic gas from a tank, delivered into the surrounds of the pneumotach and pulled through the mask using a downstream pump. ECG signals are obtained using surface electrodes embedded in a small vest. V̇o2 is determined in room air by pulling effluent gas through an O2 analyzer. TB, body temperature.
A downstream pump (AEI Technologies, Naperville, IL) connected to the outlet port of the mask pulled air through the pneumotach and mask at a flow of either 50 ml/min (P5) or 110 ml/min (P8, P12). The expired gas was drawn through a small column of Drierite (W. A. Hammond Drierite, Xenia OH) and then an O2 analyzer (AEI Technologies, Pittsburgh PA), allowing the determination of V̇o2. Anoxia (97% N2-3% CO2; Ref. 6) was delivered directly from a tank to the surrounds of the pneumotach through the open end of a 50-cc syringe placed over the end of the pneumotach. In this way, the downstream pump pulled the anoxic gas through the mask with no change in pressure. The wash-in time for the anoxia was <4 s for all animals. TB was continually monitored with fine thermocouples (Omega Engineering, Stamford, CT). Standard two-lead, surface ECG was used to measure HR. Thermocouples and ECG leads reached the exterior of the chamber by way of a hole in a rubber gasket (Terumo Medical) in the posterior end of the chamber.
Inspiratory and expiratory airflows were detected by connecting both side arms of the pneumotach to a differential pressure transducer (Validyne Engineering, Northridge, CA). Integration of the flow trace provided respiratory volume, calibrated by the injection and withdrawal of known volumes of air (0.025, 0.05 ml) at the end of each experiment. The pneumotach responded in a linear fashion to these volumes.
Experimental protocol.
The experiments were performed while the investigator was blinded to genotype. Pups were removed from the litter and immediately weighed. Animals were then instrumented with ECG leads (contained in a small vest made from tensor bandage). A rectal thermocouple was then inserted ∼1 cm and lightly glued to the base of the tail. The snout of the animal was then sealed into the mask and the animal was allowed to warm to a TB of ∼36.0°C (∼20 min). Following a 10-min baseline recording, the animal was exposed to four challenges of anoxia, separated by ≥5 min of room air such that breathing returned to baseline values before the next challenge. Anoxia was only given until apnea ensued (∼30 s), after which the syringe delivering anoxia to the pneumotach was removed such that the mask was flushed with room air. In some instances when animals had prolonged hypoxic apnea, 10 min of room air was required for ventilation to return to normal before the next episode of anoxia was administered.
Immunohistochemical analysis.
Processing and cell counts were performed while the observer was blinded to genotype. Pet-1−/− have a loss of 5-HT neurons across all 5-HT nuclei (14); we quantified 5-HT-positive cell bodies in the raphe magnus as representative of the loss of 5-HT neurons throughout the entire raphe system. This nucleus was selected because it has a cellular organization throughout the rostral-caudal axis that makes it amenable for cell counts in very young animals. Animals surviving the experiment were killed by rapid decapitation. Brains were removed, stored in 4% paraformaldehyde overnight, and then equilibrated in a solution of 25% sucrose in 0.1 M phosphate buffer. Brains were then frozen and sectioned 40-um thick. Sections were stored in a cryoprotectant solution (30% sucrose, 30% ethylene glycol in phosphate buffer) at −20°C until processing. Every third section was processed free-floating for immunofluorescence detection of 5-HT. 5-HT was detected using a rabbit anti-5-HT antibody (Chemicon International, Temecula CA) at 1:1,000. Primary antiserum was diluted in 0.1 M PBS, 0.3% Triton X-100 (PBST), 0.04% BSA, and 0.1% sodium azide and incubated with the tissue for 2–3 days at 4°C. Secondary antiserum was conjugated to CY3 (donkey anti-rabbit; Jackson Immunoresearch, West Grove, PA). Secondary antiserum was diluted 1:200 in PBST-BSA and incubated with the tissue for 60–90 min at room temperature. Between incubations, sections were rinsed in excess PBS. All individuals from the same litter were processed at the same time using aliquots of the same reagents.
Following immunofluorescence labeling, sections were mounted on glass slides in rostral-to-caudal order using references from a stereotaxic atlas (31). Sections were sampled and analyzed while the researcher was blinded to genotype. Sections were photographed using conventional epifluorescence illumination, and images were pseudocolored using Slidebook software (Intelligent Imaging Innovations, Denver, CO). For quantification of 5-HT cell number, for each rat three representative sections were selected that bracketed the rostrocaudal midpoint of the nucleus raphe magnus (Bregma −10.6 mm). On these sections, every cell residing in the raphe magnus was counted (31, 45). The triangular region from the midpoint of both pyramidal tracts to the apex distribution of 5-HT neurons was included. For each area, the average number of cells per section was determined for each individual, which were then averaged across all individuals to generate group means. Immunohistochemical analysis was performed on 21 of the 30 animals studied at P8 (Pet-1−/−: n = 11; littermates: n = 10), as well as on separate groups of P5 (Pet-1−/−: n = 12; littermates: n = 14) and P12 animals (Pet-1−/−: n = 7; littermates: n = 7) that were not subjected to anoxia. There is no difference in medullary 5-HT neuron counts between Pet-1+/+ and +/− animals (14) so these genotypes were grouped together for analysis.
Data analysis.
All analog signals were recorded and analyzed in Labchart 6 (ADInstruments, Colorado Springs, CO) using Powerlab data acquisition system (ADInstruments). Data are expressed as means ± SE. HR and breathing were analyzed using peak detection on the respiratory and R-wave traces. HR was measured continually. Values measured were HR, ventilation (V̇e), tidal volume (VT), respiratory frequency (fB), the coefficient of respiratory frequency variation (CV fB %), metabolic rate (V̇o2): [V̇o2 = (0.21 − fractional O2 exhausted from mask) × flow (ml/min)/mass (kg)], and the respiratory equivalent (V̇e/V̇o2). After each challenge, we measured 1) the time required to restore HR to 90% of baseline; 2) the duration of the hypoxic apnea (i.e., the delay in gasp initiation); and 3) the total time required to restore eupnea.
To assess whether a deficiency of 5-HT neurons altered the autonomic response to anoxia, we measured low-frequency (LF) and high frequency (HF) components of heart rate variability (HRV) in Pet-1−/− and WT at P8 during the 5-min baseline period before anoxia and a 5-min period following the first anoxic episode (∼2,500–3,000 beats for each period). The HR was sampled at 1 kHz and the interbeat intervals (R-R intervals) were computed using the peak detection function on LabChart 6.0. Text files were exported for analysis using an instrument scripted in Matlab (Mathworks, Natick MA). The R-R interval spectral estimation used the Lomb analysis technique to avoid the spectral distortion caused by constant-interval resampling (27). Spectral power was estimated by summation within LF (0.15–1.5 Hz) and HF (1.5–6 Hz) bands as suggested by Just et al. (17). The LF-to-HF ratio (LF/HF) was obtained for each segment from the quotient.
Statistical analysis.
Effects of genotype on cardiorespiratory parameters during baseline, room-air conditions were assessed with a single-factor ANOVA at each age. Effects of genotype on HR recovery, duration of hypoxic apnea, and the time required to restore eupnea were assessed using a single-factor ANOVA within each episode. Differences in 5-HT cells counts were assessed with a single-factor ANOVA. The effects of genotype and the first episode of anoxia on HRV: LF power, HF power, and LF/HF, were assessed using a two-factor, repeated-measures ANOVA (factor 1: genotype; factor 2: normoxia or anoxia). As data for LF/HF were not normally distributed, we also performed a one-factor ANOVA on the change in LF/HF (ΔLF/HF, which was normally distributed) in each group from normoxia to postanoxia. Tukey's post hoc tests were performed when significant effects were found. In some cases where data were not normally distributed, Kruskal-Wallis single-factor ANOVA was used, followed by Dunn's post hoc tests. Effects were considered significant at P < 0.05.
RESULTS
Baseline room air parameters.
Cardiorespiratory parameters during baseline conditions in Pet-1−/− and WT at the three ages studied are shown in Table 1. At P5 and P8, but not P12, HR was significantly lower in Pet-1−/− animals compared with WT (P < 0.01). No difference in HR existed between Pet-1−/− that survived or succumbed to anoxia (P = 0.67). There were no differences in V̇e between Pet-1−/− and WT at any age. However, overall, Pet-1−/− animals had reduced fB compared with WT at all ages (P < 0.05) except at P8 in Pet-1−/− animals surviving anoxia (P = 0.10). At P5, V̇o2 was lower in all Pet-1−/− animals compared with WT (P = 0.02), as it was at P8, but only in those Pet-1−/− animals susceptible to anoxia (P = 0.002). At P8, V̇e/V̇o2 was higher in Pet-1−/− animals compared with WT (P = 0.02).
Table 1.
Baseline cardiorespiratory parameters in Pet-1−/− and wild-type littermates at postnatal days 5, 8, and 12
| Mass, g | HR, min−1 | V̇e, ml · min−1 · kg−1 | VT, ml/kg | f, min−1 | CV fB, % | V̇o2, ml · min−1 · kg−1 | V̇e/V̇o2 | |
|---|---|---|---|---|---|---|---|---|
| P5 | ||||||||
| WT (n = 12) | 2.9 ± 0.2 | 554 ± 8 | 1594 ± 123 | 7.5 ± 0.5 | 212 ± 10 | 32.8 ± 5.0 | 50.1 ± 3.2 | 32.4 ± 2.8 |
| KO (n = 10) | 2.1 ± 0.1 | 481 ± 20* | 1388 ± 139 | 7.8 ± 0.4 | 176 ± 13* | 50.8 ± 9.0 | 43.8 ± 7.7* | 35.1 ± 3.9 |
| P8 | ||||||||
| WT (n = 16) | 5.3 ± 0.2 | 597 ± 9 | 1111 ± 43 | 4.4 ± 0.3 | 256 ± 8 | 34.4 ± 5.3 | 54.8 ± 2.2 | 21.1 ± 1.1 |
| KO (d) (n = 8) | 3.8 ± 0.2* | 529 ± 17* | 1154 ± 134 | 5.5 ± 0.4† | 206 ± 12* | 40.5 ± 9.8 | 40.7 ± 1.8* | 28.3 ± 2.9† |
| KO (s) (n = 6) | 3.6 ± 0.3* | 544 ± 11* | 1234 ± 105 | 5.5 ± 0.4† | 225 ± 5 | 27.6 ± 6.5 | 47.7 ± 5.2 | 28.1 ± 5.3† |
| P12 | ||||||||
| WT (n = 8) | 7.0 ± 0.5 | 631 ± 13 | 1325 ± 117 | 5.4 ± 0.5 | 246 ± 8 | 27.9 ± 3.6 | 71.0 ± 16.2 | 22.0 ± 3.3 |
| KO (n = 6) | 4.5 ± 0.7* | 615 ± 16 | 1406 ± 164 | 7.5 ± 1.4 | 197 ± 18* | 35.7 ± 5.6 | 49.6 ± 8.0 | 29.9 ± 3.3 |
Values are means ± SE. At postnatal day (P)8, Pet-1−/− (KO) animals are subdivided into those that succumb to anoxia [KO (d)] and those that survive [KO (s)]. Values displayed are heart rate (HR), ventilation (V̇e), tidal volume (VT), respiratory frequency (f), the coefficient of respiratory variation (CV %), metabolic rate (V̇o2), and the respiratory equivalent (V̇e/V̇o2).
P < 0.05, significant difference from WT values.
Significant difference from WT values when Pet-1−/− (d) and (s) are combined.
Failure of autoresuscitation in Pet-1−/− mice at a critical age.
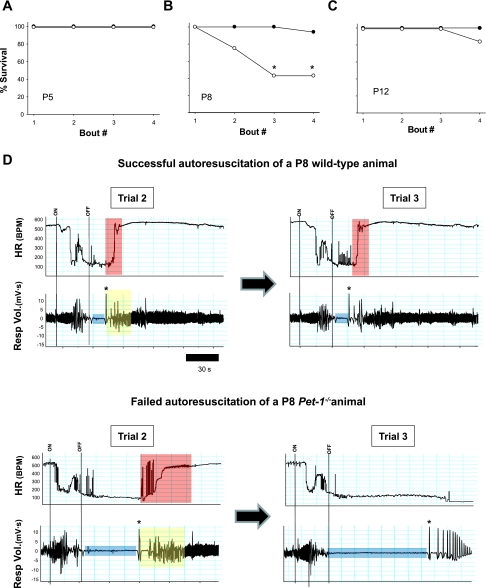
The failure of autoresuscitation and death in Pet-1−/− animals during repeated, episodic anoxia depended on age (Fig. 2, A–C). At P5, all WT (n = 12) and Pet-1−/− (n = 10) animals survived the four challenges of anoxia (Fig. 2A). At P8, only 43% of Pet-1−/− animals (6 of 14) survived all four challenges of anoxia (with all dying animals succumbing by the third challenge), while 95% (15 of 16) of WT survived all four challenges (P < 0.01; Fig. 2B). The modal number of survived challenges was two and four for Pet-1−/− and WT animals, respectively (P < 0.001). At P12, there was no death in WT animals (0 of 10) while one of six Pet-1−/− animals died on the fourth challenge. The modal number of survived challenges was four for both Pet-1−/− and WT animals (P > 0.05).
Fig. 2.
Failed auto-resuscitation in P8 Pet-1−/− mice during episodic anoxia. Mortality of Pet-1−/− animals (○) and wild-type littermates (WT; ●) at postnatal day (P)5 (A), P8 (B), and P12 (C). D: typical heart rate (HR) and respiratory volume (Resp Vol.) traces from a single WT animal (top) and a Pet-1−/− animal (bottom). For clarity, we show responses during the second and third trials only, since WT responses are consistent across all trials and Pet-1−/− animals succumb by the third episode. Beginning (ON) and end (OFF) of anoxia is indicated by vertical lines. Despite robust gasping (first gasp indicated by *), the time required for the Pet-1−/− animal to recover 90% of the prehypoxic heart rate during the second trial is ∼3 times that of the wild-type animal (compare region of heart rate trace shaded red for each animal), and completely fails during the third trial. Duration of hypoxic apnea (blue bar) and the time required to recover a normal breathing pattern (yellow bar) are also considerably longer in the Pet-1−/− animal. BPM, beats/min.
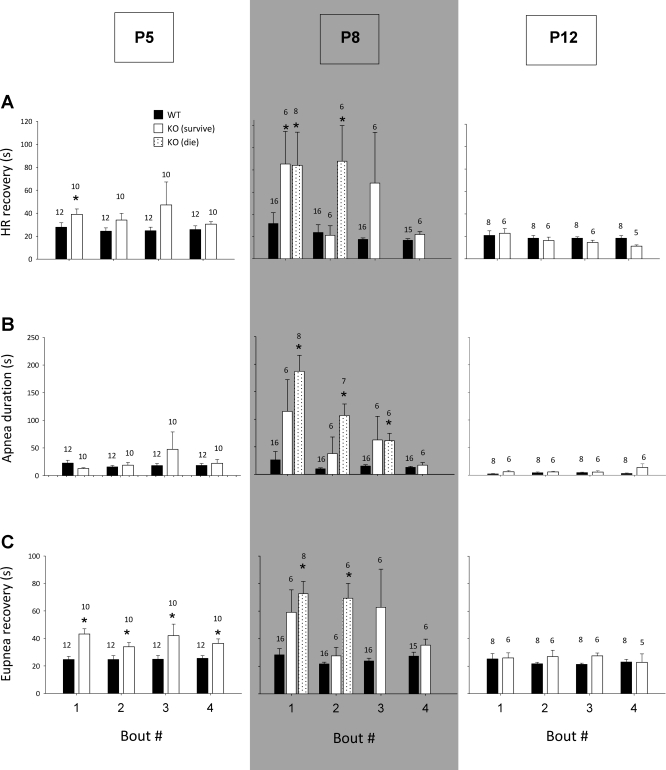
The typical WT response at P8 was characterized by a relatively short apnea and a brisk recovery of HR (Fig. 2D, top). In contrast, the typical Pet-1−/− response at P8 was characterized by a prolonged apnea, delayed HR recovery, and complete failure of HR recovery after the third challenge of anoxia (Fig. 2D, bottom). Average data across all ages for HR recovery, duration of hypoxic apnea, and time for recovery of eupnea are shown in Fig. 3, A–C, respectively. At P5, WT animals required on average 27 s to recover HR after the first episode of anoxia, while Pet-1−/− animals required 39 s (P = 0.04). HR recovery time was the same between genotypes from episodes 2 to 4. The duration of hypoxic apnea between WT and Pet-1−/− animals was not significantly different, but Pet-1−/− animals required slightly but significantly more time to recover eupnea after gasping onset (P < 0.01). At P8, WT animals took an average of 31.7 s to recover HR after the first episode of anoxia and had apneas that were on average 26.5 s long. In contrast, surviving and dying Pet-1−/− animals both required ∼85 s (i.e., 3-fold longer than WT) to recover HR after the first episode and experienced apneas that were ∼120 and ∼180 s, respectively (P < 0.001). Consequently, the time required for Pet-1−/− animals to recover eupnea was markedly increased compared with WT (P < 0.001), despite the fact that both the frequency and volume of gasps were normal once generated (not shown). During the second episode of anoxia, the responses of Pet-1−/− animals diverged: the delay in HR recovery and the duration of hypoxic apnea remained significantly prolonged relative to WT in Pet-1−/− animals eventually succumbing to anoxia (P < 0.01), while these parameters were indistinguishable from those of WT in surviving Pet-1−/− animals. By P12, the delay in HR recovery and duration of hypoxic apnea in Pet-1−/− animals were again no different than WT.
Fig. 3.
Defects in autoresuscitation exist at a critical age in Pet-1−/− mice. Heart recovery time (A), duration of hypoxic apnea (B), and eupnea recovery time (C) in Pet-1−/− (KO) and WT littermates at P5 (KO: n = 10; WT: n = 12, respectively), P8 (KO dying: n = 8; KO surviving: n = 6; WT: n = 16), and P12 (KO: n = 6; WT: n = 8) during the four trials of anoxia. There exists a critical age (P8, shaded) when KO animals have delayed heart rate recovery, prolonged hypoxic apnea, and prolonged recovery of eupnea compared with WT. In KO animals that succumb to anoxia, heart rate recovery, hypoxic apnea duration and time for recovery of eupnea are prolonged after episodes 1 and 2 of anoxia (*P < 0.05; KO vs. WT). In KO animals that survive anoxia, heart rate recovery, hypoxic apnea duration, and recovery of eupnea are not significantly different than WT. As the numbers of animals change from trial to trial in the P8 group, the number of animals represented is indicated above each histogram. Absence of data for HR and eupnea recovery in KO animals during the third and fourth trials (A and C) is because they succumbed. Note that during episodes in which they succumb, these animals still have measurable hypoxic apneas. Data are means ± SE.
Phenotypic heterogeneity in Pet-1−/− mice is correlated with 5-HT cell counts.
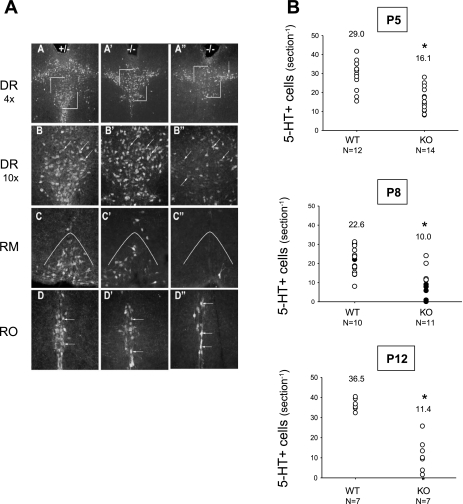
Qualitative analysis revealed that Pet-1−/− mice have a roughly equivalent loss of 5-HT neurons throughout all brainstem raphe nuclei (Fig. 4A), consistent with previous descriptions (14, 22). To assess the severity of 5-HT neuron loss in individual Pet-1−/− animals, we quantified the number of 5-HT-positive neuronal cell bodies in the raphe magnus of Pet-1−/− and WT animals at P5, P8, and P12 (Fig. 4B). On average, Pet-1−/− have 45, 56, and 68% fewer neurons than WT at P5, P8, and P12, respectively (P < 0.001 at each age). We correlated the ability of P8 Pet-1−/− animals to survive repeated anoxia with the number of 5-HT-immunopositive neurons remaining in the raphe magnus. Pet-1−/− animals that succumbed to anoxia had on average a 73.1% reduction in the number of 5-HT neurons within the raphe magnus compared with WT (P < 0.001; Fig. 4B). There was no difference in the number of 5-HT neurons in the raphe magnus between surviving Pet-1−/− animals and WT (P = 0.43; Fig. 4B).
Fig. 4.
Pet-1−/− animals have reduced 5-HT-positive neurons in pons and medulla. 5-HT-positive cell bodies were quantified in the raphe magnus to represent 5-HT system status as a whole. A: 5-HT-positive neurons at P8 in the dorsal raphe (DR), raphe magnus (RM), and raphe obscurus (RO) of a wild-type littermate (A–D), a Pet-1−/− animal (−/−) surviving 4 episodes of anoxia (A'–D'), and a Pet-1−/− animal that did not survive anoxia (A''–D''). B: numbers of 5-HT-positive cell bodies in the raphe magnus of WT and Pet-1−/− animals (KO) at P5 (top), P8 (middle), and P12 (bottom). At P8, KO animals that die (●) have significantly fewer neurons than KO animals surviving (○). At all ages, KO animals have fewer 5-HT-positive neurons than WT (*P < 0.01, KO vs. WT) and at P8, KO animals dying have ∼1/3 the 5-HT neurons as those surviving. In B, numbers above each set of points are the average cell counts.
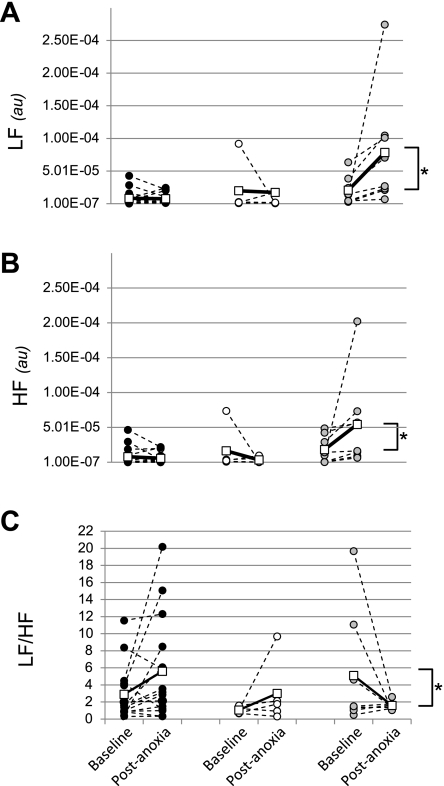
HRV in Pet-1−/− animals.
We compared the LF and HF components of HRV in surviving and dying P8 Pet-1−/− animals with WT at baseline and after the first episode of anoxia when all animals were still alive. At baseline, there was no difference between the groups with respect to LF or HF or their ratio (LF/HF; Fig. 5A–C). The effect of one episode of anoxia on LF and HF power depended on genotype and susceptibility to subsequent anoxia. While HRV did not change in WT and Pet-1−/− animals that survived anoxia, both LF and HF power increased significantly in Pet-1−/− animals eventually succumbing to anoxia (genotype-anoxia interaction: P = 0.017 and P = 0.042, respectively, for LF and HF power; Fig. 5, A and B). Unlike surviving animals in which the LF/HF tended to increase postanoxia, the LF/HF decreased in dying Pet-1−/− animals because of a bigger increase in HF power compared with LF power [overall genotype × anoxia interaction: P = 0.04 (two-factor, repeated-measures ANOVA); genotype effect on the change in LF/HF from normoxia to postanoxia: P = 0.04 (one-factor ANOVA; Fig. 5C)].
Fig. 5.
Analysis of heart rate variability in WT (●), surviving Pet-1−/− animals (○), and dying Pet-1−/− animals (grey circles) within the low frequency (LF; A), high frequency (HF) domains (B), as well as their ratio (LF/HF; C). Shown are data for each individual animal (all circles) and group averages (□) during normoxic prechallenge conditions (baseline) and following the first episode of anoxia (postanoxia). au, Arbitrary units. *P < 0.05, significant difference in the change in LF, HF, and LF/HF from baseline to postanoxia in dying Pet-1−/− animals compared with surviving animals.
DISCUSSION
The failed autoresuscitation we observed in P8 Pet-1−/− animals having reduced numbers of brainstem 5-HT neurons, characterized by a failed restoration of HR in spite of gasping, mimics the pattern of dysfunction in SIDS infants in which cardiopulmonary tracings at the time of death are available (34, 41). While the respiratory networks of mice are able to generate a normal, fictive gasping pattern in a background of Pet-1-deficiency (43), others (4) have shown that the delay in the initiation of gasping following a single episode of anoxia is unusually long in intact P4.5 and P9.5 Pet-1−/− neonates compared with WT. Our data reveal that in addition to a delay in its initiation, the gasping of Pet-1−/− animals with reduced 5-HT neurons ultimately fails to restore HR during episodic anoxia. Most Pet-1−/− animals die by the third hypoxic exposure. This phenotype is the most profound at P8, an age which may be analogous to the “critical period” when most SIDS deaths occur.
We confirmed a widespread deficit of 5-HT neurons throughout the caudal and rostral 5-HT domains in the Pet-1−/− animals, similar to previous reports (14, 22). We quantified neurons in a representative component of the caudal 5-HT domain, i.e., raphe magnus, demonstrating that the physiological heterogeneity of some Pet-1−/− animals was highly correlated with the cellular heterogeneity; i.e., animals with the fewest 5-HT neurons are the most susceptible to failed autoresuscitation. Although speculative, the ability of Pet-1−/− animals to survive anoxia may be related to the sparing of a unique, Pet-1-independent subpopulation of 5-HT neurons that make functional connections with brainstem nuclei involved in cardiorespiratory and autonomic control (22).
The mechanism(s) responsible for the failed autoresuscitation in Pet-1−/− animals are unknown. Delayed gasping in P8 Pet-1−/− animals likely contributes to their high mortality with repeated anoxia, owing to a more extreme deterioration of blood gases. In addition to their reduced respiratory frequency in room air, decreased serotonergic inputs to the pre-Bötzinger complex or other respiratory rhythm-generating neurons could underpin the delay in gasping observed in Pet-1−/− animals (32, 44). Antagonism of 5-HT2A receptors has been shown to completely abolish fictive gasping in a reduced preparation (46). Increased adenosine release associated with prolonged hypoxic apnea could be another contributing factor, as adenosine is a molecule with potent inhibitory effects on respiratory neurons (49). Differences in chemoreception between WT and Pet-1−/− animals can probably be ruled out, as gasping typically ensues only when the partial pressure of O2 in the brainstem falls below ∼10 Torr, irrespective of the CO2 level (5, 12). There was no evidence of atrio-ventricular block in Pet-1−/− animals during anoxic challenge, so in contrast to other mouse strains (10), abnormal electrical conduction within the heart cannot explain the high mortality of Pet-1−/− animals.
It has been known for some time that the success of autoresuscitation depends heavily on changes in blood pressure and perfusion that rely on autonomic adjustments to anoxic stress (11, 12). Given the widespread expression of 5-HT receptors within brainstem autonomic nuclei, and that 5-HT can alter the sympathetic and parasympathetic responses to other cardiorespiratory reflexes (35, 47, 48), autonomic control could be compromised in Pet-1−/− animals during anoxia. Pet-1−/− animals are bradycardic in room air and require a prolonged period to recover HR after the first anoxic challenge, suggesting an imbalance of sympathetic-parasympathetic outflow. While there is no difference between Pet-1−/− animals and controls with respect to LF, HF, or LF/HF at rest, the dramatic increase in HRV in both the HF and LF domains (and decrease in LF/HF) in susceptible Pet-1−/− animals after one anoxic exposure is suggestive of an unusually heightened autonomic response to anoxia, especially in the parasympathetic component. This could be a direct effect of reduced 5-HT signaling or could be secondary to prolonged hypoxic apnea, excessive stimulation of peripheral and central chemoreceptors and/or increased respiratory drive during the recovery period (15). Regardless of its origin, altered HRV in Pet-1−/− animals in response to anoxia may be relevant to SIDS, recently associated with reduced medullary 5-HT (2). In addition to failed autoresuscitation, evidence of autonomic dysregulation exists in SIDS cases, including periods of episodic bradycardia and tachycardia (8, 9, 19, 34, 36, 38, 41). Similar to dying Pet-1−/− animals, HRV analysis of a SIDS case with brainstem 5-HT abnormalities suggested increased parasympathetic to sympathetic tone during active sleep (19). However, other SIDS cases are distinct from dying Pet-1−/− animals with respect to HRV. For instance, Schechtman and colleagues (37, 38) demonstrated that HRV is decreased at rest across all examined frequency domains, and data from Franco et al. (8, 9) suggested decreased HRV at rest in just the HF domain (with increased LF/HF). With respect changes in HRV with hypoxic stress, we show that dying Pet-1−/− animals have a pronounced increase in LF and HF power post-anoxia (with decreased LF/HF). In contrast, Franco et al. −/− have shown that an increase in HF power, with a decrease in LF/HF, occurs in control, but not SIDS infants following obstructive apnea (i.e., an hypoxic event). Despite these differences, altered HRV in dying Pet-1−/− animals following oxygen deprivation implicates the brainstem 5-HT system in the autonomic adjustments necessary to survive episodic anoxia.
Why a critical period (∼P8) exists when Pet-1−/− mice are the most sensitive to episodic anoxia remains a matter of speculation. At younger ages (P5) the autonomic response may be less reliant on 5-HT neurons and more reliant on hypoxia directly (39), while in older animals there may be compensation by other neuromodulators. Previous work (16) has identified later developmental time points when mice are more susceptible to autoresuscitation failure, so we should not rule out the possibility other periods exist when 5-HT neurons play a critical role in cardiorespiratory recovery from severe oxygen stress.
This study demonstrates a causal link between brainstem 5-HT deficiency and the failure of autoresuscitation in neonatal mice during episodic anoxia at a critical age. 5-HT neurons appear to be critical for the generation of gasping and ultimately its efficacy at restoring HR with repeated anoxic challenges. These data provide insight into a mechanism whereby medullary 5-HT deficiency could increase the risk of SIDS during episodes of extreme oxygen deprivation, such as those occurring during airway obstruction and in the face down or head-covered position. Future studies should focus on potential mechanisms, be they respiratory or autonomic, by which brainstem 5-HT neurons restore HR and breathing after episodes of severe oxygen deprivation.
GRANTS
We acknowledge the generous support of the Parker B. Francis Family and its Foundation (Fellowship to K. Cummings), NIH Award R37-HD-20991 (to H. C. Kinney), CJ Foundation for SIDS, CJ Murphy Foundation, and First Candle/SIDS Alliance. Funding for this study was also provided by NIH Program Project Grant HD-36379 (NICHD PI, to H. C. Kinney, and PROJECT 2 PI, to E. E. Nattie) and National Heart, Lung, and Blood Institute Grant HL-28066 (PI, to E. E. Nattie).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonin-deficient Pet-1−/− mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol 298: R1333–R1342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA 303: 430–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol 159: 85–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erickson JT, Sposato BC. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J Appl Physiol 106: 1785–1792, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Fewell JE. Protective responses of the newborn to hypoxia. Respir Physiol Neurobiol 149: 243–255, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Fewell JE, Zhang C, Gillis AM. Influence of adenosine A(1)-receptor blockade and vagotomy on the gasping and heart rate response to hypoxia in rats during early postnatal maturation. J Appl Physiol 103: 1234–1241, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate 65: 194–197, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Franco P, Szliwowski H, Dramaix M, Kahn A. Decreased autonomic responses to obstructive sleep events in future victims of sudden infant death syndrome. Pediatr Res 46: 33–39, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Franco P, Szliwowski H, Dramaix M, Kahn A. Polysomnographic study of the autonomic nervous system in potential victims of sudden infant death syndrome. Clin Auton Res 8: 243–249, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Gershan WM, Jacobi MS, Thach BT. Mechanisms underlying induced autoresuscitation failure in BALB/c and SWR mice. J Appl Physiol 72: 677–685, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Godfrey S. Respiratory and cardiovascular changes during asphyxia and resuscitation of foetal and newborn rabbits. Q J Exp Physiol Cogn Med Sci 53: 97–118, 1968 [DOI] [PubMed] [Google Scholar]
- 12. Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest 56: 1371–1377, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harper RM. Autonomic control during sleep and risk for sudden death in infancy. Arch Ital Biol 139: 185–194, 2001 [PubMed] [Google Scholar]
- 14. Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37: 233–247, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol Heart Circ Physiol 241: H620–H629, 1981 [DOI] [PubMed] [Google Scholar]
- 16. Jacobi MS, Thach BT. Effect of maturation on spontaneous recovery from hypoxic apnea by gasping. J Appl Physiol 66: 2384–2390, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Just A, Faulhaber J, Ehmke H. Autonomic cardiovascular control in conscious mice. Am J Physiol Regul Integr Comp Physiol 279: R2214–R2221, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kinney HC. Neuropathology provides new insight in the pathogenesis of the sudden infant death syndrome. Acta Neuropathol (Berl) 117: 247–255, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, Fingers ST, Youngman M, Habbe D, Fifer WP. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J Neuropathol Exp Neurol 64: 689–694, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol 62: 1178–1191, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med 361: 795–805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci 31: 2756–2768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, Corey T, Cutz E, Hanzlick R, Keens TG, Mitchell EA. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics 114: 234–238, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol (Berl) 117: 257–265, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep 57: 1–32, 2008 [PubMed] [Google Scholar]
- 26. Melton JE, Kadia SC, Yu QP, Neubauer JA, Edelman NH. Respiratory and sympathetic activity during recovery from hypoxic depression and gasping in cats. J Appl Physiol 80: 1940–1948, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Moody GB. Spectral analysis of heart rate without resampling. Comp Cardiol 20: 715–718, 1993 [Google Scholar]
- 28. Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol 59: 377–384, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Pasquale-Styles MA, Tackitt PL, Schmidt CJ. Infant death scene investigation and the assessment of potential risk factors for asphyxia: a review of 209 sudden unexpected infant deaths. J Forensic Sci 52: 924–929, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- 32. Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poets CF. Apparent life-threatening events and sudden infant death on a monitor. Paediatr Respir Rev 5, Suppl A: S383–386, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45: 350–354, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull 56: 425–439, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep 11: 413–424, 1988 [DOI] [PubMed] [Google Scholar]
- 37. Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Heart rate variation in normal infants and victims of the sudden infant death syndrome. Early Hum Dev 19: 167–181, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP, Harper RM. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res 31: 606–612, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Slotkin TA, Seidler FJ. Adrenomedullary catecholamine release in the fetus and newborn: secretory mechanisms and their role in stress and survival. J Dev Physiol 10: 1–16, 1988 [PubMed] [Google Scholar]
- 40. Solomon IC. Excitation of phrenic and sympathetic output during acute hypoxia: contribution of medullary oxygen detectors. Respir Physiol 121: 101–117, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol 36: 113–122, 2003 [DOI] [PubMed] [Google Scholar]
- 42. St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of alpha1-adrenergic receptors and serotonin 5-HT2 receptors. J Appl Physiol 104: 665–673, 2008 [DOI] [PubMed] [Google Scholar]
- 43. St-John WM, Li A, Leiter JC. Genesis of gasping is independent of levels of serotonin in the Pet-1 knockout mouse. J Appl Physiol 107: 679–685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. St John WM. Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? J Appl Physiol 81: 1865–1877, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Steinbusch HW. Serotonin-immunoreactive neurons and their projections in the CNS. In: Handbook of Chemical Neuroanatomy, edited by Bjorklund AHT, Kuhar M. Amsterdam: Elsevier Science, 1984, p. 68–121 [Google Scholar]
- 46. Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26: 2623–2634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Y, Ramage AG. The role of central 5-HT(1A) receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J Physiol 536: 753–767, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weissheimer KV, Machado BH. Inhibitory modulation of chemoreflex bradycardia by stimulation of the nucleus raphe obscurus is mediated by 5-HT3 receptors in the NTS of awake rats. Auton Neurosci 132: 27–36, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Zaidi SI, Jafri A, Martin RJ, Haxhiu MA. Adenosine A2A receptors are expressed by GABAergic neurons of medulla oblongata in developing rat. Brain Res 1071: 42–53, 2006 [DOI] [PubMed] [Google Scholar]