Abstract
Defects in pharyngeal mechanical and neuromuscular control are required for the development of obstructive sleep apnea. Obesity and age are known sleep apnea risk factors, leading us to hypothesize that specific defects in upper airway neuromechanical control are associated with weight and age in a mouse model. In anesthetized, spontaneously breathing young and old wild-type C57BL/6J mice, genioglossus electromyographic activity (EMGGG) was monitored and upper airway pressure-flow dynamics were characterized during ramp decreases in nasal pressure (Pn, cmH2O). Specific body weights were targeted by controlling caloric intake. The passive critical pressure (Pcrit) was derived from pressure-flow relationships during expiration. The Pn threshold at which inspiratory flow limitation (IFL) developed and tonic and phasic EMGGG activity during IFL were quantified to assess the phasic modulation of pharyngeal patency. The passive Pcrit increased progressively with increasing body weight and increased more in the old than young mice. Tonic EMGGG decreased and phasic EMGGG increased significantly with obesity. During ramp decreases in Pn, IFL developed at a higher (less negative) Pn threshold in the obese than lean mice, although the frequency of IFL decreased with age and weight. The findings suggest that weight imposes mechanical loads on the upper airway that are greater in the old than young mice. The susceptibility to upper airway obstruction increases with age and weight as tonic neuromuscular activity falls. IFL can elicit phasic responses in normal mice that mitigate or eliminate the obstruction altogether.
Keywords: pharynx, obstructive sleep apnea, neuromuscular control, obesity, aging
obstructive sleep apnea is a common chronic disease with a prevalence that has been estimated at 2% of women and 4% of men in the general population (62) and has been linked to the epidemic of obesity in Western society (13, 59). It is characterized primarily by recurrent occlusion of the upper airway, which results in oxyhemoglobin desaturation and periodic arousals from sleep (42). Obesity and central adiposity are strong risk factors for this disorder (63, 64) and can account for increases in sleep apnea prevalence with age and weight, particularly in men (7, 63). Nevertheless, the mechanisms linking obstructive sleep apnea with obesity and age are not well understood.
Upper airway obstruction results from defects in pharyngeal structural and neuromuscular control (32, 38). Increases in pharyngeal mechanical loads produce elevations in pharyngeal collapsibility, which lead to worsening airflow obstruction during sleep (38). In turn, airflow obstruction can elicit neuromuscular responses that can activate upper airway muscles, which compensate and restore airway patency. Nevertheless, the impact of obesity and age on the passive mechanical and active neuromuscular control of pharyngeal patency has not been elucidated.
A major impediment to the investigation of upper airway function has been a paucity of suitable animal models. In early studies, investigators demonstrated that pressure-flow dynamics and neuromechanical control mechanisms in the isolated canine and feline upper airway are similar to those observed in sleeping humans (43, 44, 52, 53, 56). Nevertheless, large animal models are poorly suited to study the impact of body composition and genetic background on upper airway function, which has limited their applicability in modeling disease pathogenesis. More recently, investigators examined the impact of obesity on upper airway function in anesthetized rats and demonstrated increases in pharyngeal collapsibility in obese leptin receptor-deficient Zucker rats (34, 36) compared with lean wild-type controls and in old compared to young wild-type rats (41). Nevertheless, the independent effects of age and weight on upper airway function have not been elucidated in a study that has controlled for genetic background.
We hypothesized that obese, aged C57BL/6J mice would exhibit defects in upper airway mechanical and neural control compared to lean, young controls. To address this hypothesis, we developed novel methods for assessing upper airway function and modeled the effects of age and weight in anesthetized mice. Our findings suggest that both factors produce distinct disturbances in upper airway neuromechanical function that could increase sleep apnea susceptibility in humans.
METHODS
Mice
Male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME) and housed in a microisolation facility. Temperature and relative humidity were continuously regulated at 20–22°C and 40–60%, respectively. Two groups of mice were used for this study: a young (∼9-wk-old) group (young mice) and an older (∼30-wk-old) group (old mice). Dietary intake was managed to achieve the target weight goals of lean (∼24 g), obese (∼30 g), and very obese (∼35 g) in young and old mice (see Weight management protocol). Water was available ad libitum throughout the study for all groups. All study protocols were approved by the Johns Hopkins Animal Care and Use Committee (JHACUC), and all animal experiments were conducted in accordance with the JHACUC guidelines.
Experimental Procedures
Weight management protocol.
Mice arrived from the Jackson Laboratory at 7 wk of age and 17 g average body weight. Mice fed normal chow (18% protein extruded rodent diet, Harlan Laboratories, Madison, WI) naturally gained weight over time at a predictable rate (16). To augment weight, animals were fed a high-fat (35% lard) diet (Harlan Laboratories), which resulted in the development of an “obese” group of young and old mice and a “very obese” group of old mice. The old mice were allowed to gain weight naturally with age until week 27, when they were placed in separate cages in which they were weighed daily, and caloric intake was regulated accordingly. In a separate group, food was restricted in older mice to reach a “lean” target weight of ∼24 g. (Normal age-related weight trajectories preclude defining absolute weight categories for young and old mice, and the lean, obese, and very obese weight groups refer to weight ranges in our young mice.) The final ages and weights for each target group are represented in Table 1.
Table 1.
Baseline characteristics by age and weight group
| Age, wk |
Weight, g |
||||
|---|---|---|---|---|---|
| n | Mean ± SE | Range | Mean ± SE | Range | |
| Young | |||||
| Lean | 10 | 9.2 ± 0.4 | 8.0–12.0 | 23.3 ± 0.4 | 21.3–25.4 |
| Obese | 10 | 9.1 ± 0.2 | 8.1–9.9 | 29.5 ± 0.2 | 28.7–30.9 |
| Old | |||||
| Lean | 8 | 29.6 ± 0.1 | 29.1–30.1 | 23.8 ± 0.1 | 23.0–24.3 |
| Obese | 11 | 30.3 ± 0.3 | 28.9–32.1 | 29.9 ± 0.2 | 28.7–30.7 |
| Very obese | 5 | 29.9 ± 0.3 | 29.1–30.4 | 35.5 ± 0.3 | 34.7–36.6 |
Mouse groups were matched by age and weight.
Anesthesia protocol.
Surgical anesthesia was induced with 2–3% isoflurane in a closed chamber and maintained with 1–2% isoflurane throughout the remainder of the procedure. Atropine (0.001 mg ip) was injected to minimize airway secretions during the protocol. The respiratory rate and rectal temperature were continuously monitored, and the percentage of isoflurane was adjusted to maintain a stable plane of surgical anesthesia. The depth of anesthesia was continuously monitored based on a targeted respiratory rate of 60–120 breaths/min and the absence of pedal withdrawal to interdigital space pinch. The rectal temperature was monitored with a temperature probe, and body temperature was maintained at 36.5–37.5°C with a variable-temperature heating pad throughout the experiments. At the experiment's completion, the animals were euthanized by an overdose of pentobarbital (60 mg ip).
Intact murine upper airway surgery.
The trachea was cannulated as follows. A midline incision was made in the neck from the sternal notch to the larynx. The skin, subcutaneous tissue, and muscle were separated at the midline and retracted to expose the trachea. The skin was punctured between the ears dorsally, and the tracheal cannula was tunneled through subcutaneous tissues and directed out of the skin puncture site. The trachea was punctured anteriorly with a 26-gauge needle, and the tracheal cannula was inserted and secured with suture to the suprahyoid muscles. The trachea was intubated with a 2-mm-long, narrow Physiocath mouse vascular catheter (0.41 mm OD, 0.20 mm ID; catalog no. 277-0004-001, Data Sciences International, St. Paul, MN). This cannula was coupled in series to a larger-caliber (0.80 mm OD, 0.50 mm ID) 16-mm-long curved cannula (polyethylene tubing; catalog no. 112031, Critchley Electrical Products, Sidney, NSW, Australia) and, finally, to a still wider-bore (1.17 mm OD, 0.81 mm ID) 27-mm-long segment of an umbilical cannula (3.5 French single-lumen silicone umbilical venous catheter; catalog no. 4173505, Utah Medical Products, Midvale, UT), which was tunneled subcutaneously out the back of the neck. The adjacent cervical strap muscles were reopposed and sutured to prevent the cannula from dislodging. Thereafter, two Teflon-coated, fine wires (stainless steel, Teflon-coated, full hard, 0.005-in. bare, 0.008-in. coated; A-M Systems, Carlsborg, WA) were tunneled subcutaneously toward the base of the tongue. The ends were bared and looped and then sutured to the surface of the genioglossus muscle bilaterally. The mouth was sewn shut with 6-0 silk suture, and the lips were sealed with acrylate adhesive (Loctite Super Glue, Henkel Consumer Adhesives, Avon, OH).
Experimental Setup
The mouse was placed into a head-out plethysmograph in the prone position with the head positioned in a natural posture at 10–20° below the horizontal plane. A short nasal cannula was placed over the snout and secured with acrylate adhesive. The cannula was connected to a blow-by breathing circuit through which fresh oxygen and isoflurane were administered. Nasal pressure (Pn) and tracheal pressure (Ptr) were monitored with differential pressure transducers referenced to atmospheric pressure (model MP 45-1, Validyne, Northridge, CA; ±2 cmH2O), which were connected to the nasal and tracheal cannulas, respectively. For regulation of the Pn, the downstream end of the blow-by circuit was connected in series to a rotameter (0.2–2 standard ft3/min; solid acrylic flowmeter series A, Dwyer, Michigan City, IN) and vacuum source. Inspiratory airflow (V̇i) was measured through a fixed-resistance laminar flow pneumotachometer attached to a hole in the plethysmograph and monitored with a differential pressure transducer (model MP 45-1, Validyne; ±2 cmH2O), which measured the pressure difference between the inside and outside of the chamber. The system was calibrated by application of known flows (thermal mass flowmeter 4100 series, TSI, Shoreview, MN) through the pneumotachometer. Pn, Ptr, and V̇i were amplified by a Grass polygraph direct-current driver amplifier (model no. 7DAH, Grass Instruments, Quincy, MA) and digitized (LabChart Pro 7, PowerLab) for real-time display, storage, and later analysis. During the data acquisition protocol (see below), isoflurane anesthesia was titrated between 1 and 2% to control the respiratory rate between 40 and 90 breaths/min. The tracheal cannula was flushed with air for ∼1 s immediately prior to each negative pressure ramp (see below) and secretions were aspirated as needed to maintain the patency of the tracheostomy tube throughout the experiments.
Electromyography.
The two Teflon-insulated wire loop electrodes were used to record genioglossus electromyographic (EMG) activity (EMGGG). The EMGGG signal was amplified, band-pass filtered from 30 to 1,000 Hz (alternating-current preamplifier; model P511K, Grass Instruments), and digitized at a sampling rate of 1,000 Hz (LabChart Pro 7). The EMGGG was rectified, and a 55-ms time constant was applied to compute the moving average (LabChart Pro 7).
Experimental Protocol
Assessing passive upper airway function.
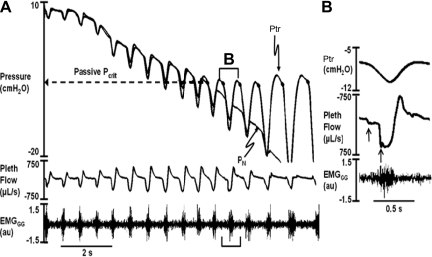
Passive upper airway function was assessed during expiration, when EMGGG fell to tonic levels. Expiratory flow dynamics were governed by pressures upstream in the trachea and downstream at the nose and by the collapsibility of the pharynx, as previously described (22, 45, 46). Pn was manipulated to vary the state of upper airway patency. The pressure at which the pharynx collapsed during expiration [passive critical pressure (Pcrit)] was determined as illustrated in Fig. 1. At elevated levels of Pn, when Pn and Ptr were greater than the Pcrit, the airway remained patent and tidal airflow remained unimpeded. The Pn was lowered in ramplike fashion from ∼+10 to approximately −30 cmH2O, and the Ptr followed the Pn signal initially. With further decreases in Pn, the expiratory Ptr and Pn signals diverged, and Ptr plateaued at end expiration, becoming insensitive to further decreases in Pn (Fig. 1A). This divergence in pressures occurred as Ptr decreased during expiration until it reached a Pcrit, at which point the airway occluded (22, 45, 46). The passive Pcrit was defined as the end-expiratory level of Ptr at which it diverged from Pn. Measurements were made on ∼10 Pn ramps in each mouse.
Fig. 1.
Representative ramp in nasal pressure (Pn) in which the airway occludes during expiration. In A, Pn was lowered progressively. Tracheal pressure (Ptr) tracked Pn initially. As Pn continued to fall, expiratory Ptr diverged and reached a plateau (dashed arrow extending from end-expiratory data points), becoming insensitive to further decreases in Pn. Pn at which Ptr diverged during expiration represents the passive critical pressure (Pcrit) when genioglossus electromyographic (EMG) activity (EMGGG) falls to tonic levels. B: airflow dynamics for the first flow-limited breath in the negative pressure ramp (B-labeled bracket in A). As Ptr decreased at the start of inspiration, airflow reached an early and midinspiratory plateau (upward arrows), despite a continued decline in Ptr, indicating inspiratory flow limitation. Sudden increase in airflow in midinspiration coincided with progressive increases in phasic EMG activity. au, Arbitrary units.
To validate our method for determining the passive Pcrit, we compared the passive Pcrit in the spontaneously breathing mouse with that obtained during complete neuromuscular blockade following the administration of pancuronium bromide (1 mg/kg ip; Hospira, Lake Forest, IL). The paralyzed C57BL/6J mice (n = 5) were ventilated (model 683, Harvard Instruments, Holliston, MA) through the tracheal cannula at a respiratory rate of 120 breaths/min and a tidal volume of 0.5 ml. The ventilator was then stopped momentarily, and Pn was lowered in a ramplike fashion to determine the passive Pcrit as described above.
Assessing phasic modulation of upper airway function.
During Pn ramps, airflow resumed during inspiration, indicating that the pharynx had reopened. Inspiratory airflow coincided with phasic increases in EMGGG, which restored airway patency by decreasing Pcrit, as previously described (22, 45, 46). During inspiration, flow dynamics were governed by pressures upstream in the nose and downstream at the trachea and by the inspiratory Pcrit (44, 52, 53). As Pn decreased progressively, pharyngeal collapse was characterized by the development of inspiratory flow limitation, as defined by an early, followed by a midinspiratory, plateau in airflow as Ptr continued to decrease (Fig. 1B, upward arrows) (15). Step increases in inspiratory airflow were accompanied by increased phasic EMGGG activity, which peaked in late inspiration in this example. Nevertheless, the airway remained flow-limited, even at maximal EMGGG activity, suggesting that phasic activity was not sufficient to eliminate the flow limitation. (Despite evidence for momentary occlusion or marked airflow limitation at the start of inspiration, the airway never remained flow-limited throughout the entire inspiration when negative Pn was applied.) We therefore examined the frequency of flow limitation and Pn at which flow limitation developed, rather than the level of flow, to characterize the phasic modulation of upper airway function (see below).
Confirming the site of upper airway collapse.
We determined the site of pharyngeal collapse by monitoring oropharyngeal pressure with a small catheter that was placed in the mouth in five lean C57BL/6J mice. During Pn ramps, the oropharyngeal pressure and Ptr paralleled each other throughout the respiratory cycle. During ramp decreases in Pn, the oropharyngeal pressure and Ptr diverged from Pn and plateaued during expiration, indicating occlusion downstream toward the nose. During inspiration, these pressures also diverged from V̇i, indicating that the flow-limiting site was upstream, toward the nose, during inspiration. These findings established that the site of pharyngeal collapse was rostral to the oropharynx (i.e., velopharynx), as previously demonstrated in the isolated upper airway of larger animals, presumably at the rim of the soft palate (52, 53).
Data Analysis
Each Pn ramp (run) was evaluated to determine the passive Pcrit and the presence of inspiratory airflow limitation. For the first flow-limited breath of each run, the tonic and peak phasic EMGGG activities were measured. The tonic and peak phasic EMGGG activities were also measured for adjacent non-flow-limited breaths, and phasic EMGGG was calculated as the difference between tonic and peak phasic levels.
All EMGGG measurements were normalized to the maximal EMGGG in each mouse and expressed as a percentage of maximal activity. A standardized maneuver was developed to assess maximal EMGGG activity following central apneic episodes. These episodes were experimentally induced by flushing the tracheal cannula before each negative pressure run. As Ptr rose during the flush, the lungs inflated and a central apnea ensued. During the recovery breaths, peak phasic activity rose to maximal levels. This maximum was assessed repeatedly throughout the experiment by flushing the tracheostomy tube before each run, and the maximal level was found to be highly reproducible throughout the experiment.
The frequency at which inspiratory airflow limitation developed during Pn ramps and the Pn threshold at which it developed were quantified to characterize upper airway function during inspiration as 1) the percentage of Pn ramps displaying inspiratory flow limitation, 2) the Pn threshold at the onset of inspiratory flow limitation, and 3) the tonic and peak phasic EMGGG levels (as above). The Pn threshold was referenced to the atmospheric pressure and the passive Pcrit, Pn − Pcrit, to account for differences in passive Pcrit (mechanical loads between groups). We recognized that these parameters could vary with ventilation under isoflurane anesthesia among the different age and weight groups. To assess for differences in ventilation, ventilation was measured during stable periods of breathing at baseline prior to Pn ramps and normalized by weight for each mouse.
Statistical Analysis
Statistical analyses were structured to test a priori hypotheses that age and weight influenced measures of upper airway function. Mixed-effects analysis of variance was utilized to model effects of the primary independent variables (age and weight group) on outcome measures of ventilation and upper airway function (Intercooled Stata 9.2, Stata, College Station, TX). Our models incorporated terms to determine the independent and interactive effects of age and weight group on the passive Pcrit, phasic parameters of upper airway function, and absolute and normalized levels of ventilation. When significant differences were detected, post hoc comparisons were performed (with Bonferroni's correction) to determine the source of these differences. Statistical significance was inferred at P < 0.05. Values are expressed as means ± SE.
RESULTS
Effect of Age and Weight on Passive Pcrit
In the C57BL/6J mice, Pcrit did not differ significantly between the paralyzed and spontaneously breathing condition (−3.5 ± 0.7 and −3.5 ± 0.9 cmH2O, respectively), suggesting that expiratory measurements were determined by the passive mechanical characteristics of the pharynx.
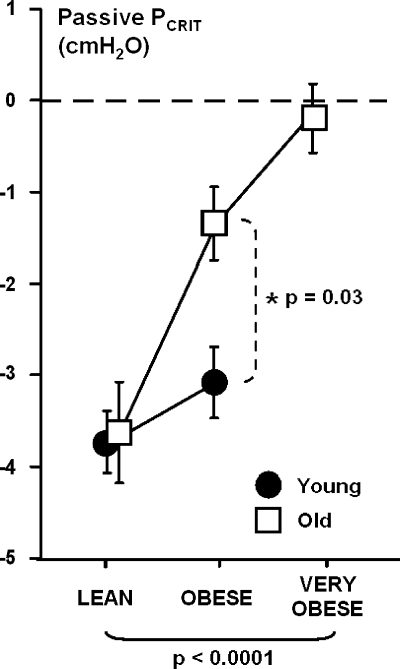
The effects of weight and age are illustrated in Fig. 2. Passive Pcrit increased significantly with weight and age, and these increases were significantly greater in the old than young mice. Increases in passive Pcrit with weight were observed in the young and old groups.
Fig. 2.
Passive Pcrit by weight group for young and old mice. Values are means ± SE. Passive Pcrit increased progressively with increasing body weight, independent of age (P < 0.0001). In addition, passive Pcrit increased more with weight in old than young obese mice (P = 0.03). Passive Pcrit increased significantly with weight in young (P = 0.003) and old (P < 0.0001) mice.
Effect of Age and Weight on Phasic Modulation of Upper Airway Function
As expected, baseline levels of ventilation increased significantly with weight (P < 0.01) but did not differ significantly with age (Table 2). After normalizing ventilation to body weight, however, we did not detect any significant differences by age or weight, suggesting that ventilation was comparable among all weight and age groups and that isoflurane anesthesia did not exert differential effects on ventilation across age and weight groups.
Table 2.
Baseline ventilatory parameters by age and weight group
| Minute Ventilation |
||||
|---|---|---|---|---|
| ml/min | μl · min−1 · g−1 | Tidal Volume, μl | Respiratory Rate, breaths/min | |
| Young | ||||
| Lean | 6.0 ± 0.5 | 256.8 ± 19.6 | 101.3 ± 5.8 | 60.8 ± 6.7 |
| Obese | 7.1 ± 0.6 | 242.7 ± 19.6 | 128.6 ± 7.1 | 56.9 ± 5.6 |
| Old | ||||
| Lean | 6.1 ± 0.5 | 256.3 ± 22.1 | 104.2 ± 5.5 | 59.2 ± 5.7 |
| Obese | 6.8 ± 0.5 | 228.7 ± 16.6 | 127.1 ± 3.9 | 53.6 ± 3.0 |
| Very obese | 9.0 ± 1.1 | 252.5 ± 29.8 | 154.6 ± 21.7 | 60.2 ± 8.8 |
Values are means ± SE. Significant increases in tidal volume and minute ventilation (ml/min) were observed with increasing weight independent of age (P < 0.01).
During Pn ramps, inspiratory airflow limitation developed in less than half of the ramps for all groups of mice (Table 3). The frequency of flow-limited inspirations during these ramps decreased significantly with increasing weight independent of age (P < 0.001). In the obese groups, inspiratory flow limitation occurred less frequently in the young than old mice (P < 0.01).
Table 3.
Frequency of flow-limited breaths during negative pressure ramps
Values are means ± SE, expressed as percentage of pressure ramps resulting in a flow-limited breath. Significant decreases were observed with increasing weight independent of age (†P < 0.001), and these decreases were less pronounced in the old than young obese mice (*P < 0.01).
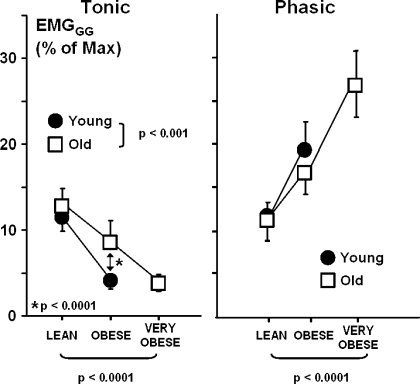
Compared with adjacent non-flow-limited breaths, flow-limited breaths were associated with a 7.3 ± 0.6% increase in phasic EMGGG activity (from 8.4 ± 0.5% to 15.7 ± 1.2%, P < 0.001) but no significant increase in tonic activity (from 8.3 ± 0.6% to 8.4 ± 0.7%, P = not significant) across all weight and age groups, suggesting that phasic neuromuscular responses were elicited by the development of inspiratory flow limitation. During flow-limited breaths, progressive decreases in tonic EMGGG were observed with obesity independent of age (P < 0.0001; Fig. 3, left). Progressive decreases in tonic EMGGG were observed with obesity in the young and old mice (P < 0.0001 in each group) and were more pronounced in the young than old mice (P < 0.0001). In contrast, phasic EMGGG activity increased significantly with weight independent of age (P < 0.0001). After stratifying by age group, we found a significant increase in phasic EMGGG in the young mice (P < 0.05) and an upward trend in phasic EMGGG in the old mice (P = 0.07; Fig. 3, right).
Fig. 3.
Tonic and phasic EMGGG activity as a percentage of maximum by weight (left) and age (right) groups. Values are means ± SE. Tonic EMGGG decreased significantly with increasing weight independent of age (P < 0.0001). Weight-related decreases in tonic EMGGG were greater in young than old mice (P < 0.0001). In contrast, phasic EMGGG activity increased significantly with weight independent of age (P < 0.0001).
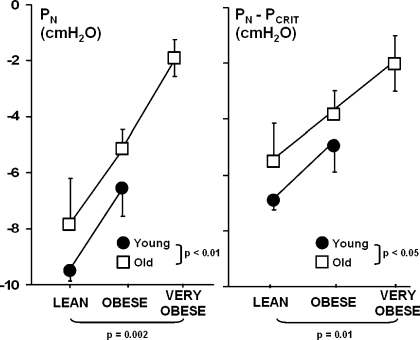
The absolute and relative Pn (Pn and Pn − Pcrit, respectively) thresholds to the development of inspiratory flow limitation are illustrated in Fig. 4. A significant increase in these thresholds occurred with weight independent of age (P ≤ 0.01). Increases in Pn thresholds indicated that flow limitation developed at higher (less negative) levels of Pn in obese than lean mice and that the pressure threshold became less negative even after accounting for concomitant increases in passive Pcrit. These thresholds were also significantly higher in the old than young mice across all weight groups (P < 0.05). Specifically, there was an overall elevation of the Pn and the Pn − Pcrit in the old compared with young mice of 1.7 ± 0.5 and 1.6 ± 0.5 cmH2O (Fig. 4, left and right) respectively. Elevations in this threshold with age persisted after adjustment for passive Pcrit (Fig. 4, right), indicating that the susceptibility to airflow obstruction was independent of pharyngeal mechanical loads.
Fig. 4.
Pn (left) and Pn − Pcrit (right) thresholds to the development of flow limitation during negative pressure ramps by weight group for young and old mice. Values are means ± SE. Pn increased progressively with increases in body weight independent of age (P = 0.002). Pn threshold was also significantly elevated in old compared with young mice independent of weight (P < 0.01). Pn − Pcrit threshold also increased progressively with increases in weight independent of age (P = 0.01). Pn − Pcrit threshold was also significantly elevated in old compared with young mice independent of body weight (P < 0.05). Specifically, there was an overall elevation of Pn and Pn − Pcrit threshold in old compared with young mice (1.7 ± 0.5 and 1.6 ± 0.5 cmH2O, respectively).
DISCUSSION
In this study of anesthetized normal, obese, and very obese mice, we found that passive Pcrit increased progressively with body weight and was greater in the old than young mice. Despite increases in passive Pcrit, inspiratory airflow limitation developed in less than half of the Pn challenges. Flow limitation developed at higher (less negative) Pn and remained elevated after adjustment for passive Pcrit in the obese compared with lean mice. The frequency of flow limitation decreased with increasing body weight, particularly in the young mice. Phasic, but not tonic, EMGGG activity increased during flow-limited compared with non-flow-limited breaths across weight and age groups. Elevations in weight were also more closely associated with decreased tonic and increased phasic EMG activity in the young than old mice. Our findings suggest that weight imposed related mechanical loads on the upper airway that worsened with age. These data also suggest that phasic neuromuscular responses compensated for the age-related decreases in tonic neuromuscular activity that predisposed to airflow limitation in the obese and very obese mice.
Passive Pharyngeal Mechanics
The passive or mechanical loads on the upper airway have been assessed under conditions of reduced or absent pharyngeal neuromuscular activity. In humans and animals, these conditions are obtained during inspiration in deeply anesthetized subjects (8–10), after elimination of airflow obstruction in sleeping subjects (19, 49), during neuromuscular blockade (20, 60), and during natural sleep or anesthesia when neuromuscular activity wanes in expiration (22, 28, 45, 46, 52, 53). In the current study, we extended our methods for assessing the passive Pcrit by determining the Ptr at which the pharynx occluded during expiration as airway pressure was reduced (22, 45). We have validated our methods by demonstrating that the expiratory closing pressure was comparable to that during complete neuromuscular blockade in our mice, indicating that alterations in passive Pcrit reflect differences in pharyngeal mechanical loads (5, 52). Compared with our previous method for measuring passive Pcrit in the isolated mouse upper airway (28), our method has also enhanced our precision in estimating passive Pcrit, thereby enhancing our ability to discriminate effects of weight and age on pharyngeal loads.
In the current study, we found that obesity produced progressive increases in pharyngeal mechanical loads (Pcrit) that were significantly greater in the old than young mice. Thus our findings may explain prior observations in the obese anesthetized New Zealand obese mice, in which greater degrees of expiratory collapse were demonstrated than in the lean New Zealand White background strain (3, 4). It has been thought that expiratory collapse may have been related to excess fat deposition in the tongue (4) or peripharyngeal tissues (18, 21), which could load the pharyngeal airway and increase its surrounding pressure (3, 27, 55). Nevertheless, polygenic differences between the New Zealand Obese and New Zealand White strains could also lead to alterations in upper airway structures that account for differences in expiratory behavior (2, 17). In contrast, we controlled for genetic factors by assessing upper airway function in a single inbred murine strain and established that weight gain leads to increases in passive Pcrit.
Aging was associated with a considerably greater elevation in Pcrit in the obese mice, despite similar levels of Pcrit in lean young and old mice. This finding suggests that age alters the response to weight gain, which leads to greater increases in pharyngeal loads in the old than young mice. Aging has been associated with an increase in central adipose tissue (27), which may infiltrate surrounding tissues, including the peripharyngeal fat pads and lateral pharyngeal walls (21, 54, 61). Specifically, adipose deposition in peripharyngeal tissues has been linked to upper airway obstruction (47, 48) and has been found to correlate with age independent of obesity (29). In addition, age-related structural changes can elongate the pharynx (54), increasing its exposure to mechanical loads from surrounding tissues (43) and predisposing to pharyngeal collapse (30). Thus, age-related changes in regional adiposity and anatomy can increase pharyngeal loads (11, 31) and account for age-related increases in collapsibility in the present study. Conversely, lean mice were protected from age-related increases in Pcrit, suggesting that increases in pharyngeal collapsibility and sleep apnea severity in older populations primarily result from concomitant increases in weight and central adiposity, rather than aging per se.
Active Responses to Airflow Obstruction
In our older mice, increases in body weight were associated with marked progressive elevations in Pcrit relative to atmospheric pressure, indicating that the airway would spontaneously occlude during expiration (14, 51). Increases in passive Pcrit in the older obese mice left the airway more susceptible to inspiratory obstruction than the young lean mice. Despite evidence for expiratory obstruction, the airway reopened during inspiration and withstood considerably more negative Pn, a finding that can only be explained by a significant fall in pharyngeal collapsibility. This decrease could be attributed to recruitment of phasic neuromuscular activity by negative airway pressures, which could prevent the development of inspiratory flow limitation during negative pressure ramps and reduce its frequency with obesity (Table 3). Despite increases in phasic neuromuscular activity, flow limitation thresholds increased with obesity (Fig. 4). These findings lead us to suspect that the elevation in inspiratory thresholds with obesity and aging could have resulted from decreases in the endurance (37) or contractility (6, 33, 41, 57) of the upper airway musculature, rather than the level of neuromuscular activity (EMGGG) per se.
Inspiratory changes in upper airway function may also result from alterations in central neuromotor control. Central serotonergic activity plays a role in the maintenance of pharyngeal patency in the obese Zucker rat, as evidenced by the 5-HT2A/2C antagonist ritanserin, which decreases upper airway neuromuscular activity and increases collapsibility (34). In contrast, a 5-HT2A/2C agonist increases the activity and reduces collapsibility (36). Similarly, after the administration of 5-HT antagonists in the English bulldog, considerable decreases in pharyngeal neuromuscular activity and cross-sectional area, as well as dynamic (inspiratory) pharyngeal collapse (58), were observed. These data suggest that serotonergic activity can stabilize airway patency during inspiration and compensate for structural loads in this model. In older rats, ultrastructural evidence for diminished serotonergic input at hypoglossal motor neurons (1) further implies that compensatory serotonergic mechanisms may deteriorate with age. Thus, disturbances in the serotonergic modulation of hypoglossal motor neurons can account for inspiratory increases in pharyngeal collapsibility in obese and aging mice (Fig. 4) and rats (41).
Limitations
A major strength of our model in the C57BL/6J mouse was that we were able to decouple effects of aging from weight gain and comorbid conditions, which can impact upper airway function in humans. Nevertheless, several limitations should be considered in interpreting our findings. 1) We adapted our approach for characterizing the dynamic modulation of upper airway function in canine (52), feline (44, 53), and murine (28) models to increase the precision of our measurements during inspiration and expiration. Nevertheless, our current methods characterize the static and dynamic determinants of pharyngeal collapse, which include defining the critical closing pressure during expiration and the susceptibility to flow limitation during inspiration. 2) As previously noted (28), inspiratory occlusion did not occur, even at markedly negative Pn, and inspiratory flow limitation occurred variably, prompting us to adopt surrogate measures of dynamic collapse in our intact mouse upper airway preparation. 3) We recognize that tonic EMG activity may have influenced our measurements of passive Pcrit to some extent. Nevertheless, we have evidence that its influence was minimal, since passive Pcrit did not differ significantly between spontaneously breathing and paralyzed mice. This finding obviated the need for administering a neuromuscular blocking agent, which allowed us to quantify the phasic modulation of upper airway function in the same mice. 4) We acknowledge that anesthesia may have exerted differential effects on upper airway dilator activity (12) and dynamic responses to age and weight. To mitigate this possibility, we adjusted the isoflurane concentration to limit the range of respiratory rate and maintain constant levels of minute ventilation (normalized to weight) among groups, reinforcing our conclusion that anesthesia did not confound our assessment of age and weight effects on upper airway function. 5) We arbitrarily restricted our studies to male mice to minimize the variability in our measurements, and we acknowledge that our findings cannot be generalized to females (35, 40). Similarly, we cannot extrapolate from observations in the wild-type mouse to genetically susceptible obese strains, such as the leptin-deficient ob−/ob− mouse (35), in which a defect in ventilatory control may impair compensatory pharyngeal neuromuscular control. 6) We acknowledge that our age groups represent postadolescent and mature adults, rather than “young” and “old” adults as described, suggesting that our findings may represent the influence of developmental, rather than aging, processes on upper airway function (11, 31). Furthermore, our findings in otherwise healthy mice do not address the potential impact of concomitant inflammatory or neuromyopathic changes in pharyngeal structures that may accelerate the decline in upper airway function (50).
Implications
Our study has implications for modeling the neuromechanical control of upper airway function in mice. Obesity and age combine to increase mechanical loads on the pharynx in our model, consistent with underlying structural defects that predispose to airflow obstruction during sleep (27). While weight gain is a “normal” concomitant of aging (16), our findings suggest that normal weight gain can play a significant role in the pathogenesis of obstructive sleep apnea. Our findings also imply that phasic modulation of upper airway function can compensate for weight- and age-related increases in pharyngeal loads in our wild-type mice (27). Neuromuscular compensatory mechanisms may be compromised, however, in leptin-deficient or -resistant mice, in which responses to respiratory loads in obesity are deficient (35). Further work in this anesthetized mouse model can help elucidate fundamental mechanisms for age- and weight-related increases in sleep apnea susceptibility, prevalence, and severity. Characterizing the amount and distribution of body fat in this model would yield greater insight into potential mechanisms for observed changes in upper airway function. This model may also serve to explore effects of genetic factors, environmental exposures, and treatments on upper airway function.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-50381 and HL-37379.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Behan M, Brownfield MS. Age-related changes in serotonin in the hypoglossal nucleus of rat: implications for sleep-disordered breathing. Neurosci Lett 267: 133–136, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bielschowsky M, Goodall CM. Origin of inbred NZ mouse strains. Cancer Res 30: 834–836, 1970 [PubMed] [Google Scholar]
- 3. Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med 179: 158–169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brennick MJ, Pickup S, Cater JR, Kuna ST. Phasic respiratory pharyngeal mechanics by magnetic resonance imaging in lean and obese Zucker rats. Am J Respir Crit Care Med 173: 1031–1037, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol 46: 772–779, 1979 [DOI] [PubMed] [Google Scholar]
- 6. Cantillon D, Bradford A. Effects of age and gender on rat upper airway muscle contractile properties. J Gerontol A Biol Sci Med Sci 55: B396–B400, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J, Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest 123: 1544–1550, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 103: 470–477, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Eastwood PR, Szollosi I, Platt PR, Hillman DR. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology 97: 786–793, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet 359: 1207–1209, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest 131: 1702–1709, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eikermann M, Malhotra A, Fassbender P, Zaremba S, Jordan AS, Gautam S, White DP, Chamberlin NL. Differential effects of isoflurane and propofol on upper airway dilator muscle activity and breathing. Anesthesiology 108: 897–906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 143: 1300–1303, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 110: 1077–1088, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Guo J, Hall KD. Predicting changes of body weight, body fat, energy expenditure and metabolic fuel selection in C57BL/6 mice. PLoS One 6: e15961, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herberg L, Coleman DL. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism 26: 59–99, 1977 [DOI] [PubMed] [Google Scholar]
- 18. Igel M, Becker W, Herberg L, Joost HG. Hyperleptinemia, leptin resistance, and polymorphic leptin receptor in the New Zealand obese mouse. Endocrinology 138: 4234–4239, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Isono S, Morrison DL, Launois SH, Feroah TR, Whitelaw WA, Remmers JE. Static mechanics of the velopharynx of patients with obstructive sleep apnea. J Appl Physiol 75: 148–154, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of the pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol 82: 1319–1326, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Kairaitis K, Parikh R, Stavrinou R, Garlick S, Kirkness JP, Wheatley JR, Amis TC. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol 95: 1560–1566, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kirkness JP, Schwartz AR, Patil SP, Pichard LE, Marx JJ, Smith PL, Schneider H. Dynamic modulation of upper airway function during sleep: a novel single-breath method. J Appl Physiol 101: 1489–1494, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 104: 1618–1624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu A, Pichard L, Schneider H, Patil SP, Smith PL, Polotsky V, Schwartz AR. Neuromechanical control of the isolated upper airway of mice. J Appl Physiol 105: 1237–1245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, Kikinis R, White DP. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 119: 72–14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med 166: 1388–1395, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Marcus CL, Fernandes do Prado LB, Lutz J, Katz ES, Black CA, Galster P, Carson KA. Developmental changes in upper airway dynamics. J Appl Physiol 97: 98–108, 2004 [DOI] [PubMed] [Google Scholar]
- 32. McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105: 197–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res 9: 389–393, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Nakano H, Magalang UJ, Lee SD, Krasney JA, Farkas GA. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am J Respir Crit Care Med 163: 1191–1197, 2001 [DOI] [PubMed] [Google Scholar]
- 35. O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159: 1477–1484, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Ogasa T, Ray AD, Michlin CP, Farkas GA, Grant BJ, Magalang UJ. Systemic administration of serotonin 2A/2C agonist improves upper airway stability in Zucker rats. Am J Respir Crit Care Med 170: 804–810, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Oliven A, Carmi N, Coleman R, Odeh M, Silbermann M. Age-related changes in upper airway muscles: morphological and oxidative properties. Exp Gerontol 36: 1673–1686, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Polotsky VY, Wilson JA, Smaldone MC, Haines AS, Hurn PD, Tankersley CG, Smith PL, Schwartz AR, O'Donnell CP. Female gender exacerbates respiratory depression in leptin-deficient obesity. Am J Respir Crit Care Med 164: 1470–1475, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Ray AD, Ogasa T, Magalang UJ, Krasney JA, Farkas GA. Aging increases upper airway collapsibility in Fischer 344 rats. J Appl Physiol 105: 1471–1476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- 43. Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol 80: 2171–2178, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Rowley JA, Williams BC, Smith PL, Schwartz AR. Neuromuscular activity and upper airway collapsibility. Mechanisms of action in the decerebrate cat. Am J Respir Crit Care Med 156: 515–521, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Schneider H, Boudewyns A, Smith PL, O'Donnell CP, Canisius S, Stammnitz A, Allan L, Schwartz AR. Modulation of upper airway collapsibility during sleep: influence of respiratory phase and flow regimen. J Appl Physiol 93: 1365–1376, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Schneider H, O'Hearn DJ, Leblanc K, Smith PL, O'Donnell CP, Eisele DW, Peter JH, Schwartz AR. High-flow transtracheal insufflation treats obstructive sleep apnea. A pilot study. Am J Respir Crit Care Med 161: 1869–1876, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med 173: 453–463, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 168: 522–530, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Schwartz AR, Patil SP, Schneider H, Smith PL. Modelling pathogenic mechanisms of upper airway dysfunction in the molecular age. Eur Respir J 32: 255–258, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J Appl Physiol 66: 1626–1634, 1989 [DOI] [PubMed] [Google Scholar]
- 52. Schwartz AR, Thut DC, Brower RG, Gauda EB, Roach D, Permutt S, Smith PL. Modulation of maximal inspiratory airflow by neuromuscular activity: effect of CO2. J Appl Physiol 74: 1597–1605, 1993 [DOI] [PubMed] [Google Scholar]
- 53. Seelagy MM, Schwartz AR, Russ DB, King ED, Wise RA, Smith PL. Reflex modulation of airflow dynamics through the upper airway. J Appl Physiol 76: 2692–2700, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Shigeta Y, Enciso R, Ogawa T, Clark GT. Changes in three dimensional simulation models of the airway which are due to increases in age or body mass index. Stud Health Technol Inform 132: 460–462, 2008 [PubMed] [Google Scholar]
- 55. Simpson L, Mukherjee S, Cooper MN, Ward KL, Lee JD, Fedson AC, Potter J, Hillman DR, Eastwood P, Palmer LJ, Kirkness J. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep 33: 467–474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thut DC, Schwartz AR, Roach D, Wise RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol 75: 2084–2090, 1993 [DOI] [PubMed] [Google Scholar]
- 57. Van LE. Effects of genetic obesity on rat upper airway muscle and diaphragm contractile properties. Eur Respir J 9: 2139–2144, 1996 [PubMed] [Google Scholar]
- 58. Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med 153: 776–786, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 29: 6–28, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med 165: 260–265, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Welch KC, Foster GD, Ritter CT, Wadden TA, Arens R, Maislin G, Schwab RJ. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep 25: 532–542, 2002 [PubMed] [Google Scholar]
- 62. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 63. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol 99: 1592–1599, 2005 [DOI] [PubMed] [Google Scholar]