Abstract
The conduction velocity (CV) of a muscle fiber is affected by the fiber's discharge history going back ∼1 s. We investigated this dependence by measuring CV fluctuations during voluntary isometric contractions of the human brachioradialis muscle. We recorded electromyogram (EMG) signals simultaneously from multiple intramuscular electrodes, identified potentials belonging to the same motor unit using EMG decomposition, and estimated the CV of each discharge from the interpotential interval. In 12 of 14 subjects, CV increased by ∼10% during the first second after recruitment and then fluctuated by about ±2% in a way that mirrored the fluctuations in the instantaneous firing rate. The CV profile could be precisely described in terms of the discharge history by a simple mathematical model. In the other two subjects, and one subject retested after cooling the arm, the CV fluctuations were inversely correlated with instantaneous firing rate. In all subjects, CV was additionally affected by very short interdischarge intervals (<25 ms): it was increased in doublets at recruitment, but decreased in doublets during continuous firing and after short interdischarge intervals in doubly innervated fibers. CV also exhibited a slow trend of about −0.05%/s that did not depend on the immediate discharge history. We suggest that measurements of CV fluctuations during voluntary contractions, or during stimulation protocols that involve longer and more complex stimulation patterns than are currently being used, may provide a sensitive approach for estimating the dynamic characteristics of ion channels in the human muscle-fiber membrane.
Keywords: muscle-fiber conduction velocity, velocity recovery function, electromyogram decomposition, doubly innervated muscle fiber
the electrical characteristics of the human muscle fiber membrane cannot be measured in situ, and so must be inferred from observations of measurable electrophysiological phenomena. One such phenomenon that can be measured fairly precisely is muscle-fiber conduction velocity (CV). CV is directly related to membrane excitability. During voluntary contraction, redistribution of ions produces dynamic changes in membrane excitability, which are manifested as changes in CV. Existing models of membrane processes (1, 6, 39) are not able to account for these changes, suggesting either that the models are incomplete (12), or that the parameter values, which were obtained from different species and under a variety of different experimental conditions, are not accurate for human muscle fibers (14). Observations of CV behavior during voluntary contractions can provide empirical data for checking models and estimating parameter values more accurately.
CV can be measured using intramuscular (8, 35) or surface electrodes (9, 32), and it can be studied either during voluntary contractions (10, 35), or in response to electrical stimulation (5, 35, 37). Although electrical stimulation allows controlled manipulation of the fiber's discharge history, most studies to date have used stimulation patterns that are much shorter and simpler than the discharge patterns that occur during voluntary contractions. The most widely used stimulation protocol is double pulse stimulation (31), in which the relative velocity of an action potential is measured as a function of the interval between it and a single conditioning action potential. This relationship is known as the velocity recovery function. In terms of the train of action potentials that occurs during a voluntary contraction, the velocity recovery function only describes the relationship between the first and second potentials in the train; it does not say anything about the subsequent potentials. Other studies have used multiple conditioning stimuli (22, 23, 40) and trains of stimuli (33), but their patterns have still not been as complex as those that occur during voluntary contractions.
During voluntary contractions, the mean value of CV has been shown to be correlated with the mean firing rate (11, 32, 33, 35), while the actual value fluctuates from discharge to discharge because of fluctuations in instantaneous firing rate (IFR) (7, 28, 35, 38). Over the longer term, CV decreases slowly over periods of hundreds of seconds (10, 15, 32, 35).
The purpose of this study was to investigate the CV behavior in a large sample of motor units (MUs) during voluntary isometric contractions of the human brachioradialis muscle. We measured the CV of MU discharges using multiple intramuscular electrodes and electromyogram (EMG) decomposition. We studied CV behavior during a variety of different discharge behaviors, including recruitment, intermittent firing, steady firing, and doublets. We also studied CV behavior in doubly innervated muscle fibers, which are activated by two different motoneurons and so experience a much more variable discharge history than normal muscle fibers (27).
We characterized the observed CV behavior using a mathematical model based on the ones proposed by Davis et al. (7) and Lööf (28). The model assumes that each discharge has a cumulative effect on the CV of future discharges, with the size of the affect decreasing exponentially with time. The model was quite accurate, being able to account for >99% of the CV variance in some cases. This suggests that the model closely reflected the dynamics of the membrane processes that affected CV.
METHODS
Subjects.
Eighteen subjects (7 women, 11 men, 22–49 yr, mean 32 yr), with no history of neuromuscular disorder, diabetes, or orthopedic impairment or surgery of the upper limb, participated in this study. Additionally, one subject (man, 52 yr) with a C6 level spinal cord injury participated. The bulk of the results were obtained from 14 of the able-bodied subjects. For the other four able-bodied subjects, only doubly innervated fibers were analyzed, and for the subject with spinal cord injury, only one unusual motor unit action potential (MUAP) train that had a large number of doublets was analyzed. The studies were approved by the Stanford University Panel on Medical Human Subjects and conformed to the Declaration of Helsinki. All subjects provided informed consent.
Protocol.
The brachioradialis muscle was identified by palpation, and the longitudinal axis of the muscle was marked. Three pairs of fine-wire electrodes (50-μm-diameter stainless steel wires, insulated except for a 1-mm exposed recording surface at the tip; Jari Electrode Supply, Gilroy, CA) were inserted along this line at 30, 60, and 90 mm from the elbow crease. For some contractions, a monopolar needle electrode (27 gauge, 1 mm of exposed recording surface) was also inserted along the line, either between or beyond the fine-wire insertion points. The subjects performed 20-s-long isometric contractions at a low level of force (∼10–20% of maximal voluntary contraction) to activate between ∼4 and 16 MUs per recording site. Six subjects also performed an additional 100-s-long contraction at a comparable level of force. For one subject, a second set of contractions was recorded after cooling the forearm with an ice pack.
All of the signals were recorded in monopolar fashion with respect to an electrode on the skin surface. The signals were amplified with filter settings of 5 Hz to 5 kHz (Nicolet Viking, Madison, WI), sampled at 10 kHz, and stored on computer. Audio feedback of the EMG signals was provided to help the subjects maintain steady isotonic contractions.
Signal processing.
The EMG signals were digitally high-pass filtered at 1 kHz and decomposed offline into trains of individual MUAPs by an experienced investigator using the EMGlab computer-aided decomposition program (30). The investigator checked and edited the results to make sure that the identified firing patterns were smooth and regular, and that all of the activity in the signal was accounted for. For each MU, separate templates were made for all of the main spikes in each signal. The templates were formed by averaging all of the detected occurrences of each spike. For each discharge of the MU, the time of occurrence of each spike was measured by aligning the appropriate template. These measurements were made to a precision of 1 μs using interpolation (29).
Potentials from doubly innervated fibers were identified by the fact that they occurred in association with two different MUAPs and were blocked or delayed in a characteristic way whenever the two MUAPs discharged close together in time (27).
The smoothed IFR of each MU and doubly innervated fiber was obtained by interpolating the reciprocal of the interdischarge intervals (IDIs) using a zero-order hold and then filtering with a causal first-order low-pass Butterworth filter with a cutoff frequency of 0.75 Hz. The MUAP waveforms were averaged from the unfiltered signals, and the mean latencies of the various spikes were measured with respect to the MUAP onset (26).
The decomposition results were examined, and a number of MUAP trains were selected for which we were reasonably confident that the discharge patterns had been completely and correctly identified, and for which there were two or more prominent MUAP spikes, either in the same or in different signals, separated by a latency of at least 4 ms. For each such pair of spikes, the latency between the spikes [the interpotential interval (IPI)] was determined for each discharge of the MU. If the IPI of any discharge was considerably different from that of the neighboring discharges of the same MU, the signals were reinspected to check for and correct possible decomposition errors. Occasionally, it was not possible to determine the precise time of occurrence of a particular spike due to excessive noise or interference. In these cases, the IPI was set equal to the average of the IPIs of the two neighboring discharges.
CV measurement.
To compare differences between subjects, the IPI values were converted into normalized CV values as shown in Fig. 1.
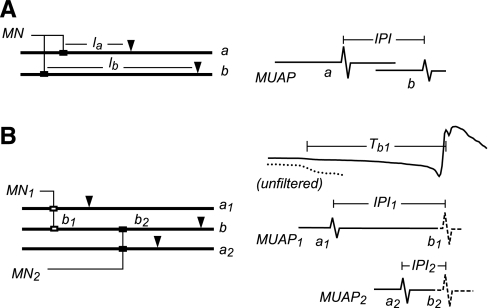
Fig. 1.
Measurement of interpotential interval (IPI). A: normal motor unit (MU). The motoneuron (MN) innervates two fibers (a, b) at possibly different endplate locations (solid rectangles). Potentials are recorded from each fiber at possibly different electrode locations (inverted triangles), giving MU action potential (MUAP) spikes a and b. The IPI was measured between the spikes. la and lb, Distances from the fiber endplates. B: doubly innervated fiber. Fiber b is innervated by MN1 at endplate b1 and by MN2 at endplate b2. MUAP1 and MUAP2 each contain spikes from singly innervated fibers (a1, a2) and from the doubly innervated fiber (b1, b2). The b spikes may be blocked from reaching the electrode if they collide with an action potential from the other endplate. The IPIs are measured separately for each MUAP and are normalized with respect to the latency of the b spike from the MUAP onset (Tb). The top trace shows the unfiltered MUAP1 with the initial part expanded vertically (dotted line) to better see the onset.
Most of the IPI measurements came from normal singly innervated fibers (Fig. 1A). The two spikes most likely came from two different muscle fibers (a and b) and were recorded at different distances from the fiber endplates (la and lb). We assumed that the difference in nerve conduction times from the branching point to the two endplates and the difference in neuromuscular transmission times at the two endplates were negligible. We further assumed that the IPI fluctuations were primarily due to fluctuations in the CV of fiber b, which would be true if the fibers had similar velocity characteristics and if lb were large compared with la. Therefore, we computed the normalized CV as follows
| (1) |
where IPIref is either the IPI when the MU first discharged after a period of rest, if that could be determined, or else the mean IPI.
In those cases in which the two spikes were recorded by different electrodes, the nonnormalized mean CV was also computed by dividing the distance between the electrodes by the mean IPI. Values that fell outside the range of 2–6 m/s were considered unreliable (either due to a long distance between the endplates or to the electrodes having been on opposites sides of the endplates) and were not tabulated.
We also measured CV in several doubly innervated fibers (Fig. 1B). Here fiber b was innervated by two different motoneurons at two widely separated endplates (b1 and b2). Each motoneuron also innervated other singly innervated fibers (a1 and a2). Potentials from the doubly innervated fiber were detected in the MUAPs of both MUs, although not every discharge of the MUAPs contained a b spike because of action potential collisions. For some fibers, it was not possible to detect an a spike for one of the MUs, and so IPIs could only be measured for the b spikes associated with the other MU.
The singly innervated a fibers and the doubly innervated b fiber had different discharge histories and so underwent different CV fluctuations. We assumed that the measured IPI fluctuations were due mostly to CV fluctuations in the doubly innervated fiber since it underwent larger fluctuations in discharge rate. We therefore estimated the normalized CV as follows:
| (2) |
where k is the MU to which the b spike was associated, IPIk is the mean IPI for that MU, and Tbk is the estimated latency of the bk spike with respect to the MUAP onset.
Model of CV.
Davis et al. (7) and Lööf (28) proposed models for the discharge history dependence of CV in which each discharge has a cumulative effect on CV that decays exponentially with time. The model of Lööf includes an additional parameter to accommodate the CV transient after rest. We augmented this model with two additional parameters to account for a linear trend and a saturation of the cumulative effect. The resulting model is given as follows:
| (3) |
where Îi is the estimated value of Ii, the IPI of the ith discharge, t1, …, tn are the discharge times, and x is a state variable that models the cumulative effect as follows:
| (4) |
where a = (s − b)/s and ti − ti−1 is the IDI of the ith discharge. This model has five parameters: s, the saturation level; b, the sensitivity to firing rate fluctuations; c, the rate of the linear trend; T, the time constant of the cumulative effect; and I0, the resting IPI at time t = 0.
The initial value of the state variable, x1, was set to 0 if the first discharge occurred >1 s after the start of recording or if the CV profile suggested that the fiber might have started from rest during the first second and this initial condition gave a good fit. Otherwise it was set to 1 − I1/I0 − ct1.
Our initial analysis suggested that it might not be possible to estimate the resting IPI value accurately for steadily firing MUs with relatively small excursions in firing rate. Therefore, we also considered a linearized version of the model that did not include the resting IPI. In this version, the state equation was given as follows:
| (5) |
with initial condition x1 = 1 − I1/I0 − ct1. In this model, I0 is given by
| (6) |
and does not have a specific physiological meaning, where I is the mean IPI, t̄ is the mean discharge time, and d̄ is the mean IDI. This model has three parameters: α and β, which take the place of a, b, and T in the full model, and c, which is equivalent to c in the full model.
For each model, the parameters were estimated by minimizing the root mean square (RMS) difference between the measured and predicted IPIs using the Matlab fminunc function. Discharges with IDIs shorter than 25 ms were excluded from the RMS calculation, as were discharges for which IPI measurements were not available.
Goodness of fit was measured both in terms of the RMS difference between I and Î (in μs) and in terms of the correlation coefficient between I and Î. If Î accounted perfectly for the history-dependent effects and there was no measurement error, then the RMS difference between I and Î should be due entirely to jitter at the neuromuscular junction. In single-fiber EMG studies, this jitter is usually measured in terms of the mean consecutive difference of IPIs (36), which, in our case, would be the mean value of |(Ii+1 − Îi+1) − (Ii − Îi)|. However, the mean consecutive difference is insensitive to slow, trendlike model errors. Therefore, we used the RMS difference instead.
Estimating the resting CV.
To test the ability to estimate the resting CV value, we chose six MUs for which it was possible to measure CV profiles during two different contractions: one in which the MU fired discontinuously, i.e., it was recruited during the recording epoch or it fired intermittently so that the CV attained its resting value for at least one discharge, and the other in which the MU fired continuously or nearly so (no firing gaps >0.7 s).
For each of these MUs, we fit both CV profiles jointly with the full model using the same values of a, b, and T for both profiles but separate values of I0, c, and x1 for each profile. We also fit each profile individually using the full model and using the linearized model. We compared the goodness of fit and the parameter values, comparing α and β of the linearized model with the corresponding steady-state values derived from the full model as follows:
| (7) |
where k = exp(−d̄/T) and d̄ is the mean IDI.
RESULTS
Accuracy of CV measurements.
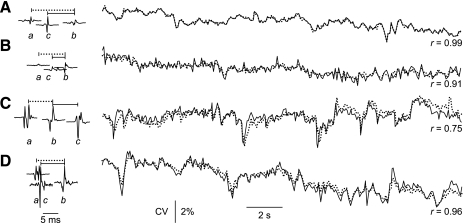
Mean CV values were measured for 281 MUs. The values ranged from 2.6 to 5.3 m/s (5th to 95th percentile; mean ± SD: 4.0 ± 0.7 m/s). Some MUAPs had more than two detectable spikes, making it possible to compare different CV measurements for the same MU. These measurements differed by as much as 15%, reflecting imprecision in the estimation of the interelectrode distances. After normalization, however, the CV profiles agreed closely. Some examples are shown in Fig. 2. In each case, CV profiles were measured from spikes a and b and from spikes c and b. Each profile was normalized to its mean value.
Fig. 2.
Accuracy of conduction velocity (CV) measurements. A–D: MUAP spikes and CV profiles for four MUs. For each MU, three different MUAP spikes were detected (a, b, c), and CV profiles were determined from the IPIs between spikes a and b (dotted lines) and between spikes c and b (solid lines). Each profile was normalized to its mean IPI value. The correlation coefficients (r) between the two profiles for each MU ranged from 0.75 to 0.99.
In general, the profiles matched quite well when the spikes had stable shapes and mean IPIs longer than ∼8 ms (e.g., Fig. 2A, r = 0.99). In this case, the IPI fluctuations can be inferred to have been due almost entirely to CV fluctuations in fiber b. Less perfect, but still quite good matches were seen with shorter mean IPIs (Fig. 2B, r = 0.91) and for spikes with variable shapes due to jitter between overlapping single-fiber potentials (Fig. 2D, r = 0.96). The overall good agreement in these and other examples gave us confidence that the measured profiles accurately reflected true CV behavior.
In Fig. 2C, the c spike came after the b spike, and so the two profiles reflected the behavior of two different fibers rather than the same fiber. The profiles still showed considerable agreement (r = 0.75), suggesting that, although the characteristics of the fibers were not identical, they were still very similar.
CV behavior.
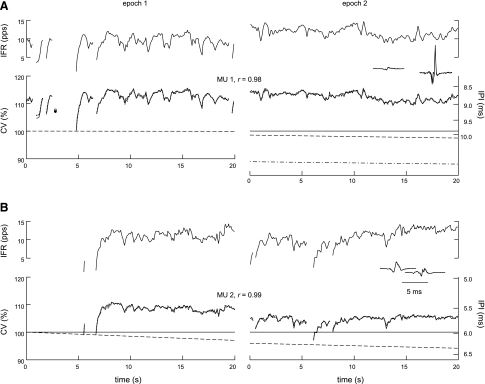
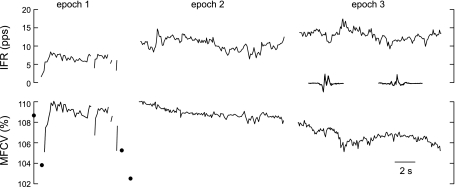
The main features of CV behavior during voluntary contraction can be seen in Fig. 3. Each panel shows CV profiles of a single MU during epochs from two different contractions. The profiles in each epoch were normalized to the estimated resting CV value at the start of the first epoch.
Fig. 3.
CV behavior in two MUs. A and B: each panel shows the IPIs (solid lines in bottom graphs, referred to the nonlinear axes on the right) and instantaneous firing rates (IFRs; top graphs) of a single MU during epochs in two different contractions. Interdischarge intervals (IDIs) longer than 250 ms are shown as gaps, and single discharges separated by 250 ms or more from both the previous and subsequent discharges are shown as solid circles. The IPIs were converted to CV values (axes on the left) by normalizing by the estimated resting IPI value at the start of the first epoch. The predicted CV profiles from the joint model (dotted lines, open circles) overlapped the actual profiles almost perfectly, with correlation coefficients (r) across both epochs ≥0.98. The estimated resting CV value changed linearly during each epoch (dashed lines). In epoch 2 of A, the resting CV value estimated by analyzing the profile individually (dash-dot line) did not match the value estimated by analyzing both profiles jointly. The MUAP spikes are shown as insets. pps, Pulses/s.
The most dramatic feature was the CV transient that occurred when a MU began to fire after a period of inactivity. This can be seen in the first epochs in Fig. 3. Both MUs had gaps of at least 1 s in their firing activity, either at the beginning or during the epoch. The gaps were long enough for the CV to recover to the resting value. Then when the MUs began firing again, the CV increased over the first second or so to steady-state values of ∼113 and 108%, respectively.
The second feature was the close correlation between CV and IFR (Fig. 3, shown in the top trace in each panel). During steady firing, CV fluctuated by about ±1 or ±2% around the steady-state value in a way that very precisely matched the fluctuations in the smoothed IFR. Whenever there was an IDI longer than ∼0.25 s (indicated by discontinuities in the traces in Fig. 3), CV decreased appreciably toward the resting value.
The third feature was a linearly decreasing trend, typically resulting in a change of −0.5 to −2% in the steady-state CV value over 20 s. The trend can be seen in Fig. 3 as a slight droop in the CV profiles compared with the IFR profiles. The slopes of the trends are indicated by the slopes of the estimated resting CV values (dashed lines).
Model of CV behavior.
The model was able to match all three main aspects of the CV behavior with a high degree of accuracy. The reconstructed CV profiles for the MUs in Fig. 3 (dotted lines) overlap the measured profiles so closely that they can barely be distinguished (r ≥ 0.98).
The reconstructed profiles for both epochs of each MU in Fig. 3 were modeled jointly using different values of the initial resting CV and trend slope for each epoch, but the same values of the other parameters. The parameter values estimated by fitting the profiles individually were essentially identical to those estimated by fitting the profiles jointly for the discontinuous (Fig. 3, A and B, epoch 1) and nearly continuous (B, epoch 2) profiles, but not for the continuous profile (A, epoch 2, dash-dot line). A sensitivity analysis showed that a wide range of parameter values could give almost the same fit for this profile. This was also true for other continuous profiles.
The linearized version of the model was not able to fit the discontinuous or nearly continuous profiles, but it was able to fit the continuous profiles as well as the joint model did, and it provided essentially the same values of α and β as the values derived from the joint model using Eq. 7.
We concluded that continuous CV profiles do not contain enough information to allow reliable estimation of the resting CV. Therefore, for the remainder of the analysis, we used the full model to analyze the discontinuous profiles and the linearized model to analyze the continuous profiles.
CV parameters in discontinuous profiles.
From the 14 extensively studied subjects, we analyzed a total of 64 CV profiles in which the MU was either recruited or had an IDI longer than 0.7 s during the recording epoch. The behavior was similar to that seen in the left-hand plots in Fig. 3. CV increased rapidly during the first 10 or so discharges until reaching a steady-state value that was, on average, 9.1 ± 3.6% (mean ± SD) higher than the resting value. After reaching the steady-state value, the CV fluctuated by ±1 or ±2%, usually exhibited a downward trend, and dropped appreciably back toward the resting value for IDIs longer than 0.25 s. All of the profiles were well fit by the full model (RMS residual: 22.9 ± 7.5 μs; r: 0.98 ± 0.02). The estimated parameter values are summarized in Table 1.
Table 1.
Summary of parameters from discontinuous profiles
| s, % | b, % | c, % | T, ms | RMS, μs | r |
|---|---|---|---|---|---|
| 24.3 ± 14.4 | 3.6 ± 0.9 | −0.06 ± 0.06 | 0.53 ± 0.23 | 22.9 ± 7.5 | 0.98 ± 0.02 |
| 9.9:51.7 | 2.5:5.0 | −0.2:+0.4 | 0.30:0.95 | 13.9:39.6 | 0.94:1.00 |
Values are means ± SD or ratio of 5th percentile to 95th percentile (N = 64). s, Saturation level; b, sensitivity to firing rate fluctuations; c, rate of the linear trend; T, time constant of the cumulative effect; RMS, root mean square.
CV parameters in continuous profiles.
From the 14 extensively studied subjects, we analyzed a total of 271 continuous CV profiles. The mean firing rate was 12.2 ± 2.3 pulses/s (pps). All of the profiles were well fit by the linearized model (RMS residual: 20.1 ± 8.0 μs; r: 0.95 ± 0.06). Two subjects stood out from the others in that most of their β values were negative, whereas almost all of the β values of the other 12 subjects were positive. In other words, the CV fluctuations in these two subjects were inversely, rather than positively, correlated with IFR. The parameter values from these two subjects were summarized separately from those of the others (Table 2).
Table 2.
Summary of parameters from continuous profiles
| Group No. | N | α | β, % | c, % | RMS, μs | r |
|---|---|---|---|---|---|---|
| 1 | 228 | 0.70 ± 0.08 | 19.2 ± 8.3 | −0.06 ± 0.04 | 19.7 ± 7.3 | 0.95 ± 0.05 |
| 0.57:0.79 | 7.3:34.4 | −0.14:−0.01 | 10.5:34.3 | 0.84:0.99 | ||
| 2 | 43 | 0.80 ± 0.22 | −8.2 ± 7.7 | −0.10 ± 0.04 | 22.2 ± 10.8 | 0.93 ± 0.07 |
| 0.36:0.94 | −22.1:3.7 | −0.18:−0.03 | 11.5:43.9 | 0.82:0.99 |
Values are means ± SD or ratio of 5th percentile to 95th percentile. α and β, Parameters.
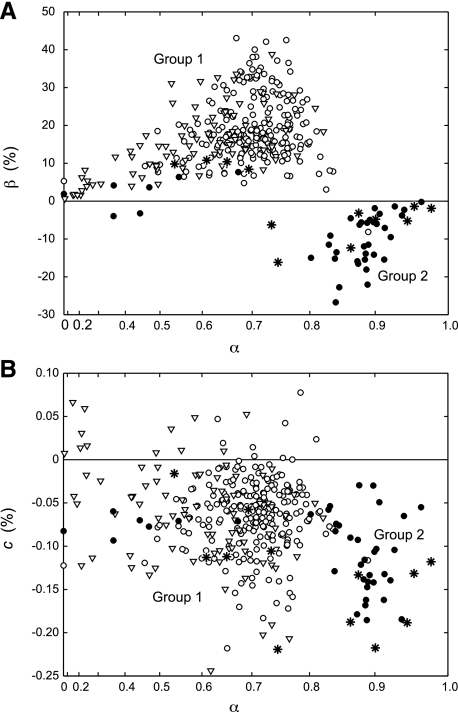
The individual parameter values for the two groups of subjects are plotted in Fig. 4. It can be seen that most of the profiles of the two outlier subjects (solid circles, group 2) also had larger values of α (Fig. 4A) and tended to have somewhat more negative values of c (Fig. 4B) compared with the other subjects (open circles, group 1).
Fig. 4.
Estimated model parameter values (α, β, c). A: α and β. B: c and α. Each symbol represents one CV profile (open circles: continuous profiles of group 1 subjects; solid circles: continuous profiles of group 2 subjects; inverted triangles: discontinuous profiles of all subjects; asterisks: continuous profiles of the subject tested at cold temperature). Note that the x-axis has been warped to distribute the points more evenly.
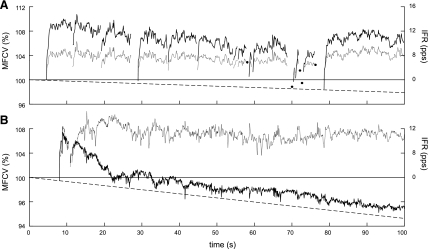
Although the CV profiles of the group 2 subjects were inversely correlated with IFR during continuous firing, they were positively correlated with IFR near recruitment. For example, the CV of the MU in Fig. 5 exhibited positive correlation during the first shown epoch when it discharged intermittently, then little correlation in the second epoch, and finally inverse correlation in the third epoch.
Fig. 5.
CV and IFR behavior of a MU from a group 2 subject during epochs from three different contractions. The CV profile was positively correlated with IFR in epoch 1, poorly correlated in epoch 2, and inversely correlated in epoch 3. MFCV, muscle fiber CV. Solid circles indicate isolated discharges as in Fig. 3.
We also analyzed 12 continuous CV profiles recorded at cold temperature. These were from a group 1 subject who had positive values of β at room temperature. Eight of the profiles at cold temperature had negative values of β, and six of them fell squarely within the group 2 cluster (Fig. 4, asterisks).
Long epochs.
We measured CV profiles for 16 continuously firing MUs during 100-s epochs. The linearized model was able to fit each of these profiles very well over the entire 100 s. In particular, the trends continued in a very nearly linear fashion for the entire 100 s. Three of these profiles were from a group 2 subject and exhibited an inverted CV/IFR correlation for the entire 100 s.
We also measured CV profiles for seven MUs that were recruited during 100-s-long epochs. Five of these profiles were from group 1 subjects. In these profiles the CV increased substantially after recruitment and remained supernormal throughout the rest of the epoch. An example is shown in Fig. 6A. It was possible to fit the entire CV profiles very well (r > 0.97) using the full model.
Fig. 6.
CV profiles during long epochs. Each panel shows the CV profile (solid line), IFR (dotted line), and estimated resting CV (dashed line) of a MU recruited during a long contraction. A: group 1 subject. B: group 2 subject. Solid circles indicate isolated discharges as in Fig. 3.
The other two discontinuous profiles were from a group 2 subject. In these profiles, the CV increased after recruitment, but then decreased again within 10–20 s back toward a steady-state value that was only ∼1 or 2% above the resting value. An example is shown in Fig. 6B. During the decrease, there was not a clear IFR dependence, but, after reaching steady state, the CV was inversely correlated with IFR. It was not possible to fit either of these profiles in its entirety using a single set of model parameters.
Doublets.
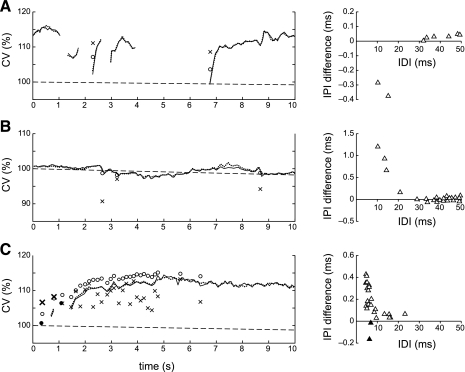
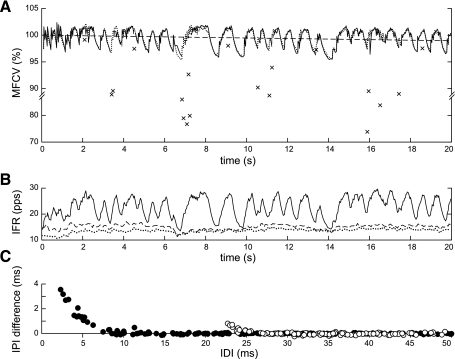
Of the total of 95,029 discharges analyzed, 58 (0.06%) followed IDIs that were shorter than 25 ms. We will refer to these discharges as doublets. An additional 31 doublets were observed in one MUAP train in the subject with spinal cord injury. The CVs of the doublets generally stood out from the rest of the CV profile and were not well estimated by the model (Fig. 7). When a doublet occurred during recruitment, its CV was greater than the value estimated by the model (Fig. 7A), and when it occurred during steady-state firing, its CV was smaller (Fig. 7B). Both behaviors can be seen in the CV profile from the subject with spinal cord injury (Fig. 7C): the first two doublets occurred during recruitment and had higher than predicted CV (boldface x's), while the later ones had lower than predicted CV (normal x's). The size of the difference between the measured and predicted values depended on the IDI (Fig. 7, right plots).
Fig. 7.
CV in doublets. In each panel, the left graph shows the measured (solid line) and predicted (dotted line) CV profiles, with the measured and predicted CV values of the doublets indicated by x's and o's, respectively. The right graph plots the difference between the measured and predicted IPI values as a function of IDI. A: when doublets occurred during recruitment, CV was higher (IPIs were lower) than predicted by the model. B: when doublets occurred during steady firing, CV was lower (IPIs were higher) than predicted. C: CV profile with multiple doublets from a subject with spinal cord injury. The first two doublets occurred during recruitment and had higher than predicted CV (boldface x's, solid triangles). The others had lower than predicted CV (normal x's, open triangles).
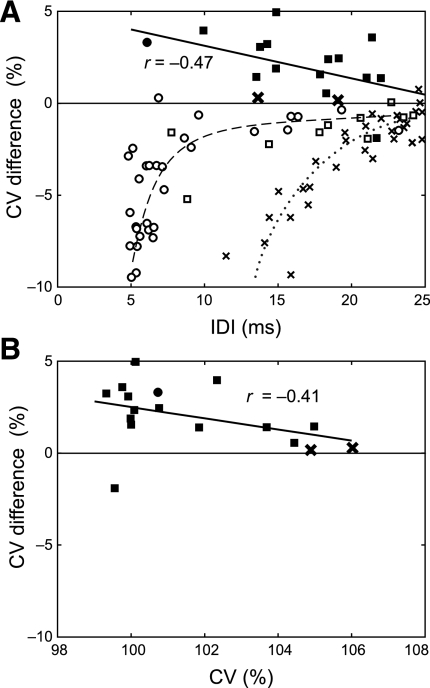
Overall, all but one of the doublets that occurred during recruitment had higher than predicted CV values, with the discrepancy tending to be larger for smaller IDIs (Fig. 8A, solid symbols) and when the preceding discharge was closer to the resting CV (Fig. 8B). The doublets that occurred during steady-state firing almost always had lower CV than predicted by the model (Fig. 8A, open symbols). For the group 1 subjects (squares) and the subject with spinal cord injury (circles), the difference was largest for IDIs <10 ms (Fig. 8A, dashed line). For the group 2 subjects (x's), the difference was appreciable until ∼20 ms (Fig. 8A, dotted line).
Fig. 8.
CV in doublets. A: difference between measured and predicted CV values of the doublets vs. IDI (squares: group 1 subjects, x's: group 2 subjects, circles: subject with spinal cord injury; solid symbols: doublet occurred during recruitment, open symbols: doublet occurred during steady-state firing). Regression curves are shown for the combined recruitment doublets (solid line), the combined group 1 and spinal cord injury steady-state doublets (dashed line, drawn by hand), and the group 2 steady-state doublets (dotted line, drawn by hand). B: difference between the measured and predicted IPI values of the doublets during recruitment vs. the CV of the preceding discharge.
Doubly innervated fibers.
We analyzed 20 doubly innervated fibers. The CV profile of one of them is shown in Fig. 9A (solid line). The IFR of this fiber (Fig. 9B, solid line) oscillated between ∼14 pps, which was the mean of the IFRs of the two coinnervating motoneurons (Fig. 9B, dashed and dotted lines), and ∼28 pps, which was their sum. This was because, whenever motoneuron 1 fired <20 ms before motoneuron 2, the action potentials in the doubly innervated fiber collided, resulting in only a single discharge of the fiber, whereas otherwise each firing of each motoneuron caused the fiber to discharge. The oscillations arose due to an interaction between the firing patterns of the two motoneurons. When they fired at very nearly the same rate (e.g., between 7 and 10 s), they tended to stay in or out of phase for a longer period, resulting in slower oscillations, and when they fired at different rates (e.g., between 10 and 12 s), they moved into and out of phase more rapidly. The CV oscillated between ∼97 and 102% of the steady-state value in a way that mirrored the IFR very closely. The model was able to fit the profile fairly well (Fig. 9A, dotted line, r = 0.92).
Fig. 9.
CV profile of a doubly innervated fiber. A: measured (solid line) and predicted (dotted line) CV profile and estimated resting CV (dashed line), all normalized to the mean value. The measured profile contains gaps where CV could not be measured because of action potential collisions. The measured CV values for discharges following short IDIs are indicated by x's. Note that the vertical axis has a change in scale. B: IFRs of the doubly innervated fiber (solid line) and the two coinnervating MNs (dashed and dotted lines). C: difference between the measured and estimated IPI values vs. IDI. The open and solid symbols correspond to discharges initiated at the different endplates. The IDI values were adjusted to compensate for the delay. For example, for the leftmost data point, the measured IDI was 5.9 ms and the estimated delay was 3.5 ms. Therefore, the point was plotted at an IDI of 2.4 ms.
Because the coinnervating motoneurons fired asynchronously, the discharge patterns of the doubly innervated fibers had many short IDIs. The CVs of the discharges that followed these short IDIs stood out below the main CV profile (x's in Fig. 9A). The behavior depended on which motoneuron fired first. When an action potential initiated at the endplate nearest the electrode (b2 in Fig. 1B) followed an action potential from the other endplate (Fig. 9C, solid circles), it exhibited a refractoriness very similar to that exhibited by doublets during continuous firing. Specifically, such potentials were blocked altogether for IPIs less than the absolute refractory period (2.4 ms in Fig. 9C), and they were delayed for IPIs up to ∼10 ms. Action potentials initiated at the farther electrode (Fig. 9C, open circles) were blocked over a much longer IPI because of collisions, and the relative refractory period was shorter and smaller because the potentials were propagating in the opposite direction of the preceding potential rather than following in its wake.
DISCUSSION
We observed four main types of CV behavior during voluntary contractions.
Trend.
The large majority of CV profiles exhibited a steady decrease in CV that was not related to the immediate discharge history. CV decreased in steadily firing fibers, even when the mean firing rate remained stable, and it was consistently lower for intermittently firing fibers each time they began to fire again. This meant that the “resting” value of CV was continuously changing, even during intervals in which the fiber was not active. The average rate of change was about −0.05%/s.
A similar decreasing trend in CV was observed by Stålberg (35) in both stimulated and voluntarily activated muscle fibers. Similar to our results, he found that CV decreased at a rate of about −0.07 to −0.05%/s in the biceps brachii and extensor digitorum communis muscles during the first 3 min of activity. The decrease continued at a slower rate for tens of minutes. A decreasing CV trend has also been reported in other muscles (10, 17, 32).
History dependence (velocity recovery).
CV fluctuated from discharge to discharge in a way that was directly related to the immediate discharge history of the fiber. In 12 of the 14 extensively studied subjects (group 1), CV increased during recruitment to a steady-state value ∼10% above the resting value and then fluctuated around that value in a way that was positively correlated with IFR. CV dropped appreciably for firing gaps longer than ∼0.25 s, and it dropped completely back to the resting value for firing gaps longer than ∼1 s. Previous studies have also reported an increase in CV at the onset of stimulation (35), a transient increase following the extra discharge elicited by an F-response during a train of stimuli (3), and a positive correlation between CV and IFR under normal conditions (10, 11, 32, 35).
Both the transient changes and the fluctuations during steady state closely mirrored the smoothed IFR. The state variable in Lööf's model (28), is, in fact, precisely proportional to the smoothed IFR. (It equals the convolution of the discharge impulse train by the impulse response of a first-order causal filter). For the IFR plots in this paper, we used a fixed filter cutoff frequency of 0.75 Hz, which corresponds to a time constant in Lööf's model of 0.21 s.
In the other two subjects (group 2), the CV behavior was positively correlated with IFR during recruitment, but inversely correlated during steady-state firing. This is seen in Fig. 6B, in which the CV increased during recruitment but then dropped back toward the resting value and became inversely correlated to IFR. During the period of inverse correlation, the CV did not respond as rapidly to changes in IFR and tended to have a steeper trend than in the group 1 subjects. Many of the CV profiles in the cooled muscle also exhibited an inverse CV/IFR correlation. Inverted CV/IFR behavior has been reported previously during ischemia (35) and after cooling (32). However, as far as we know, this behavior has not been reported before under normal conditions at room temperature. Nor has it been reported that these same MUs exhibit positive CV/IFR correlation during recruitment.
Refractoriness.
During steady firing, discharges that occurred after an IDI of <10 ms generally had CVs that were much lower than the steady-state value. This behavior was seen both in doublets (Fig. 7, B and C; Fig. 8A, open symbols) and in doubly innervated fibers (Fig. 9A). For the group 2 subjects, the refractoriness extended to ∼20 ms (Fig. 8A, dotted line).
The relationship between IDI and refractoriness was seen most clearly in the doubly innervated fibers (Fig. 9C). The asynchronous firing of the coinnervating motoneurons generated a wide range of IDIs, essentially accomplishing naturally the sweep of interstimulus intervals investigated in double-pulse stimulation protocols. In Fig. 9C, for example, the fiber can be seen to have had an absolute refractory period of 2.4 ms and a relative refractory period of ∼10 ms. For IDIs >10 ms, the CV of this fiber depended on discharge history as predicted by the model.
We only detected two doublets with IDIs <10 ms during recruitment, and both exhibited supernormality rather than refractoriness. We expect we would have seen instances of refractoriness during recruitment, if we had observed more short initial doublets.
Early supernormality.
Doublets that occurred during the first few discharges after recruitment had higher CVs than predicted by the model (Figs. 7A and 8A, solid symbols). This effect dropped off as the fiber continued to discharge. In Fig. 7C, for example, the doublets that occurred close to recruitment had CVs that stood out above the CV profile, whereas those that occurred later in the contraction stood out below the profile.
Relation to the velocity recovery function.
The term “velocity recovery” is often used to refer to the history dependence of CV in general. However, the term “velocity recovery function” refers specifically to the relative CV of a test action potential as a function of the interval between it and a single conditioning action potential (35). In terms of a voluntary contraction, the velocity recovery function characterizes the relative CVs of the first two discharges of a MU recruited from rest. However, it does not provide information about the CVs of the subsequent discharges.
The velocity recovery function has three main regions (31). First is the subnormal region, which occurs for IDIs below ∼10 ms. In this region, the CV of the test potential is less than that of the conditioning potential. During voluntary contractions, IDIs this short are only seen in doublets and in doubly innervated fibers. We consistently observed reduced CV after short IDIs in these situations. This shows that fibers are affected by refractoriness during continuous firing as well as at rest.
The second region of the velocity recovery function is the early supernormal region, which occurs for IDIs between ∼10 and 50 ms. In this region, the CV of the test potential is greater than that of the conditioning potential. The doublets that we observed during recruitment exhibited this behavior. Specifically, their CV was not only greater than the resting CV, but also greater than the value predicted by the model. However, during continuous firing, no discharges had CVs appreciably higher than predicted by the model. Thus fibers exhibited early supernormality only near rest, but not during continuous firing.
The third region of the velocity recovery function is the second supernormal region, which occurs for IDIs between ∼50 ms and 1 s. In this region, the CV of the test potential is greater than that of the conditioning potential. Most of the discharges in our study fell into this range. However, the CVs of these discharges depended on the discharge history going back ∼1 s and could not be predicted from the IDI alone. The single preceding IDI was only able to explain on average 40% and at most 70% of the variance in the continuous CV profiles, whereas the model, which used information from the entire discharge history, was able to explain on average 77% and at best 99% of the variance.
These considerations show that the velocity recovery function does not provide a full characterization of CV behavior during voluntary contractions. A more complete characterization requires the use of more than one conditioning potential (22, 23, 40). For example, Z'Graggen and Bostock (40) also found a cumulative effect in the second, but not the early, supernormal region, as in our data. [Note that their results are expressed with respect to the resting CV, whereas ours (e.g., Fig. 8) are expressed with respect to the values expected by the model.]
The results of our study suggest that it might be worthwhile for stimulation protocols to employ even longer trains of conditioning stimuli than are now being used. In particular, it might be necessary to employ trains several seconds long (or perhaps shorter trains at a higher rate) to observe the transition between positive and inverted CV/IFR behavior. In such longer trains, it will probably also be necessary to take the trend into account.
Model of CV behavior.
For MUAP trains longer than one or two discharges, it is not convenient to characterize CV behavior as an explicit function of all possible combinations of IDIs as in the velocity recovery function. Davis et al. (7) and Lööf (28) proposed compact mathematical models based on empirical observations. The models were intended to predict the effect of discharge history-dependent CV fluctuations in clinical single-fiber jitter studies. However, we found that they also serve to characterize CV behavior quite accurately.
We augmented Lööf's model in three ways. First, we included a term to model the trend. Based on observations such as Fig. 6A, which showed that the CV of an intermittently firing fiber was lower each time it began to fire, we modeled the trend as a linear time-dependent change in the resting CV value that was independent (at least over the short term) of the discharge history.
Second, we added the parameter s to model a saturation of the cumulative effect. In Lööf's model, s = ∞ (i.e., a = 1), so that the CV could theoretically increase without bound if the firing rate became high enough. The saturation parameter prevents this from happening by decreasing the increment as the state variable approaches the saturation level. Including this parameter resulted in appreciably better fits for many of the discontinuous CV profiles.
Third, we excluded CV values associated with IDIs < 25 ms from consideration in the parameter estimation procedure. The IDIs themselves were still included in Eqs. 4 and 5 (i.e., they still contributed to the cumulative effect), but the differences between the measured and predicted CV values were not included in the parameter optimization. For these short IDIs, CV is affected by refractoriness and early supernormality, which were not included in the model.
The model was able to fit both the transient and the steady-state behavior of the discontinuous CV profiles of the group 1 subjects with a very high degree of accuracy. The RMS prediction error (excluding short IDIs) was 22.9 ± 7.5 μs. This is in the range of the neuromuscular jitter measured in the extensor digitorum communis muscle (36). Neuromuscular jitter is unavoidable when measuring latency differences between potentials from two different fibers. Therefore, the model was accurate to within the physiological uncertainty. The model was also able to fit the CV profiles of the doubly innervated fibers (Fig. 9), which had much more complex discharge histories than normal singly innervated fibers.
In terms of the model parameters, when a resting fiber begins to discharge at rate f, its CV increases to a steady-state supernormality of bk/(1 − ak), with a time constant of −1/f ln ak, where k = exp (−1/fT). In steady state, the CV changes by about β/f2 (1 − α) per pps change in f with approximately the same time constant, where α and β are given by Eq. 7. The CV changes by c per second due to the trend. When the fiber stops discharging, the velocity recovers to 5% of the steady-state value in 3T seconds. For example, for f = 12 pps, and using the mean parameter values from Table 1, these quantities are given as follows: steady-state supernormality: 11.3%; time constant: 0.26 s; fluctuation sensitivity: 0.54%/pps; trend: −0.06%/s; recovery time: 1.6 s.
We found that all five model parameters could be estimated unambiguously for CV profiles that included both transient and continuous firing. However, for CV profiles that included only continuous firing, there was usually a fairly wide range of values of T and the other parameters that gave essentially the same fit. This was true even if a was constrained to equal 1 as in Lööf's original model (28). Therefore, the recovery time constant and steady-state supernormality could not be estimated reliably for CV profiles that did not contain transients. The steady-state CV behavior of these profiles was characterized by the α, β, and c parameters, which were estimated using the linearized model.
Physiological mechanisms.
Supernormality in nerve fibers has been attributed to afterdepolarization (16). One proposed explanation for the history-dependent supernormality of muscle-fiber CV is that it is due to afterdepolarization brought about by the accumulation of potassium in the t-tubule system (40). Potassium is injected into the t-tubule system during each action potential and then slowly passes back into the fiber through the inward rectifier channel or diffuses out into the extracellular space (6, 24, 34). When a fiber begins to discharge repetitively, the potassium concentration in the t-tubule systems increases to an equilibrium level at which the amount injected by each discharge balances the amount cleared between discharges. The concentration then fluctuates around this equilibrium level in response to fluctuations of the discharge rate. The increased potassium concentration in the t-tubule system depolarizes the sarcolemma membrane, bringing it closer to threshold and thus increasing CV.
Equation 4 can be considered to be a model of this process, with x representing the excess potassium concentration in the t-tubule system (or, equivalently, the depolarization of the sarcolemma membrane above the resting level), b (s − x)/s the additional potassium injected by each action potential, and T the diffusion time constant. The ability of this model to explain 99% of the observed variance in many CV profiles lends credence to the potassium accumulation explanation.
The inverted CV/IFR relationship observed in the group 2 subjects and in the cooled muscle could be due to sodium channel inactivation. If the sarcolemma becomes too depolarized, some sodium channels fail to deinactivate after an action potential, resulting in decreased CV (19). The transition from positive to inverted CV/IFR correlation (Figs. 5 and 6B) may reflect an increasing depolarization, driven by accumulated potassium in the t-tubule system, from a region in which the effect of threshold proximity is stronger than the effect of sodium-channel inactivation, to a region in which the opposite is true. Cold is known to depolarize the muscle fiber (4), and certain metabolic conditions that affect the extracellular potassium concentration can affect the depolarization level as well (41). Thus the inverted CV/IFR relationship in the group 2 subjects might have been due to their metabolic state at the time of the experiment.
An alternative explanation for the supernormality of muscle-fiber CV is that it is due to afterhyperpolarization brought about by an additional slow potassium conductance in the muscle fiber membrane (12). This explanation is predicated on the assumption that increased CV requires hyperpolarization to reduce sodium channel inactivation. However, recent measurements suggest that sodium channel deinactivation takes place at more depolarized levels than previously thought (14). These findings imply that sodium channel inactivation may not affect CV at normal membrane potential levels. This would seem to favor the explanation that the supernormality is due to the accumulation of potassium in the t-tubule system.
It has been suggested that the CV trend could be due to sarcolemmal hyperpolarization or increased membrane leakage resistance brought about by the accumulation of extracellular metabolites (20, 21, 25). This would imply that the slope of the trend should be correlated with the overall level of muscle activity (35). We did not find any clear relationship between the slope and the number of active MUs in the signal (r = −0.14), however. The trend could also be due to membrane processes, such as ion pumps or cotransporters, that are regulated by long-term intra- or extracellular conditions and operate independently of the immediate discharge history (18). A full explanation of the trend will have to explain why it is not inverted in fibers that exhibit an inverted CV/IFR relationship.
The refractoriness observed after very short IDIs is probably a reflection of sodium-channel deinactivation kinetics (19). The early supernormality observed in doublets at recruitment is thought to be a capacitive effect (13, 40).
One of the difficulties in trying to explain observed CV behavior in human muscles is that the physical parameters of human ion channels are not well known. Many of the parameter values used in membrane simulations (1, 2, 6, 39) have been based on values measured in other species, at different temperatures, and in excised conditions. The fact that those models fail to predict empirical CV behavior suggests that some of these values may not be accurate. Observations of CV behavior, both during voluntary contractions and in response to electrical stimulation, may offer a sensitive way to verify the accuracy of human ion channel parameters and to understand their implications to muscle function in health and disease.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke (R01-AR049894) and the Rehabilitation Research and Development Service of the US Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adrian RH, Peachey LD. Reconstruction of the action potential of frog sartorius muscle. J Physiol 235: 103–131, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adrian RH, Marshall MW. Action potentials reconstructed in normal and myotonic muscle fibres. J Physiol 258: 125–43, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blijham PJ, van Dijk JG, Stålberg E, Zwarts MJ. Recognising F-response interference as a source of increased jitter in stimulated single fibre EMG. Clin Neurophysiol 117: 388–391, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Brodie C, Sampson SR. Contribution of electrogenic sodium-potassium ATPase to resting membrane potential of cultured rat skeletal myotubes. Brain Res 347: 28–35, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Buchthal F, Guld C, Rosenfalck P. Propagation velocity in electrically activated muscle fibres in man. Acta Physiol Scand 34: 75–89, 1955 [DOI] [PubMed] [Google Scholar]
- 6. Cannon SC, Brown RH, Jr, Corey DP. Theoretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels. Biophys J 65: 270–288, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis GR, Ingram DA, Fincham WF, Swash M, Schwartz MS. Jitter correction: a computer algorithm for reduction of the velocity recovery function artifact. Muscle Nerve 11: 534–539, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Ekstedt J. Human single muscle fiber action potentials. Acta Physiol Scand Suppl 226: 1–96, 1964 [PubMed] [Google Scholar]
- 9. Farina D, Muhammad W, Fortunato E, Meste O, Merletti R, Rix H. Estimation of single motor unit conduction velocity from surface electromyogram signals detected with linear electrode arrays. Med Biol Eng Comput 39: 225–236, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Farina D, Gazzoni M, Camelia F. Low-threshold motor unit membrane properties vary with contraction intensity during sustained activation with surface EMG visual feedback. J Appl Physiol 96: 1505–1515, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Farina D, Falla D. Effect of muscle-fiber velocity recovery function on motor unit action potential properties in voluntary contractions. Muscle Nerve 37: 650–658, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Fortune E, Lowery MM. Simulation of the interaction between muscle fiber conduction velocity and instantaneous firing rate. Ann Biomed Eng 39: 96–109, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Frank GB. Negative after-potential of frog's skeletal muscle. J Neurophysiol 20: 602–614, 1957 [DOI] [PubMed] [Google Scholar]
- 14. Fu Y, Struyk A, Markin V, Cannon S. Gating behaviour of sodium currents in adult mouse muscle recorded with an improved two-electrode voltage clamp. J Physiol 589: 525–546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gazzoni M, Camelia F, Farina D. Conduction velocity of quiescent muscle fibers decreases during sustained contraction. J Neurophysiol 94: 387–394, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Gilliatt RW, Willison RG. The refractory and supernormal periods of the human median nerve. J Neurol Neurosurg Psychiatry 26: 136–147, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hedayatpour N, Arendt-Nielsen L, Farina D. Motor unit conduction velocity during sustained contraction of the vastus medialis muscle. Exp Brain Res 180: 509–516, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hicks A, McComas AJ. Increased sodium pump activity following repetitive stimulation of rat soleus muscles. J Physiol 414: 337–349, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001 [Google Scholar]
- 20. Jones DA. Muscle fatigue due to changes beyond the neuromuscular junction. Ciba Found Symp 82: 178–196, 1981 [DOI] [PubMed] [Google Scholar]
- 21. Juel C. Muscle action potential propagation velocity changes during activity. Muscle Nerve 11: 714–719, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Kamavuako EN, Hennings K, Farina D. Velocity recovery function of the compound muscle action potential assessed with doublet and triplet stimulation. Muscle Nerve 36: 190–196, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kamavuako EN, Farina D. Time-dependent effects of pre-conditioning activation on muscle fiber conduction velocity and twitch torque. Muscle Nerve 42: 547–555, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Kirsch GE, Nichols RA, Nakajima S. Delayed rectification in the transverse tubules: origin of the late after-potential in frog skeletal muscle. J Gen Physiol 70: 1–21, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kössler F, Lange F, Caffier G, Küchler G. External potassium and action potential propagation in rat fast and slow twitch muscles. Gen Physiol Biophys 10: 485–498, 1991 [PubMed] [Google Scholar]
- 26. Lateva ZC, McGill KC. Estimating motor-unit architectural properties by analyzing motor-unit action potential morphology. Clin Neurophysiol 112: 127–135, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Lateva ZC, McGill KC, Johanson ME. Electrophysiological evidence of adult human skeletal muscle fibres with multiple endplates and polyneuronal innervation. J Physiol 544: 549–565, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lööf Y. Improving electromyographic jitter measurements by analysis of the firing pattern. IEEE Trans Biomed Eng 37: 1105–1114, 1990 [DOI] [PubMed] [Google Scholar]
- 29. McGill KC, Dorfman LJ. High-resolution alignment of sampled waveforms. IEEE Trans Biomed Eng 31: 462–468, 1984 [DOI] [PubMed] [Google Scholar]
- 30. McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods 149: 121–133, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Mihelin M, Trontelj JV, Stålberg E. Muscle fiber recovery functions studied with double pulse stimulation. Muscle Nerve 14: 739–747, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Morimoto S, Masuda M. Dependence of conduction velocity on spike interval during voluntary muscular contraction in human motor units. Eur J Appl Physiol Occup Physiol 53: 191–195, 1984 [DOI] [PubMed] [Google Scholar]
- 33. Nishizono H, Kurata H, Miyashita M. Muscle fiber conduction velocity related to stimulation rate. Electroencephalogr Clin Neurophysiol 72: 529–534, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Peachey LD. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol 25: 209–231, 1965 [DOI] [PubMed] [Google Scholar]
- 35. Stålberg E. Propagation velocity in human muscle fibers in situ. Acta Physiol Scand Suppl 287: 1–112, 1966 [PubMed] [Google Scholar]
- 36. Stålberg E, Ekstedt J, Broman A. The electromyographic jitter in normal human muscles. Electroencephalogr Clin Neurophysiol 31: 429–438, 1971 [DOI] [PubMed] [Google Scholar]
- 37. Troni W, Cantello R, Rainero I. Conduction velocity along human muscle fibers in situ. Neurology 33: 1453–1459, 1983 [DOI] [PubMed] [Google Scholar]
- 38. Trontelj JV, Stålberg E, Mihelin M. Jitter in the muscle fibre. J Neurol Neurosurg Psychiatry 53: 49–54, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wallinga W, Meijer SL, Alberink MJ, Vliek M, Wienk ED, Ypey DL. Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys J 28: 317–329, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Z'graggen WJ, Bostock H. Velocity recovery cycles of human muscle action potentials and their sensitivity to ischemia. Muscle Nerve 39: 616–626, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Z'Graggen WJ, Aregger F, Farese S, Humm AM, Baumann C, Uehlinger DE, Bostock H. Velocity recovery cycles of human muscle action potentials in chronic renal failure. Clin Neurophysiol 121: 874–881, 2010 [DOI] [PubMed] [Google Scholar]